Abstract
Purpose
To retrospectively assess the utility of whole-lesion apparent diffusion coefficient (ADC) metrics in characterizing the Gleason 4 component of Gleason 7 prostate cancer (PCa) at radical prostatectomy.
Materials and Methods
Seventy patients underwent phased-array coil 3T-magnetic resonance imaging (MRI) before prostatectomy. A uropathologist mapped locations and Gleason 4 percentage (G4%) of Gleason 7 tumors. Two radiologists independently reviewed ADC maps, aware of tumor locations but not G4%, and placed a volume-of-interest (VOI) on all slices including each lesion on the ADC map to obtain whole-lesion mean ADC and ADC entropy. Entropy reflects textural variation and increases with greater macroscopic heterogeneity. Performance for characterizing Gleason 7 tumors was assessed with mixed-model analysis of variance (ANOVA) and logistic regression.
Results
Among 84 Gleason 7 tumors (G4% 5%–85%, median 30%; 59 Gleason 3+4, 25 Gleason 4+3), ADC entropy was significantly higher in Gleason 4+3 than Gleason 3+4 tumors (R1: 5.27 ± 0.61 vs. 4.62 ± 0.78, P =0.001; R2: 5.91 ± 0.32 vs. 5.57 ± 0.56, P =0.004); mean ADC was not significantly different between these groups (R1: 0.90 ± 0.15*10−3cm2/s vs. 0.98 ± 0.21 *10−3cm2/s, P =0.075; R2: 1.06 ± 0.19*10−3cm2/s vs. 1.14 ± 0.16*10−3cm2/s, P =0.083). The area under the receiver operating characteristic (ROC) curve (AUC) for differentiating groups was significantly higher with ADC entropy than mean ADC for one observer (R1: 0.74 vs. 0.57, P =0.027; R2: 0.69 vs. 0.61, P =0.329). For R1, correlation with G4% was moderate for ADC entropy (r =0.45) and weak for mean ADC (r =−0.25). For R2, correlation with G4% was moderate for ADC entropy (r =0.41) and mean ADC (r =−0.32). For both readers, ADC entropy (P =0.028–0.003), but not mean ADC (P =0.384–0.854), was a significant independent predictor of G4%.
Conclusion
Whole-lesion ADC entropy outperformed mean ADC in characterizing Gleason 7 tumors and may help refine prognosis for this heterogeneous PCa subset.
Keywords: prostate cancer, MRI, diffusion-weighted imaging, ADC, Gleason score
ALTHOUGH MOST CASES of prostate cancer have excellent prognosis, with little likelihood of causing symptoms or mortality, a fraction of patients harbor aggressive, lethal tumors (1). This heterogeneity in outcomes, combined with suboptimal means of establishing prognosis in individual patients and a widening array of treatment options, creates challenges for clinical management (2–4). Prostate cancer cases with a Gleason score of 7 represent a particular subset of prostate cancer diagnoses for which more precise prognostic tools would be of distinct value (5). Gleason 7 prostate cancer has been described as very common in numerous large series, and has been shown to be a separate group from lower and higher Gleason score tumors in terms of biologic features and clinical outcomes (2,6,7). While it is currently accepted that criteria for active surveillance eligibility should include Gleason score 6 tumors and exclude tumors with Gleason score ≥8 tumors, the optimal management and suitability for active surveillance of Gleason 7 tumors remains controversial (8,9).
The challenge in establishing optimal management of Gleason 7 prostate cancer is in part attributable to the heterogeneity of outcomes among this group. In particular, extensive data have established worse outcomes after radical prostatectomy in patents having Gleason 4+3 tumors compared with those having Gleason 3+4 tumor, including higher rates of lymph node metastases (10), postoperative biochemical recurrences (BCR) (6,7,11,12), and subsequent metastases and death (12,13). More recently, data have shown that rather than stratifying Gleason 7 tumors in a binary categorical fashion as Gleason 3+4 vs. Gleason 4+3, greater discriminatory power can be achieved by considering the percentage of 4 in a continuous pattern (14). For instance, several studies have shown the percentage of Gleason 4 component (G4%) to be associated with BCR (15–18), outperforming more traditional Gleason scoring as a prognostic marker in multivariate analysis (16,18). One such study demonstrated an almost linear increase in BCR for each 10% increase in presence of the higher-grade component (16). Another more recent report supported routine quantification of G4% in a continuous fashion by pathologists in reporting of both biopsy and prostatectomy specimens (14). Thus, the quantitative Gleason 4 component is likely to receive increasing attention as paradigms for prostate cancer management continue to evolve.
Multiparametric magnetic resonance imaging (MRI) has become a commonly used tool for guiding prognosis and treatment selection for prostate cancer (19). Of note, the apparent diffusion coefficient (ADC), a quantitative measure obtained from diffusion-weighted imaging (DWI), has shown a significant inverse correlation with Gleason scores from prostatectomy specimens (20,21). There is also evidence that tumor ADC is a more accurate predictor of high-risk disease at radical prostatectomy than is the biopsy-based Gleason score (22,23). Thus, ADC may become an accepted imaging biomarker that can greatly assist optimization of treatment selection.
While numerous studies have assessed correlations between ADC values and Gleason score, the existing studies have focused on differentiation of the standard summation Gleason scores (ie, categories 6 through 10) (20,21,24–26). There are minimal data investigating the utility of ADC values for differentiating Gleason 3+4 from Gleason 4+3 tumors (20,24), and no data correlating ADC with G4% among Gleason 7 tumors. This differentiation may be challenging given that the Gleason 4 component, which may comprise from 5% to 95% of the tumor, can be located anywhere within the tumor volume, and that ADC values are traditionally measured as an average value within a single-slice region-of-interest (ROI) (27). On the other hand, studies in other tissues have demonstrated the role of measuring ADC using a whole-lesion volume-based approach that not only provides a more complete representation of the entire tumor, but also facilitates computation of more advanced metrics reflecting overall lesion texture and heterogeneity (28,29). This volumetric approach to ADC quantification has shown value in studies in the brain (30,31), liver (27), and female pelvis (28,29), and may also have potential for improving the performance of ADC assessment in prostate cancer. Thus, given the importance of stratification of Gleason 7 tumors for guiding management of this large, albeit heterogeneous, population, the aim of this study was to assess the utility of whole-lesion ADC metrics in characterizing the Gleason 4 component within Gleason 7 prostate cancer at radical prostatectomy.
MATERIALS AND METHODS
Patients
This retrospective study was Health Insurance Portability and Accountability Act (HIPAA)-compliant and approved by our Institutional Review Board with a waiver of written informed consent. We searched an institutional database for all patients who underwent radical prostatectomy between January 1, 2012, and December 31, 2012, identifying ~165 cases. Of these, we identified 80 cases for whom the pathology report from the radical prostatectomy specimen indicated the presence of Gleason 7 cancer. Of these, 10 patients were then excluded for the following reasons: no preoperative prostate MRI at our institution (n =1); preoperative MRI at our institution performed at 1.5T (n =5); additional treatment for prostate cancer before surgery (n =1); pathologic slides from prostatectomy were unavailable for further detailed review for purposes of this study (n =2); and technical error in accessing the MR image files (n =1). These exclusions left a final included cohort of 70 patients (mean age 62 ± 8 years, range 46–79 years) with a mean prostate-specific antigen of 7.6 ± 6.9 ng/mL (range 1.2–45.0 ng/mL). The MRI was obtained in these 70 men for the following reasons: prior prostate biopsy positive for cancer (n =56), prior negative prostate biopsy with persistent clinical suspicion for prostate cancer (n =3), and biopsy-naïve patient with clinical suspicion for prostate cancer (n =11). The mean delay between MRI and radical prostatectomy was 40 ± 44 days (range 2–268 days).
MRI
Patients underwent MRI using a 3T whole-body system (Magnetom Trio; Siemens Healthcare; Erlangen, Germany) and a pelvic phased-array coil. Sequences of the prostate and seminal vesicles included axial turbo spin-echo (TSE) T2-weighted imaging (T2WI) (TR/TE 4960/105 msec; field of view [FOV] 180 × 180 mm; matrix 256 × 256; slice thickness 3 mm; no interslice gap; parallel imaging factor of 2; 3 signal averages) and axial single-shot echo-planar imaging (EPI) DWI (TR/TE 4100/86 msec; FOV 200 × 200 msec; matrix 100 × 100 msec; slice thickness 3 mml no interslice gap; parallel imaging factor 2; 10 signal averages) with tri-directional motion-probing gradients (b-values of 50 and 1000 s/mm2) and in-line reconstruction of ADC maps using a standard mono-exponential approach. Additional coronal and sagittal TSE T2WI, axial T1-weighted images, and dynamic contrast-enhanced images were obtained, but not evaluated as part of this study.
Reference Standard
The prostatectomy specimens were fixed overnight in formalin and then submitted in their entirety with standard step-section processing at 3–4 mm intervals. A single uropathologist (F.D., with 8 years of experience) reviewed the histologic slides and identified all foci of tumor with a Gleason score of 7. Regions of Gleason score 7 cancer within a distance of 5 mm were considered to represent portions of a single tumor focus. The pathologist manually depicted on a standardized map of the prostate the location and approximate boundaries of all identified Gleason 7 tumors. In addition, the pathologist recorded in a separate spreadsheet the G4% within each Gleason 7 tumor, based on visual assessment. G4% was reported within multiples of 5% and could therefore range from 5%–95%. Note that at our institution, tumors with G4% of under 5% are classified as having a Gleason score of 6 with a tertiary Gleason 4 component and would thus be excluded from this study (32).
Image Analysis
Two fellowship-trained abdominal radiologists (A.R. and M.T., with 5 and 1 years of experience, respectively) independently reviewed images using in-house developed software (FireVoxel; https://files.nyu.edu/hr18/public/projects.html) that allows placement of 3D volumes-of-interest (VOIs) incorporating voxels across multiple slices. The axial T2WI and ADC maps were viewed concurrently in a single session for each case. The observers had available the previously described maps constructed by the pathologist indicating the location of all Gleason 7 tumors, but were blinded to the G4% within these tumors. For each Gleason 7 tumor depicted on the maps, the observers placed a VOI of similar position and orientation on the ADC maps, encompassing all voxels demonstrating visually apparent decreased ADC in that region of the prostate. In two patients with two Gleason 7 tumors, one of the two tumors were obscured by ghosting artifact on the ADC map and excluded from further analysis. The axial T2WI was used solely to assist in anatomic localization of observations on the ADC map, rather than for directly guiding the placement of the VOIs on suspicious regions. The mean ADC and ADC entropy were then calculated for the VOI. Entropy was computed as Σ(−pi)/log(pi), in which pi represents the frequency of ADC values across the VOI (ie, the number of voxels with a given ADC value normalized to the total number of voxels within the VOI) (29). Entropy is a textural-based measure of the variation and predictability of individual values in the overall histogram distribution of values across the lesion (30). A greater entropy indicates the presence of a large number of voxels with different values in an unpredictable distribution and is believed to represent greater macroscopic structural heterogeneity of the tissue (30).
Statistics
The Pearson correlation was used to characterize the association of G4% with mean ADC and ADC entropy. Mixed-model analysis of variance (ANOVA) was used to assess the significance of these correlations and to test whether mean ADC and ADC entropy were independent predictors of G4%. Mixed-model ANOVA was also used to compare Gleason 3+4 and Gleason 4+3 tumors in terms of mean ADC and ADC entropy. Area under the receiver operating characteristic (ROC) curve (AUC) was used to describe the utility of mean ADC and ADC entropy for the detection of those Gleason 7 tumors exhibiting Gleason 4+3 pattern. Logistic regression for correlated data was used to test the utility of mean ADC and ADC entropy, alone and in combination, as predictors of Gleason 4+3 tumors within the cohort. Specifically, generalized estimating equations (GEE) in the context of binary logistic regression was used to model the binary indicator of whether or not a tumor was a Gleason 4+3 lesion as a function of mean ADC and ADC entropy. In the mixed-model and GEE analyses, statistical dependencies among results acquired from multiple tumors within the same patient were accounted for by assuming results to be symmetrically correlated when acquired from the same patient and to be independent when acquired from different patients. All statistical tests were conducted at the two-sided 5% significance level using SAS 9.3 (SAS Institute, Cary, NC).
RESULTS
Gleason 7 Tumors
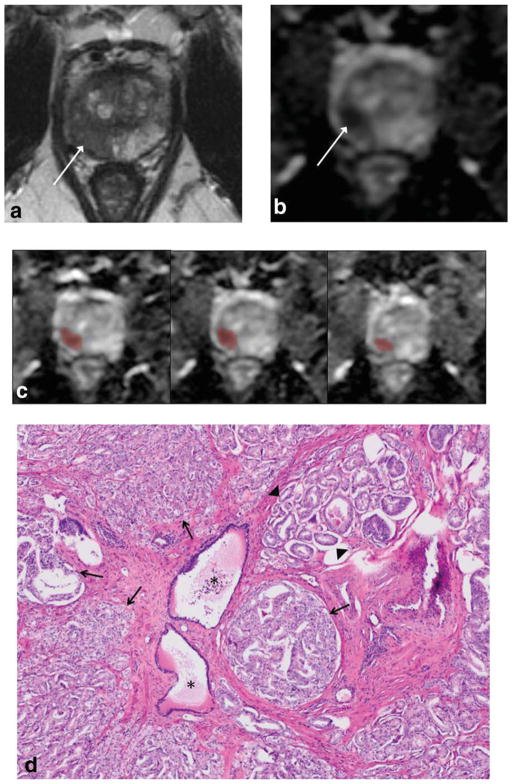
The pathologist reported and delineated 84 Gleason 7 tumors in the 70 patients: 59 patients with one, 8 patients with two, and 3 patients with three Gleason 7 tumors. Among these 84 tumors, the G4% ranged from 5%–85% (mean ± SD, 36% ± 25%; median 30%). Fifty-nine of the tumors exhibited Gleason score 3+4, and 25 tumors exhibited Gleason score 4+3. A representative case is shown in Fig. 1.
Figure 1.
A 67-year-old male who underwent radical prostatectomy for prostate cancer, with Gleason 7 tumor in right peripheral zone. a: Axial turbo spin-echo T2-weighted image shows tumor as area of decreased T2 signal (arrow). b: Axial apparent diffusion coefficient (ADC) map shows decreased ADC in tumor (arrow). c: Sample of several slices of ADC map from whole-lesion VOI assessment show placement of VOI encompassing voxels on multiple slices across area of tumor. Whole-lesion mean ADC was 0.921*10−3cm2/s for reader 1 and 1.12−3cm2/s for reader 2; whole-lesion ADC entropy was 5.79 for reader 1 and 6.49 for reader 2. d: Photomicroraph (H&E stain, ×4) of portion of tumor shows predominantly areas of Gleason pattern 4 tumor (arrows), with intermixed areas of Gleason pattern 3 tumor (arrowheads) as well as benign glands (asterisks). Overall Gleason 4 component within entire tumor was 65%.
Differentiation of Gleason 3+4 and Gleason 4+3 Tumors
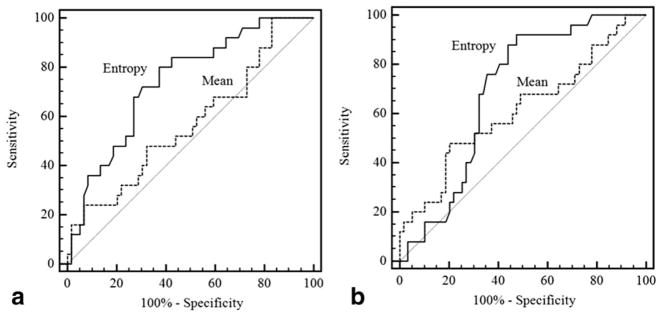
Table 1 shows findings pertaining to differentiation using whole-lesion ADC metrics of Gleason 3+4 and Gleason 4+3 tumors among the 70 patients with Gleason 7 prostate cancer. ADC entropy was significantly higher in Gleason 4+3 tumors than in Gleason 3+4 tumors for both observers (reader 1: 5.27 ± 0.61 vs. 4.62 ± 0.78, P =0.001; reader 2: 5.91 ± 0.32 vs. 5.57 ± 0.56, P =0.004). In contrast, there was no significant difference in mean ADC between these groups for either observer (reader 1: 0.90 ± 0.15*10−3cm2/s vs. 0.98 ± 0.21*10−3cm2/s, P =0.075; reader 2: 1.06 ± 0.19*10−3cm2/s vs. 1.14 ± 0.16*10−3cm2/s, P =0.083). Figure 2 shows the ROC curves comparing the AUC for differentiation of Gleason 4+3 and Gleason 3+4 tumors using the two metrics. For reader 1, there was significantly higher AUC using ADC entropy than mean ADC (0.74 vs. 0.57, P =0.027). For reader 2, a higher AUC using ADC entropy was not statistically significant (0.69 vs. 0.61, P =0.329). Table 2 shows P-values for the significance of each whole-lesion ADC metric as a significant independent predictor of the binary classification of Gleason 7 tumors (Gleason score 3+4 vs. Gleason score 4+3) when adjusted for the other metric. In this analysis, ADC entropy was a significant independent predictor of tumor classification for both observers (P ≤0.004), while mean ADC was not a significant independent predictor for either observer (P ≥0.144).
Table 1.
Comparison of Whole-Lesion ADC Metrics Between Gleason 3+4 Tumors and Gleason 4+3 Tumors, for Two Observers
| Metric | Reader 1
|
Reader 2
|
||||||
|---|---|---|---|---|---|---|---|---|
| Gleason 3+4 | Gleason 4+3 | P-value | AUC | Gleason 3+4 | Gleason 4+3 | P-value | AUC | |
| Mean ADC* | 0.98±0.21 | 0.90±0.15 | 0.075 | 0.57 | 1.14±0.16 | 1.06±0.19 | 0.083 | 0.61 |
| ADC Entropy | 4.62±0.78 | 5.27±0.61 | 0.001 | 0.74 | 5.57±0.56 | 5.91±0.32 | 0.004 | 0.69 |
x10−3mm2/s.
Figure 2.
Receiver-operator-characteristic curves for differentiation of Gleason 7 tumors with primary Gleason patterns of 3 and 4 using whole-lesion mean ADC and ADC entropy for reader 1 (a) and reader 2 (b).
Table 2.
Significance of Mean ADC and ADC Entropy as Independent Predictors of Binary Lesion Classification (Gleason 3+4 vs. Gleason 4+3 Tumor) and of Percentage of Gleason 4 Component
| Gleason 3+4 vs. Gleason 4 +3 tumors
|
Percentage Gleason 4 component
|
|||
|---|---|---|---|---|
| Reader 1 | Reader 2 | Reader 1 | Reader 2 | |
| Mean ADC | 0.640 | 0.144 | 0.854 | 0.384 |
| ADC Entropy | <0.001 | 0.004 | 0.003 | 0.028 |
All P-values are adjusted for the other whole-lesion ADC metric.
Association With Percentage Gleason 4
Table 3 shows the correlations between the whole-lesion ADC metrics and G4% in the Gleason 7 tumors. Correlations between both mean ADC and ADC entropy with G4% were statistically significant for both observers (mean ADC: P =0.004–0.023; ADC entropy, P <0.001, both observers). For reader 1, there was a moderate correlation between ADC entropy and G4% (r =0.45), compared with weak negative correlation between mean ADC and G4% (r =−0.25). For reader 2, G4% showed a moderate positive correlation with ADC entropy (r =0.41) and a moderate negative correlation with mean ADC (r =−0.32). Table 2 shows P-values for the significance of each whole-lesion ADC metric as a significant independent predictor of G4% when adjusted for the other metric. In this analysis, ADC entropy was a significant independent predictor of G4% for both observers (P =0.003–0.028), while mean ADC was not a significant independent predictor for either observer (P ≥0.384).
Table 3.
Correlation of Whole-Lesion ADC Metrics With Percentage of Gleason 4 Component Within Gleason 7 Tumors
| Metric | Reader 1
|
Reader 2
|
||
|---|---|---|---|---|
| r | P | r | P | |
| Mean ADC | −0.25 | 0.023 | −0.32 | 0.004 |
| ADC Entropy | 0.45 | <0.001 | 0.41 | <0.001 |
DISCUSSION
Gleason 7 prostate cancer represents a common subset of prostate cancer diagnoses with heterogeneous clinical behavior, leading to challenges in clinical management. In our study, we showed that two observers with varying levels of experience both identified significant differences in whole-lesion ADC entropy between Gleason 3+4 and Gleason 4+3 sub-populations of Gleason 7 cancers, with corresponding moderate performance of whole-lesion ADC entropy in their differentiation. There were also moderate correlations of ADC entropy with the G4% within the tumor. Thus, whole-lesion ADC entropy has the potential to serve as a biomarker that can assist clinical management of this group of patients.
ADC entropy is believed to be influenced by macroscopic structural heterogeneity (27,30). Its calculation is determined by the predictability of specific ADC values within the lesion and increases with a more heterogeneous distribution of ADC values (30). In our study, ADC entropy outperformed mean ADC for a number of the comparisons for the two readers, as mean ADC was not significantly different between Gleason 3+4 tumors and Gleason 4+3 tumors for either observer and, for observer 1, both showed significantly lower AUC for differentiation of these two entities as well as lower correlation with G4% in comparison with mean ADC. These findings may reflect the sensitivity of ADC entropy to greater architectural heterogeneity among tumors with a greater contribution of Gleason pattern 4 elements (33). This difference in performance between the metrics is important, given that the literature to date exploring the role of DWI in characterization of prostate cancer aggressiveness has largely used mean or median ADC values (20,21), which appears not to be the optimal metric. Furthermore, unlike in past studies, we evaluated the entire lesion in 3D on the ADC maps, thereby attaining a more complete representation of the tumor. This step is important, given that the Gleason 4 component may not be centrally located within the lesion, possibly comprising separate discontinuous areas within the lesion’s overall volume that can be distributed in “heterogeneous and unpredictable” geographic patterns (34).
While statistically significant, the observed differences in whole-lesion entropy between Gleason 7 tumors with primary patterns of 3 and 4 were not large. However, we would not have anticipated substantial differences. Past studies comparing ADC values with Gleason score have demonstrated overlap in ADC values between these categories (20,21,25), and in this study we were assessing subsets of just one such category. It would be expected that the performance for distinguishing these subsets would be inferior to that of ADC values for distinguishing the broader overall Gleason score categories. Indeed, the extent of the variation in G4% between tumors, measured in 5% increments, generally corresponds with small differences in the actual volume of Gleason pattern 4 tissue present in the tumor, which may impose inherent limits on the extent to which these differences can be distinguished via imaging. A limited number of past studies have presented data comparing ADC values between subdivisions of Gleason 7 tumors. While Vargas et al (24) showed a lower mean ADC in Gleason 4+3 tumors, compared with Gleason 3+4 tumors, a corresponding box-and-whisker plot suggests substantial overlap for most cases in their series, and no P-value directly comparing these means is provided. In a similar fashion, Hambrock et al (20) observed a slightly lower median ADC in Gleason 4+3 tumors than in Gleason 3+4 tumors, although they do not provide further data regarding the potential statistical significance of this difference or the ability of median ADC to differentiate individual patients in these two groups. Likewise, scatterplots presented by Kitajima et al (35) indicate substantial overlap in mean ADC and minimum ADC between Gleason 3+4 and Gleason 4+3 tumors, although the average values of these metrics within each group is not stated. Thus, a more focused assessment of the utility of DWI in providing a more precise characterization of Gleason 7 tumors, as we have attempted in this study, is warranted.
Precise assessment of G4% within prostate tumors is important given the emerging understanding of the unique nature of Gleason grade 4 tumor as qualitatively distinct from Gleason grade 3 tumor, rather than these representing different entities along a single spectrum (36). For instance, Gleason grade 4 cancer exhibits a widely variable pattern of gene overexpression, whereas Gleason grade 3 cancer is relatively homogeneous in its pattern of gene overexpression (37). In addition, Gleason grade 4 cancer exhibits a unique profile of downregulation and upregulation of a large number of specific genes (38), including upregulation of genes with attenuated androgen signaling, a property not observed in Gleason grade 3 cancer and that has been associated with metastatic potential (39). Furthermore, an mRNA expression signature associated with Gleason 4 prostate cancer has been linked with lethality (40). In a recent study of 1781 men with Gleason score 6, prostate cancer diagnosed in the era of prostate-specific antigen (PSA) screening, no patient demonstrated metastatic spread at the time of radical prostatectomy (41). Indeed, although clearly controversial, removing the label of “cancer” from Gleason 6 tumors has been recently suggested (42).
While the substantially higher rate of biochemical recurrence and mortality in Gleason 4+3 tumors compared with Gleason 3+4 tumors has been well known (6,12), additional data supports the importance of the actual G4% within Gleason 7 tumors. This more precise quantification offers greater statistical power for discrimination of Gleason 7 tumors in comparison with an inherently binary approach (14). In addition, a number of studies have shown that the percentage of high-grade component serves as a significant independent predictor of biochemical recurrence (15–18), outperforming the standard Gleason score (16,18) and potentially serving as the best single predictor of outcome (15). The percent of high-grade component has also been shown to correlate significantly with presence of nodal metastases (43). Given the strength of such data, a so-called “quantitative” Gleason score has recently been proposed that is largely derived from the proportion of Gleason 4 component present (14).
Our study has several limitations. First, this study was retrospective in nature; while a single uropathologist reviewed all included cases to identify the location and G4% of Gleason 7 tumors for purposes of our analysis, three different uropathologists initially processed and interpreted the specimens at the time of patient care. Also, the VOIs were placed aware of location of Gleason 7 tumors at histopathologic assessment; future studies may investigate the performance of whole-lesion ADC metrics determined blinded to this information. In addition, although we correlated whole-lesion ADC metrics with G4%, we did not correlate the metrics with clinical outcomes such as biochemical recurrence or metastatic disease. Another limitation is that while most patients underwent prostatectomy within several months of MRI, the delay between MRI and surgery was as long as ~9 months in some patients. Finally, while it is recognized that ADC entropy is influenced by macroscopic heterogeneity, the exact histologic basis for the observed differences in this metric in our study remains uncertain.
In conclusion, we demonstrated that whole-lesion ADC entropy correlates with the G4% of Gleason 7 prostate cancer and shows larger differences than mean ADC between Gleason 3+4 tumors and Gleason 4+3 tumors. Future studies may assess the potential incorporation of this whole-lesion ADC metric into the clinical management of Gleason 7 prostate cancer.
Acknowledgments
Contract grant sponsor: Joseph and Diane Steinberg Charitable Trust.
We thank James S. Babb, PhD, for assistance with statistical analysis.
References
- 1.Roobol MJ, Carlsson SV. Risk stratification in prostate cancer screening. Nat Rev Urol. 2013;10:38–48. doi: 10.1038/nrurol.2012.225. [DOI] [PubMed] [Google Scholar]
- 2.Reese AC, Pierorazio PM, Han M, Partin AW. Contemporary evaluation of the National Comprehensive Cancer Network prostate cancer risk classification system. Urology. 2012;80:1075–1079. doi: 10.1016/j.urology.2012.07.040. [DOI] [PubMed] [Google Scholar]
- 3.Sciarra A, Barentsz J, Bjartell A, et al. Advances in magnetic resonance imaging: how they are changing the management of prostate cancer. Eur Urol. 2011;59:962–977. doi: 10.1016/j.eururo.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 4.Bozzini G, Colin P, Nevoux P, Villers A, Mordon S, Betrouni N. Focal therapy of prostate cancer: energies and procedures. Urol Oncol. 2013;31:155–167. doi: 10.1016/j.urolonc.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Tefilli MV, Gheiler EL, Tiguert R, et al. Should Gleason score 7 prostate cancer be considered a unique grade category? Urology. 1999;53:372–377. doi: 10.1016/s0090-4295(98)00479-8. [DOI] [PubMed] [Google Scholar]
- 6.Sakr WA, Tefilli MV, Grignon DJ, et al. Gleason score 7 prostate cancer: a heterogeneous entity? Correlation with pathologic parameters and disease-free survival. Urology. 2000;56:730–734. doi: 10.1016/s0090-4295(00)00791-3. [DOI] [PubMed] [Google Scholar]
- 7.Rasiah KK, Stricker PD, Haynes AM, et al. Prognostic significance of Gleason pattern in patients with Gleason score 7 prostate carcinoma. Cancer. 2003;98:2560–2565. doi: 10.1002/cncr.11850. [DOI] [PubMed] [Google Scholar]
- 8.Cooperberg MR, Cowan JE, Hilton JF, et al. Outcomes of active surveillance for men with intermediate-risk prostate cancer. J Clin Oncol. 2011;29:228–234. doi: 10.1200/JCO.2010.31.4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klotz L, Zhang L, Lam A, Nam R, Mamedov A, Loblaw A. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010;28:126–131. doi: 10.1200/JCO.2009.24.2180. [DOI] [PubMed] [Google Scholar]
- 10.Herman CM, Kattan MW, Ohori M, Scardino PT, Wheeler TM. Primary Gleason pattern as a predictor of disease progression in Gleason score 7 prostate cancer: a multivariate analysis of 823 men treated with radical prostatectomy. Am J Surg Pathol. 2001;25:657–660. doi: 10.1097/00000478-200105000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Stephenson AJ, Scardino PT, Eastham JA, et al. Postoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J Clin Oncol. 2005;23:7005–7012. doi: 10.1200/JCO.2005.01.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan TY, Partin AW, Walsh PC, Epstein JI. Prognostic significance of Gleason score 3+4 versus Gleason score 4+3 tumor at radical prostatectomy. Urology. 2000;56:823–827. doi: 10.1016/s0090-4295(00)00753-6. [DOI] [PubMed] [Google Scholar]
- 13.Stark JR, Perner S, Stampfer MJ, et al. Gleason score and lethal prostate cancer: does 3 +4 =4 +3? J Clin Oncol. 2009;27:3459–3464. doi: 10.1200/JCO.2008.20.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reese AC, Cowan JE, Brajtbord JS, Harris CR, Carroll PR, Cooperberg MR. The quantitative Gleason score improves prostate cancer risk assessment. Cancer. 2012;118:6046–6054. doi: 10.1002/cncr.27670. [DOI] [PubMed] [Google Scholar]
- 15.Cheng L, Davidson DD, Lin H, Koch MO. Percentage of Gleason pattern 4 and 5 predicts survival after radical prostatectomy. Cancer. 2007;110:1967–1972. doi: 10.1002/cncr.23004. [DOI] [PubMed] [Google Scholar]
- 16.Stamey TA, McNeal JE, Yemoto CM, Sigal BM, Johnstone IM. Biological determinants of cancer progression in men with prostate cancer. JAMA. 1999;281:1395–1400. doi: 10.1001/jama.281.15.1395. [DOI] [PubMed] [Google Scholar]
- 17.Stamey TA, Yemoto CM, McNeal JE, Sigal BM, Johnstone IM. Prostate cancer is highly predictable: a prognostic equation based on all morphological variables in radical prostatectomy specimens. J Urol. 2000;163:1155–1160. doi: 10.1016/s0022-5347(05)67713-0. [DOI] [PubMed] [Google Scholar]
- 18.Cheng L, Koch MO, Juliar BE, et al. The combined percentage of Gleason patterns 4 and 5 is the best predictor of cancer progression after radical prostatectomy. J Clin Oncol. 2005;23:2911–2917. doi: 10.1200/JCO.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 19.Hoeks CM, Barentsz JO, Hambrock T, et al. Prostate cancer: multiparametric MR imaging for detection, localization, and staging. Radiology. 2011;261:46–66. doi: 10.1148/radiol.11091822. [DOI] [PubMed] [Google Scholar]
- 20.Hambrock T, Somford DM, Huisman HJ, et al. Relationship between apparent diffusion coefficients at 3. 0-T MR imaging and Gleason grade in peripheral zone prostate cancer. Radiology. 2011;259:453–461. doi: 10.1148/radiol.11091409. [DOI] [PubMed] [Google Scholar]
- 21.Verma S, Rajesh A, Morales H, et al. Assessment of aggressiveness of prostate cancer: correlation of apparent diffusion coefficient with histologic grade after radical prostatectomy. AJR Am J Roentgenol. 2011;196:374–381. doi: 10.2214/AJR.10.4441. [DOI] [PubMed] [Google Scholar]
- 22.Bittencourt LK, Barentsz JO, de Miranda LC, Gasparetto EL. Prostate MRI: diffusion-weighted imaging at 1. 5T correlates better with prostatectomy Gleason grades than TRUS-guided biopsies in peripheral zone tumours. Eur Radiol. 2012;22:468–475. doi: 10.1007/s00330-011-2269-1. [DOI] [PubMed] [Google Scholar]
- 23.Somford DM, Hambrock T, Hulsbergen-van de Kaa CA, et al. Initial experience with identifying high-grade prostate cancer using diffusion-weighted MR imaging (DWI) in patients with a Gleason score </ =3 +3 =6 upon schematic TRUS-guided biopsy: a radical prostatectomy correlated series. Invest Radiol. 2012;47:153–158. doi: 10.1097/RLI.0b013e31823ea1f0. [DOI] [PubMed] [Google Scholar]
- 24.Vargas HA, Akin O, Franiel T, et al. Diffusion-weighted endorectal MR imaging at 3 T for prostate cancer: tumor detection and assessment of aggressiveness. Radiology. 2011;259:775–784. doi: 10.1148/radiol.11102066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turkbey B, Shah VP, Pang Y, et al. Is apparent diffusion coefficient associated with clinical risk scores for prostate cancers that are visible on 3-T MR images? Radiology. 2011;258:488–495. doi: 10.1148/radiol.10100667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woodfield CA, Tung GA, Grand DJ, Pezzullo JA, Machan JT, Renzulli JF., 2nd Diffusion-weighted MRI of peripheral zone prostate cancer: comparison of tumor apparent diffusion coefficient with Gleason score and percentage of tumor on core biopsy. AJR Am J Roentgenol. 2010;194:W316–322. doi: 10.2214/AJR.09.2651. [DOI] [PubMed] [Google Scholar]
- 27.Fujimoto K, Tonan T, Azuma S, et al. Evaluation of the mean and entropy of apparent diffusion coefficient values in chronic hepatitis C: correlation with pathologic fibrosis stage and inflammatory activity grade. Radiology. 2011;258:739–748. doi: 10.1148/radiol.10100853. [DOI] [PubMed] [Google Scholar]
- 28.Downey K, Riches SF, Morgan VA, et al. Relationship between imaging biomarkers of stage I cervical cancer and poor-prognosis histologic features: quantitative histogram analysis of diffusion-weighted MR images. AJR Am J Roentgenol. 2013;200:314–320. doi: 10.2214/AJR.12.9545. [DOI] [PubMed] [Google Scholar]
- 29.Kierans AS, Bennett GL, Mussi TC, et al. Characterization of malignancy of adnexal lesions using ADC entropy: comparison with mean ADC and qualitative DWI assessment. J Magn Reson Imaging. 2013;37:164–171. doi: 10.1002/jmri.23794. [DOI] [PubMed] [Google Scholar]
- 30.Tavazzi E, Dwyer MG, Weinstock-Guttman B, et al. Quantitative diffusion weighted imaging measures in patients with multiple sclerosis. Neuroimage. 2007;36:746–754. doi: 10.1016/j.neuroimage.2007.03.056. [DOI] [PubMed] [Google Scholar]
- 31.Benedict RH, Bruce J, Dwyer MG, et al. Diffusion-weighted imaging predicts cognitive impairment in multiple sclerosis. Mult Scler. 2007;13:722–730. doi: 10.1177/1352458507075592. [DOI] [PubMed] [Google Scholar]
- 32.Pan CC, Potter SR, Partin AW, Epstein JI. The prognostic significance of tertiary Gleason patterns of higher grade in radical prostatectomy specimens: a proposal to modify the Gleason grading system. Am J Surg Pathol. 2000;24:563–569. doi: 10.1097/00000478-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Epstein JI, Allsbrook WC, Jr, Amin MB, Egevad LL. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol. 2005;29:1228–1242. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]
- 34.Aihara M, Wheeler TM, Ohori M, Scardino PT. Heterogeneity of prostate cancer in radical prostatectomy specimens. Urology. 1994;43:60–66. doi: 10.1016/s0090-4295(94)80264-5. discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 35.Kitajima K, Takahashi S, Ueno Y, et al. Do apparent diffusion coefficient (ADC) values obtained using high b-values with a 3-T MRI correlate better than a transrectal ultrasound (TRUS)-guided biopsy with true Gleason scores obtained from radical prostatectomy specimens for patients with prostate cancer? Eur J Radiol. 2013;82:1219–1226. doi: 10.1016/j.ejrad.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 36.Lavery HJ, Droller MJ. Do Gleason patterns 3 and 4 prostate cancer represent separate disease states? J Urol. 2012;188:1667–1675. doi: 10.1016/j.juro.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 37.True L, Coleman I, Hawley S, et al. A molecular correlate to the Gleason grading system for prostate adenocarcinoma. Proc Natl Acad Sci U S A. 2006;103:10991–10996. doi: 10.1073/pnas.0603678103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stamey TA, Caldwell MC, Fan Z, et al. Genetic profiling of Gleason grade 4/5 prostate cancer: which is the best prostatic control tissue? J Urol. 2003;170(6 Pt 1):2263–2268. doi: 10.1097/01.ju.0000096414.25583.0d. [DOI] [PubMed] [Google Scholar]
- 39.Tomlins SA, Mehra R, Rhodes DR, et al. Integrative molecular concept modeling of prostate cancer progression. Nat Genet. 2007;39:41–51. doi: 10.1038/ng1935. [DOI] [PubMed] [Google Scholar]
- 40.Penney KL, Sinnott JA, Fall K, et al. mRNA expression signature of Gleason grade predicts lethal prostate cancer. J Clin Oncol. 2011;29:2391–2386. doi: 10.1200/JCO.2010.32.6421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Donin NM, Laze J, Zhou M, Ren Q, Lepor H. Gleason 6 prostate tumors diagnosed in the PSA era do not demonstrate the capacity for metastatic spread at the time of radical prostatectomy. Urology. 2013;82:148–153. doi: 10.1016/j.urology.2013.03.054. [DOI] [PubMed] [Google Scholar]
- 42.Carter HB, Partin AW, Walsh PC, et al. Gleason score 6 adenocarcinoma: should it be labeled as cancer? J Clin Oncol. 2012;30:4294–4296. doi: 10.1200/JCO.2012.44.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McNeal JE, Villers AA, Redwine EA, Freiha FS, Stamey TA. Histologic differentiation, cancer volume, and pelvic lymph node metastasis in adenocarcinoma of the prostate. Cancer. 1990;66:1225–1233. doi: 10.1002/1097-0142(19900915)66:6<1225::aid-cncr2820660624>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]