Abstract
Cardiac mitochondria are composed of two distinct subpopulations: one beneath the sarcolemma (subsarcolemmal mitochondria: SSM), and another along the myofilaments (interfibrillary mitochondria: IFM). Previous studies suggest a preferential loss of IFM function with age; however, the age-related changes in oxidative stress in these mitochondrial subpopulations have not been examined. To this end, the changes in mitochondrial antioxidant capacity, oxidant output, and oxidative damage to Complex IV in IFM and SSM from young and old rats were studied. Results show no apparent differences in any parameters examined between IFM and SSM from young rats. However, relative to young, only IFM from old rats had a significantly higher rate of oxidant production and a decline in mitochondrial ascorbate levels and GSH redox status. The age-related decline in mitochondrial antioxidant capacity in IFM was accompanied by a marked loss in glutaredoxin and GSSG reductase activities, suggesting a diminished reductive capacity in IFM with age. Moreover, the loss in Complex IV activity was limited to the IFM of old rats, which was accompanied by a 4-fold increase in 4-hydroxynonenal-modified Complex IV. Thus, mitochondrial decay is not uniform and further indicates that myofibrils may be uniquely under oxidative stress in the aging heart.
Keywords: Aging, Mitochondria, Heart, Antioxidants, Oxidants, Electron transport complexes, Free radicals
INTRODUCTION
The aging heart undergoes significant functional and structural alterations leading to atrophy and a compensatory hypertrophy, followed by myocardial fibrosis [1,2]. In addition, there is an age-related decline in the capacity to withstand stress, such as ischemia/reperfusion [3–5]. In its most severe form, cardiac decay results in congestive heart failure, one of the leading causes of death in people over the age of 65. Although mechanisms underlying cardiac decay are not clear, loss of mitochondrial function and a resultant increase in oxidative stress has been proposed to be one of the key factors in myocardial aging [6–8].
The extent of age-related changes to cardiac mitochondria are poorly defined and further confounded by their heterogeneous nature. Similar to other muscle tissues, cardiac mitochondria are distributed into two localized groups. Subsarcolemmal mitochondria (SSM) are adjacent to the subsarcolemmal membrane, whereas interfibrillary mitochondria (IFM) are tightly associated with myosin fibers [9]. In addition to differences in their locality, the rates of oxidative phosphoryation of IFM and SSM are also different. In comparison to SSM, IFM exhibit higher State 3 respiratory rates and contain increased content of respiratory cytochromes and activity of electron transport chain complexes. Due to the differences in intracellular locale, SSM may be more important in supplying ATP for ion homeostasis (Ca-ATPases), whereas IFM may supply ATP required for myosin ATPase activity. Understanding the age-related changes specific to these subpopulations will be fundamentally important in identifying likely cellular processes that are affected during the aging process.
Interestingly, Hoppel and co-workers reported that the age-related loss in respiratory function is primarily localized to IFM [10]. This is reflected by the fact that aging results in a selective loss of IFM protein yield [10] and activities of Complexes III [11] and IV [10]. The aging defects on Complex III seem to involve lesions at the cytochrome c binding site, whereas the exact nature of defects involving Complex IV have not been established. The tight physical association of IFM to myosin fibrils necessitates the use of proteases during the isolation procedure and thus may be more prone to damage during isolation. However, Hoppel and co-workers demonstrated that SSM treated in an identical manner, as IFM exhibit no changes in bioenergetic parameters, such as respiratory control ratios, and in various electron transport chain activities [12].
The selective decline in IFM function raises an important issue as to whether aging results in a disproportionate increase in oxidative stress in IFM versus SSM. However, to date no information is available on the extent to which aging affects various parameters of oxidative stress in these two subpopulations of cardiac mitochondria. The redox environment of mitochondria is controlled, in part, by reductants such as NAD(P)H, ascorbate, and glutathione (GSH). In addition, GSH and thioredoxin-dependent enzymes play a major role in maintaining reducing equivalents required for enzyme function and signal transduction [13,14]. An age-related decline in any of these systems would likely result in an enhanced pro-oxidant environment and subsequent oxidative injury to critical mitochondrial proteins. Theoretically, this may provide some explanation as to the selective decline in IFM function. In this report, we show that IFM are prone to oxidative stress and injury, which is in part due to a selective loss in thiol antioxidant systems in this particular mitochondrial subpopulation.
MATERIALS AND METHODS
Animals
Rats (male Fischer 344), both young (2 to 5 months: n = 4–8) and old (24 to 28 months; n = 4–11; National Institute of Aging animal colonies), were acclimatized in the Oregon State University animal facilities for at least 1 week before experimentation. Animals were maintained on standard chow diet and water was given ad libitum. Rats were anesthetized with diethyl ether and a midline incision was made in the abdomen. Heparin (0.4 mg/ml) was injected via the femoral vein and Hank’s balanced salt buffer (pH 7.4) was perfused through the superior vena cava for 5 min to remove blood. Hearts were quickly removed for mitochondrial isolation.
Mitochondrial isolation
Two subpopulations of cardiac mitochondria were isolated according to the procedures of Palmer et al. [9] Briefly, hearts were rinsed and finely minced in buffer A (220 mmol/l mannitol, 70 mmol/l sucrose, 5 mmol/l 3-[N-Morpholino]propanesulfonic acid [MOPS], pH 7.4) containing 2 mmol/l ethylene glycol-bis(β-aminoethyl ether) tetraacetic acid (EGTA), 0.2% bovine serum albumin (BSA). The minced tissue was homogenized with a teflon-glass homogenizer and centrifuged at 500 × g at 4°C. The resulting pellet was resuspended to its original volume in buffer A and homogenized. The supernatants from these preparations were pooled and centrifuged at 3000 × g at 4°C for 10 min to obtain the subsarcolemmal mitochondrial fraction. Isolated SSM were washed twice in buffer A containing 0.5 mM EGTA and kept on ice until used. The remaining tissue pellet obtained during SSM isolation was resuspended in buffer B containing 100 mmol/l KCl, 2 mmol/l EGTA, 0.2% BSA 50 mmol/l MOPS, pH 7.4. To these samples, Nagarse (Sigma Chemical Co., St. Louis, MO, USA) was added to a final concentration of 5 mg/g wet weight of tissue and homogenized immediately with a dounce-type tissue homogenizer. This homogenate was diluted 2-fold with buffer B and centrifuged at 5000 × g for 5 min. The remaining pellet after Nagarse treatment was resuspended with buffer B and sedimented at low speed to obtain the nuclear fraction. The pellet was washed twice in buffer B to ensure maximal yield. The resulting supernatants were pooled and centrifuged at 3000 × g at 4°C for 10 min. Interfibrillary mitochondria were washed twice and resuspended in buffer B (without BSA). The protein yield was quantified using standard Lowry assay with BSA as the external standard.
Mitochondrial oxidant generation
The rate of 2′,7′-dihydrodichlorofluorescein (DCFH) oxidation was quantified to determine changes in mitochondrial oxidant generation [15]. Mitochondria (25 µg/ml) were treated with or without antimycin A (10 µmol/l) and were incubated in the presence of respiration buffer [15] containing 0.1 mmol/l ATP, 2 mmol/l ADP, 5 mmol/l malate and glutamate in 96 well plates. DCFH (25 µmol/l) was added and the change in DCFH oxidation was monitored for 60 min at 30°C using a Cytoflour 4000 fluorescence plate reader (Applied Biosystems, Foster City, CA, USA).
Ascorbate and GSH analysis
Mitochondria (750 µg protein) were used for the detection of ascorbate and GSH. Ascorbate was measured according to the method of Frei et al. [16]. Ascorbate was separated by HPLC [16] and detected at an applied potential of +0.6 V by a LC 4B electrochemical detector (Bioanalytical Systems, Inc., West Lafayette, IN, USA).
Mitochondrial GSH status was measured according to the method of Jones et al. [17]. GSH and GSSG were separated by HPLC and detected using a fluorescence spectrophotometer (Hitachi Fl-6000), with monochromators set at 335 nm for excitation and 515 nm for emission.
Mitochondrial antioxidant enzyme assays
Mitochondrial thioredoxin reductase activity was determined based on the methods of Hill and co-workers [18]. Mitochondria (10–40 µg protein) were incubated in potassium phosphate buffer (100 mmol/l; pH 7.0) in the presence of 5 mmol/l 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB), 20 mmol/l EDTA, and 0.2 mg/ml BSA for 5 min. After the initial incubation, 0.2 mmol/l NADPH was added in the presence or absence of 20 µmol/l auranofin (a specific inhibitor of thioredoxin reductase). Thioredoxin reductase activity was obtained based on the auranofin inhabitable rate of DTNB reduction.
Mitochondrial GSSG reductase activity was measured according to the method of Smith and coworkers [19]. Briefly, mitochondria (40–100 µg protein) were incubated in potassium phosphate buffer (100 mmol/l: pH 7.5) containing 0.2 mmol/l GSSG. To these samples, NADPH (0.2 mmol/l) was added and the rate of NADPH loss was monitored by following the absorbance loss at 340 nm.
Mitochondrial glutaredoxin reductase activity was determined according to the method of Gladyshev and co-workers [20]. Mitochondria (20–50 µg of protein) were added to a potassium phosphate buffer (100 mmol/l: pH 7.5) containing 0.2 mmol/l NADPH, 0.5 mmol/l GSH, and 0.4 units of GSSG reductase. To these samples, hydroxyethyldisulfide (HEDS: 2 mmol/l) was added and the decrease in absorbance of NADPH at 340 nm was monitored for 10 min. One unit of enzyme activity was defined as 1 µmol of NADPH oxidized per min.
Complex IV (cytochrome c oxidase) activity
Cytochrome c oxidase activity of isolated mitochondria was measured by using a commercially available kit from Sigma (Cytoc-ox1). Mitochondria (2 µg protein) were added to the assay buffer, which consisted of 10 mmol/l Tris-HCl, pH 7.0, and 120 mmol/l KCl. To these samples, 50 µl of reduced cytochrome C (0.22 mmol/l) were added and changes in absorbance at 550 nm were monitored for 1 min. An extinction coefficient of 21,840 M−1cm−1 was used to quantify the activity.
Blue native gel electrophoresis
Blue native polyacrylamide gel electrophoresis (BN-PAGE) was carried out on a 5–13.5% linear acrylamide gradient according to the methods of Schagger and von Jagow [21]. Mitochondria (200 µg of protein) were centrifuged at 10,000 × g for 10 min. The resulting pellet was reconstituted in 40 µl of 50 mmol/l Bis-Tris (pH 7.0), containing 750 mmol/l 6-aminocaproic acid, and 5 µl of n-dodecyl-B-D-maltoside (10% w/v). These samples were centrifuged at 100,000 × g for 15 min. Supernatants were collected and 5% (w/v) Coomassie blue brilliant G-250 (BioRad, Hercules, CA, USA) dissolved in 1 M aminocaproic acid was added at a ratio of 1:14 (dye to sample volume ratio). Bovine liver catalase (Sigma, 230 kDA and 460 kDA) was used as a molecular weight marker.
Detection of 4-HNE adduction to mitochondrial electron transport chain proteins
Electron transport chain complexes were separated using BN-PAGE and transferred onto PVDF membranes at a constant voltage of 50 V for 45 min. Immediately after the transfer, excess Coomassie dye was removed by a 30 min incubation at 50°C in stripping buffer (100 mmol/l 2-mercaptoethanol, 2% SDS, 62.5 mmol/l Tris-HCl, pH 6.7). Membranes were probed with anti-4-hydroxynonenal (4-HNE) polyclonal rabbit sera (Advanced Life Technology). After the detection with 4-HNE antisera, membranes were stripped and reprobed using monoclonal antibodies against Complex IV (Molecular Probes, Eugene, OR, USA). Immunoreactive proteins were detected using horseradish peroxidase conjugated secondary antibodies and were visualized by chemiluminescence detection (Amersham). Relative densities of the bands were digitally quantified using NIH image analysis software.
Statistical analysis
Results are shown as mean ± standard errors for the indicated sample size. Statistical significance was assessed by single factor ANOVA with Tukey’s post-hoc test. Differences were considered statistically significant at p < .05.
RESULTS
Age-associated changes in mitochondrial oxidant generation
To determine the relative and age-associated differences in oxidant appearance, IFM and SSM from young and old rats were incubated with DCFH, a general indicator of reactive oxygen and nitrogen species. As shown in Fig. 1, no significant differences in oxidant production were observed between IFM (50.80 ± 3.35 a.f.u./mg protein/min) and SSM (58.93 ± 5.98 a.f.u./mg protein/min) isolated from young rats. Likewise, we observed no differences in the respiratory control and ADP/O ratios in IFM and SSM isolated from young animals.
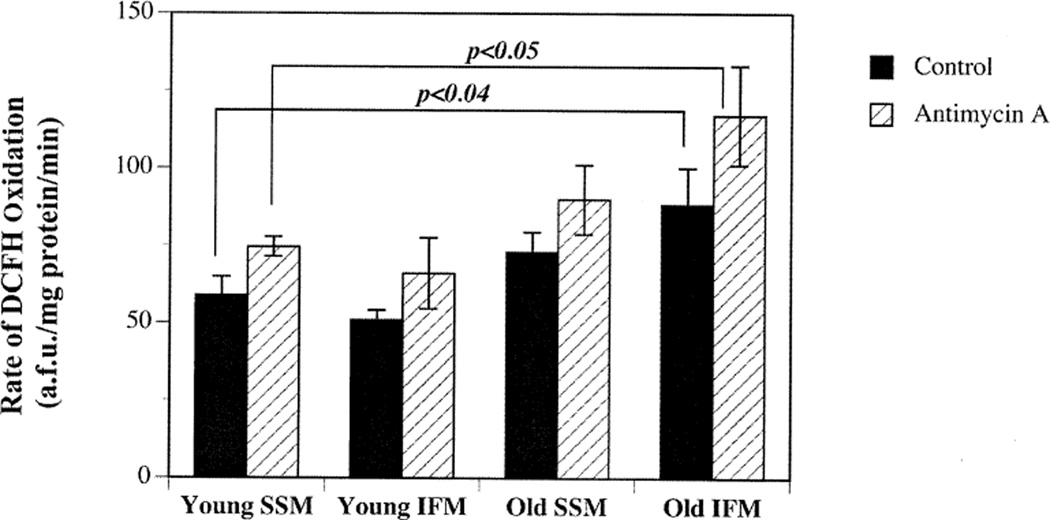
Fig. 1.
Age-related increase in mitochondrial oxidant generation is limited to IFM. The rates of mitochondrial oxidant generation in the presence or absence of antimycin A under glutamate, malate, and ADP-stimulated conditions were examined as described in Materials and Methods. Results show that the rate of mitochondrial oxidant appearance increases markedly in IFM isolated from old (open bar) when compared to young (closed bar). Results are expressed as mean ± SEM for IFM and SSM isolated from young (n = 8) and old rats (n = 11).
Comparing the rate of oxidant appearance in mitochondrial subpopulations from young and old rats showed that only the IFM were adversely affected with age. A significant 42% (p < .05) increase in the rate of oxidant appearance was seen in IFM isolated from old (77.57 ± 4.5 a.f.u./mg protein/min) versus young rats (50.80 ± 3.35 a.f.u./mg protein/min; Fig. 1). Whereas IFM exhibit an increase in the rate of oxidant appearance with age, SSM did not show similar age-related changes in the rate of DCFH oxidation (Fig. 1). In addition, the ADP/O and RCR in SSM remained unchanged with age. The age-associated increase in the rate of DCFH oxidation was not due to compromised mitochondrial integrity. The respiratory control ratios in old IFM (7.34 ± 2.54) were similar to that of young IFM (7.25 ± 0.31). However, the ADP/O ratios in old IFM (1.27 ± 0.1) were lower than young IFM (1.93 ± 0.25), suggesting that IFM from old rats utilize oxygen less efficiently for ATP production and support the DCFH findings indicating a higher propensity for oxidant generation.
Because DCFH oxidation does not specifically measure O2•− and H2O2 derived from the electron transport chain, we co-incubated the mitochondria with antimycin A. Antimycin A is a complex III inhibitor that should stimulate O2•− generation and thus we could determine whether oxidants derived from the electron transport chain could be detected by this assay. With the addition of antimycin A, the rates of DCFH oxidation increased by approximately 22% in both IFM and SSM isolated from young animals (Fig. 1). Treatment with antimycin A stimulated basal rate of DCFH oxidation in IFM obtained from both young (66.00 ± 11.45 a.f.u./mg protein/min) and old (117.08 ± 16 a.f.u./mg protein/min). The age-related differences in DCFH oxidation were conserved even in the presence of antimycin A. These results suggest that IFM in the aging heart are solely responsible for increased oxidant generation previously observed [8].
Age-associated changes in low-molecular weight antioxidant and thiol redox status
To determine whether age-associated changes in low molecular weight antioxidants could, in part, account for the differences in oxidant generation displayed by IFM and SSM, mitochondrial ascorbate and glutathione (GSH) status were determined. Results show that IFM and SSM from young animals maintained similar levels of ascorbate and GSH (Table 1).
Table 1.
Age-Associated Changes in Mitochondrial Low Molecular Weight Antioxidants
| Young | Old | |||
|---|---|---|---|---|
| IFM | SSM | IFM | SSM | |
| Ascorbate (nmol/mg protein) | 1.22 ± 0.13* | 1.47 ± 0.20 | 034 ± 0.07*† | 0.97 ± 0.30† |
| GSH (nmol/mg protein) | 3.81 ± 0.54* | 3.75 ± 0.26 | 2.32 ± 0.29*† | 3.58 ± 0.47† |
| GSSG (nmol/mg protein) | 0.37 ± 0.08* | 0.17 ± 0.09 | 0.54 ± 0.1*† | 0.12 ± 0.08† |
| Total GSH (nmol/mg protein) | 4.69 ± 0.57 | 4.11 ± 0.34 | 3.38 ± 0.2 | 3.82 ± 0.52 |
| % GSH Oxidized | 16.3 ± 2.9*† | 8.2 ± 3.6† | 32.18 ± 6.88*† | 6.2 ± 1.15† |
Values are expressed as mean ± SEM. Ascorbate and GSH levels in the freshly isolated IFM and SSM from young and old rats were measured as described in Materials and Methods. The results show a significant decline in the ascorbate and reduced GSH levels. In contrast to ascorbate, total GSH levels did not change with age or type of mitochondria. The results are expressed as mean ± SEM.
denotes significant (p < .05) difference between identical mitochondrial preparations from young and old rats while
denotes differences between IFM and SSM within the same age group.
Despite the lack of differences in antioxidant concentrations between IFM and SSM in young rats, aging resulted in a selective loss of these antioxidants in IFM isolated from old animals. On an age basis, the level of ascorbate present in old IFM was 73% lower (p < .002) than in IFM obtained from young rats (Table 1). Similarly, IFM from old rats exhibited a 40% loss (p = .02) in GSH levels when compared to IFM isolated from young rats (Table 1). In contrast to IFM, no age-dependent changes in ascorbate or GSH content were observed in SSM (Table 1). The mitochondrial GSSG levels were generally higher in IFM in comparison to SSM regardless of age (Table 1). Even in young animals, a greater proportion of total GSH (GSH + 2GSSG) in IFM were present as GSSG when compared with SSM from same animals (p < .05; Table 1). In aged rats, the difference in the GSH redox status between IFM and SSM became more pronounced; IFM exhibited a 500% increase (p < .05) in GSSG levels (Table 1) when compared to SSM from the same animals. These results suggest that the capacity to maintain thiol redox homeostasis is lower in IFM than in SSM.
The age-dependent changes in GSH redox status were only evident in the IFM. Direct comparison between IFM isolated from young and old hearts shows that the percentage of oxidized GSH almost doubled with age (p < .05; Table 1). In contrast, no age-related differences in GSH and GSSG levels were observed in SSM. The selective alteration in the GSH to GSSG redox couple suggests that IFM are either exposed to a greater level of oxidative stress and/or exhibit a more limited capacity to reduce GSSG with age. It is important to note that no significant differences in total mitochondrial GSH concentrations were observed in either IFM or SSM isolated from young and old rats. Thus, these results suggest that IFM exhibit impaired ability to maintain a reduced GSH pool rather than a loss in GSH uptake.
Age-associated changes in mitochondrial antioxidant enzyme activities
The age-related changes in mitochondrial antioxidant and thiol redox status suggest that only IFM from old rats may be in an increased pro-oxidant state. The mitochondrial redox status is largely determined by reducing equivalents, such as NAD(P)H, GSH, and ascorbate. More importantly, ascorbate and GSH are primarily maintained in a reduced state by thioredoxin (TrxR), glutaredoxin (GrxR), and GSSG reductases (GR). Therefore, we sought to determine whether alterations in any of these enzymes could account for the changes in antioxidant redox status observed. Consistent with previous results, IFM and SSM from young rats showed no apparent differences in TrxR, GrxR, or GR activities (Table 2).
Table 2.
Age-Associated Changes in Mitochondrial Thioredoxin and GSH-Related Enzymes
| Young | Old | |||
|---|---|---|---|---|
| IFM | SSM | IFM | SSM | |
| Thioredoxin reductase (nmol/min/mg protein) | 13.61 ± 2.14 | 11.98 ± 1.61 | 11.33 ± 1.16 | 24.78 ± 2.80 |
| GSSG reductase (nmol/min/mg protein) | 19.28 ± 0.86* | 19.32 ± 2.16 | 12.99 ± 0.91*† | 20.30 ± 2.00† |
| Glutaredoxin reductase (nmol/min/mg protein) | 7.02 ± 0.77* | 6.46 ± 0.49 | 3.96 ± 0.44*† | 6.78 ± 1.11† |
Values are expressed as mean ± SEM. Activities of mitochonchondrial thioredoxin, glutaredoxin, and GSSG reductase in IFM and SSM from young and old rats were measured as described in Materials and Methods. The results show a significant age-related loss in both GSH and glutaredoxin reductase activity in IFM isolated from old vs. young animals. In contrast, thioredoxin reductase activity in IFM did not show any significant loss with age. Moreover, thioredoxin reductase activity increased by 2-fold in SSM isolated from old animals. The results are expressed as mean ± SEM.
denotes significant (p < .05) difference between identical mitochondrial preparations from young and old rats, and
denotes differences between IFM and SSM within the same age group.
The selective age-related loss in ascorbate and GSH redox ratios in IFM directly implicate potential alterations in GrxR and GR. In agreement with this notion, IFM from old rats displayed a significant 33% and 44% decline in GrxR and GR activities in comparison to IFM from young rats (Table 2). Despite a marked loss in GrxR and GR activities, no apparent change in TrxR activity was observed between IFM from young and old animals (Table 2). These results indicate that the enzymes directly involved in the reduction of ascorbate and GSH are compromised in IFM with age.
The GrxR and GR activities in SSM seems to be unaffected by age (Table 2). Interestingly, we observed that SSM in old rats had a 2-fold increase in TrxR activity relative to SSM from young rats, suggesting a potential compensatory effect (Table 2). These results demonstrate that the age-related loss in the capacity to maintain a normal antioxidant and thiol redox balance declines only in IFM.
Age-associated changes in Complex IV activity
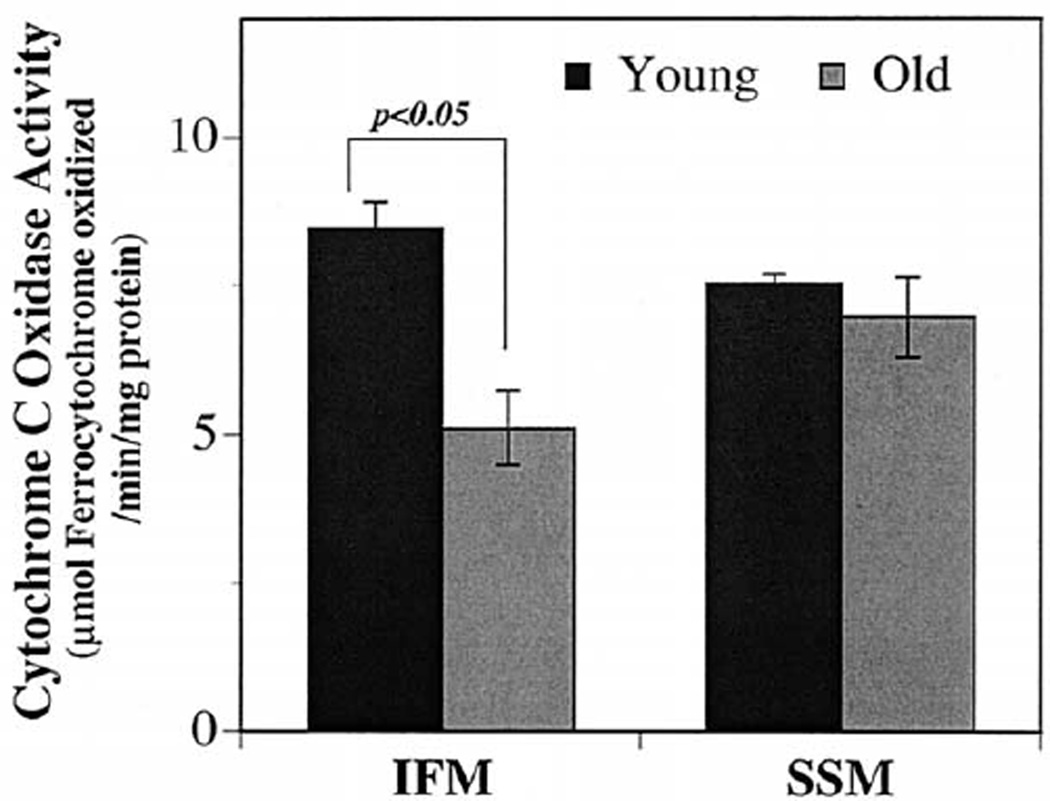
The age-related enhancement of pro-oxidant conditions seen in IFM may result in oxidative damage to mitochondrial proteins, leading to impaired mitochondrial function. To test this hypothesis, Complex IV activity in IFM and SSM from both young and old rats were determined. Results show that there were no significant differences in Complex IV activities between the two mitochondrial subpopulations isolated from young rats (Fig. 2). Comparing Complex IV activity in mitochondria from young and old rats showed that Complex IV activity declined by approximately 40% (p < .05) in IFM, whereas no age-associated differences were evident in SSM (Fig. 2). These results indicate that the increased pro-oxidant environment in IFM of the aging rat heart is associated with the decline in Complex IV activity.
Fig. 2.
Age-associated decline in Complex IV activity is observed only in IFM isolated from old rats. To assess the functional consequence to the increased oxidative damage seen in IFM isolated from old rats, the maximal Complex IV activity was determined as described in Materials and Methods. Results show a significant decline in detergent-solubilized Complex IV activity only in the IFM isolated from old (n = 4) vs. young (n = 4) rats. Results are expressed as mean ± SEM for IFM and SSM isolated from young and old rats.
Age-dependent changes in 4-HNE-modified mitochondrial electron transport chain
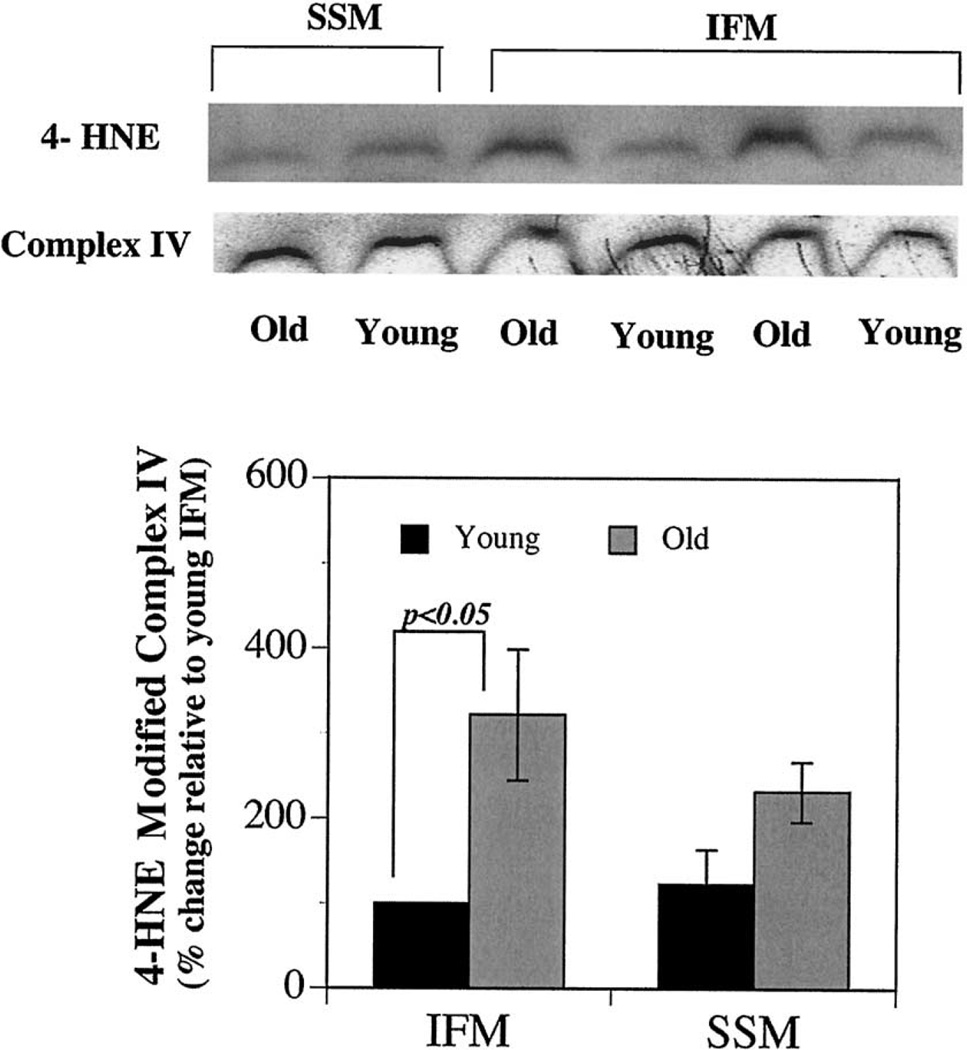
The oxidant-rich milieu combined with high levels of unsaturated lipids in mitochondria creates ideal conditions for generation of lipid-derived reactive aldehydes, such as 4-hydroxynonenal (4-HNE) and their potential to conjugate proteins, including Complex IV. This is also exemplified by in vitro experiments where incubating 4-HNE results in adduction and inactivation of Complex IV [22]. Thus, we determined whether the decrease in Complex IV activity was associated with increased oxidative damage by quantifying the extent of 4-hydroxynonenal adducted to Complex IV. Mitochondrial complexes were separated by BN-PAGE and probed for 4-HNE adducts using anti-4HNE polyclonal rabbit anti-sera. Results showed no differences in levels of 4-HNE adducted to Complex IV in the two subpopulations of mitochondria in young animals (Fig. 3). However, IFM isolated from old rats exhibited a 4-fold increase (p < .05) in 4-HNE modified Complex IV when compared with IFM from young rats (Fig. 3). No extensive age-related increases in 4-HNE modified Complex IV were observed in SSM. Thus, aging results in a selective increase in oxidative modification of Complex IV.
Fig. 3.
Age-related increase in the 4-HNE modified mitochondrial electron transport chain. Blue native gel electrophoresis (BN-PAGE) was performed to separate the mitochondrial electron transport chain complexes from young (n = 4) and old animals (n = 4). Proteins were transferred to PVDF membranes and were probed with polyclonal rabbit anti-sera as described. Results show a marked age-related increase in the steady state levels of 4-HNE modified Complex IV in IFM isolated from old compared to young. Results are expressed as % increase relative to young IFM.
DISCUSSION
Mitochondrial decay and an accompanying increase in mitochondrial oxidant generation may be an important underlying cause of cardiac decline with age [7]. Previously, we described how aging cardiac myocytes exhibit increased mitochondrial oxidant generation and lowered antioxidant capacity, which leads to a heightened accumulation of oxidative damage to DNA [8]. We have now extended these observations to show that the age-associated increase in mitochondrial oxidant production and oxidative damage is not uniform but occurs almost exclusively in IFM. The implications of these results are profound. Close physical associations of IFM with myofilaments suggest that loss of IFM may adversely affect muscle contractility by limiting ATP required for myosin ATPases. Moreover, increased oxidant generation of IFM could promote mitochondrial-derived oxidant injury to myofilaments, leading to increased fibrosis and/or fiber rearrangement. Lastly, IFM dysfunction may initiate apoptosis and myocardial loss, ultimately decreasing the contractile capacity of the heart.
It is surprising that IFM are so markedly affected with age relative to SSM. IFM and SSM cannot be distinguished either structurally or morphologically; however, they occupy unique locales within the cell [9]. It is notable that no differences in oxidative stress parameters were observed between these subpopulations obtained from young rats, suggesting that isolation procedures did not affect the analysis of oxidative stress. The protease treatment during IFM isolation may increase the possibility for selective damage to IFM, which, in turn, could confound results. However, this potential problem was addressed by Palmer and co-workers, who found that the exposure of SSM to identical isolation procedures used for IFM does not affect their function or morphology [12]. Thus, the differences in the age-related changes evident in IFM and SSM functions are likely accurate reflections of the aging process.
The exact mechanism(s) for the selective age-dependent decline in IFM are not known. Mitochondrial turnover may be different between IFM and SSM. A slower rate of degradation and/or production of IFM would enhance the probability of damage during the aging process. Aside from mitochondrial turnover, IFM may be more prone to damage due to its high respiratory activity in comparison to SSM. In young animals, the oxidative rates of IFM were found to be 1.4 to 1.7 times higher than in SSM [9,12]. The selective decline in mitochondrial antioxidant capacity, as seen by the loss of ascorbate and GSH, could further increase susceptibility for oxidative damage in IFM.
Previous studies using mixed populations of mitochondria have yielded differential results with respect to age-related increases in oxidative stress. Some studies report an increased oxidant generation with age [23], whereas others, including Drew and coworkers, using a SSM-enriched preparation, observed no change in mitochondrial oxidant production [24–26]. Apparent discrepancies in the extent of age-related changes in mitochondrial oxidant appearance and oxidative stress may reflect the differences in mitochondrial isolation procedures and resultant heterogeneity in the amount of IFM and SSM obtained in sample preparations. It is notable that when the age-related changes in the rate of cellular oxidant generation were examined in intact cardiac myocytes, a significant increase in the rates of oxidant appearance was observed in cells isolated from old compared to young rats [8]. The preferential decline in IFM may be a major contributing factor for the heightened rate of oxidant generation with age.
The selective increase in the rate of oxidant generation in IFM may be due to a number of different factors. First, the previously reported lesions in Complex III are only present in the IFM [11,27–29]. Because Complex III is one of the primary sites for electron leakage, its decline would likely result in heightened production of reactive oxygen species [11]. In addition, decreased Complex IV activity may cause blockage of electron flow and indirectly influence the oxidant generation from Complex III [10]. Second, the depletion of ascorbate and GSH, which was only observed in IFM, could result in further loss of other antioxidants such as ubiquinol and α-tocopherol [30], and increase oxidant appearance in IFM.
The decreased antioxidant levels and GSH redox status in IFM suggest an age-related alteration in mitochondrial redox environment. The maintenance of a normal mitochondrial redox state is largely dependent on thioredoxin, gluataredoxin, and GSSG reductases [14,20,31,32]. These enzymes have wide substrate specificity and are critical for maintaining antioxidants and protein thiols in a reduced state. Because de novo synthesis of ascorbate and GSH does not occur in mitochondria [33], any lesions in one or all of these enzymes may result in compromised antioxidant capacity and altered thiol/disulfide ratio. Our results indicate that the significant loss in ascorbate and GSH seen in IFM is likely due to lower glutaredoxin and GSSG reductase activities; thioredoxin reductase seems to be maintained. Although not examined directly, one potential reason for the selective loss of glutaredoxin and GSSG reductase activities may be due to differences in their susceptibility to oxidative inactivation. In support of this, a previous study by Starke and co-workers showed that both of these enzymes are more susceptible to oxidative inactivation when compared to thioredoxin reductases [34].
Results show only IFM display an age-related loss in Complex IV activity, which was associated with an increase in 4-hydroxynonenal (4-HNE) adduction to Complex IV. Due to the close physical association with cardiolipin [28,35,36], Complex IV is especially susceptible for modification by 4-HNE. Cardiolipin is an excellent substrate for the formation of 4-HNE because it is largely composed of linoleic acid (C18:2), which is easily oxidized. Previous studies with rodents show a significant age-related loss in mitochondrial cardiolipin levels, which may occur due to oxidative damage and/or removal [37,38]. The selective increase in 4-HNE modified Complex IV in IFM may also be due to the significant age-related decline in GSH levels. The conjugation of free reactive 4-HNE with GSH represents one of the major routes of elimination; hence, decreased GSH content may lead to the accumulation of 4-HNE [39]. In contrast to the current work, a study by Moghaddas and co-workers reported no apparent age-related changes in cardiolipin content, nor found any increase in 4-HNE modified electron transport chain complexes [36]. The reasons for these discrepancies are not known.
It is not clear whether the increased 4-HNE modification of Complex IV is a direct cause for the decrease in its activity. In vitro, exposures to 4-HNE deactivates a number of mitochondrial proteins, such as glucose-6-phosphate, pyruvate, and α-ketoglutarate dehydrogenases and the adenine nucleotide exchanger [5,40–42]. Similarly, multiple subunits on Complex IV (subunits II, IV, Vb, VIIa, VIIc, and VIII) are readily modified by 4-HNE and lead to its inactivation [22]. Further studies will be necessary to determine the functional implications of our current findings.
IFM dysfunction may be closely linked to the age-related alterations in myocardial relaxation rate and calcium regulation [43,44]. Disruptions in IFM may limit ATP, which is critical for the dissociation of actin from myosin, affecting both systolic contraction and diastolic relaxation [45]. Alternatively, IFM-associated loss in ATP synthesis may compromise cytosolic calcium clearance. This, in turn, could depress force-generating capacity and increase cardiac stiffness observed with age [46].
Our results show that aging results in the selective depletion of antioxidant capacity, increased oxidative modification, and a marked decline in Complex IV activity in IFM. Further studies are needed to assess whether age-dependent loss of myocytes is related specifically to increased damage seen in IFM.
Acknowledgments
We thank Dr. Elias Arnér for his expert advice on the thioredoxin reductase assay. This work was supported by a grant from the National Institute of Aging RIAG17141A and by the Environmental Health Sciences, Oregon State University.
REFERENCES
- 1.Sachs HG, Colgan JA, Lazarus ML. Ultrastructure of the aging myocardium: a morphometric approach. Am. J. Anat. 1977;150:63–71. doi: 10.1002/aja.1001500105. [DOI] [PubMed] [Google Scholar]
- 2.Muscari C, Giaccari A, Giordano E, Clo C, Guarnieri C, Caldarera CM. Role of reactive oxygen species in cardiovascular aging. Mol. Cell. Biochem. 1996;160–161:159–166. doi: 10.1007/BF00240046. [DOI] [PubMed] [Google Scholar]
- 3.Lesnefsky EJ, Moghaddas S, Tandler B, Kerner J, Hoppel CL. Mitochondrial dysfunction in cardiac disease: ischemia-reperfusion, aging, and heart failure. J. Mol. Cell. Cardiol. 2001;33:1065–1089. doi: 10.1006/jmcc.2001.1378. [DOI] [PubMed] [Google Scholar]
- 4.Lesnefsky EJ, Gudz TI, Migita CT, Ikeda-Saito M, Hassan MO, Turkaly PJ, Hoppel CL. Ischemic injury to mitochondrial electron transport in the aging heart: damage to the iron-sulfur protein subunit of electron transport complex III. Arch. Biochem. Biophys. 2001;385:117–128. doi: 10.1006/abbi.2000.2066. [DOI] [PubMed] [Google Scholar]
- 5.Lucas DT, Szweda LI. Declines in mitochondrial respiration during cardiac reperfusion: age-dependent inactivation of alpha-ketoglutarate dehydrogenase. Proc. Natl. Acad. Sci. USA. 1999;96:6689–6693. doi: 10.1073/pnas.96.12.6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sohal RS, Ku HH, Agarwal S, Forster MJ, Lal H. Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech. Ageing Dev. 1994;74:121–133. doi: 10.1016/0047-6374(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 7.Hagen TM, Moreau R, Suh JH, Visioli F. Mitochondrial decay in the aging rat heart: evidence for improvement by dietary supplementation with acetyl-L-carnitine and/or lipoic acid. Ann. N. Y. Acad. Sci. 2002;959:491–507. doi: 10.1111/j.1749-6632.2002.tb02119.x. [DOI] [PubMed] [Google Scholar]
- 8.Suh JH, Shigeno ET, Morrow JD, Cox B, Rocha AE, Frei B, Hagen TM. Oxidative stress in the aging rat heart is reversed by dietary supplementation with (R)-(alpha)-lipoic acid. FASEB J. 2001;15:700–706. doi: 10.1096/fj.00-0176com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palmer JW, Tandler B, Hoppel CL. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J. Biol. Chem. 1977;252:8731–8739. [PubMed] [Google Scholar]
- 10.Fannin SW, Lesnefsky EJ, Slabe TJ, Hassan MO, Hoppel CL. Aging selectively decreases oxidative capacity in rat heart interfibrillar mitochondria. Arch. Biochem. Biophys. 1999;372:399–407. doi: 10.1006/abbi.1999.1508. [DOI] [PubMed] [Google Scholar]
- 11.Lesnefsky EJ, Gudz TI, Moghaddas S, Migita CT, Ikeda-Saito M, Turkaly PJ, Hoppel CL. Aging decreases electron transport complex III activity in heart interfibrillar mitochondria by alteration of the cytochrome c binding site. J. Mol. Cell. Cardiol. 2001;33:37–47. doi: 10.1006/jmcc.2000.1273. [DOI] [PubMed] [Google Scholar]
- 12.Palmer JW, Tandler B, Hoppel CL. Biochemical differences between subsarcolemmal and interfibrillar mitochondria from rat cardiac muscle: effects of procedural manipulations. Arch. Biochem. Biophys. 1985;236:691–702. doi: 10.1016/0003-9861(85)90675-7. [DOI] [PubMed] [Google Scholar]
- 13.Miranda-Vizuete A, Damdimopoulos AE, Spyrou G. The mitochondrial thioredoxin system. Antioxid. Redox Signal. 2000;2:801–810. doi: 10.1089/ars.2000.2.4-801. [DOI] [PubMed] [Google Scholar]
- 14.Pedrajas JR, Porras P, Martinez-Galisteo E, Padilla CA, Miranda-Vizuete A, Barcena JA. Two isoforms of Saccharomyces cerevisiae glutaredoxin 2 are expressed in vivo and localize to different subcellular compartments. Biochem. J. 2002;364:617–623. doi: 10.1042/BJ20020570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Degli Esposti M. Measuring mitochondrial reactive oxygen species. Methods. 2002;26:335–340. doi: 10.1016/S1046-2023(02)00039-7. [DOI] [PubMed] [Google Scholar]
- 16.Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc. Natl. Acad. Sci. USA. 1989;86:6377–6381. doi: 10.1073/pnas.86.16.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones DP, Carlson JL, Samiec PS, Sternberg P, Jr, Mody VC, Jr, Reed RL, Brown LA. Glutathione measurement in human plasma. Evaluation of sample collection, storage and derivatization conditions for analysis of dansyl derivatives by HPLC. Clin. Chim. Acta. 1998;275:175–184. doi: 10.1016/s0009-8981(98)00089-8. [DOI] [PubMed] [Google Scholar]
- 18.Hill KE, McCollum GW, Burk RF. Determination of thioredoxin reductase activity in rat liver supernatant. Anal. Biochem. 1997;253:123–125. doi: 10.1006/abio.1997.2373. [DOI] [PubMed] [Google Scholar]
- 19.Smith IK, Vierheller TL, Thorne CA. Assay of glutathione reductase in crude tissue homogenates using 5,5′-dithiobis(2-nitrobenzoic acid) Anal. Biochem. 1988;175:408–413. doi: 10.1016/0003-2697(88)90564-7. [DOI] [PubMed] [Google Scholar]
- 20.Gladyshev VN, Liu A, Novoselov SV, Krysan K, Sun QA, Kryukov VM, Kryukov GV, Lou MF. Identification and characterization of a new mammalian glutaredoxin (thioltransferase), Grx2. J. Biol. Chem. 2001;276:30374–30380. doi: 10.1074/jbc.M100020200. [DOI] [PubMed] [Google Scholar]
- 21.Schagger H, von Jagow G. Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form. Anal. Biochem. 1991;199:223–231. doi: 10.1016/0003-2697(91)90094-a. [DOI] [PubMed] [Google Scholar]
- 22.Musatov A, Carroll CA, Liu YC, Henderson GI, Weintraub ST, Robinson NC. Identification of bovine heart cytochrome c oxidase subunits modified by the lipid peroxidation product 4-hydroxy-2-nonenal. Biochemistry. 2002;41:8212–8220. doi: 10.1021/bi025896u. [DOI] [PubMed] [Google Scholar]
- 23.Sawada M, Carlson JC. Changes in superoxide radical and lipid peroxide formation in the brain, heart and liver during the lifetime of the rat. Mech. Ageing Dev. 1987;41:125–137. doi: 10.1016/0047-6374(87)90057-1. [DOI] [PubMed] [Google Scholar]
- 24.Muscari C, Frascaro M, Guarnieri C, Caldarera CM. Mitochondrial function and superoxide generation from submitochondrial particles of aged rat hearts. Biochim. Biophys. Acta. 1990;1015:200–204. doi: 10.1016/0005-2728(90)90021-u. [DOI] [PubMed] [Google Scholar]
- 25.Hansford RG, Hogue BA, Mildaziene V. Dependence of H2O2 formation by rat heart mitochondria on substrate availability and donor age. J. Bioenerg. Biomembr. 1997;29:89–95. doi: 10.1023/a:1022420007908. [DOI] [PubMed] [Google Scholar]
- 26.Drew B, Phaneuf S, Dirks A, Selman C, Gredilla R, Lezza A, Barja G, Leeuwenburgh C. Effects of aging and caloric restriction on mitochondrial energy production in gastrocnemius muscle and heart. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;284:R474–R480. doi: 10.1152/ajpregu.00455.2002. [DOI] [PubMed] [Google Scholar]
- 27.Paradies G, Ruggiero FM, Gadaleta MN, Quagliariello E. The effect of aging and acetyl-L-carnitine on the activity of the phosphate carrier and on the phospholipid composition in rat heart mitochondria. Biochim. Biophys. Acta. 1992;1103:324–326. doi: 10.1016/0005-2736(92)90103-s. [DOI] [PubMed] [Google Scholar]
- 28.Paradies G, Ruggiero FM, Dinoi P, Petrosillo G, Quagliariello E. Decreased cytochrome oxidase activity and changes in phospholipids in heart mitochondria from hypothyroid rats. Arch. Biochem. Biophys. 1993;307:91–95. doi: 10.1006/abbi.1993.1565. [DOI] [PubMed] [Google Scholar]
- 29.Paradies G, Ruggiero FM, Petrosillo G, Quagliariello E. Peroxidative damage to cardiac mitochondria: cytochrome oxidase and cardiolipin alterations. FEBS Lett. 1998;424:155–158. doi: 10.1016/s0014-5793(98)00161-6. [DOI] [PubMed] [Google Scholar]
- 30.Lass A, Sohal RS. Electron transport-linked ubiquinone-dependent recycling of alpha-tocopherol inhibits autooxidation of mitochondrial membranes. Arch. Biochem. Biophys. 1998;352:229–236. doi: 10.1006/abbi.1997.0606. [DOI] [PubMed] [Google Scholar]
- 31.Lee SR, Kim JR, Kwon KS, Yoon HW, Levine RL, Ginsburg A, Rhee SG. Molecular cloning and characterization of a mitochondrial selenocysteine-containing thioredoxin reductase from rat liver. J. Biol. Chem. 1999;274:4722–4734. doi: 10.1074/jbc.274.8.4722. [DOI] [PubMed] [Google Scholar]
- 32.Pedrajas JR, Kosmidou E, Miranda-Vizuete A, Gustafsson JA, Wright AP, Spyrou G. Identification and functional characterization of a novel mitochondrial thioredoxin system in Saccharomyces cerevisiae. J. Biol. Chem. 1999;274:6366–6373. doi: 10.1074/jbc.274.10.6366. [DOI] [PubMed] [Google Scholar]
- 33.Griffith OW, Meister A. Origin and turnover of mitochondrial glutathione. Proc. Natl. Acad. Sci. USA. 1985;82:4668–4672. doi: 10.1073/pnas.82.14.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Starke DW, Chen Y, Bapna CP, Lesnefsky EJ, Mieyal JJ. Sensitivity of protein sulfhydryl repair enzymes to oxidative stress. Free Radic. Biol. Med. 1997;23:373–384. doi: 10.1016/s0891-5849(97)00009-9. [DOI] [PubMed] [Google Scholar]
- 35.Hoppel CL, Moghaddas S, Lesnefsky EJ. Interfibrillar cardiac mitochondrial comples III defects in the aging rat heart. Biogerontology. 2002;3:41–44. doi: 10.1023/a:1015251212039. [DOI] [PubMed] [Google Scholar]
- 36.Moghaddas S, Stoll MS, Minkler PE, Salomon RG, Hoppel CL, Lesnefsky EJ. Preservation of cardiolipin content during aging in rat heart interfibrillar mitochondria. J. Gerontol. A Biol. Sci. Med. Sci. 2002;57:B22–B28. doi: 10.1093/gerona/57.1.b22. [DOI] [PubMed] [Google Scholar]
- 37.McMillin JB, Taffet GE, Taegtmeyer H, Hudson EK, Tate CA. Mitochondrial metabolism and substrate competition in the aging Fischer rat heart. Cardiovasc. Res. 1993;27:2222–2228. doi: 10.1093/cvr/27.12.2222. [DOI] [PubMed] [Google Scholar]
- 38.Pepe S, Tsuchiya N, Lakatta EG, Hansford RG. PUFA and aging modulate cardiac mitochondrial membrane lipid composition and Ca2+ activation of PDH. Am. J. Physiol. 1999;276:H149–H158. doi: 10.1152/ajpheart.1999.276.1.H149. [DOI] [PubMed] [Google Scholar]
- 39.Armstrong JS, Jones DP. Glutathione depletion enforces the mitochondrial permeability transition and causes cell death in Bcl-2 overexpressing HL60 cells. FASEB J. 2002;16:1263–1265. doi: 10.1096/fj.02-0097fje. [DOI] [PubMed] [Google Scholar]
- 40.Cohn JA, Tsai L, Friguet B, Szweda LI. Chemical characterization of a protein-4-hydroxy-2-nonenal cross-link: immunochemical detection in mitochondria exposed to oxidative stress. Arch. Biochem. Biophys. 1996;328:158–164. doi: 10.1006/abbi.1996.0156. [DOI] [PubMed] [Google Scholar]
- 41.Korotchkina LG, Yang H, Tirosh O, Packer L, Patel MS. Protection by thiols of the mitochondrial complexes from 4-hydroxy-2-nonenal. Free Radic. Biol. Med. 2001;30:992–999. doi: 10.1016/s0891-5849(01)00491-9. [DOI] [PubMed] [Google Scholar]
- 42.Yan LJ, Sohal RS. Mitochondrial adenine nucleotide translocase is modified oxidatively during aging. Proc. Natl. Acad. Sci. USA. 1998;95:12896–12901. doi: 10.1073/pnas.95.22.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frolkis VV, Kobzar AL, Pugach BV. Na,K-ATPase and Ca-ATPase activities of the myocardial sarcolemma in aging rats after aorta coarctation: role of invertors. Gerontology. 1999;45:184–186. doi: 10.1159/000022084. [DOI] [PubMed] [Google Scholar]
- 44.Siri FM, Krueger J, Nordin C, Ming Z, Aronson RS. Depressed intracellular calcium transients and contraction in myocytes from hypertrophied and failing guinea pig hearts. Am. J. Physiol. 1991;261:H514–H530. doi: 10.1152/ajpheart.1991.261.2.H514. [DOI] [PubMed] [Google Scholar]
- 45.Katz AM. Energetics and the failing heart. Hosp. Pract. (Off. Ed) 1991;26:78–90. doi: 10.1080/21548331.1991.11705280. [DOI] [PubMed] [Google Scholar]
- 46.Houser SR, Margulies KB. Is depressed myocyte contractility centrally involved in heart failure? Circ. Res. 2003;92:350–358. doi: 10.1161/01.RES.0000060027.40275.A6. [DOI] [PubMed] [Google Scholar]