Abstract
Tissue engineering is well suited for the treatment of cardiac disease due to the limited regenerative capacity of native cardiac tissue and the loss of function associated with endemic cardiac pathologies, such as myocardial infarction and congenital heart defects. However, the physiological complexity of the myocardium imposes extensive requirements on tissue therapies intended for these applications. In recent years, the field of cardiac tissue engineering has been characterized by great innovation and diversity in the fabrication of engineered tissue scaffolds for cardiac repair and regeneration to address these problems. From early approaches that attempted only to deliver cardiac cells in a hydrogel vessel, significant progress has been made in understanding the role of each major component of cardiac living tissue constructs (namely cells, scaffolds, and signaling mechanisms) as they relate to mechanical, biological, and electrical in vivo performance. This improved insight, accompanied by modern material science techniques, allows for the informed development of complex scaffold materials that are optimally designed for cardiac applications. This review provides a background on cardiac physiology as it relates to critical cardiac scaffold characteristics, the degree to which common cardiac scaffold materials fulfill these criteria, and finally an overview of recent in vivo studies that have employed this type of approach.
Keywords: tissue engineering, heart repair, biomaterials, cardiac function
1. Introduction
Over the past fifteen years great advances have been made in the design, development, and optimization of scaffolds for tissue engineering. This progress has been facilitated by the discovery of a wide variety of scaffold materials and processing techniques that have been iteratively improved to produce tissue scaffolds that meet the specific requirements of nearly every tissue in the body [1]. One area of scaffold development research that has seen a particularly high level of innovation in recent years is that of cardiac tissue scaffolds [2–6].
Much of the interest in cardiac tissue engineering has been driven by the high prevalence of cardiac disease. As a group, ischemic heart diseases were responsible for 7.2 million deaths in 2004, 12.2% of all deaths that year [7]. In the United States, nearly 600 000 people die annually from cardiac diseases such as myocardial infarction, cardiac myopathies, and congenital heart defects [8]. Coronary heart disease alone (associated primarily with myocardial infarctions), costs the United States $108.9 billion annually in medication, health services, and lost productivity [9]. It’s therefore unsurprising that great efforts are being made to address this problem through tissue engineering research.
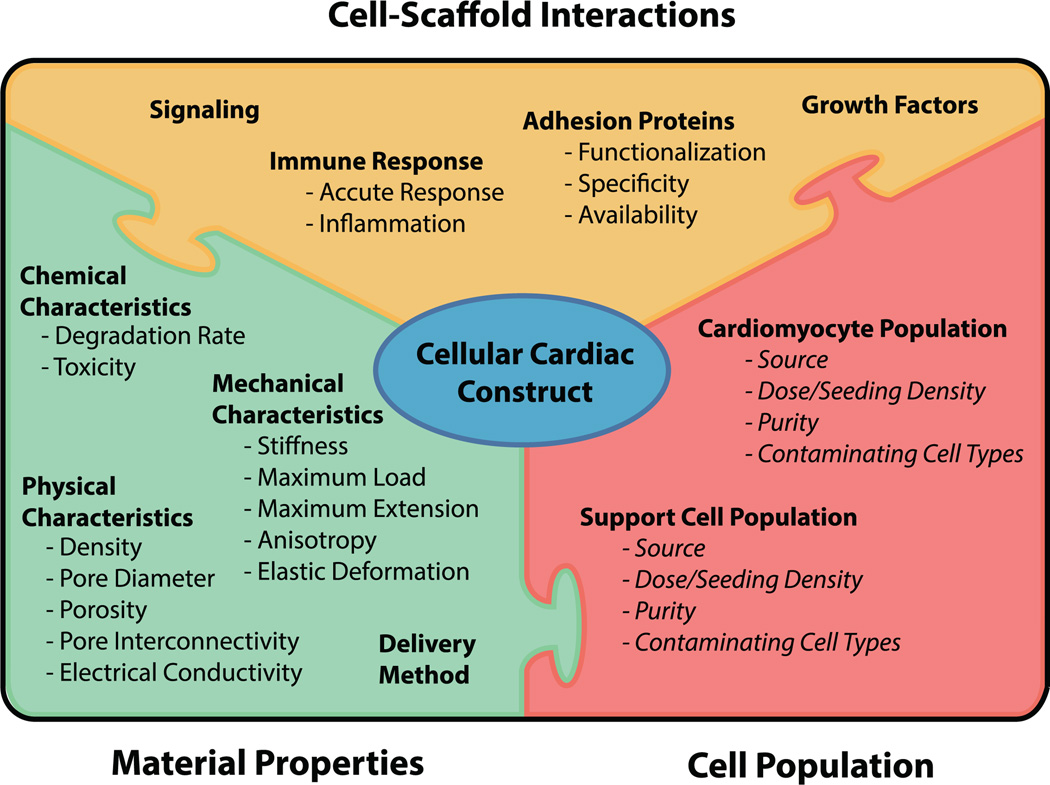
As an organ, the heart presents unique challenges to tissue engineers. In vivo, native cardiac tissue is constantly exposed to cyclic mechanical stress and strain in the form of elastic extension and relaxation, as well as electrical stimuli that must elicit an appropriate response and be transmitted to neighboring cardiac regions to initiate contraction. Tissue that lines the chambers of the heart is additionally exposed to cyclic shear stresses from circulating blood. Finally, the heterogeneous cell populations that comprise cardiac tissue (consisting primarily of cardiomyocytes, fibroblasts, smooth muscle cells, and endothelial cells) vary in alignment, population distribution, and phenotype between various sub-regions of the heart [10, 11]. In addition to these functional characteristics that define the cardiac environment, tissue scaffolds for heart regeneration must also comply with general requirements for all tissue scaffolds, including considerations for immune response, degradation time, cell-scaffold interaction, vascularization/nutrient delivery, and implantation method (figure 1).
Figure 1.
Many interconnected factors must be considered in the design of a scaffold that will be used in a cellular construct for cardiac tissue repair or regeneration. Items in italics are beyond the scope of this review. For a more detailed review of cell populations for cardiac tissue constructs, see Coulombe et al (2014) [12].
The extremely limited healing and regenerative properties of the heart also pose an enormous challenge to the tissue engineering approach. In other in vivo applications of tissue engineering, an implanted scaffold or construct can be expected to benefit from native cell infiltration and repopulation by the host tissue. These healing responses are also associated with at least a partial restoration of tissue function prior to injury. In the myocardium, the replacement rate of cardiomyocytes is estimated to be approximately 0.3 to 1% annually depending on age [13]. As a result, acellular cardiac scaffolds have little utility in restoring active contractile function, and cellular cardiac constructs must be implanted with their targeted final population of cardiomyocytes.
The numerous and varied requirements for cardiac tissue scaffolds has led to similarly diverse approaches to scaffold design. Wide arrays of natural and synthetic polymers have been utilized as cardiac tissue scaffolds, each presenting a different set of advantages and disadvantages to consider. Natural polymers tend to offer superior cell adhesions sites, immune response, and degradation rate at the cost of mechanical strength, while synthetic polymers tend to offer superior strength and are more easily tuned to desired physical characteristics at the cost of cell-scaffold interactions and immune response [1]. Copolymers of two or more natural and/or synthetic polymers have also been widely evaluated as a means of balancing the weaknesses of one polymer with the strengths of another [14].
Finally, a broad array of polymer processing techniques conducive to the generation of scaffolds for engineered tissue has allowed for the creation of innumerable novel scaffold designs. Films, meshes, powders, microbeads, and hydrogels generated and manipulated via micro- and nano-patterning, sintering, electrospinning, knitting, 3D printing, lyophilization, chemical and UV cross-linking, porogen leaching, and phase separation have provided the field of tissue engineering with an enormous variety of scaffold options, and all of these techniques have been applied to the generation tissue scaffolds for heart regeneration [2].
The goal of this review is to provide an overview of the physiological, functional parameters of the heart that define the requirements for a cardiac tissue scaffold, and to discuss the strengths and weaknesses of cardiac scaffold approaches that have been attempted to date, with a particular focus on those that have been tested in vivo on the heart. The foundation of a scaffold for any tissue in the body should lie within the physiological properties of the native tissue itself, and through this method we aim to explicitly define the challenges intrinsic to all cardiac tissue scaffolds and assess the field and its trajectory in light of the ultimate goal to develop novel clinical therapeutics.
2. Cardiac physiology
In serving as the primary pump for the circulatory system, the human heart has an enormous work output, transporting 200 to 1800 liters of blood through the body every hour of every day [15]. These levels of function and efficiency are achieved by a complex hierarchical relationship that spans from molecular level calcium dynamics associated with sarcomere force generation within cardiomyocytes to macroscopic tissue geometry, stiffness and contractility. In designing a scaffold for cardiac repair applications, it is critical to understand this framework so that scaffold characteristics can be appropriately interpreted and tuned for all levels of functional performance and host interaction.
2.1. Electrical properties
A critical quality of functional heart tissue is the transmission of electrical signals essential for synchronized cardiomyocyte contraction and heart function. In the native heart, electrical signals are initiated by the pacemaker cells in the sinoatrial node and propagate through the atria to the atrioventricular node, which passes the impulse through the rapidly conducting Purkinje fibers in the ventricular septum to the apex of the heart and into the ventricular cardiomyocytes. Electrical conduction directly from the atria to the ventricles is inhibited by the heart’s fibrous skeleton, which provides both electrical insulation and mechanical structure to ensure coordinated ventricular depolarization from apex to base of the heart. It is the controlled, anisotropic propagation of electrical signals throughout the heart that is ultimately responsible for the synchronized, controlled contractions associated with healthy heart functionality. Disturbance of these excitation patterns puts patients at risk for life-threatening arrhythmias, and for this reason, the electrical conductive properties of engineered cardiac constructs are now considered a critical functional characteristic [16].
Not only is it important that constructs intended to restore cardiac function do not interfere with the electrical conduction of the heart, but cells that respond to and pass electrical signals, such as cardiomyocytes, may benefit from growth on a material electrically similar to the native cardiac environment. Electrically conductive carbon and gold nanowires have been embedded in natural hydrogels to form scaffold materials with biomimetic electrical conduction properties, resulting in superior cardiomyocyte contraction and myoblast differentiation [4, 5]. However, if cardiomyocyte density is high enough to form an electrical syncytium, inclusion of conductive materials in the scaffold may be unnecessary.
2.2. Static mechanical properties
The primary component of the heart by weight and volume is the myocardium, the muscle tissue that is characterized by the function of cardiomyocytes. It is the periodic stiffening and contraction of the myocardium that is responsible for the primary function of the heart: pumping blood throughout the circulatory system. Diseased myocardium that exhibits decreased contractility has been clinically associated with decreased heart performance in the form of reduced ejection fraction, defined clinically as systolic dysfunction. Conversely, diseased myocardium that exhibits increased stiffness has been associated with decreased performance in the form of reduced chamber filling during diastole [17, 18]. Both reduced contractility and increased myocardial stiffness are associated with the local ischemia, injury, and remodeling that occurs in the heart after a myocardial infarction, which is a well-recognized target of tissue engineering therapies due to its prevalence.
For these functional reasons, the stiffness of a scaffold material or cellular construct for cardiac tissue repair is paramount to its performance in vivo. Scaffolds or constructs with stiffness characteristics that exceed those of surrounding native tissue may impede function by increasing stiffness in the treated region. As a result, considerations should be made in the design of any cardiac scaffold to match the stiffness of the surrounding healthy host tissue. In scarred regions, such as those present at sites of myocardial infarction, the increased stiffness of the native tissue may allow for implantation of stiffer materials for repair and regeneration without further decreasing the already impaired function. However, if healthy, optimal heart function is a goal, the material must interact with the host tissue and degrade such that the final tissue and construct complex has stiffness properties similar to those of healthy tissue. In constructs intended for implantation in the plane of native tissue, such as for repair of congenital heart defects, the stiffness properties of the scaffold, as well as any native tissue that grows to replace it, should closely mimic those of the surrounding tissue to achieve optimal function.
In addition to influencing gross mechanical characteristics, scaffold stiffness properties have also been shown to affect the phenotype of attached cells in cellular construct formats [19]. Similar to chemical, adhesion, and cell–cell interaction stimuli, material-related mechanical stimuli, such as stiffness, can influence cell behavior [20, 21]. In the case of stem cells, the effect of substrate stiffness on cell behavior is so significant that it can direct differentiation [22]. In isolated native cardiomyocytes, stiffness has been shown to affect functional characteristics of the cells, including contractility and electrical signaling [23]. Mechanical testing on myocardial tissue has found that the stiffness of the left ventricle ranges from 10–20 kPa during diastole and 200–500 kPa during systole [6]. Additionally, stiffness in the range of native cardiac tissue (10–20 kPa) tends to elicit cardiomyocyte characteristics most similar to native cardiomyocytes [24]. For these reasons, a scaffold material for cardiac tissue engineering should ideally mimic the stiffness characteristics of native tissue throughout its in vivo presence.
In optimizing scaffold stiffness properties the distinction between compressive and tensile stiffness should be considered, as evidence suggests that compressive and tensile moduli can significantly differ in soft tissues [25]. The isovolumetric contraction of cardiomyocytes seeded on a cardiac scaffold likely produces both compressive and tensile forces on the matrix as cardiomyocytes longitudinally shorten and radially expand. To achieve emulation of the properties of native cardiac tissue, engineered scaffolds and constructs should optimally feature mechanical properties similar to those of native tissue for stresses in all directions. Further research in this area may yield materials with properties that are more truly representative of cardiac tissue, and thus produce constructs with contractility and elasticity more similar to native tissue.
Another mechanical characteristic closely related to stiffness in myocardial function is elasticity. Unique from the elastic modulus of the material (i.e. stiffness), elastic deformation (the ability of the tissue to return to its original form after deformation) is a critical characteristic of the myocardium. The myocardium is highly elastic, and elastic relaxation during diastole is essential for proper heart function. In native myocardium, large elastin bundles present in the extracellular matrix lend properties to the tissue necessary for elastic recoil, a necessary feature for support during ventricular filling [11].
A final static mechanical characteristic of cardiac tissue that should be considered is tensile strength (ultimate tensile load). In a healthy heart, the myocardium experiences a wide range of pressures during normal function. Pressure in the left ventricle can range from 3 to 140 mmHg in each beat of a healthy adult heart, while pressures in the pericardium (the sac surrounding the heart within the thoracic cavity) range from −5 to 20 mmHg [26]. A well designed cardiac scaffold should take into account the expected pressures in the surrounding environment, and a scaffold implanted on the surface of or within the cavity of the heart should be able to maintain integrity in the presence environmental pressures.
2.3. Dynamic mechanical properties
Beyond static properties of cardiac tissue mechanics are dynamic properties such as anisotropy, contractility, and dimensional changes that occur with periodic myocardial contraction. Together, these properties have a great influence on cardiac efficiency, and the impairment of any one can have a deleterious effect on heart function.
The myocardium is composed of highly aligned fibers, which lend anisotropic mechanical properties to the tissue. Unlike skeletal muscle, in which muscle fibers are uniformly aligned along the length of the tissue, cardiac muscle features complex patterns of cells that converge and diverge along stress lines through the thickness of the ventricular wall and around the various regions of the heart [27]. This organization allows the heart to contract the chamber lumens and propel blood through the circulatory system with exceptional efficiency. As cardiomyocytes contract, they shorten in the direction of their alignment and expand radially outward, resulting in their host tissue following a similar paradigm. Poorly aligned cardiomyocytes may expand in the primary direction of their host tissue’s contraction, reducing contractile force and efficiency. Diffusion-tensor magnetic resonance imaging (DT-MRI) has been used to demonstrate this relationship between fiber alignment and heart function before and after myocardial infarction [28].
Recently, researchers have attempted to produce tissues with cardiomyocyte alignment similar to that found in native myocardium with some success [29– 31]. While these approaches show promise for the future, they currently produce thin monolayer tissues or larger tissue bundles separated by fenestrations as a result of the method used to induce alignment. While it is extremely challenging to control cell alignment in truly 3D culture environments, technologies such as patterned scaffolds and microparticles, embedded fiber materials, and electrically conductive scaffolds may provide viable solutions in the future.
Finally, healthy, native myocardium contracts in response to the degree it has been stretched through the Frank–Starling-mechanism, which is modulated on a beat-to-beat basis and allows the chambers of the heart to maintain relatively constant end systolic volumes. Without this characteristic, the heart may suffer decreased efficiency, decreased ejection fractions, and dilatation. An optimal cardiac construct should exert force based on the degree of stretch in a similar manner as native tissue to prevent these potential comorbidities.
3. Scaffold-host interactions
3.1. Biocompatibility
The first characteristic that should be considered in the design of a tissue scaffold for any in vivo application is that of biocompatibility, which can be generalized as the interaction between the host and the material. Biocompatibility has historically been the primary determinant of materials that are used for any medical or surgical application, as non-biocompatible materials may cause more harm than benefit through the release of toxic compounds, destructive immune responses, and functional failure in the in vivo environment [32]. An ideal scaffold material should produce either a beneficial or neutral response to contact with the host tissue or fluid substance that is present in the environment of its intended application. Blood-material interaction becomes a concern for any application in which a scaffold or construct will be exposed to blood flow, particularly for engineered vasculature, where thrombosis and embolism related to the scaffold can cause serious complications [33].
For cardiac tissue engineering applications, biocompatibility should include compatibility of the scaffold or construct with all the component tissues of the myocardium (e.g. cardiomyocytes, endothelium, fibroblasts, and perivascular cells), blood compatibility, as well as compatibility of any degradation or metabolic byproducts of the scaffold with the body as a whole. Further, the scaffold should function sufficiently at typical in vivo physiological conditions for a long enough duration to allow for repair or regeneration to the point to scaffold obsolescence.
3.2. Immune response
Associated with biocompatibility is the immune response triggered by an implanted scaffold material. It is traditionally understood that after implantation of a foreign material such as a tissue scaffold the body tends to shift towards a type 1 or type 2 T helper cell adaptive immune response, which in turn leads to macrophage infiltration [34]. On one end of the spectrum, so called ‘M1’ macrophage-mediated responses are often associated with negative healing responses, characterized by fibrosis, scar formation, and encapsulation, while the opposing ‘M2’ macrophage-mediated responses are often associated with positive healing responses characterized by tissue remodeling, angiogenesis, and restoration of functionality similar to that of native tissue. However, rather than a binary M1 or M2 macrophage response, a spectrum of possible macrophage phenotypes exist and can be activated in response to the implantation of a foreign material [35]. The population of these macrophages will ultimately lead the healing response towards regeneration or repair, though the specific mechanisms of this process are still unclear. In addition, macrophages, neutrophils, and other inflammatory cells may already be present at the site of therapeutic construct implantation for certain cardiac applications, including myocardial infarction. Implantation of a graft into a dynamic, inflamed environment populated by inflammatory cells can affect graft degradation rate and mechanical characteristics [36]. For this reason, the range of in vivo immunological environments that can be expected for a given application should be carefully considered in scaffold design.
In cardiac applications where restoration of the complex mechanical characteristics of native tissue is often necessary to restore function, eliciting an immune response that encourages tissue regeneration rather than repair is critical for success of a scaffold implant. While the specifics of the mechanisms that lead to a regenerative response are not fully understood, evidence suggests that materials that have chemical structure and degradation rates similar to those of native tissue, and that are free of pyrogens and antigens, elicit the most advantageous responses [34].
Tissue edema in response to immune activity at the site of implantation may also pose unique challenges to cardiac applications of tissue engineering techniques. Swollen cardiac tissue may result in increased stiffness, decreased flexibility and contractility, and ultimately decreased cardiac function, though observed clinical responses will depend on numerous other factors including the extent and location of the edema, as well as patient comorbidities [37]. While nearly any implanted material will generate at least a mild acute immune response, considerations must be made for any associated fluctuations in cardiac function over time as a result of this type of inflammation.
3.3. Degradation rate
In addition to influencing immune response, degradation time in vivo plays an important role in scaffold function. Ideally, a scaffold will persist long enough to allow for host infiltration and complete replacement by native extracellular matrix [38]. Implanted cells may be replaced or persist depending on the healing mechanisms of the tissue. Degradation prior to this point can cause cells within the construct to die, leading to reduced therapeutic benefit. Additionally, in applications where a scaffold or cellular construct provides mechanical support, early degradation may reduce the integrity of the host tissue or organ, potentially leading to further host injury. Scaffolds or constructs that persist for too long in the host may inhibit cellular remodeling, which can prevent integration and angiogenesis, and instead lead to encapsulation and scar formation [39]. Attempting to tune the in vivo degradation characteristics of natural materials through chemical methods (such as cross-linking) should be done with caution, as such chemically modified materials have been shown to produce undesirable host responses [12].
3.4. Fully defined conditions
A final consideration for cardiac construct design is the utilization of materials that are fully defined. Fully defined products can be described in terms of quantity and purity of all constituent materials, while products that are not fully defined contain materials, such as bovine serum, which vary in content from batch to batch. Changes in the past decade to FDA guidelines that dictate the regulation of medical devices, donated tissues, and biologic products have imposed substantial additional requirements upon tissue engineered therapies. Living tissue therapies, such as Apligraf® and Dermagraft®, which were initially approved through premarket approval (PMA) as class III medical devices in the US, would now likely be subjected to the biological product approval pathway, a regulatory pathway that generally takes 5 to 10 years before final approval (compared to less than 5 years for medical devices) and often requires more extensive testing to fully evaluate potential systemic side effects [40, 41]. Similar approval pathways exist in the EU, Japan, and many other countries [41]. The time and expense required for these regulatory processes can be mitigated if a product is fully defined. Additionally, a fully defined product will likely be subject to less variability in manufacturing, allowing for improved product yields and decreased waste. For these reasons, the regulatory and manufacturing implications of the final product should be considered at all stages of cardiac construct development. The use of biomaterials that have already received government approval may further facilitate approval of the final construct product.
4. Common cardiac scaffold materials
The enormous breadth of cardiac tissue characteristics as well as the requirements and considerations for tissue engineering scaffold materials in general has led researchers in the field of cardiac tissue engineering to explore a wide variety of options for cardiac scaffold materials. In the majority of cases, a material is chosen because it excels in one particular aspect of biomimicry, biocompatibility, or convenience. All biomaterial options have strengths and weaknesses associated with both their chemical composition as well as their structure for use in cardiac tissue constructs. In this section, the advantages and disadvantages of some of the most popular and well explored material and processing options for cardiac tissue scaffolds are reviewed. All biomaterials discussed have received US FDA approval for their use in implantable medical applications.
4.1. Natural polymers
Natural polymers include those which are produced organically, either by humans or another organism, and can be isolated for incorporation into a scaffold. Collagen, gelatin, alginate, and fibrin are some of the most commonly chosen natural polymers for cardiac scaffold applications, though other natural polymers, including silk, chitosan, and hyaluronic acid have been used as well [42–44]. Broadly, natural polymers offer the advantages of high biocompatibility, ample available cell binding cites, and biodegradability without the need for additional treatments or modifications, but carry the disadvantages of poor mechanical strength, a limited ability to tune characteristics, and rapid degradation in vivo [2]. As a group, natural polymers have seen great utility in cell-seeded construct applications throughout the body, particularly in the form of hydrogels [45].
Collagen, a fibrillar protein and the predominant structural soft-tissue extracellular matrix protein in mammals, is commercially available as a natural polymer and has been used extensively for tissue engineering applications throughout the body [46]. Type I collagen, the most prevalent type in the human body, is frequently isolated from rat tendon, bovine tendon, and bovine dermis through acid extraction, and remains soluble at low pH and low temperature [47, 48]. The type I collagen molecule is composed of a triple helical structure, which can combine hierarchically during polymerization to form superhelices, fibrils, and fibers [49, 50]. Solubilized collagen can be made into a hydrogel through neutralization at near physiological pH values (between ~6.5 and ~8.5) and temperatures (between ~4 and ~37 °C) [51, 52]. Control of the temperature and pH of collagen polymerization can produce collagen fiber networks with varying degrees of fiber thickness, with lower temperature and pH during polymerization resulting in thicker collagen fibers [51]. Thicker collagen fibers have greater tensile strength, and fiber thickness can also be used to mimic specific in vivo environments [53]. The fact that type I collagen polymerization can occur at physiological pH and temperature makes it a strong candidate for the generation of cardiac constructs with embedded cells, however the range of acceptable pH and temperature values is then constrained to the environmental stress tolerances of the embedded cells.
A limited variety of options are commonly available for tuning collagen characteristics to tissue engineering applications. Stiffness of collagen hydrogels can be controlled by adjusting the concentration of collagen, though in cell-seeded constructs, cellular activity to enzymatically remodel the matrix can have a significant effect on construct stiffness [54, 55]. Cellular activity within collagen hydrogels may also have a significant effect on degradation rate. In vivo, collagen is naturally cross-linked over time and thereby made more stable via oxidative deamination, resulting in divalent crosslinks that vary in type and prevalence with tissue age [56]. Collagen can be cross-linked in vitro through the application of UV light, chemical agents such as glutaraldehyde, or exposure to high temperatures [46, 57]. Cross-linking has been used extensively to decrease the in vivo degradation rate of collagen biomaterials, but in some cases has also been associated with increased immune response [34, 58]. Previous research has indicated that cross-linking may be necessary for collagen to mechanically support the heart during pumping, but should be limited such that it is not to the detriment of biocompatibility or composition [58–60]. It should be noted that while many conventional cross-linking techniques would be destructive to seeded cells, cross-linking methods more similar to those that occur in vivo may permit the cross-linking of cell-seeded constructs.
In addition to cross-linking methods, alternative methods of collagen stiffness modulation have recently been explored for bone tissue engineering applications, where scaffold stiffness is especially critical to therapeutic success. The application of mechanical force has been used to effect plastic compression in collagen hydrogels, which results in increased stiffness due to the expulsion of fluid resulting in increased fibrillary density [61, 62]. Incorporation of nano-sized bioactive glass in collagen hydrogels has also been shown to elicit improved mineralization behavior from seeded osteoblasts, resulting in increased construct stiffness [63]. Plastic compression and cell behavior modification techniques such as these could be translated for cardiac tissue engineering applications in the future. Collagen gel stiffness can also be modulated by the incorporation a secondary biocompatible polymer with alternative stiffness properties [64].
Gelatin, a form of denatured type I collagen in which the triple-helix structure of the molecule has been broken into single-strand molecules, has been frequently used in cardiac constructs for tissue repair as well [57]. Gelatin offers similar benefits to collagen in terms of biocompatibility and biodegradability. However, gelatin has been demonstrated to have decreased tensile strength and stiffness in comparison to collagen, likely a result of the denaturation preventing the formation of fibrils [58]. In spite of the inferior tensile mechanical properties, gelatin’s compressive mechanical properties are similar to those of collagen, resulting in a material with less mechanical anisotropy than collagen [58].
Alginate is a polysaccharide natural polymer generally isolated from brown algae and bacteria that can be polymerized with divalent cations, often calcium via CaCl2, under physiological temperature and pH to produce hydrogels [65]. However, alginate hydrogels produced through this method often lack mechanical strength and stiffness without further treatment with cross-linking methods such as free radical polymerization in the form of UV treatment [65]. The degradation rate and mechanical properties of alginate hydrogels can also be modulated by adjusting the molecular weight of the constituent alginate [66]. Without modification, alginate lacks the appropriate monomers for cell adhesion. However, this provides some ability to tune cell attachment to the alginate scaffold through the incorporation of arginine-glycine-aspartate (RGD) peptides, which is one of the most common cell adhesion motifs [67].
In the category of natural polymers, defined here as materials that can be isolated and purified from an organic source, is also native organ-specific extracellular matrix (ECM) which can either be collected from a donor tissue or generated in vitro. Native ECM from most tissues is composed primarily of type I collagen, with various forms of other structural and functional proteins such as elastin, glycosaminoglycans, and laminins [39]. The ECM of the heart specifically is known to lend critical mechanical, morphological, and electrical characteristics to the tissue [11]. For these reasons, cardiac ECM has significant potential for use in tissue engineered constructs and has been explored as a cardiac scaffold in many different forms. Whole hearts have been decellularized and repopulated with neonatal cardiomyocytes to produce whole organs that respond to drugs and electrical stimulation [68]. Alternatively, decellularized heart tissue has been lyophilized, milled, and solubilized into a polymer solution containing many of the native cardiac ECM components [69, 70].
4.2. Synthetic polymers
Synthetic polymers include all scaffold materials which cannot be found in nature, but nonetheless exhibit biocompatibility. Many synthetic polymers that have been used as scaffold materials have been adopted from prior use in resorbable medical devices, such as sutures and meshes for tissue repair. Synthetic polymers offer a great advantage in that they can be highly controlled and easily tuned in terms of their critical characteristics, which includes superior mechanical properties compared to most natural polymers. However, these advantages are paralleled by the additional challenge of ensuring that, in the case of degradable synthetic polymers, all products and byproducts are safe and non-toxic for the extent of all pathways through which they pass. Non-degradable synthetic polymers must persist in the body indefinitely without negatively impacting tissue function or patient health and comfort. Finally, synthetic polymers intended for tissue engineering applications where integration into native tissue is desired should provide cell adhesions sites, which many natural polymers feature without modification. For this reason, synthetic polymers are frequently used in combination with natural polymer hydrogels. In this case, the natural polymer can serve as a cell vehicle and provide attachments sites, while the synthetic polymer can provide critical mechanical and degradation characteristics [64].
A prominent cardiac tissue engineering synthetic polymer with broad medical applications throughout the body is polyglycolic acid (PGA). PGA was first utilized in the 1970s as biodegradable suture material due to its mechanical strength, stiffness, and predictable bioabsorption properties [71]. Since that time, PGA has been used to engineer such varied tissues as liver, cartilage, bone, and intestine [72]. Conventionally, PGA is most frequently used in the form of fibrous knit, woven, and non-woven meshes produced by extruding PGA fibers, though it has also been manipulated into sponges, foams, and nanofibers for specific tissue engineering applications [2, 73]. The tensile elastic modulus of PGA fibers ranges from 6 to 7 GPa, with a tensile strength (load at break) between 60 and 99.7 MPa [74]. While these fibers can have utility in lending mechanical durability to cardiac tissue constructs, alone they offer poor cell attachment and infiltration due to their hydrophobicity and surface molecules [2, 75]. For this reason, natural hydrogels, such as collagen and alginate, are frequently used with PGA scaffolds such that the natural polymers can provide a vehicle for the cells, allowing the PGA mesh to provide mechanical qualities such as stiffness and strength [76].
Another prominent synthetic polymer is poly(lactic acid) (PLA), which includes the chiral molecule lactide and exists in two forms: L-lactide and D-lactide, allowing for several forms of PLA. Of these distinct polymers, poly-L-lactide (PLLA) is used most frequently in bioresorbable medical applications because the L-lactic acid conformation is a naturally occurring byproduct of anaerobic metabolism in humans and can be excreted as carbon dioxide and water [77, 78]. Poly-DL-lactide (PLLDA) is also used in some cases due to its increased strength. Compared to the tensile properties of PGA, PLLA is less stiff and less strong, with an elastic modulus between 2.7 and 4.14 GPa and a tensile strength between 15.5 and 150 MPa [74]. However, PLLA offers a slower degradation time in vivo due to its greater hydrophobicity compared to PGA, with a typical degradation time of 30 to 50 weeks compared to the 2 to 4 weeks of PGA [77].
PGA and PLA are frequently copolymerized to form poly(lactic-co-glycolic acid) (PLGA). PLGA can be formed with varying ratios of PLA and PGA to manipulate mechanical and degradation characteristics for a targeted application. Of these copolymers, a 50:50 (molar ratio) blend of PGA and PLA results in relatively fast biodegradability and is frequently used for drug delivery and scaffold materials, while more skewed ratios, such as 90% PGA and 10% PLA, are used for applications where mechanical strength and slow degradation are more important, such as in VICRYL® (Ethicon, Inc.) mesh and sutures [79, 80].
Finally, poly(glycerol sebacate) (PGS) is a relatively new polymer (first described by Wang et al in 2002) that has seen utility in tissue engineering applications, and most recently in cardiac applications [81–83]. In these studies, sheets and sponges formed via salt leaching of PGS are used as cell scaffolds, either without any treatment or with a gelatin coating [82, 83]. A synthetic polyester like PLA, PGA, and their copolymers, PGS was designed to feature a high degree of elasticity with a low level of cross-linking to mimic some of the mechanical and molecular characteristics of native collagen, with a particular emphasis on elasticity [81]. Composed of glycerol and sebacic acid, both of which occur within the human body, PGS can be degraded through natural metabolic processes [84]. The tensile modulus of bulk strips of PGS can range from 25 to 1200 kPa depending on processing parameters, allowing the polymer to have similar stiffness characteristics to those of native myocardium (between 10 and 500 kPa) [6, 84]. These mechanical characteristics makes PGS particularly well suited to in vivo cardiac applications intended to achieve additional therapeutic benefit through mechanical restriction [83].
5. Analysis of in vivo outcomes
5.1. Overview of progress to date
While the foundation of cardiac construct design lies in understanding the characteristics and principles that govern native cardiac tissue, the complexities of wound healing and regeneration are so great that it is difficult to comprehensively assess the functionality of a tissue engineering construct outside of an in vivo environment, even with the advanced in vitro models presently available [85]. Instead, in vivo studies of construct integration, remodeling, immune response, and functional restoration in models of cardiac disease provide superior information on the potential clinical utility of engineered cardiac constructs. It is for this reason that the focus of this discussion will be cardiac constructs that have been evaluated in vivo. By the nature of this empirical testing, in vivo evaluation of engineered cardiac tissue is an iterative process both within laboratory groups and more broadly in the field. Therefore, we review these studies in chronological order and emphasize that the successful approaches and components are carried forward and adopted by other groups. Table 1 summarizes these studies and we conclude this section with our assessment of future directions for ongoing development of engineered cardiac tissue.
Table 1.
Approaches to functional in vivo characterization of biomaterial scaffolds for cardiac repair.
| Publication | Scaffold material |
Seeded cells (Species) | Cardiac animal model (Species) |
In vivo functional assessment |
In vivo timepoints (days post-implant) |
|---|---|---|---|---|---|
| Leor et al [90] |
Lyophilized alginate hydrogel |
Fetal cardiomyocytes (Rat) |
Coronary artery occlusion (Rat) |
Echocardiography (FS, LVWT, LVID) |
65 ± 5 |
| Zimmerman et al [92] |
Rat type I collagen and Matrigel® hydrogel |
Neonatal cardiomyocytes (Rat) |
Unmodified (Rat) | Echocardiography (FAC, FS, HR, LVWT, LVID) |
14 and 28 |
| Matsub- ayashi et al [101] |
PCLA sponge | Aortic smooth muscle cells (Rat) |
LAD coronary artery occlusion (Rat) |
Echocardiography (FAC, LVA) | 7, 21, 35, and 56 |
| Chang et al [102] |
Porous acellular bovine pericardium |
None | Transmural defect in right ventricle (Rat) |
Epicardial electrogram | 28 |
| Kellar et al [103] |
VICRYL® mesh | Dermal fibroblasts (Human) |
LAD coronary artery occlusion(Mouse) |
Conductance catheter with distal electrode (CO, EF, HR, LVV, SV, SW, LVP, SW, elastance, end-diastolic pressure-volume relationship) |
14 |
| Kochupura et al [104] |
Porcine urinary bladder ECM |
None | Full thickness right ventricle free wall patch excision (Dog) |
Conductance catheter (HR, RVP) High density mapping (DS, RR, SAC) |
56 |
| Gaballa et al [105] |
Porcine type I collagen foam |
None | LV free wall injury via cryoprobe (Rat) |
Conductance catheter (AP, LVID, LVOD, LVP) |
42 |
| Zimmerman et al [94] |
Rat type I collagen and Matrigel® hydrogel |
Neonatal cardiomyocytes (Rat) |
LAD coronary artery occlusion (Rat) |
Echocardiography (FAS, FS, HR, LVV, LVWT, PV Loop Analysis) |
28 |
| Fujimoto et al [106] |
Polyester urethane urea |
None | LAD coronary artery occlusion (Rat) |
Echocardiography (FAC, LVA) | 28, 56 |
| Piao et al [107] |
Poly-glycolide- co-caprolactone (PGCL) |
Bone marrow-derived mononuclear cells (Rat) |
LAD coronary artery occlusion (Rat) |
Echocardiography (FS, LVID, LVP, LVWT ) |
28 |
| Simpson et al [108] |
Rat type I collagen hydrogel |
Bone marrow-derived mesenchymal stem cells (Human) |
LAD coronary artery occlusion (Rat) |
Echocardiography (FS, L/S, LVID, LVWT) |
2–3, 28 |
| Potapova et al [109] |
Porcine urinary bladder ECM |
Mesenchymal stem cells (Human) |
Full thickness right ventricle free wall patch excision (Dog) |
Conductance catheter (HR, RVP) High density mapping (DS, RR, SAC, SW) |
56 |
| Dvir et al [98] |
Alginate-sulfate/ alginate |
Neonatal cardiomyocytes (Rat) |
LAD coronary artery occlusion (Rat) |
Echocardiography (FAC, FS, LV-ID) |
28 |
| Chen et al [83] |
PGS | Stem cell-derived cardiomyocytes (Human) |
Unmodified (Rat) | Conductance catheter (dP/dt Max, EF, LVP) |
14 |
| Habib et al [100] |
PF | Neonatal cardiomyocytes (Rat), Stem cell-derived cardiomyocytes (Human) |
LAD coronary artery occlusion (Rat) |
Echocardiography (FS, LVID, WM) |
30 |
AP, arterial pressure; CO, cardiac output; dP/dt Max, maximum rate of increase in ventricular pressure; DS, diastolic shear; EF, ejection fraction; FAC, fractional area change; FAS, fractional area shortening; FS, fractional shortening; L/S, major axis/minor axis ratio; LAD, left anterior descending (coronary artery); LVA, left ventricle area; LVID, left ventricle inner diameter; LVOD, left ventricle outer diameter; LVP, left ventricle pressure; LVV, left ventricle volume; LVWT, left ventricle wall thickness; RR, recoil rate; RVP, right ventricle pressure; SAC, systolic area contraction; SV, stroke volume; SW, stroke work; WM, wall motion.
Cell seeded scaffold approaches to cardiac scaffold design have been evaluated in vivo since the late 1990’s. On the one hand, biomaterials research has been motivated by the desire to create grafts that could integrate and grow with a patient, while scientists focused on developing cell-based therapies have been motivated by the desire to localize and control the environment of implanted cells. Early approaches tended to use readily available biopolymers, such as gelatin, without attempts to optimize the construct or scaffold to mimic the characteristics of native tissue. Neonatal and adult rat cardiomyocytes were frequently used as the cell population because of their availability. When tested in vivo in cardiac environments, these constructs tended to rapidly degrade and failed to demonstrate any significant improvement in cardiac morphology and function [86, 87].
It is important to note that these studies did not focus on designing and building an optimal scaffold for cardiac tissue repair, but rather on exploring the feasibility of using a tissue engineered graft in a cardiac application. For this reason, the rapid deterioration and functional insignificance of the constructs is not entirely unexpected. However, these studies did succeed in demonstrating scaffolds as a delivery method for cells in the heart, and in doing so laid the ground work for future in vivo designs. Such early feasibility studies were complimented by studies that attempted to optimize cell dose and viability in vivo [88]. Zhang et al evaluated the viability of syngeneic neonatal rat cardiomyocytes injected in serum-free medium into necrotic tissue and inflammatory tissue through the first week in vivo for varying cardiomyocyte doses. Based on their results, they estimated a 90% loss of cardiomyocytes injected into the left ventricular wall after one week, with half of the loss occurring in the first 24 h, and an increased fraction of cell death with increased cell doses. Cell death was also increased when cells were injected in ischemic regions of the heart compared to vascularized regions. Heat shock of the cells was the most effective means to promote cell survival, reducing cell death by half after one day.
At the same time, alternative scaffold materials were being evaluated, including synthetic materials and natural non-mammalian materials. Ozawa et al examined two synthetic biodegradable materials, PCLA and PGA, versus natural gelatin as both acellular scaffolds and as constructs seeded with vascular smooth muscle cells (SMCs) [89]. Both types of grafts were studied in a rat myocardial defect model, and in vitro characterization of SMC growth in the biomaterials was also performed. It was found that in vivo, the acellular scaffolds were rapidly infiltrated with fibroblasts, which ultimately resulted in the formation of a scarred patch in place of the scaffold. In vivo efficacy of the seeded PGA scaffold was likely hindered by the limited ability of the SMCs to infiltrate and proliferate in the scaffold in vitro, which may have been caused in part by gross physical characteristics (such as porosity) of the material. While SMCs successfully attached to and proliferated in the gelatin scaffold, its low mechanical strength and integrity limited its utility as a scaffold material in its own right. Further, a previous study performed by the same group indicated that the gelatin scaffold may have been immunogenic [87]. Leor et al were the first group to evaluate alginate cardiac tissue patches in vivo in a study in 2000 [90]. Neonatal rat cardiomyocytes were embedded in a lyophilized alginate scaffold material composed of sodium alginate cross-linked by calcium gluconate with average pore diameters of 100 µm, prepared using a method reported by Shapiro et al [91]. This alginate scaffold platform was designed to feature a high surface to volume ratio as well as high porosity to promote cell attachment and vascularization, and more detailed physical and mechanical properties were reported by Shapiro et al for various preparation parameters. Leor et al seeded cells onto dry scaffolds (6 mm diameter × 1 mm height) at a density of 3 × 105 cells per scaffold and cultured them for four days prior to implantation. The constructs were implanted into a rat model of myocardial infarction 7 d after ligation of the coronary artery and acellular alginate scaffolds were used as controls. Echocardiography data collected 65 ± 5 d after implantation showed significantly increased left ventricular end diastolic and end systolic volumes, indicating ventricular dilation and functional deterioration, in animals treated with the acellular scaffolds, while these effects were not present in animals treated with the cell-seeded constructs. Histological assessment of specimens collected at the same time point showed surviving cardiomyocytes with myofiber development (and varying degrees of alignment), the presence of connexin-43-positive gap junctions, and penetrating vascular lumens. At this final time point, the scaffold was almost entirely absent and a collagen-rich matrix surrounded the transplanted cells, demonstrating successful use of alginate as a biodegradable matrix for cardiomyocyte transplantation and preservation of global heart function.
In a progressive study for its time, Zimmermann et al combined a natural scaffold with cardiomyocytes in a geometry that would impart stress to the cells in order to influence their alignment and contractile function. This marriage of materials and cell biology to promote favorable cell-matrix interactions and tissue function has now become a cornerstone in cardiac tissue engineering. In their initial study, Zimmermann et al created annular constructs of syngeneic neonatal rat cardiomyocytes embedded in type I collagen and Matrigel® and evaluated these ‘engineered heart tissue’ (EHT) rings when sutured around healthy rat hearts in vivo [92]. Echocardiography conducted at 7, 14, and 28 d after construct implantation showed that there was no significant change in left ventricular function associated with the construct. Importantly, after 14 d, constructs implanted in rats that did not receive immunosuppression were almost completely absorbed and sarcomeric structure was degraded, while constructs in immunosuppressed rats were intact and highly vascularized. The rapid construct degradation in the rats without immunosuppression was attributed to residual xenogeneic immunogenic factors (such as Matrigel® and media components) in the construct that elicited a strong response in the host, despite attempts to mitigate this issue by thoroughly washing the construct and using syngeneic animals. These findings emphasize the relationship between the critical characteristics of scaffold materials, in this case immune response and degradation rate. Indeed, the immunogenicity of mouse-derived Matrigel® was shown in a study by Leung et al where macrophage infiltration was high and syngeneic neonatal rat cardiomyocyte survival was low in the presence of Matrigel®, but omission of Matrigel® alleviated a large majority of the engraftment and immunogenicity problems [93].
In a follow-up study in 2006, Zimmermann and Eschenhagen used the same technology and approach to conclusively demonstrate a therapeutic benefit to implantation of EHT constructs in vivo after myocardial infarction [94]. Their multifaceted approach included addressing diffusion limitations inherent to all engineered tissues by arranging five annular constructs in a layered configuration, as well as providing strain stimuli to the constructs during culture via a custom stretching apparatus. ‘Multiloop’ constructs were then implanted onto rat hearts that were subjected to a coronary artery ligation model of myocardial infarction 14 d prior. Four weeks after implantation, echocardiography, MRI, and catheterization data all showed an improvement in left ventricular diastolic and systolic function in rats that received viable EHT constructs compared to sham controls and rats that received non-contractile grafts. Thus, deleterious remodeling and decline in heart function were prevented by cardiac construct implantation. Further, epicardial mapping showed a more uniform conduction of electrical activity across the infarct region in EHT-treated hearts, suggesting reduced susceptibility to arrhythmia. Finally, histological assessment of the implanted tissue showed thick, continuous bands of cardiac muscle that spanned the infarct had formed within the EHT construct.
The great successes of the Zimmerman studies have stimulated a new generation of questions regarding the design and application of constructs for cardiac repair and regeneration. Much work in cardiac tissue engineering during the past decade has attempted to answer these questions with more detailed exploration of the vascular and electromechanical integration of cardiac constructs and consideration of immunological reactions.
Generating highly vascularized cardiac constructs will be necessary for cell survival, particularly in thicker constructs, and where metabolic demand is high. Lesman et al approached this problem by focusing on the optimization of scaffold degradation rate and porosity, and selecting heterogeneous cell populations intended to promote vascularization. Human embryonic stem cell-derived cardiomyocytes (hESC-CMs) were seeded on a synthetic scaffold composed of PLGA (50% PLA and 50% PGA), manipulated through salt-leaching to feature pore diameters ranging from 200 to 600 µm with an overall porosity of 93% [95]. Based on prior work, the scaffold was known to have an in vivo degradation time (subcutaneously in a rat) of approximately 6 months [96]. During preparation, 4 × 105 hESC-CMs alone or a co-population of 4 × 105 hESC-CMs with 4 × 105 human umbilical vein endothelial cells (HUVECs) and 2 × 105 mouse embryonic fibroblasts (MEFs) were seeded onto each 5 × 5 × 1 mm3 scaffold in a 50/50 mixture of culture medium and Matrigel®. Co-culture cell types, ratios, and scaffold design were optimized to promote angiogenesis in a prior in vitro study [97]. Constructs were cultured for two weeks prior to transplantation to the anterior left ventricular wall of healthy rat hearts. After two weeks in vivo, fibrotic tissue between the grafts and native tissue was present in some hearts, while in others it was absent and the graft and native tissue were in close proximity. Traces of scaffold material were identified in the explanted tissue, and myocardial tissue (in varying states of organization) was present in all grafts. Finally, grafts containing the co-culture trio of hESC-CMs, HUVECs, and MEFs exhibited significantly more indications of angiogenesis in the form of increased endothelial cells, lumen area, and lumen quantity. Similarly, in ‘scaffold-free’ engineered cardiac tissue where cells alone secrete and remodel the tissue extracellular matrix, tri-culture of hESC-CMs, HUVECs, and human bone-marrow-derived mesenchymal stem cells increase vascularization versus tissues with MEFs or without vascular cells (REF both Stevens 2009 and Kreutziger 2011). While limitations exist in the design and control over tissue properties in these scaffold-free tissues, biocompatibility and a natural matrix specific to the composite cell types are two major benefits. These promising results of vascular development due to heterogeneous cell populations included in engineered cardiac tissue demand assessment in a disease model where heart function and vascular perfusion are measured.
Dvir et al approached vascularization of cardiac constructs by eliciting angiogenesis from the native host cells and vascular system using a pre-vascularization approach on the omentum of the abdomen [98]. Neonatal rat cardiomyocytes were seeded on a porous alginate scaffold with a mean pore size of 100 µm. 2.5 × 106 cells were seeded into each 5 mm diameter × 2 mm thick scaffold with growth factor-reduced Matrigel® and a mixture of prosurvival/proangiogenic factors including insulin-like growth factor-1 (IGF-1), stromal-cell derived factor 1 (SDF-1), and vascular endothelial growth factor (VEGF). Alginate-sulfate, known to promote the adhesion of heparin-binding proteins in compensation for alginate’s otherwise poor adhesion properties, was incorporated in the scaffolds to encourage controlled release of these factors [99]. After 48 h of in-vitro culture, the constructs were implanted onto the surface of the rat omentum, a highly vascularized tissue that surrounds abdominal organs, for 7 d with the intention of forming a vascular network and promoting muscle development within the cardiac construct prior to implantation on infarcted rat hearts. Histological analysis at 7 d indicated that anastomosed vessels with smooth muscle cell encoatment and distinctive cardiac muscle structures were present in the constructs only with IGF-1/SDF-1/VEGF supplementation. These pre-vascularized constructs were implanted on the epicardial surface of injured rat hearts 7 d after coronary ligation. Four weeks after implantation, fractional area change (indicative of ventricular dilation) and a decline in fractional shortening (indicative of decreased function) were minimized in animals implanted with the pre-vascularized cardiac grafts compared to those receiving sham treatment, with intermediate results for a construct cultured exclusively in vitro and an acellular construct pre-vascularized on the omentum. The original alginate scaffold appears to be absent in histological images (suggesting complete degradation) and ex vivo electrical stimulation showed enhanced electrical properties across the scar in hearts treated with pre-vascularized cardiac constructs. This recent work to develop vascular networks in engineered cardiac constructs benefited from scaffold materials that were tuned in terms of in vivo degradation rate, pore size, porosity, and growth factor binding.
Important progress has also been made in applications of synthetic materials for cardiac regeneration. Chen et al have demonstrated the in vivo feasibility of PGS as a scaffold for a hESC-CM-seeded construct to both deliver cells to the heart and provide mechanical support to the ventricular wall [83]. PGS was chosen due to its long-term biomimetic elastic properties, tunable degradation rate, and cell compatibility. Evaluation and optimization of cytocompatibility, wettability, cell attachment, cell viability, and cell delivery was completed using two forms of PGS, synthesized at either 110 °C or 120 °C, and compared to PLLDA. Uncoated PGS scaffolds seeded with hESC-CMs were implanted onto the left ventricular surface of healthy rat hearts. After two weeks, no significant difference in left ventricle ejection fraction, max pressure, or end diastolic pressure was identified, suggesting that further in vivo assessment should ensue. The rigorous consideration and analysis of scaffold biocompatibility, degradation rate, mechanical characteristics, and ability to sustain cell viability has produced a scaffold material with a much more robust set of known and controlled features than the vast majority of those that have come before it.
Beyond the development of macroscale patches fabricated in vitro, scaffolds that polymerize in situ present an exciting hybrid between injection-based cell therapy and traditional scaffold-based approaches to cardiac repair. Habib et al have developed a biodegradable, photopolymerizable cardiac scaffold adding a photoinitiator to PEGylated bovine fibrinogen (PF) [100]. Neonatal rat ventricular cardiomyocytes (NRVCMs) suspended in the PF material were injected into the infarct regions of rat hearts 7 d after coronary artery ligation. The suspension was then polymerized by the application of 365 nm,<100 mW cm −2 UV light for 1 to 3 min. After 30 d, the constructs were fully degraded and ventricular walls in rats treated with the constructs were significantly thicker than those treated with a saline control. Most impressively, physiological assessment indicated that the fractional shorting in hearts treated with the NRVCM PF combination was slightly improved over baseline, and the relative increase was significantly greater than that observed in the saline group, which experienced typical post-infarct deterioration.
5.2. Directions for future development
The in vivo assessment of cardiac tissue scaffolds has advanced our understanding of material compatibility with the cardiac environment and its physiological functions. Cardiac tissue engineering scaffolds have clearly benefited from the investment of materials science research with an increasing variety of scaffold materials having unique and tunable characteristics. Indeed, many recent studies (including those not reviewed here) aim to optimize pore size, stiffness, mechanical strength, degradation rate, and biocompatibility among others, and an awareness of the limitations of these materials has prompted innovation such as providing cell attachment sites (e.g. RGD peptides or natural polymers) and tailored signaling from the materials to the tissue (e.g. angiogenic growth factor release). Due to the complexity of these novel materials and the in vivo environment, we believe that in vivo evaluation early in the development of a material is critical for its success as a cardiac scaffold. To receive the greatest benefit from this type of testing, in vivo evaluations should be designed to replicate the physiological, immunological, and temporal conditions of the intended application as closely as possible. In vivo models that incompletely reproduce the physiology of an injured or diseased heart have limited value in evaluating therapeutic success in a clinical application.
As the field moves towards clinical applications, there is much yet to study in the design of engineered cardiac tissues. Current studies often include poorly-defined xenogeneic components (such as serum and Matrigel®), are of short duration, involve immune suppression in animals, and demonstrate low levels of functional improvement (not approaching the functional capacity of healthy hearts). Yet we are optimistic about the future of scaffold design for cardiac tissue engineering and the ability of this field to overcome the challenges that remain to make these therapies become a clinical reality.
6. Conclusion
Heart repair and regeneration through the application of tissue engineering technology presents great opportunities, as well as great challenges due to the enormous complexity of the cardiac environment and heart function. The choice of scaffold material greatly influences the in vivo function of the final cell-seeded construct through many characteristics, such as biocompatibility, immunogenicity, static and dynamic mechanical characteristics, degradation rate, available adhesion molecules, pore size, and porosity (figure 1), and therefore can direct the success or failure of the therapeutic product. In all of these characteristics, an optimal cardiac scaffold should meet the requirements of the target application in the heart and meet or exceed those characteristics necessary for functional improvement of the host heart. Additional constraints may also be imposed on constructs intended for clinical applications due to manufacture requirements related to the definition, quantification, and variability of incorporated components.
As reviewed here, the field has developed an appreciation for the many physical, mechanical, and chemical characteristics that are necessary for success of an engineered cardiac tissue, and has made great progress in addressing these requirements through the development of innovative biomaterials. While bio-compatibility and functionality continue to present the greatest challenges to cardiac scaffold development, robust in vivo study designs offer numerous metrics by which to evaluate each of these critical qualities. A wide variety of available scaffold materials and processing techniques provides the field with a broad set of tools to create and iteratively improve cardiac scaffolds that can begin to meet the many requirements imposed by the application. As these tools continue to be applied and further developed, it can be expected that new construct designs will approach the physiology of native tissue and result in more optimal restoration of cardiac function.
References
- 1.Dhandayuthapani B, Yoshida Y, Maekawa T, Kumar DS. Polymeric scaffolds in tissue engineering application: a review. Int. J. Polym. Sci. 2011;2011:e290602. [Google Scholar]
- 2.Silvestri A, Boffito M, Sartori S, Ciardelli G. Biomimetic materials and scaffolds for myocardial tissue regeneration. Macromol. Biosci. 2013;13:984–1019. doi: 10.1002/mabi.201200483. [DOI] [PubMed] [Google Scholar]
- 3.Annabi N, Tsang K, Mithieux SM, Nikkhah M, Ameri A, Khademhosseini A, Weiss AS. Highly elastic micropatterned hydrogel for engineering functional cardiac tissue. Adv. Funct. Mater. 2013;23:4950–4959. doi: 10.1002/adfm.201300570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahadian S, et al. Hybrid hydrogels containing vertically aligned carbon nanotubes with anisotropic electrical conductivity for muscle myofiber fabrication. Sci. Rep. 2014;4:4271. doi: 10.1038/srep04271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dvir T, Timko BP, Brigham MD, Naik SR, Karajanagi SS, Levy O, Jin H, Parker KK, Langer R, Kohane DS. Nanowired 3D cardiac patches. Nat. Nanotechnol. 2011;6:720–725. doi: 10.1038/nnano.2011.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Q-Z, Bismarck A, Hansen U, Junaid S, Tran MQ, Harding SE, Ali NN, Boccaccini AR. Characterisation of a soft elastomer poly(glycerol sebacate) designed to match the mechanical properties of myocardial tissue. Biomaterials. 2008;29:47–57. doi: 10.1016/j.biomaterials.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. The Global Burden of Disease: 2004 Update. Geneva: World Health Organization; 2008. [Google Scholar]
- 8.Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. Natl Vital Stat. Rep. 2013;61:1–117. [PubMed] [Google Scholar]
- 9.Go AS, et al. Heart disease and stroke statistics--2014 update: a report from the american heart association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xin M, Olson EN, Bassel-Duby R. Mending broken hearts: cardiac development as a basis for adult heart regeneration and repair. Nat. Rev. Mol. Cell Biol. 2013;14:529–541. doi: 10.1038/nrm3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parker KK, Ingber DE. Extracellular matrix, mechanotransduction and structural hierarchies in heart tissue engineering. Phil. Trans. R. Soc. Lond. B Biol. Sci. 2007;362:1267–1279. doi: 10.1098/rstb.2007.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coulombe KLK, Bajpai VK, Andreadis ST, Murry CE. Heart regeneration with engineered myocardial tissue. Annu. Rev. Biomed. Eng. 2014;16:1–28. doi: 10.1146/annurev-bioeng-071812-152344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergmann O, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bajaj P, Schweller RM, Khademhosseini A, West JL, Bashir R. 3D biofabrication strategies for tissue engineering and regenerative medicine. Annu. Rev. Biomed. Eng. 2014;16:247–276. doi: 10.1146/annurev-bioeng-071813-105155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Åstrand P-O, Cuddy TE, Saltin B, Stenberg J. Cardiac output during submaximal and maximal work. J. Appl. Physiol. 1964;19:268–274. doi: 10.1152/jappl.1964.19.2.268. [DOI] [PubMed] [Google Scholar]
- 16.Bursac N, Loo Y, Leong K, Tung L. Novel anisotropic engineered cardiac tissues: studies of electrical propagation. Biochem. Biophys. Res. Commun. 2007;361:847–853. doi: 10.1016/j.bbrc.2007.07.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stienen GJM. Pathomechanisms in heart failure: the contractile connection. J. Muscle Res. Cell Motil. 2015;36:47–60. doi: 10.1007/s10974-014-9395-8. [DOI] [PubMed] [Google Scholar]
- 18.Chatterjee K, Massie B. Systolic and diastolic heart failure: differences and similarities. J. Card. Fail. 2007;13:569–576. doi: 10.1016/j.cardfail.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Discher DE, Janmey P, Wang Y. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 20.Hersch N, Wolters B, Dreissen G, Springer R, Kirchgeβner N, Merkel R, Hoffmann B. The constant beat: cardiomyocytes adapt their forces by equal contraction upon environmental stiffening. Biol. Open. 2013;2:351–361. doi: 10.1242/bio.20133830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levental I, Georges PC, Janmey P. Soft biological materials and their impact on cell function. Soft Matter. 2006;3:299–306. doi: 10.1039/b610522j. [DOI] [PubMed] [Google Scholar]
- 22.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 23.Jacot JG, McCulloch AD, Omens JH. Substrate stiffness affects the functional maturation of neonatal rat ventricular myocytes. Biophys. J. 2008;95:3479–3487. doi: 10.1529/biophysj.107.124545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engler AJ, Carag-Krieger C, Johnson CP, Raab M, Tang H-Y, Speicher DW, Sanger JW, Sanger JM, Discher DE. Embryonic cardiomyocytes beat best on a matrix with heartlike elasticity: scar-like rigidity inhibits beating. J. Cell Sci. 2008;121:3794–3802. doi: 10.1242/jcs.029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKee CT, Last JA, Russell P, Murphy CJ. Indentation versus tensile measurements of young’s modulus for soft biological tissues. Tissue Eng. Part B Rev. 2011;17:155–164. doi: 10.1089/ten.teb.2010.0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tyberg JV, Taichman GC, Smith ER, Douglas NW, Smiseth OA, Keon WJ. The relationship between pericardial pressure and right atrial pressure: an intraoperative study. Circulation. 1986;73:428–432. doi: 10.1161/01.cir.73.3.428. [DOI] [PubMed] [Google Scholar]
- 27.Sedmera D, Pexieder T, Vuillemin M, Thompson RP, Anderson RH. Developmental patterning of the myocardium. Anat. Rec. 2000;258:319–337. doi: 10.1002/(SICI)1097-0185(20000401)258:4<319::AID-AR1>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 28.Zhang S, Crow JA, Yang X, Chen J, Borazjani A, Mullins KB, Chen W, Cooper RC, McLaughlin RM, Liao J. The correlation of 3D DT-MRI fiber disruption with structural and mechanical degeneration in porcine myocardium. Ann. Biomed. Eng. 2010;38:3084–3095. doi: 10.1007/s10439-010-0073-8. [DOI] [PubMed] [Google Scholar]
- 29.Bian W, Jackman CP, Bursac N. Controlling the structural and functional anisotropy of engineered cardiac tissues. Biofabrication. 2014;6:024109. doi: 10.1088/1758-5082/6/2/024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macadangdang J, Lee HJ, Carson D, Jiao A, Fugate J, Pabon L, Regnier M, Murry C, Kim D-H. Capillary force lithography for cardiac tissue engineering. J. Vis. Exp. JoVE. 2014;88:e50039. doi: 10.3791/50039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang P-Y, Yu J, Lin J-H, Tsai W-B. Modulation of alignment, elongation and contraction of cardiomyocytes through a combination of nanotopography and rigidity of substrates. Acta Biomater. 2011;7:3285–3293. doi: 10.1016/j.actbio.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 32.Ramakrishna S, Mayer J, Wintermantel E, Leong KW. Biomedical applications of polymer-composite materials: a review. Compos. Sci. Technol. 2001;61:1189–1224. [Google Scholar]
- 33.Thomas AC, Campbell GR, Campbell JH. Advances in vascular tissue engineering. Cardiovasc. Pathol. 2003;12:271–276. doi: 10.1016/s1054-8807(03)00086-3. [DOI] [PubMed] [Google Scholar]
- 34.Badylak SF, Gilbert TW. Immune response to biologic scaffold materials. Semin. Immunol. 2008;20:109–116. doi: 10.1016/j.smim.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 36.Freytes DO, Santambrogio L, Vunjak-Novakovic G. Optimizing dynamic interactions between a cardiac patch and inflammatory host cells. Cells Tissues Organs. 2012;195:171–182. doi: 10.1159/000331392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laine GA, Allen SJ. Left ventricular myocardial edema. Lymph flow, interstitial fibrosis, and cardiac function. Circ. Res. 1991;68:1713–1721. doi: 10.1161/01.res.68.6.1713. [DOI] [PubMed] [Google Scholar]
- 38.Theoret C. Tissue engineering in wound repair: the three ‘R’ s—repair, replace, regenerate. Vet. Surg. 2009;38:905–913. doi: 10.1111/j.1532-950X.2009.00585.x. [DOI] [PubMed] [Google Scholar]
- 39.Brown BN, Badylak SF. Extracellular matrix as an inductive scaffold for functional tissue reconstruction. Transl. Res. J. Lab. Clin. Med. 2014;163:268–285. doi: 10.1016/j.trsl.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pashuck ET, Stevens MM. Designing regenerative biomaterial therapies for the clinic. Sci. Transl. Med. 2012;4:160sr4. doi: 10.1126/scitranslmed.3002717. [DOI] [PubMed] [Google Scholar]
- 41.Yano K, Tsuyuki K, Watanabe N, Kasanuki H, Yamato M. The regulation of allogeneic human cells and tissue products as biomaterials. Biomaterials. 2013;34:3165–3173. doi: 10.1016/j.biomaterials.2013.01.048. [DOI] [PubMed] [Google Scholar]
- 42.Miyagawa S, Roth M, Saito A, Sawa Y, Kostin S. Tissue-engineered cardiac constructs for cardiac repair. Ann. Thorac. Surg. 2011;91:320–329. doi: 10.1016/j.athoracsur.2010.09.080. [DOI] [PubMed] [Google Scholar]
- 43.Pok S, Vitale F, Eichmann SL, Benavides OM, Pasquali M, Jacot JG. Biocompatible carbon nanotube-chitosan scaffold matching the electrical conductivity of the heart. ACS Nano. 2014;8:9822–9832. doi: 10.1021/nn503693h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patra C, Talukdar S, Novoyatleva T, Velagala SR, Mühlfeld C, Kundu B, Kundu SC, Engel FB. Silk protein fibroin from antheraea mylitta for cardiac tissue engineering. Biomaterials. 2012;33:2673–2680. doi: 10.1016/j.biomaterials.2011.12.036. [DOI] [PubMed] [Google Scholar]
- 45.Malafaya PB, Silva GA, Reis RL. Natural-origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv. Drug Deliv. Rev. 2007;59:207–233. doi: 10.1016/j.addr.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 46.Parenteau-Bareil R, Gauvin R, Berthod F. Collagen-based biomaterials for tissue engineering applications. Materials. 2010;3:1863–1887. [Google Scholar]
- 47.Dalgleish R. The human type I collagen mutation database. Nucleic Acids Res. 1997;25:181–187. doi: 10.1093/nar/25.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rajan N, Habermehl J, Coté M-F, Doillon CJ, Mantovani D. Preparation of ready-to-use, storable and reconstituted type I collagen from rat tail tendon for tissue engineering applications. Nat. Protocol. 2007;1:2753–2758. doi: 10.1038/nprot.2006.430. [DOI] [PubMed] [Google Scholar]
- 49.Van der Rest M, Garrone R. Collagen family of proteins. FASEB J. 1991;5:2814–2823. [PubMed] [Google Scholar]
- 50.Gautieri A, Vesentini S, Redaelli A, Buehler MJ. Hierarchical structure and nanomechanics of collagen microfibrils from the atomistic scale up. Nano Lett. 2011;11:757–766. doi: 10.1021/nl103943u. [DOI] [PubMed] [Google Scholar]
- 51.Sung KE, Su G, Pehlke C, Trier SM, Eliceiri KW, Keely PJ, Friedl A, Beebe DJ. Control of 3D collagen matrix polymerization for reproducible human mammary fibroblast cell culture in microfluidic devices. Biomaterials. 2009;30:4833–4841. doi: 10.1016/j.biomaterials.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raub CB, Suresh V, Krasieva T, Lyubovitsky J, Mih JD, Putnam AJ, Tromberg BJ, George SC. Noninvasive assessment of collagen gel microstructure and mechanics using multiphoton microscopy. Biophys. J. 2007;92:2212–2222. doi: 10.1529/biophysj.106.097998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Doillon CJ, Dunn MG, Bender E, Silver FH. Collagen fiber formation in repair tissue: development of strength and toughness. Coll. Relat. Res. 1985;5:481–492. doi: 10.1016/s0174-173x(85)80002-9. [DOI] [PubMed] [Google Scholar]
- 54.Willits RK, Skornia SL. Effect of collagen gel stiffness on neurite extension. J. Biomater. Sci. Polym. Ed. 2004;15:1521–1531. doi: 10.1163/1568562042459698. [DOI] [PubMed] [Google Scholar]
- 55.Raub C, Putnam A, Tromberg B, George S. Predicting bulk mechanical properties of cellularized collagen gels using multiphoton microscopy. Acta Biomater. 2010;6:4657–4665. doi: 10.1016/j.actbio.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Avery NC, Sims TJ, Bailey AJ. Quantitative determination of collagen cross-links. Methods Mol. Biol. 2009;522:103–121. doi: 10.1007/978-1-59745-413-1_6. [DOI] [PubMed] [Google Scholar]
- 57.Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem. Rev. 2001;101:1869–1880. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 58.Grover CN, Cameron RE, Best SM. Investigating the morphological, mechanical and degradation properties of scaffolds comprising collagen, gelatin and elastin for use in soft tissue engineering. J. Mech. Behav. Biomed. Mater. 2012;10:62–74. doi: 10.1016/j.jmbbm.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 59.Chiu L, Radisic M, Vunjak-Novakovic G. Bioactive scaffolds for engineering vascularized cardiac tissues. Macromol. Biosci. 2010;10:1286–1301. doi: 10.1002/mabi.201000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jorge-Herrero E, Fernández P, Turnay J, Olmo N, Calero P, García R, Freile I, Castillo-Olivares JL. Influence of different chemical cross-linking treatments on the properties of bovine pericardium and collagen. Biomaterials. 1999;20:539–545. doi: 10.1016/s0142-9612(98)90205-8. [DOI] [PubMed] [Google Scholar]
- 61.Serpooshan V, Julien M, Nguyen O, Wang H, Li A, Muja N, Henderson JE, Nazhat SN. Reduced hydraulic permeability of 3D collagen scaffolds attenuates gel contraction and promotes the growth and differentiation of mesenchymal stem cells. Acta Biomater. 2010;6:3978–3987. doi: 10.1016/j.actbio.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 62.Serpooshan V, Muja N, Marelli B, Nazhat SN. Fibroblast contractility and growth in plastic compressed collagen gel scaffolds with microstructures correlated with hydraulic permeability. J. Biomed. Mater. Res. A. 2011;96:609–620. doi: 10.1002/jbm.a.33008. [DOI] [PubMed] [Google Scholar]
- 63.Marelli B, Ghezzi CE, Mohn D, Stark WJ, Barralet JE, Boccaccini AR, Nazhat SN. Accelerated mineralization of dense collagen-nano bioactive glass hybrid gels increases scaffold stiffness and regulates osteoblastic function. Biomaterials. 2011;32:8915–8926. doi: 10.1016/j.biomaterials.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 64.Chen G, Sato T, Ushida T, Hirochika R, Shirasaki Y, Ochiai N, Tateishi T. The use of a novel PLGA fiber/collagen composite web as a scaffold for engineering of articular cartilage tissue with adjustable thickness. J. Biomed. Mater. Res. A. 2003;67:1170–1180. doi: 10.1002/jbm.a.10164. [DOI] [PubMed] [Google Scholar]
- 65.Sun J, Tan H. Alginate-based biomaterials for regenerative medicine applications. Materials. 2013;6:1285–1309. doi: 10.3390/ma6041285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kong HJ, Kaigler D, Kim K, Mooney DJ. Controlling rigidity and degradation of alginate hydrogels via molecular weight distribution. Biomacromolecules. 2004;5:1720–1727. doi: 10.1021/bm049879r. [DOI] [PubMed] [Google Scholar]
- 67.Shachar M, Tsur-Gang O, Dvir T, Leor J, Cohen S. The effect of immobilized RGD peptide in alginate scaffolds on cardiac tissue engineering. Acta Biomater. 2011;7:152–162. doi: 10.1016/j.actbio.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 68.Ott HC, Matthiesen TS, Goh S-K, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat. Med. 2008;14:213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 69.Duan Y, Liu Z, O’Neill J, Wan LQ, Freytes DO, Vunjak-Novakovic G. Hybrid gel composed of native heart matrix and collagen induces cardiac differentiation of human embryonic stem cells without supplemental growth factors. J. Cardiovasc. Transl. Res. 2011;4:605–615. doi: 10.1007/s12265-011-9304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Singelyn JM, et al. Catheter-deliverable hydrogel derived from decellularized ventricular extracellular matrix increases endogenous cardiomyocytes and preserves cardiac function post-myocardial infarction. J. Am. Coll. Cardiol. 2012;59:751–763. doi: 10.1016/j.jacc.2011.10.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boland ED, Wnek GE, Simpson DG, Pawlowski KJ, Bowlin GL. Tailoring tissue engineering scaffolds using electrostatic processing techniques: a study of poly(glycolic acid) electrospinning. J. Macromol. Sci. A. 2001;38:1231–1243. [Google Scholar]
- 72.Ma PX, Langer R. Degradation, structure and properties of fibrous nonwoven poly(glycolic acid) scaffolds for tissue engineering. MRS Proc. 1995:394. [Google Scholar]
- 73.Rampichová M, Koštáková E, Filová E, Prosecká E, Plencner M, Ocheretná L, Lytvynets A, Lukáš D, Amler E. Non-woven PGA/PVA fibrous mesh as an appropriate scaffold for chondrocyte proliferation. Physiol. Res. 2010;59:773–781. doi: 10.33549/physiolres.931888. [DOI] [PubMed] [Google Scholar]
- 74.Van de Velde K, Kiekens P. Biopolymers: overview of several properties and consequences on their applications. Polym. Test. 2002;21:433–442. [Google Scholar]
- 75.Beckstead BL, Pan S, Bhrany AD, Bratt-Leal AM, Ratner BD, Giachelli CM. Esophageal epithelial cell interaction with synthetic and natural scaffolds for tissue engineering. Biomaterials. 2005;26:6217–6228. doi: 10.1016/j.biomaterials.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 76.Hiraoka Y, Kimura Y, Ueda H, Tabata Y. Fabrication and biocompatibility of collagen sponge reinforced with poly(glycolic acid) fiber. Tissue Eng. 2003;9:1101–1112. doi: 10.1089/10763270360728017. [DOI] [PubMed] [Google Scholar]
- 77.Gorth D, Webster TJ. 10—Matrices for tissue engineering and regenerative medicine. In: Webster MLJ, editor. Biomaterials for Artificial Organs (Woodhead Publishing Series in Biomaterials. Sawston: Woodhead Publishing; 2011. pp. 270–286. [Google Scholar]
- 78.Singhal AR, Agrawal CM, Athanasiou KA. Salient degradation features of a 50:50 PLA/PGA scaffold for tissue engineering. Tissue Eng. 1996;2:197–207. doi: 10.1089/ten.1996.2.197. [DOI] [PubMed] [Google Scholar]
- 79.Miller RA, Brady JM, Cutright DE. Degradation rates of oral resorbable implants (polylactates and polyglycolates): rate modification with changes in PLA/PGA copolymer ratios. J. Biomed. Mater. Res. 1977;11:711–719. doi: 10.1002/jbm.820110507. [DOI] [PubMed] [Google Scholar]
- 80.Agrawal CM, Ong JL, Appleford MR, Mani G. Introduction to Biomaterials: Basic Theory with Engineering Applications. Cambridge: Cambridge University Press; 2013. [Google Scholar]
- 81.Wang Y, Ameer GA, Sheppard BJ, Langer R. A tough biodegradable elastomer. Nat. Biotechnol. 2002;20:602–606. doi: 10.1038/nbt0602-602. [DOI] [PubMed] [Google Scholar]
- 82.Gao J, Crapo PM, Wang Y. Macroporous elastomeric scaffolds with extensive micropores for soft tissue engineering. Tissue Eng. 2006;12:917–925. doi: 10.1089/ten.2006.12.917. [DOI] [PubMed] [Google Scholar]
- 83.Chen Q-Z, et al. An elastomeric patch derived from poly(glycerol sebacate) for delivery of embryonic stem cells to the heart. Biomaterials. 2010;31:3885–3893. doi: 10.1016/j.biomaterials.2010.01.108. [DOI] [PubMed] [Google Scholar]
- 84.Rai R, Tallawi M, Grigore A, Boccaccini AR. Synthesis, properties and biomedical applications of poly(glycerol sebacate) (PGS): a review. Prog. Polym. Sci. 2012;37:1051–1078. [Google Scholar]
- 85.Novakovic GV, Eschenhagen T, Mummery C. Myocardial tissue engineering: in vitro models. Cold Spring Harb. Perspect. Med. 2014;4:a014076. doi: 10.1101/cshperspect.a014076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li R-K, Jia Z-Q, Weisel RD, Mickle DAG, Choi A, Yau TM. Survival and function of bioengineered cardiac grafts. Circulation. 1999;100:II-63–II-69. doi: 10.1161/01.cir.100.suppl_2.ii-63. [DOI] [PubMed] [Google Scholar]
- 87.Sakai T, Li R-K, Weisel RD, Mickle DAG, Kim ETJ, Jia Z-Q, Yau TM. The fate of a tissue-engineered cardiac graft in the right ventricular outflow tract of the rat. J. Thorac. Cardiovasc. Surg. 2001;121:932–942. doi: 10.1067/mtc.2001.113600. [DOI] [PubMed] [Google Scholar]
- 88.Zhang M, Methot D, Poppa V, Fujio Y, Walsh K, Murry CE. Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J. Mol. Cell. Cardiol. 2001;33:907–921. doi: 10.1006/jmcc.2001.1367. [DOI] [PubMed] [Google Scholar]
- 89.Ozawa T, Mickle DAG, Weisel RD, Koyama N, Ozawa S, Li R-K. Optimal biomaterial for creation of autologous cardiac grafts. Circulation. 2002;106:I-176–I-182. [PubMed] [Google Scholar]
- 90.Leor J, Aboulafia-Etzion S, Dar A, Shapiro L, Barbash IM, Battler A, Granot Y, Cohen S. Bioengineered cardiac grafts a new approach to repair the infarcted myocardium? Circulation. 2000;102:III-56–III-61. doi: 10.1161/01.cir.102.suppl_3.iii-56. [DOI] [PubMed] [Google Scholar]
- 91.Shapiro L, Cohen S. Novel alginate sponges for cell culture and transplantation. Biomaterials. 1997;18:583–590. doi: 10.1016/s0142-9612(96)00181-0. [DOI] [PubMed] [Google Scholar]
- 92.Zimmermann W-H, Didié M, Wasmeier GH, Nixdorff U, Hess A, Melnychenko I, Boy O, Neuhuber WL, Weyand M, Eschenhagen T. Cardiac grafting of engineered heart tissue in syngenic rats. Circulation. 2002;106:I-151–I-157. [PubMed] [Google Scholar]
- 93.Leung BM, Miyagi Y, Li R-K, Sefton MV. Fate of modular cardiac tissue constructs in a syngeneic rat model. J. Tissue Eng. Regen. Med. 2013 doi: 10.1002/term.1724. at press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zimmermann W-H, et al. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat. Med. 2006;12:452–458. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]
- 95.Lesman A, Habib M, Caspi O, Gepstein A, Arbel G, Levenberg S, Gepstein L. Transplantation of a tissue-engineered human vascularized cardiac muscle. Tissue Eng. A. 2010;16:115–125. doi: 10.1089/ten.TEA.2009.0130. [DOI] [PubMed] [Google Scholar]
- 96.Holder WD, Gruber HE, Moore AL, Culberson CR, Anderson W, Burg KJL, Mooney DJ. Cellular ingrowth and thickness changes in poly-L-lactide and polyglycolide matrices implanted subcutaneously in the rat. J. Biomed. Mater. Res. 1998;41:412–421. doi: 10.1002/(sici)1097-4636(19980905)41:3<412::aid-jbm11>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 97.Caspi O, Lesman A, Basevitch Y, Gepstein A, Arbel G, Habib IHM, Gepstein L, Levenberg S. Tissue engineering of vascularized cardiac muscle from human embryonic stem cells. Circ. Res. 2007;100:263–272. doi: 10.1161/01.RES.0000257776.05673.ff. [DOI] [PubMed] [Google Scholar]
- 98.Dvir T, et al. Prevascularization of cardiac patch on the omentum improves its therapeutic outcome. Proc. Natl Acad. Sci. 2009;106:14990–14995. doi: 10.1073/pnas.0812242106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Freeman I, Kedem A, Cohen S. The effect of sulfation of alginate hydrogels on the specific binding and controlled release of heparin-binding proteins. Biomaterials. 2008;29:3260–3268. doi: 10.1016/j.biomaterials.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 100.Habib M, Shapira-Schweitzer K, Caspi O, Gepstein A, Arbel G, Aronson D, Seliktar D, Gepstein L. A combined cell therapy and in-situ tissue-engineering approach for myocardial repair. Biomaterials. 2011;32:7514–7523. doi: 10.1016/j.biomaterials.2011.06.049. [DOI] [PubMed] [Google Scholar]
- 101.Matsubayashi K, Fedak PWM, Mickle DAG, Weisel RD, Ozawa T, Li R-K. Improved left ventricular aneurysm repair with bioengineered vascular smooth muscle grafts. Circulation. 2003;108:II-219–II-225. doi: 10.1161/01.cir.0000087450.34497.9a. [DOI] [PubMed] [Google Scholar]
- 102.Chang Y, Chen S-C, Wei H-J, Wu T-J, Liang H-C, Lai P-H, Yang H-H, Sung H-W. Tissue regeneration observed in a porous acellular bovine pericardium used to repair a myocardial defect in the right ventricle of a rat model. J. Thorac. Cardiovasc. Surg. 2005;130:705–711. doi: 10.1016/j.jtcvs.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 103.Kellar RS, Shepherd BR, Larson DF, Naughton GK, Williams SK. Cardiac patch constructed from human fibroblasts attenuates reduction in cardiac function after acute infarct. Tissue Eng. 2005;11:1678–1687. doi: 10.1089/ten.2005.11.1678. [DOI] [PubMed] [Google Scholar]
- 104.Kochupura PV, Azeloglu EU, Kelly DJ, Doronin SV, Badylak SF, Krukenkamp IB, Cohen IS, Gaudette GR. Tissue-engineered myocardial patch derived from extracellular matrix provides regional mechanical function. Circulation. 2005;112:I-144–I-149. doi: 10.1161/CIRCULATIONAHA.104.524355. [DOI] [PubMed] [Google Scholar]
- 105.Gaballa MA, Sunkomat JNE, Thai H, Morkin E, Ewy G, Goldman S. Grafting an acellular 3D collagen scaffold onto a non-transmural infarcted myocardium induces neoangiogenesis and reduces cardiac remodeling. J. Heart Lung Transplant. 2006;25:946–954. doi: 10.1016/j.healun.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 106.Fujimoto KL, Tobita K, Merryman WD, Guan J, Momoi N, Stolz DB, Sacks MS, Keller BB, Wagner WR. An elastic, biodegradable cardiac patch induces contractile smooth muscle and improves cardiac remodeling and function in subacute myocardial infarction. J. Am. Coll. Cardiol. 2007;49:2292–2300. doi: 10.1016/j.jacc.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Piao H, et al. Effects of cardiac patches engineered with bone marrow-derived mononuclear cells and PGCL scaffolds in a rat myocardial infarction model. Biomaterials. 2007;28:641–649. doi: 10.1016/j.biomaterials.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 108.Simpson D, Liu H, Fan T-HM, Nerem R, Dudley SC. A tissue engineering approach to progenitor cell delivery results in significant cell engraftment and improved myocardial remodeling. Stem Cells. 2007;25:2350–2357. doi: 10.1634/stemcells.2007-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Potapova IA, et al. Enhanced recovery of mechanical function in the canine heart by seeding an extracellular matrix patch with mesenchymal stem cells committed to a cardiac lineage. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H2257–H2263. doi: 10.1152/ajpheart.00219.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]