Abstract
Translation initiation of the full-length mRNA of the human immunodeficiency virus can occur via several different mechanisms to maintain production of viral structural proteins throughout the replication cycle. HIV-1 viral protein synthesis can occur by the use of both a cap-dependant and IRES-driven mechanism depending on the physiological conditions of the cell and the status of the ongoing infection. For both of these mechanisms there is a need for several viral and cellular co-factors for optimal translation of the viral mRNA. In this review we will describe the mechanism used by the full-length mRNA to initiate translation highlighting the role of co-factors within this process. A particular emphasis will be given to the role of the DDX3 RNA helicase in HIV-1 mRNA translation initiation.
Introduction
The human immunodeficiency virus type-1 (HIV-1) is the prototype member of the lentivirus genus of the Retroviridae and the etiologic agent of acquired immunodeficiency syndrome (AIDS). Retroviruses are a unique family of RNA viruses that utilize virally encoded reverse transcriptase (RT) to replicate through a proviral DNA intermediate.1 The provirus is permanently integrated into the host cell chromosome and, like a cellular gene, is expressed by the host cell transcription, RNA processing, and translation machinery. Upon transcription, the retroviral pre-mRNA is spliced into viral mRNAs that exhibit all characteristics of cellular mRNAs as they bear a 5′cap structure and a 3′poly(A) tail. In the case of HIV-1, alternative splicing gives rise to over 30 different mRNA species that are then exported to the cytoplasm by different pathways. HIV-1 mRNAs have been grouped into 3 classes according to their degree of splicing: (i) full-length transcripts, which correspond to mRNAs that do not undergo the splicing process, encode for the Gag and Gag–Pol polyproteins; (ii) singly spliced transcripts which generate the viral proteins Env, Vif and Vpu; (iii) fully spliced transcripts which express Rev, Tat, Vpr and Nef. As for all viruses known to date, HIV-1 protein synthesis relies exclusively on the host cell translation machinery for ribosomes, tRNAs, amino acids and all required initiation, elongation and termination factors. In this review, we provide an overview of what has been documented regarding the mechanism of translation initiation of the full-length HIV-1 mRNA.
The HIV-1 Full-Length or Genomic RNA (gRNA)
Upon HIV-1 entry, gRNA reverse transcription and viral DNA integration, the integrated proviral genomic DNA is transcribed by the host RNA polymerase II (Pol II) to generate a primary transcription product that interacts with the cellular RNA processing machinery to be spliced, exported to the cytoplasm, and translated by the host protein synthesis machinery.2 However, a proportion of the pre-mRNA subverts typical RNA processing conserving their introns. To export its unspliced and partially spliced mRNAs, HIV-1 uses a specific mechanism involving the cellular CRM1 export pathway and the viral protein Rev (Regulator of virion expression).3,4 The HIV-1 Rev protein, a nuclear-cytoplasmic shuttling RNA binding protein, interacts with a highly structured RNA element located within the env gene known as the Rev response element (RRE). The Rev-RRE complex is then exported to the cytoplasm.5-7
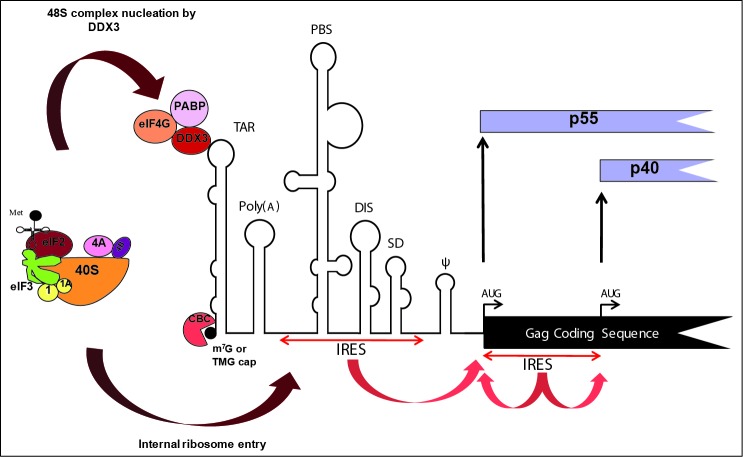
The unspliced or full-length HIV-1 RNA fulfills a dual role as a mRNA (HIV-1 mRNA) where it codes for the Gag and Gag/Pol polyproteins and as the genomic RNA (gRNA) to be encapsidated into newly synthesized particles.2 The HIV-1 mRNA harbors a highly structured 5′ untranslated region (5′UTR) or leader8 with distinct and well characterized RNA motifs that are involved in many steps of the viral replication cycle (see Fig. 1 ).8 The first structural element is an ∼60 nucleotides long stem loop named the trans-activation response element (TAR) which is recognized by the viral Tat protein and is essential for viral RNA (vRNA) transcription.9 TAR is followed by the polyadenylation (poly(A)) stem loop that contains a polyadenylation signal which is ignored when located within the 5′leader but used for 3′ end processing when it is read in the 3′untranslated region (3′UTR).10 Following the poly(A) stem loop comes the primer binding site (PBS) which is important for the recruitment of the tRNA(Lys3) that serves as primer to initiate the process of gRNA reverse transcription.11 Downstream of the PBS are the dimerization initiation site (DIS), the splice donor (SD), and the packaging signals (Ψ). All hairpin loops are either used during RNA processing (SD) or during viral gRNA dimerization and encapsidation (DIS and Ψ).12,13 These RNA signals differ in RNA structure among different lentiviruses, but their presence and function are conserved among them.8,14,15 For example, the HIV-2 TAR is much longer and folded into a fork motif whereas it is a stem loop in HIV-1.16-18
Figure 1.
Schematic cartoon of the HIV-1 genomic RNA (gRNA). HIV-1 full-length mRNA is capped at the 5′terminus by a 7-methyl-guanylic acid residue (m7G) or a trimethylguanosine (TMG) and is structured in well-defined RNA motifs: TAR hairpin, Poly (A) Signal, Primary Binding Site (PBS), Dimerization Initiation Site (DIS), the major Splice Donor Site (SD) and RNA Packaging Signal (ψ). The mRNA codes for 2 proteins: p55 and p40 that can be synthesized by either ribosome entry from the 5′cap or from IRESes located both in the 5′UTR and the coding region (as indicated on the Figure). Ribosomal entry at the 5′end occurs by the recruitment of a trimeric complex composed of DDX3, PABP and eIF4G whereas internal entry takes place by direct binding of the 43S preinitiation complex to the IRES elements. Note that the 5′cap can also be bound by the CBC complex.
Translation Initiation
Translation initiation of most eukaryotic mRNAs occurs by a scanning mechanism whereby the 40S ribosomal subunit is recruited to the vicinity of the cap structure, a 7-methyl-guanylic acid residue located at the 5′ terminus of all eukaryotic mRNAs, and scans in the 5′ to 3′ direction until an initiation codon is encountered.19,20 The 40S ribosomal subunit is recruited to the mRNA as part of the 43S initiation complex, composed of the subunit bound to eIF2-GTP/Met-tRNAi (initiator tRNA), eIF1A, eIF1 and eIF3. eIF4F, a multi-subunit complex composed of eIF4E, eIF4A and eIF4G is important in this recruitment process.19,20 eIF4E directly binds to the 5′cap structure, while eIF4A, a member of the DEA(D/H)-box RNA helicase family, participates by unwinding secondary structure in the 5′-untranslated region (5′UTR) of the mRNA. eIF4A's helicase activity is stimulated by eIF4G and eIF4B.21 In this process, eIF4G serves as a scaffold for the coordinated assembly of the translation initiation complex as it contains binding sites for eIF4A, eIF4E, and eIF3. Therefore, eIF4G associates the mRNA cap (via eIF4E) and the 40S ribosomal subunit (via eIF3) to attach the template mRNA to the translation machinery. eIF4G also binds the poly(A)-binding protein (PABP) and this promotes mRNA circularization by linking the 5′cap (via eIF4E-eIF4G) and the 3′poly(A) tail (via PABP-eIF4G) of the mRNA that is the physical conformation favorable for translation.19,20
An alternative cap-independent mechanism of translation initiation was evidenced by studying translation of the uncapped picornaviral mRNAs.22 Picornaviral mRNAs contain an RNA structural element, termed internal ribosome entry site (IRES), to promote the recruitment of the 43 S initiation complex in a cap-independent manner.23,24 In addition, some picornaviruses encode viral proteases that directly target eIF4G and PABP during infection while others modify the availability of eIF4E.25-28 Both events result in the disruption of cellular cap-dependent translation initiation during viral infection in order to redirect the host translational machinery toward viral mRNAs.29 More recent studies showed that this alternative mechanism of translation initiation is not confined to picornaviruses as the mRNAs from other viral families including some members of the Retroviridae, also utilize IRES-dependent translation initiation.30,31 In sharp contrast to picornavirus mRNAs, retroviral mRNAs possess a 5′cap structure and can initiate translation either by a canonical cap-dependent mechanism or via an IRES.32,33
RNA Structures Modulate Protein Synthesis of the Full-Length HIV-1 mRNA
The HIV-1 5′UTR contains several well-characterized RNA structural elements critical for different stages of the HIV-1 replication cycle, such as the TAR, Poly(A) loop, PBS domain, the SD, DIS and Ψ (see Fig. 1 ). Due to their stability, these RNA structures can also modulate viral mRNA translation initiation. As such, the TAR domain is a 57 nucleotides long, highly stable stem-loop structure (ΔG = −29.6kcal/mol) located at the most 5′ end of all HIV-1 transcripts.30 In fact, the TAR stem loop structure occludes the cap moiety raising the question of its accessibility for eIF4E during the assembly of the translation initiation complex over the viral mRNA.34,35 As it was previously demonstrated that the presence of a stem-loop of weaker stability (ΔG = −13.6kcal/mol) at the 5′end of a synthetic mRNA is enough to inhibit ribosome recruitment at the 5′end,36 it did not come as a surprise that the presence of TAR alone, or in the context of the HIV-1 5′-UTR, is inhibitory to cap-dependent translation in the RRL and in Xenopus oocytes.34,35,37,38 Addition of the trans-dominant negative eIF4AR362Q mutant39 into the RRL strongly repressed expression from the HIV-1 5′-UTR indicating that both eIF4G and eIF4A are required to overcome RNA structures present in the HIV-1 5′-UTR.40 However, translational inhibition imposed by TAR was less pronounced in cells and this was confirmed by the rescue of translational inhibition in the RRL upon addition of cytoplasmic HeLa cell extracts.34,41 This observation is consistent with the fact that several cellular proteins are known to bind the HIV-1 TAR structure promoting translational activation of HIV-1 mRNA.42
Studies have shown that the HIV-1 5′UTR can adopt 2 spatial conformations which are the branched multiple hairpins (BMH) and the long distance interaction (LDI) structures.43 The BMH was shown to expose the dimerization and packaging signals in order to favor encapsidation and viral replication.44-46 The riboswitch between LDI and BMH depends on viral infection as it is enhanced both by the genomic RNA concentration and the presence of the viral NC protein.43,47 However, experiments showed that despite the fact that the BMH structure occludes the AUG start site codon,48 the structure by itself does not modify the translation rate driven by the HIV-1 5′UTR.41,44 Furthermore, introduction of mutations designed to alter the LDI-BMH equilibrium do not significantly impact on the ability of the HIV-1 5′UTR to drive translation initiation in both the context of a mono- or bi- cistronic mRNA.41,44
Interestingly, expression of the viral Gag protein inhibits its own synthesis while stabilizing the conformation of the BMH structure.49 As the BMH structure does not by itself impede translation from the viral mRNA41,44 it may be the presence of the Gag protein on the viral mRNA that affects ribosome attachment or scanning along the 5′UTR. Gag protein exerts a bimodal effect on its own production with enhancement of expression at low concentration and inhibition at high concentration.49 Another possibility would be that translation repression of the HIV-1 mRNA is induced by the Gag mediated dimerization of HIV-1 gRNA. The duplex formed by the genomic RNA and the Gag protein via the NC domain probably establishes a strong hindrance for scanning or recruitment of the translation initiation complex. Therefore, at high concentration, the Gag protein would be directly responsible for inhibiting translation from the viral mRNA favoring its packaging into de novo synthesized viral particles49 This possibility is in agreement with findings showing that viral RNA dimerization inhibits viral mRNA translation while promoting gRNA encapsidation.47,50 Interestingly, RNA dimerization would be favored by a long distance RNA-RNA interaction established between nucleotides present within the Gag coding region and the 5′UTR sequence.47
Cap-Dependent Initiation on the HIV-1 Genomic RNA
Ribosomal scanning from the 5′cap
In vitro translation experiments conducted in the rabbit reticulocyte lysate (RRL) reported that addition of both cap-analog and the cleavage of eIF4G by the FMDV L protease were inhibitory to translation of a capped monocistronic HIV-1 reporter gene providing evidence that 5′ ribosomal scanning takes place on the 5′UTR of HIV-1.33 This was followed by data from the Berkhout laboratory showing that expression of the 2A protease from poliovirus impaired translation initiation on the HIV-1 genomic RNA ex-vivo. Moreover, insertion of upstream AUG codons (uAUGs) at different locations within the HIV-1 5′UTR was severely inhibitory to translation confirming the use of a 5′ dependent ribosomal scanning mechanism.51 Initiation from the capped end also gave an explanation to the fact that the folding and length of the TAR structure (which is different between HIV type 1 and type 2) had such a great influence on the overall efficiency of translation.34 This ribosomal scanning hypothesis also fits well with the fact that most natural HIV-1 isolates do not tolerate uAUGs within their 5′UTR despite its great length.8 However, the mechanism by which the 43S preinitiation complex can deal with these stable RNA structures that are known to impair both initial ribosome binding and scanning remains unclear.52,53 In most cases, the presence of eIF4A and eIF4B/eIF4H on the 43S preinitiation complex can resolve these structures in an ATP dependent manner.54 However, as stated above, the TAR structure immediately downstream to the cap structure and the complex structure of the whole HIV-1 5′ UTR appear to be too stable to be simply unwound by the eIF4A/eIF4B/eIF4H complexes. Therefore, it leaves the question of how the 43S preinitiation complex can bind and scan this structured HIV-1 5′UTR.
The need for additional helicases in HIV-1 translation
A hint to the above question, came from recent studies showing that complex RNA structures can be unwound by recruiting cellular RNA helicases to the 43S complex to assist ribosomal scanning.55 These helicases belong to the growing family of DExD/H box (DEAD, DEAH, DexH and DExD) proteins that are characterized by the presence of a highly conserved helicase core domain involved in ATP binding, ATP hydrolysis and nucleic acid binding.56,57 These enzymes participate in virtually all aspects of RNA metabolism by remodeling ribonucleoprotein (RNP) or RNA-RNA complexes. As such, some of them have been specifically involved in the process of translation initiation. This is the case for Ded1, RHA (DHX9) and the DEAH-box protein DHX29 in mammals which cooperate with eIF4A/eIF4B/eIF4H to enhance the processivity of ribosomal scanning on structured transcripts.55,58-60 In this process, these enzymes are delivered to the 5′ end of the mRNA either by binding to a specific post-transcriptional element (PCE) in the case of RHA, as part of the eIF4F complex for Ded1, or together with the 40S ribosomal subunit for DHX29.60-62 In the case of HIV-1, Boris-Lawrie and colleagues have addressed the role of DHX9/RHA in viral translation and they showed that the latter was required to promote RNP remodeling through the 5′UTR to ensure ribosome progression.61 This occurs by the initial binding of RHA to the PCE element found in the HIV-1 5′UTR and appears to require an ATP-dependent activity.61 The yeast ortholog of Ded1, which is called DDX3 in mammals, has also been implicated in HIV-1 translation. DDX3 is a member of the DEAD-box family of RNA helicases and has been involved in many biological processes such as cell cycle progression, apoptosis, cancer, innate immune response and in the replication of many viruses that infect humans31 such as the major pathogens hepatitis C and B viruses together with the HIV-1.31,63
There are 2 related DDX3 genes DDX3X and DDX3Y in the human genome. DDX3X or DBX (hereafter called DDX3) was initially mapped to the X chromosome-linked genes with functional homologs in the Y chromosome and was found in the Xp11.3-11.23 region.64,65 DDX3Y or DBY is specifically expressed in testis where it plays an essential function in spermatogenesis;66 however, due to this specialized role, it will not be further discussed in this review. Initially, DDX3 was shown to be essential for HIV-1 replication by acting as a cofactor of Rev and CRM1 during the nuclear export of RRE-containing vectors.67 DDX3 was later found to associate with some key members of the translation initiation machinery and to participate in translation of a subset of cellular and viral mRNAs carrying structured 5′-UTRs.59,68,69 However, unlike its yeast ortholog, DDX3 is dispensable for general translation and cannot be considered as a functional translation initiation factor.59,70 In a recent report, Liu and colleagues have investigated the role of DDX3 in HIV-1 translation in the context of a full-length provirus and with HIV-1 derived reporter genes. They have performed overexpression and siRNA based knock-down of the endogenous protein to find that HIV-1 required the presence of the helicase for translation.71 In addition, by using bicistronic constructs containing the HIV-1 IRES in the intercistronic spacer, they suggested that DDX3 could act as an ITAF to promote IRES-driven translation. However, no mechanistic insight was provided to explain the need for the enzyme and they rather suggested a role in assisting ribosomal scanning on the viral 5′ leader.
In a different study, Soto-Rifo et al. knocked-down DDX3 in HeLa cells expressing a full-length HIV-1 proviral clone coding for the renilla reporter gene, finding that while general translation was unaffected by depletion of DDX3, HIV-1 Gag synthesis from the vRNA was dramatically impaired.72 In the same study, several DDX3 mutants were used to document the need for the ATP binding and hydrolysis motifs for DDX3 function in translation initiation. By inserting unstructured RNA spacers between the cap and the beginning of the TAR structure, it was shown that displacement of TAR by only 15 nucleotides away from the m7GTP was sufficient to abolish the requirement for DDX3 in protein synthesis. Together, these observations indicate that DDX3 is needed for the initial ribosomal binding step rather than for the scanning process. This was further demonstrated by using a biochemical assay in which DDX3 was shown to bind to both the TAR RNA duplex and 2 additional sites on the HIV-1 5′UTR that correspond to single stranded RNA regions giving biological evidence to the proposed mechanism of local strand separation proposed by Jankowsky and colleagues.73 DDX3 was also demonstrated to locally unwind the base of the TAR motif in order to render the m7GTP accessible for ribosome binding. In this model, DDX3 would substitute for eIF4E to nucleate the entry of a 43S preinitiation complex. Interestingly, recent work from the Boris-Lawrie laboratory has shown that translation of the HIV-1 genomic RNA can occur in an eIF4E independent manner by recruiting the CBP 80 at the 5′ end of the transcript.74 Thus, this leaves the question of whether 43S complex formation occurs in a single step, e.g., DDX3 binds and nucleates the assembly of the full complex composed of eIF4F, eIF4B, eIF3, eIF1/1a, eIF2 and the 40S ribosome, or is the assembly of such a complex taking place in a sequential manner? Similarly, the other important question to answer will be to determine the composition of the DDX3 mediated 43S preinitiation complex: does it contain the whole set of eIFs or are some lacking? Furthermore, one has to consider that TAR is the preferential binding site for Tat.75 As such, a direct interaction between DDX3 and Tat has been recently shown both in vitro and in vivo.76,77 Strikingly, Tat/DDX3 interaction results in both the enhancement of viral transcription and translation.76,77 In the latter case, which is more relevant to the topic of this review, the Tat/DDX3 complex was found to be associated to both the translation initiation complexes and to polysomes. The knockdown of DDX3 resulted in the release of Tat from the polysomal fraction, highlighting its role in this interaction.76
These findings are in agreement with data showing that the interaction of cellular RNA-binding proteins with the viral RNA impacts RNA structure-function.41 The RNA-binding protein lupus autoantigen La78 was the first identified cellular protein shown to bind to the HIV-1 5′leader and to relieve TAR-induced translational repression in an in vitro system.38 Such an effect required the dimerization domain of La79 and, later, Hühn et al. (1997) reported that La exhibited a dsRNA unwinding enzyme activity that could potentially be used to destabilize the TAR structure;80 nevertheless the effect of La on HIV-1 expression in cell culture or its role on HIV-1 replication has not been further investigated. Staufen 1 and TAR-RNA-binding protein (TRBP) are other examples of cellular proteins that are capable of relieving the TAR-induced translational repression by directly interacting with the RNA TAR structure.37,81
eIF4E independent translation of the full-length HIV-1 mRNA
During mRNA synthesis and processing several different yet dynamic mRNA-ribonucleoprotein complexes (mRNPs) are formed. One of the first protein complexes that co-transcriptionally assembles over the 5′cap structure is known as the nuclear cap-binding complex (CBC).82 The CBC consists of a cap-binding subunit protein 20 (CBP 20) which binds the cap and cap-binding protein 80 (CBP 80) which stabilizes this interaction. The CBC plays an important role in various aspects of nuclear mRNA metabolism such as pre-mRNA splicing and nuclear export and it remains associated to the mRNA during intron removal, deposition of the exon junction complex (EJC) and nuclear export. In the cytoplasm, the CBC plays an analogous role to eIF4E as both can interact directly with eIF4G to serve as a platform for the loading of the 43S preinitiation complex to mRNA,83,84 to undergo what is referred to the pioneer round of translation.84 This induces extensive rearrangements of the CBC-bound mRNPs with the removal of EJCs and EJC-associated factors by the translating ribosomes, and the exchange of nuclear poly(A)-binding protein N1 (PABPN1) with cytoplasmic PABPC1 at the poly(A) tail.84 CBP80 also promotes the replacement of CBC by eIF4E in polysome associated mRNA by a mechanism that involves the karyopherin importin-β–promoted dissociation of CBC from the mRNA.83,84 Dissociation of CBC allows the binding of eIF4E to the 5′cap and the beginning of protein synthesis. eIF4E alone has a lower affinity for the cap than CBC but assembly of eIF4F increases the affinity of eIF4E for the cap.85 A recent report suggests that in the case of HIV-1, spliced viral mRNAs undergo CBC exchange for eIF4E, while unspliced HIV-1 mRNAs remain associated with the CBC in the cytoplasm74 during mRNA translation and viral gRNA encapsidation.74 These observations drove the authors to propose a model by which translation initiation of the full-length HIV-1 mRNA would take place by a non-canonical cap-dependent translation initiation mechanism reliant on CBC instead of elF4E.74 However, an alternative interpretation to these findings is that CBC remains associated to the viral mRNA yet 40S ribosomal subunit recruitment occurs in a 5′end independent fashion as highlighted below. This possibility that was not considered by the authors also fits all the findings that they describe.74
Cap-Independent Translation Initiation from the HIV-1 mRNA
Characterization of IRES sequences
Two IRESes have been identified on the HIV-1 full-length mRNA, one within its 5′UTR, and the other within the Gag coding region.86,87 The IRES within the 5′UTR has been evidenced in the laboratory adapted HIV-1 infectious recombinant proviral clone NL4.3, the CXCR4 (X4)-tropic primary isolate HIV-LAI and in the 5′UTR of viral RNA isolated from clinical samples.86,88,89
The characterization of the HIV-1 IRES was done by using a series of bicistronic constructs that were verified to contain no cryptic promoter or spurious splice sites as they are often a caveat in this area of research86,88-90 In addition, translation of HIV-1 in a bicistronic context was shown to be resistant to picornaviral proteases that specifically inhibit cap-dependent translation initiation.86,89,91 More recently, Monette et al. have used transfection-infection experiments of DNA encoding a bicistronic RNA containing the HIV-1 5′UTR proving that its activity is not disrupted by poliovirus infection in 293 T cells.
Structural analysis of the HIV-1 leader evidenced that a short RNA duplex of predicted free energy (ΔG) of −10.4 is formed in the wild type leader region by base-pairing of sequences flanking the primer-binding site (PBS). In Brasey et al. (2003), this 7-bp duplex was strengthened in the context of the pNL4.3 infectious clone to generate a plasmid encoding for viral RNA with increased stability in the PBS domain. The ΔG predicted for these new RNA structures ranged between −23.5 kcal/mol (wild-type) and −38.4 kcal/mol. The last value greatly exceeded the stability of the wild-type HIV-1 5′UTR, including that of the extended trans activation responsive (TAR) region hairpin structure (−29.6 kcal/mol), which is known to strongly inhibit translation initiation. Following transfection in C33A cells, no effect on Pr55Gag and CA-p24 protein expression could be observed suggesting the presence of the IRES. Additionally, translation activity from the HIV-1 5′UTR IRES proved to be resistant to the introduction of mutations designed to alter specific RNA structural elements within the functional region,41,89,90 suggesting that structure-function relationship for the HIV-1 IRES is not as rigid as that described for other viral IRESes.92-98 The biological significance of these findings still has to be addressed. Moreover, while studying the HIV-1 5′UTR IRES, Gendron et al. defined a region upstream from the PBS that they named IRENE, for IRES negative element, which negatively impacts on activity of the HIV-1 leader IRES.88 The molecular mechanisms by which this cis-acting RNA element can inhibit IRES-driven translation are unknown and have not been studied further. Besides IRENE, other cis-acting repressive sequences or inhibitory sequences (CRS/INS)99-104 reported to be scattered throughout the Gag, Pol, and Env regions of HIV-1 mRNA 99-104 are also able to restrict the expression of the HIV-1 structural proteins by a, yet, undefined mechanism. Although no consensus sequence has been defined for these CRS/INS elements, they were shown to contain AU-rich elements (AREs).101,102 One such inhibitory element, known as the INS-1, is found within the matrix (p17) Gag coding region and is reported to function as an inhibitor of both HIV-1 mRNA cap-dependent and IRES-mediated translation initiation.99,102 Inhibition of translation was not restricted to the context of HIV-1 mRNAs as insertion of the INS-1 within a heterologous non-viral mRNA could also inhibit its expression.99,102,105 Although the molecular mechanism by which INS-1 restricts HIV-1 mRNA translation initiation remains undefined, several reports suggest that host proteins are involved.106-109
Along the same line, Brasey et al. showed that the Gag ORF inhibited protein expression mediated by the HIV-1 5′UTR in the context of a HeLa cell line.86 Such results were very puzzling at the time considering that the HIV-1 5′UTR drives the expression of Gag protein. Therefore, how could the HIV-1 5′UTR IRES drive efficient expression of Gag while the Gag ORF inhibits IRES function? This question was recently solved by Plank et al. who showed that the inhibitory effect of the Gag ORF was not observed in the context of a lymphocytic cell line.90 These observations suggest that the repression of translation is restricted to certain cell types but not in the natural hosts of HIV-1 infection. Together, these observations would suggest that HIV-1 tropism is partially regulated by a yet poorly characterized molecular mechanism involving translational control of viral gene expression. A similar scenario has been established for other viruses harboring IRESes. Translational control of viral tropism has been characterized for HCV as well as for several members of the picornaviridae family of viruses.110-114
In addition to the IRES present within the HIV-1 5′UTR Buck and colleagues also identified an IRES within the Gag ORF.87 This second IRES drives expression of both the Gag-protein and of a shorter N-truncated isoform of it (p40) in which the entire matrix domain is missing (See Fig. 1 ). Although the function of Gag p40 is unknown, it is produced during infection and its deletion impaired the growth kinetics of HIV-1 in culture.87 The presence of shorter N-truncated isoforms that result from alternative initiation at codons located in the Gag coding region is conserved in HIV-2 and the simian homolog (SIV).115,116 Investigation into the translational mechanism used for production of p40 revealed the exclusive use of the IRES located in the Gag ORF whose activity is totally independent from the cap structure and eIF4E, and is resistant to the FMDV L protease treatment.33 However, the most peculiar characteristics of this IRES lies in its ability to promote expression on the upstream AUG located at its 5′border.87,117 This allows efficient protein synthesis from synthetic leaderless mRNAs in which the AUG codon is located immediately at the 5′end of the RNA. Surprisingly, translation mediated by this synthetic mRNA is highly efficient117 (de Breyne et al., unpublished) and occurs at the authentic AUG codon with no associated leaky scanning indicating that ribosome recruitment on the Gag coding region IRES is mediated by an unconventional mechanism. This characteristic is conserved in HIV-2 and in SIV.115,117
All these features contrast with other IRESes described to date and suggest that the Gag coding region contains 2 binding sites for ribosome attachment which are dedicated to each initiation site.
Activity of the HIV-1 5′leader IRES is regulated by IRES trans-acting factors (ITAFs)
The HIV-1 5′UTR IRES demonstrated activity in various biological systems such as HeLa based translation extracts,86 in cells such as HeLa, U20S, HEK293-T, Jurkat T cells,71,86,88,90,118,119 and in Xenopus laevis oocytes (see Fig. 2 ).41,120 Nonetheless, the HIV-1 IRES is quite inefficient in promoting translation in the RRL.121 However, this poor activity can be rescued by addition of HeLa cell extracts prepared from G2/M arrested cells to the lysate41 suggesting a requirement for cellular proteins and/or IRES trans-acting factors (ITAFs).41 Although the precise mechanism by which ITAFs facilitate the recruitment of ribosomal subunits remains unknown, they appear to facilitate binding of eIFs and ribosomes, or may act by assisting the RNA to undertake a structure able to recruit the initiation complex.94,122-125 Using pull-down experiments Vallejos et al.41 identified a series of proteins found in G2/M HeLa extracts that interact with the viral 5′UTR. However, these findings are very preliminary as their role in translation initiation was not evaluated in a functional assay.41 The impacts of some well known ITAFs have been assayed on translation initiation of the HIV-1 5′UTR IRES.71,118,120 As such, Liu et al. showed that eIF5A, the human Rev-interacting protein (hRIP) and DDX3 were all capable of stimulating translational activity from the HIV-1 5′UTR IRES when located in a bicistronic context in HEK293T cells. Interestingly, the same report discarded Sam68, a member of the signal transduction and activation of RNA (STAR) protein family, as a possible ITAF for the HIV-1 5′UTR IRES.71 Using a similar strategy, Monette et al. identified hnRNP A1 as a cellular factor that enhances HIV-1 5′UTR IRES function.118 They further presented evidence that HIV-1 mRNA is loaded with hnRNP A1 in the cell nucleus and that the protein shuttles to the cytoplasm associated to the viral mRNA where it can exert its function at the level of IRES-mediated translation initiation.118 As for nascent cellular mRNAs, the HIV-1 mRNA is expected to first encounter RNA-binding proteins in the nucleus.82 In fact, the full-length viral mRNA reaches the cytoplasm as part of a distinct ribonucleoprotein (RNP) complex with nuclear RNA-binding factors.10,126 Therefore, it is plausible that both viral and cellular proteins such as Rev and hRNP A1 are initially loaded on the viral mRNA in the nucleus before being used for cytoplasmic RNA expression.118,127 In line with this possibility, a recent report confirms the cotranscriptional formation of a stable export complex that includes both Rev and CRM1.128 It is noteworthy that Rev and associated proteins not only determine the export of the full-length mRNA, they also play a role in its translation. In fact, Rev promotes polysomal association of the full-length mRNA and stimulates its translation.127,129 Furthermore Rev is also required for the binding of PABP to the Rev-dependent viral mRNAs and relieves the translational inhibition imposed by Gag ORF.100,101,130 HIV-1 IRES-mediated translation initiation can also be altered by cytoplasmic modifications of specific ITAFs. For instance, the mitogen-activated protein (MAP) kinase-interacting kinases (Mnks) trigger the phosphorylation of hRNP A1 and this event seems to be required for HIV-1 IRES activity, as inhibition of Mnk phosphorylation results in a decrease of HIV-1 IRES mediated protein expression.118 Consistent with this observation, HIV-1 infection induces a moderate increase in the phosphorylation status of hnRNP A1 and this may contribute to its ability to influence HIV-1 IRES-mediated translation initiation.
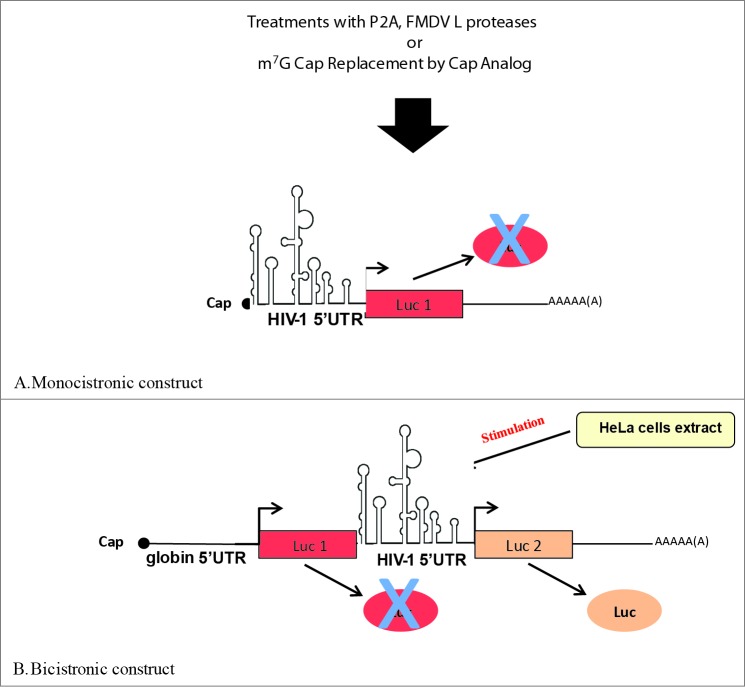
Figure 2.
Different levels of requirement for initiation factors on the HIV-1 5′UTR. The HIV-1 mRNA can use both a cap-dependant mechanism in a monocistronic context (A) or an IRES-driven mechanism (B). Addition of cap-dependant inhibitors (P2A and FMDV L proteases, Cap-analog) results in a partial inhibition of gene expression in a monocitronic context but not in a bicistronic setting. Addition of HeLa cell extract was shown to greatly stimulate initiation from the HIV-1 IRES.
Conversely, activity of the HIV-1 5′UTR IRES can also be inhibited by cellular proteins. For example, HuR, a nucleus-cytoplasm shuttling protein that belongs to a conserved family of RNA-binding factors that were originally identified in Drosophila as embryonic lethal abnormal vision (ELAV) proteins, was identified as a negative modulator of HIV-1 IRES activity.120 Translational inhibition of the HIV-1 IRES activity by HuR is not due to a direct protein-RNA interaction as the binding of HuR to the HIV-1 5′UTR could not be established;120 therefore the molecular mechanism in place remains to be determined.
Why would HIV-1 mRNA require an IRES?
As the HIV-1 mRNAs bear a 5′cap, it can be argued that IRES-dependent initiation would seem to be unnecessary during viral mRNA translation. However, several lines of evidence suggest that cap-dependent translation initiation is disrupted during HIV-1 replication.
The studies of cellular mRNAs harboring a dual initiation mode such as that exhibited by the HIV-1 mRNA show that under normal physiological conditions, cap-dependent translation would be the predominant mechanism used for translation. However, under various cellular and environmental stresses, global protein synthesis declines and expression of diverse stress-responsive factors driven by IRESes are preferentially upregulated.122-124,131 As such, cellular mRNAs can switch from cap-dependent to cap-independent translation as an adaptive response of stress resistance 122-124,131; this may also be the case for translation initiation from the HIV-1 mRNA.
As is true for all viruses, HIV-1 is an obligate intracellular parasite and must use the cellular machinery for viral protein production. As a consequence, early in infection, the viral mRNAs have to compete with all actively translated cellular mRNAs for the use of ribosomes and, above all, for the limited pool of eukaryotic translation initiation factors (eIFs) necessary for translation initiation. Success in this competition will ensure the reprogramming of the host machinery toward the production of virally encoded proteins and, ultimately, the release of infectious virions.132,133 In the case of the HIV-1, the 5′UTR has been shown to drive cap-dependent translation initiation under normal physiological conditions33,51 and to mediate IRES-dependent protein synthesis during stress conditions known to hinder cap-dependent translation initiation.32,86,88,91,118 Interestingly, in the course of viral replication, HIV-1 has been shown to change cellular homeostasis altering several components of the host translational apparatus which results in the inhibition of cap-dependent translation. For instance, HIV-1 induces an endogenous cellular increase of reactive oxygen intermediates leading to an oxidative stress that has been demonstrated to facilitate NF-kappa B-dependent activation of HIV transcription and to stimulate the HIV-1 5′UTR IRES activity.88 Additionally, the expression of the viral protein R (Vpr), which plays a role in the regulation of reverse transcription, nuclear transport, transcription, cell cycle and apoptosis, is also able to promote cell cycle arrest in the G2/M phase.134 In general, during the G2/M phase of the cell cycle, both eIF4E and 4E-BP are hypophosphorylated, leading to the disruption of the eIF4F complex, to the sequestration of eIF4E by 4E-BP and to the suppression of cap-dependent translation initiation.135 Consistently, during Vpr induced G2/M cell cycle arrest, functional eIF4E is diminished as the eIF4E-4EBP interaction is favored.74 Conversely, virus induced G2/M cell cycle arrest stimulates both HIV-1 transcription and Gag mRNA translation,136 resulting in an overall increase of viral production.137 This finding strongly correlates with the observation that HIV-1 IRES-driven expression is barely affected by G2/M arrest.86
The initiation factor eIF4G is also a substrate for several distinct cellular and viral proteases in the late steps of HIV-1 infection. Indeed, activation of apoptosis by Vpr induces the activation of caspase 3 which, in turn, mediates the proteolysis of eIF4G.138,139 Furthermore, the HIV-1 viral protease which is required for processing of viral proteins and has also several cellular targets,140-148, can also cleave the initiation factors eIF4G, PABP and eIF3d.140-142,148 Although the effect of eIF3d cleavage on translation has not been determined,148 proteolysis of eIF4G and PABP lead to repression of cap-dependent translation in RRL, in cell free extracts, and in cultured cells.141,142,149,150 However, under these conditions that hinder cap-dependent translation initiation, protein synthesis driven by the EMCV- and PV-IRESes, and by the HIV-1 5′UTR remained barely affected both in cell free extracts and in cells (See Fig. 2 ).149,150 These experiments would suggest that during the late stages of the HIV-1 replication cycle, cap-dependent translation initiation is targeted. In consequence under conditions that hamper cap-dependent translation initiation the presence of a functional IRES would favor viral mRNA translation over that of cellular mRNAs.
In addition, the presence of a modified 5′cap structure at the extremity of the HIV-1 full-length RNA could also justify the requirement of an IRES. Indeed, the peroxisome proliferator-activated receptor-interacting protein with methyltransferase domain (PIMT) is a protein known to interact with the Rev/RRE complex. PIMT hypermethylates the m7G RNA-cap to generate a pool of trimethylguanosine (TMG; m2, 2,7 guanosine)-cap containing viral mRNAs.151-153 TMG-capped-RNAs are known to translate poorly,154 due to the inefficient recognition of the trimethyl by the cap-binding protein eIF4E.155-158 As a consequence, it would be predicted that TMG-HIV-1 RNAs would be inefficiently translated resulting in a decrease of viral expression. However, this is not the case and TMG-capping of viral mRNAs associates with an increased expression of HIV-1 structural proteins.151 Although the mechanism by which these TMG-capped viral mRNAs recruit the translational machinery was not studied, it is tempting to speculate that translation initiation from the TMG-HIV-1 RNAs could take place by a cap-independent IRES-mediated mechanism.
Perspectives
Over the past 10 years, there has been an increasing number of manuscripts concerning the translational control of HIV-1 gene expression. This has been a very exciting growing field that has contributed to a better understanding of HIV-1 protein synthesis. Knowledge regarding the different mechanisms of translation initiation used by HIV-1 is growing. Noteworthy, several novel strategies have emerged to specifically target IRES mediated translation of viral mRNAs in the context of eukaryotic cells. For example several strategies have been developed to target the HCV IRES.159-162 Findings suggest that translation initiation of viral mRNAs can be specifically targeted within a cell without hampering canonical cellular mRNA translation. It is therefore plausible to predict that the knowledge concerning the HIV-1 cap-independent translation initiation might prove paramount in the development of new therapeutic strategies that specifically target HIV-1 IRES-dependent protein synthesis.
Viral RNA localization
New observations are starting to shed light on important issues regarding the cytoplasmic localization of the HIV-1 genomic RNA while it is being translated. This knowledge that has generated novel perspectives on the understanding of HIV-1 translation is summarized below. The formation of a preinitiation complex between DDX3, eIF4G, PABP and the HIV-1 genomic RNA has implications on the subcellular localization of the genomic RNA during translation. Indeed, DDX3 has been found to be a component of at least 2 kinds of cytoplasmic foci that are neuronal granules implicated in CAMKIIalpha163 and Beta-actin164 transport and cytoplamic stress granules and P-bodies.165 In the latter case, the overexpression of DDX3 was shown to lead to spontaneous assembly indicating a scaffolding role for the enzyme in stress granules assembly.59,165 By using RNA fluorescence in situ hybridization coupled to indirect immunofluorescence, Soto-Rifo and colleagues were able to visualize the formation of a core DDX3/PABP/eIF4G trimeric complex on the HIV-1 genomic RNA that is localized to compartmentalized cytoplasmic foci.166 This was further confirmed by biochemical data showing direct interactions between DDX3 with both PABP and eIF4G. Surprisingly, these foci were devoid of any of the cap- binding proteins (eIF4E and CBP80) and contained only traces of eIF4A, eIF4B and eIF3. These observations, together with the fact that they could form in the absence of any cellular stress suggested that they were not bona fide stress granules. In agreement with this, a mutated version of DDX3 in which the 38YIPPHLR43 motif required for stress granule assembly was deleted could still be recruited in these cytoplasmic foci. This is also consistent with earlier work showing that expression of HIV-1 prevents stress granule formation induced by arsenite treatment.167 These data lead to a model in which these cytoplasmic spots would be the location for assembly of a minimal preinitiation complex on the HIV-1 genomic RNA. Such a complex is constituted by the assembly of DDX3/eIF4G/PABP at the 5′ end of the HIV-1 genomic RNA where it locally unwinds the basis of TAR to create a single stranded RNA region accessible for ribosomal binding.72 Due to its essential role in HIV-1 translation and replication, it is not surprising that several anti-HIV drugs are currently being developed against DDX3 over the last few years.168 This represents a good alternative as it overcomes the problem of drug resistance that is often observed. Some of these molecules are relatively small, easy to synthesize and have been demonstrated to be very competent to inactivate DDX3 resulting in a good reduction of viral load in peripheral blood mononuclear cells rendering their use quite promising for novel therapeutic approaches.169
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Coffin JM, Hughes SH, Varmus HE. Retroviruses. New York, 1997. [Google Scholar]
- 2. Coffin JM, Hughes SH, Varmus HE, eds. Retroviruses. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press, 1997. [PubMed] [Google Scholar]
- 3. Neville M, Stutz F, Lee L, Davis LI, Rosbash M. The importin-beta family member Crm1p bridges the interaction between Rev and the nuclear pore complex during nuclear export. Curr Biol 1997; 7:767-75; PMID:9368759; http://dx.doi.org/10.1016/S0960-9822(06)00335-6 [DOI] [PubMed] [Google Scholar]
- 4. Bogerd HP, Echarri A, Ross TM, Cullen BR. Inhibition of human immunodeficiency virus Rev and human T-cell leukemia virus Rex function, but not Mason-Pfizer monkey virus constitutive transport element activity, by a mutant human nucleoporin targeted to Crm1. J Virol 1998; 72:8627-35; PMID:9765402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Felber BK, Hadzopoulou-Cladaras M, Cladaras C, Copeland T, Pavlakis GN. rev protein of human immunodeficiency virus type 1 affects the stability and transport of the viral mRNA. Proc Natl Acad Sci U S A 1989; 86:1495-9; PMID:2784208; http://dx.doi.org/10.1073/pnas.86.5.1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Malim MH, Hauber J, Le SY, Maizel JV, Cullen BR. The HIV-1 rev trans-activator acts through a structured target sequence to activate nuclear export of unspliced viral mRNA. Nature 1989; 338:254-7; PMID:2784194; http://dx.doi.org/10.1038/338254a0 [DOI] [PubMed] [Google Scholar]
- 7. Rosen CA, Terwilliger E, Dayton A, Sodroski JG, Haseltine WA. Intragenic cis-acting art gene-responsive sequences of the human immunodeficiency virus. Proc Natl Acad Sci U S A 1988; 85:2071-5; PMID:2832844; http://dx.doi.org/10.1073/pnas.85.7.2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Berkhout B. Structure and function of the human immunodeficiency virus leader RNA. Prog Nucleic Acid Res Mol Biol 1996; 54:1-34; PMID:8768071; http://dx.doi.org/10.1016/S0079-6603(08)60359-1 [DOI] [PubMed] [Google Scholar]
- 9. Roebuck KA, Saifuddin M. Regulation of HIV-1 transcription. Gene Expr 1999; 8:67-84; PMID:10551796 [PMC free article] [PubMed] [Google Scholar]
- 10. Cochrane AW, McNally MT, Mouland AJ. The retrovirus RNA trafficking granule: from birth to maturity. Retrovirology 2006; 3:18; PMID:16545126; http://dx.doi.org/10.1186/1742-4690-3-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arts EJ, Le Grice SF. Interaction of retroviral reverse transcriptase with template-primer duplexes during replication. Prog Nucleic Acid Res Mol Biol 1998; 58:339-93; PMID:9308371; http://dx.doi.org/10.1016/S0079-6603(08)60041-0 [DOI] [PubMed] [Google Scholar]
- 12. Paillart JC, Dettenhofer M, Yu XF, Ehresmann C, Ehresmann B, Marquet R. First snapshots of the HIV-1 RNA structure in infected cells and in virions. J Biol Chem 2004; 279:48397-403; PMID:15355993; http://dx.doi.org/10.1074/jbc.M408294200 [DOI] [PubMed] [Google Scholar]
- 13. D’Souza V, Summers MF. How retroviruses select their genomes. Nat Rev Microbiol 2005; 3:643-55; PMID:16064056; http://dx.doi.org/10.1038/nrmicro1210 [DOI] [PubMed] [Google Scholar]
- 14. McCann EM, Lever AM. Location of cis-acting signals important for RNA encapsidation in the leader sequence of human immunodeficiency virus type 2. J Virol 1997; 71:4133-7; PMID:9094696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Damgaard CK, Dyhr-Mikkelsen H, Kjems J. Mapping the RNA binding sites for human immunodeficiency virus type-1 gag and NC proteins within the complete HIV-1 and -2 untranslated leader regions. Nucleic Acids Res 1998; 26:3667-76; PMID:9685481; http://dx.doi.org/10.1093/nar/26.16.3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Watts JM, Dang KK, Gorelick RJ, Leonard CW, Bess JW, Jr., Swanstrom R, Burch CL, Weeks KM. Architecture and secondary structure of an entire HIV-1 RNA genome. Nature 2009; 460:711-6; PMID:19661910; http://dx.doi.org/10.1038/nature08237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Berkhout B. Structural features in TAR RNA of human and simian immunodeficiency viruses: a phylogenetic analysis. Nucleic Acids Res 1992; 20:27-31; PMID:1738599; http://dx.doi.org/10.1093/nar/20.1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Berkhout B, Schoneveld I. Secondary structure of the HIV-2 leader RNA comprising the tRNA-primer binding site. Nucleic Acids Res 1993; 21:1171-8; PMID:8464701; http://dx.doi.org/10.1093/nar/21.5.1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jackson RJ, Hellen CU, Pestova TV. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat Rev Mol Cell Biol 2010; 11:113-27; PMID:20094052; http://dx.doi.org/10.1038/nrm2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell 2009; 136:731-45; PMID:19239892; http://dx.doi.org/10.1016/j.cell.2009.01.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nielsen KH, Behrens MA, He Y, Oliveira CL, Jensen LS, Hoffmann SV, Pedersen JS, Andersen GR. Synergistic activation of eIF4A by eIF4B and eIF4G. Nucleic Acids Res 2011; 39:2678-89; PMID:21113024; http://dx.doi.org/10.1093/nar/gkq1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jackson RJ, Kaminski A. Internal initiation of translation in eukaryotes: the picornavirus paradigm and beyond. RNA 1995; 1:985-1000; PMID:8595564 [PMC free article] [PubMed] [Google Scholar]
- 23. Jang SK, Kräusslich HG, Nicklin MJ, Duke GM, Palmenberg AC, Wimmer E. A segment of the 51602; nontranslated region of encephalomyocarditis virus RNA directs internal entry of ribosomes during in vitro translation. J Virol 1988; 62:2636-43; PMID:2839690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pelletier J, Sonenberg N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature 1988; 334:320-5; PMID:2839775; http://dx.doi.org/10.1038/334320a0 [DOI] [PubMed] [Google Scholar]
- 25. Kräusslich HG, Nicklin MJ, Toyoda H, Etchison D, Wimmer E. Poliovirus proteinase 2A induces cleavage of eucaryotic initiation factor 4F polypeptide p220. J Virol 1987; 61:2711-8; PMID:3039165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Joachims M, Van Breugel PC, Lloyd RE. Cleavage of poly(A)-binding protein by enterovirus proteases concurrent with inhibition of translation in vitro. J Virol 1999; 73:718-27; PMID:9847378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lamphear BJ, Kirchweger R, Skern T, Rhoads RE. Mapping of functional domains in eukaryotic protein synthesis initiation factor 4G (eIF4G) with picornaviral proteases. Implications for cap-dependent and cap-independent translational initiation. J Biol Chem 1995; 270:21975-83; PMID:7665619; http://dx.doi.org/10.1074/jbc.270.37.21975 [DOI] [PubMed] [Google Scholar]
- 28. Gingras AC, Svitkin Y, Belsham GJ, Pause A, Sonenberg N. Activation of the translational suppressor 4E-BP1 following infection with encephalomyocarditis virus and poliovirus. Proc Natl Acad Sci U S A 1996; 93:5578-83; PMID:8643618; http://dx.doi.org/10.1073/pnas.93.11.5578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lloyd RE. Translational control by viral proteinases. Virus Res 2006; 119:76-88; PMID:16303201; http://dx.doi.org/10.1016/j.virusres.2005.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Balvay L, Lopez Lastra M, Sargueil B, Darlix JL, Ohlmann T. Translational control of retroviruses. Nat Rev Microbiol 2007; 5:128-40; PMID:17224922; http://dx.doi.org/10.1038/nrmicro1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. de Breyne S, Soto-Rifo R, López-Lastra M, Ohlmann T. Translation initiation is driven by different mechanisms on the HIV-1 and HIV-2 genomic RNAs. Virus Res 2013; 171:366-81; PMID:23079111; http://dx.doi.org/10.1016/j.virusres.2012.10.006 [DOI] [PubMed] [Google Scholar]
- 32. Monette A, Valiente-Echeverría F, Rivero M, Cohen EA, Lopez-Lastra M, Mouland AJ. Dual mechanisms of translation initiation of the full-length HIV-1 mRNA contribute to gag synthesis. PLoS One 2013; 8:e68108; PMID:23861855; http://dx.doi.org/10.1371/journal.pone.0068108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ricci EP, Soto Rifo R, Herbreteau CH, Decimo D, Ohlmann T. Lentiviral RNAs can use different mechanisms for translation initiation. Biochem Soc Trans 2008; 36:690-3; PMID:18631141; http://dx.doi.org/10.1042/BST0360690 [DOI] [PubMed] [Google Scholar]
- 34. Soto-Rifo R, Limousin T, Rubilar PS, Ricci EP, Décimo D, Moncorgé O, Trabaud MA, André P, Cimarelli A, Ohlmann T. Different effects of the TAR structure on HIV-1 and HIV-2 genomic RNA translation. Nucleic Acids Res 2012; 40:2653-67; PMID:22121214; http://dx.doi.org/10.1093/nar/gkr1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Parkin NT, Cohen EA, Darveau A, Rosen C, Haseltine W, Sonenberg N. Mutational analysis of the 51602; non-coding region of human immunodeficiency virus type 1: effects of secondary structure on translation. EMBO J 1988; 7:2831-7; PMID:3181141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pestova TV, Kolupaeva VG. The roles of individual eukaryotic translation initiation factors in ribosomal scanning and initiation codon selection. Genes Dev 2002; 16:2906-22; PMID:12435632; http://dx.doi.org/10.1101/gad.1020902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dugré-Brisson S, Elvira G, Boulay K, Chatel-Chaix L, Mouland AJ, DesGroseillers L. Interaction of Staufen1 with the 51602; end of mRNA facilitates translation of these RNAs. Nucleic Acids Res 2005; 33:4797-812; PMID:16126845; http://dx.doi.org/10.1093/nar/gki794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Svitkin YV, Pause A, Sonenberg N. La autoantigen alleviates translational repression by the 51602; leader sequence of the human immunodeficiency virus type 1 mRNA. J Virol 1994; 68:7001-7; PMID:7933082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pause A, Méthot N, Svitkin Y, Merrick WC, Sonenberg N. Dominant negative mutants of mammalian translation initiation factor eIF-4A define a critical role for eIF-4F in cap-dependent and cap-independent initiation of translation. EMBO J 1994; 13:1205-15; PMID:8131750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. de Breyne S, Chamond N, Décimo D, Trabaud MA, André P, Sargueil B, Ohlmann T. In vitro studies reveal that different modes of initiation on HIV-1 mRNA have different levels of requirement for eukaryotic initiation factor 4F. FEBS J 2012; 279:3098-111; PMID:22759308; http://dx.doi.org/10.1111/j.1742-4658.2012.08689.x [DOI] [PubMed] [Google Scholar]
- 41. Vallejos M, Deforges J, Plank TD, Letelier A, Ramdohr P, Abraham CG, Valiente-Echeverría F, Kieft JS, Sargueil B, López-Lastra M. Activity of the human immunodeficiency virus type 1 cell cycle-dependent internal ribosomal entry site is modulated by IRES trans-acting factors. Nucleic Acids Res 2011; 39:6186-200; PMID:21482538; http://dx.doi.org/10.1093/nar/gkr189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Braddock M, Powell R, Blanchard AD, Kingsman AJ, Kingsman SM. HIV-1 TAR RNA-binding proteins control TAT activation of translation in Xenopus oocytes. FASEB J 1993; 7:214-22; PMID:8422967 [DOI] [PubMed] [Google Scholar]
- 43. Huthoff H, Berkhout B. Two alternating structures of the HIV-1 leader RNA. RNA 2001; 7:143-57; PMID:11214176; http://dx.doi.org/10.1017/S1355838201001881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Abbink TE, Ooms M, Haasnoot PC, Berkhout B. The HIV-1 leader RNA conformational switch regulates RNA dimerization but does not regulate mRNA translation. Biochemistry 2005; 44:9058-66; PMID:15966729; http://dx.doi.org/10.1021/bi0502588 [DOI] [PubMed] [Google Scholar]
- 45. Berkhout B, Ooms M, Beerens N, Huthoff H, Southern E, Verhoef K. In vitro evidence that the untranslated leader of the HIV-1 genome is an RNA checkpoint that regulates multiple functions through conformational changes. J Biol Chem 2002; 277:19967-75; PMID:11896057; http://dx.doi.org/10.1074/jbc.M200950200 [DOI] [PubMed] [Google Scholar]
- 46. Ooms M, Huthoff H, Russell R, Liang C, Berkhout B. A riboswitch regulates RNA dimerization and packaging in human immunodeficiency virus type 1 virions. J Virol 2004; 78:10814-9; PMID:15367648; http://dx.doi.org/10.1128/JVI.78.19.10814-10819.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lu K, Heng X, Garyu L, Monti S, Garcia EL, Kharytonchyk S, Dorjsuren B, Kulandaivel G, Jones S, Hiremath A, et al. NMR detection of structures in the HIV-1 51602;-leader RNA that regulate genome packaging. Science 2011; 334:242-5; PMID:21998393; http://dx.doi.org/10.1126/science.1210460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Abbink TE, Berkhout B. A novel long distance base-pairing interaction in human immunodeficiency virus type 1 RNA occludes the Gag start codon. J Biol Chem 2003; 278:11601-11; PMID:12458192; http://dx.doi.org/10.1074/jbc.M210291200 [DOI] [PubMed] [Google Scholar]
- 49. Anderson EC, Lever AM. Human immunodeficiency virus type 1 Gag polyprotein modulates its own translation. J Virol 2006; 80:10478-86; PMID:17041220; http://dx.doi.org/10.1128/JVI.02596-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Baudin F, Marquet R, Isel C, Darlix JL, Ehresmann B, Ehresmann C. Functional sites in the 51602; region of human immunodeficiency virus type 1 RNA form defined structural domains. J Mol Biol 1993; 229:382-97; PMID:8429553; http://dx.doi.org/10.1006/jmbi.1993.1041 [DOI] [PubMed] [Google Scholar]
- 51. Berkhout B, Arts K, Abbink TE. Ribosomal scanning on the 51602;-untranslated region of the human immunodeficiency virus RNA genome. Nucleic Acids Res 2011; 39:5232-44; PMID:21393254; http://dx.doi.org/10.1093/nar/gkr113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kozak M. Influence of mRNA secondary structure on binding and migration of 40S ribosomal subunits. Cell 1980; 19:79-90; PMID:7357609; http://dx.doi.org/10.1016/0092-8674(80)90390-6 [DOI] [PubMed] [Google Scholar]
- 53. Kozak M. Effects of long 51602; leader sequences on initiation by eukaryotic ribosomes in vitro. Gene Expr 1991; 1:117-25; PMID:1820209 [PMC free article] [PubMed] [Google Scholar]
- 54. Hinnebusch AG. Molecular mechanism of scanning and start codon selection in eukaryotes. [first page of table of contents.]. Microbiol Mol Biol Rev 2011; 75:434-67; PMID:21885680; http://dx.doi.org/10.1128/MMBR.00008-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Parsyan A, Svitkin Y, Shahbazian D, Gkogkas C, Lasko P, Merrick WC, Sonenberg N. mRNA helicases: the tacticians of translational control. Nat Rev Mol Cell Biol 2011; 12:235-45; PMID:21427765; http://dx.doi.org/10.1038/nrm3083 [DOI] [PubMed] [Google Scholar]
- 56. Jankowsky E, Bowers H. Remodeling of ribonucleoprotein complexes with DExH/D RNA helicases.Nucleic Acids Res 2006; 34:4181-8; PMID:16935886; http://dx.doi.org/10.1093/nar/gkl410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Linder P, Jankowsky E. From unwinding to clamping - the DEAD box RNA helicase family. Nat Rev Mol Cell Biol 2011; 12:505-16; PMID:21779027; http://dx.doi.org/10.1038/nrm3154 [DOI] [PubMed] [Google Scholar]
- 58. Hartman TR, Qian S, Bolinger C, Fernandez S, Schoenberg DR, Boris-Lawrie K. RNA helicase A is necessary for translation of selected messenger RNAs. Nat Struct Mol Biol 2006; 13:509-16; PMID:16680162; http://dx.doi.org/10.1038/nsmb1092 [DOI] [PubMed] [Google Scholar]
- 59. Lai MC, Lee YH, Tarn WY. The DEAD-box RNA helicase DDX3 associates with export messenger ribonucleoproteins as well as tip-associated protein and participates in translational control. Mol Biol Cell 2008; 19:3847-58; PMID:18596238; http://dx.doi.org/10.1091/mbc.E07-12-1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pisareva VP, Pisarev AV, Komar AA, Hellen CU, Pestova TV. Translation initiation on mammalian mRNAs with structured 5’UTRs requires DExH-box protein DHX29. Cell 2008; 135:1237-50; PMID:19109895; http://dx.doi.org/10.1016/j.cell.2008.10.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bolinger C, Sharma A, Singh D, Yu L, Boris-Lawrie K. RNA helicase A modulates translation of HIV-1 and infectivity of progeny virions. Nucleic Acids Res 2010; 38:1686-96; PMID:20007598; http://dx.doi.org/10.1093/nar/gkp1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hilliker A, Gao Z, Jankowsky E, Parker R. The DEAD-box protein Ded1 modulates translation by the formation and resolution of an eIF4F-mRNA complex. Mol Cell 2011; 43:962-72; PMID:21925384; http://dx.doi.org/10.1016/j.molcel.2011.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Schröder M. Viruses and the human DEAD-box helicase DDX3: inhibition or exploitation? Biochem Soc Trans 2011; 39:679-83; PMID:21428961; http://dx.doi.org/10.1042/BST0390679 [DOI] [PubMed] [Google Scholar]
- 64. Lahn BT, Page DC. Functional coherence of the human Y chromosome. Science 1997; 278:675-80; PMID:9381176; http://dx.doi.org/10.1126/science.278.5338.675 [DOI] [PubMed] [Google Scholar]
- 65. Park SH, Lee SG, Kim Y, Song K. Assignment of a human putative RNA helicase gene, DDX3, to human X chromosome bands p11.3–>p11.23. Cytogenet Cell Genet 1998; 81:178-9; PMID:9730595; http://dx.doi.org/10.1159/000015022 [DOI] [PubMed] [Google Scholar]
- 66. Foresta C, Ferlin A, Moro E. Deletion and expression analysis of AZFa genes on the human Y chromosome revealed a major role for DBY in male infertility. Hum Mol Genet 2000; 9:1161-9; PMID:10767340; http://dx.doi.org/10.1093/hmg/9.8.1161 [DOI] [PubMed] [Google Scholar]
- 67. Yedavalli VS, Neuveut C, Chi YH, Kleiman L, Jeang KT. Requirement of DDX3 DEAD box RNA helicase for HIV-1 Rev-RRE export function. Cell 2004; 119:381-92; PMID:15507209; http://dx.doi.org/10.1016/j.cell.2004.09.029 [DOI] [PubMed] [Google Scholar]
- 68. Lai MC, Chang WC, Shieh SY, Tarn WY. DDX3 regulates cell growth through translational control of cyclin E1. Mol Cell Biol 2010; 30:5444-53; PMID:20837705; http://dx.doi.org/10.1128/MCB.00560-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tarn WY, Chang TH. The current understanding of Ded1p/DDX3 homologs from yeast to human. RNA Biol 2009; 6:17-20; PMID:19106629; http://dx.doi.org/10.4161/rna.6.1.7440 [DOI] [PubMed] [Google Scholar]
- 70. Abaeva IS, Marintchev A, Pisareva VP, Hellen CU, Pestova TV. Bypassing of stems versus linear base-by-base inspection of mammalian mRNAs during ribosomal scanning. EMBO J 2011; 30:115-29; PMID:21113134; http://dx.doi.org/10.1038/emboj.2010.302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Liu J, Henao-Mejia J, Liu H, Zhao Y, He JJ. Translational regulation of HIV-1 replication by HIV-1 Rev cellular cofactors Sam68, eIF5A, hRIP, and DDX3. J Neuroimmune Pharmacol 2011; 6:308-21; PMID:21360055; http://dx.doi.org/10.1007/s11481-011-9265-8 [DOI] [PubMed] [Google Scholar]
- 72. Soto-Rifo R, Rubilar PS, Limousin T, de Breyne S, Décimo D, Ohlmann T. DEAD-box protein DDX3 associates with eIF4F to promote translation of selected mRNAs. EMBO J 2012; 31:3745-56; PMID:22872150; http://dx.doi.org/10.1038/emboj.2012.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yang Q, Del Campo M, Lambowitz AM, Jankowsky E. DEAD-box proteins unwind duplexes by local strand separation. Mol Cell 2007; 28:253-63; PMID:17964264; http://dx.doi.org/10.1016/j.molcel.2007.08.016 [DOI] [PubMed] [Google Scholar]
- 74. Sharma A, Yilmaz A, Marsh K, Cochrane A, Boris-Lawrie K. Thriving under stress: selective translation of HIV-1 structural protein mRNA during Vpr-mediated impairment of eIF4E translation activity. PLoS Pathog 2012; 8:e1002612; PMID:22457629; http://dx.doi.org/10.1371/journal.ppat.1002612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Berkhout B, Silverman RH, Jeang KT. Tat trans-activates the human immunodeficiency virus through a nascent RNA target. Cell 1989; 59:273-82; PMID:2478293; http://dx.doi.org/10.1016/0092-8674(89)90289-4 [DOI] [PubMed] [Google Scholar]
- 76. Lai MC, Wang SW, Cheng L, Tarn WY, Tsai SJ, Sun HS. Human DDX3 interacts with the HIV-1 Tat protein to facilitate viral mRNA translation. PLoS One 2013; 8:e68665; PMID:23840900; http://dx.doi.org/10.1371/journal.pone.0068665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yasuda-Inoue M, Kuroki M, Ariumi Y. DDX3 RNA helicase is required for HIV-1 Tat function. Biochem Biophys Res Commun 2013; 441:607-11; PMID:24183723; http://dx.doi.org/10.1016/j.bbrc.2013.10.107 [DOI] [PubMed] [Google Scholar]
- 78. Chang YN, Kenan DJ, Keene JD, Gatignol A, Jeang KT. Direct interactions between autoantigen La and human immunodeficiency virus leader RNA. J Virol 1994; 68:7008-20; PMID:7933083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Craig AW, Svitkin YV, Lee HS, Belsham GJ, Sonenberg N. The La autoantigen contains a dimerization domain that is essential for enhancing translation. Mol Cell Biol 1997; 17:163-9; PMID:8972196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hühn P, Pruijn GJ, van Venrooij WJ, Bachmann M. Characterization of the autoantigen La (SS-B) as a dsRNA unwinding enzyme. Nucleic Acids Res 1997; 25:410-6; PMID:9016572; http://dx.doi.org/10.1093/nar/25.2.410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Dorin D, Bonnet MC, Bannwarth S, Gatignol A, Meurs EF, Vaquero C. The TAR RNA-binding protein, TRBP, stimulates the expression of TAR-containing RNAs in vitro and in vivo independently of its ability to inhibit the dsRNA-dependent kinase PKR. J Biol Chem 2003; 278:4440-8; PMID:12475984; http://dx.doi.org/10.1074/jbc.M208954200 [DOI] [PubMed] [Google Scholar]
- 82. Aguilera A. Cotranscriptional mRNP assembly: from the DNA to the nuclear pore. Curr Opin Cell Biol 2005; 17:242-50; PMID:15901492; http://dx.doi.org/10.1016/j.ceb.2005.03.001 [DOI] [PubMed] [Google Scholar]
- 83. Maquat LE, Hwang J, Sato H, Tang Y. CBP80-promoted mRNP rearrangements during the pioneer round of translation, nonsense-mediated mRNA decay, and thereafter. Cold Spring Harb Symp Quant Biol 2010; 75:127-34; PMID:21447822; http://dx.doi.org/10.1101/sqb.2010.75.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Maquat LE, Tarn WY, Isken O. The pioneer round of translation: features and functions. Cell 2010; 142:368-74; PMID:20691898; http://dx.doi.org/10.1016/j.cell.2010.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Topisirovic I, Svitkin YV, Sonenberg N, Shatkin AJ. Cap and cap-binding proteins in the control of gene expression. Wiley Interdiscip Rev RNA 2011; 2:277-98; PMID:21957010; http://dx.doi.org/10.1002/wrna.52 [DOI] [PubMed] [Google Scholar]
- 86. Brasey A, Lopez-Lastra M, Ohlmann T, Beerens N, Berkhout B, Darlix JL, Sonenberg N. The leader of human immunodeficiency virus type 1 genomic RNA harbors an internal ribosome entry segment that is active during the G2/M phase of the cell cycle. J Virol 2003; 77:3939-49; PMID:12634354; http://dx.doi.org/10.1128/JVI.77.7.3939-3949.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Buck CB, Shen X, Egan MA, Pierson TC, Walker CM, Siliciano RF. The human immunodeficiency virus type 1 gag gene encodes an internal ribosome entry site. J Virol 2001; 75:181-91; PMID:11119587; http://dx.doi.org/10.1128/JVI.75.1.181-191.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gendron K, Ferbeyre G, Heveker N, Brakier-Gingras L. The activity of the HIV-1 IRES is stimulated by oxidative stress and controlled by a negative regulatory element. Nucleic Acids Res 2011; 39:902-12; PMID:20935056; http://dx.doi.org/10.1093/nar/gkq885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Vallejos M, Carvajal F, Pino K, Navarrete C, Ferres M, Huidobro-Toro JP, Sargueil B, López-Lastra M. Functional and structural analysis of the internal ribosome entry site present in the mRNA of natural variants of the HIV-1. PLoS One 2012; 7:e35031; PMID:22496887; http://dx.doi.org/10.1371/journal.pone.0035031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Plank TD, Whitehurst JT, Kieft JS. Cell type specificity and structural determinants of IRES activity from the 51602; leaders of different HIV-1 transcripts. Nucleic Acids Res 2013; 41:6698-714; PMID:23661682; http://dx.doi.org/10.1093/nar/gkt358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Amorim R, Costa SM, Cavaleiro NP, da Silva EE, da Costa LJ. HIV-1 transcripts use IRES-initiation under conditions where Cap-dependent translation is restricted by poliovirus 2A protease. PLoS One 2014; 9:e88619; PMID:24520405; http://dx.doi.org/10.1371/journal.pone.0088619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Fernández-Miragall O, López de Quinto S, Martínez-Salas E. Relevance of RNA structure for the activity of picornavirus IRES elements. Virus Res 2009; 139:172-82; PMID:18692097; http://dx.doi.org/10.1016/j.virusres.2008.07.009 [DOI] [PubMed] [Google Scholar]
- 93. Filbin ME, Kieft JS. Toward a structural understanding of IRES RNA function. Curr Opin Struct Biol 2009; 19:267-76; PMID:19362464; http://dx.doi.org/10.1016/j.sbi.2009.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Fitzgerald KD, Semler BL. Bridging IRES elements in mRNAs to the eukaryotic translation apparatus. Biochim Biophys Acta 2009; 1789:518-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Pfingsten JS, Costantino DA, Kieft JS. Structural basis for ribosome recruitment and manipulation by a viral IRES RNA. Science 2006; 314:1450-4; PMID:17124290; http://dx.doi.org/10.1126/science.1133281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kieft JS. Viral IRES RNA structures and ribosome interactions. Trends Biochem Sci 2008; 33:274-83; PMID:18468443; http://dx.doi.org/10.1016/j.tibs.2008.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Martínez-Salas E. The impact of RNA structure on picornavirus IRES activity. Trends Microbiol 2008; 16:230-7; PMID:18420413; http://dx.doi.org/10.1016/j.tim.2008.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Martínez-Salas E, Sáiz JC, Dávila M, Belsham GJ, Domingo E. A single nucleotide substitution in the internal ribosome entry site of foot-and-mouth disease virus leads to enhanced cap-independent translation in vivo. J Virol 1993; 67:3748-55; PMID:8389904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Cochrane AW, Jones KS, Beidas S, Dillon PJ, Skalka AM, Rosen CA. Identification and characterization of intragenic sequences which repress human immunodeficiency virus structural gene expression. J Virol 1991; 65:5305-13; PMID:1895385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hadzopoulou-Cladaras M, Felber BK, Cladaras C, Athanassopoulos A, Tse A, Pavlakis GN. The rev (trs/art) protein of human immunodeficiency virus type 1 affects viral mRNA and protein expression via a cis-acting sequence in the env region. J Virol 1989; 63:1265-74; PMID:2783738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Schwartz S, Campbell M, Nasioulas G, Harrison J, Felber BK, Pavlakis GN. Mutational inactivation of an inhibitory sequence in human immunodeficiency virus type 1 results in Rev-independent gag expression. J Virol 1992; 66:7176-82; PMID:1433510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Wolff H, Brack-Werner R, Neumann M, Werner T, Schneider R. Integrated functional and bioinformatics approach for the identification and experimental verification of RNA signals: application to HIV-1 INS. Nucleic Acids Res 2003; 31:2839-51; PMID:12771211; http://dx.doi.org/10.1093/nar/gkg390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Huffman KM, Arrigo SJ. Identification of cis-acting repressor activity within human immunodeficiency virus type 1 protease sequences. Virology 1997; 234:253-60; PMID:9268156; http://dx.doi.org/10.1006/viro.1997.8655 [DOI] [PubMed] [Google Scholar]
- 104. Maldarelli F, Martin MA, Strebel K. Identification of posttranscriptionally active inhibitory sequences in human immunodeficiency virus type 1 RNA: novel level of gene regulation. J Virol 1991; 65:5732-43; PMID:1656066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Mikaélian I, Krieg M, Gait MJ, Karn J. Interactions of INS (CRS) elements and the splicing machinery regulate the production of Rev-responsive mRNAs. J Mol Biol 1996; 257:246-64; PMID:8609621; http://dx.doi.org/10.1006/jmbi.1996.0160 [DOI] [PubMed] [Google Scholar]
- 106. Afonina E, Neumann M, Pavlakis GN. Preferential binding of poly(A)-binding protein 1 to an inhibitory RNA element in the human immunodeficiency virus type 1 gag mRNA. J Biol Chem 1997; 272:2307-11; PMID:8999938; http://dx.doi.org/10.1074/jbc.272.4.2307 [DOI] [PubMed] [Google Scholar]
- 107. Black AC, Luo J, Chun S, Bakker A, Fraser JK, Rosenblatt JD. Specific binding of polypyrimidine tract binding protein and hnRNP A1 to HIV-1 CRS elements. Virus Genes 1996; 12:275-85; PMID:8883365; http://dx.doi.org/10.1007/BF00284648 [DOI] [PubMed] [Google Scholar]
- 108. Olsen HS, Cochrane AW, Rosen C. Interaction of cellular factors with intragenic cis-acting repressive sequences within the HIV genome. Virology 1992; 191:709-15; PMID:1448921; http://dx.doi.org/10.1016/0042-6822(92)90246-L [DOI] [PubMed] [Google Scholar]
- 109. Zolotukhin AS, Michalowski D, Bear J, Smulevitch SV, Traish AM, Peng R, Patton J, Shatsky IN, Felber BK. PSF acts through the human immunodeficiency virus type 1 mRNA instability elements to regulate virus expression. Mol Cell Biol 2003; 23:6618-30; PMID:12944487; http://dx.doi.org/10.1128/MCB.23.18.6618-6630.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Jahan N, Wimmer E, Mueller S. Polypyrimidine tract binding protein-1 (PTB1) is a determinant of the tissue and host tropism of a human rhinovirus/poliovirus chimera PV1(RIPO). PLoS One 2013; 8:e60791; PMID:23593313; http://dx.doi.org/10.1371/journal.pone.0060791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Gromeier M, Bossert B, Arita M, Nomoto A, Wimmer E. Dual stem loops within the poliovirus internal ribosomal entry site control neurovirulence. J Virol 1999; 73:958-64; PMID:9882296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Conrad KD, Niepmann M. The role of microRNAs in hepatitis C virus RNA replication. Arch Virol 2013; PMID:24158346 [DOI] [PubMed] [Google Scholar]
- 113. Fehr C, Conrad KD, Niepmann M. Differential stimulation of hepatitis C virus RNA translation by microRNA-122 in different cell cycle phases. Cell Cycle 2012; 11:277-85; PMID:22189820; http://dx.doi.org/10.4161/cc.11.2.18699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Durand T, Di Liberto G, Colman H, Cammas A, Boni S, Marcellin P, Cahour A, Vagner S, Féray C. Occult infection of peripheral B cells by hepatitis C variants which have low translational efficiency in cultured hepatocytes. Gut 2010; 59:934-42; PMID:20442199; http://dx.doi.org/10.1136/gut.2009.192088 [DOI] [PubMed] [Google Scholar]
- 115. Herbreteau CH, Weill L, Décimo D, Prévôt D, Darlix JL, Sargueil B, Ohlmann T. HIV-2 genomic RNA contains a novel type of IRES located downstream of its initiation codon. Nat Struct Mol Biol 2005; 12:1001-7; PMID:16244661; http://dx.doi.org/10.1038/nsmb1011 [DOI] [PubMed] [Google Scholar]
- 116. Nicholson MG, Rue SM, Clements JE, Barber SA. An internal ribosome entry site promotes translation of a novel SIV Pr55(Gag) isoform. Virology 2006; 349:325-34; PMID:16494914; http://dx.doi.org/10.1016/j.virol.2006.01.034 [DOI] [PubMed] [Google Scholar]
- 117. Weill L, James L, Ulryck N, Chamond N, Herbreteau CH, Ohlmann T, Sargueil B. A new type of IRES within gag coding region recruits three initiation complexes on HIV-2 genomic RNA. Nucleic Acids Res 2010; 38:1367-81; PMID:19969542; http://dx.doi.org/10.1093/nar/gkp1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Monette A, Ajamian L, López-Lastra M, Mouland AJ. Human immunodeficiency virus type 1 (HIV-1) induces the cytoplasmic retention of heterogeneous nuclear ribonucleoprotein A1 by disrupting nuclear import: implications for HIV-1 gene expression. J Biol Chem 2009; 284:31350-62; PMID:19737937; http://dx.doi.org/10.1074/jbc.M109.048736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Wilker EW, van Vugt MA, Artim SA, Huang PH, Petersen CP, Reinhardt HC, Feng Y, Sharp PA, Sonenberg N, White FM, et al. 14-3-3sigma controls mitotic translation to facilitate cytokinesis. Nature 2007; 446:329-32; PMID:17361185; http://dx.doi.org/10.1038/nature05584 [DOI] [PubMed] [Google Scholar]
- 120. Rivas-Aravena A, Ramdohr P, Vallejos M, Valiente-Echeverría F, Dormoy-Raclet V, Rodríguez F, Pino K, Holzmann C, Huidobro-Toro JP, Gallouzi IE, et al. The Elav-like protein HuR exerts translational control of viral internal ribosome entry sites. Virology 2009; 392:178-85; PMID:19647848; http://dx.doi.org/10.1016/j.virol.2009.06.050 [DOI] [PubMed] [Google Scholar]
- 121. Miele G, Mouland A, Harrison GP, Cohen E, Lever AM. The human immunodeficiency virus type 1 51602; packaging signal structure affects translation but does not function as an internal ribosome entry site structure. J Virol 1996; 70:944-51; PMID:8551634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. King HA, Cobbold LC, Willis AE. The role of IRES trans-acting factors in regulating translation initiation. Biochem Soc Trans 2010; 38:1581-6; PMID:21118130; http://dx.doi.org/10.1042/BST0381581 [DOI] [PubMed] [Google Scholar]
- 123. Komar AA, Hatzoglou M. Cellular IRES-mediated translation: the war of ITAFs in pathophysiological states. Cell Cycle 2011; 10:229-40; PMID:21220943; http://dx.doi.org/10.4161/cc.10.2.14472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Stoneley M, Willis AE. Cellular internal ribosome entry segments: structures, trans-acting factors and regulation of gene expression. Oncogene 2004; 23:3200-7; PMID:15094769; http://dx.doi.org/10.1038/sj.onc.1207551 [DOI] [PubMed] [Google Scholar]
- 125. Semler BL, Waterman ML. IRES-mediated pathways to polysomes: nuclear versus cytoplasmic routes. Trends Microbiol 2008; 16:1-5; PMID:18083033; http://dx.doi.org/10.1016/j.tim.2007.11.001 [DOI] [PubMed] [Google Scholar]
- 126. Cochrane A. How does the journey affect the message(RNA)? RNA Biol 2009; 6:169-70; PMID:19246987; http://dx.doi.org/10.4161/rna.6.2.8095 [DOI] [PubMed] [Google Scholar]
- 127. D’Agostino DM, Felber BK, Harrison JE, Pavlakis GN. The Rev protein of human immunodeficiency virus type 1 promotes polysomal association and translation of gag/pol and vpu/env mRNAs. Mol Cell Biol 1992; 12:1375-86; PMID:1545819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Nawroth I, Mueller F, Basyuk E, Beerens N, Rahbek UL, Darzacq X, Bertrand E, Kjems J, Schmidt U. Stable assembly of HIV-1 export complexes occurs cotranscriptionally. RNA 2014; 20:1-8; PMID:24255166; http://dx.doi.org/10.1261/rna.038182.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Groom HC, Anderson EC, Dangerfield JA, Lever AM. Rev regulates translation of human immunodeficiency virus type 1 RNAs. J Gen Virol 2009; 90:1141-7; PMID:19264599; http://dx.doi.org/10.1099/vir.0.007963-0 [DOI] [PubMed] [Google Scholar]
- 130. Valiente-Echeverría F, Vallejos M, Monette A, Pino K, Letelier A, Huidobro-Toro JP, Mouland AJ, López-Lastra M. A cis-acting element present within the Gag open reading frame negatively impacts on the activity of the HIV-1 IRES. PLoS One 2013; 8:e56962; PMID:23451120; http://dx.doi.org/10.1371/journal.pone.0056962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Lewis SM, Holcik M. For IRES trans-acting factors, it is all about location. Oncogene 2008; 27:1033-5; PMID:17767196; http://dx.doi.org/10.1038/sj.onc.1210777 [DOI] [PubMed] [Google Scholar]
- 132. López-Lastra M, Ramdohr P, Letelier A, Vallejos M, Vera-Otarola J, Valiente-Echeverría F. Translation initiation of viral mRNAs. Rev Med Virol 2010; 20:177-95; PMID:20440748; http://dx.doi.org/10.1002/rmv.649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Walsh D, Mohr I. Viral subversion of the host protein synthesis machinery. Nat Rev Microbiol 2011; 9:860-75; PMID:22002165; http://dx.doi.org/10.1038/nrmicro2655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Emerman M. HIV-1, Vpr and the cell cycle. Curr Biol 1996; 6:1096-103; PMID:8805364; http://dx.doi.org/10.1016/S0960-9822(02)00676-0 [DOI] [PubMed] [Google Scholar]
- 135. Pyronnet S, Dostie J, Sonenberg N. Suppression of cap-dependent translation in mitosis. Genes Dev 2001; 15:2083-93; PMID:11511540; http://dx.doi.org/10.1101/gad.889201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Thierry S, Marechal V, Rosenzwajg M, Sabbah M, Redeuilh G, Nicolas JC, Gozlan J. Cell cycle arrest in G2 induces human immunodeficiency virus type 1 transcriptional activation through histone acetylation and recruitment of CBP, NF-kappaB, and c-Jun to the long terminal repeat promoter. J Virol 2004; 78:12198-206; PMID:15507606; http://dx.doi.org/10.1128/JVI.78.22.12198-12206.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Goh WC, Rogel ME, Kinsey CM, Michael SF, Fultz PN, Nowak MA, Hahn BH, Emerman M. HIV-1 Vpr increases viral expression by manipulation of the cell cycle: a mechanism for selection of Vpr in vivo. Nat Med 1998; 4:65-71; PMID:9427608; http://dx.doi.org/10.1038/nm0198-065 [DOI] [PubMed] [Google Scholar]
- 138. Marissen WE, Guo Y, Thomas AA, Matts RL, Lloyd RE. Identification of caspase 3-mediated cleavage and functional alteration of eukaryotic initiation factor 2alpha in apoptosis. J Biol Chem 2000; 275:9314-23; PMID:10734073; http://dx.doi.org/10.1074/jbc.275.13.9314 [DOI] [PubMed] [Google Scholar]
- 139. Marissen WE, Lloyd RE. Eukaryotic translation initiation factor 4G is targeted for proteolytic cleavage by caspase 3 during inhibition of translation in apoptotic cells. Mol Cell Biol 1998; 18:7565-74; PMID:9819442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Alvarez E, Castelló A, Menéndez-Arias L, Carrasco L. HIV protease cleaves poly(A)-binding protein. Biochem J 2006; 396:219-26; PMID:16594896; http://dx.doi.org/10.1042/BJ20060108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Ohlmann T, Prévôt D, Décimo D, Roux F, Garin J, Morley SJ, Darlix JL. In vitro cleavage of eIF4GI but not eIF4GII by HIV-1 protease and its effects on translation in the rabbit reticulocyte lysate system. J Mol Biol 2002; 318:9-20; PMID:12054764; http://dx.doi.org/10.1016/S0022-2836(02)00070-0 [DOI] [PubMed] [Google Scholar]
- 142. Ventoso I, Blanco R, Perales C, Carrasco L. HIV-1 protease cleaves eukaryotic initiation factor 4G and inhibits cap-dependent translation. Proc Natl Acad Sci U S A 2001; 98:12966-71; PMID:11606767; http://dx.doi.org/10.1073/pnas.231343498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Adams LD, Tomasselli AG, Robbins P, Moss B, Heinrikson RL. HIV-1 protease cleaves actin during acute infection of human T-lymphocytes. AIDS Res Hum Retroviruses 1992; 8:291-5; PMID:1540415; http://dx.doi.org/10.1089/aid.1992.8.291 [DOI] [PubMed] [Google Scholar]
- 144. Nie Z, Phenix BN, Lum JJ, Alam A, Lynch DH, Beckett B, Krammer PH, Sekaly RP, Badley AD. HIV-1 protease processes procaspase 8 to cause mitochondrial release of cytochrome c, caspase cleavage and nuclear fragmentation. Cell Death Differ 2002; 9:1172-84; PMID:12404116; http://dx.doi.org/10.1038/sj.cdd.4401094 [DOI] [PubMed] [Google Scholar]
- 145. Rivière Y, Blank V, Kourilsky P, Israël A. Processing of the precursor of NF-kappa B by the HIV-1 protease during acute infection. Nature 1991; 350:625-6; PMID:2017258; http://dx.doi.org/10.1038/350625a0 [DOI] [PubMed] [Google Scholar]
- 146. Shoeman RL, Höner B, Stoller TJ, Mothes E, Kesselmeier C, Traub P, Graves MC. Cleavage of the intermediate filament subunit protein vimentin by HIV-1 protease: utilization of a novel cleavage site and identification of higher order polymers of pepstatin A. Adv Exp Med Biol 1991; 306:533-7; PMID:1812754; http://dx.doi.org/10.1007/978-1-4684-6012-4_72 [DOI] [PubMed] [Google Scholar]
- 147. Solis M, Nakhaei P, Jalalirad M, Lacoste J, Douville R, Arguello M, Zhao T, Laughrea M, Wainberg MA, Hiscott J. RIG-I-mediated antiviral signaling is inhibited in HIV-1 infection by a protease-mediated sequestration of RIG-I. J Virol 2011; 85:1224-36; PMID:21084468; http://dx.doi.org/10.1128/JVI.01635-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Jäger S, Cimermancic P, Gulbahce N, Johnson JR, McGovern KE, Clarke SC, Shales M, Mercenne G, Pache L, Li K, et al. Global landscape of HIV-human protein complexes. Nature 2012; 481:365-70; PMID:22190034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Castelló A, Franco D, Moral-López P, Berlanga JJ, Alvarez E, Wimmer E, Carrasco L. HIV- 1 protease inhibits Cap- and poly(A)-dependent translation upon eIF4GI and PABP cleavage. PLoS One 2009; 4:e7997; PMID:19956697; http://dx.doi.org/10.1371/journal.pone.0007997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Perales C, Carrasco L, Ventoso I. Cleavage of eIF4G by HIV-1 protease: effects on translation. FEBS Lett 2003; 533:89-94; PMID:12505164; http://dx.doi.org/10.1016/S0014-5793(02)03764-X [DOI] [PubMed] [Google Scholar]
- 151. Yedavalli VS, Jeang KT. Trimethylguanosine capping selectively promotes expression of Rev-dependent HIV-1 RNAs. Proc Natl Acad Sci U S A 2010; 107:14787-92; PMID:20679221; http://dx.doi.org/10.1073/pnas.1009490107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Zhu Y, Qi C, Cao WQ, Yeldandi AV, Rao MS, Reddy JK. Cloning and characterization of PIMT, a protein with a methyltransferase domain, which interacts with and enhances nuclear receptor coactivator PRIP function. Proc Natl Acad Sci U S A 2001; 98:10380-5; PMID:11517327; http://dx.doi.org/10.1073/pnas.181347498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Enünlü I, Pápai G, Cserpán I, Udvardy A, Jeang KT, Boros I. Different isoforms of PRIP-interacting protein with methyltransferase domain/trimethylguanosine synthase localizes to the cytoplasm and nucleus. Biochem Biophys Res Commun 2003; 309:44-51; PMID:12943661; http://dx.doi.org/10.1016/S0006-291X(03)01514-6 [DOI] [PubMed] [Google Scholar]
- 154. Darzynkiewicz E, Stepinski J, Ekiel I, Jin Y, Haber D, Sijuwade T, Tahara SM. Beta-globin mRNAs capped with m7G, m2.7(2)G or m2.2.7(3)G differ in intrinsic translation efficiency. Nucleic Acids Res 1988; 16:8953-62; PMID:3174438; http://dx.doi.org/10.1093/nar/16.18.8953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Cai A, Jankowska-Anyszka M, Centers A, Chlebicka L, Stepinski J, Stolarski R, Darzynkiewicz E, Rhoads RE. Quantitative assessment of mRNA cap analogues as inhibitors of in vitro translation. Biochemistry 1999; 38:8538-47; PMID:10387101; http://dx.doi.org/10.1021/bi9830213 [DOI] [PubMed] [Google Scholar]
- 156. Carberry SE, Darzynkiewicz E, Stepinski J, Tahara SM, Rhoads RE, Goss DJ. A spectroscopic study of the binding of N-7-substituted cap analogues to human protein synthesis initiation factor 4E. Biochemistry 1990; 29:3337-41; PMID:2334695; http://dx.doi.org/10.1021/bi00465a027 [DOI] [PubMed] [Google Scholar]
- 157. Carberry SE, Rhoads RE, Goss DJ. A spectroscopic study of the binding of m7GTP and m7GpppG to human protein synthesis initiation factor 4E. Biochemistry 1989; 28:8078-83; PMID:2605173; http://dx.doi.org/10.1021/bi00446a017 [DOI] [PubMed] [Google Scholar]
- 158. Rutkowska-Wlodarczyk I, Stepinski J, Dadlez M, Darzynkiewicz E, Stolarski R, Niedzwiecka A. Structural changes of eIF4E upon binding to the mRNA 51602; monomethylguanosine and trimethylguanosine Cap. Biochemistry 2008; 47:2710-20; PMID:18220364; http://dx.doi.org/10.1021/bi701168z [DOI] [PubMed] [Google Scholar]
- 159. Dallas A, Ilves H, Ma H, Chin DJ, Maclachlan I, Klumpp K, Johnston BH. Inhibition of hepatitis C virus in chimeric mice by short synthetic hairpin RNAs: sequence analysis of surviving virus shows added selective pressure of combination therapy. J Virol 2014; 88:4647-56; PMID:24478422; http://dx.doi.org/10.1128/JVI.00105-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Dibrov SM, Parsons J, Carnevali M, Zhou S, Rynearson KD, Ding K, Garcia Sega E, Brunn ND, Boerneke MA, Castaldi MP, et al. Hepatitis C virus translation inhibitors targeting the internal ribosomal entry site. J Med Chem 2014; 57:1694-707; PMID:24138284; http://dx.doi.org/10.1021/jm401312n [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Parsons J, Castaldi MP, Dutta S, Dibrov SM, Wyles DL, Hermann T. Conformational inhibition of the hepatitis C virus internal ribosome entry site RNA. Nat Chem Biol 2009; 5:823-5; PMID:19767736; http://dx.doi.org/10.1038/nchembio.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Kikuchi K, Umehara T, Fukuda K, Kuno A, Hasegawa T, Nishikawa S. A hepatitis C virus (HCV) internal ribosome entry site (IRES) domain III-IV-targeted aptamer inhibits translation by binding to an apical loop of domain IIId. Nucleic Acids Res 2005; 33:683-92; PMID:15681618; http://dx.doi.org/10.1093/nar/gki215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Kanai Y, Dohmae N, Hirokawa N. Kinesin transports RNA: isolation and characterization of an RNA-transporting granule. Neuron 2004; 43:513-25; PMID:15312650; http://dx.doi.org/10.1016/j.neuron.2004.07.022 [DOI] [PubMed] [Google Scholar]
- 164. Elvira G, Wasiak S, Blandford V, Tong XK, Serrano A, Fan X, del Rayo Sánchez-Carbente M, Servant F, Bell AW, Boismenu D, et al. Characterization of an RNA granule from developing brain. Mol Cell Proteomics 2006; 5:635-51; PMID:16352523; http://dx.doi.org/10.1074/mcp.M500255-MCP200 [DOI] [PubMed] [Google Scholar]
- 165. Shih JW, Wang WT, Tsai TY, Kuo CY, Li HK, Wu Lee YH. Critical roles of RNA helicase DDX3 and its interactions with eIF4E/PABP1 in stress granule assembly and stress response. Biochem J 2011; PMID:21883093 [DOI] [PubMed] [Google Scholar]
- 166. Soto-Rifo R, Rubilar PS, Ohlmann T. The DEAD-box helicase DDX3 substitutes for the cap-binding protein eIF4E to promote compartmentalized translation initiation of the HIV-1 genomic RNA. Nucleic Acids Res 2013; 41:6286-99; PMID:23630313; http://dx.doi.org/10.1093/nar/gkt306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Abrahamyan LG, Chatel-Chaix L, Ajamian L, Milev MP, Monette A, Clément JF, Song R, Lehmann M, DesGroseillers L, Laughrea M, et al. Novel Staufen1 ribonucleoproteins prevent formation of stress granules but favour encapsidation of HIV-1 genomic RNA. J Cell Sci 2010; 123:369-83; PMID:20053637; http://dx.doi.org/10.1242/jcs.055897 [DOI] [PubMed] [Google Scholar]
- 168. Garbelli A, Radi M, Falchi F, Beermann S, Zanoli S, Manetti F, Dietrich U, Botta M, Maga G. Targeting the human DEAD-box polypeptide 3 (DDX3) RNA helicase as a novel strategy to inhibit viral replication. Curr Med Chem 2011; 18:3015-27; PMID:21651478; http://dx.doi.org/10.2174/092986711796391688 [DOI] [PubMed] [Google Scholar]
- 169. Radi M, Falchi F, Garbelli A, Samuele A, Bernardo V, Paolucci S, Baldanti F, Schenone S, Manetti F, Maga G, et al. Discovery of the first small molecule inhibitor of human DDX3 specifically designed to target the RNA binding site: towards the next generation HIV-1 inhibitors. Bioorg Med Chem Lett 2012; 22:2094-8; PMID:22300661; http://dx.doi.org/10.1016/j.bmcl.2011.12.135 [DOI] [PubMed] [Google Scholar]