Abstract
Oxidative stress has been implicated as a causal factor in the aging process of the heart and other tissues. To determine the extent of age-related myocardial oxidative stress, oxidant production, antioxidant status, and oxidative DNA damage were measured in hearts of young (2 months) and old (28 months) male Fischer 344 rats. Cardiac myocytes isolated from old rats showed a nearly threefold increase in the rate of oxidant production compared to young rats, as measured by the rates of 2,7-dichlorofluorescin diacetate oxidation. Determination of myocardial antioxidant status revealed a significant twofold decline in the levels of ascorbic acid (P = 0.03), but not α-tocopherol. A significant age-related increase (P = 0.05) in steady-state levels of oxidative DNA damage was observed, as monitored by 8-oxo-2′-deoxyguanosine levels. To investigate whether dietary supplementation with (R)-α-lipoic acid (LA) was effective at reducing oxidative stress, young and old rats were fed an AIN-93M diet with or without 0.2% (w/w) LA for 2 wk before death. Cardiac myocytes from old, LA-supplemented rats exhibited a markedly lower rate of oxidant production that was no longer significantly different from that in cells from unsupplemented, young rats. Lipoic acid supplementation also restored myocardial ascorbic acid levels and reduced oxidative DNA damage. Our data indicate that the aging rat heart is under increased mitochondrial-induced oxidative stress, which is significantly attenuated by lipoic acid supplementation.
Keywords: aging, cardiac myocytes, oxidative stress, lipoic acid
Aging is associated with an increased incidence of cardiac arrhythmias and diastolic and systolic dysfunction, which may ultimately lead to heart failure. Heart failure alone is the leading cause of hospitalization, permanent disability, and death in persons over the age of 65 (1) in the U.S. Because of the enormous suffering and health care burden that cardiac dysfunction causes, much effort has gone into understanding the mechanisms leading to age-related myocardial decline. It has been difficult, however, to separate the effects of aging per se from those of age-associated diseases (atherosclerosis, diabetes, hypertension) on cardiac performance. Thus, the relative contribution of ‘aging’ to myocardial dysfunction is not well defined.
Even though the mechanisms leading to alterations in cardiac performance are not well understood, there is reason to suspect increased oxidative stress to significantly contribute to myocardial dysfunction with age. It is generally agreed that isolated mitochondrial preparations from old compared to young hearts produce more reactive oxygen species (ROS), reflecting an age-related decline in coupling of electron transport to ATP production. These changes in mitochondria may lead to the reported increase in superoxide and hydrogen peroxide production in mitochondria prepared from old vs. young rats (2–4). Thus, it is conceivable that dietary interventions with antioxidants, which could augment endogenous antioxidant compounds to either prevent the formation or quench the higher levels of oxidants, could provide an effective means to improve or maintain myocardial function with age.
(R)-α-lipoic acid (LA) is a thiol compound found naturally in plants and animals (4). Lipoamide dehydrogenases, found only in mitochondria, reduce free LA to dihydrolipoic acid, which is a potent antioxidant. Thus, LA supplementation may increase cellular and mitochondrial antioxidant status, thereby effectively attenuating any putative increase in oxidative stress with age (5).
Aside from acting as a potent antioxidant in its own right, LA increases or maintains levels of other low molecular weight antioxidants such as ubiquinone, glutathione (GSH), and ascorbic acid. LA may exert these effects by ‘sparing’ or reducing, ubiquinone (6), GSH, and vitamin C (7) or, in the case of GSH, by increasing the cellular uptake of cysteine (8), which is the rate-limiting substrate for GSH biosynthesis.
The purpose of the present study was to 1) examine the age-related changes to myocardial oxidant production, low molecular weight antioxidant status, and indices of oxidative damage, and 2) determine whether dietary supplementation of lipoic acid could improve those indices of oxidative stress. Overall, our results show that the aging rat myocardium exhibits increased oxidant production, significantly lower ascorbic acid levels, and a marked increase in steady-state levels of oxidative DNA damage. LA supplementation significantly reverses the age-related decline in myocardial ascorbic acid content, and lowers the rate of oxidant production and the steady-state levels of oxidative DNA damage. Our results thus indicate that dietary supplementation with lipoic acid may be an effective means to lower increased myocardial oxidative stress with age.
MATERIALS AND METHODS
The following chemicals were used: EGTA (ethylene glycolbis (α-aminoethyl ether) N,N,N′,N′-tetraacetic acid), heparin (sodium salt), rhodamine 123, dithiothreitol, L-ascorbic acid, meta-phosphoric acid, and 8-oxo-dG standard (Sigma, St. Louis, Mo.); 2′,7′-dichlorofluoroscein diacetate (DCFH) (Molecular Probes, Eugene, Oreg.); collagenase, type 2 (Worthington, Lakewood, N.J.); nuclease P1, alkaline phosphatase, sodium iodide, proteinase K, and RNAse A (Roche Pharmaceuticals, Indianapolis, Ind.). All other reagents were of reagent grade or better.
Animals
Rats (Fischer 344, virgin male, outbred albino), both young (2–5 months) and old (24–28 months; National Institute of Aging animal colonies), were acclimatized in the Oregon State University animal facilities for at least 1 wk prior to experimentation. Animals were placed on an AIN-93M standard diet; some rats were given an AIN-93M diet supplemented with 0.2% (w/w) (R)-α-lipoic acid (Asta Medica, Germany) for 2 wk prior to death. Water was given ad libitum throughout.
In experiments examining tissue antioxidant and oxidative DNA damage levels, rats were anesthetized with diethyl ether and a midline incision was made in the abdomen. Animals were killed by cutting through the diaphragm, followed by severing of the superior vena cava. Hearts were quickly removed and cut into small pieces; the pieces were placed individually in cryotubes and snap frozen in liquid nitrogen.
Cardiac myocyte isolation
For analysis of cellular oxygen consumption, average mitochondrial membrane potential, and oxidant production, the heart was dispersed into single cells by perfusion with 1% collagenase (9). Typically, the isolation procedure yielded 5.0–7.5 × 106 calcium-tolerant ventricular cells per heart, which exhibited typical rod-shaped appearance and morphological striations. Cell viability, as measured by trypan blue exclusion, was typically between 60 and 80%. These values agree with those cited in the literature (10).
It was necessary to modify the above procedure in order to isolate cells from old rats. Old rats had to be injected with twice the amount of heparin to remove all blood from the heart. The amount of collagenase perfused through the heart was also increased to 1.2% (w/v) and the perfusion flow rate was raised from 2.8 to 7.0 ml/min. These changes were necessary because of the increased fibrotic nature of the heart in old vs. young rats. Even with these modifications, cell yield and viability were more variable than that for young rats: 3.0–7.0 × 106 cells isolated per heart from old rats, with viability ranging from 45 to 80%. Because of the possibility that low cell viability and yield would result in data that do not accurately represent the in vivo situation, no isolated cell preparations were used that had an initial viability below 70% and a yield of <5.0 × 106 cells per heart.
DCFH measurement
Formation of oxidants in isolated cardiac myocytes was determined by assaying the fluorescence of 2′,7′-dichlorofluorescein (DCF), the oxidation product of DCFH. Duplicate samples were routinely monitored. Fluorescence was measured with a Hitachi F-2500 fluorescent spectrophotometer (Hitachi Instruments, Tokyo, Japan) using standard fluorescein filters and Hitachi-supplied software. To determine whether age-related changes to cellular oxygen consumption affected apparent changes in oxidant production, we measured cellular oxygen consumption and expressed data as the fluorescence change/mol O2 consumed/106 cells.
Ascorbic acid analysis
Ascorbate was measured essentially as in ref 11. Briefly, tissue homogenates were mixed with an equal volume of meta-phosphoric acid (10% w/v) containing 1 mmol/l of the metal chelator diethylenetriaminepentaacetic acid (DTPA) and centrifuged to remove the precipitated proteins. Ascorbic acid was separated by high-performance liquid chromatography (HPLC) (11) and detected at an applied potential of +0.6 V by a LC 4B (Bioanalytical Systems, Inc., West Lafayette, Ind.) electrochemical detector.
Vitamin E analysis
Myocardial vitamin E levels were determined as in ref 12. Briefly, myocardial tissue (50 mg) was homogenized in 10 mM phosphate-buffered saline containing 1 mmol DTPA. 50 μl of the homogenate was extracted in hexane:methanol (5:1), and the hexane phase was collected and dried under a constant stream of nitrogen. The sample was reconstituted with methanol and analyzed by HPLC with electrochemical detection at an applied potential of +0.5 V by a LC 4B electrochemical detector (12).
Oxidative DNA damage
Analysis of oxidative damage to nuclear DNA was measured by the method of Helbock et al. (13). DNA was extracted using the chaotropic sodium iodide method by a DNA Extractor WB kit (Wako BioProducts, Richmond, Va.). DNA hydrolysates were analyzed by HPLC with electrochemical coulometric detection, as described (13).
Oxidative lipid damage
Analysis of oxidative damage to lipids was quantified by measuring F2− isoprostanes in lipids using a highly precise and accurate assay employing gas chromatography/mass spectrometry (14).
Statistical analysis
Statistical significance was determined by the unpaired Student’s t test, using Stat-View statistical software. Results are expressed as the mean ± SE. A P value of less than 0.05 was considered significant.
RESULTS
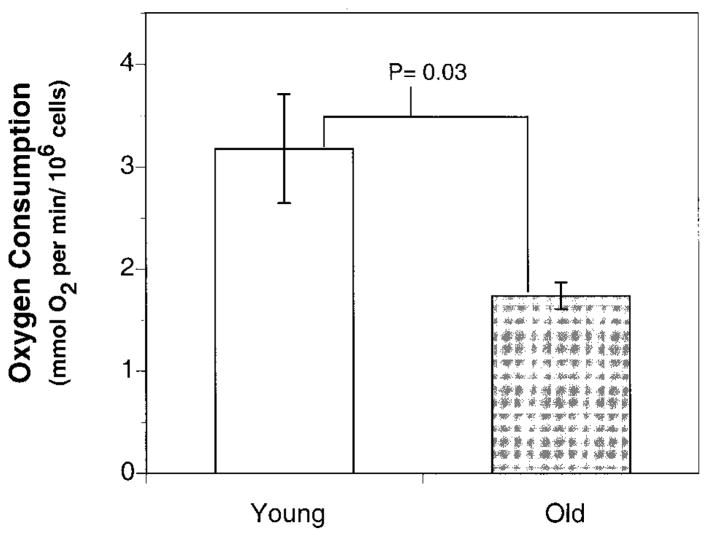
To determine the effects of aging on the general metabolic rate, O2 consumption in freshly isolated cardiac myocytes from young and old rats was determined. O2 consumption in cells from young rats was 3.18 ± 0.53 mmol O2/min per 106 cells. In contrast, O2 consumption in cardiac myocytes from old rats was 1.74 ± 0.13 mmol O2/min per 106 cells, a 45% decline compared to young rats (Fig. 1). These results suggest an age-associated decrease in the basal metabolic rate of the cardiac myocytes.
Figure 1.
O2 consumption in isolated cardiac myocyte declines with age. O2 consumption in isolated cardiac myocytes from young and old rats was monitored using a Clark type oxygen electrode (Yellow Springs Instruments, Yellow Springs, Ohio). Cardiac myocytes (1×106) were added to 3 ml of Krebs-Henseleit buffer supplemented with 20 mM glucose, pH 7. 4, that had been pre-equilibrated to 37°C, and oxygen consumption was monitored for at least 15 min (25). Results show that O2 consumption, an indicator of cellular metabolic rate, declines sharply with age. Results are expressed as mean ± SE for cells from young rats (n=5) and the mean ± SE (n=5) for cells from old rats.
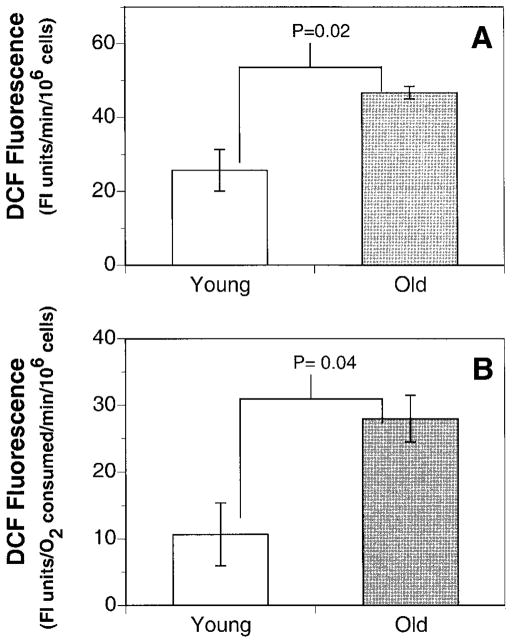
To examine how the decline in mitochondrial function affected cellular oxidant production, the rates of DCFH oxidation in freshly isolated cardiac myocytes from old and young rats were determined. In cardiac myocytes isolated from old rats, total cellular oxidant production was 125% higher than in myocytes from young rats (Fig. 2). This increase in oxidant production was even more pronounced when normalized to the rates of oxygen consumption. Thus, the rate of DCFH fluorescence per mmol O2 consumed was threefold higher in cardiac myocytes from old rats compared to young rats. These results strongly suggest that, with age, cardiac myocytes are under increased oxidative stress, possibly due to a decline in mitochondrial function.
Figure 2.
Cardiac myocytes produce significantly more oxidants with age. A) Cells from old rats (24 months) produce ~45% more oxidants, as measured by DCFH fluorescence, than cells from young (3 months) rats. B) The rate of oxidant production is even more marked when oxidant production is normalized to the cellular rate of O2 consumption. Results are expressed as the mean ± SE of 3 experiments.
To investigate the effects of this age-associated increase in oxidative stress on cellular antioxidant capacities, ascorbate and α-tocopherol levels in the freshly isolated hearts from old and young rats were measured. Consistent with the data on oxidant production (Fig. 2), we observed a 57% decrease in the tissue ascorbate concentration of old rats (1.40±0.87 nmol/mg protein) compared to young rats (3.08±1.10 pmol/mg tissue (P=0.02; Table 1). In contrast, no age-related decrease in vitamin E levels was observed (0.97±0.29 and 1.16±0.62 nmol/mg protein in hearts from young and old). This result suggests that there is a differential consumption of water-soluble vs. lipid-soluble antioxidants in vivo due to aging.
TABLE 1.
Age-associated changes in antioxidant levels and oxidative damage in the hearta
| Young | Old | |
|---|---|---|
| Ascorbate (nmol/mg protein ± SE) | 3.08 ± 1.10 | 1.40 ± 0.87* |
| α-Tocopherol (nmol/mg protein ± SE) | 0.97 ± 0.29 | 1.16 ± 0.62 |
| C + Sc8-oxo-dG (8-oxo-dG/1 × 105 dG bases) | 2.01 ± 0.25 | 4.00 ± 0.40 # |
| C + ScF2-isoprostanes (ng/g wet tissue) | 3.47 ± 0.02 | 3.74 ± 0.41 |
Ascorbate and α-tocopherol levels in the freshly isolated heart from young and old rats were measured using electrochemical detection following separation by HPLC. The results show a significant decline in the ascorbate levels. In contrast, lipid-soluble antioxidant α-tocopherol did not change with age. As a measure of oxidative damage to DNA and lipids, 8-oxo-dG and F2-isoprostanes were measured using coulometric electrochemical detection and GC-MS. The results show a twofold increase in steady-state levels of 8-oxo-dG. However, there was no change in steady-state levels of ± SE; F2-isoprotanes with age. The results are expressed as mean statistical analysis was done by using the Student’s t test.
P = 0.02;
P = 0.002.
To further explore this notion of different levels of oxidative stress with aging in the aqueous and lipid compartments, we examined oxidative damage to DNA, as assessed by the levels of 8-oxo-dG, and lipids, as assessed by levels of F2-isoprostanes. Consistent with the pattern of antioxidant depletion with age, we found a twofold increase in oxidative DNA damage in the heart of old rats (4.00±4.80-oxo-dG/105 dG bases) compared to young rats (2.01±0.25 8-oxo-dG/105 dG bases (P= 0.002; Fig. 4). In contrast, the levels of F2-isoprostanes in hearts from old rats (3.74±0.41 ng/g wet tissue) were not significantly different from those in young rats (3.47±0.02 ng/g wet tissue). These results suggest that in the heart, macromolecules in an aqueous environment may be more susceptible to damage due to age-associated increase in oxidative stress than membrane lipids.
Figure 4.
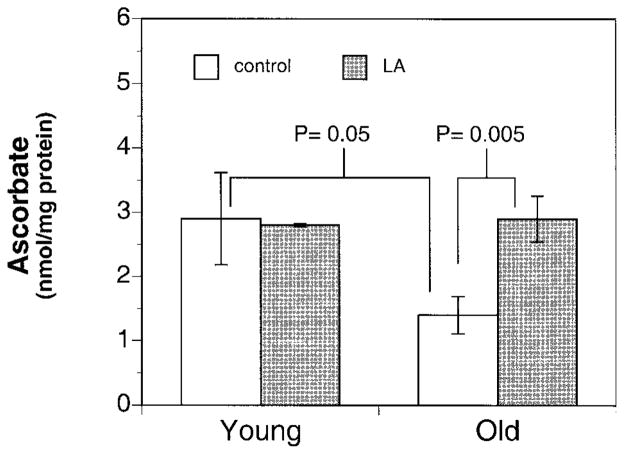
Dietary supplementation of LA leads to restoration of ascorbate back to the levels found in the young. Ascorbate levels in freshly isolated hearts from old and young animals were determined after a 2-wk supplementation of the AIN-93M diet with or without 0.2% (w/w) (R)-α–lipoic acid. Ascorbate levels in the old supplemented animals (n=12) were significantly higher (P=0.03) than old unsupplemented animals (n=10), to a level no longer significantly different from young unsupplemented animals. Results are expressed as mean ± SE for heart isolated from young (3), young LA (n=3), old (n=10), and old LA (n=12). One-way analysis of variance test was used for statistical analysis with the level of significance defined as P < 0.05.
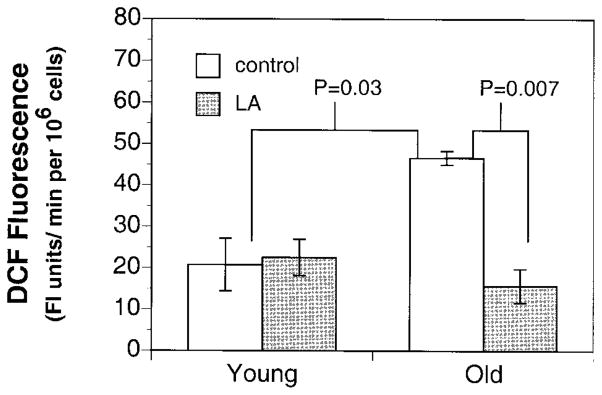
In light of the above evidence of increased oxidative stress in the aging heart, we examined whether supplementation with (R)-α-lipoic acid can reverse the decline in mitochondrial function and reduce oxidative stress. To this end, Fischer 344 rats of varying ages (young: 2–4 months; old: 24–28 months) were fed an AIN-93M diet with or without 0.2% (w/w) (R)-α-lipoic acid for 2 wk. Results show a significant decrease in the cellular oxidant production in the old LA-supplemented vs. old unsupplemented animals (Fig. 3). In agreement with these data, we also found a significant twofold improvement in ascorbate levels in the hearts from old LA-treated vs. untreated animals (2.90±0.36 vs. 1.4±2.9 nmol/mg protein, respectively; P=0.03). In fact, cardiac ascorbate levels in LA-fed old rats were not different from those in young rats (2.90±0.72 nmol/mg protein; Fig. 4). However, a similar increase in tissue ascorbate levels was not achieved by LA feeding to young rats. Furthermore, there was no effect of LA treatment on the levels of α-tocopherol.
Figure 3.
A 2 wk regimen of LA markedly lowers the age-related increase in cellular oxidant production. Myocardial oxidant production in both young (2–4 months; n=9) and old (24–28 months; n=8) rats treated with or without 0.2% (w/w) lipoic acid was measured. Results show that lipoic acid treatment significantly reversed the age-related increase in oxidant production back to levels not different from the young. Results are expressed as mean ± SE for heart isolated from young (n=4), young LA (n=5), old (n=4), and old LA (n=4). One-way ANOVA was used for statistical analysis with the level of significance defined as P < 0.05.
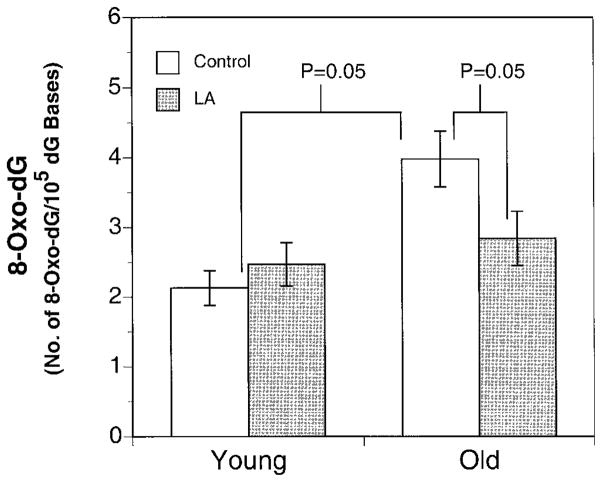
To investigate whether the decreased ROS production and increased ascorbate levels in LA-treated old rats translated into a decrease in oxidative damage to DNA and lipids, 8-oxo-dG and F2-isoprostane levels were measured. Indeed, we found a 30% decrease in cardiac 8-oxo-dG levels in the supplemented old rats (2.84±0.39 8-oxo-dG/105 dG bases) compared to their age-matched controls (4.00±0.40 8-oxo-dG/105 dG bases (P=0.05; Fig. 5). In the young animals, LA supplementation did not alter the steady-state levels of 8-oxo-dG in the heart. As expected, treatment with LA had no effect on the steady-state levels of F2-isoprostanes in young or old rats. Thus, by decreasing the rate of oxidant production and increasing the level of antioxidant protection in the aqueous phase, LA treatment caused a marked reversal in steady-state levels of 8-oxo-dG in old rats to levels found in the young rats.
Figure 5.
LA supplementation leads to marked reduction in the steady-state levels of 8-oxo-dG. The steady-state levels of 8-oxo-dG in freshly isolated hearts from old and young rats after 2 wk supplementation with an AIN-93M diet with or without 0.2% (w/w) (R)-α-lipoic acid by using coulometric detection after separation with HPLC. The results show that LA supplementation significantly lowered the age-related accumulation of 8-oxo-dG in hearts from old rats. Results are expressed as mean ± SE for heart isolated from young (n=8), young LA (n=3), old (n=10), and old LA (n=9). One-way ANOVA was used for statistical analysis with the level of significance defined as P < 0.05.
DISCUSSION
Previous studies examining the age-related changes in mitochondria isolated from old animals have reported that mitochondrial oxidant production increases with age (3). However, a recent study by Hansford and co-workers suggested that this increase may be attributed to an artifact stemming from assay conditions due to excessively high levels of substrate (succinate) used (15). In addition, Hagen and co-workers have found that selective loss of defective mitochondria during the isolation process can further complicate the interpretation of results from studies using isolated mitochondria (16). However, we have avoided these potential problems here by examining the age-associated changes in metabolic function and antioxidant status in intact cardiac myocytes isolated from young and old animals.
In the present study, we observed an apparent increase in DCF fluorescence in myocytes from old when compared to young animals. This increase in oxidant levels may be attributed to at least three possibilities: 1) increased oxidant production mainly from decaying mitochondria, 2) decline in cellular antioxidant status with no increase in oxidant flux, and 3) both an increase in oxidant production and a decline in anti-oxidant status. The present work cannot distinguish between these possibilities. However, it is notable that we observed a significant decline in myocardial ascorbate levels and oxygen consumption with age. Lower oxygen consumption would seemingly be a compensatory mechanism to also lower mitochondrial oxidant flux. Along with loss of ascorbate, our results would suggest that the decline in antioxidant status may be a significant contributing factor in the apparent age-related increase in myocardial oxidative stress.
In contrast to the age-related decrease in the levels of ascorbate and an increase in the levels of 8-oxo-dG, the levels of α-tocopherol and F2-isoprostanes did not change with age. Because these moieties are located primarily in lipophilic environments, these data suggest that the effects of endogenous oxidants may be confined to the aqueous milieu of the heart. In support of this notion, we had previously found that F2-isoprostane formation is reduced by α-tocopherol (17). Ascorbate however, does not affect the formation of F2-isoprostanes, at least in vitro in microsomes (18).
One factor contributing to such compartmentalization may be due to the presence of redox-active transition metal ions, which can catalyze the conversion of hydrogen peroxide and superoxide into the more highly reactive hydroxyl radical via Fenton chemistry. Previous in vitro experiments by Lodge and co-workers show that dihydrolipoic acid can inhibit copper-induced LDL oxidation by direct chelation of free copper ions (19). In addition, LA appears to inhibit iron-induced ascorbate oxidation, possibly by reducing redox active iron (J. H. Suh et al., unpublished observation). We are currently examining the nature of the interaction between transition metals and LA. Thus, in addition to its antioxidant effect, dihydrolipoic acid may protect against lipid peroxidation by chelating free metal ions in vivo.
Ascorbate may be consumed more rapidly than α-tocopherol in vivo. First, the reduction potential of ascorbate (280 mV) is much lower than that of α-tocopherol (500 mV) (21). Thus, ascorbate is a more effective antioxidant capable of inhibiting lipid peroxidation against a number of different oxidant species (11). Its low reduction potential also allows it to regenerate α-tocopherol whereas the reverse is not possible (20). Second, the concentration of ascorbate is two- to threefold higher than α-tocopherol; thus, ascorbate may spare α-tocopherol from oxidation.
In contrast to a previous study showing an increase in urinary F2-isoprostanes with age, our results show that myocardial levels of F2-isoprostanes did not increase (22). Myocardial F2-isoprostanes may not accumulate due to rapid repair and/or clearance, whereas plasma or urinary F2-isoprostanes would be reflective of released F2-isoprostanes from all tissues and renal clearance.
The beneficial effects of dietary supplementation of (R)-α-lipoic acid toward ameliorating the age-related changes in cardiac myocytes may be attributed to its ability to act as an antioxidant as well as its role as in modulating metabolism. Along with the reduction in oxidant production, (R)-α-lipoic acid supplementation restores myocardial ascorbate levels from old rats. Previous work by Lykkesfeldt and co-workers have reported that there is no change in hepatic ascorbate synthesis with age (23), which suggests that (R)-α-lipoic acid may restore myocardial ascorbate levels by stimulating hexose transporter activity and/or by direct regeneration of dehydroascorbate. Furthermore, LA can indirectly increase GSH levels by increasing cysteine uptake, which is a rate-limiting step for GSH biosynthesis. This increase in thiol antioxidant levels can in turn enhance the rate of ascorbate recycling.
Dietary supplementation of LA also leads to significantly lower steady-state levels of 8-oxo-dG in hearts from old animals. This reduction suggests that the age-related accumulation of 8-oxo-dG in vivo may be due to increased oxidative insult rather than decreased repair capacity. In support of this, a recent study by Souza-Pinto and co-workers has reported that there is an age-dependent increase in 8-oxo-dG glycosylate/AP lyase activity in rat mitochondria with age (24).
The causes for this age-related decline in myocardial mitochondrial function are not completely understood. One possibility is that with age there may be a loss of essential cofactors such as LA, which may limit optimal mitochondrial performance. Supplementation with LA may replenish needed cofactors for α-keto acid dehydrogenases (pyruvate oxidoreductase and 2-oxo-glutarate oxidoreductase) used in pyruvate and fatty acid metabolism. It has been shown that the proportion of the active form of pyruvate oxidoreductase declines with age (25), possibly due to modification of the lipoamide moiety of the E2 subunit (26). Thus, feeding old rats LA may reverse the age-associated decline in LA-dependent oxidoreductase activity. This may also explain why we only observed a beneficial effect of LA only in old and not in young animals.
Other necessary cofactors necessary for mitochondrial function, such as carnitine and cardiolipin, also decline with age (27, 28). Carnitine loss, for example, can limit the transport of fatty acids into mitochondria for β-oxidation, which is the major source for ATP synthesis. In addition, decline in cardiolipin has been shown to decrease substrate transport in isolated mitochondrial preparations and lower cytochrome c oxidase activity.
One possible physiological consequence of these decreased cofactors would be loss of ATP production, which may lead to cardiac stiffness. To maintain myocardial function, a constant supply of ATP is required and small reserves are maintained. This suggests that when energy supply is interrupted (ischemia) or impaired (aging), ATP levels decline rapidly. Like systolic contraction, diastolic relaxation also requires high levels of ATP, because ATP acts as an allosteric effector to disassociate actin from myosin (29). Thus, any decrement in mitochondrial ATP synthesis affects cardiac stiffness appreciably. A decline in ATP synthesis also compromises Ca2+ reuptake into the sarcoplasmic reticulum from the cytosol, again affecting myocardial relaxation (30, 31). The Na+/Ca2+ transporter is also energy dependent, and a decline in myocardial ATP levels would thus slow cardiac relaxation by decreasing the rate of Ca2+ removal from the cytosol (30, 31). It is notable that a general attribute of myocardial aging is a prolonged cytosolic calcium transient and slower myocardial relaxation rate (32, 33).
The exact physiological consequences associated with these cellular changes remains to be elucidated. Although these changes may not affect the heart’s function under normal conditions, it is possible that a loss in bioenergetic capacity along with antioxidant protection may severely limit the hearts ability to respond to physical stress. More studies are needed to carefully correlate the limitation brought about by age-associated changes in the mitochondrial function.
In conclusion, our present findings suggest that (R)-α-lipoic acid supplementation may be a safe and effective means of improving systemic decline in over all metabolic function and also increase protection against both endogenous and external production of ROS. However, long-term feeding studies with LA are needed to determine whether benefits of LA seen in old animals can be sustained over time.
Acknowledgments
This work was supported in part by National Institute on Aging Grant RIAG17141A (T.M.H.), an American Heart Association predoctoral fellowship 9910089Z (J.H.S.), and National Institutes of Health grants DK48831, GM15431, GM42056, DK26657, and CA77839 (J.D.M). J.D.M. is the recipient of a Burroughs Wellcome Fund Clinical Scientist Award in Translational Research.
References
- 1.Cohn JN, Bristow MR, Chien KR, Colucci WS, Frazier OH, Leinwand LA, Lorell BH, Moss AJ, Sonnenblick EH, Walsh RA, et al. Report of the National Heart, Lung, and Blood Institute Special Emphasis Panel on Heart Failure Research. Circulation. 1997;95:766–770. doi: 10.1161/01.cir.95.4.766. [DOI] [PubMed] [Google Scholar]
- 2.Sawada M, Carlson JC. Changes in superoxide radical and lipid peroxide formation in the brain, heart and liver during the lifetime of the rat. Mech Ageing Dev. 1987;41:125–137. doi: 10.1016/0047-6374(87)90057-1. [DOI] [PubMed] [Google Scholar]
- 3.Nohl H, Hegner D. Do mitochondria produce oxygen radicals in vivo? Eur J Biochem. 1978;82:563–567. doi: 10.1111/j.1432-1033.1978.tb12051.x. [DOI] [PubMed] [Google Scholar]
- 4.Sohal RS, Ku HH, Agarwal S, Forster MJ, Lal H. Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech Ageing Dev. 1994;74:121–133. doi: 10.1016/0047-6374(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 5.Haramaki N, Han D, Handelman GJ, Tritschler HJ, Packer L. Cytosolic and mitochondrial systems for NADH—and NADPH-dependent reduction of alpha-lipoic acid. Free Radic Res Med. 1997;22:535–542. doi: 10.1016/s0891-5849(96)00400-5. [DOI] [PubMed] [Google Scholar]
- 6.Kozlov AV, Gille L, Staniek K, Nohl H. Dihydrolipoic acid maintains ubiquinone in the antioxidant active form by two-electron reduction of ubiquinone and one-electron reduction of ubisemiquinone. Arch Biochem Biophys. 1999;363:148–154. doi: 10.1006/abbi.1998.1064. [DOI] [PubMed] [Google Scholar]
- 7.Roy S, Packer L. Redox regulation of cell functions by alpha-lipoate: biochemical and molecular aspects. Biofactors. 1998;7:263–267. doi: 10.1002/biof.5520070324. [DOI] [PubMed] [Google Scholar]
- 8.Han D, Handelman G, Marcocci L, Sen CK, Roy S, Kobuchi H, Tritschler HJ, Flohe L, Packer L. Lipoic acid increases de novo synthesis of cellular glutathione by improving cystine utilization. Biofactors. 1997;6:321–338. doi: 10.1002/biof.5520060303. [DOI] [PubMed] [Google Scholar]
- 9.Tytgat J. How to isolate cardiac myocytes. Cardiovasc Res. 1994;28:280–283. doi: 10.1093/cvr/28.2.280. [DOI] [PubMed] [Google Scholar]
- 10.Farmer BB, Mancina M, Williams ES, Watanabe AM. Isolation of calcium tolerant myocytes from adult rat hearts: review of the literature and description of a method. Life Sci. 1983;33:1–18. doi: 10.1016/0024-3205(83)90706-3. [DOI] [PubMed] [Google Scholar]
- 11.Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci USA. 1989;86:6377–6381. doi: 10.1073/pnas.86.16.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chou PP, Jaynes PK, Bailey JL. Determination of vitamin E in microsamples of serum by liquid chromatography with electrochemical detection. Clin Chem. 1985;31:880–882. [PubMed] [Google Scholar]
- 13.Helbock HJ, Beckman KB, Shigenaga MK, Walter PB, Woodall AA, Yeo HC, Ames BN. DNA oxidation matters: the HPLC-electrochemical detection assay of 8-oxo-deoxyguanosine and 8-oxo-guanine. Proc Natl Acad Sci USA. 1998;95:288–293. doi: 10.1073/pnas.95.1.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morrow JD, Awad JA, Kato T, Takahashi K, Badr KF, Roberts LJD, Burk RF. Formation of novel non-cyclooxygenase-derived prostanoids (F2-isoprostanes) in carbon tetrachloride hepatotoxicity. An animal model of lipid peroxidation. J Clin Invest. 1992;90:2502–2507. doi: 10.1172/JCI116143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansford RG, Hogue BA, Mildaziene V. Dependence of H2O2 formation by rat heart mitochondria on substrate availability and donor age. J Bioenerg Biomembr. 1997;29:89–95. doi: 10.1023/a:1022420007908. [DOI] [PubMed] [Google Scholar]
- 16.Hagen TM, Yowe DL, Bartholomew JC, Wehr CM, Do KL, Park JY, Ames BN. Mitochondrial decay in hepatocytes from old rats: membrane potential declines, heterogeneity and oxidants increase. Proc Natl Acad Sci USA. 1997;94:3064–3069. doi: 10.1073/pnas.94.7.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Awad JA, Morrow JD, Hill KE, Roberts LJ, Burk RF. Detection and localization of lipid peroxidation in selenium and vitamin E deficient rats using F2-isoprostanes. J Nutr. 1994;124:810–816. doi: 10.1093/jn/124.6.810. [DOI] [PubMed] [Google Scholar]
- 18.Morrow JD, Roberts LJ, Daniel VC, Awad JA, Mirochnitchenko O, Swift LL, Burk RF. Comparison of formation of D2/E2-isoprostanes and F2-isoprostanes in vitro and in vivo—effects of oxygen tension and glutathione. Arch Biochem Biophys. 1998;353:160–171. doi: 10.1006/abbi.1998.0645. [DOI] [PubMed] [Google Scholar]
- 19.Lodge JK, Traber MG, Packer L. Thiol chelation of Cu2+ by dihydrolipoic acid prevents human low density lipoprotein peroxidation. Free Radic Biol Med. 1998;25:287–297. doi: 10.1016/s0891-5849(98)00048-3. [DOI] [PubMed] [Google Scholar]
- 20.Buettner GR. The pecking order of free radicals and antioxidants: lipid peroxidation, alpha-tocopherol, and ascorbate. Arch Biochem Biophys. 1993;300:535–543. doi: 10.1006/abbi.1993.1074. [DOI] [PubMed] [Google Scholar]
- 21.Nakagawa Y, Cotgreave IA, Moldeus P. Relationships between ascorbic acid and alpha-tocopherol during di-quat-induced redox cycling in isolated rat hepatocytes. Biochem Pharmacol. 1991;42:883–888. doi: 10.1016/0006-2952(91)90049-b. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z, Ciabattoni G, Créminon C, Lawson J, Fitzgerald GA, Patrono C, Maclouf J. Immunological characterization of urinary 8-epi-prostaglandin F2 excretion in man. J Pharmacol Exp Ther. 1995;363:148–154. [PubMed] [Google Scholar]
- 23.Lykkesfeldt J, Hagen TM, Vinarsky V, Ames BN. Age-associated decline in ascorbic acid concentration, recycling, and biosynthesis in rat hepatocytes—reversal with (R)-alpha-lipoic acid supplementation. FASEB J. 1998;12:1183–1189. doi: 10.1096/fasebj.12.12.1183. [DOI] [PubMed] [Google Scholar]
- 24.Souza-Pinto NC, Croteau DL, Hudson EK, Hansford RG, Bohr VA. Age-associated increase in 8-oxo-deoxyguanosine glycosylase/AP lyase activity in rat mitochondria. Nucleic Acids Res. 1999;27:1935–1942. doi: 10.1093/nar/27.8.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakai N, Sato Y, Oshida Y, Yoshimura A, Fujitsuka N, Sugiyama S, Shimomura Y. Effects of aging on the activities of pyruvate dehydrogenase complex and its kinase in rat heart. Life Sci. 1997;60:2309–2314. doi: 10.1016/s0024-3205(97)00286-5. [DOI] [PubMed] [Google Scholar]
- 26.Humphries KM, Szweda LI. Selective inactivation of alpha-ketoglutarate dehydrogenase and pyruvate dehydrogenase: reaction of lipoic acid with 4-hydroxy-2-nonenal. Biochemistry. 1998;37:15835–15841. doi: 10.1021/bi981512h. [DOI] [PubMed] [Google Scholar]
- 27.Hagen TM, Ingersoll RT, Wehr CM, Lykkesfeldt J, Vinarsky V, Bartholomew JC, Song MH, Ames BN. Acetyl-L-carnitine fed to old rats partially restores mitochondrial function and ambulatory activity. Proc Natl Acad Sci USA. 1998;95:9562–9566. doi: 10.1073/pnas.95.16.9562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoch FL. Cardiolipins and biomembrane function. Biochim Biophys Acta. 1992;1113:71–133. doi: 10.1016/0304-4157(92)90035-9. [DOI] [PubMed] [Google Scholar]
- 29.Katz AM. Energetics and the failing heart. Hosp Pract. 1991;26:78–90. doi: 10.1080/21548331.1991.11705280. [DOI] [PubMed] [Google Scholar]
- 30.Morgan JP, Erny RE, Allen PD, Grossman W, Gwathmey JK. Abnormal intracellular calcium handling, a major cause of systolic and diastolic dysfunction in ventricular myocardium from patients with heart failure. Circulation. 1990;81:III21–III32. [PubMed] [Google Scholar]
- 31.Negretti N, O’Neill SC, Eisner DA. The relative contributions of different intracellular and sarcolemmal systems to relaxation in rat ventricular myocytes. Cardiovasc Res. 1993;27:1826–1830. doi: 10.1093/cvr/27.10.1826. [DOI] [PubMed] [Google Scholar]
- 32.Frolkis VV, Frolkis RA, Mkhitarian LS, Shevchuk VG, Fraifeld VE, Vakulenko LG, Syrovy I. Contractile function and Ca2+ transport system of myocardium in ageing. Gerontology. 1988;34:64–74. doi: 10.1159/000212932. [DOI] [PubMed] [Google Scholar]
- 33.Siri FM, Krueger J, Nordin C, Ming Z, Aronson RS. Depressed intracellular calcium transients and contraction in myocytes from hypertrophied and failing guinea pig hearts. Am J Physiol. 1991;261:H514–H530. doi: 10.1152/ajpheart.1991.261.2.H514. [DOI] [PubMed] [Google Scholar]