Abstract
OBJECTIVE
To assess perinatal outcomes with Carpenter-Coustan criteria for gestational diabetes mellitus (GDM), those with normal glucose testing, and those who would be added to GDM by The International Association of the Diabetes and Pregnancy Study Groups (IADPSG) criteria.
METHODS
This was a retrospective cohort study of women who underwent screening and diagnostic testing for GDM. Patients were divided into nonoverlapping groups: GDM by Carpenter-Coustan (Carpenter-Coustan), IADPSG GDM criteria but not Carpenter-Coustan (IADPSG), and normal GDM screening or testing (control). Outcomes included newborn birth weight, birth weight z-score, Ponderal Index, and large for gestational age. Data were analyzed with one-way analysis of variance, t tests, or χ2.
RESULTS
There were 8,390 women who met inclusion criteria: 338 Carpenter-Coustan; 281 IADPSG; and 7,771 women in the control group. Mean birth weight (3,411 compared with 3,240 g, P<.01), birth weight z-score (0.477 compared with 0.059, P<.01), Ponderal Index (2.79 compared with 2.73 g/cm3, P=.014), and large for gestational age (19.9% compared with 8.8%, relative risk 2.25, 95% confidence interval [CI] 1.76–2.88) were higher in IADPSG compared with women in the control group. The IADPSG group had greater birth weight (3,411 compared with 3,288 g, P<.01) than Carpenter-Coustan neonates with no difference in large for gestational age (19.9% compared with 16.0%, relative risk 1.25 95% CI 0.88–1.75), Ponderal Index (2.78 compared with 2.79 g/cm3, P=1), or birth weight z-score (0.477 compared with 0.330, P=.30).
CONCLUSIONS
Newborns of women who would be added to the diagnosis of GDM by IADPSG criteria have greater measures of fetal overgrowth than those in the control group and greater birth weight in comparison with Carpenter-Coustan GDM neonates.
In the United States, gestational diabetes (GDM) is diagnosed using a two-step process: screening with a 50-g glucose load followed by a diagnostic 100-g, 3-hour oral glucose tolerance test (OGTT).1 Currently, the Carpenter-Coustan criteria for GDM require two or more abnormal values of the 3-hour OGTT.2 However, these criteria were established primarily through secondary analyses of glucose testing in pregnant women.3 It was not until the Hyperglycemia and Adverse Pregnancy Outcomes trial that perinatal outcomes were primarily correlated with the results of a universal OGTT (fasting 75-g, 2-hour OGTT).4
Based on the Hyperglycemia and Adverse Pregnancy Outcomes trial results, revised criteria for the diagnosis of GDM were proposed by The International Association of the Diabetes and Pregnancy Study Groups (IADPSG). The subsequent “IADPSG criteria for GDM” required only one abnormal value on a fasting 75-g OGTT to diagnose GDM.5 These criteria were discussed at a National Institutes of Health consensus conference but not adopted.6 The use of only Carpenter-Coustan criteria was subsequently reaffirmed by the American College of Obstetricians and Gynecologists.1 In the National Institutes of Health report, a primary concern regarding the IADPSG criteria was the lack of data regarding maternal or newborn outcome in women fulfilling the IADPSG criteria (but not Carpenter-Coustan criteria).6 Prior investigations have attempted to address this issue but with methodologic limitations.7 Hence, the purpose of this retrospective analysis was to compare clinical outcomes of those women who would be added to the diagnosis of GDM by IADPSG criteria and compare them with those diagnosed by Carpenter-Coustan criteria and those with normal glucose testing or screening.
MATERIALS AND METHODS
This is a retrospective cohort study comparing pregnancy and neonatal outcomes of patients who underwent a 100-g OGTT or a 50-g oral glucose challenge test at MetroHealth Medical Center (Cleveland, Ohio). A query of our electronic medical record system was performed to identify all patients who had a 3-hour OGTT completed between January 1, 2007, and June 30, 2012. A second query was done to identify all patients who had a 50-g glucose screen completed during the same time period. Additionally, the perinatal database at MetroHealth was then queried to obtain maternal demographic data and perinatal outcome data for all singleton deliveries between July 1, 2007, and June 30, 2012. The perinatal database contains delivery records from the Department of Obstetrics and Gynecology, MetroHealth Medical Center, Cleveland, Ohio, that have been entered prospectively into a computerized data base for research purposes. Each entry is done by trained data entry personnel and is then independently compared with the patient’s electronic medical record by an independent reviewer. Dates of glucose screening and testing were compared with delivery dates to verify that the pregnancy outcomes from the perinatal database were correctly linked with glucose screening and testing results.
Patients were included in the study if they delivered a singleton gestation between July 1, 2007, and June 30, 2012, and had glucose screening or glucose tolerance testing completed after 24 weeks of gestation. Patients were excluded if they had an abnormal glucose screen without a subsequent glucose tolerance test, missing outcome data, or preterm delivery (defined as delivery less than 37 0/7 weeks of gestation). For patients with more than one pregnancy in the study period that met inclusion and exclusion criteria, only the first pregnancy was included. For those patients who had more than one glucose screen or more than one 100-g, 3-hour glucose tolerance test during a pregnancy, only the test performed at a later gestational age was used for analysis.
Results of 3-hour OGTTs were used to classify patients into one of three nonoverlapping groups: 1) patients diagnosed as GDM by Carpenter-Coustan criteria (and treated for GDM) (Carpenter-Coustan); 2) patients diagnosed as GDM only by IADPSG criteria and not Carpenter-Coustan criteria (and not treated for GDM) (IADPSG); and 3) patients with a normal 3-hour OGTT result that would not be diagnosed with GDM by either IADPSG or Carpenter-Coustan criteria (normal glucose tolerance). A fourth additional group consisted of patients with a normal 1-hour glucose screen (less than 135 mg/dL) that did not have a 100-g OGTT (normal glucose screening). For the purposes of analysis, the normal glucose tolerance and normal glucose screening groups were analyzed separately and then later as a combined group, “control” (control=normal glucose tolerance+normal glucose screening). This approach was taken as data support that women with a normal 50-g glucose screen may not have similar pregnancy outcomes compared with women with a positive screen, but normal 3-hour OGTT results.8
The IADPSG criteria were established using the original Hyperglycemia and Adverse Pregnancy Outcomes trial data, in which a maternal glucose value was considered abnormal if the 75-g OGTT fasting, 1 hour, or 2-hour mark was associated with at least a 1.75-fold increase in the odds ratio for one of several adverse perinatal outcomes (greater than 90th percentiles of cord blood C-peptide, large for gestational age [LGA], or percent body fat of newborn).5 For this study, results of a fasting 100-g OGTT were available for review. Several studies have compared blood glucose values after 100-g and 75-g glucose loads.9–11 The largest of these, done by Mello et al,9 showed that in 484 Italian women between 26 and 31 weeks of gestation, blood glucose 1 hour after a 100-g glucose load was 2.3 mg/dL higher than 1 hour after a 75-g glucose load, but this difference was not statistically significant. Outcomes of interest were then compared among the groups with the IADPSG used as the reference for all comparisons. Newborn outcomes included birth weight, birth weight z-score (calculated using gestational age-specific mean birth weights and standard deviations from our previously published local birth weight criteria),12 gestational age at delivery, LGA (greater than 90th percentile), Ponderal Index (measurement of relative amount of adiposity present in a newborn calculated as weight per length cubed and typically expressed in g/cm3 for newborns),13 Apgar scores, and neonatal intensive care unit admission. Pregnancy outcomes included mode of delivery and pregnancy-induced hypertension (defined as gestational hypertension, preeclampsia, eclampsia, or hemolysis, elevated liver enzymes and low platelet count). Continuous variables were compared between the groups using one-way analysis of variance with Tahmane method for multiple comparisons. Categorical variables were compared between groups using χ2. Relative risks were calculated where appropriate. Data were analyzed using IBM SPSS Statistics 20. A P value <.05 was considered significant. Primary outcomes were not prespecified and a pre-study power calculation was not done, but based on 2,500 annual deliveries with 7% of patients with GDM by Carpenter-Coustan criteria, an additional 4% of patients added to GDM by IADPSG criteria, 89% of patients as controls, and a 25% exclusion rate in all groups, to detect a difference between 18% LGA in IADPSG patients and 10% LGA in those in the control group, the study would have a power of 99.3%. This retrospective study was approved by the Metro-Health institutional review board.
RESULTS
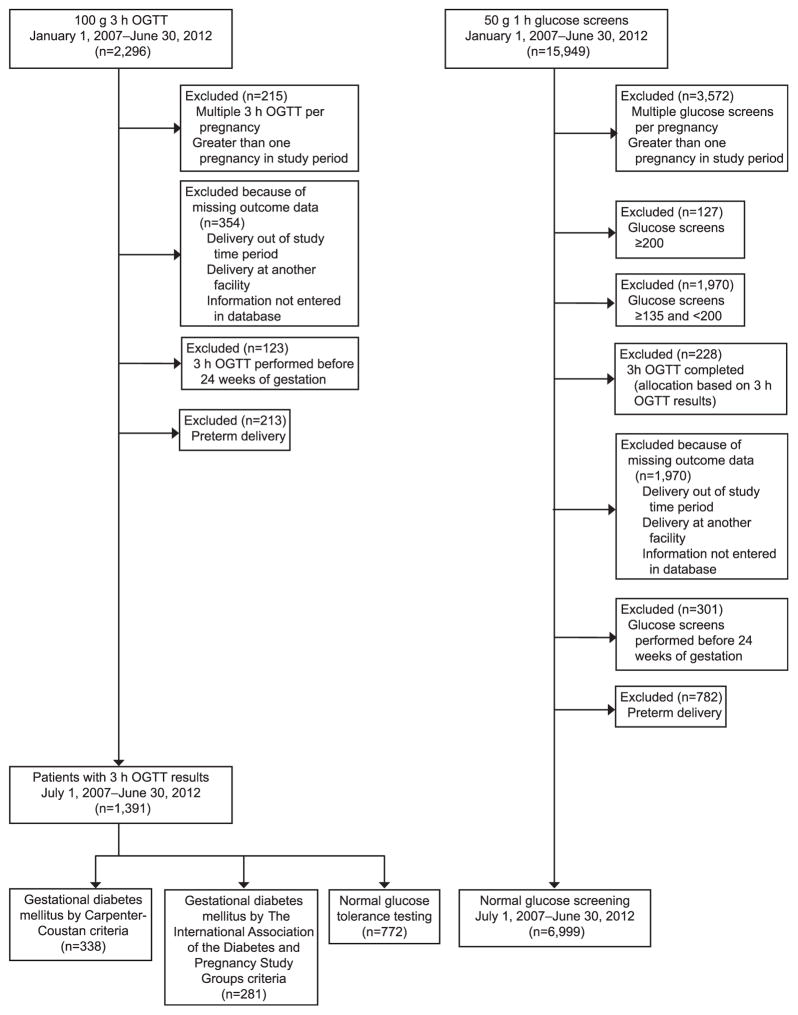
Figure 1 details the derivation of the groups and Table 1 presents their demographic characteristics. Among women who completed a 3-hour OGTT, women added to the diagnosis of GDM by IADPSG criteria were not significantly different in regard to age, rate of nulliparity, or race with either Carpenter-Coustan GDM or normal patients. Maternal body mass index (at the time of delivery) was greater in IADPSG patients compared with women with normal glucose tolerance (IADPSG 35.6 kg/m2 compared with normal glucose tolerance 32.7 kg/m2, P<.001). When comparing women who would be added to GDM by IADPSG criteria with those with a normal 1-hour screen, those in the IADPSG group were noted to be heavier, older, less likely to be nulliparous, and more likely to be Caucasian.
Fig. 1.
Patient allocation flow sheet. OGTT, oral glucose tolerance test.
Ethridge. Perinatal Outcomes With Gestational Diabetes. Obstet Gynecol 2014.
Table 1.
Maternal Demographics
| Parameter (Units) | GDM by Carpenter-Coustan (n=338) | IADPSG (n=281) | Normal Glucose Tolerance (n=772) | Normal Glucose Screening (n=6,999) | Normal Glucose Tolerance and Normal Glucose Screening* (n=7,771) |
|---|---|---|---|---|---|
| Maternal age (y) | 28.98 | 28.54 | 27.54 | 24.69 | 25.0 |
| P | .949 | Reference | .138 | <.001 | <.001 |
| Maternal BMI (kg/m2) | 35.85 | 35.57 | 32.74 | 32.30 | 32.3 |
| P | .999 | Reference | <.001 | <.001 | <.001 |
| Nulliparous (%) | 38.5 | 35.9 | 38.0 | 48.3 | 47.3 |
| P | .519 | Reference | .551 | <.001 | <.001 |
| African American (%) | 32.8 | 30.2 | 28.8 | 47.5 | 45.6 |
| P | .49 | Reference | .637 | <.001 | <.001 |
| Caucasian (%) | 41.1 | 47.0 | 46.1 | 35.3 | 36.4 |
| P | .144 | Reference | .804 | <.001 | <.001 |
| Hispanic (%) | 15.7 | 16.0 | 17.1 | 13.5 | 13.9 |
| P | .91 | Reference | .677 | .228 | .305 |
GDM, gestational diabetes mellitus; IADPSG, The International Association of the Diabetes and Pregnancy Study Groups; BMI, body mass index.
Less than 135 mg/dL who did not have a 100-g oral glucose tolerance test.
Data are mean unless otherwise specified.
P values shown for continuous variables are post hoc analysis of variance with Tahmane method for multiple comparisons with IADPSG as reference (four-group analysis of variance was used for Carpenter-Coustan, IADPSG, normal glucose tolerance, and normal glucose screening columns, and a three-group analysis of variance was used for the normal glucose tolerance+normal glucose screening column).
P values for categorical variables are χ2.
Pregnancy and newborn outcome data are shown in Table 2. As anticipated, glucose intolerance (as indicated by mean 1-hour 50-g glucose screen) was significantly different among the groups, with Carpenter-Coustan (160.0 mg/dL) being the greatest followed by IADPSG (152.3 mg/dL), then normal glucose tolerance (148.2 mg/dL), and then normal glucose screening (101.1 mg/dL). All of the patients in the cohort delivered at 37 weeks of gestation or later, but those diagnosed with GDM by Carpenter-Coustan criteria had an earlier gestational age at delivery by approximately 3 days than IADPSG patients (38.63 compared with 39.09 weeks, P<.001). Newborns of IADPSG patients had significantly higher mean birth weight compared with Carpenter-Coustan (3,411 compared with 3,288 g, P<.01) and those in the control group (normal glucose tolerance and normal glucose screening; 3,411 compared with 3,240 g, P<.01). Additionally birth weight z-scores in IADPSG patients were significantly higher than those in the control group (normal glucose tolerance+normal glucose screening) (0.477 compared with 0.059, P<.01). No difference was observed in birth weight z-score between Carpenter-Coustan and IADPSG patients (0.330 compared with 0.477, P=.304). Large for gestational age (19.9% compared with 16.0%, relative risk [RR] 1.25, 95% confidence interval [CI] 0.88–1.75) and Ponderal index (2.78 compared with 2.79 g/cm3, P=1) were not different between IADPSG and Carpenter-Coustan patients, and the remainder of clinical outcomes analyzed also showed no significant differences between the Carpenter-Coustan and IADPSG groups.
Table 2.
Newborn and Pregnancy Outcome Data
| Parameter (Units) | GDM by Carpenter-Coustan (n=338) | IADPSG (n=281) | Normal Glucose Tolerance (n=772) | Normal Glucose Screening (n=6,999) | Normal Glucose Tolerance and Normal Glucose Screening* (n=7,771) |
|---|---|---|---|---|---|
| 50-g glucose screen (mg/dL) | 160.0 | 152.3 | 148.2 | 101.1 | 105.6 |
| P | <.001 | Reference | <.001 | <.001 | <.001 |
| Gestational age at delivery (wk) | 38.63 | 39.09 | 39.25 | 39.22 | 39.25 |
| P | <.001 | Reference | .265 | .147 | .076 |
| Birth weight (g) | 3,288 | 3,411 | 3,310 | 3,233 | 3,240 |
| P | .005 | Reference | .01 | <.001 | <.001 |
| Birth weight z-score | 0.330 | 0.477 | 0.209 | 0.043 | 0.059 |
| P | .304 | Reference | <.001 | <.001 | <.001 |
| LGA (%) | 16.0 | 19.9 | 13.6 | 8.3 | 8.8 |
| P | .200 | Reference | .012 | <.001 | <.001 |
| Macrosomia, 4,000 g or greater (%) | 5.9 | 9.6 | 7.3 | 4.5 | 5.0 |
| P | .084 | Reference | .210 | <.001 | <.001 |
| Ponderal Index (g/cm3) | 2.778 | 2.786 | 2.754 | 2.718 | 2.722 |
| P | 1 | Reference | .775 | .018 | .014 |
| NICU admission (%) | 5.3 | 8.5 | 6.3 | 6.8 | 6.7 |
| P | .113 | Reference | .215 | .250 | .236 |
| 1-min Apgar score less than 7 (%) | 8.0 | 8.5 | 7.0 | 7.9 | 7.8 |
| P | .803 | Reference | .397 | .710 | .667 |
| 5-min Apgar score less than 7 (%) | 0.59 | 0.36 | 0.52 | 1.4 | 1.3 |
| P† | 1 | Reference | 1 | .186 | .267 |
| Pregnancy-induced hypertension (%) | 6.8 | 8.2 | 6.5 | 6.4 | 6.4 |
| P | .514 | Reference | .334 | .221 | .223 |
| Spontaneous vaginal delivery (%) | 61.2 | 68.7 | 69.4 | 74.3 | 73.8 |
| P | .054 | Reference | .816 | .037 | .057 |
| Vaginal delivery total (%) | 64.2 | 70.8 | 72.4 | 77.0 | 76.6 |
| P | .081 | Reference | .611 | .016 | .026 |
| Primary cesarean delivery (%) | 19.5 | 17.8 | 14.8 | 13.7 | 13.8 |
| P | .582 | Reference | .231 | .053 | .059 |
| Repeat cesarean delivery (%) | 16.3 | 11.4 | 12.8 | 9.3 | 9.6 |
| P | .082 | Reference | .532 | .229 | .323 |
| Cesarean delivery total (%) | 35.8 | 29.2 | 27.6 | 23.0 | 23.4 |
| P | .081 | Reference | .611 | .016 | .026 |
| Shoulder dystocia (%) | 1.2 | 0.71 | 0.91 | 0.83 | 0.84 |
| P† | .694 | Reference | 1 | 1 | 1 |
| Postpartum hemorrhage (%) | 1.5 | 1.4 | 1.3 | 1.2 | 1.2 |
| P† | 1 | Reference | 1 | .575 | .579 |
| 3rd-degree perineal laceration (%) | 2.1 | 1.1 | 1.4 | 1.5 | 1.5 |
| P† | .361 | Reference | .771 | .801 | .801 |
| 4th-degree perineal laceration (%) | 0 | 0.36 | 0.26 | 0.19 | 0.19 |
| P† | .454 | Reference | 1 | .424 | .434 |
GDM, gestational diabetes mellitus; IADPSG, The International Association of the Diabetes and Pregnancy Study Groups; LGA, large for gestational age; NICU, neonatal intensive care unit.
Less than 135 mg/dL who did not have a 100-g oral glucose tolerance test.
Fisher’s exact test P value.
Data are mean unless otherwise specified.
P values shown for continuous variables are post hoc analysis of variance with the Tahmane method for multiple comparisons with IADPSGas as reference (four-group analysis of variance was used for Carpenter-Coustan, IADPSG, normal glucose tolerance, and normal glucose screening columns, and a three-group analysis of variance was used for the normal glucose tolerance+normal glucose screening column).
P values for categorical variables are χ2 unless otherwise noted.
The IADPSG group had a significantly higher percentage of large for gestational age newborns compared with normal glucose tolerance (19.9% compared with 13.6%, RR 1.47, 95% CI 1.09–1.97), normal glucose screening (19.9% compared with 8.3%, RR 2.40, 95% CI 1.87–3.07), or the combined control group (normal glucose tolerance+normal glucose screening) (19.9% compared with 8.8%, RR 2.25, 95% CI 1.76–2.88). Ponderal Index was significantly higher in women diagnosed by the IADPSG criteria compared with women with normal glucose screening (2.79 compared with 2.72 g/cm3, P=.018). The observed rate of neonatal admission to the neonatal intensive care unit for IADPSG neonates was 8.5%, which was not significantly different than any other group. Also, the frequency of pregnancy-induced hypertension in IADPSG patients (8.2%) was not significantly different than any of the other groups. The overall frequency of vaginal delivery was significantly lower for IADPSG patients (70.8% compared with 77.0%, RR 0.92, 95% CI 0.85–0.99) with a higher rate of primary cesarean delivery (17.8% compared with 13.7%, RR 1.30, 95% CI 1.00–1.68) when compared with normal glucose screening. Stillbirth was a rare event in this study occurring once in Carpenter-Coustan, zero times in IADPSG, twice in normal glucose tolerance, and 11 times in normal glucose screening. The study lacked adequate power to evaluate this rare outcome and the low occurrence rate precluded statistical analysis of stillbirth among the groups.
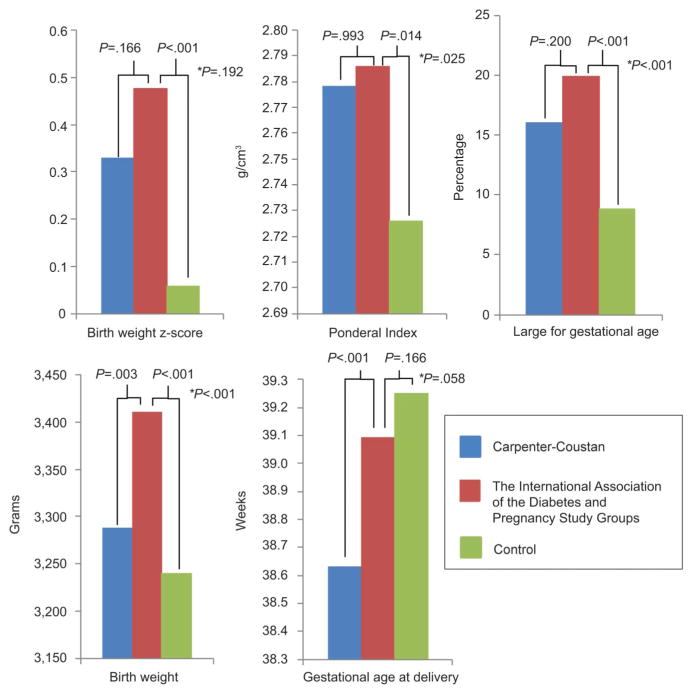
When IADPSG patients were compared with the combined control group (normal glucose tolerance+normal glucose screening) instead of just those with normal screening, the statistical findings regarding glucose intolerance, gestational age at delivery, birth weight, birth weight z-score, percent large for gestational age, Ponderal Index, neonatal intensive care unit admission, Apgar scores, pregnancy-induced hypertension, and vaginal delivery rate did not change. The difference in primary cesarean delivery rate (higher in IADPSG) was no longer statistically different with the combined control group (17.8% compared with 13.8%, RR 1.29, 95% CI 0.99–1.67). Glucose tolerance tests were performed after 34 weeks of gestation in 12.1%, 13.8%, and 14.8% of Carpenter-Coustan, IADPSG, and normal glucose tolerance groups, respectively. These rates were not statistically different among the groups (P=.812). Of those in the normal glucose screening group, 4.6% had 1-hour glucose screens done after 34 weeks of gestation. Exclusion of patients who had their OGTTs performed after 34 weeks of gestation did not change any of the statistical findings of significance in the study (data not shown). Figure 2 graphically demonstrates the findings of birth weight, birth weight z-score, Ponderal Index, percent LGA, and gestational age at delivery.
Fig. 2.
Neonatal outcomes compared among women with diabetes according to the Carpenter-Coustan criteria, women who would be added by The International Association of the Diabetes and Pregnancy Study Groups (IADPSG) criteria, and women without gestational diabetes. Women with gestational diabetes per the Carpenter-Coustan criteria were treated with home fingerstick blood sugar testing, diet, and insulin if needed. Patients added to the gestational diabetes mellitus group by IADPSG criteria were typically untreated. The control group included patients with a normal 100-g, 3-hour oral glucose tolerance test or normal 50-g 1-hour glucose screen. P values shown for continuous variables are post hoc analysis of variance with the Tahmane method for multiple comparisons (three-group analysis of variance was used). P values for the categorical variable are χ2. *P value for Carpenter-Coustan group compared with control group.
Ethridge. Perinatal Outcomes With Gestational Diabetes. Obstet Gynecol 2014.
The perinatal database was also queried for outcomes of shoulder dystocia, postpartum hemorrhage, third-degree perineal laceration, and fourth-degree perineal laceration. Each of these were rare events occurring across the entire study population at rates of 0.8%, 1.2%, 1.5%, and 0.2%, respectively. These rates did not statistically differ between the groups (χ2 P values=.910, .931, .772, and .742, respectively). The study was not appropriately powered to study outcomes as rare as these. Specific rates of each of these complications in each group and respective P values are shown in Table 2.
DISCUSSION
To address concern regarding the lack of outcome data supporting the proposed IADPSG criteria for GDM, we undertook this retrospective analysis of pregnancy outcomes in women with normal glucose screening, those with standard criteria for GDM (Carpenter-Coustan), and those who would be added to the diagnosis of GDM by the IADPSG criteria. We found that neonates of women who would be added to the diagnosis of GDM by IADPSG criteria (excluding those with Carpenter-Coustan criteria for GDM) have higher newborn birth weight, LGA, and higher birth weight z-score compared with those with normal testing. We also observed a higher birth weight for IADPSG patients compared with those with Carpenter-Coustan GDM.
In reviewing the results of this retrospective analysis, it is important to understand that the women in the study who would be added to the diagnosis of GDM by IADPSG criteria were typically not treated for diabetes. Previous studies by Crowther et al and Landon et al have shown that treatment of even mild GDM results in lower birth weight, less macrosomia, and fewer LGA neonates compared with no treatment.14,15 Consistent with prior publications, other markers of neonatal well-being (Apgar scores and neonatal intensive care unit admission rates) did not differ statistically in the group of women who would be added to GDM by IADPSG criteria when compared with treated Carpenter-Coustan diabetic women or women with normal glucose screening or testing. Our study also failed to find a difference in the frequency of hypertensive disorders of pregnancy in treated Carpenter-Coustan diabetic women compared with IADPSG diabetic women. However, the overall rate of pregnancy-induced hypertension in our study was markedly lower than prior publications.14,15 We also did not observe a difference in cesarean delivery rates between treated Carpenter-Coustan diabetic women and IADPSG diabetic women. When comparing IADPSG patients with those in a control group, the IADPSG patients had a higher cesarean delivery rate (29.4% compared with 23.4%, RR 1.25, 95% CI 1.03–1.50).
The strengths of this study include the relatively large size of the cohort and control groups. Limitations of this study include lack of adequate power to evaluate infrequent events and the racial makeup of the cohort (44.6% African American) may limit its generalizability to other populations. This study is also limited by the fact that only a small proportion of the population (those with abnormal screening) had an OGTT performed, which is in contrast to IADPSG recommendations of 75-g glucose tolerance testing of the entire pregnant population. Another limitation of this study is the use of the 100-g glucose tolerance test results in place of the 75-g glucose tolerance test recommended by the IADPSG.
Bodmer-Roy and colleagues reported a similar analysis comparing pregnancy outcomes of those women that would be diagnosed with GDM by IADPSG, and not by Canadian Diabetes Association criteria with a control group with normal glucose tolerance or normal glucose screening. They observed no difference in LGA, macrosomia, birth-weight, or neonatal intensive care unit admission, but there was a nonsignificant increased risk of pre-eclampsia and neonatal respiratory problems at birth. Cesarean delivery was more frequent in the IADPSG group compared with their control group. However, this study was limited by several factors including the use of a non-U.S. population that was older and more often Caucasian and included premature deliveries.7 Most importantly, the Bodmer-Roy study analyzed only IADPSG patients who would not meet Canadian Diabetes Association criteria for GDM or glucose intolerance, which are more stringent and inclusive than Carpenter-Coustan criteria (Coustan DR. Pregnancy outcomes in women with and without gestational diabetes mellitus according to the International Association of the Diabetes and Pregnancy Study Groups Criteria [letter]. Obstet Gynecol 2013;121:377).
In conclusion, this study demonstrated that untreated women who would be added to the diagnosis of GDM by IADPSG criteria have similar neonatal outcomes of birth weight z-score, LGA, macrosomia, and Ponderal Index as treated gestational diabetic women diagnosed by Carpenter-Coustan criteria. Each of these outcomes was significantly higher when compared with those in a control group, indicating that women who would be added to the diagnosis of GDM by IADPSG criteria are different from the normal population.
Footnotes
Presented at the 73rd Scientific Sessions of the American Diabetes Association, June 21–25, 2013, Chicago, Illinois.
Financial Disclosure
The authors did not report any potential conflicts of interest.
LEVEL OF EVIDENCE: II
References
- 1.Gestational diabetes mellitus. Practice Bulletin No. 137. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2013;122:406–16. doi: 10.1097/01.AOG.0000433006.09219.f1. [DOI] [PubMed] [Google Scholar]
- 2.Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768–73. doi: 10.1016/0002-9378(82)90349-0. [DOI] [PubMed] [Google Scholar]
- 3.O’Sullivan JB, Mahan C. Criteria for oral glucose tolerance test in pregnancy. Diabetes. 1964;13:278–85. [PubMed] [Google Scholar]
- 4.HAPO Study Cooperative Research Group. Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 5.International Association of Diabetes and Pregnancy Study Groups Consensus Panel. Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, et al. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676–82. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vandorsten JP, Dodson WC, Espeland MA, Grobman WA, Guise JM, Mercer BM, et al. NIH consensus development conference: diagnosing gestational diabetes mellitus. NIH Consens State Sci Statements. 2013;29:1–31. [PubMed] [Google Scholar]
- 7.Bodmer-Roy S, Morin L, Cousineau J, Rey E. Pregnancy outcomes in women with and without gestational diabetes mellitus according to the International Association of the Diabetes and Pregnancy Study Groups criteria. Obstet Gynecol. 2012;120:746–52. doi: 10.1097/AOG.0b013e31826994ec. [DOI] [PubMed] [Google Scholar]
- 8.Landon MB, Mele L, Spong CY, Carpenter MW, Ramin SM, Casey B, et al. The relationship between maternal glycemia and perinatal outcome. Obstet Gynecol. 2011;117:218–24. doi: 10.1097/aog.0b013e318203ebe0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mello G, Elena P, Ognibene A, Cioni R, Tondi F, Pezzati P, et al. Lack of concordance between the 75-g and 100-g glucose load tests for the diagnosis of gestational diabetes mellitus. Clin Chem. 2006;52:1679–84. doi: 10.1373/clinchem.2005.058040. [DOI] [PubMed] [Google Scholar]
- 10.Weiss PA, Haeusler M, Kainer F, Pürstner P, Haas J. Toward universal criteria for gestational diabetes: relationships between seventy-five and one hundred gram glucose loads and between capillary and venous glucose concentrations. Am J Obstet Gynecol. 1998;178:830–5. doi: 10.1016/s0002-9378(98)70500-9. [DOI] [PubMed] [Google Scholar]
- 11.Soonthornpun S, Soonthornpun K, Aksonteing J, Thamprasit A. A comparison between a 75-g and 100-g oral glucose tolerance test in pregnant women. Int J Gynaecol Obstet. 2003;81:169–73. doi: 10.1016/s0020-7292(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 12.Amini SB, Catalano PM, Hirsch V, Mann LI. An analysis of birth weight by gestational age using a computerized perinatal database, 1975–1992. Obstet Gynecol. 1994;83:342–52. [PubMed] [Google Scholar]
- 13.Miller HC, Hassanein K. Diagnosis of impaired fetal growth in newborn infants. Pediatrics. 1971;48:511–22. [PubMed] [Google Scholar]
- 14.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) Trial Group. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352:2477–86. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]
- 15.Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361:1339–48. doi: 10.1056/NEJMoa0902430. [DOI] [PMC free article] [PubMed] [Google Scholar]