Abstract
Depression is often linked to early-life adversity and circadian disturbances. Here, we assessed the long-term impact of early-life adversity, particularly preweaning mother–infant separation, on the circadian system’s responsiveness to a time giver or synchronizer (Zeitgeber). Mother-reared (MR) and peer-reared (PR) rhesus monkeys were subjected to chronic jet-lag, a forced desynchrony protocol of 22 hr T-cycles [11:11 hr light:dark (LD) cycles] to destabilize the central circadian organization. MR and PR monkeys subjected to the T-cycles showed split locomotor activity rhythms with periods of ~22 hr (entrained) and ~24 hr (free-running), simultaneously. Continuous melatonin treatment in the drinking water (20 μg/mL) gradually increased the amplitude of the entrained rhythm at the expense of the free-running rhythm, reaching complete entrainment by 1 wk. Upon release into constant dim light, a rearing effect on anticipation for both the predicted light onset and food presentation was observed. In MR monkeys, melatonin did not affect the amplitude of anticipatory behavior. Interestingly, however, PR macaques showed light onset and food anticipatory activities in response to melatonin treatment. These results demonstrate for the first time a rearing-dependent effect of maternal separation in macaques, imprinting long-term plastic changes on the circadian system well into late adulthood. These effects could be counteracted by the synchronizer molecule melatonin. We conclude that the melatonergic system is targeted by early-life adversity of maternal separation and that melatonin supplementation ameliorates the negative impact of stress on the circadian system.
Keywords: adverse rearing, circadian rhythms, depression, early stress, melatonin, rhesus monkey
Introduction
Patients with a history of childhood abuse show persistent hyperactivity of the hypothalamo-pituitary-adrenal (HPA) axis [1, 2], as well as abnormalities in brain regions involved in emotional disorders [3, 4]. Magnetic resonance spectroscopic imaging from adult macaques (10 yr of age) revealed an enduring impact of adverse rearing on cerebral metabolites and neurotransmitters in brain regions implicated in trauma-related psychiatric disorders [5]. Hence, stressful conditions in early life can alter experience-dependent maturation of brain structures underlying emotional functioning and endocrine responses to stress [6]. The associated behavioral changes, such as an increased response to stress and depression, are usually long lasting, even during adulthood [7–10].
Dramatic alterations in the responsiveness to stress due to early-life adversity can reflect plastic changes in or modified interactions between the circadian system and the cortico-limbic system [11]. Additionally, the potential hormonal mediator, melatonin, enabling the cross-talk between both system maybe affected [12, 13]. Melatonin is a hormone that is rhythmically synthesized and released from the pineal gland. The circadian rhythm of melatonin synthesis is regulated by the mammalian master circadian-clock, the suprachiasmatic nucleus (SCN), and entrained by light [14]. Importantly, melatonin itself is a Zeitgeber, capable of transmitting temporal information back to the circadian system [15, 16]. Indeed, melatonin is a major synchronizer of endocrine rhythms [17–19]. The synchronizing effects of melatonin on endocrine rhythms as demonstrated in capuchin monkeys is mediated through its modulatory action on circadian core clock gene products [20]. Vice versa, hormones implicated in HPA abnormalities in depressed patients can affect pineal melatonin synthesis. Hence, a feedback, between the HPA axis and the pineal gland, at the CNS level also exists [21]. Indeed, it is well documented that chronic stress, aging and pathological conditions such as major depression are frequently associated with circadian disturbances [22], persistent hyperactivation of the HPA axis which potentially can result in disturbances in circadian rhythms of pituitary and adrenal secretions, abnormal magnitude and duration of endocrine responses to stress and decreased sensitivity to glucocorticoid feedback [23–27].
Preweaning mother–infant separation in nonhuman primates has been shown to affect rhythms in hormone secretion, [28, 29]. However, whether these acute circadian effects associated with early-life maternal separation or stress are also permanent is unknown. Thus, in this study we investigated the hypothesis that preweaning mother–infant separation (peer-reared, PR) results in permanent changes to the circadian system responsiveness to stress.
To test this, we subjected mother reared (MR) and PR macaques to a forced desynchrony protocol, chronic jetlag, using an imposed light–dark cycle (T-cycles) of 22 hr consisting of 11 hr of light and 11 hr of darkness. We then measured the effect of early-life adversity on the adaptability to phase-lock onto the 22 hr LD cycles, hence, to assess the plasticity of the circadian system. Furthermore, we measured the responsiveness of the circadian system to the entraining properties of melatonin to phase-lock the rhythm of locomotor activity on to the 22 hr T-cycles [30].
Materials and methods
Animals
This study used both MR and PR monkeys (Macaca mulatta) 18–20 yr of age, with a life expectancy in capture ranging between 15 and 25 yr. These animals were originally reared at the Harlow Primate Laboratory, Madison, WI, and transferred to Northwestern University (Chicago, IL, USA) at 6–8 yr of age. Off study, monkeys were fed twice daily, once during the morning and once during the afternoon hours of the light portion of their 12:12 hr light: dark cycles. The animal’s diet consisted of monkey chow, fruits and vegetables, and water was available ad libitum unless stated otherwise. Entries into the primate rooms were limited to an assigned research technician, staff veterinarian and caretaker. All room entries were scheduled around feeding times to minimize disturbances. Animal care and all experimental procedures were approved by the Northwestern University Animal Care and Use Committee and are in accordance with the guidelines of the National Institute of Health.
Mother rearing
Infants were reared with their biological mothers until 7 months of age [31, 32]. Mothers and their offspring infants were provided with Purina Monkey Chow once daily. Water was available ad libitum. Infants were separated from their mothers at 7 months of age and subsequently group housed (mixed sex) with peers of three animals each.
Peer rearing
Infants were separated from their biological mothers within 2–3 days after birth instead of the usual 7–9 months of age, and housed in individual wire mesh cages in a nursery [31, 32]. Infants had daily (30 min) contact with peers (peer stimulation) and object stimulation. Animals were trained to self-feed with infant formula (Similac) from bottles. At the age of 6 wk, infants were housed in peer groups of three. Infants at 7 months of age were then reorganized such that every peer-reared animal was group housed with a new group of peers to mimic a similar situation to mother-reared infants group housed with peers at 7 months of age [31, 32].
Measurements of spontaneous locomotor activity
Spontaneous locomotor activity was recorded using actiwatches (minimitters) that were fitted into custom-designed collars for each individual animal [33]. To avoid introducing novelty to the experiment, animals were habituated to the collars by wearing them around their necks at all times, during and off experiments for the last 8 yr. Activity data recorded by actiwatches fitted into the collars reflect true activity records and not activity bouts as a result of the animals’ interaction with their collars. This was confirmed as similar actograms were obtained using telemetric recordings by abdominal implanted minimeters (unpublished observation). Data were collected every 3 wk, requiring the removal of actiwatches positioned in the collars of each individual primate without anesthetic interventions. Activity data were downloaded using Mini-Mitter actiwatch reader into ACTIWARE-SLEEP 3.4 software (MiniMitter Co., Inc., Bend, OR, USA) and analyzed using CLOCKLAB 2.61 (Actimetrics, Evanston, IL, USA).
Experimental design and protocols
Lighting conditions
Animals previously exposed to 12:12 hr LD cycles were subjected to T-cycles of 11:11 hr LDim cycles. The light intensity on top of the cage during the light period and dim-light periods were 80 lux and 20 lux, respectively. During free-running constant dim light conditions (stage 3), the light intensity on top of the cage was kept at 20 lux.
Forced desynchrony protocol
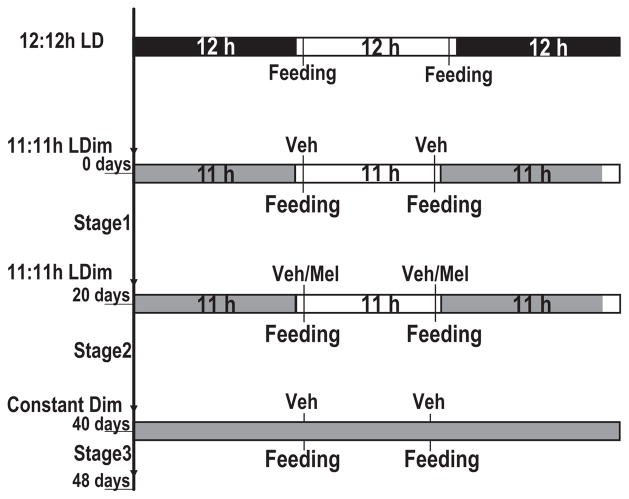
In this study, we used two groups of nonhuman primates (Macaca mulatta), each consisting of six MR and six PR adult animals. Animals were habituated to single housing conditions since their arrival in the laboratory at the age of 6–8 yr. Visual interaction between animals was prevented with the help of curtains. All animals were housed in the same room and subjected to the same conditions with respect to light:dark cycles, time of feeding, handling and background noise. Animals previously exposed to 12:12 hr LD cycles, regular off-study lighting conditions, were subjected to T-cycles of 22 hr with 11 hr of light (80 lux) and 11 hr of Dim light (20 lux; 11:11 hr LDim) for a period of 6 wk followed by 8 days of constant dim light (Fig. 1). Animals were maintained under a restricted diet, based on the individuals body weight. Food was provided twice during the light portion of the 11:11 hr LDim cycles, 1 hr after light onset (transition from 20 to 80 lux) and 1 hr before light offset (transition from 80 to 20 lux; Fig. 1). Excess diluted apple juice with either vehicle or melatonin was supplied freshly in cage-attached water bottles during feeding times 1 hr after light onset and 1 hr before dim light onset. During the first 3 wk of 11:11 hr LDim all animals received vehicle in diluted apple juice (stage 1; Fig. 1). During stage 2 of 11:11 hr LDim cycles, animals within each group were divided into two subgroups treated with either melatonin (n = 3 for both MR and PR) or vehicle (n = 3 for both MR and PR) to which the animals had free access (ad libitum). During stage 3, animals were released into constant dim light conditions (20 lux) with feeding times scheduled according to the previous light dark cycle (11:11 hr LDim; stage 3; Fig. 1). Actiwatches were removed and cages were changed at the end of each experimental stage. Visual daily health assessments were kept to a minimum and restricted to feeding times. Lighting conditions and data acquisition were controlled remotely, thus minimizing further disturbances. Treatments, with melatonin or vehicle, were subsequently crossed over in a second experiment under identical conditions as described above following a 45 days rest period under 12:12 hr LD cycles.
Fig. 1.
Experimental paradigm. Animals previously exposed to 12:12 hr light:dark (LD) cycles with feeding restricted to the photophase were subjected to T-cycles of 22 hr with 11:11 hr light: DimLight for 6 wk. Animals were fed twice during the photophase, 1 hr after lights ‘ON’ and 1 hr prior to lights ‘OFF’. During stage 2, animals were either treated with melatonin chronically or vehicle alone in drinking water provided freshly during feeding time. Finally, animals were subjected to constant dim light conditions. During stage 3, the feeding cycle was maintained according to the previous 11:11 hr LDim cycles.
Melatonin treatments
Animals treated with either vehicle [ethanol dissolved in diluted apple juice (water:apple juice, 0.05:100 v/v) to a final concentration of 0.001%] or melatonin dissolved in vehicle (20 μg/ml; Sigma-Aldrich, St Louis, MO, USA) received their treatments via water bottles attached to their cages. The melatonin dose was approximately 1.2 mg/kg (oral) calculated based on total drinking (600 mL) during an 11 hr dim light period for a 10 kg animal. Vehicle and melatonin were prepared fresh for every feeding time point (1 hr after light onset and 1 hr before dim light onset) to provide a relatively consistent amount of vehicle or melatonin intake as well as minimizing disturbance.
Drinking behavior
The amount of water consumed per animal was monitored throughout the experiment by measuring water consumption by the end of the light phase and dim light phase of the 11:11 hr LDim cycles.
Data analysis
The number of animals from which data were used for analysis varied based on the stage and trial analyzed. A total of 11 animals were used to analyze stage 1 and stage 3 activity data for trial 1 as one animal had to be terminally removed due to health-related problems. As for stage 2 of trial 1, only data from ten animals were available for analysis due to actiwatch failure of one individual. Consequently to the replacement of the malfunctioning actiwatch at the end of stage 2 of trial 1, data from that animal were included for stage 3 analysis, thus, n = 11. Data from a total number of ten animals only were used for analysis for all stages during trial 2 as another individual had to be removed due to health-related problems. Circadian period of locomotor activity rhythms were analyzed using clocklab (Actimetrics, IL, USA). For the analysis of food anticipatory activity and the anticipation of light onset, individual daily locomotor activity profiles were exported from clocklab and averaged in 10 min bins starting 70 min prior to the second time of food presentation during the photophase, or 100 min prior to light onset, respectively. Anticipatory activity was not analyzed beyond 20–30 min prior to feeding as the increase in activity beyond this time point may reflect the reaction of the animals to noises in the neighboring room originating from the experimenter in preparation to enter the animal holding room. As cycling light onset and offset times were regulated via an automated lighting system, anticipatory activity could be analyzed within 10 min of the scheduled daily light onset. Group average profiles were then generated with Microsoft Excel. To determine the period of the light-dependent and light-independent activity rhythms, we used the chi-square periodogram procedure [34] with a 1 min step size using the ClockLab software package (Actimetrics, Evanston, IL, USA) developed in MatLab. The reliability (robustness) of a rhythm was defined as the value of the Sokolove-Bushell Qp statistics indicated here as the amplitude. Locomotor activity rhythms with a confidence level of 99.9% were considered as statistically significant. Oblique lines in periodograms indicate significance (P = 0.01). Qp values above the line are statistically significant.
Statistics
Results for anticipatory activity are presented as the mean ± S.E.M. The significance of differences between 10 min activity bins for every rearing and treatment condition was determined by one-way analysis of variance followed by Newman–Keuls post hoc-test. Drinking behavior was analyzed using a two-way ANOVA to determine the existence of rearing-dependent differences in drinking behavior under two treatment conditions (vehicle and melatonin) and the Student’s t-test to determine rearing independent effects of treatment for both day and nighttime.
Results
Forced splitting of the circadian activity rhythm in rhesus monkey (Macaca mulatta)
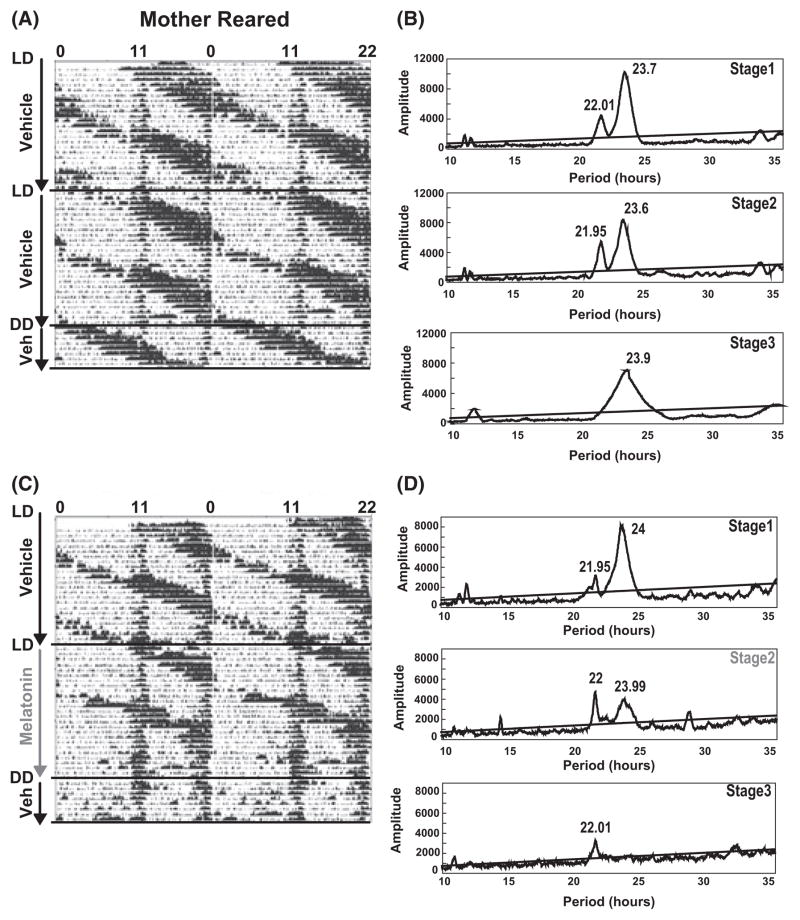
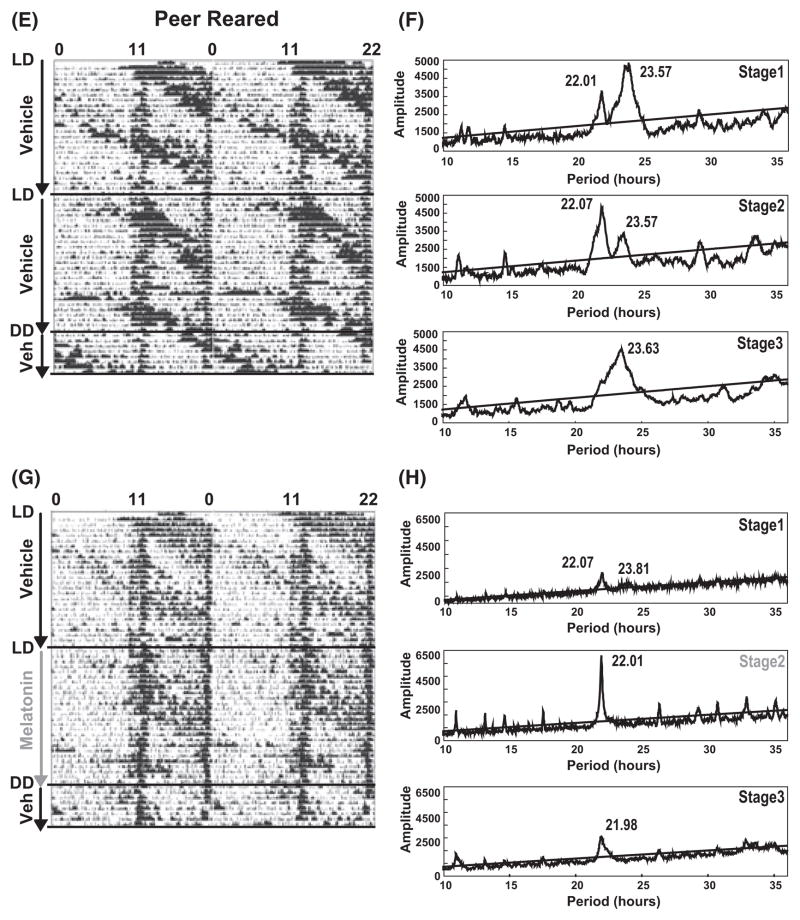
We first assessed the efficacy of a forced desynchronization protocol, chronic jet-lag, in splitting circadian rhythms of spontaneous locomotor activity into two rhythms. Animals were subjected to T-cycles of 22 hr period consisting of 11 hr of lights ‘ON’ and 11 hr of ‘dim light’. All animals, regardless of rearing conditions, subjected to the forced desynchrony protocol showed within the first 3 wk (stage 1 of trial 1 and 2) a dissociation of the entrained activity rhythm into two rhythms with periods of both 22.02 hr ± 0.03 hr (Trial 1: n = 11, Trial 2: n = 10) and 23.57 hr ± 0.05 hr (Trial 1: n = 11, Trial 2: n = 10). By 6 wk (stage 2 of trial 1 and 2), animals could be divided based on the period of activity rhythms into two groups. In one group, animals synchronized to the 22 hr T-cycles as confirmed by chi-square analysis of periodograms and visual confirmation of actograms with a circadian period of 22.01 hr ± 0.03 hr [three of ten animals treated with vehicle for 6 wk; cumulative for both trial 1 (n = 5) and trial 2 (n = 5)]. The second group of individuals showed split activity rhythms with circadian periods of 22.01 ± 0.032 hr and 23.67 hr ± 0.13 hr in seven of ten animals treated with vehicle for 6 wk [cumulative for both trial 1 (n = 5) and trial 2 (n = 5)] (Fig. 2A,E). However, when animals were released for 1 wk into constant dim light conditions, their activity rhythms were free-running with either a period of 24.5 hr ± 0.6 hr [five of 11 animals; cumulative for both trial 1 (n = 6) and trial 2 (n = 5)] or maintained their split activity rhythms with periods of 22.04 hr ± 0.02 hr and 23.97 hr ± 0.13 hr [six of 11 animals; cumulative for both trial 1 (n = 6) and trial 2 (n = 5)]. Thus, there lacks true entrainment of the locomotor activity rhythm to the 22 hr T-cycles for the vehicle group.
Fig. 2.
Melatonin resynchronizes forced-split locomotor activity. Animals were subjected to T-cycles of 22 hr with 11 hr of light and 11 hr of dim light. (A and E; Left) Representative actogram for mother-reared and peer-reared individuals treated with vehicle while subjected to 11:11 hr light:DimLight (LDim) conditions for 6 wk (stages 1 and 2). The individual was continued on vehicle alone treatment for an additional 8 days under constant dim light conditions (stage 3). (B and F) Corresponding chi-square analysis for the periodogram for stages (1–3) demonstrating the split spontaneous locomotor activity rhythms in stages 1 and 2 and lack of entrainment to the 11:11 hr LDim cycles as demonstrated in stage 3 where chi-square analysis reveals a single significant dominant peak with a period of 23.9 hr. (C and G) Representative actograms for mother-reared and peer-reared individuals subjected to 11:11 hr LDim conditions for 6 wk (stages 1 and 2) during which the individual was treated first with vehicle (ethanol; stage 1) followed by melatonin during stage 2. Finally, during stage 3, melatonin was replaced by vehicle under conditions of constant dim light for 8 days. (D and H) Corresponding chi-square analysis for periodograms of stages 1–3, demonstrating two spontaneous locomotor activity rhythms in stages 1 and 2 in case of vehicle treatment and the entraining effect of melatonin treatment in stage 2 as shown by chi-square analysis. Locomotor activity rhythms with a confidence level of 99.9% were considered as statistically significant. The periods (inserts) were indicated where rhythmicity was significant. Oblique lines indicate significance (P = 0.01). Qp values above the line are statistically significant.
Melatonin entraines desynchronized locomotor activity rhythms
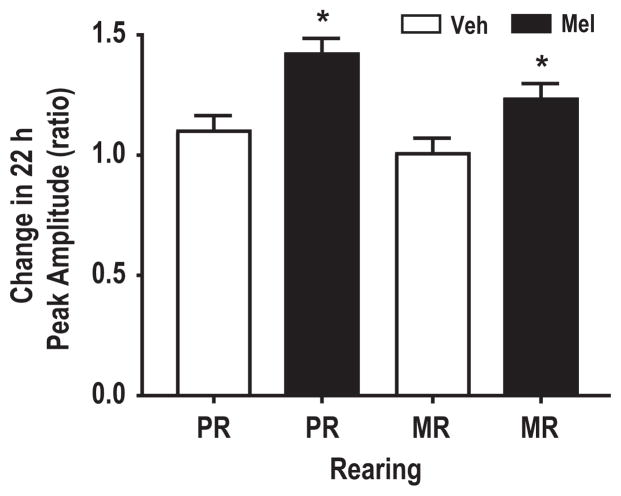
In contrast to vehicle, treatment of macaques with the chronobiotic, melatonin, consolidated the split free-running activity rhythm by entraining the free-running rhythm to the 22 hr T-cycles. The entrainment by melatonin was measured as a change in the ratio between the peak amplitude of the 22 hr light-dependent rhythm for stage 2 (melatonin) and stage 1 (vehicle). Nota bene, during stage 2, MR and PR macaques received either melatonin or vehicle, while during stage 1 both rearing groups were subjected to only vehicle in their drinking water (Fig. 2). The melatonin-mediated entrainment was interpreted as an increase in the amplitude of the 22 hr locomotor activity rhythm. The results show a similar increase in the amplitude for both rearing groups [MR; 1.27 ± 0.06 (n = 5) for melatonin versus 1.0 ± 0.06 (n = 5) for vehicle, P < 0.05, and PR; 1.46 ± 0.14 (n = 5) for melatonin versus 1.11 ± 0.08 (n = 5) for vehicle, P < 0.05; Fig. 3]. The number of individuals for every treatment group reflect pooled data from two successive crossover experiments. Chi-square analysis of locomotor activity under constant dim light conditions showed a single significant rhythm with a period of approximately 22 hr in nine of 11 animals treated with melatonin (Fig. 2D,F, stage 3). Two MR individuals did not show an entrainment in response to melatonin treatment. The results demonstrate that a single consolidated spontaneous locomotor activity rhythm in nonhuman primates (Macaca mulatta) is forced to dissociate into both light-dependent and light-independent (free-running) components in response to 22 hr T-cycles (11:11 hr LDim) exposure. Furthermore, melatonin is able to reconsolidate the split activity rhythms.
Fig. 3.
Melatonin increases the amplitude of the 22 hr peak activity rhythm. Animals treated with melatonin for 3 wk in 22 hr T-cycles showed an increased amplitude of the 22 hr spontaneous activity rhythm as compared to 3 wk vehicle treatment under similar conditions. *P < 0.005 student t-test. Error bars represent S.E.M. Only animals with peak activity rhythms of 22 hr in the presence or absence of free-running activity rhythms during both stage 1 and stage 2 were included in the analysis. Veh, Vehicle; Mel, melatonin.
Rearing conditions determine the effect of melatonin on the anticipation of light and food-presentation
Anticipation to light onset
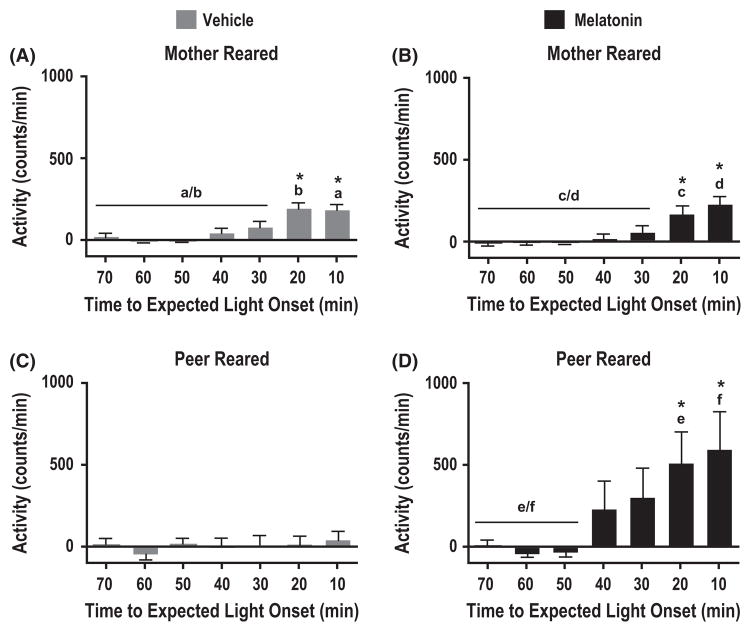
When assessing the phase relationship between the predicted light onset and the actual onset of activity for animals set to free-run under constant dim light conditions (stage 3), only those individuals that entrained to the 22 hr T-cycles exhibited a stable phase relationship. Entrainment, or the anticipation of the predicted light-onset, was measured as an increase in locomotor activity prior to the expected dim light to light transition. Mother-reared individuals showed anticipatory activity around the time of expected light onset when treated with either vehicle alone (Fig. 4A; F(6,210) = 14.95, P < 0.0001, n = 5; Newman–Keuls post hoc analysis: 10–0.0 min and 20–10 min versus 70–30 min, P < 0.05) or melatonin (Fig. 4B; F(6,168) = 9.91, P < 0.0001, n = 5; Newman–Keuls post hoc analysis: 10–0.0 and 20–10 min versus 70–30 min, P < 0.05). Furthermore, the amplitude of the anticipatory activity to light-onset did not change in MR individuals in response to the melatonin treatment (Student t-test, matching the same time intervals between vehicle and melatonin, P > 0.05). On the other hand, peer-reared animals treated with vehicle alone, did not show any evidence for entrainment to light-onset (Fig. 4C; F(6,119) = 0.38, P > 0.9, n = 5). In contrast, treatment of PR macaques with melatonin resulted in robust anticipatory activity prior to the expected light-onset (Fig. 4D; F(6,168) = 3.48, P < 0.005, n = 6; Newman–Keuls post hoc analysis: 10–0.0 min and 20–10 min versus 70–40 min, P < 0.05). Comparison between vehicle and melatonin treatments among PR individuals showed a significant effect of melatonin on the anticipation to light onset (Student t-test, matching the same time intervals between vehicle and melatonin starting from 30 min prior to light onset, P < 0.05).
Fig. 4.
Effect of maternal separation on the responsiveness to melatonin in the anticipation for light onset. Anticipation of the predicted light onset during stage 3 was recorded by measuring the spontaneous locomotor activity prior to the predicted light onset time according to the original 11:11 hr light:dark cycle in both peer- and mother-reared animals. Spontaneous locomotor activity profile for the last 70 min prior to expected light onset in peer-reared (C, D) and mother-reared (A, B) animals continuously treated with vehicle (ethanol; A, C) (stages 1–3) or treated with melatonin in stage 2 (B, D). *P < 0.05, One-way ANOVA and Newman–Keuls post hoc analysis. Error bars represent s.e.m. Identical letters are assigned to identify time intervals compared by Newman–Keuls post hoc analysis. Data presented are normalized to baseline activity.
Food anticipation
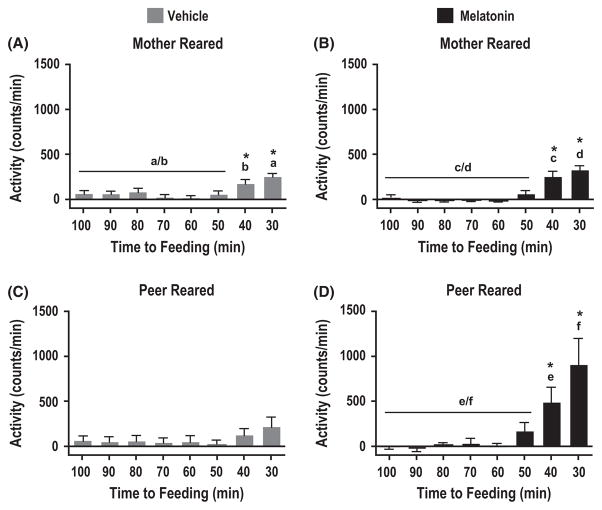
To assess whether the food entrainable oscillator is affected differentially by melatonin across rearing conditions, we compared changes in spontaneous locomotor activity in anticipation to feeding between MR and PR individuals. Only MR but not PR rhesus monkeys treated with vehicle alone showed a significant increase in the amplitude of locomotor activity at 30 and 40 min prior to food presentation under constant dim light conditions (Fig. 5A,C; MR: F(7,192) = 10.61, P < 0.0001; Newman–Keuls Post hoc analysis: 40–30 min versus 100–50 min, P < 0.05, n = 5 and PR: F(7,192) = 1.29, P > 0.25, n = 5). Consistent with light anticipatory activity (compare Fig. 4A,B) mother-reared animals did not show a significant enhanced response to melatonin treatment (n = 5) in the amplitude of locomotor activity prior to feeding as compared to the vehicle group (n = 5; Fig. 5A,B, F(3,96) = 2.58, P > 0.05). Similar to MR individuals, PR animals treated with melatonin demonstrated food anticipatory activity when placed in constant dim light (Fig. 5D, F(7,192) = 5.47, P < 0.0001; Newman–Keuls post hoc analysis: 40–30 min versus 100–50 min, P < 0.05, n = 6). However, the amplitude of spontaneous locomotor activity in anticipation of food significantly increased in response to chronic melatonin treatment (Fig. 5C,D, F(3,96) = 4.26, P < 0.01, n = 6; Newman–Keuls post hoc analysis: 30 min melatonin versus 30 min vehicle and 40 min melatonin versus 40 min vehicle, P < 0.05).
Fig. 5.
Effect of maternal separation on the responsiveness to melatonin in food anticipation. Analysis of food anticipation for the second feeding time point (1 hr prior to lights off) according to the previous (stage 2) 11:11 hr light: DimLight cycles for animals subjected to constant dim light conditions, measured as changes in spontaneous locomotor activity. Mean spontaneous locomotor activity profiles were averaged into 10 min bins for both peer-reared and mother-reared groups either treated with vehicle in stages 1–3 (A, C) or with melatonin in stage 2 (B, D). *P < 0.05, One-way ANOVA and Newman–Keuls post hoc analysis. Error bars represent s.e.m. Identical letters are assigned to identify time intervals compared by Newman–Keuls post hoc analysis. Data presented are normalized to baseline activity.
Drinking behavior
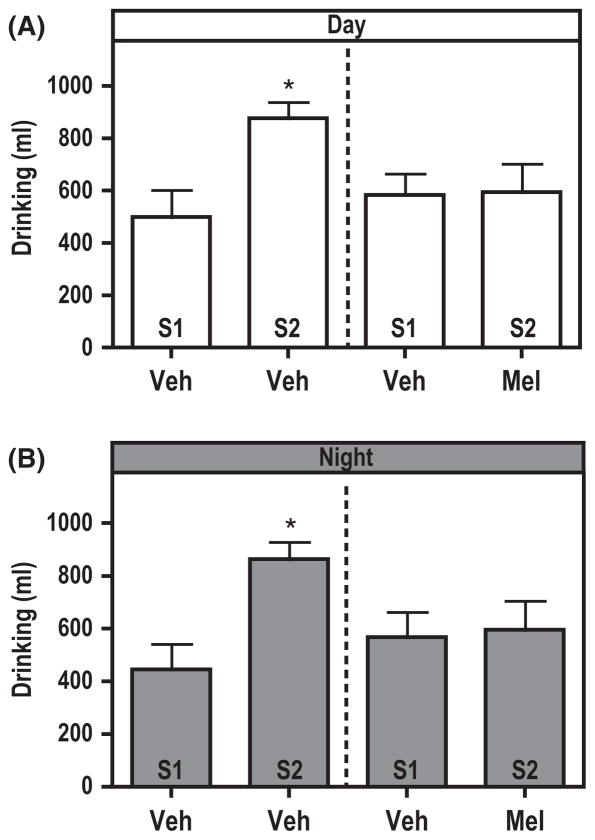
The drinking profile through the experiment for MR and PR individuals treated with vehicle only during both the light and dim light portions of the 11:11 hr LDim cycles were similar (compare stage 2 with stage 1). Furthermore, there was no phase-dependent difference in the amount of fluid consumption for both MR and PR groups. Two-way ANOVA showed no interaction in vehicle or melatonin drinking between rearing conditions and time of day (vehicle: P > 0.8 and melatonin: P > 0.99) as well as no interaction in overall liquid consumption between rearing conditions and treatment (P > 0.5; Fig. 6). To assess the effect of melatonin on drinking behavior irrespective of rearing conditions, we compared the amount of fluid consumption across the light and dim light conditions. The results show no effect of melatonin or vehicle on the amount of fluid consumption between light and dim light conditions within stage 1 and stage 2 of the experiment (Fig. 6, Student t-test: vehicle-stage 1, P > 0.7, vehicle-stage 2, P > 0.89 and melatonin-stage 2, P > 0.9). However, when comparing the amount of fluid consumption across experimental stages 1–2, a dual effect can be noticed. For one, chronic jet-lag altered the amount of fluid consumption and second, an effect of melatonin on drinking behavior could be confirmed. Drinking during stage 2 (vehicle) as compared to stage 1 (vehicle) was significantly increased for both the dim light and light periods (Fig. 6, Student t-test: Daytime: vehicle-stage 1 versus vehicle-stage 2, P < 0.02, and Nighttime: vehicle-stage 1 versus vehicle-stage 2, P < 0.005, n = 21 for stage 1 Trial 1 and 2 together and n = 9 for both stage 2, Trail 1 and 2 together). Animals treated during stage 2 with melatonin showed no significant differences in the amount of fluid intake across the light and dim light periods, similar to the average amount of fluid consumption for vehicle-treated individuals during stage 1 (Fig. 6), Student t-test Light condition: vehicle-stage 1 versus melatonin-stage 2, P > 0.9, and Dim light condition: vehicle-stage 1 versus melatonin-stage 2, P > 0.85, n = 21 for stage 1 of Trial 1 and 2 together and n = 11 for stage 2 of Trail 1 and 2 together).
Fig. 6.
Melatonin maintains water consumption at baseline levels. Independent of rearing conditions the amount of liquid consumption containing vehicle for daytime (A) and nighttime (B) drinking during stage 2 (S2) increased significantly in relation to daytime and nighttime drinking for stage 1 (S1; Daytime: Veh-S1 versus Veh-S2, t = 3.07, P < 0.02 and Nighttime: Veh-S1 versus Veh-S2, t = 3.54, P < 0.005). Melatonin during stage 2 for both daytime (A) and nighttime (B) maintained the amount of drinking at baseline levels (stage 1; Daytime: Veh-Stage 1 versus Mel-S2, t = 0.08, P > 0.9 and Nighttime; Veh-S1 versus Mel-S2, t = 0.18, P > 0.85). *P < 0.05, Student t-test. Error bars represent s.e.m. Abbreviations: veh, vehicle; S, stage; Mel, melatonin.
Discussion
This study provides evidence that early-life adversity influences a diurnal primate’s (Macaca mulatta) circadian system, particularly in the circadian responsiveness to the chronobiotic melatonin. To assess circadian rhythm plasticity, we developed a protocol to measure the degree of circadian stability in MR and PR monkeys based on the splitting of the locomotor activity rhythm in response to forced desynchrony. This protocol allows assessment of destabilized or desynchronized circadian responses to the entraining properties of melatonin. Entrainment was measured by the ability of melatonin treatment to reconsolidate split activity rhythms into a single rhythm phase-locked onto the light-onset of the 22 hr T-cycles. Irrespective of an organism’s activity profile, nocturnal [35] or diurnal (this study), melatonin entrains the non-light dependent activity component of free-running rhythms. This entrainment property of melatonin appears in this study as a dynamic change in the period of locomotor activity, from a tau of ~24 hr to an entrained single rhythm with a tau of 22 hr. Indeed, the results show that during forced desynchrony, melatonin facilitates the entrainment to light-onset and food-presentation when analyzed under constant dim light conditions. Furthermore, this effect of melatonin is specifically pronounced in PR animals as compared to MR animals. Importantly, the maintained split locomotor activity rhythms in constant dim light conditions following a 6 wk exposure to 22 hr T-cycles (Fig. 2A,E) confirms, that the light-dependent component is the output of a true oscillator entrained to the 22 hr T-cycles rather than a masking effect of light.
Studies in rodents and primates demonstrated that stress induced by loss of an attachment bond in pigtailed monkeys (M. nemestrina; Kaufman and Rosenblum, 1967) and social defeat in rats can influence the circadian system [36]. Stress-related events can phase-shift overt circadian rhythms of body temperature, heart rate and/or locomotor activity [36, 37]. These investigations among others, clearly demonstrate that a stressor can at least momentarily induce changes in circadian rhythmicity. However, questions to whether early life stress related to maternal separation induces long-lasting physiological changes leading to circadian disturbances following exposure to stressors later in life are just recently being investigated [38].
Psychological trauma resulting from preweaning mother–infant separation in monkeys is long lasting and likely permanent [5]. Thus, it can be regarded as a candidate model to study the effect of stress associated with mother–infant separation on circadian biology at maturity [37, 39–41]. During adulthood, the effects of postnatal maternal separation, and reduced maternal or parental care, become particularly evident upon stress exposure [5, 7, 42–45]. In this study, the forced desynchrony paradigm was designed to expose the animals to a daily 2 hr phase-advance for 6 wk. This repetitive phase-shift of 2 hr simulates chronic jet-lag and consequently represents a stressful experimental paradigm, namely a stressor. Hence, our experimental paradigm aimed to exacerbate existing physiological differences likely to be linked to preweaning mother–infant separation. However, additional studies are required to determine whether maternally deprived individuals, as used in this study, are indeed in a state of depression or stress triggered by the experimental protocol.
During ontogeny, neural wiring and chemistry are influenced by environmental factors. Modifications in brain neurochemistry targeted by the environment are often manifested by a differential response to pharmacological drugs, such as melatonin [44, 46–48]. Interestingly, melatonin mediated phase-shifts of locomotor activity rhythms are similar in both MR and PR individuals (MR: 1.92 ± 0.26 hr versus vehicle 0.13 ± 0.04 hr, n = 4, P < 0.001 and PR: 1.95 ± 0.36 hr versus vehicle 0.02 ± 0.14 hr, n = 3, P < 0.001, unpublished data). The phase-shifts were induced gradually via five consecutive daily oral doses of melatonin (30 μg/kg) at CT10. Hence, the differences reported here between MR and PR individuals in their response to melatonin, under conditions of forced desynchrony, suggests that the responsiveness of the circadian system to melatonin per se may not be affected. That is, at least not within a stressor-free context. Alternatively, stress and/or chronic jet-lag alters the responsiveness of the circadian system to zeitgebers in individuals predisposed for depression. Thus, the likelihood to measure developmental changes in the integrity of the SCN neuronal framework increases with the presence of a stressful event (forced desynchronization; chronic jet-lag). In support of this, it has been suggested that the molecular clock in subjects suffering from mood disorders are incapable to adapt to environmental changes [49, 50]. Similarly, PR monkeys fail to entrain to the 22 hr T-cycles as compared to MR animals [Compare (a) with (c) for light anticipation: Fig. 4, and for food anticipation: Fig. 5].
One could argue that increased drinking during stage 2 of vehicle treated monkeys is the result of an increased activity or distribution of activity due to the split locomotor activity rhythm. This, however, is unlikely since the activity rhythm was also split during stage 1 of the experiment yet fluid consumption was reduced. Alternatively, the increase in water consumption during stage 2 of vehicle-treated animals may reflect the chronic stress associated with the exposure to the forced desynchrony protocol. Indeed, melatonin has been shown to influence different physiological systems including renal functions through activation of melatonin receptors [51, 52]. Furthermore, melatonin mediated alteration in renal functions may involve the modulation of thirst mechanisms [53, 54]. Interestingly, the amount of water consumed by animals treated with melatonin during stage 2 was significantly reduced as compared to water consumption in vehicle-treated animals. Thus, the effect of melatonin observed on drinking behavior during stage 2 could be attributed to a homeostatic effect. Water consumption was similar during both the day and nighttime (Fig. 6). Importantly, the data presented here, suggest that rearing-specific effects of melatonin on the entrainment of locomotor activity onset to the 22 hr T-cycles is unlikely due to rearing differences in total melatonin intake.
In summary, maternal separation in macaques (Macaca mulatta) imprints long-term plastic changes in the circadian system that extends well into late adulthood. We demonstrated a differential effect of melatonin on the entrainment to T-cycles between MR and PR individuals with a significant increased response to melatonin-mediated entrainment in preweaned mother–infant separated macaques (PR). Together these results suggest that maternal separation imposes long-term changes on the plasticity of the circadian system. The effects of melatonin on anticipatory activity for food and light onset/offset are not due to its sleep promoting properties as food presentation and anticipatory locomotor activity was always measured prior to treatments and thus is unlikely to be a consequence or a contributing factor.
The increased responsiveness of PR individuals to melatonin facilitating adaptation to the imposed lighting conditions and feeding schedules strongly encourages further research for the use of a melatonin treatment protocol in patients vulnerable to depression due to early life adversity. We suggest that melatonin may act as an ameliorant to the negative impact of stress on the circadian system.
Acknowledgments
We thank Dr. Steven Dubovsky (Department of Psychiatry, University at Buffalo) for critical comments on the manuscript, and Iwona Stepien and Aaron Lambert for technical assistance. We are in debt to Dr. Diana P. Berger and all veterinary staff on site for their corporation with respect to periodic animal health assessment and insightful suggestions and knowledge about macaque behavior. All experiments were conducted at Northwestern University, Center for Comparative Medicine, and approved by the University’s Animal Care and Use Committee. This study was supported by MH 63466 to MLD.
Footnotes
Author contributions
Both authors (OR and MLD) designed, analyzed and interpreted the data. OR and MLD wrote the manuscript.
References
- 1.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- 2.Heim C, Newport DJ, Bonsall R, et al. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am J Psychiatry. 2001;158:575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- 3.Kaufman J, Charney D. Effects of early stress on brain structure and function: implications for understanding the relationship between child maltreatment and depression. Dev Psychopathol. 2001;13:451–471. doi: 10.1017/s0954579401003030. [DOI] [PubMed] [Google Scholar]
- 4.Kaufman J, Plotsky PM, Nemeroff CB, et al. Effects of early adverse experiences on brain structure and function: clinical implications. Biol Psychiatry. 2000;48:778–790. doi: 10.1016/s0006-3223(00)00998-7. [DOI] [PubMed] [Google Scholar]
- 5.Mathew SJ, Shungu DC, Mao X, et al. A magnetic resonance spectroscopic imaging study of adult nonhuman primates exposed to early-life stressors. Biol Psychiatry. 2003;54:727–735. doi: 10.1016/s0006-3223(03)00004-0. [DOI] [PubMed] [Google Scholar]
- 6.Spinelli S, Chefer S, Suomi SJ, et al. Early-life stress induces long-term morphologic changes in primate brain. Arch Gen Psychiatry. 2009;66:658–665. doi: 10.1001/archgenpsychiatry.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bifulco AT, Brown GW, Harris TO. Childhood loss of parent, lack of adequate parental care and adult depression: a replication. J Affect Disord. 1987;12:115–128. doi: 10.1016/0165-0327(87)90003-6. [DOI] [PubMed] [Google Scholar]
- 8.Harris T, Brown GW, Bifulco A. Loss of parent in childhood and adult psychiatric disorder: the role of social class position and premarital pregnancy. Psychol Med. 1987;17:163–183. doi: 10.1017/s0033291700013064. [DOI] [PubMed] [Google Scholar]
- 9.Seckl JR, Meaney MJ. Glucocorticoid programming. Ann N Y Acad Sci. 2004;1032:63–84. doi: 10.1196/annals.1314.006. [DOI] [PubMed] [Google Scholar]
- 10.Weaver IC, Diorio J, Seckl JR, et al. Early environmental regulation of hippocampal glucocorticoid receptor gene expression: characterization of intracellular mediators and potential genomic target sites. Ann N Y Acad Sci. 2004;1024:182–212. doi: 10.1196/annals.1321.099. [DOI] [PubMed] [Google Scholar]
- 11.Girotti M, Weinberg MS, Spencer RL. Diurnal expression of functional and clock-related genes throughout the rat HPA axis: system-wide shifts in response to a restricted feeding schedule. Am J Physiol Endocrinol Metab. 2009;296:E888–E897. doi: 10.1152/ajpendo.90946.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Couto-Moraes R, Palermo-Neto J, Markus RP. The immune-pineal axis: stress as a modulator of pineal gland function. Ann N Y Acad Sci. 2009;1153:193–202. doi: 10.1111/j.1749-6632.2008.03978.x. [DOI] [PubMed] [Google Scholar]
- 13.Crupi R, Mazzon E, Marino A, et al. Melatonin treatment mimics the antidepressant action in chronic corticosterone-treated mice. J Pineal Res. 2010;49:123–129. doi: 10.1111/j.1600-079X.2010.00775.x. [DOI] [PubMed] [Google Scholar]
- 14.Klein DC. Photoneural regulation of the mammalian pineal gland. Ciba Found Symp. 1985;117:38–56. doi: 10.1002/9780470720981.ch4. [DOI] [PubMed] [Google Scholar]
- 15.Bothorel B, Barassin S, Saboureau M, et al. In the rat, exogenous melatonin increases the amplitude of pineal melatonin secretion by a direct action on the circadian clock. Eur J Neurosci. 2002;16:1090–1098. doi: 10.1046/j.1460-9568.2002.02176.x. [DOI] [PubMed] [Google Scholar]
- 16.Stehle J, Vanecek J, Vollrath L. Effects of melatonin on spontaneous electrical activity of neurons in rat suprachiasmatic nuclei: an in vitro iontophoretic study. J Neural Transm. 1989;78:173–177. doi: 10.1007/BF01252503. [DOI] [PubMed] [Google Scholar]
- 17.Arendt J. Melatonin and the pineal gland: influence on mammalian seasonal and circadian physiology. Rev Reprod. 1998;3:13–22. doi: 10.1530/ror.0.0030013. [DOI] [PubMed] [Google Scholar]
- 18.Tamarkin L, Baird CJ, Almeida OF. Melatonin: a coordinating signal for mammalian reproduction? Science. 1985;227:714–720. doi: 10.1126/science.3881822. [DOI] [PubMed] [Google Scholar]
- 19.Torres-Farfan C, Richter HG, Rojas-Garcia P, et al. mt1 Melatonin receptor in the primate adrenal gland: inhibition of adrenocorticotropin-stimulated cortisol production by melatonin. J Clin Endocrinol Metab. 2003;88:450–458. doi: 10.1210/jc.2002-021048. [DOI] [PubMed] [Google Scholar]
- 20.Valenzuela FJ, Torres-Farfan C, Richter HG, et al. Clock gene expression in adult primate suprachiasmatic nuclei and adrenal: is the adrenal a peripheral clock responsive to melatonin? Endocrinology. 2008;149:1454–1461. doi: 10.1210/en.2007-1518. [DOI] [PubMed] [Google Scholar]
- 21.Kellner M, Yassouridis A, Manz B, et al. Corticotropin-releasing hormone inhibits melatonin secretion in healthy volunteers–a potential link to low-melatonin syndrome in depression? Neuroendocrinology. 1997;65:284–290. doi: 10.1159/000127186. [DOI] [PubMed] [Google Scholar]
- 22.Wirz-Justice A. Biological rhythm disturbances in mood disorders. Int Clin Psychopharmacol. 2006;21(Suppl 1):S11–S15. doi: 10.1097/01.yic.0000195660.37267.cf. [DOI] [PubMed] [Google Scholar]
- 23.Aoyama H, Mori N, Mori W. Anti-glucocorticoid effects of melatonin on adult rats. Acta Pathol Jpn. 1987;37:1143–1148. doi: 10.1111/j.1440-1827.1987.tb00431.x. [DOI] [PubMed] [Google Scholar]
- 24.Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev. 1991;12:118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- 25.Pierpaoli W, Maestroni GJ. Melatonin: a principal neuroimmunoregulatory and anti-stress hormone: its anti-aging effects. Immunol Lett. 1987;16:355–361. doi: 10.1016/0165-2478(87)90169-6. [DOI] [PubMed] [Google Scholar]
- 26.Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res. 1986;396:64–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- 27.Sapolsky RM, Plotsky PM. Hypercortisolism and its possible neural bases. Biol Psychiatry. 1990;27:937–952. doi: 10.1016/0006-3223(90)90032-w. [DOI] [PubMed] [Google Scholar]
- 28.Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev Psychopathol. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- 29.Sanchez MM, Noble PM, Lyon CK, et al. Alterations in diurnal cortisol rhythm and acoustic startle response in non-human primates with adverse rearing. Biol Psychiatry. 2005;57:373–381. doi: 10.1016/j.biopsych.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 30.Carpentieri AR, Pujolras MA, Chiesa JJ, et al. Effect of melatonin and diazepam on the dissociated circadian rhythm in rats. J Pineal Res. 2006;40:318–325. doi: 10.1111/j.1600-079X.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- 31.Clarke AS, Ebert MH, Schmidt DE, et al. Biogenic amine activity in response to fluoxetine and desipramine in differentially reared rhesus monkeys. Biol Psychiatry. 1999;46:221–228. doi: 10.1016/s0006-3223(99)00027-x. [DOI] [PubMed] [Google Scholar]
- 32.Kraemer GW, Ebert MH, Schmidt DE, et al. Strangers in a strange land: a psychobiological study of infant monkeys before and after separation from real or inanimate mothers. Child Dev. 1991;62:548–566. [PubMed] [Google Scholar]
- 33.Zhdanova IV, Geiger DA, Schwagerl AL, et al. Melatonin promotes sleep in three species of diurnal nonhuman primates. Physiol Behav. 2002;75:523–529. doi: 10.1016/s0031-9384(02)00654-6. [DOI] [PubMed] [Google Scholar]
- 34.Sokolove PG, Bushell WN. The chi square periodogram: its utility for analysis of circadian rhythms. J Theor Biol. 1978;72:131–160. doi: 10.1016/0022-5193(78)90022-x. [DOI] [PubMed] [Google Scholar]
- 35.Redman J, Armstrong S, Ng KT. Free-running activity rhythms in the rat: entrainment by melatonin. Science. 1983;219:1089–1091. doi: 10.1126/science.6823571. [DOI] [PubMed] [Google Scholar]
- 36.Meerlo P, de Boer SF, Koolhaas JM, et al. Changes in daily rhythms of body temperature and activity after a single social defeat in rats. Physiol Behav. 1996;59:735–739. doi: 10.1016/0031-9384(95)02182-5. [DOI] [PubMed] [Google Scholar]
- 37.Reite M, Short R, Seiler C, et al. Attachment, loss, and depression. J Child Psychol Psychiatry. 1981;22:141–169. doi: 10.1111/j.1469-7610.1981.tb00539.x. [DOI] [PubMed] [Google Scholar]
- 38.Engert V, Buss C, Khalili-Mahani N, et al. Investigating the association between early life parental care and stress responsivity in adulthood. Dev Neuropsychol. 2010;35:570–581. doi: 10.1080/87565641.2010.494752. [DOI] [PubMed] [Google Scholar]
- 39.Kaufman IC, Rosenblum LA. Depression in infant monkeys separated from their mothers. Science. 1967;155:1030–1031. doi: 10.1126/science.155.3765.1030. [DOI] [PubMed] [Google Scholar]
- 40.Laudenslager M, Capitanio JP, Reite M. Possible effects of early separation experiences on subsequent immune function in adult macaque monkeys. Am J Psychiatry. 1985;142:862–864. doi: 10.1176/ajp.142.7.862. [DOI] [PubMed] [Google Scholar]
- 41.Laudenslager ML, Boccia ML, Berger CL, et al. Total cortisol, free cortisol, and growth hormone associated with brief social separation experiences in young macaques. Dev Psychobiol. 1995;28:199–211. doi: 10.1002/dev.420280402. [DOI] [PubMed] [Google Scholar]
- 42.Andrews B, Brown GW, Creasey L. Intergenerational links between psychiatric disorder in mothers and daughters: the role of parenting experiences. J Child Psychol Psychiatry. 1990;31:1115–1129. doi: 10.1111/j.1469-7610.1990.tb00850.x. [DOI] [PubMed] [Google Scholar]
- 43.Harris TO, Brown GW, Bifulco AT. Depression and situational helplessness/mastery in a sample selected to study childhood parental loss. J Affect Disord. 1990;20:27–41. doi: 10.1016/0165-0327(90)90047-c. [DOI] [PubMed] [Google Scholar]
- 44.Kraemer GW, Clarke AS. The behavioral neurobiology of self-injurious behavior in rhesus monkeys. Prog Neuropsychopharmacol Biol Psychiatry. 1990;14(Suppl):S141–S168. doi: 10.1016/0278-5846(90)90092-u. [DOI] [PubMed] [Google Scholar]
- 45.Kraemer GW, Ebert MH, Schmidt DE, et al. A longitudinal study of the effect of different social rearing conditions on cerebrospinal fluid norepinephrine and biogenic amine metabolites in rhesus monkeys. Neuropsychopharmacology. 1989;2:175–189. doi: 10.1016/0893-133x(89)90021-3. [DOI] [PubMed] [Google Scholar]
- 46.Clarke AS, Kraemer GW, Kupfer DJ. Effects of rearing condition on HPA axis response to fluoxetine and desipramine treatment over repeated social separations in young rhesus monkeys. Psychiatry Res. 1998;79:91–104. doi: 10.1016/s0165-1781(98)00032-8. [DOI] [PubMed] [Google Scholar]
- 47.Insel TR, Scanlan J, Champoux M, et al. Rearing paradigm in a nonhuman primate affects response to beta-CCE challenge. Psychopharmacology. 1988;96:81–86. doi: 10.1007/BF02431537. [DOI] [PubMed] [Google Scholar]
- 48.Rosenblum LA, Andrews MW. Influences of environmental demand on maternal behavior and infant development. Acta Paediatr Suppl. 1994;397:57–63. doi: 10.1111/j.1651-2227.1994.tb13266.x. [DOI] [PubMed] [Google Scholar]
- 49.Grandin LD, Alloy LB, Abramson LY. The social zeitgeber theory, circadian rhythms, and mood disorders: review and evaluation. Clin Psychol Rev. 2006;26:679–694. doi: 10.1016/j.cpr.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 50.McClung CA. Circadian genes, rhythms and the biology of mood disorders. Pharmacol Ther. 2007;114:222–232. doi: 10.1016/j.pharmthera.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song Y, Chan CW, Brown GM, et al. Studies of the renal action of melatonin: evidence that the effects are mediated by 37 kDa receptors of the Mel1a subtype localized primarily to the basolateral membrane of the proximal tubule. FASEB J. 1997;11:93–100. doi: 10.1096/fasebj.11.1.9034171. [DOI] [PubMed] [Google Scholar]
- 52.Song Y, Pang CS, Ayre EA, et al. Melatonin receptors in the chicken kidney are up-regulated by pinealectomy and linked to adenylate cyclase. Eur J Endocrinol. 1996;135:128–133. doi: 10.1530/eje.0.1350128. [DOI] [PubMed] [Google Scholar]
- 53.Nava F, Calapai G, Facciola G, et al. Melatonin effects on inhibition of thirst and fever induced by lipopolysaccharide in rat. Eur J Pharmacol. 1997;331:267–274. doi: 10.1016/s0014-2999(97)01049-2. [DOI] [PubMed] [Google Scholar]
- 54.Richardson BA, Studier EH, Stallone JN, et al. Effects of melatonin on water metabolism and renal function in male Syrian hamsters (Mesocricetus auratus) J Pineal Res. 1992;13:49–59. doi: 10.1111/j.1600-079x.1992.tb00054.x. [DOI] [PubMed] [Google Scholar]