Abstract
Cannabis is the most widely used illicit substance in the U.S. Women report greater positive subjective effects of cannabis, and greater cannabis withdrawal compared to men. Female rodents are more sensitive than males to some acute effects of Δ9-tetrahydrocannabinol (THC), and females also develop greater tolerance to THC in some assays. The purpose of this study was to determine whether gonadal hormones modulate THC dependence in rats. Adult rats were gonadectomized (GDX) or sham-GDX, and hormone was replaced in half of the GDX rats of each sex (testosterone in males; estradiol and/or progesterone in females). THC (30 mg/kg) or vehicle was administered twice daily for 6.5 days, followed on the seventh day by vehicle or rimonabant challenge and assessment for withdrawal-related behaviors. Sham-GDX females developed greater tolerance than males to THC-induced hypothermia, and GDX females given progesterone showed greater tolerance to THC-induced locomotor suppression. Rimonabant precipitated withdrawal, as evidenced by increased somatic signs (forepaw tremors, licking), and increased startle amplitude. Testosterone in GDX males decreased withdrawal-induced licking. Estradiol and progesterone in GDX females increased withdrawal-induced chewing, and progesterone increased withdrawal-induced sniffing. These results suggest that estradiol and progesterone may promote the development of dependence, whereas testosterone may protect against dependence. While the present study indicates that testosterone and estradiol produce opposite effects on THC-induced behavior, estradiol appears to play a broader role than testosterone in modulating THC’s behavioral effects.
Keywords: Δ9-tetrahydrocannabinol, dependence, estradiol, rimonabant, testosterone
1.0 Introduction
Cannabis is the most widely used illicit substance in the U.S., and past month and daily use has been escalating each year since 2007 (Substance Abuse and Mental Health Services Administration, 2014). As an increasing number of states decriminalize recreational use, the upward trend in regular cannabis use is expected to continue. Consequently, the number of people dependent on cannabis is likely to rise. Cessation of cannabis use is accompanied by withdrawal symptoms in 44–91% of users (Hasin et al., 2008; Levin et al., 2010). A recent study found that women had higher ratings of positive subjective effects of cannabis compared to men (Cooper & Haney, 2014), and women were also more likely to report withdrawal effects when cannabis use was terminated (Cooper & Haney, 2014; Levin et al., 2010). Greater subjective effects from use and greater tolerance development may contribute to the more rapid transition from first use to cannabis use disorder observed in women compared to men (Khan et al., 2013).
Sex differences in various effects of Δ9-tetrahydrocannabinol (THC) have been reported in rodents. For example, acute THC was more potent in producing antinociceptive and locomotor suppressing effects in adult female rats compared to males (Craft et al., 2012; Tseng and Craft, 2001). Furthermore, females developed greater tolerance than males to the antinociceptive, locomotor suppressing, and cataleptic effects of THC (Wakley et al., 2014b). Sex differences have also been found in some behavioral correlates of rimonabant-precipitated withdrawal from THC in rats (Marusich et al., 2014). These and other results suggest that female rats are generally more sensitive than males to the effects of THC.
Gonadal hormones modulate the effects of cannabinoids in adult rodents. Estradiol (E2) in gonadectomized (GDX) female rats increased THC-induced antinociception without significantly altering THC-induced locomotor suppression (Craft and Leitl, 2008; Wakley et al., 2014a), whereas testosterone (T) in GDX males lessened the locomotor suppressant effects of THC, but had no significant effect on antinociception (Craft and Leitl, 2008). E2 also increased self-administration of the cannabinoid agonist WIN55,212-2 in GDX females (Fattore et al., 2010). In contrast, E2 lessened cannabinoid-induced hypothermia and hyperphagia in GDX female guinea pigs (Kellert et al., 2009), and attenuated the acquisition and performance decrements caused by cannabinoids on an operant task in GDX female rats (Daniel et al., 2002). Adolescent GDX female rats showed increased THC-induced errors but decreased THC-induced rate decrements compared to intact females in an operant task (Winsauer et al., 2011). In contrast, adult GDX female rats showed fewer rate-decreasing and error-increasing effects of acute THC administration compared to intact females (Winsauer and Sutton, 2014). These results imply that the role of sex hormones may be assay- and age-dependent, and that E2 and T play different roles in modulating sensitivity to cannabinoid effects.
The purpose of this study was to examine the impact of gonadal hormones [T in males, and E2 and progesterone (P4) in females] on THC dependence in adult rats. Following termination of chronic THC dosing, spontaneous withdrawal was assessed in some rats; however, because spontaneous withdrawal may be difficult to detect in rodents (Compton et al., 1990), rimonabant, a CB1 receptor antagonist/inverse agonist, was used to precipitate withdrawal in other rats. Precipitated withdrawal often results in increased somatic signs including forepaw flutters/tremors and head twitches (Cook et al., 1998; Marusich et al., 2014; Tsou et al., 1995). Because acute THC produces activity suppression, antinociception, and hypothermia (Martin et al., 1991; Wiley et al., 2007), locomotor activity, antinociception, and body temperature were assessed along with somatic signs of withdrawal. Finally, sensorimotor reactivity and gating and habituation in a locomotor activity procedure were used to measure affective and rudimentary cognitive processes during withdrawal. We hypothesized that gonadal hormones contribute to sex differences in cannabinoid dependence; specifically, that estradiol enhances and/or testosterone inhibits the development of cannabinoid dependence in females and males, respectively.
2.0 Materials and Methods
2.1 Subjects
Ninety-six male and 160 female Sprague-Dawley rats (Harlan, Dublin VA) were housed in polycarbonate cages in a temperature-controlled (20–22ºC) environment with a 12–12 hr light-dark cycle (lights on at 0600 hr). Each rat was pair-housed with a rat of the same sex and in the same drug, surgery, and hormone replacement group. Rats were 57–63 days old at the beginning of the experiment. Rats had ad libitum access to food (Purina® Certified 5002 Rodent Chow, Barnes Supply, Durham NC) and water while in their home cages. All experiments were carried out in accordance with guidelines published in the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011) and were approved by the Institutional Animal Care and Use Committee at RTI.
2.2 Apparatus
Locomotor activity was measured in standard Plexiglas locomotor activity chambers. Beam breaks were recorded by San Diego Instruments Photobeam Activity System software (San Diego, CA, USA) containing two 4-beam infrared arrays. Tail flick latency was measured with a standard, automated tail flick apparatus. An infrared heat stimulus focused heat on the tail, and turned off when the rat flicked its tail (Stoelting, Wood Dale, IL). A digital thermometer (Physitemp Instruments, Inc., Clifton, NJ, USA) was used to measure rectal temperature. Startle sessions were conducted in Kinder Scientific (Poway, CA, USA) clear Plexiglas rectangular chamber which rested on a force sensing plate inside a sound attenuating cabinet. Acoustic stimuli were produced by a noise generator. For behavioral observations during withdrawal, rats were placed in a rectangular Plexiglas chamber (56 cm x 29 cm x 20 cm). Other apparatus details are the same as those described previously (Marusich et al., 2014).
2.3 Surgery
Surgical procedures were similar to those used previously (Craft and Leitl, 2008; Stoffel et al., 2003). Briefly, rats were anesthetized with a cocktail of ketamine and xylazine or isoflurane. For GDX males, the testes and epididymis were removed, and for sham-orchidectomy, the testes were exposed but not exteriorized. For GDX females, the ovaries were removed, and for sham-ovariectomy, each ovary was exposed but not exteriorized.
2.4 Steroid hormone replacement
Chronic steroid replacement was delivered by Silastic capsules implanted immediately after gonadectomy or sham-gonadectomy (Stoffel et al., 2003; Wakley et al., 2015). T capsules were filled with a 10 mm length of T, and E2 capsules were filled with a 1 mm length of E2. Males were implanted with two blank capsules or one T capsule/100 g body weight, rounding to the closest 100 grams. Females were implanted with one blank capsule, or one E2 capsule regardless of body weight (Stoffel et al., 2003; Wakley et al., 2015). Capsules were in place for 20–21 days. These hormone treatments have previously been shown to restore reproductive behavior and organ weights similar to those for intact male and female rats (Stoffel et al., 2003), and produce stable hormone levels for at least 6 weeks after surgery (Wakley et al., 2015). After study completion, all capsules were removed to verify that the correct type (hormone or blank) and number of capsules was implanted, and that capsules remained intact. Additionally, half of the GDX females received 500 μg of P4 dissolved in 0.1 ml safflower oil s.c. every 3 days to mimic the pre-ovulatory P4 surge observed in normally cycling females (Feder, 1981). The remaining females and all males received a P4 vehicle (safflower oil) injection on the same days.
2.5 Procedures
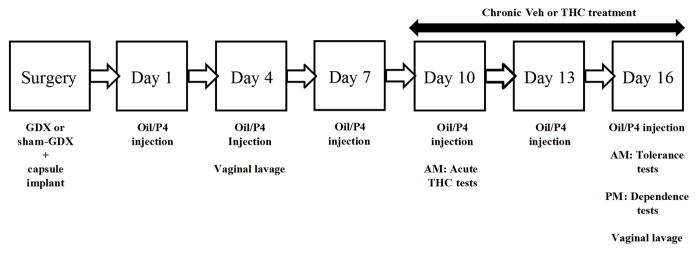
All rats underwent surgery six to ten days after arrival. Males were divided into three surgery and hormone groups and females were divided into five surgery and hormone groups; rats within each surgery and hormone group were then divided into one of four drug treatment groups (n=8 rats of each sex per group; see Table 1). Figure 1 shows the experimental timeline. Rats were given injections of safflower oil or P4 at approximately 0700 hr beginning 5 (n=50) or 6 (n=206) days post-surgery (Day 1). All rats were injected with vehicle or 30 mg/kg THC twice daily for 6.5 days beginning on Day 10, with injections occurring at approximately 0700 hr and 1500 hr on Days 10–15. The vehicle/vehicle (Veh/Veh) group was administered vehicle twice daily. The vehicle/rimonabant group (Veh/Rim) received vehicle twice daily for 6.5 days and 10 mg/kg rimonabant for the second injection on Day 16. The THC/vehicle (THC/Veh) group received 30 mg/kg THC twice daily for 6.5 days and vehicle for the second injection on Day 16. The THC/rimonabant (THC/Rim) group received 30 mg/kg THC twice daily for 6.5 days and 10 mg/kg rimonabant for the second injection on Day 16. All injections were given s.c. except the morning injection on Day 10 and both injections on Day 16, which were given i.p. Previous research on THC has primarily used the i.p. route of administration, which produces characteristic cannabinoid effects (Wiley et al., 2007).
Table 1.
Surgery, capsule, P4/oil conditions, and sample size for each treatment group. The first three groups contained males and females, and the last two groups contained only females. Sample size is the number of rats remaining at the end of the study and following capsule checks (see 2.11 Data Analysis).
| Conditions | Veh/Veh | Veh/Rim | THC/Veh | THC/Rim |
|---|---|---|---|---|
| Surgery | Sham | Sham | Sham | Sham |
| Capsule | Blank | Blank | Blank | Blank |
| P4/Oil | Oil | Oil | Oil | Oil |
| Sample Size ♂ | n=8 | n=8 | n=7 | n=8 |
| Sample Size ♀ | n=8 | n=8 | n=8 | n=8 |
|
| ||||
| Surgery | GDX | GDX | GDX | GDX |
| Capsule | Blank | Blank | Blank | Blank |
| P4/Oil | Oil | Oil | Oil | Oil |
| Sample Size ♂ | n=7 | n=8 | n=8 | n=8 |
| Sample Size ♀ | n=8 | n=8 | n=7 | n=8 |
|
| ||||
| Surgery | GDX | GDX | GDX | GDX |
| Capsule | Hormone | Hormone | Hormone | Hormone |
| P4/Oil | Oil | Oil | Oil | Oil |
| Sample Size ♂ | n=8 | n=8 | n=8 | n=8 |
| Sample Size ♀ | n=6 | n=7 | n=7 | n=8 |
|
| ||||
| Surgery | GDX | GDX | GDX | GDX |
| Capsule | Blank | Blank | Blank | Blank |
| P4/Oil | P4 | P4 | P4 | P4 |
| Sample Size ♀ | n=8 | n=8 | n=8 | n=8 |
|
| ||||
| Surgery | GDX | GDX | GDX | GDX |
| Capsule | Hormone | Hormone | Hormone | Hormone |
| P4/Oil | P4 | P4 | P4 | P4 |
| Sample Size ♀ | n=7 | n=8 | n=8 | n=7 |
Abbreviations: ♂: male; ♀: female; GDX: gonadectomized; P4: progesterone; THC: Δ9-tetrahydrocannabinol.
Figure 1.
Experimental timeline of surgery, injections, and tests for rats that started chronic treatment 15 days after surgery. For rats that started chronic treatment 14 days after surgery, the entire schedule was shifted one day earlier. GDX: gonadectomized; P4: progesterone; THC: Δ9-tetrahydrocannabinol; Veh: vehicle.
2.6 Acute THC effects (Day 10)
On Day 10 after oil or P4 injection, baseline body temperature and tail flick latency were measured, followed by administration of the first drug injection. Thirty min later, rats were placed in the locomotor activity chambers for 15 min. Immediately thereafter, body temperature and tail flick latency were measured again.
2.7 Tolerance (Day 10 vs. morning of Day 16)
On Day 16, baseline body temperature and tail flick latency were measured, followed by the final vehicle or THC injection. Thirty min later, rats were placed in the locomotor activity chambers for 15 min. Immediately thereafter, body temperature and tail flick latency were measured.
2.8 Dependence (afternoon of Day 16)
Four hr after tolerance evaluation, vehicle or rimonabant challenge was administered. Five min later, rats were placed in the locomotor activity chambers for 15 min, immediately followed by body temperature and tail flick latency measurement. Rats were then placed in the observation arena for 30 min and their overt behavior was scored. All observations were made by one trained technician who was blind to the challenge condition. The number of times the following behaviors occurred was recorded: forepaw tremors/flutters, head twitches, “wet dog” shakes (entire body), grooming, sniffing, scratching with hind paw, ptosis, writhing, piloerection, retropulsion, audible vocalizations, licking objects (e.g. wall of chamber), upright tail, and any other unusual behavior such as chewing (jaw opening and closing as if chewing food, but with no audible sound).
Following observations, rats were exposed to an auditory startle session similar to that used previously (Marusich et al., 2014). Sessions were composed of four trial types. On pulse trials, rats were exposed to a 120-dB acoustic stimulus. Prepulse + pulse trials consisted of an 85-dB prepulse followed by a 40 ms pulse. The other two types of trials consisted of an 85-dB prepulse alone or to 69-dB background noise.
2.9 Determination of estrous cyclicity
For females, vaginal cytology samples were collected at approximately 1400 hr on Days 4 and 16. Estrous cycle stage was determined cytologically following vaginal lavage. Proestrus was identified as a predominance (approximately 75% or more) of nucleated epithelial cells. A predominance of cornified epithelial cells was designated as estrus. Diestrus was recognized by scattered, nucleated or cornified epithelial cells and leukocytes, or a relative lack of any type of cells (Freeman, 1988).
2.10 Drugs
THC [National Institute on Drug Abuse (NIDA), Bethesda, MD, USA] and rimonabant (NIDA) were suspended in a vehicle of 7.8 % Polysorbate 80 N.F. (VWR, Radnor, PA, USA) and 92.2% sterile saline USP (Butler Schein, Dublin, OH, USA). Doses of THC and rimonabant were administered at a volume of 1 ml/kg. Ketamine (Fort Dodge, Fort Dodge, IA) and xylazine (Lloyd Laboratories, Shenandoah, IA) were diluted with sterile saline USP, and the cocktail was administered at a volume of 3 ml/kg. P4 (Steraloids, Newport, RI, USA) was dissolved in safflower oil USP (Spectrum Chemical, Gardena, CA, USA). E2 and T (Steraloids, Newport, RI, USA) were administered through Silastic capsules assembled in-house (c.f. Stoffel et al., 2003).
2.11 Data Analysis
2.11.1 Effects of THC
Antinociception was expressed as the percent maximum possible effect (%MPE) using a 10-s maximum cut off latency: [(test latency-baseline latency)/(10-baseline latency)] x 100. Rectal temperatures were expressed as the difference between control temperature and temperature following injection (ΔºC). Locomotor activity was measured as the total number of photocell beam interruptions. For behavioral observation sessions, the mean (± SEM) number of incidents was calculated for each behavior. There was no effect of sex, hormone, or drug group on audible vocalizations, head twitches or piloerection, therefore, these behaviors were not analyzed further. The remaining behaviors were grouped into four domains, and the alpha level was controlled within each domain: CNS activity (α = 0.025; grooming, sniffing), CNS excitability (α = 0.0063; forepaw tremor, scratching, upright tail, writhing, licking, retropulsion, wet dog shakes, unusual behavior in the form of chewing), and autonomic effects (α = 0.05; ptosis) (Bowen et al., 1996). Startle score was defined as the average of the maximum Newtons of pressure exerted during pulse-alone trials. Prepulse inhibition (PPI) was calculated for prepulse + pulse trials as a percentage of pulse-alone scores [(mean startle amplitude for pulse-alone trials - mean startle amplitude for prepulse + pulse trials)/mean startle amplitude for pulse-alone trials] x 100. For the acute (Day 10) and tolerance (Day 16 - Day 10) components, data for rats from all challenge groups that received a given repeated treatment (i.e., vehicle or THC) were grouped for analysis.
2.11.2 Effects of sex and gonadal hormones
Between-subjects ANOVAs were used for all analyses. Sex differences were assessed by comparing sham-GDX females to sham-GDX males. GDX male groups were compared to assess effects of T. GDX female groups were compared to assess effects of the ovarian hormones E2 and P4. Significant ANOVAs were followed by Tukey post hoc tests (α = 0.05 unless otherwise specified) to specify differences among means.
2.11.3 Body Weight
Body weight change was calculated as the difference between chronic dosing days (Day 16 - Day 10). Data were analyzed separately for each sex using between-subjects ANOVAs, with factors of chronic treatment (vehicle or THC) and surgery-hormone condition (in males, sham-GDX vs. GDX+0 vs. GDX+T; in females, sham-GDX vs. GDX+0 vs. GDX+E2 vs. GDX+P4 vs. GDX+E2/P4). Body weight results are presented in the supplemental material. Estrous stage was used to confirm that GDX surgery was complete, and that E2 and P4 produced the expected effects. Data from 10 rats were dropped before analysis: 6 received an incorrect capsule, 1 GDX+E2/P4 female had vaginal cytology samples that indicated incomplete surgery or capsule malfunction, 1 sham-GDX THC/Veh male and 1 GDX+E2 Veh/Veh female died, and 1 sham-GDX THC/Rim female was euthanized following seizures.
3.0 Results
3.1 Tolerance (Day 10 vs. morning of Day 16)
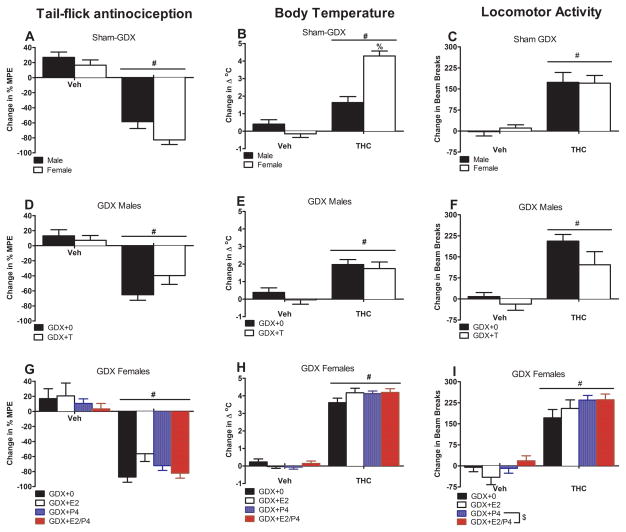
Tolerance development is depicted as change in THC effect from Day 10 to Day 16 of treatment (Day 16 effect – Day 10 effect). Figure 2 (panel A) shows that sham-GDX males and females developed tolerance to the antinociceptive effects of THC [F(1,59)=117.08, p<0.05], with a slightly but not significantly greater decrease in effect in females compared to males [F(1,59)=1.92, p=0.17]. Sham-GDX males and females also developed tolerance to the hypothermic effects of THC, but the change in effect was greater in females [sex x chronic treatment: F(1,59)=34.44, p<0.05] (Figure 2, panel B). Tolerance also developed to the locomotor suppressive effects of THC with no sex differences in sham-GDX rats [F(1,59)=48.99, p<0.05] (Figure 2, panel C).
Figure 2.
Tolerance to THC’s effects on tail flick antinociception (left column), body temperature (center column), and locomotor activity (right column) plotted as change in effect from Day 10 to Day 16. Mean (±SEM) values are shown for sham-GDX males and females (n=7–8/group; panels A–C), GDX males (n=7–8/group; panels D–F), and GDX females (n=6–8/group; panels G–I). # significant difference from Veh group (main effect). ). $ significant P4 effect (main effect). % significant sex difference for the same drug group (interaction). GDX: gonadectomized; E2: estradiol; MPE: maximum possible effect; P4: progesterone; T: testosterone: THC: Δ9-tetrahydrocannabinol; Veh: vehicle.
Figure 2 (panels D-E) shows that in GDX males, tolerance developed to THC’s antinociceptive [F(1,59)=52.31, p<0.05] and hypothermic [F(1,59)=32.32, p<0.05] effects, and T did not significantly alter tolerance development. Tolerance also developed to the locomotor suppressive effects of THC with no T effect in GDX males [F(1,59)=31.92, p<0.05] (Figure 2, panel F). In GDX females (Figure 2, panels G-H), tolerance also developed to THC’s antinociceptive [F(1,113)=162.52, p<0.05] and hypothermic [F(1,113)=923.75, p<0.05] effects, and neither E2 nor P4 significantly influenced tolerance development. Tolerance also developed to the locomotor suppressive effects of THC with E2 effect in GDX females [F(1,110)=196.39, p<0.05] (Figure 2, panel I). In contrast, P4 increased tolerance to the locomotor suppressant effects of THC in GDX females [F(1,110)=4.91, p<0.05].
3.2 Dependence (afternoon of Day 16)
3.2.1 Tail Flick Antinociception and Body Temperature
Dependence was assessed on Day 16, beginning 30 min after vehicle or rimonabant injection. On the tail flick test, sham-GDX females had overall higher %MPE values compared to sham-GDX males [F(1,55)=10.59, p<0.05], but %MPE values did not differ among the 4 dependence groups (Veh/Veh, Veh/Rim, THC/Veh, THC/Rim) (data not shown). Neither T in GDX males nor E2 or P4 in GDX females altered %MPE values in any dependence group (data not shown).
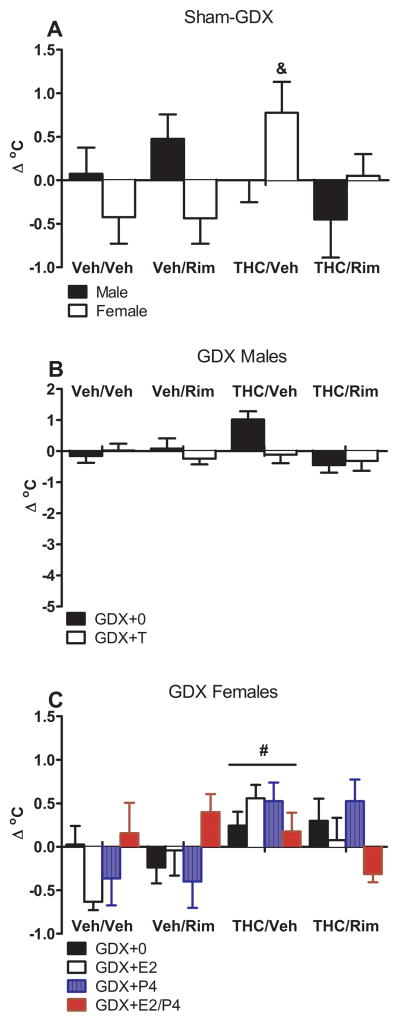
Figure 3 (panel A) shows that sham-GDX females administered chronic THC (THC/Veh) showed a greater increase in body temperature than other sham-GDX dependence groups [dependence group x sex: F(1,55)=3.20, p<0.05]. In GDX males, T did not affect change in body temperature during dependence testing, and this measure did not differ among the four dependence groups (Figure 3, panel B). Similar to sham-GDX females, GDX females treated with chronic THC (THC/Veh) were hyperthermic compared to other dependence groups [F(3,105)=4.55, p<0.05] (Figure 3, panel C). In GDX+0 females, THC/Rim increased temperature, whereas Veh/Rim decreased temperature [dependence group x E2: F(3,105)=3.14, p<0.05]. P4 appeared to reverse E2 modulation of body temperature in GDX females with the direction of the modulation varying across dependence treatment groups [dependence group x E2 x P4: F(3,105)=3.70, p<0.05].
Figure 3.
THC dependence, assessed as change in body temperature following vehicle or rimonabant challenge on Day 16. Mean (±SEM) values are shown for sham-GDX males and females (n=7–8/group; panel A), GDX males (n=7–8/group; panels B), and GDX females (n=6–8/group; panel C). # significant difference from Veh/Veh group (main effect). & significant difference from Veh/Veh group for the same sex (interaction). GDX: gonadectomized; E2: estradiol; P4: progesterone; Rim: rimonabant; THC: Δ9-tetrahydrocannabinol; Veh: vehicle.
3.2.2 Locomotor Activity
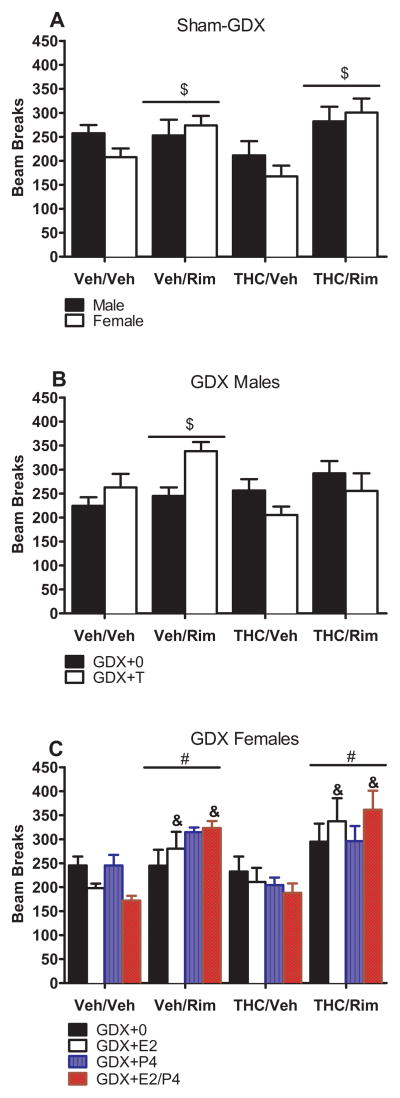
Figure 4 shows locomotor activity after the challenge injection with vehicle or rimonabant. In sham-GDX rats, rimonabant challenge (Veh/Rim and THC/Rim) increased activity compared to the THC/Veh group in both sexes [F(3,55)=5.92, p<0.05] (Figure 4, panel A). In GDX males, rimonabant challenge did not significantly change activity, although T tended to increase activity in chronic-vehicle treated rats and decrease it in chronic THC-treated rats [dependence group x T: F(3,53)=3.86, p<0.05] (Figure 4, panel B). In GDX females, rimonabant (Veh/Rim and THC/Rim) increased activity, and this effect was greater in E2-treated females [dependence group: F(3,102)=14.04, p<0.05; dependence group x E2: F(3,102)=3.47, p<0.05] (Figure 4, panel C).
Figure 4.
THC dependence, assessed as locomotor activity following vehicle or rimonabant challenge on Day 16. Mean (±SEM) number of beam breaks is shown for sham-GDX males and females (n=7–8/group; panel A), GDX males (n=6–8/group; panel B), and GDX females (n=6–8/group; panel C). # significant difference from Veh/Veh group (main effect). $ significant difference from THC/Veh group (main effect). & significant difference from Veh/Veh group for the same hormone group (interaction). GDX: gonadectomized; E2: estradiol; P4: progesterone; Rim: rimonabant; T; testosterone; THC: Δ9-tetrahydrocannabinol; Veh: vehicle.
3.2.3 Behavioral Observations of Withdrawal
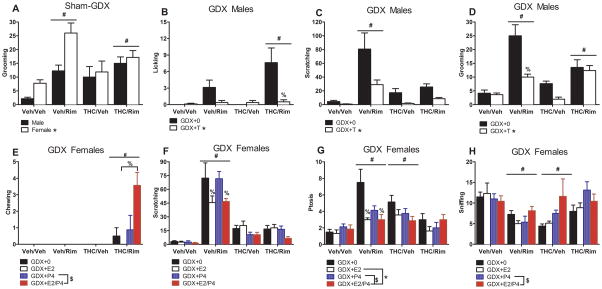
Figure 5 shows somatic signs observed after rimonabant injection. In chronic THC-treated, rimonabant-challenged sham-GDX rats, precipitated withdrawal was evident as increased forepaw tremors [F(3,54)=6.34, p<0.0063], licking [F(3,54)=11.27, p<0.0063], upright tail [F(3,54)=10.50, p<0.0063], and chewing [F(3,54)=4.73, p<0.0063]; females tended to show more tremors, licking and chewing than males, but these sex differences were not significant (data not shown). While retropulsion was also increased in the Rim/THC groups, this effect was not significant [F(3,54)=3.91, p>0.0063] (data not shown). Rimonabant also increased grooming [F(3,54)=10.29, p<0.025], and females showed more grooming than males [F(1,54)=9.10, p<0.025], but only in the chronic vehicle-treated group (Veh/Rim) (Figure 5, panel A). Rimonabant also increased scratching [F(3,54)=21.58, p<0.0063] to a slightly greater extent in females than in males (data not shown). THC and rimonabant significantly increased ptosis in the Veh/Rim and THC/Veh groups, with no sex differences [dependence group: F(3,54)=3.88, p<0.05] (data not shown). Lastly, males displayed writhing whereas females did not [F(1,54)=10.06, p<0.0063] (data not shown), but this sex difference was not specific to the precipitated withdrawal group or the injection of rimonabant. There were no significant sex differences or differences among dependence groups in sniffing or wet dog shakes (data not shown).
Figure 5.
THC dependence in sham-GDX rats (n=7–8/group), GDX males (n=7–8/group), and GDX females (n=6–8/group) assessed as behavioral signs of withdrawal following vehicle or rimonabant challenge on Day 16. Mean (±SEM) incidents of behavior are shown. Behaviors that did not show both drug effects and sex/hormone effects are not shown to elucidate interactions between sex/hormone and drug group. * significant sex, T or E2 effect (main effect). # significant difference from Veh/Veh group (main effect). $ significant P4 effect (main effect). % significant difference from GDX+0 for the same drug group (interaction). E2: estradiol. P4: progesterone; Rim: rimonabant; THC: Δ9-tetrahydrocannabinol; Veh: vehicle.
In chronic THC-treated, rimonabant-challenged male GDX rats, precipitated withdrawal was evident as increased forepaw tremors [F(3,55)=12.09, p<0.0063], licking [F(3,55)=5.78, p<0.0063], and retropulsion [F(3,55)=6.05, p<0.0063] (data not shown). T significantly decreased licking [dependence group x T: F(3,55)= 5.06, p<0.0063] (Figure 5, panel B). Rimonabant increased wet dog shakes [F(3,55)=9.43, p<0.0063] (data not shown), scratching [F(3,55)=12.87, p<0.0063], and ptosis [F(3,55)=2.89, p<0.05] in the Veh/Rim group (data not shown), and increased grooming in both Veh/Rim and THC/Rim groups [F(3,55)=21.34, p<0.025]. T slightly decreased wet dog shakes, and significantly decreased scratching [T: F(1,55)=11.45, p<0.0063], and grooming [T: F(1,55)=15.38, p<0.025; T x dependence group: F(3,55)=5.55, p<0.025] (Figure 5, panels C-D). There were no significant differences among dependence groups or between GDX+0 and GDX+T males in chewing, upright tail, writhing, or sniffing (data not shown).
In chronic THC-treated, rimonabant-challenged GDX female rats, precipitated withdrawal was evident as increased forepaw tremors [F(3,105)=21.44, p<0.0063], licking [F(3,105)=14.65, p<0.0063], retropulsion [F(3,105)=16.93, p<0.0063], chewing [F(3,105)=14.20, p<0.0063], and upright tail [F(3,105)=30.57, p<0.0063] (data not shown). P4 and E2 increased precipitated withdrawal-induced chewing [P4: F(1,105)=14.20, p<0.0063; dependence group x E2: F(3,105)=5.22, p<0.0063] (Figure 5, panel E). Several other behaviors were increased by THC or rimonabant alone. THC increased grooming [F(3,105)=15.00, p<0.025] (data not shown), and rimonabant increased both grooming and scratching [F(3,105)=73.59, p<0.0063]; rimonabant-induced increases in scratching were dampened by E2 [dependence group x E2: F(3,105)=4.64, p<0.0063] (Figure 5, panel F). THC and rimonabant increased ptosis except when combined [dependence group: F(3,105)=11.67, p<0.05]; both E2 and P4 dampened these effects [P4: F(1,105)=4.62, p<0.05; E2: F(1,105)=6.19, p<0.05; dependence group x E2: F(3,105)=4.99, p<0.05] (Figure 5, panel G). Rimonabant also increased writhing [F(3,105)=7.29, p<0.0063] and wet dog shakes [F(3,105)=7.64, p<0.0063] (data not shown). Finally, THC and rimonabant, but not the combination (THC/Rim), decreased sniffing [F(3,105)=7.21, p<0.025], while P4 increased sniffing [P4: F(1,105)=5.52, p<0.025] (Figure 5, panel H).
3.2.4 Startle
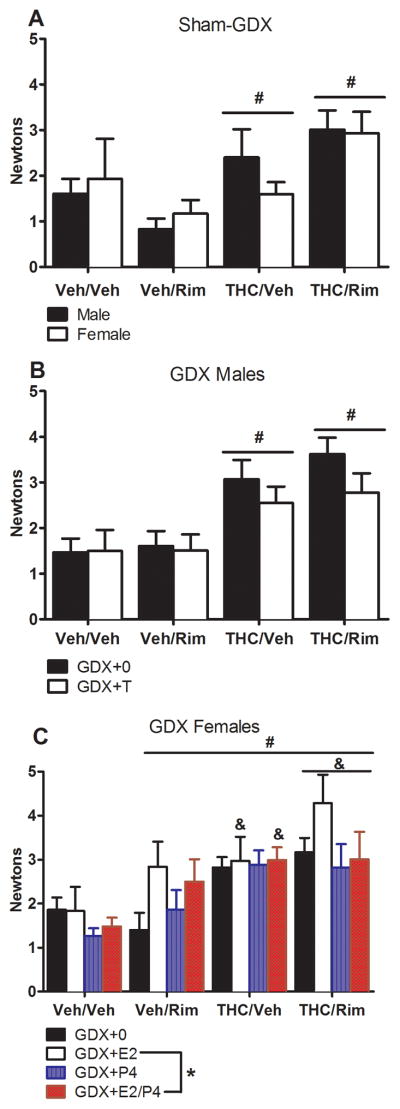
Termination of chronic THC administration and rimonabant-precipitated withdrawal increased startle reactivity in sham-GDX rats [F(3,54)=5.68, p<0.05] (Figure 6, panel A), with no sex differences. Termination of chronic THC and precipitated withdrawal increased startle reactivity in GDX males [F(3,55)=10.48, p<0.05]; this effect was slightly but not significantly reduced by T [dependence group x T: F(3,55)=0.55, p=0.65] (Figure 6, panel B). In GDX females, termination of chronic THC and precipitated withdrawal also increased startle reactivity [F(3,105)=10.86, p<0.05] as did E2 [E2: F(1,105)=4.79, p<0.05] (Figure 6, panel C). There were no effects of T in GDX males or E2 or P4 in GDX females on PPI, nor were there differences among dependence groups in PPI for GDX rats (data not shown).
Figure 6.
THC dependence assessed as startle response following vehicle or rimonabant challenge on Day 16. Mean (±SEM) Newtons are shown for sham-GDX males and females (n=7–8/group; panel A), GDX males (n=7–8/group; panel B), and GDX females (n=6–8/group; panel C). * significant E2 effect (main effect). # significant difference from Veh/Veh group (main effect). & significant difference from Veh/Veh group for the same hormone group (interaction). GDX: gonadectomized; E2: estradiol; P4: progesterone; Rim: rimonabant; T; testosterone; THC: Δ9-tetrahydrocannabinol; Veh: vehicle.
4.0 Discussion
Tolerance developed to acute effects of THC, similar to what has been reported previously (Bass and Martin, 2000; Wakley et al., 2014b); however, in contrast with past studies (Wakley et al., 2014b; Wiley et al., 2007), sham-GDX females developed greater tolerance than sham-GDX males to the hypothermic effects of THC, and no significant sex difference in antinociceptive tolerance was found. These discrepancies in sex differences in tolerance are likely due to procedural differences among studies including chronic doses given, pretreatment times, routes of injection, and single vs. multi-dose testing (Wakley et al., 2014b; Wiley et al., 2007). The greater tolerance to hypothermic effects observed in sham-GDX females may be due to the greater hypothermia produced by acute THC in sham-GDX females. That is, initially 30 mg/kg THC produced greater hypothermia in females than in males, so giving a functionally larger chronic dose in females than in males would be expected to lead to greater tolerance in females than in males (Barrett et al., 2001). Additionally, GDX+P4 females developed greater tolerance than GDX+0 females to the locomotor suppressant effects of THC, suggesting that P4 may modulate the development of tolerance to effects of THC.
Both spontaneous and precipitated withdrawal increased startle amplitude in sham-GDX and GDX rats of both sexes, suggesting that THC dependence is associated with anxiogenesis. This is consistent with past reports of increased startle amplitude and decreased open arm time in an elevated plus maze for THC-dependent rats (Harte-Hargrove and Dow-Edwards, 2012; Huang et al., 2010; Marusich et al., 2014). Precipitated withdrawal was observed in the form of increased forepaw tremors and licking in accord with past research (Aceto et al., 1996; Cook et al., 1998; Lichtman et al., 2001; Marusich et al., 2014; Tsou et al., 1995). Although gonadal hormone modulation of THC-induced dependence was not consistent across measures, the interactions found suggest that E2 and P4 may contribute to greater dependence in females, while T may protect against dependence in males. These results are consistent with a previous study that found opposite effects of THC withdrawal on anxiety-related behaviors in male and female rats, with females showing greater anxiety (Harte-Hargrove and Dow-Edwards, 2012). Interestingly, E2 and P4 lessened spontaneous withdrawal-induced ptosis, indicating that ovarian hormones may normalize effects of THC in some instances.
While precipitated withdrawal increased grooming and wet dog shakes in a previous study (Tsou et al., 1995), these somatic signs appeared to be produced by rimonabant alone in the present study. Similar to the findings for THC withdrawal, effects of rimonabant were modulated by gonadal hormones in some instances. These results suggest that effects of ovarian hormones on rimonabant-induced behavior vary across different somatic signs, whereas T may lessen the impact of rimonabant on somatic signs.
Only a few previous studies have included analysis of P4 as a factor in drug effects. P4 decreased THC’s acute antinociceptive potency in both a paw pressure and tail withdrawal assay, and P4 continued to decrease THC’s effects following chronic administration in the paw pressure assay (Wakley et al., 2015). In contrast, P4 had no effect on THC-induced antinociception in GDX female rats in a similar study (Wakley et al., 2014a). These disparate results may be due to different THC dosing regimens (cumulative dosing v. one dose per day). P4 also produced dose-dependent decreases in WIN 55,212-2-induced antinociception in GDX mice (Kalbasi Anaraki et al., 2008). In contrast, P4 increased the cataleptic effects of WIN 55,212-2 in GDX mice (Kalbasi Anaraki et al., 2008), but not GDX rats (Wakley et al., 2014a). In a related study, P4 decreased cannabinoid receptor density in striatum, but increased density in the mesencephalon (Rodriguez de Fonseca et al., 1994). In the present study, P4 was associated with greater tolerance to effects of THC, greater precipitated withdrawal, and mixed effects in spontaneous THC withdrawal. These results combined suggest that P4 modulation of cannabinoid effects is complicated, and further research is needed to elucidate the impact of this ovarian hormone.
One potential explanation for sex and gonadal hormone differences in cannabinoid effects is the active THC metabolite 11-OH-THC, which females produce more readily than males. Blocking THC metabolism equalized THC-induced antinociception in male and female rats (Tseng et al., 2004). Additionally, following repeated THC administration 11-OH-THC levels in blood increased in female rats, but decreased in males (Wiley and Burston, 2014). Higher levels of 11-OH-THC would likely increase the development of tolerance and dependence through increased desensitization and/or down-regulation of CB1 receptors; however, this hypothesis is not supported by current research on sex differences in CB1 receptor density following chronic THC administration, which shows inconsistent sex differences (Burston et al., 2010). Further research is needed to elucidate the role of gonadal hormones in THC metabolism.
5.0 Conclusion
In summary, GDX+P4 females and sham-GDX females showed greater tolerance than males to select effects of THC. Sex and gonadal hormones inconsistently affected the various measures of THC dependence, but taken together the results suggest that E2 and P4 may contribute to somewhat greater dependence in females, while T may protect against dependence in males. Thus it would be predicted that T and E2/P4 would have opposite effects on humans’ experience of cannabis, with ovarian hormones making women more sensitive to the development of tolerance and dependence. Results are consistent with those found for humans in which women report greater abuse-related effects of cannabis (Cooper & Haney, 2014), and experience more severe cannabis withdrawal than men (Levin et al., 2010). Women using oral contraceptives may be at greatest risk for cannabis dependence due to their elevated levels of estrogens and progestins.
Supplementary Material
Acknowledgments
This research was supported by NIH/NIDA Grant DA-016644. The funding source had no other role other than financial support. The authors thank Kateland Antonazzo, Ricardo Cortes, Nikita Pulley, and Alexa Wakley for technical assistance.
Footnotes
Disclosures
All authors contributed in a significant way to the manuscript, and have read and approved the final manuscript. The authors have no conflict of interest.
References
- Aceto MD, Scates SM, Lowe JA, Martin BR. Dependence on delta 9-tetrahydrocannabinol: studies on precipitated and abrupt withdrawal. Journal of Pharmacology and Experimental Therapeutics. 1996;278:1290–1295. [PubMed] [Google Scholar]
- Barrett AC, Cook CD, Terner JM, Craft RM, Picker MJ. Importance of sex and relative efficacy at the mu opioid receptor in the development of tolerance and cross-tolerance to the antinociceptive effects of opioids. Psychopharmacology. 2001;158:154–164. doi: 10.1007/s002130100821. [DOI] [PubMed] [Google Scholar]
- Bass CE, Martin BR. Time course for the induction and maintenance of tolerance to Delta(9)- tetrahydrocannabinol in mice. Drug and Alcohol Dependence. 2000;60:113–119. doi: 10.1016/s0376-8716(99)00150-7. [DOI] [PubMed] [Google Scholar]
- Bowen SE, Wiley JL, Evans EB, Tokarz ME, Balster RL. Functional observational battery comparing effects of ethanol, 1,1,1-trichloroethane, ether, and flurothyl. Neurotoxicology and Teratology. 1996;18:577–585. doi: 10.1016/0892-0362(96)00064-5. [DOI] [PubMed] [Google Scholar]
- Burston JJ, Wiley JL, Craig AA, Selley DE, Sim-Selley LJ. Regional enhancement of cannabinoid CB1 receptor desensitization in female adolescent rats following repeated Δ9-tetrahydrocannabinol exposure. British Journal of Pharmacology. 2010;161:103–112. doi: 10.1111/j.1476-5381.2010.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton DR, Dewey WL, Martin BR. Cannabis dependence and tolerance production. Advances in Alcohol and Substance Abuse. 1990;9:129–147. doi: 10.1300/J251v09n01_08. [DOI] [PubMed] [Google Scholar]
- Cook SA, Lowe JA, Martin BR. CB1 receptor antagonist precipitates withdrawal in mice exposed to delta9-tetrahydrocannabinol. Journal of Pharmacology and Experimental Therapeutics. 1998;285:1150–1156. [PubMed] [Google Scholar]
- Cooper ZD, Haney M. Investigation of sex-dependent effects of cannabis in daily cannabis smokers. Drug and Alcohol Dependence. 2014;136:85–91. doi: 10.1016/j.drugalcdep.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft RM, Leitl MD. Gonadal hormone modulation of the behavioral effects of delta9-tetrahydrocannabinol in male and female rats. European Journal of Pharmacology. 2008;578:37–42. doi: 10.1016/j.ejphar.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Craft RM, Wakley AA, Tsutsui KT, Laggart JD. Sex differences in cannabinoid 1 vs. cannabinoid 2 receptor-selective antagonism of antinociception produced by delta9-tetrahydrocannabinol and CP55,940 in the rat. Journal of Pharmacology and Experimental Therapeutics. 2012;340:787–800. doi: 10.1124/jpet.111.188540. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Winsauer PJ, Brauner IN, Moerschbaecher JM. Estrogen improves response accuracy and attenuates the disruptive effects of delta-9-THC in ovariectomized rats responding under a multiple schedule of repeated acquisition and performance. Behavioral Neuroscience. 2002;116:989–998. [PubMed] [Google Scholar]
- Fattore L, Spano MS, Altea S, Fadda P, Fratta W. Drug- and cue-induced reinstatement of cannabinoid-seeking behaviour of male and female rats: influence of ovarian hormones. British Journal of Pharmacology. 2010;160:724–735. doi: 10.1111/j.1476-5381.2010.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder HH. Estrous cyclicity in mammals. In: Adler NT, editor. Neuroendocrinology of reproduction. New York, NY: Plenum Press; 1981. pp. 279–348. [Google Scholar]
- Freeman ME. The ovarian cycle of the rat. In: Knobil E, Neill J, editors. The physiology of reproduction. New York, NY: Raven Press; 1988. pp. 1893–1928. [Google Scholar]
- Harte-Hargrove LC, Dow-Edwards DL. Withdrawal from THC during adolescence: sex differences in locomotor activity and anxiety. Behavioral Brain Research. 2012;231:48–59. doi: 10.1016/j.bbr.2012.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Keyes KM, Alderson D, Wang S, Aharonovich E, Grant BF. Cannabis withdrawal in the United States: results from NESARC. Journal of Clinical Psychiatry. 2008;69:1354–1363. doi: 10.4088/jcp.v69n0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Liu-Chen LY, Kirby LG. Anxiety-like effects of SR 141716-precipitated delta9-tetrahydrocannabinol withdrawal in mice in the elevated plus-maze. Neuroscience Letters. 2010;475:165–168. doi: 10.1016/j.neulet.2010.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalbasi Anaraki D, Sianati S, Sadeghi M, Ghasemi M, Paydar MJ, Ejtemaei Mehr S, Dehpour AR. Modulation by female sex hormones of the cannabinoid-induced catalepsy and analgesia in ovariectomized mice. European Journal of Pharmacology. 2008;586:189–196. doi: 10.1016/j.ejphar.2008.02.055. [DOI] [PubMed] [Google Scholar]
- Kellert BA, Nguyen MC, Nguyen C, Nguyen QH, Wagner EJ. Estrogen rapidly attenuates cannabinoid-induced changes in energy homeostasis. European Journal of Pharmacology. 2009;622:15–24. doi: 10.1016/j.ejphar.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SS, Secades-Villa R, Okuda M, Wang S, Pérez-Fuentes G, Kerridge BT, Blanco C. Gender differences in cannabis use disorders: results from the National Epidemiologic Survey of Alcohol and Related Conditions. Drug and Alcohol Dependence. 2013;130:101–108. doi: 10.1016/j.drugalcdep.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin KH, Copersino ML, Heishman SJ, Liu F, Kelly DL, Boggs DL, Gorelick DA. Cannabis withdrawal symptoms in non-treatment-seeking adult cannabis smokers. Drug and Alcohol Dependence. 2010;111:120–127. doi: 10.1016/j.drugalcdep.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH, Fisher J, Martin BR. Precipitated cannabinoid withdrawal is reversed by Delta(9)-tetrahydrocannabinol or clonidine. Pharmacology, Biochemistry, and Behavior. 2001;69:181–188. doi: 10.1016/s0091-3057(01)00514-7. [DOI] [PubMed] [Google Scholar]
- Martin BR, Compton DR, Thomas BF, Prescott WR, Little PJ, Razdan RK, et al. Behavioral, biochemical, and molecular modeling evaluations of cannabinoid analogs. Pharmacology, Biochemistry, and Behavior. 1991;40:471–478. doi: 10.1016/0091-3057(91)90349-7. [DOI] [PubMed] [Google Scholar]
- Marusich JA, Lefever TW, Antonazzo KR, Craft RM, Wiley JL. Evaluation of sex differences in cannabinoid dependence. Drug and Alcohol Dependence. 2014;137:20–28. doi: 10.1016/j.drugalcdep.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Guidelines for the care and use of mammals in neuroscience and behavioral research. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Cebeira M, Ramos JA, Martín M, Fernández-Ruiz JJ. Cannabinoid receptors in rat brain areas: sexual differences, fluctuations during estrous cycle and changes after gonadectomy and sex steroid replacement. Life Sciences. 1994;54:159–170. doi: 10.1016/0024-3205(94)00585-0. [DOI] [PubMed] [Google Scholar]
- Stoffel EC, Ulibarri CM, Craft RM. Gonadal steroid hormone modulation of nociception, morphine antinociception and reproductive indices in male and female rats. Pain. 2003;103:285–302. doi: 10.1016/s0304-3959(02)00457-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014. pp. 14–4863. NSDUH Series H-48, HHS Publication No. (SMA) [Google Scholar]
- Tseng AH, Craft RM. Sex differences in antinociceptive and motoric effects of cannabinoids. European Journal of Pharmacology. 2001;430:41–47. doi: 10.1016/s0014-2999(01)01267-5. [DOI] [PubMed] [Google Scholar]
- Tseng A, Harding J, Craft R. Pharmacokinetic factors in sex differences in Delta(9)-tetrahydrocannabinol-induced behavioral effects in rats. Behavioural Brain Research. 2004;154:77–83. doi: 10.1016/j.bbr.2004.01.029. [DOI] [PubMed] [Google Scholar]
- Tsou K, Patrick S, Walker JM. Physical withdrawal in rats tolerant to Δ9-tetrahydrocannabinol precipitated by a cannabinoid receptor antagonist. European Journal of Pharmacology. 1995;280:R13–R15. doi: 10.1016/0014-2999(95)00360-w. [DOI] [PubMed] [Google Scholar]
- Wakley AA, McBride AA, Vaughn LK, Craft RM. Cyclic ovarian hormone modulation of supraspinal Δ9-tetrahydrocannabinol-induced antinociception and cannabinoid receptor binding in the female rat. Pharmacology, Biochemistry, and Behavior. 2014a;124:269–277. doi: 10.1016/j.pbb.2014.06.007. [DOI] [PubMed] [Google Scholar]
- Wakley AA, Wiley JL, Craft RM. Sex differences in antinociceptive tolerance to delta-9-tetrahydrocannabinol in the rat. Drug and Alcohol Dependence. 2014b;143:22–28. doi: 10.1016/j.drugalcdep.2014.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakley AA, Wiley JL, Craft RM. Gonadal hormones do not alter the development of antinociceptive tolerance to delta-9-tetrahydrocannabinol in adult rats. Pharmacology, Biochemistry, and Behavior. 2015;133:111–121. doi: 10.1016/j.pbb.2015.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Burston JJ. Sex differences in Δ9 -tetrahydrocannabinol metabolism and in vivo pharmacology following acute and repeated dosing in adolescent rats. Neuroscience Letters. 2014;576:51–55. doi: 10.1016/j.neulet.2014.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Burston JJ, Leggett DC, Alekseeva OO, Razdan RK, Mahadevan A, Martin BR. CB1 cannabinoid receptor-mediated modulation of food intake in mice. British Journal of Pharmacology. 2005;145:293–300. doi: 10.1038/sj.bjp.0706157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, O’Connell MM, Tokarz ME, Wright MJ., Jr Pharmacological effects of acute and repeated administration of delta9-tetrahydrocannabinol in adolescent and adult rats. Journal of Pharmacology and Experimental Therapeutics. 2007;320:1097–1105. doi: 10.1124/jpet.106.108126. [DOI] [PubMed] [Google Scholar]
- Winsauer PJ, Daniel JM, Filipeanu CM, Leonard ST, Hulst JL, Rodgers SP, et al. Long-term behavioral and pharmacodynamic effects of delta-9-tetrahydrocannabinol in female rats depend on ovarian hormone status. Addiction Biology. 2011;16:64–81. doi: 10.1111/j.1369-1600.2010.00227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsauer PJ, Sutton JL. Chronic administration during early adulthood does not alter the hormonally-dependent disruptive effects of delta-9-tetrahydrocannabinol (Δ9-THC) on complex behavior in female rats. Pharmacology, Biochemistry, and Behavior. 2014;117:118–127. doi: 10.1016/j.pbb.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.