Abstract
A persistently positive positron emission tomography (PET) scan during therapy for diffuse large B-cell lymphoma (DLBCL) is predictive of treatment failure. A response-adapted strategy consisting of an early treatment change to four cycles of R-ICE (rituximab, ifosfamide, carboplatin, etoposide) was studied in the Eastern Cooperative Oncology Group E3404 trial. Previously untreated patients with DLBCL stage III, IV, or bulky II, were eligible. PET scan was performed after 3 cycles of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone) and scored as positive or negative by central review during the fourth cycle. PET-positive patients received 4 cycles of R-ICE, PET-negative patients received 2 more cycles of R-CHOP. A ≥45% 2-year progression-free survival (PFS) for mid-treatment PET-positive patients was viewed as promising. Of 74 patients, 16% were PET positive, 79% negative. The PET positivity rate was much lower than the 33% expected. Two-year PFS was 70%; 42% (90% confidence interval [CI], 19-63%) for PET-positives and 76% (90% CI 65-84%) for PET-negatives. Three-year overall survival (OS) was 69% (90% CI 43-85%) and 93% (90% CI 86-97%) for PET-positive and –negative cases, respectively. The 2-year PFS for mid-treatment PET-positive patients intensified to R-ICE was 42%, with a wide confidence interval due to the low proportion of positive mid-treatment PET scans. Treatment modification based on early PET scanning should remain confined to clinical trials.
Keywords: Diffuse Large B-Cell Lymphoma, Interim PET Scan, R-CHOP, R-ICE, Response-Adapted Therapy
Introduction
Only a proportion of patients with diffuse large B-cell lymphoma (DLBCL) will be cured with the currently standard R-CHOP regimen (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone) as first-line therapy (Coiffier et al, 2002;Coiffier, 2003;Habermann et al, 2003). Although many trials of early dose intensification strategies have been conducted, results have been variable and it has been difficult to perceive any overall benefit. However, it has been a recurring observation that any benefit from early treatment modification and intensification applies mainly to the prognostic groups at highest risk for relapse (Haioun et al, 2000;Milpied et al, 2004;Stiff et al, 2013). Several dose-intensification strategies, with or without autologous stem cell transplant, have been studied (Pfreundschuh et al, 2004;Haioun et al, 2000;Milpied et al, 2004). The most established approach to DLBCL patients who have relapsed after initial therapy has been salvage chemotherapy with regimens that differ significantly from the initial induction regimen. Platinum-containing combinations, such as DHAP (dexamethasone, cytarabine, cisplatin), ESHAP (etoposide, methylprednisolone, cytarabine, cisplatin) and ICE (ifosfamide, carboplatin, etoposide), have been shown capable of inducing a second remission. With the addition of rituximab to the ICE regimen (R-ICE), a 53% second complete remission rate has been reported in such patients (Kewalramani et al, 2004). Long-term disease-free survival without additional therapy has been described for relapsed patients treated with ESHAP (Velasquez et al, 1994). Although such salvage regimens are principally used as a prelude to high-dose therapy and stem cell rescue, their activity against relapsed disease makes them attractive for incorporation into the initial treatment regimen. A more individually accurate prognostic indicator than the International Prognostic Index (IPI) would allow the selection of high-risk patients for treatment modification, while reducing the likelihood of exposing those patients who would have done well with R-CHOP to unnecessary toxicities.
Early studies of positron emission tomography (PET) scanning after just a few cycles of therapy suggested that such imaging was a sensitive marker of the rate of tumour response and a strong indicator of ultimate outcome, and has been found to be prognostically significant across IPI subsets (Spaepen et al, 2002;Kostakoglu et al, 2002;Jerusalem et al, 1999;Juweid et al, 2002;Haioun et al, 2003;Spaepen et al, 2001). The current study was undertaken with the hypothesis that the results of a mid-treatment PET scan, dichotomized as negative or positive, could be used to select patients at high-risk of relapse for response-adapted treatment modification. Based on the existing data at the time, it was estimated that the PET scan remains positive after 2 to 4 cycles of therapy in approximately one third of patients with DLBCL, and that 2-year progression-free survival (PFS) for such patients was 25% or less. Studies prior to 2006 generally did not use standardized interpretation criteria. Although early introduction of an alternative treatment has often implied high-dose therapy and autologous stem cell transplantation, a more practical, less toxic, and as yet little-explored approach would be early conversion to a regimen typically used as salvage therapy for relapsed disease. R-ICE was selected as a very active salvage regimen with a favourable non-haematological toxicity profile. It was hypothesized that early introduction of an alternative treatment with four cycles of R-ICE would reduce the relapse rate of patients with a persistently positive PET scan after an initial 3 cycles of R-CHOP.
Methods
Study Design
Informed consent was required. A pre-treatment PET scan was performed as part of the initial assessment. All patients then received 4 cycles of R-CHOP chemotherapy, consisting of rituximab 375 mg/m2 iv, cyclophosphamide 750 mg/m2 iv, doxorubicin 50 mg/m2 iv, vincristine 1.4 mg/m2 iv (max 2 mg), all on day 1, and prednisone 100 mg/m2po days 1 to 5 of a 21-day cycle. The mid-treatment PET scan was performed during cycle 3 between days 11 and 20. Treatment after cycle 4 was based on the result of the central review of the mid-treatment PET scan, which was available prior to the start of cycle 5. Patients whose PET scan was interpreted as negative received two more cycles of R-CHOP for a total of 6 cycles.
Patients whose PET scan was interpreted as positive received no further R-CHOP, but received 4 cycles of R-ICE instead, for a total of 8 cycles of treatment (4 cycles R-CHOP plus 4 cycles R-ICE). R-ICE consisted of rituximab 375 mg/m2 iv day 1, ifosfamide 5000 mg/m2 iv over 24 h day 2, carboplatin calculated on a target area under the concentration versus time curve of 5 mg/ml/min (max 800 mg) iv day 2, etoposide 100 mg/m2 iv days 1-3, MESNA 5000 mg/m2 iv over 24 h day 2 and filgrastim 5 μg/kg/day sc day 4 until neutrophil recovery. PET/computerized tomography (CT) was performed again at the end of treatment. Follow up visits and CT imaging were performed every 4 months for the first 2 years, then 6 monthly to 5 years, and yearly without routine imaging after that.
Eligibility criteria
Previously untreated patients between the ages of 18 and 70 years with DLBCL stage III, IV or bulky stage II (bulky defined as any lesion ≥ 10 cm) were eligible. A histological diagnosis with central pathology review and measurable disease defined by CT imaging were required. Other eligibility criteria included adequate organ reserve, defined as absolute neutrophil count ≥ 1.5 × 109/l, platelet count > 100 × 109/l, creatinine < 176.8 μmol/l, total bilirubin < 34.2 μmo/l (≤ 51.3 μmol/l if due to liver involvement), documentation of left ventricular ejection fraction > 50% in patients over 50 years of age and Eastern Cooperative Oncology Group (ECOG) Performance Status ≤ 3. Exclusion criteria included positivity for human immunodeficiency virus, pregnancy or breastfeeding, primary large B-cell mediastinal lymphoma, primary central nervous system lymphoma, primary testicular diffuse large B-cell lymphoma, immunodeficiency-related lymphomas and prior malignancy, unless in situ or treated with curative intent at least 5 years prior to enrollment.
PET scan interpretation
Submission of all PET scans for central review was required. The pre-treatment and mid-treatment PET scans were reviewed and a positive or negative interpretation was assigned to the mid-treatment scan by the single central reviewer. This interpretation took place during the fourth cycle of R-CHOP chemotherapy, with scoring as outlined in Table I. Specifically, the protocol specified a binary visual interpretation, which the central reviewer based on modifications of the International Harmonization Project (Juweid et al, 2007), customized for ECOG E3404 interim scans and deemed the “ECOG criteria”: (1) only sites of abnormality at baseline were evaluated; (2) abnormal activity required both a focal appearance and intensity greater than average liver; (3) all positive nodal sites had to have an anatomic correlate; (4) activity in bone marrow and spleen was considered abnormal only if focal and clearly discernible; (5) symmetric abnormal foci in the mediastinum and hilum were considered abnormal only if the remainder of the scan was positive; and (6) new foci were considered positive only if the remainder of the scan was positive or a new lesion was focal, very intense and associated with a lesion on CT. All of the mid-treatment PET scans were centrally reviewed. Institutional results were used for 6 of 70 end-of-treatment PET scans because images could not be acquired for central review.
Table I. Scoring system for interpretation of the mid-treatment PET scan.
| NEGATIVE | |
| 0 | no abnormal activity (tumour cold compared with background) |
| 1+ | minimal activity (tumour less than surrounding tissue) |
| 2+ | equivocal (tumour = surrounding tissue) |
| POSITIVE | |
| 3+ | moderate activity (focal and tumour > liver) |
| 4+ | strong activity (focal and tumour ≫ liver) |
Statistical methods
The primary endpoint was the 2-year PFS for patients who remained PET-positive after 3 cycles of R-CHOP and were then treated with 4 cycles of R-ICE. PFS was defined as time from study entry to disease progression or death, whichever came first. The non-promising 2-year PFS rate was considered to be 25% and the promising rate 45%. A one-stage design with 33 PET-positive eligible patients was planned, in order to have 88% power to detect a difference in 2-year PFS of 25% vs. 45%, with one-sided alpha of 0.1. The 80% confidence interval (CI) of 2-year PFS that corresponded to a test with a one-sided alpha of 0.1 was calculated. The treatment would be considered promising if the 80% CI excluded the non-promising rate of 25%. It was expected that 33% of patients would be PET-positive after 3 cycles of R-CHOP, so the planned total accrual was 99 patients. Overall survival (OS) was a secondary endpoint, and was defined as the time from study entry until death from any cause. The PFS and OS in subgroups of patients who were PET-positive and PET-negative at mid-treatment PET scan were calculated from the time of mid-treatment PET and from study entry (separate analysis). As post-hoc analyses, the negative predictive value (NPV) of the mid-treatment PET scan and the concordance of responses, as determined by CT alone, with PET scan was also evaluated. NPV of the PET scan was defined as the proportion of patients with a negative scan result who remained progression-free at 2 years.
CT response was assessed using The International Working Group response criteria (Cheson et al, 1999), which are based on CT only. Exact binomial confidence intervals were used to describe response rates. All confidence intervals reported are two-sided. Toxicities were assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. The method of Kaplan and Meier (Kaplan & Meier, 1958) was used to characterize PFS and OS. The Fisher-Freeman-Halton test was used to compare the NPV of PET scan among different IPI risk groups. Mid-treatment response, PFS and OS for the entire cohort were analyzed based on all eligible and treated patients (n=80). End-of-treatment response, PFS and OS, based on mid-treatment PET results, were analyzed based on eligible patients proceeding to further therapy after the fourth cycle of R-CHOP (n=74). All analyses were done with SAS 9.2 (SAS Institute Inc., Cary, NC, USA). Figures were drawn using R 2.11.1 (http://www.r-project.org/).
Results
Patient characteristics
The study was activated on 7 April 2006 and was terminated on 5 August 2009. Final accrual was 100 patients. Data as of 19 June 2013 are reported, with median follow-up of 4.6 years. Tissue was submitted for central pathology review for 80 patients (80%), of whom 66 were confirmed to be DLBCL. Institutional pathology reports were reviewed centrally for the 20 cases for whom no or inadequate tissue had been submitted; in 15 of these cases it was concluded that the diagnosis was indeed most probably DLBCL and those cases were considered to be eligible. One patient, although DLBCL on subsequent central review, did not start protocol treatment because of institutional uncertainty over the diagnosis and was excluded from the analysis. A total of 80 patients were classified as eligible and treated, and are included in the analysis.
Table II shows patient characteristics at the time of study registration. Of the 80 eligible and treated patients, 58% were male, 91% were white and the median age was 62 (range, 20-74) years. Most patients had stage III/IV disease (90%). IPI distribution was: 14% low risk (0-1), 31% low/intermediate risk (2), 36% intermediate/high risk (3) and 19% high risk (4-5).
Table II. Patient characteristics.
| N | % | |
|---|---|---|
| Number of eligible and treated patients included in primary analysis | 80 | |
| Age, years | ||
| Median (Range) | 62 (20-74) | |
| Gender | ||
| Male | 46 | 58 |
| Female | 34 | 43 |
| Race | ||
| White | 73 | 91 |
| Black | 2 | 3 |
| Orient | 3 | 4 |
| Institution refusal/unknown | 2 | 3 |
| National Cancer Institute Ethnicity | ||
| Hispanic or Latino | 4 | 5 |
| Non-hispanic | 68 | 85 |
| Institution refusal/unknown | 8 | 10 |
| Modified Ann Arbor Stage at Study Entry | ||
| II/IIE | 8 | 10 |
| III/IIIE/IIIS | 30 | 38 |
| IV | 42 | 53 |
| Eastern Cooperative Oncology Group Performance Status | ||
| 0 | 42 | 53 |
| 1 | 29 | 36 |
| 2 | 8 | 10 |
| 3 | 1 | 1 |
| Extra-Nodal Sites (n) | ||
| 0 | 26 | 33 |
| 1 | 21 | 26 |
| 2 | 17 | 21 |
| >2 | 16 | 20 |
| Bone Marrow Involvement | ||
| Yes | 13 | 16 |
| No | 67 | 84 |
| B Symptom | ||
| Yes | 23 | 29 |
| No | 57 | 71 |
| Any Lymph Node or Aggregate with Diameter > 10cm? | ||
| Yes | 26 | 33 |
| No | 51 | 64 |
| Unknown | 3 | 4 |
| International Prognostic Index (IPI) | ||
| Low risk (0-1) | 11 | 14 |
| Low/intermediate (2) | 25 | 31 |
| Intermediate/high risk (3) | 29 | 36 |
| High risk (≥4) | 15 | 19 |
Treatment compliance
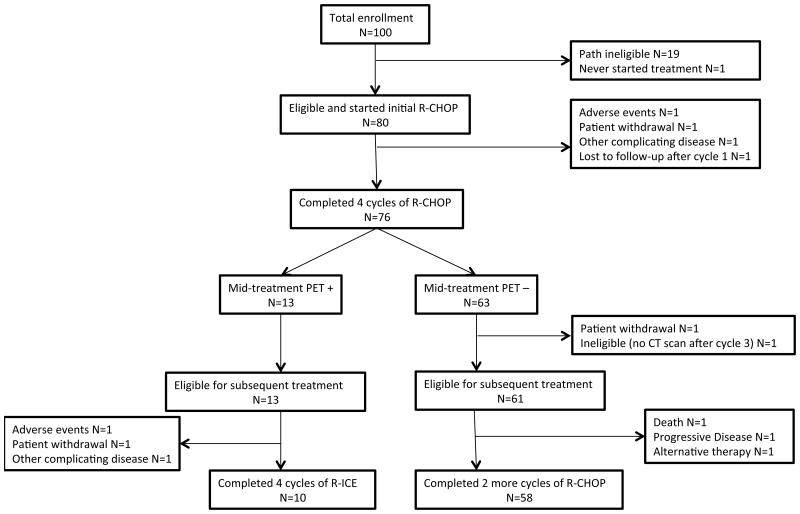
Among 80 eligible and treated patients, 76 (95%) completed 3 cycles of R-CHOP, and had a mid-treatment PET scan that was centrally reviewed. Figure 1 shows patient flow and the reasons for going off-treatment. Among the 74 eligible patients proceeding to treatment beyond cycle 4 of R-CHOP, 68 (92%) completed protocol treatment. This included 10 (77%) patients assigned to R-ICE and 58 (95%) patients assigned to further R-CHOP. Three patients assigned to R-ICE went off treatment due to adverse events, patient withdrawal and other complicating disease, respectively. Three patients assigned to further R-CHOP did not complete treatment due to disease progression, death on study and alternative therapy, respectively.
Figure 1. Patient Flow Chart.
CT, computerized tomography; PET+,; PET-, negative result on positron emission tomography; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; R-ICE, rituximab, ifosfamide, carboplatin, etoposide.
Response
Mid-Treatment
The mid-treatment PET scan was available for central review for 76 of the 80 (95%) eligible patients and was scored as negative in 63 (79%) and positive in 13 (16%). The mid-treatment PET positivity rate was only approximately half of what had been expected, and of what the statistical design required. Mid-treatment response was also determined by CT alone. Among the 80 eligible patients there were 21 (26%) complete responses (CR), 20 (25%) unconfirmed CR (CRu), 30 (38%) partial response (PR) and 2 (3%) stable disease (SD). Seven patients (9%) were inevaluable for response because of: early treatment discontinuation (4); no CT scan (1); insufficient measurements on CT scan (2). The CR+CRu rate was 51% (41/80; 90% CI, 41-61%). Overall response rate (ORR) was 89% (71/80; 90% CI, 81-94%). Table IIIa shows the mid-treatment response by CT criteria and the mid-treatment PET result.
Table IIIa.
Mid-Treatment Response by CT and PET scan
| PET scan | ||||
|---|---|---|---|---|
| Response by CT only | Not done | Positive | Negative | Total |
| Complete Response | 0 | 0 | 21 | 21 (26%) |
| Complete Response uncertain | 0 | 7 | 13 | 20 (25%) |
| Partial Response | 0 | 6 | 24 | 30 (38%) |
| Stable Disease | 0 | 0 | 2 | 2 (3%) |
| Inevaluable | 4 | 0 | 3 | 7 (9%) |
|
| ||||
| Total | 4 (5%) | 13 (16%) | 63 (79%) | 80 (100%) |
End of Treatment
Seventy-four patients were eligible for subsequent treatment following initial R-CHOP. End-of-treatment PET scan was done in 70 (95%) patients, and submitted for central review for 64 (86%) patients. For those whose scan was done but not submitted for central review, institutional results were used for the analysis. Results are summarized in Table IIIb. End-of-treatment PET scan was positive in 10 (14%), negative in 60 (81%) and not done in 4 (5%). Of the 13 mid-treatment PET-positive patients, 4 remained PET-positive at the end of treatment. Of the 61 mid-treatment PET-negative patients, 6 had become PET-positive at the end of treatment. When assessed by CT criteria only, there were 48 (65%) CR, 13 (18%) CRu, 5 (7%) PR, 7 (9%) PD, and 1 inevaluable for response. CR+CRu rate was 82% (61/74; 90% CI, 74-89%), ORR was 89% (66/74; 90% CI, 81-95%).
Table IIIb.
End-of-Treatment Response by CT and PET scan
| PET scan | ||||
|---|---|---|---|---|
| Response by CT only | Not done | Positive | Negative | Total |
| Complete Response | 2 | 3 | 43 | 48 (65%) |
| Complete Response uncertain | 0 | 2 | 11 | 13 (18%) |
| Partial Response | 1 | 0 | 4 | 5 (7%) |
| Progressive Disease | 0 | 5 | 2 | 7 (9%) |
| Inevaluable | 1 | 0 | 0 | 1 (1%) |
|
| ||||
| Total | 4 (5%) | 10 (14%) | 60 (81%) | 74 (100%) |
CT, computerized tomography; PET, positron emission tomography
Survival
Progression-Free Survival
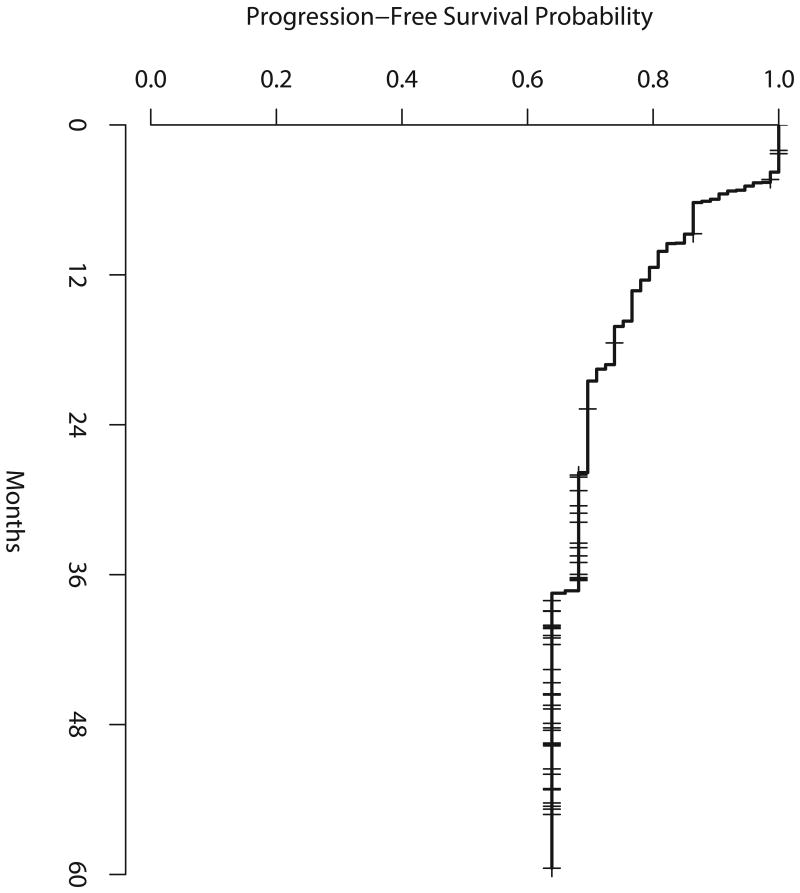
Figure 2a shows PFS from study entry for all eligible and treated patients. Median PFS has not been reached. Two-year PFS was 70% (90% CI, 60-78%), 3-year PFS was 69% (59-77%) and 4-year PFS was 64% (90% CI, 53-73%). Among the 13 patients who were scored as mid-treatment PET-positive, 2-year PFS from study entry was 42% (80% CI, 24-59%). The 80% CI included the null hypothesis of 25%, implying that the results did not meet the pre-specified criteria for concluding that the study regimen was promising, realizing that only 13 mid-treatment PET-positive patients were accrued, not the 33 required for this analysis.
Figure 2a. Progression-free survival for all eligible and treated patients.
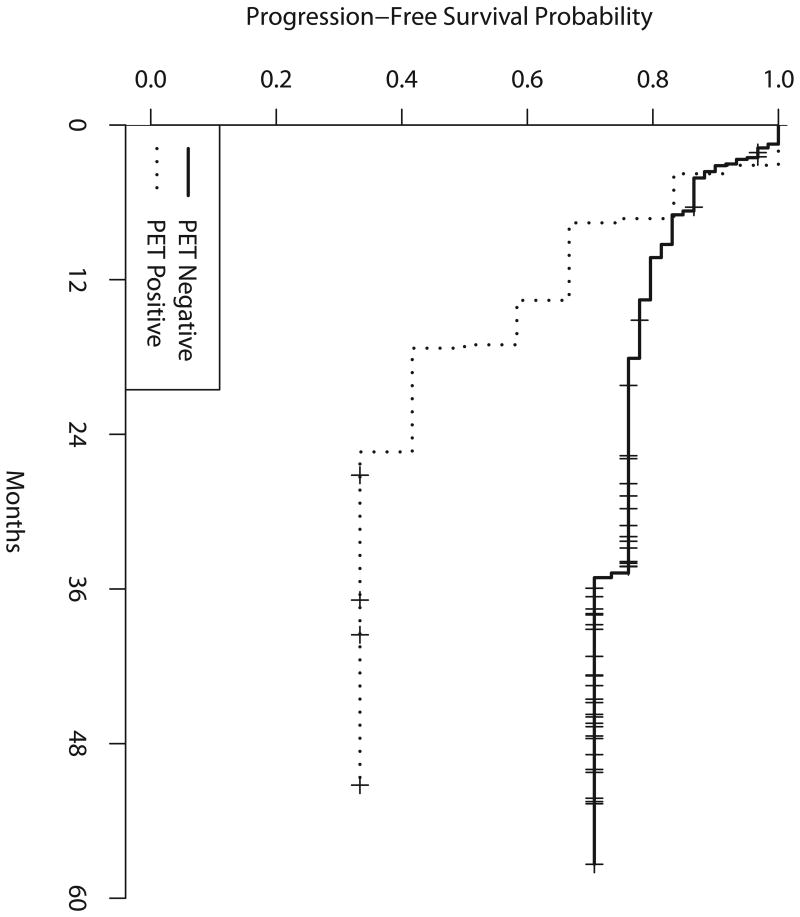
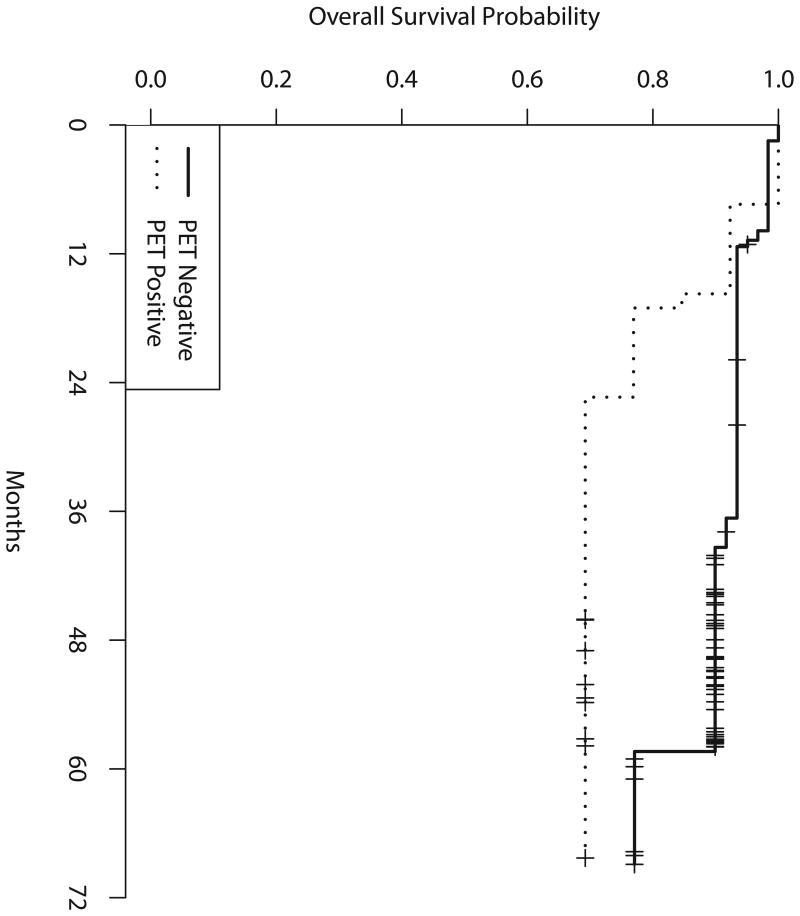
Figure 2b shows PFS from the time of the mid-treatment PET scan, according to mid-treatment PET results. Median PFS was 17 months for PET-positive patients, from the time of mid-treatment PET (19 months from study entry), and has not been reached for PET-negative patients. Two-year PFS was 42% (90% CI, 19-63%) for PET-positive patients and 76% (90% CI, 65-84%) for PET-negative patients. Table IV shows the 2-, 3- and 4-year PFS for all eligible and treated patients as well as by group according to mid-treatment PET results. Among the 7 patients who were mid-treatment PET-positive and converted to end-of-treatment PET-negative, 3 progressed within 2 years and 4 remained in remission at 2 years.
Figure 2b. Progression-free survival by mid-treatment positron emission tomography (PET) scan (from time of mid-treatment PET scan).
Table IV.
Survival Probabilities by Mid-Treatment PET
| Mid-Treatment PET-positive (n=13) |
Mid-Treatment PET negative (n=61) |
|
|---|---|---|
| PFS* (90% CI) | ||
| 2-year | 0.42 (0.19-0.63) | 0.76 (0.65-0.84) |
| 3-year | 0.33 (0.13-0.55) | 0.71 (0.59-0.80) |
| 4-year | 0.33 (0.13-0.55) | 0.71 (0.59-0.80) |
| OS* (90% CI) | ||
| 2-year | 0.77 (0.51-0.90) | 0.93 (0.86-0.97) |
| 3-year | 0.69 (0.43-0.85) | 0.93 (0.86-0.97) |
| 4-year | 0.69 (0.43-0.85) | 0.90 (0.81-0.95) |
PFS and OS were calculated by group from the time of the mid-treatment PET scan.
PET, positron emission tomography; PFS, progression-free survival; OS, overall survival; 90% CI, 90% confidence interval.
Overall Survival
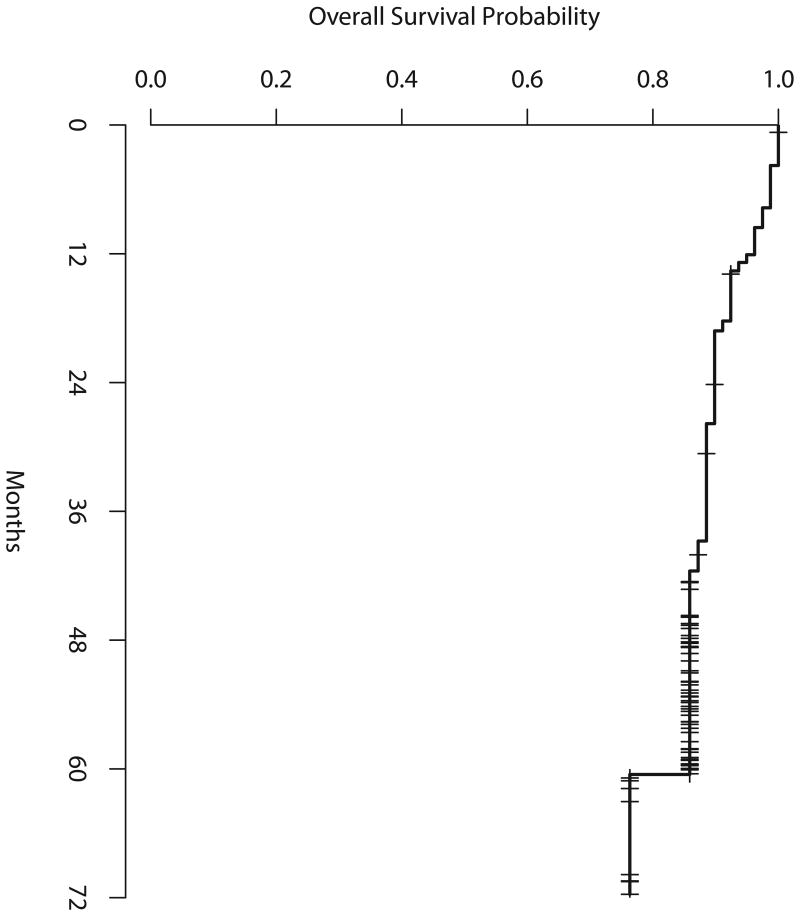
Figure 3a shows the overall survival (OS), from the time of study entry, for all eligible and treated patients. Median survival has not been reached. Figure 3b shows the OS from the time of the mid-treatment PET scan, according to mid-treatment PET results. Two-year OS among all eligible patients was 90% (90% CI 83-94%), 3-year OS 89% (90% CI 81-93%) and 4-year OS 86% (90% CI 78-91%). Among mid-treatment PET-negative patients, 2-year OS was 93% (90% CI 86-97%), 3-year OS 93% (90% CI 86-97%), 4-year OS 90% (90% CI 81-95%) and 5-year OS 77% (90% CI 49-91%). Among mid-treatment PET-positive patients, 2-year OS was 77% (90% CI 51-90%), 3-year 69% (90% CI 43-85%) and 4-year OS 69% (90% CI 43-85%), as shown in Table IV.
Figure 3a. Overall survival among all eligible and treated patients.
Figure 3b. Overall survival by mid-treatment positron emission tomography (PET) scan (from time of mid-treatment PET scan).
Negative Predictive Value of the Mid-Treatment PET Scan and End-of-Treatment PET Scan
The negative predictive value (NPV) of a mid-treatment PET scan was defined as the proportion of patients with a negative scan result who remained progression-free at 2 years. Out of the 57 mid-treatment PET-negative patients who had known 2-year PFS status (i.e., not censored before 2 years), 43 remained progression-free at 2 years. The NPV for mid-treatment PET was 75% (43/57; 90% CI of 64-84%). Out of the 57 end-of-treatment PET-negative patients who had known 2-year PFS status (i.e., not censored before 2 years), 45 remained progression-free at 2 years. The NPV for end-of-treatment PET was 79% (45/57; 90% CI, 68-87%). NPV did not vary according to IPI risk group (p=1.0 and 0.89 for mid-treatment PET and end-of treatment PET, respectively) as shown in Tables Va and Vb, indicating that at either time point among PET-negative patients, IPI risk at study entry was not associated with PFS status at 2 years.
Table Va. NPV of Mid-Treatment PET According to IPI Risk Group.
| IPI risk group | Patients with negative mid-treatment PET (N=57) |
Patients progression-free at 2 years (N=43) |
NPV of mid-Treatment PET Scan (90% CI) |
|---|---|---|---|
| Low (0-1) | 11 | 8 | 73% (44-92%) |
| Low intermediate (2) | 14 | 11 | 79% (53-94%) |
| High intermediate (3) | 19 | 14 | 74% (52-89%) |
| High (4-5) | 13 | 10 | 77% (51-93%) |
|
| |||
| Total | 57 | 43 | 75% (64-84%) |
Patients who were mid-treatment PET-negative and who had known 2-year progression-free survival status (i.e., not censored before 2 years) were included in the table.
Table Vb. NPV of End-of-Treatment PET According to IPI Risk Group.
| IPI risk group | Patients with negative end-of-treatment PET (N=57) |
Patients progression-free at 2 years (N=45) |
NPV of end-of-treatment PET Scan (90% CI) |
|---|---|---|---|
| Low (0-1) | 10 | 7 | 70% (39-91%) |
| Low intermediate (2) | 16 | 13 | 81% (58-95%) |
| High intermediate (3) | 19 | 15 | 79% (58-92%) |
| High (4-5) | 12 | 10 | 83% (56-97%) |
|
| |||
| Total | 57 | 45 | 79% (68-87%) |
Patients who were end-of-treatment PET-negative and who had known 2-year progression-free survival status (i.e., not censored before 2 years) were included in the table.
NPV, negative predictive value, PET positron emission tomography; IPI, International Prognostic Index
Toxicity
There was no unexpected toxicity during the initial 4 cycles of R-CHOP. Table VI shows the distribution of toxicities for subsequent therapy, separating those patients who received 4 cycles of R-ICE from those receiving a further 2 cycles of R-CHOP. Toxicities were primarily haematological, and as expected for these regimens. There was one death, from bowel perforation and neutropenic sepsis, in the R-CHOP group.
Table VI. Treatment-Related Toxicity for Subsequent Therapy Following Initial R-CHOP.
| Toxicity (Grade 3/4) | PET-negative (R-CHOP) (N=61) |
PET-positive (R-ICE) (N=13) |
|---|---|---|
| Anaemia | 5% | 54% |
| Neutropenia | 15% | 69% |
| Thrombocytopenia | 5% | 92% |
| Neutropenic fever | 3% | 0% |
| Nausea/Vomiting | 0 | 8% |
| Fatigue | 2% | 8% |
| Death (n) | 1 | 0 |
PET, positron emission tomography; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; R-ICE, rituximab, ifosfamide, carboplatin, etoposide.
Discussion
We report the results of a multi-centre prospective phase II trial of the early introduction of alternative treatment, consisting of four cycles of R-ICE, in patients with previously untreated DLBCL whose PET scan remained persistently positive after an initial 3 cycles of R-CHOP. The primary endpoint of the study was the 2-year PFS in such patients, considering an improvement to 45% from an estimated less than 25% to be promising. The 2-year PFS was 42%, but conclusions are limited by the fact that only 13 patients (rather than the projected 33) had mid-treatment PET-positive imaging. Although considered, study accrual was not expanded to obtain the required number of mid-treatment PET-positive patients, mainly because of concerns over the prognostic accuracy of early PET scanning in DLBCL and over the inter-observer reproducibility of the dichotomous scoring of such scans as positive or negative.
Given the lower than expected rate of mid-treatment PET positivity seen in our study, a panel of three expert nuclear medicine reviewers was convened to re-interpret the first 38 mid-treatment scans obtained from the trial. The binary ECOG study criteria, which were modifications of the Harmonization Criteria and the London criteria, were applied (Juweid et al, 2007). Of 38 interim scans, agreement was complete in 68% and 71% by ECOG and London criteria, respectively. The range of PET positivity on the interim scans varied from 16% to 34% depending on the reviewer. Only moderate consistency was observed among reviewers upon statistical analysis. In 1/3 of cases, interpretations were discrepant among reviewers, using either criteria; when those cases were re-examined by all the reviewers together, no consensus could be reached in three quarters of the discrepant cases. (Horning et al, 2010) Those results, as well as more recent reports (Mylam et al, 2014), highlight the need for more consistent and accurate scoring systems, and significant effort has recently been devoted to the development of more quantitative interpretation algorithms. (Itti et al, 2013;Meignan et al, 2014)
During the course of the trial, several small studies examining the prognostic value of early PET scan reported 2-year PFS of 17 to 34% for patients scored as positive (Spaepen et al, 2002;Mikhaeel et al, 2005;Haioun et al, 2005;Fruchart et al, 2006;Kostakoglu et al, 2006;Querellou et al, 2006;Ng et al, 2007;Terasawa et al, 2009). NPVs of 73-86% 2-year PFS have been reported, with less consistent Positive Predictive Values (PPVs) of 18-74% (Barrington et al, 2014;Yang et al, 2011;Zinzani et al, 2011;Pregno et al, 2012;Safar et al, 2012;Micallef et al, 2011;Itti et al, 2013;Cashen et al, 2011;Yoo et al, 2011;Yang et al, 2013). Quantitative assessment of reduction in maximum standardized uptake value (SUVmax) after 4 cycles of therapy from baseline values has been explored. Using what was calculated to be an optimum cut-off of 72.9% reduction in SUVmax , 2-year event-free survival was 79% in patients with more than, and 32% in those with less than, this amount of reduction. (Itti et al, 2009) The timing of the interim PET scan in our trial was chosen based on data available at the time. The majority of patients studied had received three or four prior cycles of treatment, and concerns had been raised over false positive results if the scan was performed after only 2 cycles. As much time as possible was placed between the most recent chemotherapy administration and the interim PET, in order to avoid the” stunning effect” of chemotherapy as much as possible, while still allowing a practical time window for the scan and never delaying the start of the next scheduled cycle. Over multiple studies, using a variety of interpretation techniques, the NPV of interim PET in DLBCL has consistently been more reliable than its PPV. It is however the PPV that is most relevant to attempts at selective dose-intensification.
The efficacy of dose-intensification also remains unclear (Stiff et al, 2013). Although the overall outcomes of the current trial and of a study in which all but a few patients received R-CHOP followed by R-ICE were excellent (Moskowitz et al, 2010), it cannot be concluded from these trial designs that the addition of R-ICE improved the outcome. The results of a trial with a very similar design to our E3404 study, reported in abstract form (Sehn et al, 2014) are very comparable to our results. The trial design differed in that interim PET was performed after 4 rather than 3 cycles of R-CHOP, Harmonization criteria were used for PET interpretation and consolidative irradiation was allowed. Interim PET-negative patients had an excellent result, while PET-positive patients fared considerably less well. Although the 4-year PFS of 59% was believed to compare favourably to historical data, 78% of interim PET-positive patients progressed through or remained PET-positive after four cycles of R-ICE. Despite variability in PET interpretation, it does appear that patients with a positive mid-treatment PET have a less favourable outcome, not or not fully overcome by treatment intensification, and indicative of innate or early acquired resistance to chemotherapy. In order to further explore the concept of response-adapted therapy in DLBCL, more standardized and reproducible image interpretation, and a clearer understanding of what the true PPV of the test is, will be needed. Specific issues that need to be addressed include the criteria used for PET interpretation, whether qualitative, quantitative or a combination of such criteria, in order to achieve greater reproducibility of readings; clearer data on the natural history of a positive or negative scan without treatment modification; and the optimum timing of interim scanning. At present, we would conclude that treatment modification in DLBCL based on early PET scan should remain confined to clinical trials.
Acknowledgments
This study was coordinated by the ECOG-ACRIN Cancer Research Group (Robert L. Comis, MD and Mitchell D. Schnall, MD, PhD, Group Co-Chairs) and supported in part by Public Health Service Grants CA21115, CA23318, CA66636, CA180820, CA180794, CA16116, CA180802, CA180816, CA21076, CA180799, CA13650, CA180790 and from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute. Biospecimens were provided by the ECOG-ACRIN Pathology Coordinating Office and Reference Laboratory.
Footnotes
Author contributions: Lode J. Swinnen: performed the research, designed the research study, analysed the data, wrote the paper
Hailun Li: designed the research study, analysed the data, contributed to the writing of the paper
Andrew Quon: central radiology reviewer, designed the research study, analysed the data, contributed to the writing of the paper
Randy Gascoyne: central pathology reviewer, contributed to the writing of the paper
Fangxin Hong: designed the research study, analysed the data, contributed to the writing of the paper
Erik A. Ranheim: performed the research, contributed to the writing of the paper
Thomas M. Habermann: performed the research, contributed to the writing of the paper
Brad S. Kahl: performed the research, analysed the data, contributed to the writing of the paper
Sandra J. Horning: designed the research study, analysed the data, contributed to the writing of the paper
References
- Barrington SF, Mikhaeel NG, Kostakoglu L, Meignan M, Hutchings M, Mueller SP, Schwartz LH, Zucca E, Fisher RI, Trotman J, Hoekstra OS, Hicks RJ, O'Doherty MJ, Hustinx R, Biggi A, Cheson BD. Role of Imaging in the Staging and Response Assessment of Lymphoma: Consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol. 2014;32:3048–58. doi: 10.1200/JCO.2013.53.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashen AF, Dehdashti F, Luo J, Homb A, Siegel BA, Bartlett NL. 18F-FDG PET/CT for early response assessment in diffuse large B-cell lymphoma: poor predictive value of international harmonization project interpretation. J Nucl Med. 2011;52:386–392. doi: 10.2967/jnumed.110.082586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-Lopez A, Hagenbeek A, Cabanillas F, Klippensten D, Hiddemann W, Castellino R, Harris NL, Armitage JO, Carter W, Hoppe R, Canellos GP. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- Coiffier B. GELA study comparing CHOP and R-CHOP in elderly patients with DLCL: 3-year median follow-up with an analysis according to co-morbidity factors. 2003:596. [Google Scholar]
- Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G, Gaulard P, Reyes F, Lederlin P, Gisselbrecht C. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- Fruchart C, Reman O, Le SN, Musafiri D, Cheze S, Macro M, Switsers O, Aide N, Liegard M, Levaltier X, Peny AM, Leporrier M, Bardet S. Prognostic value of early 18 fluorodeoxyglucose positron emission tomography and gallium-67 scintigraphy in aggressive lymphoma: a prospective comparative study. Leuk Lymphoma. 2006;47:2547–2557. doi: 10.1080/10428190600942959. [DOI] [PubMed] [Google Scholar]
- Habermann TM, Weller EA, Morrison VA, Gascoyne RD, Cassileth PA, Cohn JB, Dakhil SR, Woda B, Fisher RI, Peterson BA, Horning SJ. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:3121–3127. doi: 10.1200/JCO.2005.05.1003. [DOI] [PubMed] [Google Scholar]
- Haioun C, Lepage E, Gisselbrecht C, Salles G, Coiffier B, Brice P, Bosly A, Morel P, Nouvel C, Tilly H, Lederlin P, Sebban C, Briere J, Gaulard P, Reyes F. Survival benefit of high-dose therapy in poor-risk aggressive non-Hodgkin's lymphoma: final analysis of the prospective LNH87-2 protocol--a groupe d'Etude des lymphomes de l'Adulte study. J Clin Oncol. 2000;18:3025–3030. doi: 10.1200/JCO.2000.18.16.3025. [DOI] [PubMed] [Google Scholar]
- Haioun C, Itti E, Rahmouni A, Brice P, Rain JD, elhadj K, Gaulard P, Garderet L, Lepage E, Reyes F, Meignan M. [18F]fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) in aggressive lymphoma: an early prognostic tool for predicting patient outcome. (Abstract) Proc Am Soc Clin Oncol. 2003;22:565. doi: 10.1182/blood-2005-01-0272. [DOI] [PubMed] [Google Scholar]
- Haioun C, Itti E, Rahmouni A, Brice P, Rain JD, Belhadj K, Gaulard P, Garderet L, Lepage E, Reyes F, Meignan M. [18F]fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) in aggressive lymphoma: an early prognostic tool for predicting patient outcome. Blood. 2005;106:1376–1381. doi: 10.1182/blood-2005-01-0272. [DOI] [PubMed] [Google Scholar]
- Horning SJ, Juweid ME, Schoder H, Wiseman G, McMillan A, Swinnen LJ, Advani R, Gascoyne R, Quon A. Interim positron emission tomography scans in diffuse large B-cell lymphoma: an independent expert nuclear medicine evaluation of the Eastern Cooperative Oncology Group E3404 study. Blood. 2010;115:775–777. doi: 10.1182/blood-2009-08-234351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itti E, Lin C, Dupuis J, Paone G, Capacchione D, Rahmouni A, Haioun C, Meignan M. Prognostic value of interim 18F-FDG PET in patients with diffuse large B-Cell lymphoma: SUV-based assessment at 4 cycles of chemotherapy. J Nucl Med. 2009;50:527–533. doi: 10.2967/jnumed.108.057703. [DOI] [PubMed] [Google Scholar]
- Itti E, Meignan M, Berriolo-Riedinger A, Biggi A, Cashen AF, Vera P, Tilly H, Siegel BA, Gallamini A, Casasnovas RO, Haioun C. An international confirmatory study of the prognostic value of early PET/CT in diffuse large B-cell lymphoma: comparison between Deauville criteria and DeltaSUVmax. Eur J Nucl Med Mol Imaging. 2013;40:1312–1320. doi: 10.1007/s00259-013-2435-6. [DOI] [PubMed] [Google Scholar]
- Jerusalem G, Warland V, Najjar F, Paulus P, Fassotte MF, Fillet G, Rigo P. Whole-body 18F-FDG PET for the evaluation of patients with Hodgkin's disease and non-Hodgkin's lymphoma. Nucl Med Commun. 1999;20:13–20. doi: 10.1097/00006231-199901000-00004. [DOI] [PubMed] [Google Scholar]
- Juweid ME, Wiseman GA, Menda Y. FDG-PET predicts with high accuray the 1-year progression-free survival (1-year PFS) of patients with aggressive non-Hodgkin's lymphoma (NHL) following anthracycline-based first-line chemotherapy (FLC). (Abstract) J Nucl Med. 2002;43:78p. [Google Scholar]
- Juweid ME, Stroobants S, Hoekstra OS, Mottaghy FM, Dietlein M, Guermazi A, Wiseman GA, Kostakoglu L, Scheidhauer K, Buck A, Naumann R, Spaepen K, Hicks RJ, Weber WA, Reske SN, Schwaiger M, Schwartz LH, Zijlstra JM, Siegel BA, Cheson BD. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007;25:571–578. doi: 10.1200/JCO.2006.08.2305. [DOI] [PubMed] [Google Scholar]
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- Kewalramani T, Zelenetz AD, Nimer SD, Portlock C, Straus D, Noy A, O'Connor O, Filippa DA, Teruya-Feldstein J, Gencarelli A, Qin J, Waxman A, Yahalom J, Moskowitz CH. Rituximab and ICE as second-line therapy before autologous stem cell transplantation for relapsed or primary refractory diffuse large B-cell lymphoma. Blood. 2004;103:3684–3688. doi: 10.1182/blood-2003-11-3911. [DOI] [PubMed] [Google Scholar]
- Kostakoglu L, Goldsmith SJ, Leonard JP, Christos P, Furman RR, Atasever T, Chandramouly A, Verma S, Kothari P, Coleman M. FDG-PET after 1 cycle of therapy predicts outcome in diffuse large cell lymphoma and classic Hodgkin disease. Cancer. 2006;107:2678–2687. doi: 10.1002/cncr.22276. [DOI] [PubMed] [Google Scholar]
- Kostakoglu L, Leonard JP, Kuji I, Coleman M, Vallabhajosula S, Goldsmith SJ. Comparison of fluorine-18 fluorodeoxyglucose positron emission tomography and Ga-67 scintigraphy in evaluation of lymphoma. Cancer. 2002;94:879–888. [PubMed] [Google Scholar]
- Meignan M, Barrington S, Itti E, Gallamini A, Haioun C, Polliack A. Report on the 4th International Workshop on Positron Emission Tomography in Lymphoma held in Menton, France. Leuk Lymphoma. 2014;55:31–7. doi: 10.3109/10428194.2013.802784. [DOI] [PubMed] [Google Scholar]
- Mylam KJ, Kostakoglu L, Hutchings M, Coleman M, Lamonica D, Czuczman MS, Diehl LF, Nielsen AL, Jensen P, Loft A, Hendel HW, Iyer V, Leppä S, Jyrkkiö S, Holte H, Eriksson M, Gillstrøm D, Hansen PB, Seppänen M, Hjorthaug K, de Nully Brown P, Pedersen LM. Leuk Lymphoma. 2014;20:1–28. doi: 10.3109/10428194.2014.975800. [DOI] [PubMed] [Google Scholar]
- Micallef IN, Maurer MJ, Wiseman GA, Nikcevich DA, Kurtin PJ, Cannon MW, Perez DG, Soori GS, Link BK, Habermann TM, Witzig TE. Epratuzumab with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy in patients with previously untreated diffuse large B-cell lymphoma. Blood. 2011;118:4053–4061. doi: 10.1182/blood-2011-02-336990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhaeel NG, Hutchings M, Fields PA, O'Doherty MJ, Timothy AR. FDG-PET after two to three cycles of chemotherapy predicts progression-free and overall survival in high-grade non-Hodgkin lymphoma. Ann Oncol. 2005;16:1514–1523. doi: 10.1093/annonc/mdi272. [DOI] [PubMed] [Google Scholar]
- Milpied N, Deconinck E, Gaillard F, Delwail V, Foussard C, Berthou C, Gressin R, Lucas V, Colombat P, Harousseau JL. Initial treatment of aggressive lymphoma with high-dose chemotherapy and autologous stem-cell support. N Engl J Med. 2004;350:1287–1295. doi: 10.1056/NEJMoa031770. [DOI] [PubMed] [Google Scholar]
- Moskowitz CH, Schoder H, Teruya-Feldstein J, Sima C, Iasonos A, Portlock CS, Straus D, Noy A, Palomba ML, O'Connor OA, Horwitz S, Weaver SA, Meikle JL, Filippa DA, Caravelli JF, Hamlin PA, Zelenetz AD. Risk-adapted dose-dense immunochemotherapy determined by interim FDG-PET in Advanced-stage diffuse large B-Cell lymphoma. J Clin Oncol. 2010;28:1896–1903. doi: 10.1200/JCO.2009.26.5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng AP, Wirth A, Seymour JF, Lee M, Hogg A, Januszewicz H, Wolf M, Prince HM, Macmanus M, Hicks RJ. Early therapeutic response assessment by (18)FDG-positron emission tomography during chemotherapy in patients with diffuse large B-cell lymphoma: isolated residual positivity involving bone is not usually a predictor of subsequent treatment failure. Leuk Lymphoma. 2007;48:596–600. doi: 10.1080/10428190601099965. [DOI] [PubMed] [Google Scholar]
- Pfreundschuh M, Trumper L, Kloess M, Schmits R, Feller AC, Rube C, Rudolph C, Reiser M, Hossfeld DK, Eimermacher H, Hasenclever D, Schmitz N, Loeffler M. Two-weekly or 3-weekly CHOP chemotherapy with or without etoposide for the treatment of elderly patients with aggressive lymphomas: results of the NHL-B2 trial of the DSHNHL. Blood. 2004;104:634–641. doi: 10.1182/blood-2003-06-2095. [DOI] [PubMed] [Google Scholar]
- Pregno P, Chiappella A, Bello M, Botto B, Ferrero S, Franceschetti S, Giunta F, Ladetto M, Limerutti G, Menga M, Nicolosi M, Priolo G, Puccini B, Rigacci L, Salvi F, Vaggelli L, Passera R, Bisi G, Vitolo U. Interim 18-FDG-PET/CT failed to predict the outcome in diffuse large B-cell lymphoma patients treated at the diagnosis with rituximab-CHOP. Blood. 2012;119:2066–2073. doi: 10.1182/blood-2011-06-359943. [DOI] [PubMed] [Google Scholar]
- Querellou S, Valette F, Bodet-Milin C, Oudoux A, Carlier T, Harousseau JL, Chatal JF, Couturier O. FDG-PET/CT predicts outcome in patients with aggressive non-Hodgkin's lymphoma and Hodgkin's disease. Ann Hematol. 2006;85:759–767. doi: 10.1007/s00277-006-0151-z. [DOI] [PubMed] [Google Scholar]
- Safar V, Dupuis J, Itti E, Jardin F, Fruchart C, Bardet S, Vera P, Copie-Bergman C, Rahmouni A, Tilly H, Meignan M, Haioun C. Interim [18F]fluorodeoxyglucose positron emission tomography scan in diffuse large B-cell lymphoma treated with anthracycline-based chemotherapy plus rituximab. J Clin Oncol. 2012;30:184–190. doi: 10.1200/JCO.2011.38.2648. [DOI] [PubMed] [Google Scholar]
- Sehn LH, Hardy ELG, Gill KK, Al-Tourah AJ, Shustik J, Macpherson NA, Yee A, Lam W, Savage KJ, Klasa R, Villa D, Gerrie AS, Shenkier T, Slack GW, Gascoyne RD, Benard F, Wilson D, Tonseth P, Connors JM. Phase 2 Trial of Interim PET Scan-Tailored Therapy in Patients with Advanced Stage Diffuse Large B-Cell Lymphoma (DLBCL) in British Columbia (BC) Blood (ASH Annual Meeting Abstracts) 2014;124:392. [Google Scholar]
- Spaepen K, Stroobants S, Dupont P, Van SS, Thomas J, Vandenberghe P, Vanuytsel L, Bormans G, Balzarini J, De Wolf-Peeters C, Mortelmans L, Verhoef G. Prognostic value of positron emission tomography (PET) with fluorine-18 fluorodeoxyglucose ([18F]FDG) after first-line chemotherapy in non-Hodgkin's lymphoma: is [18F]FDG-PET a valid alternative to conventional diagnostic methods? J Clin Oncol. 2001;19:414–419. doi: 10.1200/JCO.2001.19.2.414. [DOI] [PubMed] [Google Scholar]
- Spaepen K, Stroobants S, Dupont P, Vandenberghe P, Thomas J, de GT, Balzarini J, De Wolf-Peeters C, Mortelmans L, Verhoef G. Early restaging positron emission tomography with ( 18)F-fluorodeoxyglucose predicts outcome in patients with aggressive non-Hodgkin's lymphoma. Ann Oncol. 2002;13:1356–1363. doi: 10.1093/annonc/mdf256. [DOI] [PubMed] [Google Scholar]
- Stiff PJ, Unger JM, Cook JR, Constine LS, Couban S, Stewart DA, Shea TC, Porcu P, Winter JN, Kahl BS, Miller TP, Tubbs RR, Marcellus D, Friedberg JW, Barton KP, Mills GM, LeBlanc M, Rimsza LM, Forman SJ, Fisher RI. Autologous transplantation as consolidation for aggressive non-Hodgkin's lymphoma. N Engl J Med. 2013;369:1681–1690. doi: 10.1056/NEJMoa1301077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasawa T, Lau J, Bardet S, Couturier O, Hotta T, Hutchings M, Nihashi T, Nagai H. Fluorine-18-fluorodeoxyglucose positron emission tomography for interim response assessment of advanced-stage Hodgkin's lymphoma and diffuse large B-cell lymphoma: a systematic review. J Clin Oncol. 2009;27:1906–1914. doi: 10.1200/JCO.2008.16.0861. [DOI] [PubMed] [Google Scholar]
- Velasquez WS, McLaughlin P, Tucker S, Hagemeister FB, Swan F, Rodriguez MA, Romaguera J, Rubenstein E, Cabanillas F. ESHAP--an effective chemotherapy regimen in refractory and relapsing lymphoma: a 4-year follow-up study. J Clin Oncol. 1994;12:1169–1176. doi: 10.1200/JCO.1994.12.6.1169. [DOI] [PubMed] [Google Scholar]
- Yang DH, Ahn JS, Byun BH, Min JJ, Kweon SS, Chae YS, Sohn SK, Lee SW, Kim HW, Jung SH, Kim YK, Kim HJ, Bom HS, Lee JJ. Interim PET/CT-based prognostic model for the treatment of diffuse large B cell lymphoma in the post-rituximab era. Ann Hematol. 2013;92:471–479. doi: 10.1007/s00277-012-1640-x. [DOI] [PubMed] [Google Scholar]
- Yang DH, Min JJ, Song HC, Jeong YY, Chung WK, Bae SY, Ahn JS, Kim YK, Bom HS, Chung IJ, Kim HJ, Lee JJ. Prognostic significance of interim (1)(8)F-FDG PET/CT after three or four cycles of R-CHOP chemotherapy in the treatment of diffuse large B-cell lymphoma. Eur J Cancer. 2011;47:1312–1318. doi: 10.1016/j.ejca.2010.12.027. [DOI] [PubMed] [Google Scholar]
- Yoo C, Lee DH, Kim JE, Jo J, Yoon DH, Sohn BS, Kim SW, Lee JS, Suh C. Limited role of interim PET/CT in patients with diffuse large B-cell lymphoma treated with R-CHOP. Ann Hematol. 2011;90:797–802. doi: 10.1007/s00277-010-1135-6. [DOI] [PubMed] [Google Scholar]
- Zinzani PL, Gandolfi L, Broccoli A, Argnani L, Fanti S, Pellegrini C, Stefoni V, Derenzini E, Quirini F, Baccarani M. Midtreatment 18F-fluorodeoxyglucose positron-emission tomography in aggressive non-Hodgkin lymphoma. Cancer. 2011;117:1010–1018. doi: 10.1002/cncr.25579. [DOI] [PubMed] [Google Scholar]