Abstract
Type 2 diabetes (T2DM) results when increases in beta cell function and/or mass cannot compensate for rising insulin resistance. Numerous studies have documented the longitudinal changes in metabolism that occur during the development of glucose intolerance and lead to T2DM. However, the role of changes in insulin secretion, both amount and temporal pattern has been understudied. Most of the insulin secreted from pancreatic beta cells of the pancreas is released in a pulsatile pattern, which is disrupted in T2DM. Here we review the evidence that changes in beta cell pulsatility occur during the progression from glucose intolerance to T2DM in humans, and contribute significantly to the etiology of the disease. We review the evidence that insulin pulsatility improves the efficacy of secreted insulin on its targets, particularly hepatic glucose production, but also examine evidence that pulsatility alters or is altered by changes in peripheral glucose uptake. Finally, we summarize our current understanding of the biophysical mechanisms responsible for oscillatory insulin secretion. Understanding how insulin pulsatility contributes to normal glucose homeostasis and is altered in metabolic disease states may help improve the treatment of T2DM.
Keywords: Islets, oscillations, insulin pulsatility, insulin resistance, diabetes
INTRODUCTION
Type 2 diabetes (T2DM) is associated with both a reduction in beta-cell mass and impaired beta-cell function. Less attention has been paid to beta cell function, which may begin to decline prior to the reduction in beta cell mass or the development of T2DM [1]. For example, the early loss of first phase secretion has long been considered a hallmark of T2DM [2][3][4]. From a therapeutic standpoint, improving insulin secretion pharmacologically is a more realistic alternative to stimulating beta cell mass expansion, in part because the latter is likely to occur on a much slower time scale than improvements in beta cell function. Even in rodents, where robust changes in beta cell mass can occur, beta cell function changes more rapidly and more markedly than mass [5]. Compensatory changes in beta cell function would be expected to be even more important in humans, where mass expansion is two orders of magnitude slower [6][7][8].
As is the case for other hormones, insulin is secreted from the pancreas in a pulsatile manner in both experimental animals and humans, and in patients with T2DM and other metabolic disorders the pattern of pulsatile release is disturbed. Thus, soon after the first report that fasting plasma insulin and glucagon levels oscillate in non-human primates in vivo ([9]), Turner’s group in the UK demonstrated that pulsatility occurs in healthy human subjects [10] and found disturbed pulsatility in subjects with T2DM [11].
The main focus of this review is the pulsatile insulin secretion of humans, particularly ‘fast oscillations’ in plasma insulin that have a period reported to range from 5–15 minutes. Readers with a special interest in ultradian insulin oscillations (period ≈ 80–180 minutes) are directed to other reviews [12].
Insulin levels oscillate in fasted humans
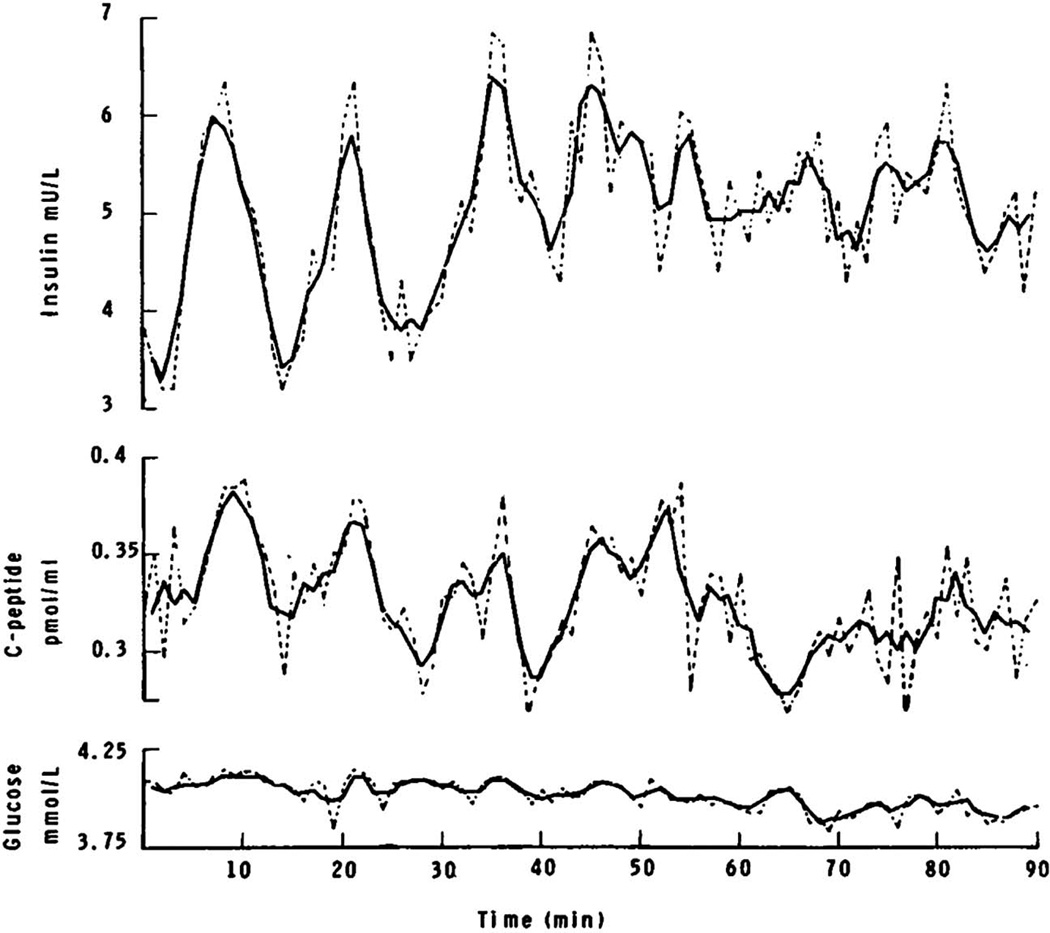
Lang and colleagues were the first to report insulin oscillations in the peripheral circulation of fasted but otherwise healthy human subjects. The oscillations they observed had a mean period of 15 minutes or so [10]. Peripheral blood was sampled once per minute for a total duration of 1–2 hours. An example from their paper shows, at least initially, clear oscillations in insulin, C peptide, and glucose concentrations in peripheral blood, as shown in Fig. 1. The continuous lines depict three-minute moving averages of the data, while the dashed lines show the raw, unsmoothed data. Small oscillations in glucose are also apparent in the lower part of the figure, but are difficult to resolve.
FIG. 1.
Oscillations in plasma insulin, C-peptide, and glucose measured in a peripheral vein in a fasted human subject. Dashed lines show unsmoothed data, continuous lines show three point moving averages of the data. Reprinted from [10] with permission of the New England Journal of Medicine.
Using records such as this, Lang et al applied autocorrelation to aid in pulse detection. Autocorrelation involves creating a mirror image of smoothed time series data, translating it stepwise along the original data, and then calculating a correlation coefficient for each time point in the interval. Plots of these coefficients reveal peaks that occur at multiples of the dominant oscillation period(s). Although Lang et al and other early studies of in vivo insulin pulsatility [13] reported an oscillation period of 10–15 minutes, more recent studies have determined the in vivo period of insulin oscillations to be closer to 5 minutes. In a later section, we will discuss why limitations in the technical approaches that were available at the time are likely to explain the prolonged insulin periods originally reported in this literature.
Individuals with T2DM have impaired insulin pulsatility
Using the same approach, Lang et al [11] reported that individuals with diabetes (their mean fasting glucose was 7 mM) displayed shorter and highly irregular oscillations having a mean period of 8.8 minutes (vs. controls having a period of 10.7 minutes). Later studies confirmed the impaired insulin pulsatility of T2DM patients (e.g.[14], [15], [16]; but see [17]).
O’Rahilly et al [18] extended these studies in individuals with T2DM to their first-degree relatives who lacked fasting hyperglycemia, to see whether pulsatility defects were an early event in the progression to diabetes. Control subjects were matched by age, gender and BMI to the relatives of patients with diabetes. While the control subjects exhibited regular insulin oscillations, these were lacking in relatives of T2DM subjects. However, the relatives studied were already glucose intolerant and insulin resistant and had reduced first phase insulin secretion, so the loss of pulsatility may have been secondary to reduced pulse amplitude, which may have reduced the signal-to-noise ratio.
In a similar study, Schmitz et al studied the transition from normal to abnormal glucose tolerance to fasting hyperglycemia to determine whether the loss of first phase and pulsatile secretion was due to intrinsic beta cell defects or glucotoxicity [19]. Insulin action and insulin pulsatility were measured in healthy offspring of T2DM patients vs. controls matched by age, gender and BMI.
Approximate entropy (ApEn), a measure of the likelihood that a similar pattern of observed activity will be repeated in a given time interval was used to gauge the level of irregularity of plasma insulin pulses; consistent with the general sense that entropy quantifies disorder, high ApEn indicates less regular pulses. Both first-degree relatives and their matched controls had normal OGTTs and no differences were noted in their plasma glucose or insulin levels. However, the relatives of T2DM patients had decreased insulin sensitivity, increased ApEn, and lacked regular autocorrelations. The data were interpreted as supporting the hypothesis that the relatives of diabetic subjects have an intrinsic beta cell defect that precedes the development of hyperglycemia as diabetes progresses. These data do not, however, indicate whether the beta cell defect precedes the onset of insulin resistance.
To test whether type 1 diabetes is also linked to changes in pulsatile secretion, Bingley et al (1992) studied insulin pulsatility in subjects who tested positive for a type 1 biomarker, islet cell antibody (ICA; [20]). Plasma glucose levels were similar in ICA-positive subjects and controls after an overnight fast. However, whereas control subjects exhibited a dominant pulse period, as determined by autocorrelation, regular insulin oscillations were lacking in ICA positive subjects, and only faster, highly irregular oscillations were observed. However, the authors had no way to assess beta cell mass, which could have been reduced in subjects who were ICA positive.
Abnormal insulin pulsatility and increased insulin resistance
From an evolutionary standpoint, it is likely that the highly complex and tightly controlled nature of pulsatile insulin release confers a survival advantage to the organism because maintenance of the machinery for pulsatility is costly. Modeling of beta cell exocytosis has shown that one possible advantage of pulsatile insulin secretion is that it may permit more insulin to be secreted by allowing the “readily releasable pool” of insulin granules (or RRP), sufficient time to be refilled during the resting intervals between pulses of secretion[21].
Pulsatile insulin may also act more efficiently on insulin target tissues [11]. Thus, exposing insulin receptors to continuous insulin might down regulate or internalize the insulin receptor, leading to reduced insulin action, and this might be mitigated by releasing insulin in an intermittent fashion [22–24]. Post-receptor signaling defects may also contribute, as discussed later. Goodner et al measured insulin binding to isolated hepatocytes to test the plausibility of this concept [22]. After being exposed to insulin, insulin receptors are rapidly internalized and only reappear at the cell surface after a recovery period. Once insulin receptors have been autophosphorylated, release bound insulin, and are then dephosphorylated, they return to the cell surface, a process that is broadly compatible with the timing of insulin pulsatility [24].
Interestingly, recent work suggests the possibility that zinc, which is co-released with insulin by the beta cell [25] may suppress hepatic insulin clearance by inhibiting insulin receptor internalization by the liver [26]. However, it is unlikely this mechanism confounds studies of the effects of insulin pulses on hepatic insulin clearance as large amplitude insulin pulses, whether they are experimentally imposed [27] or secreted endogenously result in more extensive insulin clearance than smaller pulses as demonstrated in dogs [28], rats [29] and humans [30], as discussed below.
Using hyperinsulinemic euglycemic clamp, it was reported that 20 hours of insulin exposure (vs. a saline control) at a constant glucose level decreased insulin action in healthy volunteers, as evidenced by decreased insulin-induced glucose disposal and a reduction in insulin sensitivity.
To test whether the distribution of abdominal body fat and peripheral insulin sensitivity were associated with changes in insulin pulsatility, Peiris et al [31] measured oscillations in plasma insulin during fasting in subjects whose peripheral insulin sensitivity was determined using glucose clamp. Insulin pulse interval appeared to be positively correlated with peripheral insulin sensitivity, such that insulin sensitivity increased as the measured period of insulin pulses increased from 7 to 12 minutes. No correlation was found between basal insulin levels and peripheral insulin sensitivity. These results suggest that sufficiently long rest periods between pulses are required to avoid decreased insulin sensitivity.
Another study of the possible linkage between insulin pulse frequency and peripheral insulin action compared T2DM and control subjects after an overnight fast [15]. Blood from the dorsal hand vein was sampled once every two minutes for 90 minutes, and the resulting insulin time series analyzed was using the PULSAR algorithm. Hepatic glucose production and glucose utilization were determined using hyperinsulinemic euglycemic clamp and an infusion of 3H-glucose tracer. Levels of fasting glucose and insulin were greater in subjects with diabetes, while glucose clearance and insulin sensitivity were higher in controls, indicating that T2DM subjects had increased peripheral insulin resistance and greater hepatic glucose output. The number of pulses observed was positively associated with decreased glucose clearance, especially in subjects with diabetes, as in [31]. In addition to being more frequent, the insulin pulses observed were more irregular in T2DM subjects. The results were interpreted in support of the hypothesis that abnormal insulin secretion and impaired insulin action are linked.
An alternative explanation for the findings of the latter two studies is that the observed inverse relationship between measured pulse frequency and insulin sensitivity was driven by the detection of a higher proportion of pulses in insulin resistant individuals in which insulin pulse amplitude might reasonably be expected to be enhanced. This explanation gains credence given the difficulties of insulin pulse detection at the time of the studies. Another consideration is that hepatic glucose production could have been affected by glucagon pulses, which were not assessed in the above studies. We discuss this further below.
Difficulties measuring insulin pulses under fasting conditions in the peripheral circulation, especially using older methods
Most of the studies of peripheral insulin pulsatility discussed so far were carried out in fasted human subjects whose plasma insulin levels approached the limits of detection of the insulin assays available at the time (prior to 1990). Also, peripheral insulin levels are low due to extensive hepatic insulin extraction, the relatively small amount of insulin secreted under basal conditions, and because plasma insulin is diluted by the peripheral circulation. In addition to the technical difficulties involved in measuring the low insulin levels, the analytical techniques and computing power available to detect and quantify insulin pulsatility were not as advanced 20 years ago as they are today.
These factors must therefore be kept in mind when considering the significance of the older, albeit clearly pioneering, research to our current understanding of insulin pulsatility. In particular, older reports concluding that insulin oscillates in the periphery with periods exceeding 4–6 minutes or concluding that pulsatility is more irregular or varies in period in T2DM subjects are not definitive at best. Also, the possibility that insulin pulse frequency contributes to the development of insulin resistance in T2DM patients, while highly interesting, remain to be confirmed using more up to date methodology. Those readers interested in more detailed and in depth discussions of the strengths and weaknesses of the different approaches used to detect and quantify insulin pulses in vivo and in vitro are referred to [32][33][34][35].
In our view, the most reliable data obtained to date that most convincingly demonstrate the functional importance of pulsatile insulin secretion come from studies where insulin was directly measured in the hepatic portal circulation using quantitatively validated methods to detect the pulses and determine their period. In most of the newer studies period is generally invariant and in the range of 4–6 minutes [36][37][38][35]. It is of interest that insulin is secreted from isolated islets with a similar pulse period [39][40][41][42].
Pulsatile insulin regulates hepatic function more efficiently
Insulin pulses in the hepatic portal circulation are larger than those in the peripheral circulation; these larger pulses suppress hepatic glucose production by activating hepatic insulin receptors. [43][30]. Many studies have shown that insulin administered in a pulsatile pattern augments insulin modulation of hepatic glucose ([44][45][30][46][37]; but see [47][48]). Bratusch-Marrain et al showed that pulsed insulin more effectively suppressed hepatic glucose output than continuous insulin in Type 1 patients, which they interpreted as showing that pulsatile insulin increased the insulin sensitivity of the liver[44].
More recent studies have continued to explore the ramifications of pulsatile insulin action on liver function. In [30], pulsatile insulin secretion regulated hepatic insulin extraction, and in so doing, systemic insulin delivery. Large pulses of insulin presented to the liver via the hepatic portal circulation (under conditions where endogenous insulin secretion was inhibited experimentally by an infusion of exogenous somatostatin) were more extensively extracted than small insulin pulses or constant insulin, thus limiting the variation in peripheral pulse amplitude homeostatically. In contrast, insulin pulses of reduced amplitude were less extensively extracted by the liver, which resulted in relatively greater systemic insulin levels. These features account for the strong attenuation of insulin pulses in the periphery, which is much greater than expected based on dilution in the circulation alone.
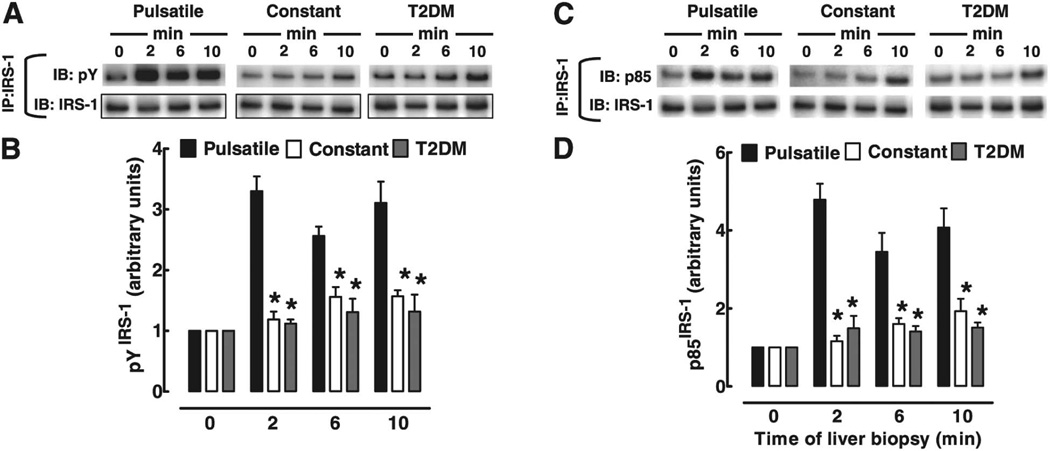
Matveyenko and colleagues studied the relative effectiveness of infusing either pulsatile or continuous insulin into the portal circulation, keeping the total amounts of insulin fixed, and then compared downstream insulin receptor signaling in the livers of animals at different time points [37]. They found that liver insulin receptor signaling was potentiated by pulsatile compared to continuous insulin administration. Not only did pulsed insulin blunt the rise in plasma glucose seen with continuous insulin in T2DM, but post-insulin receptor signaling in biopsied liver (as evidenced by immunoblotting liver lysates with phosphospecific antibodies) was clearly facilitated by pulsatile vs. continuous insulin delivery. As shown in Fig. 2, measuring liver phospho-IRS1 and PI3K activation revealed increased activation when pulsatile rather than continuous insulin was imposed in the portal circulation. In addition, greater activation of the more distal insulin signaling molecules Akt and Foxo was also seen. An important advantage of the study was its use of portal catheterization to circumvent difficulties inherent in trying to alter hepatic insulin responses by changing systemic insulin levels. While a previous study failed to find differences in the ability of pulsatile and continuous insulin to inhibit hepatic glucose output [48], the higher insulin levels that were used completely suppressed hepatic glucose output, which might have prevented the authors from differentiating the two methods of insulin administration.
FIG. 2.
The time course of IRS-1 activation by insulin in liver of rats exposed to either full amplitude insulin pulses, diminished pulses (to mimic T2DM), or constant insulin for up to 10 minutes. After portal vein infusion of these insulin patterns, livers were biopsied and IRS-1 activation was analyzed by immunoprecipitation with IRS-1 antibody, followed by immunoblotting with pY or p85/PI3K antibodies. Full amplitude insulin pulses potentiated insulin signaling in the liver, as evidenced by increases in pY-IRS-1 and p85-IRS-1. Similar results were obtained for IRS-2 activation (not shown). Statistics were ANOVA followed by Fisher’s post hoc test; p<0.05 was deemed significant. Reprinted from [37] with permission of Diabetes.
The relationship between pulsatile insulin and insulin resistance
We have discussed evidence that pulsatile insulin delivery is more efficacious, at least for the liver. In addition to receptor down-regulation, discussed previously, negative feedback within the insulin signaling pathway is a likely candidate, and may involve S6 kinase and/or PKC targeting of IRS-1 [23][49]. Rest periods that occur between bursts of granule exocytosis during pulsatile secretion would allow the negative feedback present in the insulin signaling pathway sufficient time to decay away.
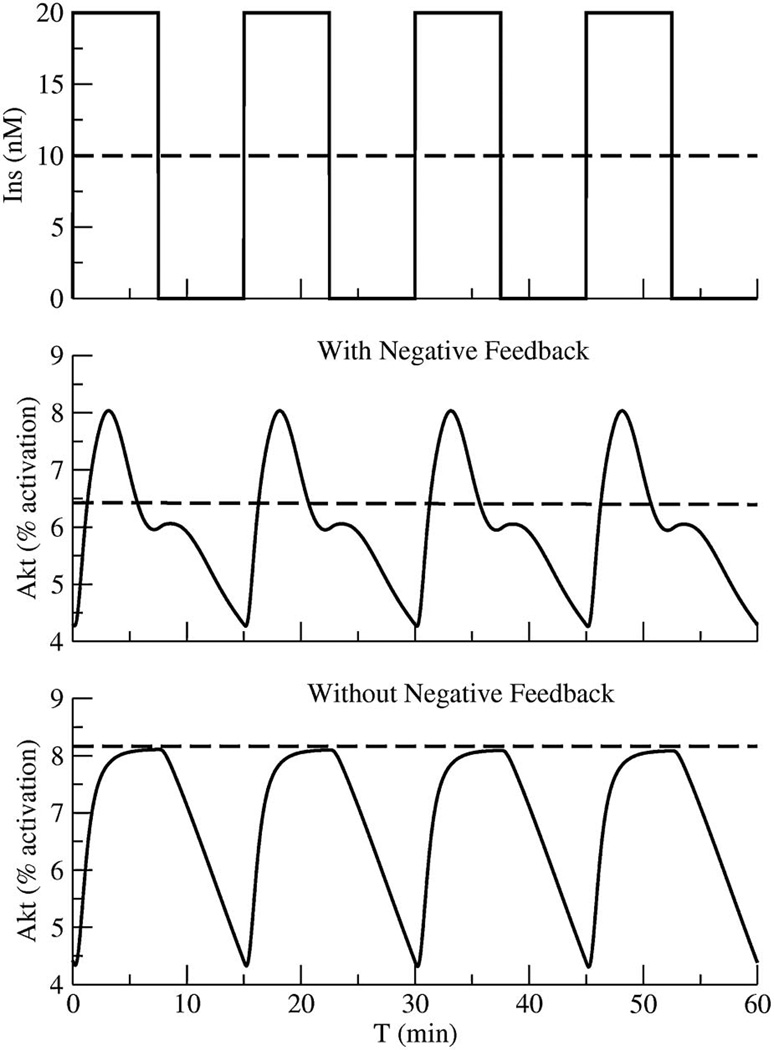
We show here, for the first time, with a mathematical model for insulin action that in the presence of negative feedback, it is advantageous to present insulin to its targets in a pulsatile manner. Square waves of insulin were compared to continuous insulin using the model of Sedaghat et al [49]. As shown in Fig. 3, pulses of insulin produce pulses in the percentage of phospho-Akt, an index of insulin receptor pathway activation.
FIG. 3.
A model of the insulin receptor signaling pathway demonstrating that due to frictional resistance, insulin receptor signaling is potentiated by insulin applied in a pulsatile rather than continuous manner. As shown, negative feedback in the signaling pathway facilitates insulin pulse responses because frictional resistance is allowed to fade between applied pulses of insulin. Details are provided in the text.
The model with negative feedback shows higher peak Akt activation in response to pulses (solid lines) than to continuous insulin (dashed lines). The increased peak Akt activation from negative feedback results from recovery of the insulin signaling in the target tissue from the negative feedback during the silent phases that follow each pulse of insulin response. Note that despite differences in peak Akt activation, activation averaged over the pulses is not much affected. In the absence of negative feedback, in contrast, the increase in the peak is nearly abolished, and the mean response is lower than the pulsed input. We view the lower peak response to steady insulin as a “frictional effect” in the sense that it is immediately relieved when pulses resume after a hiatus.
It is not clear if this frictional effect is related to chronic insulin resistance, but both are thought to involve inhibition of IRS-1 by serine phosphorylation, as mentioned above. There is evidence that chronic hyperinsulinemia, which can be imposed experimentally by transplanting extra islets into mice [50] or by increasing insulin secretion by blocking leptin receptors on the beta cells of mice [51] can lead to insulin resistance, but it is not clear whether the insulin resistance would be relieved by lowering insulin or how rapidly this would occur. Shimomura et al exposed isolated hepatocytes to high insulin to produce insulin resistance [52], and found that it was reversed 24 hours after washing out the insulin. Similarly, Alemzadeh et al [53] reduced insulin resistance in normoglycemic, highly insulin resistant subjects by partially suppressing insulin secretion with diazoxide. The subjects studied were also on a weight-loss regimen, which raises the possibility that some of the improvement in insulin action observed was due to the increased weight loss in diazoxide rather than inhibition of insulin secretion per se.
Physiological glucose modifies the amplitude but generally not the frequency of insulin pulse bursts
In principle, an increase in plasma glucose could increase plasma insulin by increasing the amplitude (sometimes referred to as the “burst mass” or “pulse mass”), altering the frequency of the insulin pulses, increasing plateau fraction or all three. Matthews et al [54] observed that elevated glucose increases the mass but not the frequency of plasma insulin bursts, and this has been confirmed in numerous studies ([55][56][57]). The action of glucose mainly to increase insulin pulse amplitude has also been observed in isolated human islets, where small numbers of islets could be perifused while insulin was sampled in their effluent [39].
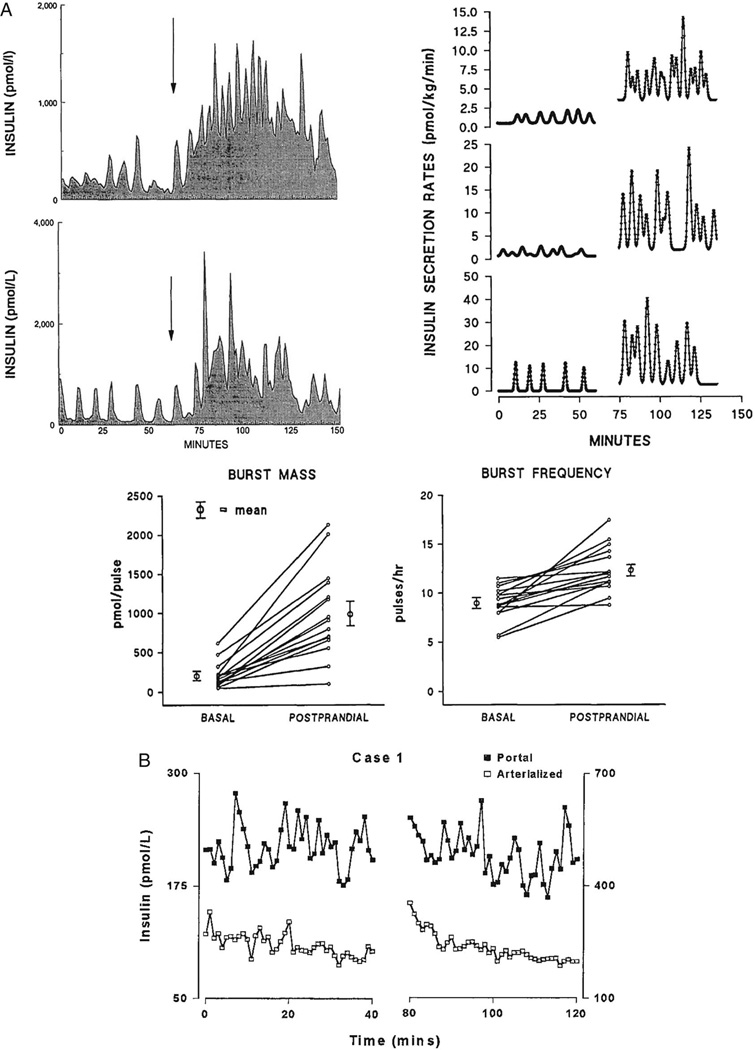
In an in vivo study of canine insulin secretion, where plasma glucose was increased following glucose ingestion, both the amplitude and the frequency of portal vein insulin pulses were increased by the sugar ([58]; Fig. 4A). As can be seen in Fig. 2 of their paper, which shows portal vein insulin profiles for two representative dogs, the insulin pulses of the portal system were large and the effect of glucose ingestion was dramatic (however, ingestion of course invokes not only the direct actions of glucose on the pancreatic islets, but also incretins secreted by the gut which in turn potentiate secretion). Their Fig. 3, using deconvolution to calculate underlying secretory rates, confirms the profound effect of glucose to amplify secretory burst mass in this example. Quantification of each subject’s response to glucose, for both insulin pulse frequency and mass, in their Fig. 4 shows that both frequency and mass increase postprandially. However, it is possible that the assay that was employed did not distinguish smaller insulin pulses that might have been present under low glucose conditions, which would have underestimated the basal insulin pulse frequency. If this was the case, the net effect of ingested glucose on pulse frequency (but not amplitude) might have been much smaller.
FIG. 4.
Glucose increases insulin pulse amplitude. A. Glucose ingestion (arrow) causes an increase in insulin secretory burst mass and frequency in the portal circulation of catheterized dogs (top left). The data from two representative dogs are shown. Deconvolution shows potentiation of insulin secretory pulse amplitude and frequency (top right), which are plotted for a total of 15 dogs in the lower part of the figure. Reprinted from [58] with permission of Diabetes. B. Insulin pulses in the portal circulation of human subjects (as well as in the periphery) are also increased in amplitude after glucose is increased. Samples taken during first 40 minutes of the experiment under basal conditions are shown on the left, while samples collected during the second 40 minutes of samples once hyperglycemia was established are shown on the right of the figure. Note the profound differences in scale of for the respective ordinates shown. Reprinted from [59] with permission of The Journal of Clinical Endocrinology and Metabolism.
Using indwelling intrahepatic shunts to sample the portal circulation of human subjects, Song et al (2000) carried out a study of insulin pulsatility that included measurements from the portal vein [59]. As shown in Fig. 4B, simultaneous sampling of both the portal and arterial circulation under glucose clamp demonstrated that levels of insulin in the portal vein were far greater than in the periphery, and moreover that hyperglycemic clamp resulted in an increase in the amplitude of the insulin pulses [53]. Deconvolution was used to determine the underlying insulin secretory rates, as in other studies, and showed that increasing glucose increased the size of insulin pulses in both the portal and arterial circulation.
Ultradian oscillations and their entrainment by small glucose fluctuations are disrupted in T2DM
Besides “fast” oscillations having a period of 6–15 minutes, slower “ultradian” insulin oscillations having a period of 80–180 minutes have also been observed in longer duration studies. In a study of 16 T2DM patients vs. 14 matched control subjects, where blood was sampled at 15–20 minute intervals for up to 24 hours and three meals were provided between 9 AM and 11 PM, changes in ultradian pulsatility were observed in the diabetic subjects. While ≈ 8 pulses of insulin were detected during the measurement period (1 pulse every 2 hours) in both controls and diabetics, the pulses of the diabetic subjects were of diminished amplitude, occurred less frequently paired with a glucose pulse, and were more irregular [60]. Polonsky’s group has shown that entrainment, the process whereby small, subthreshold changes in plasma glucose regulate the insulin pulsatility of islets and contribute to their synchronization, is lost in T2DM patients [61, 62].
In a study of both healthy and diabetic obese subjects, diurnal insulin pulsatility was identified as the occurrence of 10 insulin oscillations over a 12 hour period. A decrease in the number of pulses was observed in the obese diabetic subjects, and an increase in pulse amplitude was noted after weight loss occurred. The insulin pulses of obese controls had the largest amplitude, while subjects with overt T2DM had pulses of the lowest amplitude. In addition, the pulsatile insulin secretion elicited by the administration of secretagogues was reduced in frank diabetic as well as subclinical T2DM subjects with fasting hyperglycemia [14]. The insulin pulses observed for healthy obese subjects were regular in appearance, while those of T2DM patients were markedly disorganized. The cross-sectional nature of this study, however, precludes assessment of whether the changes in the ultradian oscillations were a cause or consequence of the pre-diabetic and diabetic states.
Pulsatility of other islet hormones
Although the focus of this review is pulsatile insulin release and its metabolic consequences, glucagon and insulin have reciprocal actions on blood glucose, and reciprocal oscillations in glucagon have also been reported. These oscillations have been observed in isolated islets in vitro, albeit at high glucose levels (20 mM); see [63][42]. As insulin [64][65]and somatostatin [66][67] restrain glucagon secretion, glucagon pulses likely occur because constant glucagon secretion is periodically inhibited by pulses of secretion from beta and delta cells. There is evidence that glucagon secretion from the alpha cell can be intrinsically pulsatile [68][69], but this would not be manifest in secretion from islets because, in contrast to beta cells, alpha cells are not coupled to each other by gap junctions. Anti-synchronous oscillations of glucagon and insulin have also been observed in vivo [70][9][71]. Human glucagon and insulin levels were reported to exist in an inverse relationship in control subjects, but not subjects exhibiting impaired fasting/postprandial glucose together with elevated glucagon [72]. Assuming the hormones normally oscillate in an anti-synchronous manner in vivo, as has been proposed [73], this raises the question of what would be the physiological benefit, as the two hormones seem to be working at cross purposes. Recent data [74] have revealed that glucagon can directly contribute to the loss of insulin secretion by stimulating secretion from the liver of kisspeptin, a peptide that inhibits insulin secretion. The loss of the anti-synchronous oscillations of the two hormones would then be both a symptom of the impairment in insulin secretion and a contributor to that process.
In addition to glucagon, somatostatin, which is secreted from delta cells of the islet is also pulsatile, and occurs in synchrony with insulin [63][42]. Such oscillations would not be possible unless the delta cells were stimulated by the beta cells, possibly through GABA or ATP [75] as the delta cells, like the alpha cells, are not coupled to each other. Another islet hormone, amylin, which is co-stored in the beta cell with insulin in large dense core granules also shows pulsatile secretion, with a mean period of around 5 minutes [76]. ATP, also co-released with insulin, is hydrolyzed to adenosine, which appears to play a role in maintaining reciprocal glucagon and insulin as the out-of-phase pattern is lost in adenosine A1 receptor null mice [77]. A common theme of all these oscillations is that they appear to be orchestrated by the core machinery driving insulin oscillations in the beta cells, rather than being intrinsic to the otherwise uncoupled alpha and delta cells.
Mechanisms governing the intrinsic pulsatility of an islet
It has been known since the 1970s that pulsatility is intrinsic to the pancreatic islet, when Dean and Matthews showed that even after being completely isolated, individual mouse islets exposed to glucose concentrations > 7 mM were electrically active. This activity consisted of bursts of action potentials riding on plateau depolarizations that occurred every 15 seconds or so in the steady state [78]. Single dispersed beta cells are also pulsatile [79]. Later work established that beta-cell electrical activity is dependent on the influx of Ca2+ ions [80][81], that its oscillations increase in duty cycle or plateau fraction as glucose increases [80, 82, 83], and that it leads to the rise in beta cell intracellular free Ca2+ that drives the periodic exocytosis of insulin from the beta cells [79].
The advent of free Ca2+ measurements in islets using the dye fura-2 established that islets with oscillatory periods in the range of 3–5 minutes could be commonly observed (slower electrical changes had been seen earlier as well; see [84, 85]). The slower patterns are of particular interest here as their periodicity corresponds to that of plasma insulin seen in vivo, as described above.
Many hypotheses have been proposed to account for islet pulsatility, and some of these have been formalized as mathematical models. Readers are referred to earlier reviews for an overview of the different mechanisms proposed for islet oscillations ([86, 87],[88],[89],[90–92],[93]).
Islet modelers face many challenges. First, islets have a wide dynamic range as glucose varies, and islet activity can range from seconds to many minutes [41]. This flexibility possibly contributes to the ability of the beta cell to regulate insulin secretion very precisely in a dynamic milieu. Second, while models of oscillations of human islets are now becoming available [92, 94], much less is known about the basic biophysical properties of human islets, including their electrical activity. This stems from the greater heterogeneity of human subjects along with the fragility of human islets and their limited availability to researchers; even those that have become more widely available at the time of this writing can be of highly variable quality. Thus, it is still necessary to carefully model mouse islets, with an eye to extending and adapting these models to humans as more high quality data appear in the literature.
As pulses in plasma insulin result from the synchronized activity of many intrinsically oscillatory islets, and humans exhibit pulsatile insulin in the circulation similar to that of rodents, it is expected that isolated human islets, like those of mouse or rat, should show glucose-dependent slow oscillations. However, and surprisingly, this issue has been somewhat controversial. Thus, while oscillations in intracellular free Ca, and in some instance, bursts of electrical activity have been reported in isolated human islets ([95][96, 97][98]; Merrins and Satin, unpublished), it has also been claimed that human islets lack regular oscillations [99]. As the cellular architecture of the human islet lacks the stereotypical pattern seen in rodents, where there is a pronounced alpha cell mantle and beta cell core, it has been claimed that human and rodent islets function in fundamentally different ways. Indeed, the specific ion channels expressed in human beta cells are different from those of mouse, with rapidly inactivating T type Ca channels and voltage gated Na channels playing prominent roles in human, but not mouse, beta cells [100, 101]. Nonetheless, observations of isolated human islets confirm that, despite important differences in ion channels and spiking patterns, they exhibit slow oscillations and synchrony similar to those of rodents [73, 95, 96, 98].
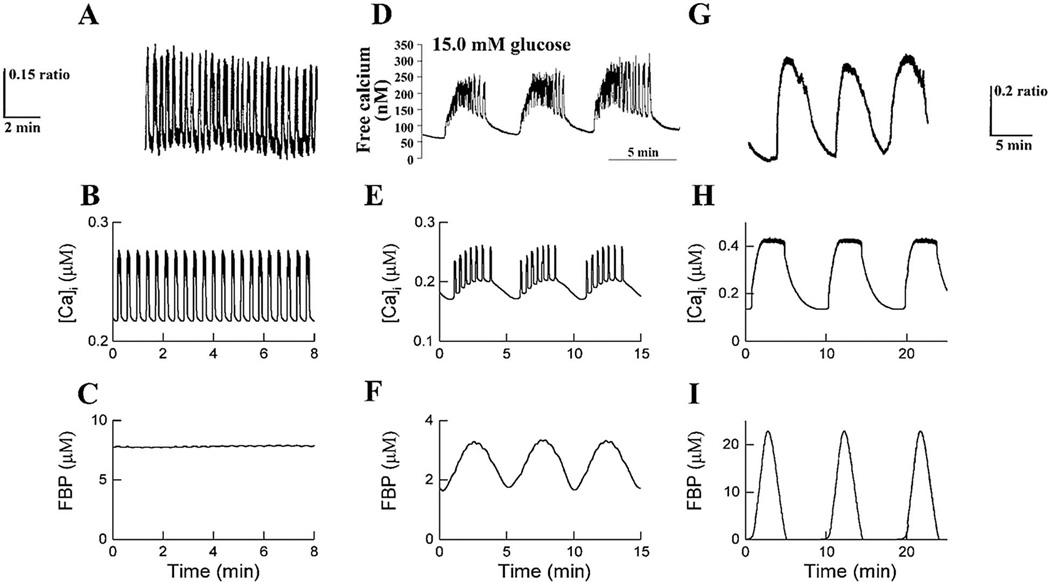
Our current model of islet oscillations, which we call the Dual Oscillator Model (DOM), consists of a slow metabolic oscillator based on the glycolytic enzyme phosphofructokinase (PFK), and an electrical or ionic oscillator, which is mediated by negative feedback of intracellular free Ca on KCa channels and changes in ATP/ADP acting on the ATP-sensitive potassium channels of the beta cell (KATP channels; FIG. 5.;[41, 102]). Tornheim originally proposed that slow oscillations in the activity of the M-isoform of PFK1 could result from the allosteric activation of PFKm by its product, fructose-1-6-bisphosphate (‘FBP’; [103]). According to this hypothesis, the cyclic activity of PFK leads to slow oscillations in ATP/ADP, which cyclically regulate KATP channel activity in the beta cell plasma membrane. The oscillations in KATP conductance in turn produce oscillatory membrane depolarization and action potentials, resulting in the increase in Ca that triggers insulin granule exocytosis. Our model combines this scenario with a model for ion channels and Ca handling by the endoplasmic reticulum. An additional component of the DOM not addressed in the scenario of Tornheim is the mitochondria, which couple glycolytic oscillations to oscillations in oxidative phosphorylation and ATP production [102, 104].
FIG. 5.
Three types of oscillations typically observed in islets. Top row of panels is from islet measurements of Ca2+. Middle row shows simulated Ca2+ oscillations using the Dual Oscillator Model (DOM). Bottom row shows simulations of the glycolytic intermediate fructose 1,6 bisphosphate (FBP), indicating that glycolysis is stationary (c) or oscillatory (f, i). Reprinted from [41] with permission of the American Journal of Physiology.
In the DOM, cytosolic Ca oscillations are not mandatory for slow oscillations in metabolism to occur, and we have investigated this possibility in detail both theoretically and experimentally (e.g. [105][106][107][108]). Therefore, while other experimental observations have been cited in support of the hypothesis that free Ca oscillations drive beta cell metabolic oscillations [109][110], this cannot be the whole story. We note that the interplay between oscillatory free Ca and metabolism is complex [107], and that Ca must be clamped to a permissive level in order to see metabolic oscillations in the absence of Ca oscillations [105], which may account for why this feature has not been universally observed. In contrast to the predictions of the DOM, however, the mitochondrial substrates ketoisocaproate (KIC) and leucine have been found by some investigators to trigger slow oscillations in mouse islets even in glucose-free solutions [111][112]. However, other workers have observed that some glucose is required for islets to exhibit slow oscillations in response to KIC, for reasons that remain unclear ([113]; Zhang and Satin, unpublished).
The DOM shows that not only are the ion channels responsible for generating electrical spiking activity governed by the metabolic drive through the KATP channels, but that the metabolic oscillator by itself would be unable to generate large amplitude Ca oscillations without the nonlinear threshold properties of the electrical subsystem [107]. The model is able to account for a wide variety of phenomena not explained by other beta cell models, including compound (mixed fast and slow) Ca and membrane potential oscillations and metabolic oscillations when Ca is held fixed. Pure fast oscillations in free Ca or electrical activity, also known as ‘electrical bursting’, occur in the model because of feedback interactions between the Ca and K channels of the beta cells when metabolism (represented as the level of FBP in the leftmost panel of Fig. 5) is fixed. Pure slow oscillations, reflecting metabolic oscillations, produce smooth oscillations in FBP and downstream variables, shown in the rightmost panel of Fig. 5. Concurrent activity of both oscillators results in ‘compound’ or ‘mixed’ oscillations [40, 102, 113, 114], as shown in the center panel of Fig. 5. Shifts in the glucose thresholds of the ionic and metabolic oscillators can account for the wide variety of oscillatory patterns described in the islet literature (see [41, 115])
For more comprehensive reviews of the DOM, readers are directed to other recent papers [41, 102]. Experimental validation of the DOM can be found in [105, 106, 115–117]. Some alternative islet models are described in [90, 93].
The Metronome Hypothesis
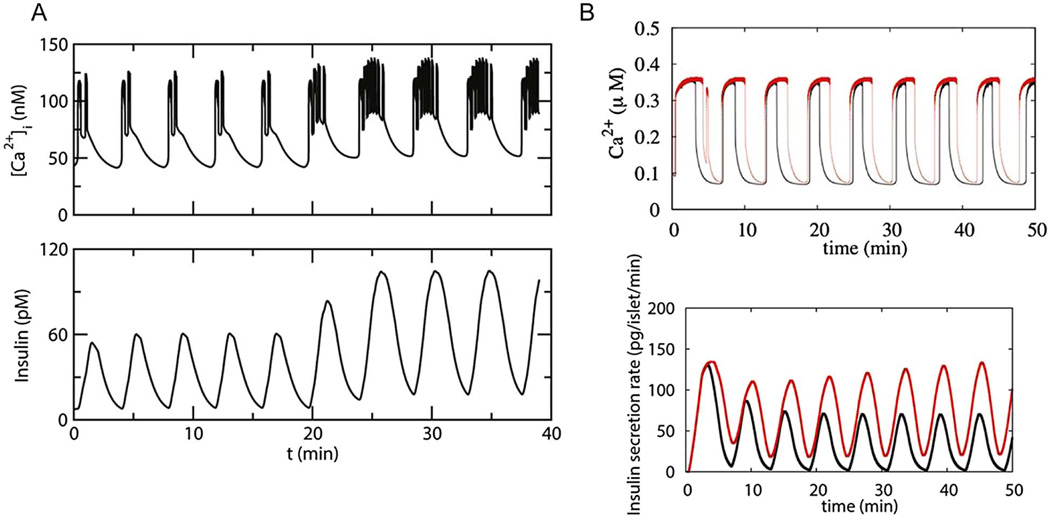
Our work, and that of others [115][40, 118], has shown that in isolated mouse islets, increasing the concentration of glucose applied to the islet tends to evoke one of three canonical Ca oscillatory patterns: fast oscillations exhibiting a period of 15–60 seconds; slow oscillations exhibiting a period of ≥ 2 minutes; and mixed or compound oscillations consisting of fast oscillations superimposed upon the slower oscillations [78] [114]. We here use the DOM to propose distinct roles for the fast and slow oscillations. We call this the “metronome model” because the metabolic oscillations control the pacing of the secretory oscillations while the electrical oscillations control how much insulin is secreted during each slow cycle. This is illustrated in Fig. 6A, where secretion in response to a compound oscillation is shown. This arrangement also explains why increased glucose metabolism increases the amplitude of insulin pulses more than their frequency, as indicated by the data reviewed above.
FIG. 6.
The Metronome Hypothesis. A. Simulation obtained using a model of an isolated ‘compound bursting’ islet showing that increasing the plateau fraction of an islet Ca2+ oscillation as glucose is raised results in a dramatic increase in insulin pulse amplitude, but little or no change in oscillation frequency. Note that there was a small increase in the Ca2+ baseline as well. B. Simulation showing that increasing the duration of the islet Ca2+ oscillations in a slow islet lacking faster oscillations superimposed on the slower ones also results in potentiation of the insulin pulse amplitude, but no change in pulse frequency.
In the DOM, the slow outer oscillation is determined by the cyclic activation of the glycolytic oscillator, PFKM [41, 103, 116], which sets the period of the insulin oscillations. The plateau fraction of the fast inner oscillations determines the free Ca concentration during each episode of fast bursting. Increasing glucose increases the amplitude of the insulin pulses because insulin is a slow function of the Ca level set by the fast oscillation. In contrast, the width and frequency of the metabolic pulses is relatively insensitive to the level of glucose, as shown in Fig. 6A. Glucose is raised at 20 min., resulting in an increase in plateau fraction but little change in oscillation period, while insulin secretion pulse amplitude is approximately doubled. Absolute Ca does increase somewhat, as observed in some experiments [115], but the main effect is an increase in plateau fraction.
We have confirmed this result with a more complex version of the DOM that includes more details of mitochondrial metabolism and in which insulin secretion is not merely a function of Ca, but is determined by incorporating a model for insulin granule trafficking and exocytosis[21]. In Fig. 6B the pure slow oscillations simulated in lower glucose are shown in black, those in higher glucose in red. Increasing glucose does not change the period much (≈5 min for both high and low glucose), nor the amplitude of the Ca pulses produced. However, by increasing the duration of the Ca active phase, the pulse amplitude of insulin secretion goes up markedly in higher glucose. The incorporation of the exocytosis model into the DOM allows the simulation to reproduce the rising second phase of secretion in higher glucose, which is due to increased granule trafficking from the reserve pool to the plasma membrane. This illustrates the idea that an increase in the size of the RRP is the most important factor controlling the amplitude of the burst mass. The fraction of the RRP that is secreted depends on Ca, which determines the release probability per granule in the model. This does not change much because peak Ca does not change much in this case, but mean Ca does increase as a result of the increased plateau fraction of the electrical bursts and results in release of more vesicles.
The demonstration of amplitude rather than frequency modulation of insulin secretion by glucose in Fig. 6, using two different versions of the DOM, two different models for the Ca dependence of secretion and two distinct slow patterns is an encouraging sign that the phenomenon is robust and not dependent on the particular assumptions made for each simulation.
In contrast to the slow oscillations exhibited in Fig. 6, fast oscillations would not leave sufficient time between bursts for the RRP to be replenished and would result in non-pulsatile insulin secretion (see Fig. 3 in [21]). The rest period between pulses was likewise crucial for the simulations of insulin action in Fig. 3, in that case because of the time needed for negative feedback in the insulin signaling cascade to recover. The two sets of simulations together lead to a picture in which pulsatility integrates the nanoscale behavior of insulin granules in the beta cells with the nanoscale behavior of metabolic enzymes in the liver, allowing the two tissues to coordinate their activities and regulate glucose homeostasis at the whole body level.
How are islets synchronized across the pancreas in vivo?
The integrative consequences of pulsatility described above depend on coordinated activity by the whole beta-cell population in the pancreas, but it is not obvious how this coordination is achieved. Beta cells within a single islet have an intrinsic oscillatory mechanism that is dependent on glucose metabolism and ion channels and is modulated by a spectrum of neuroendocrine factors (reviewed in [119]). Within the islet, beta cells are electrically coupled to neighboring beta cells through gap junctions [120–122], ensuring a high degree of synchronicity among the interconnected cells.
Within the intact human pancreas, however, are hundreds of thousands of islets, whose pulsations must be highly synchronized for pancreatic insulin output and plasma insulin levels to be pulsatile [123]. A lack of synchronization between the islets would obliterate the regular pulsatile pattern that has been observed in vivo, and in the perfused pancreas where pulsatility is well maintained. It is likely that islets are the intrinsic pacemakers of pulsatile secretion, even in vivo, if they are highly coordinated, as the periods we observed for isolated islets are highly correlated with the in vivo periods of insulin pulsatility, at least in mouse [124].
It has been proposed that islet to islet communication within the pancreas is enabled by an intrapancreatic nerve network that is also connected to the pancreatic ganglia [125, 126]. Many investigators have tested for the presence of such a network using indirect approaches such as injecting pharmacological inhibitors of cholinergic [126] or adrenergic [127] transmitter release, or other blockers into animals while monitoring plasma insulin oscillations, mostly with no success [125]. One positive result was the finding that the pulse pattern could be disrupted by exposure to the Na channel blocker tetrodotoxin, which eliminates nerve action potentials [125]. Still, the nature of the endogenous pacemaker or nerve net is not known.
However, we have found that we can synchronize the oscillatory activity of groups of islets exposed to 11.1 mM glucose in vitro by briefly applying a cholinergic agonist. Thus, exposing islets to a 15 second pulse of carbachol completely synchronized the oscillations of a group of islets for as long as 30 minutes [123]. Within the framework of the Dual Oscillator Model, the release of Ca from the beta cell endoplasmic reticulum can result in islet synchronization because rapid changes in Ca can reset the slow glycolytic oscillator, and after such a reset it takes considerable time for the oscillations of the individual islets to drift apart. We showed through modeling that even if only a fraction of the pancreatic islet population were regularly exposed to episodic acetylcholine release, say due to the activity of intrapancreatic cholinergic neurons, the effect could entrain the entire islet population [128]. However, this result remains to be tested in vivo. The model showed that maintenance of synchrony by neural pulses is greatly facilitated if the intrinsic frequencies of the individual islets are similar. We have found, remarkably, that this is indeed the case when islets from a given mouse are compared [124], but the mechanism behind this remains to be elucidated. We also point out that other coupling factors released by beta cells may be important paracrine factors in islets, including ATP [129][130].
Therapeutic implications of insulin pulsatility for pharmacotherapy of Type 2 diabetes and for improving the efficacy of insulin administration
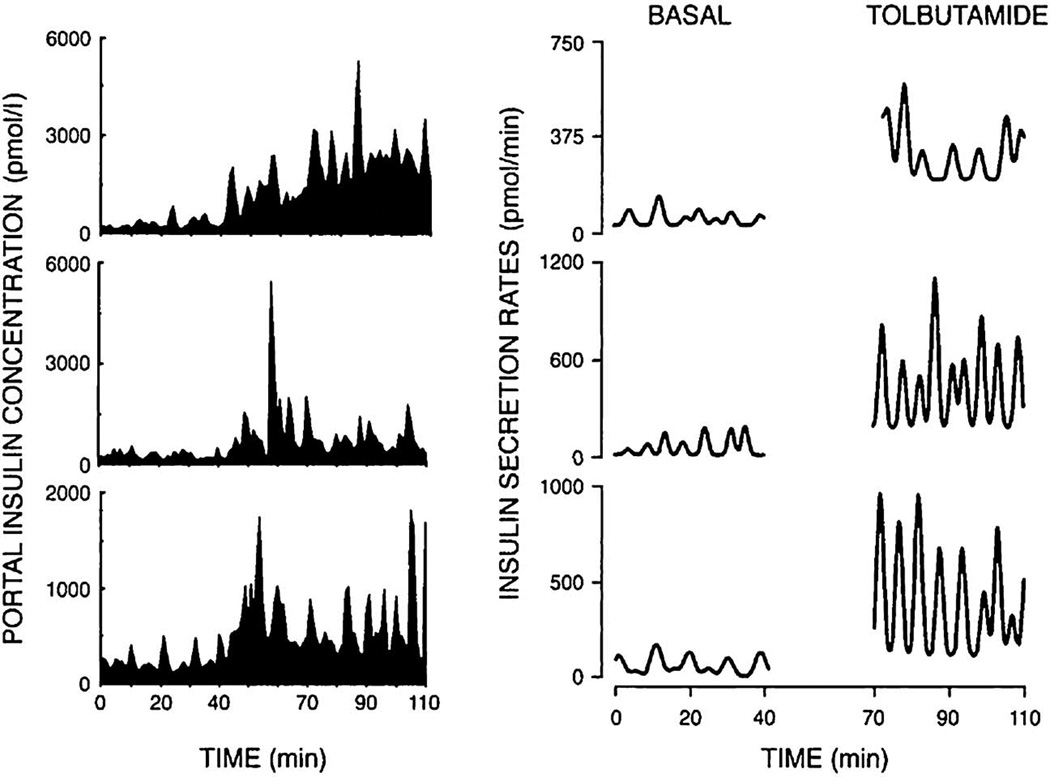
Several well-known classes of pharmacologic agents that are used to treat patients with T2DM have been shown to increase plasma insulin pulsatility in both human and animal subjects. Sulfonylureas, for example, which increase insulin secretion by closing KATP channels in the beta cell plasma membrane, acutely increase the amplitude, but not the frequency, of insulin pulse bursts or the basal insulin levels when studied in dogs [131], or in humans with T2DM [56]. An example of the modulation resulting from tolbutamide infusion is shown in Fig. 7. In dogs having a portal vein catheter, the IV infusion of tolbutamide, or its oral ingestion, increased the amplitude of portal vein insulin pulses without significantly modifying insulin pulse frequency [132]. Deconvolution to reveal pulses in insulin secretory rate further delineated the amplitude increase after tolbutamide.
FIG. 7.
Tolbutamide infused into the portal vein of three representative dogs results in increased insulin pulse amplitude (left hand side of the figure). Deconvolution of the data reveals that tolbutamide greatly increased the amplitudes of the insulin secretory rates. Tolbutamide infusion occurred from 40–110 minutes.
Pulse burst amplitude, but not pulse frequency, was also increased in T2DM subjects who were treated with rosiglitazone [133]. This may have been a consequence of reduced overall insulin secretion, which might prevent depletion of the RRP. Similar results were obtained in Zucker diabetic fatty rats, which also exhibited improved glucose entrainment after such treatment [61]. Rosiglitazone, a thiazolidinedione, is a PPAR gamma agonist that increases peripheral insulin sensitivity [133]. Furthermore, it has been shown that treating healthy human subjects with repaglinide, a meglitinide that provokes rapid insulin secretion and damps postprandial glucose, increases insulin pulse burst amplitude, but not frequency [55]. All of the drugs mentioned did not affect the regularity of insulin pulse production.
Drugs that potentiate endogenous levels of the incretin GLP-1 [57][134] or are themselves GLP-1 agonists [134] have currently found wide acceptance in diabetes pharmacotherapy. Several studies have now appeared documenting the ability of GLP-1 to potentiate insulin pulse mass and pulse regularity without altering insulin pulse frequency [135]. These observations suggest that modulation of insulin pulsatility may contribute to the actions of GLP-1 agonists or DPP4 inhibitors for treating T2DM in humans [136]. Mechanistically, this may be the case because GLP-1 increases the size of the RRP of insulin granules in the beta cell, the granules that serve as the substrate for the recurrent pulses of insulin that constitute the pulse burst [137]. This would be complementary to the action of rosiglitazone to preserve the RRP, but by enhancing supply of vesicles to the plasma membrane, rather than reducing release of vesicles. Alternatively, long term GLP-1 treatment could also increase pulse burst mass by increasing beta cell mass [138].
Pharmacologically inhibiting beta cell insulin secretion for a period of time (e.g. overnight) has also been shown to potentiate secretion when the inhibition is removed, a phenomenon known as ‘beta cell rest’ [139][140]. This can be accomplished using somatostatin or its analogs, or drugs that cause the sustained opening of KATP channels, such as diazoxide. Laedtke et al (2000) suppressed insulin secretion overnight in T2DM subjects using somatostatin, while preventing hyperglycemia by insulin infusion. They found that resting beta cells in this way increased insulin burst mass and burst orderliness, without affecting pulse frequency. Beta cell rest might work similarly to rosiglitazone treatment, by increasing the size of the RRP of insulin granules that contributes to insulin pulse mass or by generally disinhibiting exocytosis. Rest may, however, have other effects as well [141].
Besides these documented actions of known drugs and peptides, our recent work to decipher the underlying mechanism of the slow oscillations in calcium and metabolism of isolated mouse islets, and other work, suggests additional ways to possibly increase the diminished insulin pulses of T2DM patients to control levels. We summarize several potential approaches below.
First, while closing KATP channels by increased glucose metabolism triggers insulin secretion by raising intracellular free Ca [41, 87, 142], insulin release is also controlled by a ‘KATP-independent’ amplification pathway, which likely reflects the release of mitochondrial metabolites or an increase in beta cell cyclic AMP [143][144] to potentiate secretion at a site distal to the rise in intracellular free Ca [3, 145]. While the definitive nature of this metabolite or pathway has been elusive, its identification would potentially provide a way to amplify secretion, and hence increase pulse burst amplitude without incurring further rises in Ca or metabolism and concomitant damaging reactive oxygen species production.
Second, it may be feasible to improve the regularity of the pulse bursts by strengthening the glycolytic oscillations that we propose drive the slow oscillations [41]. This could be accomplished by removing inhibitory influences on PFKM, such as excess intracellular citrate or ATP binding to PFKM. This could be done using selective site-specific antagonists, or by modifying PFKM activity through altering the activity of the PFK2/FBPase2 modulatory pathway [117]. As defective synchronization of islets within the pancreas can also contribute to the production of abnormal insulin pulses, a better understanding of intrapancreatic islet synchronization might also provide novel drug targets for future treatments. Our own work suggests that muscarinic agonists might have promise based on our ex vivo experiments [123].
The very nature of pulsatile secretion and its biological advantages could have translational significance for diabetes treatment. Thus, providing insulin in a pulsatile fashion using a device to make pulses of exogenous insulin or by applying insulin secretagogues such as tolbutamide in a pulsatile pattern, would be expected to affect insulin target tissues more effectively by more closely mimicking the changes in plasma insulin observed in normal individuals. As mentioned earlier in the review, this could result in increased peripheral as well as hepatic insulin sensitivity, increased preservation of beta cell function, and increased glucagon suppression by plasma insulin or better liver insulin extraction. While it is difficult to envisage a way in which insulin injected subcutaneously could produce plasma insulin pulses due to the lags inherent in this method of administration, recent simulations suggest the approach may be feasible [146]. Alternatively, intravenous insulin injection using an artificial pancreas control system could be used to produce physiological insulin pulse patterns in individuals with diabetes where traditional therapy is insufficient [147].
Summary and Conclusions
Insulin secretion occurs in a pulsatile manner in the plasma of both humans and animals, with fast pulses exhibiting a period in the range of 5 – 15 minutes, and slower ultradian oscillations having periods ranging from 80 – 180 minutes. Insulin pulsatility is disrupted in diabetes, most clearly as reduced pulse amplitude, and this appears to be an early marker of diabetes, as it is observed not only in pre-diabetics but also in first-degree relatives of patients with diabetes who lack significant metabolic abnormalities. Conversely, pulsatile insulin is more effective at mediating the metabolic effects of insulin, most clearly suppression of hepatic glucose production but possibly also enhanced uptake by peripheral tissues. The pulsatility of insulin secretion is intrinsic to the islet and may involve close coupling between slow metabolic oscillations mediated by glycolysis and faster oscillations involving beta cell ion channels and Ca mediated negative feedback. Some key coordination mechanism must also be present to synchronize islets within the intact pancreas, but the nature of this mechanism, which is likely neural, remains obscure. The essential features of the human islet oscillatory mechanism are likely to closely resemble those of mouse islets, but a complete analysis of the biophysical mechanisms of human islet oscillations has been hampered by the lack of readily available human islets of high quality. Lastly, new pharmacological agents to treat diabetes may be developed that take advantage of our improved understanding of the mechanisms controlling physiological insulin pulse patterns and their connection to insulin granule release.
Acknowledgments
The authors gratefully acknowledge research support from the National Institutes of Health as follows: RO1 DK46409 (L. Satin), RO1 DK059579 (P. Butler), and the intramural research program of the NIDDK (A. Sherman, J. Ha). We are also grateful to Mariana R. Ortiz for providing excellent editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Leslie S. Satin, Department of Pharmacology, Brehm Diabetes Research Center, University of Michigan Medical School, Ann Arbor, MI, 48105.
Peter C. Butler, Department of Medicine, David Geffen School of Medicine, UCLA, Los Angeles, CA. 90095-7073.
Joon Ha, Laboratory of Biological Modeling, National Institutes of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD. 20892.
Arthur S. Sherman, Laboratory of Biological Modeling, National Institutes of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD. 20892.
References Cited
- 1.Rahier J, Guiot Y, Goebbels RM, et al. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab. 2008;10(Suppl 4):32–42. doi: 10.1111/j.1463-1326.2008.00969.x. [DOI] [PubMed] [Google Scholar]
- 2.Ward WK, Beard JC, Porte D., Jr Clinical aspects of islet B-cell function in non-insulin-dependent diabetes mellitus. Diabetes Metab Rev. 1986;2:297–313. doi: 10.1002/dmr.5610020305. [DOI] [PubMed] [Google Scholar]
- 3.Straub SG, Sharp GWG. Glucose-stimulated signaling pathways in biphasic insulin secretion. Diabetes Metab Res Rev. 2002;18:451–463. doi: 10.1002/dmrr.329. [DOI] [PubMed] [Google Scholar]
- 4.Nesher R, Cerasi E. Modeling phasic insulin release: immediate and time-dependent effects of glucose. Diabetes. 2002;51(Suppl 1):S53–S59. doi: 10.2337/diabetes.51.2007.s53. [DOI] [PubMed] [Google Scholar]
- 5.Topp BG, Atkinson LL, Finegood DT. Dynamics of insulin sensitivity, -cell function, and -cell mass during the development of diabetes in fa/fa rats. Am J Physiol Endocrinol Metab. 2007;293:E1730–E1735. doi: 10.1152/ajpendo.00572.2007. [DOI] [PubMed] [Google Scholar]
- 6.Teta M, Long SY, Wartschow LM, et al. Very slow turnover of beta-cells in aged adult mice. Diabetes. 2005;54:2557–2567. doi: 10.2337/diabetes.54.9.2557. [DOI] [PubMed] [Google Scholar]
- 7.Kushner JA. The role of aging upon β cell turnover. J Clin Invest. 2013;123:990–995. doi: 10.1172/JCI64095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saisho Y, Butler AE, Manesso E, et al. β-cell mass and turnover in humans: effects of obesity and aging. Diabetes Care. 2013;36:111–117. doi: 10.2337/dc12-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodner CJ, Walike BC, Koerker DJ, et al. Insulin, glucagon, and glucose exhibit synchronous, sustained oscillations in fasting monkeys. Science. 1977;195:177–179. doi: 10.1126/science.401543. [DOI] [PubMed] [Google Scholar]
- 10.Lang DA, Matthews DR, Peto J, Turner RC. Cyclic oscillations of basal plasma glucose and insulin concentrations in human beings. N Engl J Med. 1979;301:1023–1027. doi: 10.1056/NEJM197911083011903. [DOI] [PubMed] [Google Scholar]
- 11.Lang DA, Matthews DR, Burnett M, Turner RC. Brief, irregular oscillations of basal plasma insulin and glucose concentrations in diabetic man. Diabetes. 1981;30:435–439. doi: 10.2337/diab.30.5.435. [DOI] [PubMed] [Google Scholar]
- 12.Polonsky KS. Evolution of beta-cell dysfunction in impaired glucose tolerance and diabetes. Exp Clin Endocrinol Diabetes Off J Ger Soc Endocrinol Ger Diabetes Assoc. 1999;107(Suppl 4):S124–S127. doi: 10.1055/s-0029-1212166. [DOI] [PubMed] [Google Scholar]
- 13.Hansen BC, Jen KC, Belbez Pek S, Wolfe RA. Rapid oscillations in plasma insulin, glucagon, and glucose in obese and normal weight humans. J Clin Endocrinol Metab. 1982;54:785–792. doi: 10.1210/jcem-54-4-785. [DOI] [PubMed] [Google Scholar]
- 14.Gumbiner B, Van Cauter E, Beltz WF, et al. Abnormalities of insulin pulsatility and glucose oscillations during meals in obese noninsulin-dependent diabetic patients: effects of weight reduction. J Clin Endocrinol Metab. 1996;81:2061–2068. doi: 10.1210/jcem.81.6.8964829. [DOI] [PubMed] [Google Scholar]
- 15.Hunter SJ, Atkinson AB, Ennis CN, et al. Association between insulin secretory pulse frequency and peripheral insulin action in NIDDM and normal subjects. Diabetes. 1996;45:683–686. doi: 10.2337/diab.45.5.683. [DOI] [PubMed] [Google Scholar]
- 16.Meier JJ, Pennartz C, Schenker N, et al. Hyperglycaemia is associated with impaired pulsatile insulin secretion: effect of basal insulin therapy. Diabetes Obes Metab. 2013;15:258–263. doi: 10.1111/dom.12022. [DOI] [PubMed] [Google Scholar]
- 17.Lin J-M, Fabregat ME, Gomis R, Bergsten P. Pulsatile insulin release from islets isolated from three subjects with type 2 diabetes. Diabetes. 2002;51:988–993. doi: 10.2337/diabetes.51.4.988. [DOI] [PubMed] [Google Scholar]
- 18.O’Rahilly S, Turner RC, Matthews DR. Impaired pulsatile secretion of insulin in relatives of patients with non-insulin-dependent diabetes. N Engl J Med. 1988;318:1225–1230. doi: 10.1056/NEJM198805123181902. [DOI] [PubMed] [Google Scholar]
- 19.Schmitz O, Pørksen N, Nyholm B, et al. Disorderly and nonstationary insulin secretion in relatives of patients with NIDDM. Am J Physiol. 1997;272:E218–E226. doi: 10.1152/ajpendo.1997.272.2.E218. [DOI] [PubMed] [Google Scholar]
- 20.Bingley PJ, Matthews DR, Williams AJ, et al. Loss of regular oscillatory insulin secretion in islet cell antibody positive non-diabetic subjects. Diabetologia. 1992;35:32–38. doi: 10.1007/BF00400849. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen MG, Sherman A. Newcomer insulin secretory granules as a highly calcium-sensitive pool. Proc Natl Acad Sci U S A. 2009;106:7432–7436. doi: 10.1073/pnas.0901202106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodner CJ, Sweet IR, Harrison HC., Jr Rapid reduction and return of surface insulin receptors after exposure to brief pulses of insulin in perifused rat hepatocytes. Diabetes. 1988;37:1316–1323. doi: 10.2337/diab.37.10.1316. [DOI] [PubMed] [Google Scholar]
- 23.Hirashima Y, Tsuruzoe K, Kodama S, et al. Insulin down-regulates insulin receptor substrate-2 expression through the phosphatidylinositol 3-kinase/Akt pathway. J Endocrinol. 2003;179:253–266. doi: 10.1677/joe.0.1790253. [DOI] [PubMed] [Google Scholar]
- 24.Hori SS, Kurland IJ, DiStefano JJ3rd. Role of endosomal trafficking dynamics on the regulation of hepatic insulin receptor activity: models for Fao cells. Ann Biomed Eng. 2006;34:879–892. doi: 10.1007/s10439-005-9065-5. [DOI] [PubMed] [Google Scholar]
- 25.Robertson RP, Zhou H, Slucca M. A role for zinc in pancreatic islet β-cell crosstalk with the α-cell during hypoglycaemia. Diabetes Obes Metab. 2011;13(Suppl 1):106–111. doi: 10.1111/j.1463-1326.2011.01448.x. [DOI] [PubMed] [Google Scholar]
- 26.Tamaki M, Fujitani Y, Hara A, et al. The diabetes-susceptible gene SLC30A8/ZnT8 regulates hepatic insulin clearance. J Clin Invest. 2013;123:4513–4524. doi: 10.1172/JCI68807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matveyenko AV, Veldhuis JD, Butler PC. Adaptations in pulsatile insulin secretion, hepatic insulin clearance, and beta-cell mass to age-related insulin resistance in rats. Am J Physiol Endocrinol Metab. 2008;295:E832–E841. doi: 10.1152/ajpendo.90451.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pørksen N, Munn SR, Steers JL, et al. Effects of somatostatin on pulsatile insulin secretion: elective inhibition of insulin burst mass. Am J Physiol. 1996;270:E1043–E1049. doi: 10.1152/ajpendo.1996.270.6.E1043. [DOI] [PubMed] [Google Scholar]
- 29.Matveyenko AV, Veldhuis JD, Butler PC. Mechanisms of Impaired Fasting Glucose and Glucose Intolerance Induced by a −50% Pancreatectomy. Diabetes. 2006;55:2347–2356. doi: 10.2337/db06-0345. [DOI] [PubMed] [Google Scholar]
- 30.Meier JJ, Veldhuis JD, Butler PC. Pulsatile insulin secretion dictates systemic insulin delivery by regulating hepatic insulin extraction in humans. Diabetes. 2005;54:1649–1656. doi: 10.2337/diabetes.54.6.1649. [DOI] [PubMed] [Google Scholar]
- 31.Peiris AN, Stagner JI, Vogel RL, et al. Body fat distribution and peripheral insulin sensitivity in healthy men: role of insulin pulsatility. J Clin Endocrinol Metab. 1992;75:290–294. doi: 10.1210/jcem.75.1.1619021. [DOI] [PubMed] [Google Scholar]
- 32.Pørksen N, Hollingdal M, Juhl C, et al. Pulsatile insulin secretion: detection, regulation, and role in diabetes. Diabetes. 2002;51(Suppl 1):S245–S254. doi: 10.2337/diabetes.51.2007.s245. [DOI] [PubMed] [Google Scholar]
- 33.Veldhuis JD, Keenan DM, Pincus SM. Motivations and methods for analyzing pulsatile hormone secretion. Endocr Rev. 2008;29:823–864. doi: 10.1210/er.2008-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pørksen N. The in vivo regulation of pulsatile insulin secretion. Diabetologia. 2002;45:3–20. doi: 10.1007/s001250200001. [DOI] [PubMed] [Google Scholar]
- 35.Pørksen N, Munn S, Steers J, et al. Impact of sampling technique on appraisal of pulsatile insulin secretion by deconvolution and cluster analysis. Am J Physiol. 1995;269:E1106–E1114. doi: 10.1152/ajpendo.1995.269.6.E1106. [DOI] [PubMed] [Google Scholar]
- 36.Kjems LL, Kirby BM, Welsh EM, et al. Decrease in beta-cell mass leads to impaired pulsatile insulin secretion, reduced postprandial hepatic insulin clearance, and relative hyperglucagonemia in the minipig. Diabetes. 2001;50:2001–2012. doi: 10.2337/diabetes.50.9.2001. [DOI] [PubMed] [Google Scholar]
- 37.Matveyenko AV, Liuwantara D, Gurlo T, et al. Pulsatile portal vein insulin delivery enhances hepatic insulin action and signaling. Diabetes. 2012;61:2269–2279. doi: 10.2337/db11-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pørksen N, Munn S, Steers J, et al. Pulsatile insulin secretion accounts for 70% of total insulin secretion during fasting. Am J Physiol. 1995;269:E478–E488. doi: 10.1152/ajpendo.1995.269.3.E478. [DOI] [PubMed] [Google Scholar]
- 39.Ritzel RA, Veldhuis JD, Butler PC. Glucose stimulates pulsatile insulin secretion from human pancreatic islets by increasing secretory burst mass: dose-response relationships. J Clin Endocrinol Metab. 2003;88:742–747. doi: 10.1210/jc.2002-021250. [DOI] [PubMed] [Google Scholar]
- 40.Beauvois MC, Merezak C, Jonas J-C, et al. Glucose-induced mixed [Ca2+]c oscillations in mouse beta-cells are controlled by the membrane potential and the SERCA3 Ca2+-ATPase of the endoplasmic reticulum. Am J Physiol Cell Physiol. 2006;290:C1503–C1511. doi: 10.1152/ajpcell.00400.2005. [DOI] [PubMed] [Google Scholar]
- 41.Bertram R, Sherman A, Satin LS. Metabolic and electrical oscillations: partners in controlling pulsatile insulin secretion. Am J Physiol Endocrinol Metab. 2007;293:E890–E900. doi: 10.1152/ajpendo.00359.2007. [DOI] [PubMed] [Google Scholar]
- 42.Hellman B, Salehi A, Gylfe E, et al. Glucose generates coincident insulin and somatostatin pulses and antisynchronous glucagon pulses from human pancreatic islets. Endocrinology. 2009;150:5334–5340. doi: 10.1210/en.2009-0600. [DOI] [PubMed] [Google Scholar]
- 43.Field JB, Rojdmark S, Harding P, et al. Role of liver in insulin physiology. Diabetes Care. 1980;3:255–260. doi: 10.2337/diacare.3.2.255. [DOI] [PubMed] [Google Scholar]
- 44.Bratusch-Marrain PR, Komjati M, Waldhäusl WK. Efficacy of pulsatile versus continuous insulin administration on hepatic glucose production and glucose utilization in type I diabetic humans. Diabetes. 1986;35:922–926. doi: 10.2337/diab.35.8.922. [DOI] [PubMed] [Google Scholar]
- 45.Paolisso G, Scheen AJ, Albert A, Lefebvre PJ. Effects of pulsatile delivery of insulin and glucagon in humans. Am J Physiol. 1989;257:E686–E696. doi: 10.1152/ajpendo.1989.257.5.E686. [DOI] [PubMed] [Google Scholar]
- 46.Komjati M, Bratusch-Marrain P, Waldhäusl W. Superior efficacy of pulsatile versus continuous hormone exposure on hepatic glucose production in vitro. Endocrinology. 1986;118:312–319. doi: 10.1210/endo-118-1-312. [DOI] [PubMed] [Google Scholar]
- 47.Dobbins RL, Davis SN, Neal DW, et al. Pulsatility does not alter the response to a physiological increment in glucagon in the conscious dog. Am J Physiol. 1994;266:E467–E478. doi: 10.1152/ajpendo.1994.266.3.E467. [DOI] [PubMed] [Google Scholar]
- 48.Grubert JM, Lautz M, Lacy DB, et al. Impact of continuous and pulsatile insulin delivery on net hepatic glucose uptake. Am J Physiol Endocrinol Metab. 2005;289:E232–E240. doi: 10.1152/ajpendo.00567.2004. [DOI] [PubMed] [Google Scholar]
- 49.Sedaghat AR, Sherman A, Quon MJ. A mathematical model of metabolic insulin signaling pathways. Am J Physiol Endocrinol Metab. 2002;283:E1084–E1101. doi: 10.1152/ajpendo.00571.2001. [DOI] [PubMed] [Google Scholar]
- 50.Declercq J, Kumar A, Van Diepen JA, et al. Increased beta-cell mass by islet transplantation and PLAG1 overexpression causes hyperinsulinemic normoglycemia and hepatic insulin resistance in mice. Diabetes. 2010;59:1957–1965. doi: 10.2337/db09-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gray SL, Donald C, Jetha A, et al. Hyperinsulinemia precedes insulin resistance in mice lacking pancreatic beta-cell leptin signaling. Endocrinology. 2010;151:4178–4186. doi: 10.1210/en.2010-0102. [DOI] [PubMed] [Google Scholar]
- 52.Shimomura I, Matsuda M, Hammer RE, et al. Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol Cell. 2000;6:77–86. [PubMed] [Google Scholar]
- 53.Alemzadeh R, Langley G, Upchurch L, et al. Beneficial effect of diazoxide in obese hyperinsulinemic adults. J Clin Endocrinol Metab. 1998;83:1911–1915. doi: 10.1210/jcem.83.6.4852. [DOI] [PubMed] [Google Scholar]
- 54.Matthews DR, Lang DA, Burnett MA, Turner RC. Control of pulsatile insulin secretion in man. Diabetologia. 1983;24:231–237. doi: 10.1007/BF00282705. [DOI] [PubMed] [Google Scholar]
- 55.Juhl CB, Pørksen N, Hollingdal M, et al. Repaglinide acutely amplifies pulsatile insulin secretion by augmentation of burst mass with no effect on burst frequency. Diabetes Care. 2000;23:675–681. doi: 10.2337/diacare.23.5.675. [DOI] [PubMed] [Google Scholar]
- 56.Juhl CB, Pørksen N, Pincus SM, et al. Acute and short-term administration of a sulfonylurea (gliclazide) increases pulsatile insulin secretion in type 2 diabetes. Diabetes. 2001;50:1778–1784. doi: 10.2337/diabetes.50.8.1778. [DOI] [PubMed] [Google Scholar]
- 57.Pørksen N, Grøfte B, Nyholm B, et al. Glucagon-like peptide 1 increases mass but not frequency or orderliness of pulsatile insulin secretion. Diabetes. 1998;47:45–49. doi: 10.2337/diab.47.1.45. [DOI] [PubMed] [Google Scholar]
- 58.Pørksen N, Munn S, Steers J, et al. Effects of glucose ingestion versus infusion on pulsatile insulin secretion. The incretin effect is achieved by amplification of insulin secretory burst mass. Diabetes. 1996;45:1317–1323. doi: 10.2337/diab.45.10.1317. [DOI] [PubMed] [Google Scholar]
- 59.Song SH, McIntyre SS, Shah H, et al. Direct measurement of pulsatile insulin secretion from the portal vein in human subjects. J Clin Endocrinol Metab. 2000;85:4491–4499. doi: 10.1210/jcem.85.12.7043. [DOI] [PubMed] [Google Scholar]
- 60.Polonsky KS, Given BD, Hirsch LJ, et al. Abnormal patterns of insulin secretion in non-insulin-dependent diabetes mellitus. N Engl J Med. 1988;318:1231–1239. doi: 10.1056/NEJM198805123181903. [DOI] [PubMed] [Google Scholar]
- 61.O’Meara NM, Sturis J, Van Cauter E, Polonsky KS. Lack of control by glucose of ultradian insulin secretory oscillations in impaired glucose tolerance and in non-insulin-dependent diabetes mellitus. J Clin Invest. 1993;92:262–271. doi: 10.1172/JCI116560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O’Meara NM, Sturis J, Herold KC, et al. Alterations in the patterns of insulin secretion before and after diagnosis of IDDM. Diabetes Care. 1995;18:568–571. doi: 10.2337/diacare.18.4.568. [DOI] [PubMed] [Google Scholar]
- 63.Hellman B, Salehi A, Grapengiesser E, Gylfe E. Isolated mouse islets respond to glucose with an initial peak of glucagon release followed by pulses of insulin and somatostatin in antisynchrony with glucagon. Biochem Biophys Res Commun. 2012;417:1219–1223. doi: 10.1016/j.bbrc.2011.12.113. [DOI] [PubMed] [Google Scholar]
- 64.Franklin I, Gromada J, Gjinovci A, et al. Beta-cell secretory products activate alpha-cell ATP-dependent potassium channels to inhibit glucagon release. Diabetes. 2005;54:1808–1815. doi: 10.2337/diabetes.54.6.1808. [DOI] [PubMed] [Google Scholar]
- 65.Leung YM, Ahmed I, Sheu L, et al. Insulin regulates islet alpha-cell function by reducing KATP channel sensitivity to adenosine 5’-triphosphate inhibition. Endocrinology. 2006;147:2155–2162. doi: 10.1210/en.2005-1249. [DOI] [PubMed] [Google Scholar]
- 66.Gromada J, Høy M, Olsen HL, et al. Gi2 proteins couple somatostatin receptors to low-conductance K+ channels in rat pancreatic alpha-cells. Pflüg Arch Eur J Physiol. 2001;442:19–26. doi: 10.1007/s004240000474. [DOI] [PubMed] [Google Scholar]
- 67.Gromada J, Høy M, Buschard K, et al. Somatostatin inhibits exocytosis in rat pancreatic alpha-cells by G(i2)-dependent activation of calcineurin and depriming of secretory granules. J Physiol. 2001;535:519–532. doi: 10.1111/j.1469-7793.2001.00519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berts A, Gylfe E, Hellman B. Ca2+ oscillations in pancreatic islet cells secreting glucagon and somatostatin. Biochem Biophys Res Commun. 1995;208:644–649. doi: 10.1006/bbrc.1995.1387. [DOI] [PubMed] [Google Scholar]
- 69.Quoix N, Cheng-Xue R, Mattart L, et al. Glucose and pharmacological modulators of ATP-sensitive K+ channels control [Ca2+]c by different mechanisms in isolated mouse alpha-cells. Diabetes. 2009;58:412–421. doi: 10.2337/db07-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Menge BA, Grüber L, Jørgensen SM, et al. Loss of inverse relationship between pulsatile insulin and glucagon secretion in patients with type 2 diabetes. Diabetes. 2011;60:2160–2168. doi: 10.2337/db11-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lang DA, Matthews DR, Burnett M, et al. Pulsatile, synchronous basal insulin and glucagon secretion in man. Diabetes. 1982;31:22–26. doi: 10.2337/diab.31.1.22. [DOI] [PubMed] [Google Scholar]
- 72.Rohrer S, Menge BA, Grüber L, et al. Impaired crosstalk between pulsatile insulin and glucagon secretion in prediabetic individuals. J Clin Endocrinol Metab. 2012;97:E791–E795. doi: 10.1210/jc.2011-3439. [DOI] [PubMed] [Google Scholar]
- 73.Hellman B. Pulsatility of insulin release--a clinically important phenomenon. Ups J Med Sci. 2009;114:193–205. doi: 10.3109/03009730903366075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Song W-J, Mondal P, Wolfe A, et al. Glucagon regulates hepatic kisspeptin to impair insulin secretion. Cell Metab. 2014;19:667–681. doi: 10.1016/j.cmet.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gylfe E, Tengholm A. Neurotransmitter control of islet hormone pulsatility. Diabetes Obes Metab. 2014;16(Suppl 1):102–110. doi: 10.1111/dom.12345. [DOI] [PubMed] [Google Scholar]
- 76.Juhl CB, Pørksen N, Sturis J, et al. High-frequency oscillations in circulating amylin concentrations in healthy humans. Am J Physiol Endocrinol Metab. 2000;278:E484–E490. doi: 10.1152/ajpendo.2000.278.3.E484. [DOI] [PubMed] [Google Scholar]
- 77.Salehi A, Parandeh F, Fredholm BB, et al. Absence of adenosine A1 receptors unmasks pulses of insulin release and prolongs those of glucagon and somatostatin. Life Sci. 2009;85:470–476. doi: 10.1016/j.lfs.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 78.Dean PM, Matthews EK. Glucose-induced electrical activity in pancreatic islet cells. J Physiol. 1970;210:255–264. doi: 10.1113/jphysiol.1970.sp009207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tengholm A, Gylfe E. Oscillatory control of insulin secretion. Mol Cell Endocrinol. 2009;297:58–72. doi: 10.1016/j.mce.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 80.Meissner HP, Schmelz H. Membrane potential of beta-cells in pancreatic islets. Pflüg Arch Eur J Physiol. 1974;351:195–206. doi: 10.1007/BF00586918. [DOI] [PubMed] [Google Scholar]
- 81.Malaisse WJ, Herchuelz A, Devis G, et al. Regulation of calcium fluxes and their regulatory roles in pancreatic islets. Ann N Y Acad Sci. 1978;307:562–582. doi: 10.1111/j.1749-6632.1978.tb41982.x. [DOI] [PubMed] [Google Scholar]
- 82.Beigelman PM, Ribalet B, Atwater I. Electric activity of mouse pancreatic beta-cells. II. Effects of glucose and arginine. J Physiol (Paris) 1977;73:201–217. [PubMed] [Google Scholar]
- 83.Henquin JC. Regulation of insulin release by ionic and electrical events in B cells. Horm Res. 1987;27:168–178. doi: 10.1159/000180806. [DOI] [PubMed] [Google Scholar]
- 84.Cook DL. Isolated islets of Langerhans have slow oscillations of electrical activity. Metabolism. 1983;32:681–685. doi: 10.1016/0026-0495(83)90124-5. [DOI] [PubMed] [Google Scholar]
- 85.Henquin JC, Meissner HP, Schmeer W. Cyclic variations of glucose-induced electrical activity in pancreatic B cells. Pflüg Arch Eur J Physiol. 1982;393:322–327. doi: 10.1007/BF00581418. [DOI] [PubMed] [Google Scholar]
- 86.Ashcroft FM, Rorsman P. Electrophysiology of the pancreatic beta-cell. Prog Biophys Mol Biol. 1989;54:87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- 87.Cook DL, Satin LS, Hopkins WF. Pancreatic B cells are bursting, but how? Trends Neurosci. 1991;14:411–414. doi: 10.1016/0166-2236(91)90033-q. [DOI] [PubMed] [Google Scholar]
- 88.Sherman A. Lessons from models of pancreatic beta cells for engineering glucose-sensing cells. Math Biosci. 2010;227:12–19. doi: 10.1016/j.mbs.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Satin LS, Tavalin SJ, Smolen PD. Inactivation of HIT cell Ca2+ current by a simulated burst of Ca2+ action potentials. Biophys J. 1994;66:141–148. doi: 10.1016/S0006-3495(94)80759-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fridlyand LE, Tamarina N, Philipson LH. Bursting and calcium oscillations in pancreatic beta-cells: specific pacemakers for specific mechanisms. Am J Physiol Endocrinol Metab. 2010;299:E517–E532. doi: 10.1152/ajpendo.00177.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fridlyand LE, Tamarina N, Philipson LH. Modeling of Ca2+ flux in pancreatic beta-cells: role of the plasma membrane and intracellular stores. Am J Physiol Endocrinol Metab. 2003;285:E138–E154. doi: 10.1152/ajpendo.00194.2002. [DOI] [PubMed] [Google Scholar]
- 92.Fridlyand LE, Jacobson DA, Philipson LH. Ion channels and regulation of insulin secretion in human β-cells: a computational systems analysis. Islets. 2013;5:1–15. doi: 10.4161/isl.24166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cha CY, Powell T, Noma A. Analyzing electrical activities of pancreatic β cells using mathematical models. Prog Biophys Mol Biol. 2011;107:265–273. doi: 10.1016/j.pbiomolbio.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 94.Pedersen MG. A biophysical model of electrical activity in human β-cells. Biophys J. 2010;99:3200–3207. doi: 10.1016/j.bpj.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Martín F, Soria B. Glucose-induced [Ca2+]i oscillations in single human pancreatic islets. Cell Calcium. 1996;20:409–414. doi: 10.1016/s0143-4160(96)90003-2. [DOI] [PubMed] [Google Scholar]
- 96.Kindmark H, Köhler M, Nilsson T, et al. Measurements of cytoplasmic free Ca2+ concentration in human pancreatic islets and insulinoma cells. FEBS Lett. 1991;291:310–314. doi: 10.1016/0014-5793(91)81309-v. [DOI] [PubMed] [Google Scholar]
- 97.Kindmark H, Köhler M, Arkhammar P, et al. Oscillations in cytoplasmic free calcium concentration in human pancreatic islets from subjects with normal and impaired glucose tolerance. Diabetologia. 1994;37:1121–1131. doi: 10.1007/BF00418376. [DOI] [PubMed] [Google Scholar]
- 98.Riz M, Braun M, Pedersen MG. Mathematical modeling of heterogeneous electrophysiological responses in human β-cells. PLoS Comput Biol. 2014;10:e1003389. doi: 10.1371/journal.pcbi.1003389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cabrera O, Berman DM, Kenyon NS, et al. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci U S A. 2006;103:2334–2339. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Braun M, Ramracheya R, Bengtsson M, et al. Voltage-gated ion channels in human pancreatic beta-cells: electrophysiological characterization and role in insulin secretion. Diabetes. 2008;57:1618–1628. doi: 10.2337/db07-0991. [DOI] [PubMed] [Google Scholar]
- 101.Braun M. The αβδ of ion channels in human islet cells. Islets. 2009;1:160–162. doi: 10.4161/isl.1.2.9405. [DOI] [PubMed] [Google Scholar]
- 102.Bertram R, Sherman A, Satin LS. Electrical bursting, calcium oscillations, and synchronization of pancreatic islets. Adv Exp Med Biol. 2010;654:261–279. doi: 10.1007/978-90-481-3271-3_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tornheim K. Are metabolic oscillations responsible for normal oscillatory insulin secretion? Diabetes. 1997;46:1375–1380. doi: 10.2337/diab.46.9.1375. [DOI] [PubMed] [Google Scholar]
- 104.Bertram R, Satin LS, Pedersen MG, et al. Interaction of glycolysis and mitochondrial respiration in metabolic oscillations of pancreatic islets. Biophys J. 2007;92:1544–1555. doi: 10.1529/biophysj.106.097154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Merrins MJ, Fendler B, Zhang M, et al. Metabolic oscillations in pancreatic islets depend on the intracellular Ca2+ level but not Ca2+ oscillations. Biophys J. 2010;99:76–84. doi: 10.1016/j.bpj.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ren J, Sherman A, Bertram R, et al. Slow oscillations of KATP conductance in mouse pancreatic islets provide support for electrical bursting driven by metabolic oscillations. Am J Physiol Endocrinol Metab. 2013;305:E805–E817. doi: 10.1152/ajpendo.00046.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Goel P, Sherman A. The Geometry of Bursting in the Dual Oscillator Model of Pancreatic $\beta$-cells. SIAM J Appl Dyn Syst. 2009;8:1664–1693. doi: 10.1137/08074427X. [DOI] [Google Scholar]
- 108.Nunemaker CS, Dishinger JF, Dula SB, et al. Glucose metabolism, islet architecture, and genetic homogeneity in imprinting of [Ca2+](i) and insulin rhythms in mouse islets. PloS One. 2009;4:e8428. doi: 10.1371/journal.pone.0008428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Luciani DS, Misler S, Polonsky KS. Ca2+ controls slow NAD(P)H oscillations in glucose-stimulated mouse pancreatic islets. J Physiol. 2006;572:379–392. doi: 10.1113/jphysiol.2005.101766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li J, Shuai HY, Gylfe E, Tengholm A. Oscillations of sub-membrane ATP in glucose-stimulated beta cells depend on negative feedback from Ca(2+) Diabetologia. 2013;56:1577–1586. doi: 10.1007/s00125-013-2894-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Heart E, Smith PJS. Rhythm of the beta-cell oscillator is not governed by a single regulator: multiple systems contribute to oscillatory behavior. Am J Physiol Endocrinol Metab. 2007;292:E1295–E1300. doi: 10.1152/ajpendo.00648.2006. [DOI] [PubMed] [Google Scholar]
- 112.Leclercq-Meyer V, Marchand J, Woussen-Colle MC, et al. Multiple effects of leucine on glucagon, insulin, and somatostatin secretion from the perfused rat pancreas. Endocrinology. 1985;116:1168–1174. doi: 10.1210/endo-116-3-1168. [DOI] [PubMed] [Google Scholar]
- 113.Dahlgren GM, Kauri LM, Kennedy RT. Substrate effects on oscillations in metabolism, calcium and secretion in single mouse islets of Langerhans. Biochim Biophys Acta. 2005;1724:23–36. doi: 10.1016/j.bbagen.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 114.Zhang M, Goforth P, Bertram R, et al. The Ca2+ dynamics of isolated mouse beta-cells and islets: implications for mathematical models. Biophys J. 2003;84:2852–2870. doi: 10.1016/S0006-3495(03)70014-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nunemaker CS, Bertram R, Sherman A, et al. Glucose modulates [Ca2+]i oscillations in pancreatic islets via ionic and glycolytic mechanisms. Biophys J. 2006;91:2082–2096. doi: 10.1529/biophysj.106.087296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Merrins MJ, Van Dyke AR, Mapp AK, et al. Direct measurements of oscillatory glycolysis in pancreatic islet β-cells using novel fluorescence resonance energy transfer (FRET) biosensors for pyruvate kinase M2 activity. J Biol Chem. 2013;288:33312–33322. doi: 10.1074/jbc.M113.508127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Merrins MJ, Bertram R, Sherman A, Satin LS. Phosphofructo-2-kinase/fructose-2,6-bisphosphatase modulates oscillations of pancreatic islet metabolism. PloS One. 2012;7:e34036. doi: 10.1371/journal.pone.0034036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gilon P, Ravier MA, Jonas J-C, Henquin J-C. Control mechanisms of the oscillations of insulin secretion in vitro and in vivo. Diabetes. 2002;51(Suppl 1):S144–S151. doi: 10.2337/diabetes.51.2007.s144. [DOI] [PubMed] [Google Scholar]
- 119.Satin LS, Kinard TA. Neurotransmitters and their receptors in the islets of Langerhans of the pancreas: what messages do acetylcholine, glutamate, and GABA transmit? Endocrine. 1998;8:213–223. doi: 10.1385/ENDO:8:3:213. [DOI] [PubMed] [Google Scholar]
- 120.Benninger RKP, Zhang M, Head WS, et al. Gap junction coupling and calcium waves in the pancreatic islet. Biophys J. 2008;95:5048–5061. doi: 10.1529/biophysj.108.140863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Benninger RKP, Head WS, Zhang M, et al. Gap junctions and other mechanisms of cell-cell communication regulate basal insulin secretion in the pancreatic islet. J Physiol. 2011;589:5453–5466. doi: 10.1113/jphysiol.2011.218909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Head WS, Orseth ML, Nunemaker CS, et al. Connexin-36 gap junctions regulate in vivo first- and second-phase insulin secretion dynamics and glucose tolerance in the conscious mouse. Diabetes. 2012;61:1700–1707. doi: 10.2337/db11-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang M, Fendler B, Peercy B, et al. Long lasting synchronization of calcium oscillations by cholinergic stimulation in isolated pancreatic islets. Biophys J. 2008;95:4676–4688. doi: 10.1529/biophysj.107.125088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nunemaker CS, Zhang M, Wasserman DH, et al. Individual mice can be distinguished by the period of their islet calcium oscillations: is there an intrinsic islet period that is imprinted in vivo? Diabetes. 2005;54:3517–3522. doi: 10.2337/diabetes.54.12.3517. [DOI] [PubMed] [Google Scholar]
- 125.Stagner JI, Samols E. Perturbation of insulin oscillations by nerve blockade in the in vitro canine pancreas. Am J Physiol. 1985;248:E516–E521. doi: 10.1152/ajpendo.1985.248.5.E516. [DOI] [PubMed] [Google Scholar]
- 126.Stagner JI, Samols E. Role of intrapancreatic ganglia in regulation of periodic insular secretions. Am J Physiol. 1985;248:E522–E530. doi: 10.1152/ajpendo.1985.248.5.E522. [DOI] [PubMed] [Google Scholar]
- 127.Cyrus J, Stagner J, Samols E. Abolition of dopaminergic insulin secretion by beta-adrenergic antagonism. Endocrinology. 1982;111:214–218. doi: 10.1210/endo-111-1-214. [DOI] [PubMed] [Google Scholar]
- 128.Fendler B, Zhang M, Satin L, Bertram R. Synchronization of pancreatic islet oscillations by intrapancreatic ganglia: a modeling study. Biophys J. 2009;97:722–729. doi: 10.1016/j.bpj.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Grapengiesser E, Dansk H, Hellman B. Pulses of external ATP aid to the synchronization of pancreatic beta-cells by generating premature Ca(2+) oscillations. Biochem Pharmacol. 2004;68:667–674. doi: 10.1016/j.bcp.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 130.Hellman B, Dansk H, Grapengiesser E. Pancreatic beta-cells communicate via intermittent release of ATP. Am J Physiol Endocrinol Metab. 2004;286:E759–E765. doi: 10.1152/ajpendo.00452.2003. [DOI] [PubMed] [Google Scholar]
- 131.Schmitz O, Rungby J, Edge L, Juhl CB. On high-frequency insulin oscillations. Ageing Res Rev. 2008;7:301–305. doi: 10.1016/j.arr.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 132.Pørksen NK, Munn SR, Steers JL, et al. Mechanisms of sulfonylurea’s stimulation of insulin secretion in vivo: selective amplification of insulin secretory burst mass. Diabetes. 1996;45:1792–1797. doi: 10.2337/diab.45.12.1792. [DOI] [PubMed] [Google Scholar]
- 133.Juhl CB, Hollingdal M, Pørksen N, et al. Influence of rosiglitazone treatment on beta-cell function in type 2 diabetes: evidence of an increased ability of glucose to entrain high-frequency insulin pulsatility. J Clin Endocrinol Metab. 2003;88:3794–3800. doi: 10.1210/jc.2002-021181. [DOI] [PubMed] [Google Scholar]
- 134.Drucker DJ. Incretin action in the pancreas: potential promise, possible perils, and pathological pitfalls. Diabetes. 2013;62:3316–3323. doi: 10.2337/db13-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ritzel R, Schulte M, Pørksen N, et al. Glucagon-like peptide 1 increases secretory burst mass of pulsatile insulin secretion in patients with type 2 diabetes and impaired glucose tolerance. Diabetes. 2001;50:776–784. doi: 10.2337/diabetes.50.4.776. [DOI] [PubMed] [Google Scholar]
- 136.Schmitz O, Brock B, Hollingdal M, et al. High-frequency insulin pulsatility and type 2 diabetes: from physiology and pathophysiology to clinical pharmacology. Diabetes Metab. 2002;28:4S14–4S20. [PubMed] [Google Scholar]
- 137.Kwan EP, Xie L, Sheu L, et al. Interaction between Munc13-1 and RIM is critical for glucagon-like peptide-1 mediated rescue of exocytotic defects in Munc13-1 deficient pancreatic beta-cells. Diabetes. 2007;56:2579–2588. doi: 10.2337/db06-1207. [DOI] [PubMed] [Google Scholar]
- 138.Tortosa F, Dotta F. Incretin hormones and beta-cell mass expansion: what we know and what is missing? Arch Physiol Biochem. 2013;119:161–169. doi: 10.3109/13813455.2013.795175. [DOI] [PubMed] [Google Scholar]
- 139.Brown RJ, Rother KI. Effects of beta-cell rest on beta-cell function: a review of clinical and preclinical data. Pediatr Diabetes. 2008;9:14–22. doi: 10.1111/j.1399-5448.2007.00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Laedtke T, Kjems L, Pørksen N, et al. Overnight inhibition of insulin secretion restores pulsatility and proinsulin/insulin ratio in type 2 diabetes. Am J Physiol Endocrinol Metab. 2000;279:E520–E528. doi: 10.1152/ajpendo.2000.279.3.E520. [DOI] [PubMed] [Google Scholar]
- 141.Remedi MS, Nichols CG. Chronic antidiabetic sulfonylureas in vivo: reversible effects on mouse pancreatic beta-cells. PLoS Med. 2008;5:e206. doi: 10.1371/journal.pmed.0050206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ashcroft FM, Rorsman P. Electrophysiology of the pancreatic beta-cell. Prog Biophys Mol Biol. 1989;54:87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- 143.Dyachok O, Idevall-Hagren O, Sågetorp J, et al. Glucose-induced cyclic AMP oscillations regulate pulsatile insulin secretion. Cell Metab. 2008;8:26–37. doi: 10.1016/j.cmet.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 144.Tengholm A. Cyclic AMP dynamics in the pancreatic β-cell. Ups J Med Sci. 2012;117:355–369. doi: 10.3109/03009734.2012.724732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Henquin J-C. The dual control of insulin secretion by glucose involves triggering and amplifying pathways in β-cells. Diabetes Res Clin Pract. 2011;93(Suppl 1):S27–S31. doi: 10.1016/S0168-8227(11)70010-9. [DOI] [PubMed] [Google Scholar]
- 146.Skjaervold NK, Ostling D, Hjelme DR, et al. Blood glucose control using a novel continuous blood glucose monitor and repetitive intravenous insulin boluses: exploiting natural insulin pulsatility as a principle for a future artificial pancreas. Int J Endocrinol. 2013;2013:245152. doi: 10.1155/2013/245152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Juhl CB, Gjedsted J, Nielsen MF, Schmitz O. Increased action of pulsatile compared to non-pulsatile insulin delivery during a meal-like glucose exposure simulated by computerized infusion in healthy humans. Metabolism. 2012;61:1177–1181. doi: 10.1016/j.metabol.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 148.Quon MJ, Campfield LA. A mathematical model and computer simulation study of insulin receptor regulation. J Theor Biol. 1991;150:59–72. doi: 10.1016/s0022-5193(05)80475-8. [DOI] [PubMed] [Google Scholar]