ABSTRACT
Summit maximum thermoregulatory metabolic rate (Msum) and maximum exercise metabolic rate (MMR) both increase in response to acute cold or exercise training in birds. Because lipids are the main fuel supporting both thermogenesis and exercise in birds, adjustments to lipid transport and catabolic capacities may support elevated energy demands from cold and exercise training. To examine a potential mechanistic role for lipid transport and catabolism in organismal cross-training effects (exercise effects on both exercise and thermogenesis, and vice versa), we measured enzyme activities and mRNA and protein expression in pectoralis muscle for several key steps of lipid transport and catabolism pathways in house sparrows (Passer domesticus) during acute exercise and cold training. Both training protocols elevated pectoralis protein levels of fatty acid translocase (FAT/CD36), cytosolic fatty acid-binding protein, and citrate synthase (CS) activity. However, mRNA expression of FAT/CD36 and both mRNA and protein expression of plasma membrane fatty acid-binding protein did not change for either training group. CS activities in supracoracoideus, leg and heart, and carnitine palmitoyl transferase (CPT) and β-hydroxyacyl CoA-dehydrogenase activities in all muscles did not vary significantly with either training protocol. Both Msum and MMR were significantly positively correlated with CPT and CS activities. These data suggest that up-regulation of trans-sarcolemmal and intramyocyte lipid transport capacities and cellular metabolic intensities, along with previously documented increases in body and pectoralis muscle masses and pectoralis myostatin (a muscle growth inhibitor) levels, are common mechanisms underlying the training effects of both exercise and shivering in birds.
KEY WORDS: Phenotypic flexibility, Cold training, Exercise training, Fatty acid translocase, Fatty acid binding protein, Carnitine palmitoyl transferase, Citrate synthase, β-Hydroxyacyl CoA-dehydrogenase
Summary: Cold and exercise training in house sparrows increase trans-sarcolemmal and intramyocyte lipid transport capacities and cellular metabolic intensities associated with elevated shivering thermogenesis and exercise capacities.
INTRODUCTION
Cross-training effects (i.e. exercise-training effects on both exercise and thermogenic performance and cold-training effects on both thermogenic and exercise performance) have been observed in both birds (Petit and Vézina, 2014; Zhang et al., 2015b) and mammals (Schaeffer et al., 2001; Boström et al., 2012). For example, Zhang et al. (Zhang et al., 2015a) documented that cold- and exercise-training protocols increased maximal organismal metabolic capacities for both the trait of training (e.g. cold) as well as the alternate trait (e.g. exercise) in house sparrows. These changes in organismal metabolic capacities were associated with increases in pectoralis muscle mass and reduced pectoralis protein levels of the muscle growth inhibitor myostatin in both training groups (Zhang et al., 2015a). In addition to increased muscle masses, increases in organismal metabolic capacities may also be associated with increases in cellular metabolic intensities or increases in supply of lipid fuels (the primary substrate during both exercise and shivering in birds; McWilliams et al., 2004; Guglielmo, 2010) to active muscles (Swanson, 2010; Zhang et al., 2015b,c). However, whether changes in metabolic pathways supporting elevated cellular metabolic intensity or lipid transport and catabolism are associated with the whole-animal cross-training effects between exercise and shivering in birds has not yet been studied.
In birds, exogenous lipids from adipose tissues are the main fuel for both prolonged shivering thermogenesis (Swanson, 2010) and flight (McWilliams et al., 2004). Consequently, capacities for non-esterified fatty acid (NEFA) uptake into the myocyte, their subsequent transport to and across mitochondrial membranes, and β-oxidation are potential regulatory steps for both shivering (Swanson, 2010; Zhang et al., 2015b) and exercise performance (Kiens, 2006; Guglielmo, 2010). Once circulating NEFA reach the myocyte, plasma membrane-bound fatty acid binding protein (FABPpm) and fatty acid translocase (FAT/CD36) cooperate to transport NEFA across the endothelium, the interstitial space, and the sarcolemma to enter muscle cells (Kiens, 2006; Glatz et al., 2010). The effects of exercise training on skeletal muscle FAT/CD36 and FABPpm levels are incompletely resolved, with studies showing either elevated (Tunstall et al., 2002; Talanian et al., 2010) or stable (Kiens et al., 2004; Price et al., 2010) FAT/CD36 and FABPpm levels after training in both mammals and birds. Moreover, migration (McFarlan et al., 2009) and winter acclimatization (Zhang et al., 2015b) are both associated with higher FAT/CD36 and/or FABPpm levels in birds.
Intramyocyte fatty acid transport in skeletal muscles is mediated, at least in part, by cytosolic fatty acid binding protein (FABPc) (Kiens, 2006; Guglielmo, 2010). Moreover, FABPc also acts as an intramyocyte fatty acid receptor and may potentially limit fatty acid uptake (Glatz et al., 2010). Most exercise training protocols have failed to change FABPc levels in both birds (Price et al., 2010) and mammals (Kiens et al., 2004; Lee et al., 2007), but studies of migration (Guglielmo et al., 2002; McFarlan et al., 2009) and winter acclimatization (Zhang et al., 2015b) showed that increases in pectoralis FABPc levels are consistent contributors to enhanced flight and shivering performance in birds. Because mobilization, transport and catabolism of exogenous fatty acids are more important for support of prolonged muscular activity in birds than in mammals (Jenni-Eiermann et al., 2002; Guglielmo, 2010; Swanson, 2010), examination of the effects of both cold and exercise training on FABPc levels in birds would be worthwhile.
List of symbols and abbreviations.
- BMR
basal metabolic rate
- CPT
carnitine palmitoyl transferase
- CS
citrate synthase
- FABPc
cytosolic fatty acid-binding protein
- FABPpm
plasma membrane fatty acid-binding protein
- FAT/CD36
fatty acid translocase
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HOAD
β-hydroxyacyl CoA-dehydrogenase
- Mb
body mass
- MMR
maximum metabolic rate (maximum exercise metabolic rate)
- Msum
summit metabolic rate (maximum thermogenic metabolic rate)
- NEFA
non-esterified fatty acid
After reaching the mitochondrial membrane, fatty acids are transported into mitochondria by the carnitine palmitoyl transferase (CPT) system (Guglielmo, 2010; Swanson, 2010; Price et al., 2011) and FAT/CD36 (Campbell et al., 2004; Schenk and Horowitz, 2006). CPT activities or levels were up-regulated during exercise training in skeletal muscle of both mammals (Starritt et al., 2000; Tunstall et al., 2002; Bruce et al., 2006) and birds (Price et al., 2010). Moreover, pectoralis CPT activities were also elevated during migration (Guglielmo et al., 2002; McFarlan et al., 2009), but not for winter acclimation (Zhang et al., 2015b) or for temperature and photoperiod manipulations in captive winter-acclimatized birds (Swanson et al., 2014b).
After transport to the mitochondrial matrix, fatty acids are catabolized through β-oxidation and the citric acid cycle. In these two pathways, β-hydroxyacyl CoA-dehydrogenase (HOAD) and citrate synthase (CS) are key regulatory enzymes, respectively (Swanson, 2010). The effects of a variety of exercise training protocols on skeletal muscle HOAD and CS activities/levels have been intensively studied. HOAD (Tremblay et al., 1994; Burgomaster et al., 2008) and CS (Siu et al., 2003; Talanian et al., 2007) activities generally increase with exercise training or cold exposure in birds and mammals, although a few studies documented stable HOAD (Burgomaster et al., 2006) or CS (Price et al., 2010) activities with exercise training. For free-living birds, studies have also documented either increased (Guglielmo et al., 2002; McFarlan et al., 2009) or stable (Liknes and Swanson, 2011) HOAD activity with both migration and winter acclimatization in birds. In addition, migratory and winter-induced variations of CS activities in birds are not universal. Increases in skeletal muscle CS activities during migration occur for many migratory (Guglielmo et al., 2002; McFarlan et al., 2009) and winter-acclimatized (Vézina and Williams, 2005; Liknes and Swanson, 2011) birds. Seasonally stable CS activities, however, also occur commonly for migratory (Driedzic et al., 1993; Weber and Piersma, 1996) and winter-acclimatized (O'Connor, 1995; Swanson, 2010) birds.
In the present study, we investigated whether lipid transport and catabolism in skeletal muscles might be associated with the previously documented cross-training effects on organismal metabolic capacities for exercise and thermogenesis of house sparrows (Passer domesticus) (Zhang et al., 2015a). We measured mRNA expression and protein levels of FAT/CD36, FABPpm and FABPc to examine potential effects of acute cold and exercise training on trans-sarcolemmal and intramyocyte lipid transport in pectoralis. We also measured CPT, CS and HOAD activities in skeletal muscles and heart to examine trans-mitochondrial membrane transport, lipid oxidation capacity and cellular metabolic intensity. We hypothesized that: (1) both trans-sarcolemmal and intramyocyte lipid transport capacities will increase for both cold and exercise training; and (2) both training protocols will also result in increased CPT, CS and HOAD activities. To our knowledge, this is the first study to concurrently measure multiple key regulatory steps of fatty acid transport and catabolism as a function of acute exercise or cold training to provide an integrative picture of lipid transport and catabolism as potential mechanisms underlying observed training effects on both exercise and thermogenesis in birds.
MATERIALS AND METHODS
All procedures in this study were approved by the University of South Dakota Institutional Animal Care and Use Committee (protocol 79-01-11-14C).
Bird capture, training protocols and tissue dissection
We used house sparrows as our study species, since the same population of house sparrows exhibited winter increases in summit metabolic rate (maximum thermogenic metabolic rate; Msum) (Swanson and Liknes, 2006) and a positive correlation between Msum and CS activities in pectoralis (Swanson et al., 2014a). Bird collection, training protocols and tissue dissection methods have been discussed in detail by Zhang et al. (2015a). Briefly, house sparrows were captured by mist net from wild populations during September 2012 (cold-training experiments, N=18) and March 2013 (exercise-training experiments, N=16). We were limited by equipment and personnel so that we could not conduct both training protocols and the subsequent metabolic measurements for both cold and exercise at the same time. We chose spring and fall because those seasons typically show intermediate organismal metabolic capacities between those in summer and winter for temperate resident birds (Liknes et al., 2014; Petit and Vézina, 2014), so these seasons should provide roughly similar metabolic capacities for wild house sparrows. All sparrows were housed individually in 59 cm×45 cm×36 cm stainless-steel cages at room temperature (23±2°C, 12 h:12 h light:dark photoperiod), with ad libitum food and water in the University of South Dakota Department of Biology Animal Facility for 2 weeks prior to experimental training protocols. Two weeks of indoor captivity acclimation were sufficient for body mass (Mb) and Msum to reach stable levels for wild birds in a previous study (Swanson and King, 2013). After the 2-week acclimation period, we measured pre-treatment basal metabolic rate (BMR), Msum and MMR for each bird (Zhang et al., 2015b). We then randomly assigned birds into acute cold training, acute cold training control, exercise training and exercise training control groups. Both training protocols included six 3-day training sessions with 1 day of rest in between sessions. Acute cold training was completed by placing birds in a metabolic chamber with a 79% helium and 21% oxygen (helox) atmosphere for 45 min with the initial training session at 10°C. We dropped the temperature by 2°C for each subsequent training session, so the last session was at 0°C. Acute cold training control birds were also placed in the same metabolic chamber in air for 45 min at 30°C during training sessions. Exercise training was performed by forcing birds to fly non-stop between two perches located 6 m apart for 45 min. Exercise training control birds were placed inside a cloth bag for 45 min to generate a handling stress without exercise. Following completion of the 3 week training sessions, we euthanized birds by cervical dislocation and quickly excised pectoralis, supracoracoideus and mixed leg muscles and heart on ice. Each muscle was divided into two samples, with one placed in RNAlater (Ambion, Grand Island, NY, USA) for real-time quantitative reverse transcription PCR (qRT-PCR) and the other flash frozen in liquid nitrogen. Both samples were stored frozen at −80°C for later analyses. Pre- and post-treatment measurements of BMR, Msum and MMR for these same individual birds were previously reported in Zhang et al. (2015a).
Enzyme assays
CPT, CS and HOAD activities were measured spectrophotometrically following protocols from Swanson et al. (2014b). Small samples of each muscle were minced and weighed to the nearest 0.1 mg after removing muscles from the −80°C ultracold freezer. Samples were then mixed with 10–40 volumes mass−1 homogenizing buffer. Homogenizing buffer contained 100 mmol l−1 phosphate and 2 mmol l−1 EDTA at pH 7.3 for CS and HOAD measurements; for CPT, homogenizing buffer contained 10 mmol l−1 Tris (hydroxymethyl) aminomethane and 1 mmol l−1 EDTA at pH 7.5. The diluted samples were homogenized (Tekmar model ST-1810 Tissuemizer, Cincinnati, OH, USA) and then sonicated (Cole-Parmer 4710 series ultrasonic homogenizer, Chicago, IL, USA) on ice for three 10 s bursts with 30 s between bursts.
All spectrophotometric assays were performed at 39°C with a Beckman DU 7400 spectrophotometer (Beckman Coulter, Fullerton, CA, USA) at 412 nm (CPT, CS) or 340 nm (HOAD). The CPT assay buffer contained 50 mmol l−1 Tris buffer, 5 mmol l−1 carnitine, 0.15 mmol l−1 5,5′-dithio-bis-(2-nitrobenzoic acid) (DTNB), 0.035 mmol l−1 palmitoyl CoA, and 10 µl of homogenate at pH 8.0. The CS assay buffer contained 100 mmol l−1 triethanolamine–HCl, 2.5 mmol l−1 EDTA, 0.1 mmol l−1 DTNB, 0.2 mmol l−1 acetyl-CoA, 0.5 mmol l−1 oxaloacetate and 5 µl of homogenate at pH 7.5. The HOAD assay buffer contained 100 mmol l−1 triethanolamine–HCl, 5 mmol l−1 EDTA, 0.225 mmol l−1 NADH2, 0.1 mmol l−1 acetoacetyl-CoA and 20 µl of homogenate at pH 7.0. Total volume for all three enzyme assays was 1.0 ml. Background activities for each enzyme were measured for 2 min before starting the reaction by adding substrate. Each sample was run in duplicate and we used average values for subsequent calculations. We subtracted background activities from activities after substrate addition and report mean mass-specific activities as µmol min−1 g wet mass−1 and mean total activities (mass-specific activity×wet muscle mass) as µmol min−1. We excluded enzyme activity data from the initial three cold-trained and two cold-training control birds from subsequent analyses because of poor quality assay reagents for these samples, which we realized only after assays were complete.
qRT-PCR and western blot
We homogenized roughly 200 mg of pectoralis with a Tekmar model ST-1810 Tissuemizer in 300 µl RLT buffer (QIAGEN, Valencia, CA, USA) with 1% β-mercaptoethanol. Total RNA was extracted using the RNeasy Fibrous Tissue Mini Kit (QIAGEN) and quantified using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). A 50 ng sample of purified RNA was used for qRT-PCR reactions with a TaqMan RNA-to-CT 1-Step Kit and StepOnePlus Real-Time PCR System (Applied Biosystems, Carlsbad, CA, USA). We performed qRT-PCR using the custom qRT-PCR probe and primer sets (Applied Biosystems and Eurofins, Huntsville, AL, USA) containing the sequences listed in Table 1 from Zhang et al. (2015b). We used glyceraldehyde phosphate dehydrogenase (GAPDH, Applied Biosystems) as a housekeeping gene. For all qRT-PCR reactions, we used 6 μl of total RNA in 25 μl reactions. We performed the qRT-PCR at 48°C for 15 min, then 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. Each sample was run in duplicate and normalized to the expression of GAPDH. We optimized protocols for all four genes to verify efficiency for these probe and primers sets for sparrows. For unknown reasons, the FABPc probe and primers sets failed to amplify mRNA using the StepOnePlus Real-Time PCR System. Slopes and efficiencies for other genes were: GAPDH (−3.46, 94.6%), FABPpm (−3.5, 93.1%) and FAT/CD36 (−3.37, 98.0%). We quantified changes in mRNA expression using the 2−ΔΔCT method (Livak and Schmittgen, 2001; Arendt et al., 2012), comparing all samples to a reference sample. We used the mean value for all groups for each tissue for each gene as the reference sample and set the value for this reference sample equal to 1. We then normalized mRNA expression to this reference sample to determine relative amounts of mRNA expression for all other samples for the same tissue and species. We present mRNA expression data as relative expression levels (i.e. mean fold change±s.e.m.).
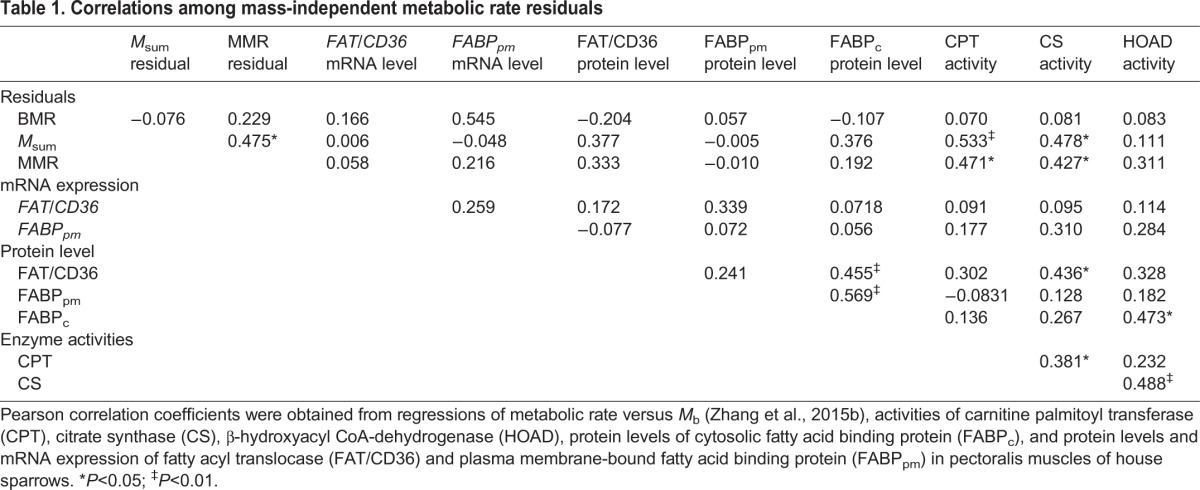
Table 1.
Correlations among mass-independent metabolic rate residuals
We conducted western blots on pectoralis muscles to analyze fatty acid transporter protein levels and GAPDH (as a housekeeping protein). Pectoralis muscles were homogenized on ice with a Cole-Parmer (Chicago, IL, USA) 4710 series ultrasonic homogenizer for three 10-s bursts with 30 s between bursts in 300 μl of homogenizing buffer (50 mmol l−1 Tris, pH 7; 100 mmol l−1 NaCl; 2% SDS). We used a modified DC Lowry improved protein assay to determine protein concentration. All samples were run on NuPAGE® Novex® 4–12% Bis-Tris protein gels with the same random sample included on every gel to serve as a standard for detecting gel-to-gel variation. We conducted western blotting using primary antibodies for FAT/CD36 (rabbit polyclonal; Novus Biologicals, Littleton, CO, USA; 1:1000 dilution), FABPpm (rabbit polyclonal, from Christopher G. Guglielmo; 1:10,000 dilution), FABPc (rabbit polyclonal, from Christopher G. Guglielmo; 1:8000 dilution) (McFarlan et al., 2009), and GAPDH (chicken polyclonal; Millipore, Temecula, CA, USA; 1:8000 dilution). Proteins were then transferred to the membranes and washed with a mixture of Tris-buffered saline and Tween 20 (TBS-T) with 5% milk (20 mmol Tris, 137 mmol l−1 NaCl, 100 mmol l−1 HCl, 0.01% Tween 20, pH 7.5) overnight at 4°C. Membranes were incubated with horseradish peroxidase-conjugated secondary antibodies: anti-rabbit (1:1000; Santa Cruz Biotechnology, Dallas, TX, USA) for FAT/CD36, FABPpm and FABPc, and anti-chicken (1:1500; Abcam, Cambridge, MA, USA) for GAPDH. The protein samples were visualized using enhanced chemiluminescence (GE Healthcare ECL Plus western blotting detection reagents; Buckinghamshire, UK) and analyzed using Quantity One software (Bio-Rad Laboratories, Hercules, CA, USA). Fatty acid transporter protein levels were normalized by dividing by GAPDH protein levels for the same tissue sample. We used normalized protein values for subsequent statistical analyses.
Statistics
We present data as means±standard error (s.e.m.). We compared enzyme activities and mRNA and protein expression between training and control groups by Student's t-test. If parametric assumptions of normal distribution (Kolmogorov–Smirnov test) or homogenous variances (Levene's test) were violated, we log10-transformed data prior to comparisons. If log10-transformed data still did not meet parametric assumptions, we used Mann–Whitney U-tests for comparisons. We further calculated Pearson correlation coefficients to examine relationships among enzyme activities, fatty acid transporter mRNA and protein levels, and metabolic rates. Metabolic rate data were obtained from Zhang et al. (2015a) for the same individual birds. To remove the effects of Mb from analyses of relationships among metabolic variables, we calculated residuals from allometric regressions for BMR, Msum and MMR and used least-squares linear regression of residuals. We used best subsets multiple regression for multiple regressions of lipid transporter levels and enzyme activities on Msum and MMR. We performed all statistical analyses with SigmaStat 3.5 (Systat Software, Point Richmond, CA, USA). We accepted statistical significance for all tests at P<0.05.
RESULTS
Trans-sarcolemmal and intramyocyte lipid transport
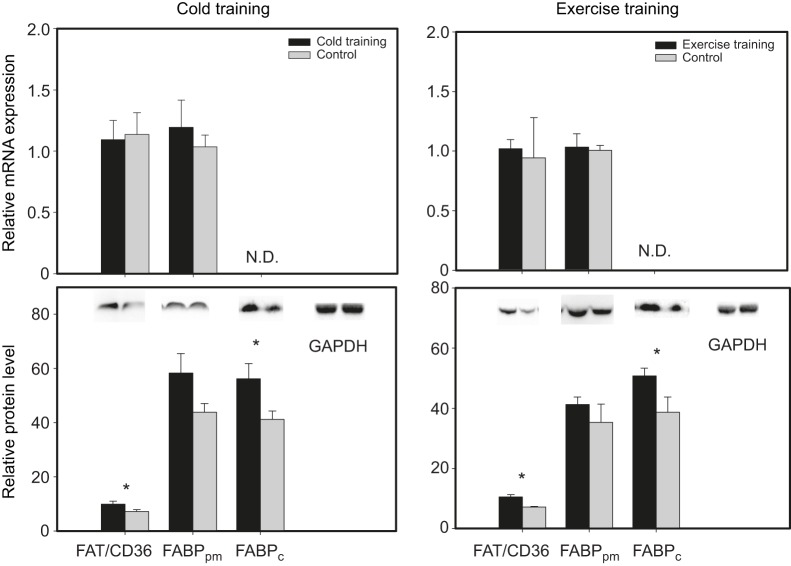
No significant differences were detected in pectoralis mRNA expression of FAT/CD36 or FABPpm for either training protocol when comparing training and control groups (Fig. 1). Cold-trained sparrows exhibited significantly higher pectoralis FAT/CD36 (45.8%, t16=3.081, P=0.007) and FABPc (36.4%, t16=2.338, P=0.03) protein levels than control sparrows (Fig. 1). However, FABPpm protein levels in pectoralis of cold-trained sparrows did not differ significantly from control birds. Similarly, exercise-trained birds also had significantly higher pectoralis protein levels of FAT/CD36 (42.7%, t14=3.993, P=0.001) and FABPc (34.6%, t14=2.433, P=0.03), but not FABPpm, than non-exercise-trained control birds (Fig. 1).
Fig. 1.
Cold and exercise training effects on relative mRNA expression and protein levels from qRT-PCR and western blot assays for plasma membrane-bound fatty acid binding protein (FABPpm), fatty acyl translocase (FAT/CD36) and cytosolic fatty acid binding protein (FABPc) in pectoralis muscles of house sparrow (Passer domesticus). Error bars represent s.e.m. Sample sizes for the different treatment groups were: cold training, N=9; cold training control, N=9; exercise training, N=8; exercise training control, N=8. *Significant differences between treatment groups.
Mitochondrial membrane lipid transport
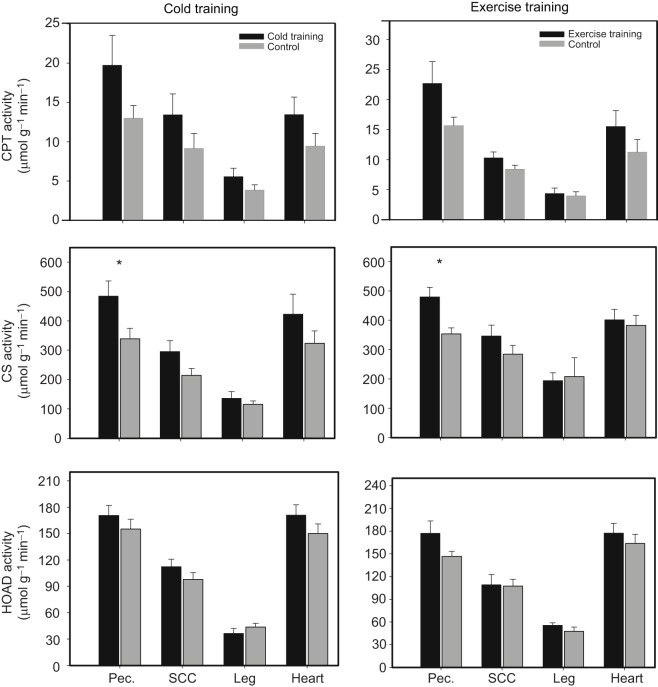
For both training groups, mass-specific CPT activities did not exhibit any significant differences between training and control groups for any tissue (Fig. 2). For cold-trained birds, total CPT activity showed non-significant trends towards higher activities in cold-trained relative to control birds in both pectoralis (69.8%, t11=1.954, P=0.08) and heart (50.2%, t11=1.862, P=0.09). For exercise-trained birds, total CPT activity showed a similar non-significant trend towards higher activity in pectoralis (56.3%, t14=1.912, P=0.08) in exercise-trained compared with control sparrows.
Fig. 2.
Cold and exercise training effects on mass-specific activities for carnitine palmitoyl transferase (CPT), citrate synthase (CS) and β-hydroxyacyl CoA-dehydrogenase (HOAD) in pectoralis (Pec.), supracoracoideus (SCC), mixed leg muscles and heart of house sparrow (Passer domesticus). Error bars represent s.e.m. Sample sizes for the different treatment groups were: cold training, N=6; cold training control, N=7; exercise training, N=8; exercise training control, N=8. *Significant differences between treatment groups.
Cellular lipid catabolic capacity and metabolic intensity
For cold-trained birds, mass-specific CS activity was significantly higher in trained than in control sparrows in pectoralis (42.9%, t11=2.385, P=0.04), but not in other tissues (Fig. 2). Cold-trained sparrows also had a significantly higher total CS activity in pectoralis (56.4%, t11=2.509, P=0.03) than control birds, with a non-significant trend towards higher total HOAD activity in heart (22.7%, t11=2.036, P=0.07) than in the control group.
For exercise-trained birds, mass-specific pectoralis CS exhibited significantly higher activity than in control birds (35.6%, t14=3.24, P=0.006; Fig. 2). Mass-specific CS activities did not differ significantly between exercise-trained and control sparrows for any other tissue (Fig. 2). Mass-specific HOAD activity showed a non-significant trend towards higher levels for exercise-trained relative to control birds in pectoralis (21.6%, t14=1.917, P=0.08), but activities in other tissues were not significantly different for trained and control birds (Fig. 2). Exercise-trained birds also showed significantly elevated total CS (44.8%, t14=3.998, P=0.001) and HOAD (29.5%, t14=2.419, P=0.03) activities in pectoralis relative to control birds, but total CS or HOAD activities did not vary significantly with exercise training in other tissues. Higher mass-specific (29.5%, t12=3.1, P=0.009) HOAD activity in leg for exercise-trained than for cold-trained birds was the only significant difference in activity for either enzyme between the two training groups. No significant differences in either mass-specific CS or HOAD activities were apparent between birds in the two control groups.
Correlations
Both FAT/CD36 and FABPpm mRNA expression were not significantly correlated with their protein levels for pooled data. FAT/CD36 protein levels were significantly positively correlated with FABPc protein levels and CS activity in pectoralis (Table 1). Pectoralis FABPc protein levels were significantly positively correlated with pectoralis FABPpm protein levels and HOAD activity (Table 1). Significant positive correlations were also observed for pectoralis between mass-specific CS activities and both CPT and HOAD mass-specific activities. Mass-independent Msum and MMR residuals (data from Zhang et al., 2015a) were both positively correlated with mass-specific CPT and CS activities (Table 1). Best subsets multiple regression indicated that CPT activity was the only variable retained in the regression model, and can significantly predict both Msum (r2=0.55, P<0.001) and MMR (r2=0.28, P=0.005).
DISCUSSION
We have previously documented that both acute cold and exercise training protocols increased organismal metabolic capacities (for both Msum and MMR) in sparrows (Zhang et al., 2015a). Results from the present study suggest that up-regulation of some aspects of lipid transport in pectoralis muscle, including trans-sarcolemmal and intramyocyte lipid transport capacities, are matched to the higher energetic demands associated with acute cold and exercise training. Increases of pectoralis mass-specific cellular metabolic intensity (as measured by CS activity) are also associated with high shivering and exercise performance in both training groups. Trans-mitochondrial lipid transport and cellular lipid oxidation capacity, however, appear less important to training-induced increases in organismal metabolic capacities for house sparrows. Patterns of training-induced variation in plasma membrane and intracellular fatty acid transporter levels and cellular metabolic intensity in this study were almost identical for both cold and exercise training. Together with similar responses of body mass, pectoralis muscle mass and myostatin (a muscle growth regulator) levels to both training protocols (Zhang et al., 2015a), these data further suggest that similar response mechanisms underlie acute training responses to both exercise and thermogenesis in small birds.
Trans-sarcolemmal lipid transport
Both training protocols in this study did not significantly alter pectoralis FAT/CD36 and FABPpm mRNA expression. Our data are consistent with studies showing stable mRNA expression for FAT/CD36 and/or FABPpm in skeletal muscle after exercise training in birds (Price et al., 2010) and mammals (Tunstall et al., 2002; Yang et al., 2005). In contrast, skeletal muscle mRNA expression of FAT/CD36 and/or FABPpm may be up-regulated after exercise training (Tunstall et al., 2002; Kiens et al., 2004) in mammals, and also with migratory status in birds (McFarlan et al., 2009). Captive birds photostimulated to migratory condition may show either increased (Zajac et al., 2011) or stable (Price et al., 2010) pectoralis FAT/CD36 mRNA expression. Many studies, including the present study, failed to detect any correlation between FAT/CD36 or FABPpm mRNA expression and protein levels or with changes in the rate of fatty acid transport (Luiken et al., 2002; Chabowski et al., 2006). Moreover, post-transcriptional processing for FABPpm, and especially FAT/CD36, may be a preferred strategy to alter protein levels (Bonen et al., 1999). Therefore it may be more instructive to examine fatty acid transporter protein levels rather than mRNA expression to study the association of trans-sarcolemmal lipid transport with changing energetic demands (Glatz et al., 2010).
Both training protocols in the present study induced up-regulation of FAT/CD36, but not FABPpm, protein levels and both protocols produced FAT/CD36 increases of similar magnitude (46% and 43% increases for cold and exercise training, respectively). Exercise training generally increases both FAT/CD36 and FABPpm protein levels and sarcolemmal fatty acid transport rates in mammals (Bonen et al., 1999; Bradley et al., 2012). Furthermore, FAT/CD36 knock-out mice also had lower myocyte fatty acid uptake during shivering under cold stress (Putri et al., 2015). Migratory birds increase pectoralis FABPpm protein levels during migration compared with winter (McFarlan et al., 2009). Similarly, winter black-capped chickadees (Poecile atricapillus) had higher protein levels of FABPpm in both pectoralis and heart compared with their summer counterparts (Zhang et al., 2015b). Winter-acclimatized American goldfinches (Spinus tristis), however, showed seasonally stable pectoralis protein levels for both FAT/CD36 and FABPpm (Zhang et al., 2015b). Pectoralis FAT/CD36 protein levels increased during migration relative to summer for warbling vireos (Vireo gilvus) and yellow warblers (Setophaga petchia) and increased during spring relative to fall migration for yellow-rumped warblers (Setophaga coronata), consistent with changes in organismal metabolic capacities (Swanson and Dean, 1999; Zhang et al., 2015a). Pectoralis FABPpm protein levels also showed a similar seasonal pattern of variation for yellow and yellow-rumped warblers, but not for warbling vireos, which showed seasonally stable protein levels (Zhang et al., 2015c). Thus protein levels of both FAT/CD36 and FABPpm in skeletal muscles generally increase under conditions of elevated energy demands in both birds and mammals, but such increases are not universal.
Reasons for the stable pectoralis FABPpm protein levels for both training groups in the present study, while FAT/CD36 levels increased with training, are unclear. However, translocation of FABPpm from intramyocyte stores to the sarcolemma to promote elevated fatty acid transport capacity, without alternating its protein levels, may occur (reviewed in Glatz et al., 2010). Moreover, the importance of FABPpm by itself to trans-sarcolemmal fatty acid transport has been questioned. Instead, the rate of trans-sarcolemmal fatty acid uptake appears correlated with the combined levels of FAT/CD36 and FABPpm (Chabowski et al., 2007), suggesting a cooperative role for the two trans-sarcolemmal lipid transporters. In skeletal muscles, FAT/CD36 co-immunoprecipitates with the CPT system, suggesting a role in facilitating transport of fatty acids into mitochondria (Campbell et al., 2004; Schenk and Horowitz, 2006). For this study, we measured FAT/CD36 protein levels from both sarcolemmal and mitochondrial membranes. Hence up-regulation of FAT/CD36 from both training protocols could partially result from increases in fatty acid transport capacity across the mitochondrial membranes. Finally, Zhang et al. (2015b) also documented non-parallel variation in FABPpm and FAT/CD36 under changing energy demands, so our data are consistent with that study.
Intramyocyte lipid transport
Both cold and exercise training increased pectoralis FABPc protein levels by 43–46% in sparrows in this study, suggesting that intramyocyte lipid transport is an important target for increasing organismal metabolic capacity. Exercise training-induced variation in intramyocyte lipid transport capacity, indicated by FABPc protein levels, often does not occur in mammals (Kiens et al., 2004; Lee et al., 2007; Jeppesen et al., 2012), but increases in FABPc mRNA expression may occur (Fu et al., 2009). Because exercise-trained mammals show stable FABPc protein levels despite increased lipid oxidation potential in muscle, it has been suggested that intramyocyte levels of FABPc in untrained mammals are sufficient for trafficking fatty acids during exercise (Kiens, 2006). However, this is not the case for birds, which are more reliant on exogenous fatty acids from adipose tissue to support high-intensity muscular activity (McWilliams et al., 2004). FABPc is consistently reported as an important regulatory target for both migratory (Guglielmo et al., 2002; McFarlan et al., 2009) and winter (Zhang et al., 2015b) phenotypes in birds.
FABPc serves not only as an intramyocyte fatty acid transporter, but also as a fatty acid receptor and may be co-regulated with fatty acid-binding proteins on the cell membrane (Luiken et al., 2003). For example, similar increases in FABPc and FAT/CD36 protein contents were observed in human skeletal muscle and these contribute to higher lipid oxidation capacities (Roepstorff et al., 2004). In the present study, increases of FAT/CD36 and FABPc protein levels for both training protocols were also similar, with cold training producing increases of 45% and 46% and exercise training producing increases of 35% and 43% for FAT/CD36 and FABPc, respectively. FABPc may also be more important to overall lipid transport capacity than membrane-associated lipid transporters because very little fatty acid exists as free or unbound molecules inside muscle cells (Kiens, 2006), whereas at least some fatty acid transport across membranes occurs by simple diffusion (Hamilton et al., 2002). Mammals have lower ability to transport and catabolize exogenous lipid as a fuel for high-intensity aerobic exercise than birds (Jenni-Eiermann et al., 2002; Guglielmo, 2010). Differential abilities for regulation of FABPc under high-intensity muscular activity could be one reason underlying differences in capacities for lipid transport between mammals and birds. In summary, increases in intramyocyte fatty acid transport capacity through up-regulation of FABPc is consistently an important target for increasing organismal metabolic capacities associated with both flight and shivering in birds.
Mitochondrial membrane lipid transport
Mass-specific CPT levels did not exhibit any significant differences in skeletal muscles or heart with either cold or exercise training, although non-significant trends towards higher total CPT activity in pectoralis were evident for birds from both training protocols. Pectoralis CPT activity may be elevated (Guglielmo et al., 2002; McFarlan et al., 2009; Zajac et al., 2011) or stable (Price et al., 2010) during migration or in photostimulated migratory birds. Skeletal muscle CPT activities were, however, statistically invariant for winter-acclimatized (Zhang et al., 2015b) and cold-acclimated (Swanson et al., 2014b) birds. Positive effects of exercise training on skeletal muscle CPT activities or levels have been observed in mammals (Starritt et al., 2000; Tunstall et al., 2002; Bruce et al., 2006), but not birds (Price et al., 2010). Exercise may, however, result in enhanced acetylation of carnitine by acetyl CoA when acetyl CoA formation increases during elevated energy demands (Odland et al., 1998; van Loon et al., 2001). As a result, a possible reason for stable mass-specific CPT activities after training in the present study could be a reduction in free carnitine, which is the essential substrate of CPT (van Loon et al., 2001).
The absence of variation in mass-specific CPT activity with cold and exercise training in the present study may also be related to variation in allosteric regulation. For example, CPT activity was not altered, but its sensitivity to the inhibitor malonyl-CoA was progressively reduced during exercise in humans (Holloway et al., 2006). More importantly, increased rates of fatty acid oxidation after exercise training in humans were correlated with an increase in FAT/CD36 associated with CPT, but not with CPT alone (Schenk and Horowitz, 2006). Alternatively, CPT may occur in sufficient levels in the mitochondria of control birds so that training-induced elevation is not necessary. Such a result would be consistent with the several studies documenting stable CPT activity with winter, cold or migration (Price et al., 2010; Swanson et al., 2014b; Zhang et al., 2015b,c). Finally, statistically stable pectoralis CPT activities in both training groups may have resulted from low statistical power (power analyses revealed values of 0.60 and 0.47 for cold and exercise training, respectively, both of which are less than the recommended value of 0.8), as both cold and exercise training produced nearly significant increases in CPT activity in pectoralis. If such is the case, an increased sample size could result in significant increases in CPT activities for both cold and exercise training groups. Such an increase in CPT would be consistent with previous studies showing up-regulation of pectoralis CPT activity with migration (Guglielmo et al., 2002; McFarlan et al., 2009) in birds. In summary, even though CPT is considered to be a key regulatory enzyme for fatty acid transport into the mitochondria and for overall rates of lipid oxidation (Stephens et al., 2007), it is not a consistent target of regulation for metabolic capacity changes associated with exercise and thermogenesis in birds.
Cellular lipid catabolic capacity and metabolic intensity
Neither cold nor exercise training significantly influenced skeletal muscle HOAD activities, and only exercise training produced a non-significant trend for higher mass-specific HOAD activity in pectoralis. Pectoralis HOAD activity increases (Guglielmo et al., 2002; McFarlan et al., 2009; Zajac et al., 2011) or remains stable (Price et al., 2010) during migratory and photostimulated migratory states in birds. Pectoralis HOAD activities also vary inconsistently with seasonal acclimatization, with both winter up-regulation (Swanson, 2010) and seasonal stability (O'Connor, 1995; Liknes and Swanson, 2011) reported. A lack of consistency in HOAD activities with training also occurs for mammals, as both increasing (Tremblay et al., 1994; Burgomaster et al., 2008) and stable (Helge and Kiens, 1997; Burgomaster et al., 2006) values have been documented. Given the absence of training-induced changes in pectoralis HOAD activities in this study, the lack of consistent variation in HOAD activity with increasing energy demands in birds generally, and the absence of seasonal differences in HOAD activities previously documented for house sparrows (Liknes and Swanson, 2011), it seems likely that pectoralis HOAD activities in untrained birds in this study are sufficient to also meet the β-oxidation requirements of both cold and exercise-trained birds.
In contrast, both training protocols produced significantly higher mass-specific CS activities in pectoralis compared with their control groups. Increasing pectoralis CS activities have been documented for migratory (6–110%) (Guglielmo et al., 2002; McFarlan et al., 2009) and for winter phenotypes (52%) (Liknes and Swanson, 2011) in birds. However, migratory (Driedzic et al., 1993) and seasonal (Dawson and Marsh, 1989; Swanson, 2010) stability in pectoralis CS activities are also common in birds. The increases in mass-specific pectoralis CS activities for both training protocols in this study, along with the significant positive correlations between pectoralis CS activities and organismal metabolic capacities, are consistent with other house sparrow data, as total pectoralis CS activity increases in winter relative to summer (Liknes and Swanson, 2011) and pectoralis CS activity is positively correlated with Msum (Swanson et al., 2014a). Collectively, these studies suggest that increases in pectoralis CS activities are important to increased organismal capacities for exercise and shivering in house sparrows.
For supracoracoideus, leg and heart, mass-specific enzyme activities did not show any significant differences for either cold or exercise training groups relative to their controls. This result suggests that cellular metabolic intensities in these muscles are not important targets to increase lipid transport and oxidation and organismal metabolic capacities in sparrows. Seasonally acclimatized house sparrows also showed variable responses for CS and HOAD activities in supracoracoideus and leg muscles, with winter increases in CS activity in supracoracoideus, but not leg, and in HOAD activity in leg, but not supracoracoideus (Liknes and Swanson, 2011). The supracoracoideus muscle powers the flight upstroke and contracts against the pectoralis during isometric shivering in birds. However, both training groups failed to show much difference in supracoracoideus activities for all three enzymes. This result is consistent with other recent work suggesting that the supracoracoideus does not limit either isometric shivering (Liknes and Swanson, 2011) or migration flight performance (Piersma and Dietz, 2007) in small birds.
Correlations
Positive correlations of pectoralis FAT/CD36 and FABPpm with FABPc, in addition to positive correlations of pectoralis CS with CPT and HOAD, suggest parallel variation and integration of the various steps in fatty acid transport and catabolism pathways in house sparrows in this study. Such relationships are consistent with the concept of symmorphosis and suggest that correlated variation is important for cold and exercise training in birds (Suarez, 1998; Swanson, 2010). However, similar correlations were not observed for either migration (McFarlan et al., 2009) or winter acclimatization (Zhang et al., 2015b) in birds, so support for the concept of symmorphosis of metabolic pathways under conditions of increased energy demands is mixed for birds generally. Positive correlations of pectoralis CS activity with both Msum and MMR for sparrows in the present study suggest that cellular metabolic intensity contributes to training effects for house sparrow. This is not necessarily the case for birds generally, as previous studies showed that Msum was also positively correlated with pectoralis CS activity in house sparrows, but not for other birds (Swanson et al., 2013; Swanson et al., 2014a). In addition, MMR was only significantly positively correlated with pectoralis CS activities in male red junglefowl (Gallus gallus), but not in female red junglefowl or house sparrows (Hammond et al., 2000; Buttemer et al., 2010). The positive correlation of CPT activity with both Msum and MMR for sparrows suggests that CPT activity is important to metabolic capacities in birds through regulation of lipid flux into mitochondria (Guglielmo, 2010). However, the absence of significant training-induced variation in CPT activity suggests that despite its importance to overall metabolic capacities, CPT is not a prominent target for regulation of flexible metabolic responses in sparrows.
Conclusions
To our knowledge, this is the first study to examine lipid transport and catabolism as mechanisms underlying training effects on both thermogenesis and exercise in birds. Some previous studies suggested that fuel selection, metabolic pathways and physiological responses may differ between exercise and shivering in mammals (Haman et al., 2005; Gagnon et al., 2014). However, the present study suggests similar muscular adjustments between exercise and shivering in birds. These differences between birds and mammals may be related, at least partially, to the absence of brown fat in birds (Mezentseva et al., 2008), so that skeletal muscles support both exercise and thermogenesis in birds to a greater degree than in mammals. Along with the data of Zhang et al. (Zhang et al., 2015a), these data suggest that coordinated regulation of trans-sarcolemmal and intramyocyte lipid transport capacities, cellular metabolic intensities, pectoralis muscle masses and pectoralis myostatin (a muscle growth inhibitor) levels, are common mechanisms underlying the training effects of both exercise and cold on organismal metabolic capacities in birds.
Acknowledgements
We thank Donis Drappeau, Jake Johnson and Will Culver for technical support in the laboratory and field. We thank Jianqiu Zou and Yi-Fan Li for their advice on western blots. We also thank T. J. Agin for his suggestions on statistical analyses. Two anonymous reviewers provided constructive comments on a previous version of this manuscript, and we thank them for their efforts.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Y.Z. and D.L.S. conceived the study and designed the experiments; Y.Z., T.C. and K.E. collected the data; Y.Z. and D.L.S. analyzed the data; Y.Z. and D.L.S. wrote the manuscript; Y.Z., T.C., K.E. and D.L.S. interpreted data and revised the manuscript. All authors assume responsibility for the content of the paper.
Funding
This study was supported by the US National Science Foundation (grant number IOS-1021218 to D.L.S.). Research reported in this manuscript was also supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences (NIGMS) of the National Institutes of Health (NIH) under grant number P20GM103443. Its contents are solely the responsibility of the authors and do not necessarily represent official views of NIGMS or NIH. Deposited in PMC for release after 12 months.
References
- Arendt D. H., Smith J. P., Bastida C. C., Prasad M. S., Oliver K. D., Eyster K. M., Summers T. R., Delville Y. and Summers C. H. (2012). Contrasting hippocampal and amygdalar expression of genes related to neural plasticity during escape from social aggression. Physiol. Behav. 107, 670-679. 10.1016/j.physbeh.2012.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonen A., Miskovic D. and Kiens B. (1999). Fatty acid transporters (FABPpm, FAT, FATP) in human muscle. Can. J. Appl. Physiol. 24, 515-523. 10.1139/h99-033 [DOI] [PubMed] [Google Scholar]
- Boström P., Wu J., Jedrychowski M. P., Korde A., Ye L., Lo J. C., Rasbach K. A., Boström E. A., Choi J. H., Long J. Z. et al. (2012). A PGC1α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481, 463-468. 10.1038/nature10777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley N. S., Snook L. A., Jain S. S., Heigenhauser G. J. F., Bonen A. and Spriet L. L. (2012). Acute endurance exercise increases plasma membrane fatty acid transport proteins in rat and human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 302, E183-E189. 10.1152/ajpendo.00254.2011 [DOI] [PubMed] [Google Scholar]
- Bruce C. R., Thrush A. B., Mertz V. A., Bezaire V., Chabowski A., Heigenhauser G. J. F. and Dyck D. J. (2006). Endurance training in obese humans improves glucose tolerance and mitochondrial fatty acid oxidation and alters muscle lipid content. Am. J. Physiol. Endocrinol. Metab. 291, E99-E107. 10.1152/ajpendo.00587.2005 [DOI] [PubMed] [Google Scholar]
- Burgomaster K. A., Heigenhauser G. J. F. and Gibala M. J. (2006). Effect of short-term sprint interval training on human skeletal muscle carbohydrate metabolism during exercise and time-trial performance. J. Appl. Physiol. 100, 2041-2047. 10.1152/japplphysiol.01220.2005 [DOI] [PubMed] [Google Scholar]
- Burgomaster K. A., Howarth K. R., Phillips S. M., Rakobowchuk M., MacDonald M. J., McGee S. L. and Gibala M. J. (2008). Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J. Physiol. 586, 151-160. 10.1113/jphysiol.2007.142109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttemer W., Bech C. and Chappell M. (2010). Citrate synthase activity does not account for age-related differences in maximum aerobic performance in House Sparrows (Passer domesticus). Aust. Zool. 35, 378-382. 10.7882/AZ.2010.026 [DOI] [Google Scholar]
- Campbell S. E., Tandon N. N., Woldegiorgis G., Luiken J. J. F. P., Glatz J. F. C. and Bonen A. (2004). A novel function for Fatty Acid Translocase (FAT)/CD36: involvement in long chain fatty acid transfer into the mitochondria. J. Biol. Chem. 279, 36235-36241. 10.1074/jbc.M400566200 [DOI] [PubMed] [Google Scholar]
- Chabowski A., Chatham J. C., Tandon N. N., Calles-Escandon J., Glatz J. F. C., Luiken J. J. F. P. and Bonen A. (2006). Fatty acid transport and FAT/CD36 are increased in red but not in white skeletal muscle of ZDF rats. Am. J. Physiol. Endocrinol. Metab. 291, E675-E682. 10.1152/ajpendo.00096.2006 [DOI] [PubMed] [Google Scholar]
- Chabowski A., Górski J., Luiken J. J. F. P., Glatz J. F. C. and Bonen A. (2007). Evidence for concerted action of FAT/CD36 and FABPpm to increase fatty acid transport across the plasma membrane. Prostaglandins. Leukot. Essent. Fatty Acids 77, 345-353. 10.1016/j.plefa.2007.10.017 [DOI] [PubMed] [Google Scholar]
- Dawson W. R. and Marsh R. L. (1989). Metabolic acclimatization to cold and season in birds. In Physiology of Cold Adaptation in Birds (ed. Bech C. and Reinertsen R. E.), pp. 83-94. New York: Plenum. [Google Scholar]
- Driedzic W. R., Crowe H. L., Hicklin P. W. and Sephton D. H. (1993). Adaptations in pectoralis muscle, heart mass, and energy metabolism during premigratory fattening in semipalmated sandpipers (Calidris pusilla). Can. J. Zool. 71, 1602-1608. 10.1139/z93-226 [DOI] [Google Scholar]
- Fu M.-h. H., Maher A. C., Hamadeh M. J., Ye C. and Tarnopolsky M. A. (2009). Exercise, sex, menstrual cycle phase, and 17β-estradiol influence metabolism-related genes in human skeletal muscle. Physiol. Genomics 40, 34-47. 10.1152/physiolgenomics.00115.2009 [DOI] [PubMed] [Google Scholar]
- Gagnon D. D., Rintamäki H., Gagnon S. S., Oksa J., Porvari K., Cheung S. S., Herzig K.-H. and Kyröläinen H. (2014). Fuel selection during short-term submaximal treadmill exercise in the cold is not affected by pre-exercise low-intensity shivering. Appl. Physiol. Nutr. Metab. 39, 282-291. 10.1139/apnm-2013-0061 [DOI] [PubMed] [Google Scholar]
- Glatz J. F. C., Luiken J. J. F. P. and Bonen A. (2010). Membrane fatty acid transporters as regulators of lipid metabolism: implications for metabolic disease. Physiol. Rev. 90, 367-417. 10.1152/physrev.00003.2009 [DOI] [PubMed] [Google Scholar]
- Guglielmo C. G. (2010). Move that fatty acid: fuel selection and transport in migratory birds and bats. Integr. Comp. Biol. 50, 336-345. 10.1093/icb/icq097 [DOI] [PubMed] [Google Scholar]
- Guglielmo C. G., Haunerland N. H., Hochachka P. W. and Williams T. D. (2002). Seasonal dynamics of flight muscle fatty acid binding protein and catabolic enzymes in a migratory shorebird. Am. J. Physiol. 282, R1405-R1413. 10.1152/ajpregu.00267.2001 [DOI] [PubMed] [Google Scholar]
- Haman F., Péronnet F., Kenny G. P., Massicotte D., Lavoie C. and Weber J.-M. (2005). Partitioning oxidative fuels during cold exposure in humans: muscle glycogen becomes dominant as shivering intensifies. J. Physiol. 566, 247-256. 10.1113/jphysiol.2005.086272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J. A., Kamp F. and Guo W. (2002). Mechanism of cellular uptake of long-chain fatty acids: do we need cellular proteins? Mol. Cell. Biochem. 239, 17-23. 10.1023/A:1020542220599 [DOI] [PubMed] [Google Scholar]
- Hammond K. A., Chappell M. A., Cardullo R. A., Lin R.-s. and Johnsen T. S. (2000). The mechanistic basis of aerobic performance variation in red junglefowl. J. Exp. Biol. 203, 2053-2064. [DOI] [PubMed] [Google Scholar]
- Helge J. W. and Kiens B. (1997). Muscle enzyme activity in humans: role of substrate availability and training. Am. J. Physiol. 272, R1620-R1624. [DOI] [PubMed] [Google Scholar]
- Holloway G. P., Bezaire V., Heigenhauser G. J. F., Tandon N. N., Glatz J. F. C., Luiken J. J. F. P., Bonen A. and Spriet L. L. (2006). Mitochondrial long chain fatty acid oxidation, fatty acid translocase/CD36 content and carnitine palmitoyltransferase I activity in human skeletal muscle during aerobic exercise. J. Physiol. 571, 201-210. 10.1113/jphysiol.2005.102178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenni-Eiermann S., Jenni L., Kvist A., Lindstrom A., Piersma T. and Visser G. H. (2002). Fuel use and metabolic response to endurance exercise: a wind tunnel study of a long-distance migrant shorebird. J. Exp. Biol. 205, 2453-2460. [DOI] [PubMed] [Google Scholar]
- Jeppesen J., Jordy A. B., Sjøberg K. A., Füllekrug J., Stahl A., Nybo L. and Kiens B. (2012). Enhanced fatty acid oxidation and FATP4 protein expression after endurance exercise training in human skeletal muscle. PLoS ONE 7, e29391 10.1371/journal.pone.0029391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiens B. (2006). Skeletal muscle lipid metabolism in exercise and insulin resistance. Physiol. Rev. 86, 205-243. 10.1152/physrev.00023.2004 [DOI] [PubMed] [Google Scholar]
- Kiens B., Roepstorff C., Glatz J. F. C., Bonen A., Schjerling P., Knudsen J. and Nielsen J. N. (2004). Lipid-binding proteins and lipoprotein lipase activity in human skeletal muscle: influence of physical activity and gender. J. Appl. Physiol. 97, 1209-1218. 10.1152/japplphysiol.01278.2003 [DOI] [PubMed] [Google Scholar]
- Lee J. K., Lee J. S., Park H., Cha Y.-S., Yoon C. S. and Kim C. K. (2007). Effect of l-carnitine supplementation and aerobic training on FABPc content and β-HAD activity in human skeletal muscle. Eur. J. Appl. Physiol. 99, 193-199. 10.1007/s00421-006-0333-3 [DOI] [PubMed] [Google Scholar]
- Liknes E. T. and Swanson D. L. (2011). Phenotypic flexibility in passerine birds: seasonal variation of aerobic enzyme activities in skeletal muscle. J. Therm. Biol. 36, 430-436. 10.1016/j.jtherbio.2011.07.011 [DOI] [Google Scholar]
- Liknes E. T., Guglielmo C. G. and Swanson D. L. (2014). Phenotypic flexibility in passerine birds: seasonal variation in fuel storage, mobilization and transport. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 174, 1-10. 10.1016/j.cbpa.2014.03.017 [DOI] [PubMed] [Google Scholar]
- Livak K. J. and Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-ΔΔC(T)) method. Methods 25, 402-408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Luiken J. J. F. P., Dyck D. J., Han X.-X., Tandon N. N., Arumugam Y., Glatz J. F. C. and Bonen A. (2002). Insulin induces the translocation of the fatty acid transporter FAT/CD36 to the plasma membrane. Am. J. Physiol. Endocrinol. Metab. 282, E491-E495. 10.1152/ajpendo.00419.2001 [DOI] [PubMed] [Google Scholar]
- Luiken J. J. F. P., Koonen D. P. Y., Coumans W. A., Pelsers M. M. A. L., Binas B., Bonen A. and Glatz J. F. C. (2003). Long-chain fatty acid uptake by skeletal muscle is impaired in homozygous, but not heterozygous, heart-type-FABP null mice. Lipids 38, 491-496. 10.1007/s11745-003-1089-6 [DOI] [PubMed] [Google Scholar]
- McFarlan J. T., Bonen A. and Guglielmo C. G. (2009). Seasonal upregulation of fatty acid transporters in flight muscles of migratory white-throated sparrows (Zonotrichia albicollis). J. Exp. Biol. 212, 2934-2940. 10.1242/jeb.031682 [DOI] [PubMed] [Google Scholar]
- McWilliams S. R., Guglielmo C., Pierce B. and Klaassen M. (2004). Flying, fasting, and feeding in birds during migration: a nutritional and physiological ecology perspective. J. Avian Biol. 35, 377-393. 10.1111/j.0908-8857.2004.03378.x [DOI] [Google Scholar]
- Mezentseva N. V., Kumaratilake J. S. and Newman S. A. (2008). The brown adipocyte differentiation pathway in birds: an evolutionary road not taken. BMC Biol. 6, 17 10.1186/1741-7007-6-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor T. P. (1995). Seasonal acclimatization of lipid mobilization and catabolism in house finches (Carpodacus mexicanus). Physiol. Zool. 68, 985-1005. [Google Scholar]
- Odland L. M., Howlett R. A., Heigenhauser G. J., Hultman E. and Spriet L. L. (1998). Skeletal muscle malonyl-CoA content at the onset of exercise at varying power outputs in humans. Am. J. Physiol. 274, E1080-E1085. [DOI] [PubMed] [Google Scholar]
- Petit M. and Vézina F. (2014). Phenotype manipulations confirm the role of pectoral muscles and haematocrit in avian maximal thermogenic capacity. J. Exp. Biol. 217, 824-830. 10.1242/jeb.095703 [DOI] [PubMed] [Google Scholar]
- Piersma T. and Dietz M. W. (2007). Twofold seasonal variation in the supposedly constant, species-specific, ratio of upstroke to downstroke flight muscles in red knots Calidris canutus. J. Avian Biol. 38, 536-540. 10.1111/j.0908-8857.2007.04253.x [DOI] [Google Scholar]
- Price E. R., McFarlan J. T. and Guglielmo C. G. (2010). Preparing for migration? The effects of photoperiod and exercise on muscle oxidative enzymes, lipid transporters, and phospholipids in white-crowned sparrows. Physiol. Biochem. Zool. 83, 252-262. 10.1086/605394 [DOI] [PubMed] [Google Scholar]
- Price E. R., Staples J. F., Milligan C. L. and Guglielmo C. G. (2011). Carnitine palmitoyl transferase activity and whole muscle oxidation rates vary with fatty acid substrate in avian flight muscles. J. Comp. Physiol. B 181, 565-573. 10.1007/s00360-010-0542-2 [DOI] [PubMed] [Google Scholar]
- Putri M., Syamsunarno M. R. A. A., Iso T., Yamaguchi A., Hanaoka H., Sunaga H., Koitabashi N., Matsui H., Yamazaki C., Kameo S. et al. (2015). CD36 is indispensable for thermogenesis under conditions of fasting and cold stress. Biochem. Biophys. Res. Commun. 457, 520-525. 10.1016/j.bbrc.2014.12.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepstorff C., Helge J. W., Vistisen B. and Kiens B. (2004). Studies of plasma membrane fatty acid-binding protein and other lipid-binding proteins in human skeletal muscle. Proc. Nutr. Soc. 63, 239-244. 10.1079/PNS2004332 [DOI] [PubMed] [Google Scholar]
- Schaeffer P. J., Hokanson J. F., Wells D. J. and Lindstedt S. L. (2001). Cold exposure increases running VO2max) and cost of transport in goats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280, R42-R47. [DOI] [PubMed] [Google Scholar]
- Schenk S. and Horowitz J. F. (2006). Coimmunoprecipitation of FAT/CD36 and CPT I in skeletal muscle increases proportionally with fat oxidation after endurance exercise training. Am. J. Physiol. 291, E254-E260. 10.1152/ajpendo.00051.2006 [DOI] [PubMed] [Google Scholar]
- Siu P. M., Donley D. A., Bryner R. W. and Alway S. E. (2003). Citrate synthase expression and enzyme activity after endurance training in cardiac and skeletal muscles. J. Appl. Physiol. 94, 555-560. 10.1152/japplphysiol.00821.2002 [DOI] [PubMed] [Google Scholar]
- Starritt E. C., Howlett R. A., Heigenhauser G. J. and Spriet L. L. (2000). Sensitivity of CPT I to malonyl-CoA in trained and untrained human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 278, E462-E468. [DOI] [PubMed] [Google Scholar]
- Stephens F. B., Constantin-Teodosiu D. and Greenhaff P. L. (2007). New insights concerning the role of carnitine in the regulation of fuel metabolism in skeletal muscle. J. Physiol. 581, 431-444. 10.1113/jphysiol.2006.125799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez R. K. (1998). Oxygen and the upper limits to animal design and performance. J. Exp. Biol. 201, 1065-1072. [DOI] [PubMed] [Google Scholar]
- Swanson D. L. (2010). Seasonal metabolic variation in birds: functional and mechanistic correlates. In Current Ornithology, Vol. 17 (ed. Thompson C. F.), pp. 75-129. New York: Springer. [Google Scholar]
- Swanson D. L. and Dean K. L. (1999). Migration-induced variation in thermogenic capacity in migratory passerines. J. Avian Biol. 30, 245-254. 10.2307/3677350 [DOI] [Google Scholar]
- Swanson D. L. and King M. O. (2013). Short-term captivity influences maximal cold-induced metabolic rates and their repeatability in summer-acclimatized American goldfinches Spinus tristis. Curr. Zool. 59, 439-448. [Google Scholar]
- Swanson D. L. and Liknes E. T. (2006). A comparative analysis of thermogenic capacity and cold tolerance in small birds. J. Exp. Biol. 209, 466-474. 10.1242/jeb.02024 [DOI] [PubMed] [Google Scholar]
- Swanson D. L., Zhang Y. and King M. O. (2013). Individual variation in thermogenic capacity is correlated with flight muscle size but not cellular metabolic capacity in American goldfinches (Spinus tristis). Physiol. Biochem. Zool. 86, 421-431. 10.1086/671447 [DOI] [PubMed] [Google Scholar]
- Swanson D. L., Zhang Y. and King M. (2014a). Mechanistic drivers of flexibility in summit metabolic rates of small birds. PLoS ONE 9, e101577 10.1371/journal.pone.0101577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson D., Zhang Y., Liu J.-S., Merkord C. L. and King M. O. (2014b). Relative roles of temperature and photoperiod as drivers of metabolic flexibility in dark-eyed juncos. J. Exp. Biol. 217, 866-875. 10.1242/jeb.096677 [DOI] [PubMed] [Google Scholar]
- Talanian J. L., Galloway S. D. R., Heigenhauser G. J. F., Bonen A. and Spriet L. L. (2007). Two weeks of high-intensity aerobic interval training increases the capacity for fat oxidation during exercise in women. J. Appl. Physiol. 102, 1439-1447. 10.1152/japplphysiol.01098.2006 [DOI] [PubMed] [Google Scholar]
- Talanian J. L., Holloway G. P., Snook L. A., Heigenhauser G. J. F., Bonen A. and Spriet L. L. (2010). Exercise training increases sarcolemmal and mitochondrial fatty acid transport proteins in human skeletal muscle. Am. J. Physiol. 299, E180-E188. 10.1152/ajpendo.00073.2010 [DOI] [PubMed] [Google Scholar]
- Tremblay A., Simoneau J.-A. and Bouchard C. (1994). Impact of exercise intensity on body fatness and skeletal muscle metabolism. Metabolism 43, 814-818. 10.1016/0026-0495(94)90259-3 [DOI] [PubMed] [Google Scholar]
- Tunstall R. J., Mehan K. A., Wadley G. D., Collier G. R., Bonen A., Hargreaves M. and Cameron-Smith D. (2002). Exercise training increases lipid metabolism gene expression in human skeletal muscle. Am. J. Physiol. 283, E66-E72. 10.1152/ajpendo.00475.2001 [DOI] [PubMed] [Google Scholar]
- van Loon L. J. C., Greenhaff P. L., Constantin-Teodosiu D., Saris W. H. M. and Wagenmakers A. J. M. (2001). The effects of increasing exercise intensity on muscle fuel utilisation in humans. J. Physiol. 536, 295-304. 10.1111/j.1469-7793.2001.00295.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vézina F. and Williams T. D. (2005). Interaction between organ mass and citrate synthase activity as an indicator of tissue maximal oxidative capacity in breeding European starlings: implications for metabolic rate and organ mass relationships. Funct. Ecol. 19, 119-128. 10.1111/j.0269-8463.2005.00942.x [DOI] [Google Scholar]
- Weber T. P. and Piersma T. (1996). Basal metabolic rate and the mass of tissues differing in metabolic scope: migration-related covariation between individual knots Calidris canutus. J. Avian Biol. 27, 215-224. 10.2307/3677225 [DOI] [Google Scholar]
- Yang Y., Creer A., Jemiolo B. and Trappe S. (2005). Time course of myogenic and metabolic gene expression in response to acute exercise in human skeletal muscle. J. Appl. Physiol. 98, 1745-1752. 10.1152/japplphysiol.01185.2004 [DOI] [PubMed] [Google Scholar]
- Zajac D. M., Cerasale D. J., Landman S. and Guglielmo C. G. (2011). Behavioral and physiological effects of photoperiod-induced migratory state and leptin on Zonotrichia albicollis: II. Effects on fatty acid metabolism. Gen. Comp. Endocrinol 174, 269-275. 10.1016/j.ygcen.2011.08.024 [DOI] [PubMed] [Google Scholar]
- Zhang Y., King M. O., Harmon E. and Swanson D. L. (2015a). Summer-to-winter phenotypic flexibility of fatty acid transport and catabolism in skeletal muscle and heart of small birds. Physiol. Biochem. Zool. 88, 535-549. 10.1086/682154 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Eyster K., Liu J.-S. and Swanson D. L. (2015b). Cross-training in birds: cold and exercise training produce similar changes in maximal metabolic output, muscle masses and myostatin expression in house sparrows, Passer domesticus. J. Exp. Biol. 218, 2190-2200. 10.1242/jeb.121822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., King M. O., Harmon E., Eyster K. and Swanson D. L. (2015c). Migration-induced variation of fatty acid transporters and cellular metabolic intensity in passerine birds. J. Comp. Physiol. B 185, 797-810. 10.1007/s00360-015-0921-9 [DOI] [PubMed] [Google Scholar]