Abstract
Introduction
Manganese (Mn) is an essential nutrient but higher exposure has been associated with poorer neurodevelopment in children.
Methods
We measured Mn levels in prenatal (Mnpre) (n=197) and postnatal (Mnpost) dentin (n=193) from children's shed teeth using laser ablation inductively coupled plasma mass spectroscopy and examined the relationship with children's scores on the Mental Development Index (MDI) and Psychomotor Development Index (PDI) on the Bayley Scales of Infant Development at 6, 12, and 24-months. We explored non-linear associations and interactions by sex, blood lead concentrations and maternal iron status during pregnancy.
Results
A two-fold increase of Mnpost levels in dentin was associated with small decreases in MDI at 6-months and 12-months of age. We also observed a non-linear relationship between Mnpost levels and PDI at 6-months. We found effect modification by sex for Mnpost levels and neurodevelopment at 6-months with stronger effects among girls for both MDI (−1.5 points; 95% Confidence Interval (CI): −2.4, −0.6) and PDI (−1.8 points; 95% CI: −3.3, −0.3). Girls whose mothers had lower hemoglobin levels experienced larger decreases in MDI and PDI associated with Mnpre levels than girls whose mothers had higher hemoglobin levels (pinteraction=0.007 and 0.09, respectively). We did not observe interactions with blood lead concentrations or any relationships with neurodevelopment at 24-months.
Conclusions
Using Mn measurements in tooth dentin, a novel biomarker that provides prenatal and early postnatal levels, we observed negative transient associations between postnatal Mn levels and early neurodevelopment with effect modification by sex and interactions with prenatal hemoglobin.
Keywords: Bayley Scales of Infant Development, manganese, neurodevelopment, pesticides
1. Introduction
Manganese (Mn) is an essential nutrient but can be neurotoxic at high levels (Menezes-Filho et al. 2009; Roels et al. 2012). Inhalation exposures bypass intestinal and hepatic Mn control processes (Teeguarden et al. 2007). Fetuses and infants might be more vulnerable to the negative effects of high Mn concentrations due to the ability of Mn to cross the placenta and differences in Mn homeostatic mechanisms in young children, who absorb and retain a larger fraction of ingested Mn than adults (Aschner and Aschner 2004; Yoon et al. 2011).
Previous studies have reported associations between Mn exposure and cognitive and behavioral problems in children (Rodriguez-Barranco et al. 2013) with adverse associations sometimes noted at both highest and lowest exposure levels. A cross-sectional study of 448 infants and toddlers observed an inverted U-shaped relationship between Mn blood concentrations at 12-months and concurrent mental development, with deficits at the lowest and highest quintiles (Claus Henn et al. 2010). In cross-sectional studies of school-aged children, higher Mn levels in hair, blood and drinking water have been associated with lower full-scale and verbal intelligence quotients (Kim et al. 2009; Riojas-Rodriguez et al. 2010; Bouchard et al. 2011; Menezes-Filho et al. 2011; Wasserman et al. 2011); lower verbal learning and memory scores (Torres-Agustin et al. 2013); and poorer motor coordination (Lucchini et al. 2012). In prospective studies, higher cord blood Mn levels have been associated with poorer neonatal behavior in a non-linear fashion (Yu et al. 2014); poorer cognition and language in 2-year-olds (Lin et al. 2013); and poorer attention, non-verbal memory, and hand skills in 3-year-olds (Takser et al. 2003). A recent study reported an inverted U-shaped association between maternal prenatal blood Mn and both mental and psychomotor development in 6-month-olds (Chung et al. 2015). Only one previous small study (n=27) measured Mn levels in enamel of deciduous teeth and reported positive correlations with behavioral disinhibition in 3-year-olds (Ericson et al. 2007).
Several studies have observed stronger adverse associations in girls than boys (Riojas-Rodriguez et al. 2010; Torres-Agustin et al. 2011; Roels et al. 2012; Menezes-Filho et al. 2014). In addition, steeper negative slopes have been reported for children with high exposure to both lead and Mn (Claus Henn et al. 2012; Lin et al. 2013; Kim et al. 2009). Iron status may also modify the relationship between Mn exposure and neurodevelopment; Mn and iron share the same absorption pathways (Park et al. 2013; Smith et al. 2013), blood Mn levels in pregnant women at delivery have been related to iron metabolizing genes (Claus Henn et al. 2011), and higher blood Mn concentrations have been reported among iron deficient infants and children (Smith et al. 2013; Kim et al. 2014), who are at risk of poorer cognitive development with iron deficiency (Chang et al. 2013; Radlowski and Johnson 2013).
Though blood is the most common matrix in which Mn exposure is quantified (Rodriguez-Barranco et al. 2013), the short half-life (<30 days) in blood means measurements reflect only recent exposures (Smith et al. 2007). Maternal Mn concentrations may not accurately reflect fetal exposure: Mn concentrations are typically two-fold higher in cord than in maternal delivery blood (Takser et al. 2004; Zota et al. 2009; Gunier et al. 2014). Another biomarker, Mn in hair, is only representative of exposure during hair growth, and measurements can be compromised by exogenous contamination (Eastman et al. 2013).
In this study, we use a novel exposure matrix, Mn levels in prenatal and postnatal dentin from children's shed teeth (Arora et al. 2012), to examine perinatal Mn in association with infant neurodevelopment in the Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS) study, a prospective birth cohort of children. Measurement of Mn in dentin offers a promising biomarker of early life exposure that provides integrated measures of exposure over the prenatal and postnatal periods of tooth development, which reflect longer term exposure than a single measurement in blood or hair, and may better reflect fetal Mn levels than Mn in maternal blood (Gunier et al. 2014). We previously reported that higher Mn levels in prenatal dentin were associated with residential proximity to use of Mn-containing fungicides and storage of farmworkers’ clothing and shoes inside the home (Gunier et al. 2013).
2. Materials and Methods
2.1 Study population
In 1999-2000, we enrolled 601 pregnant women receiving prenatal care at clinics serving the Salinas Valley. Women who were ≥ 18 years of age and < 20 weeks gestational age, qualified for California's low-income health insurance program, spoke English or Spanish, and planned to deliver at the county hospital were eligible to participate. Of 536 live born children whose mothers remained enrolled at delivery, we included those with neurodevelopmental assessments at 6, 12, and/or 24-months who provided a shed incisor at or after age 7 (n=204). We excluded 4 children with medical conditions that could affect performance (e.g. seizures, autism) and 3 twins, yielding a final sample of 197 children. Neurodevelopmental assessments included 182, 188, and 186 children at 6, 12 and 24-months, respectively. Mothers of children included in analyses were older at delivery (mean=26.7 years) than mothers of excluded children (mean=24.7 years); otherwise; the two populations were similar. Written informed consent was obtained from all women and protocols were approved by the University of California, Berkeley Institutional Review Board.
2.2. Neurodevelopmental outcomes
We used the Bayley Scales of Infant Development-Second Edition to assess children's development at 6, 12, and 24-months (Bayley 1993). The Mental Developmental Index (MDI) characterizes cognitive abilities and the Psychomotor Developmental Index (PDI) characterizes fine and gross motor coordination. Trained psychometricians who were blind to Mn exposure administered scales in children's dominant home language (Spanish or English) at the CHAMACOS research office or in a mobile testing facility. We assessed the children on average (mean ± SD) at 6.6 ± 0.9 months, 12.8 ± 1.6-months and 24.6 ± 1.0 months of age. Both MDI and PDI scores were age-standardized to a mean of 100 with a SD of 15. We excluded scores that were more than 4 SD from the mean (< 5 scores per scale per age point).
2.3. Manganese exposure measurements
Beginning at age 7, participants were asked to mail or bring in teeth as they were shed. We analyzed incisors that were free of obvious defects such as caries and extensive tooth wear. Analysis methods have been described in detail elsewhere (Arora et al. 2012). Briefly, teeth were sectioned in a vertical plane and microscopy was used to visualize the neonatal line in sectioned teeth samples. The neonatal line is formed by changes in the direction and degree of tooth mineralization occasioned by the transition from intrauterine to extra uterine life and can be used to distinguish between tooth formation during the prenatal and postnatal periods (Sabel et al. 2008). Formation of prenatal dentin of incisors begins at approximately 3 months gestation and continues until birth; postnatal dentin formation of incisors occurs from birth until approximately 2.5 months of age (Ash and Nelson 2003). We determined the concentrations and spatial distribution of Mn using laser ablation inductively coupled plasma mass spectroscopy. Because multiple measurements were taken in prenatal and postnatal dentin, we calculated the area under the curve (AUC) of Mn levels across all sampling points to estimate cumulative Mn exposure during the prenatal and postnatal periods. Tooth Mn levels were normalized to measured tooth calcium levels (55Mn:43Ca ratio) to provide a measure independent of variations in tooth mineral density. Our final Mn exposure values are 55Mn:43Ca AUC × 10,000 for points measured during the prenatal (Mnpre) and postnatal (Mnpost) periods separately. The coefficient of variation for five teeth measured on three different days ranged from 4.5% to 9.5% indicating good reproducibility of 55Mn:43Ca dentin measurements. The limit of detection (LOD) was 0.001 55Mn:43Ca. Values below the LOD (n=4 postnatal measurements) were assigned the LOD/2. In addition, there were four children missing Mnpost measurements due to tooth wear.
2.4. Maternal interviews and assessments
We interviewed mothers twice during pregnancy (mean=14 and 27 weeks gestation), at delivery, and at child ages 6, 12, and 24-months, collecting information on numerous demographic and lifestyle factors. We administered the Peabody Picture Vocabulary Test (PPVT) at the 6-month visit to assess mothers’ verbal intelligence (Dunn and Dunn 1981) and the Center for Epidemiologic Studies Depression Scale (CES-D) (Radloff 1977) at the 12-month visit to evaluate maternal depression. We completed full or abbreviated versions of the Infant-Toddler Home Observation for Measurement of the Environment (HOME) instrument (Caldwell and Bradley 1984) at 6-, 12-month, and 24-month visits to assess the quality of cognitive stimulation and emotional support provided by the child's family. We abstracted maternal pregnancy (mean = 25 weeks gestation) blood hemoglobin concentrations (g/dL) from medical records as an indicator of maternal iron status.
2.5. Other chemical measurements
We measured prenatal exposure to p, p’-dichlorodiphenyltrichloroethylene (DDT) and p, p’-dichlorodiphenyldichloroethylene (DDE) in maternal blood samples collected at the second pregnancy visit (n=104) or just prior to delivery (n=37) using gas chromatography-high resolution mass spectrometry methods described previously (Barr et al. 2003). We used lipid-adjusted log10-transformed DDT and DDE concentrations in our analyses. We measured dialkyl phosphate metabolites (DAPs) of organophosphate insecticides in maternal urine samples collected twice at the two prenatal interviews using high-resolution gas chromatography-tandem mass spectrometry with isotope dilution quantification (Olsson et al. 2003). We averaged the two prenatal DAP measurements and used log10-transformed creatinine adjusted concentrations in our analyses. We measured blood lead concentrations (μg/dL) using graphite furnace atomic absorption spectrophotometry in cord blood samples (n=139) or in maternal blood samples collected at delivery (n=15) or the second prenatal visit (n=15). We collected blood samples from children at 12- (n=158) and 24-months (n=172), which were analyzed for lead at the Monterey County Public Health Laboratory.
2.6. Data analysis
We log2-transformed Mn teeth levels to reduce heteroskedasticity and the influence of outliers; results reflect the change in Bayley scores for a doubling in Mn dentin levels. We used a paired t-test to compare Mnpre and Mnpost levels and calculated the intraclass correlation coefficient to assess the within-person variability of Mn levels over time. The MDI and PDI values were normally distributed and were modeled as continuous outcomes.
We selected model covariates a priori based on factors associated with infant neurodevelopment in previous analyses [i.e., child's exact age at assessment, sex, maternal PPVT score (continuous) and maternal education (< high school vs. ≥ high school)]. We considered the following variables as additional covariates in our models, categorized as in Table 1: maternal age at delivery, parity, years in the U.S., smoking and alcohol consumption (yes/no), Cesarean delivery (yes/no), breastfeeding duration (months), and maternal depression (≥16 on CES-D). In addition, we considered covariates collected at each visit including housing density (number of persons per room), HOME score (continuous), household poverty level (relative to federal poverty), father presence in the home (yes/no), maternal work status, psychometrician, location of assessment (field office or recreational vehicle), and season of assessment. We imputed missing values (<10% missing) at a visit point using data from the nearest available visit. We retained covariates that were associated in bivariate analyses (p < 0.2) with an outcome or exposure at any time point in the multivariate models, and used the same covariates in all models. We fit all models for Mnpost with Mnpre included as a confounder. We also used generalized estimating equations (GEE) to perform longitudinal data analyses of the relationship between Mn and Bayley scores at all 3 time points.
Table 1.
Geometric mean and standard deviation of prenatal and postnatal manganese levels (AUC 55Mn:43Ca) in dentin by demographic characteristics, CHAMACOS Study.
| Prenatal Dentin |
Postnatal Dentin |
|||||
|---|---|---|---|---|---|---|
| Characteristic | N (%) | GM (GSD) | N (%) | GM (GSD) | ||
| All participants | 197 (100.0) | 0.47 (1.5) | 193 (100.0) | 0.14 (2.8) | ||
| Mothers | ||||||
| Age (years) | ||||||
| 18 - 24 | 74 (37.6) | 0.43 (1.5) | 73 (37.8) | 0.13 (3.0) | ||
| 25 - 29 | 74 (37.6) | 0.51 (1.5) | 71 (36.8) | 0.17 (1.9) | ||
| 30 - 34 | 31 (15.7) | 0.51 (1.4) | 31 (16.1) | 0.17 (3.7) | ||
| 35 - 45 | 18 (9.1) | 0.45 (1.4) | * | 18 (9.3) | 0.09 (4.1) | * |
| Race/Ethnicity | ||||||
| Latina | 192 (97.5) | 0.48 (1.5) | 188 (97.4) | 0.14 (2.9) | ||
| Other | 5 (2.5) | 0.33 (1.5) | * | 5 (2.6) | 0.11 (1.4) | |
| Education | ||||||
| < High school | 154 (78.2) | 0.50 (1.5) | 151 (78.2) | 0.14 (2.8) | ||
| ≥ High school | 43 (21.8) | 0.39 (1.5) | ** | 42 (21.8) | 0.13 (3.0) | |
| Income (% poverty) | ||||||
| < 100 | 118 (59.9) | 0.49 (1.4) | 115 (59.6) | 0.14 (3.6) | ||
| 100 - 200 | 71 (36.0) | 0.44 (1.5) | 70 (36.3) | 0.15 (1.6) | ||
| > 200 | 8 (4.1) | 0.47 (1.3) | 8 (4.2) | 0.13 (2.5) | ||
| Country of birth | ||||||
| Mexico | 174 (88.3) | 0.49 (1.5) | 170 (88.1) | 0.15 (3.0) | ||
| Other | 23 (11.7) | 0.36 (1.4) | ** | 23 (11.9) | 0.12 (1.8) | * |
| Time in U.S. (years) | ||||||
| ≤ 5 | 42 (21.3) | 0.50 (1.4) | 42 (21.8) | 0.13 (4.4) | ||
| 6 - 10 | 54 (27.4) | 0.48 (1.7) | 52 (26.9) | 0.17 (1.9) | ||
| ≥ 11 | 101 (51.3) | 0.46 (1.4) | 99 (51.3) | 0.13 (2.7) | ||
| Parity | ||||||
| 0 | 66 (33.5) | 0.47 (1.5) | 65 (33.7) | 0.15 (2.6) | ||
| ≥ 1 | 131 (66.5) | 0.47 (1.5) | 128 (66.3) | 0.14 (2.9) | ||
| Smoking during pregnancy | ||||||
| No | 187 (94.9) | 0.49 (1.5) | 183 (94.8) | 0.14 (2.9) | ||
| Yes | 10 (5.1) | 0.32 (1.8) | * | 10 (5.2) | 0.14 (1.8) | |
| Alcohol during pregnancy | ||||||
| No | 187 (98.4) | 0.48 (1.5) | 183 (98.4) | 0.15 (2.7) | ||
| Yes | 3 (1.6) | 0.32 (2.4) | 3 (1.6) | 0.11 (1.2) | ||
| HOME score at 6-months | ||||||
| < 33 | 98 (49.8) | 0.51 (1.5) | 95 (49.2) | 0.14 (3.9) | ||
| ≥ 33 | 99 (50.2) | 0.44 (1.5) | * | 98 (50.8) | 0.15 (1.8) | |
| Maternal PPVT score | ||||||
| < 91 | 96 (48.7) | 0.48 (1.5) | 93 (48.2) | 0.13 (3.3) | ||
| ≥ 91 | 101 (51.3) | 0.47 (1.5) | 100 (51.8) | 0.15 (2.4) | ||
| Children | ||||||
| Sex | ||||||
| Boy | 84 (42.6) | 0.47 (1.5) | 81 (42.0) | 0.13 (3.1) | ||
| Girl | 113 (57.4) | 0.48 (1.5) | 112 (58.0) | 0.15 (2.6) | ||
| Birth weight (g) | ||||||
| < 2500 | 8 (4.1) | 0.41 (1.7) | 8 (4.2) | 0.15 (2.0) | ||
| 2500 - 3500 | 94 (47.7) | 0.47 (1.5) | 91 (47.2) | 0.16 (1.9) | ||
| > 3500 | 95 (48.2) | 0.49 (1.4) | 94 (48.7) | 0.12 (3.7) | ||
| Gestational duration (weeks) | ||||||
| < 37 | 17 (8.6) | 0.40 (1.8) | 17 (8.8) | 0.16 (2.1) | ||
| 37 - 42 | 180 (91.4) | 0.48 (1.5) | 176 (91.2) | 0.14 (2.9) | ||
p<0.05
p<0.01
Since altered neurodevelopment was hypothesized to occur at both high and low levels of Mn exposure following an inverted U-shaped dose response, we used generalized additive models (GAMs) with a three-degrees-of-freedom cubic spline function to evaluate the shape of the relationships and to test for non-linearity. We ran models using tertiles of Mn teeth levels for outcomes with pGAM < 0.05 for deviation from linearity using the middle tertile as the reference category. In separate sensitivity analyses, we controlled for prenatal DAPs, DDT, or DDE; excluded preterm infants (n=17); and excluded outliers identified with studentized residuals greater than three for MDI at 6-months (n=4 for Mnpre and n=3 for Mnpost), PDI at 6-months (n=2 for both Mnpre and Mnpost) and MDI at 12-months (n=1 for both Mnpre and Mnpost). To control for potential selection bias due to loss to follow-up, we ran models with weights determined as the inverse probability of inclusion in our analyses at each time point (Hogan et al. 2004). We determined probability of inclusion using multiple logistic regression models with baseline covariates as potential predictors.
We evaluated effect modification by sex using interaction terms and stratified models and interactions of blood lead concentrations and Mn (prenatal with Mnpre, child with Mnpost) using linear models stratified by blood lead concentrations (< 2 μg/dL vs. ≥ 2 μg/dL). We used these categories for blood lead concentrations because many of the blood lead measurements were performed as screening tests and concentrations were reported as < 2 μg/dL for 30% of our participants. We assessed potential interactions with prenatal iron level using models for Mnpre stratified by maternal hemoglobin levels (< 11.6 g/dL vs. ≥ 11.6 g/dL) during pregnancy with the cut-off point based on normal reference ranges (Abbassi-Ghanavati et al. 2009).
3. Results
3.1. Sociodemographic characteristics, neurodevelopment scores, and tooth Mn concentrations
Mothers in this sample were mostly Latinas (98%) born in Mexico (88%), most of whom had not completed high school (78%) and lived below the poverty level (60%) but did not smoke (95%) or drink alcohol (98%) during pregnancy (Table 1). More of the children were girls (57%) than boys. Mean (± SD) Bayley MDI scores were 96 (± 7), 101 (± 9), and 86 (± 12) at 6, 12, and 24-months, respectively. Mean (± SD) PDI scores were 96 (± 10), 106 (± 14), and 98 (± 10) at 6, 12, and 24-months (Table 2).
Table 2.
Distributions of manganese (AUC 55Mn:43Ca) in prenatal and postnatal dentin, CHAMACOS Study.
| Variable | N | Min. | p25 | p50 | p75 | Max. | Mean± SD |
|---|---|---|---|---|---|---|---|
| Prenatal Dentin | 197 | 0.07 | 0.39 | 0.51 | 0.57 | 1.34 | 0.51 ± 0.19 |
| Postnatal Dentin | 193 | <LOD | 0.11 | 0.15 | 0.22 | 2.50 | 0.20 ± 0.23 |
| MDI 6 months | 181 | 67 | 92 | 96 | 100 | 121 | 95.9 ± 7.3 |
| PDI 6 months | 182 | 64 | 90 | 97 | 104 | 120 | 96.4 ± 10.4 |
| MDI 12 months | 188 | 72 | 96 | 102 | 107 | 126 | 101.4 ± 9.1 |
| PDI 12 months | 187 | 66 | 97 | 105 | 116 | 134 | 105.8 ± 13.6 |
| MDI 24 months | 185 | 58 | 76 | 84 | 94 | 117 | 85.9 ± 11.7 |
| PDI 24 months | 186 | 72 | 92 | 100 | 106 | 125 | 98.1 ± 10.1 |
Limit of Detection (LOD) for Mn Dentin = 0.001 AUC 55Mn:43Ca.
The geometric means (geometric standard deviation) of Mn in prenatal and postnatal dentin (55Mn:43Ca AUC × 104) were 0.47 (1.5) and 0.14 (2.8), respectively (Table 1). Because there are no matrix-matched certified reference materials for dentin-Mn, we use Mn counts normalized to Ca. The geometric mean prenatal Mn level of 0.47 55Mn:43Ca AUC × 104 corresponds to approximately 0.04 μg Mn/g dentin based on calibration using dissolved dentin in solution (Arora et al. 2012) and analysis of National Institute of Standards and Technology Standard Reference Material 1486 bone meal pressed into a high-density pellet (Arora et al. 2011). In 193 children with both measurements, Mnpre levels were significantly higher than Mnpost values (p < 0.001). There was moderate within-person correlation between Mnpre and Mnpost (ICC=0.48). Both Mnpre and Mnpost were higher in children of Mexican-born women and had an inverted U-shaped relationship with maternal age at delivery (data not shown). Mnpre was also higher in children whose mothers did not complete high school or smoke during pregnancy, and in children with below-median HOME scores at 6-months (Table 1).
3.2. Relationship between Mn exposure and neurodevelopment
There were no linear or non-linear relationships between Mnpre and MDI or PDI at 6, 12, or 24-months (Table 3) and there were no interactions between sex and Mnpre for neurodevelopment at any time point. We found that a two-fold increase in Mnpost was associated with a −0.8 point decrease (95% CI: −1.4, −0.2) in MDI at 6-months and −0.9 points at 12-months of age (95% CI: −1.8, −0.1), after excluding outliers and including Mnpre in the model (Table 3). The association between Mnpost and PDI at 6-months was not significant (−0.7 points; 95% CI: −1.6, 0.1). However, there was a deviation from linearity for this relationship (pGAM<0.001). We observed an inverted U-shaped relationship with PDI at 6-months of age, with a −3.4 point decrease (95% CI: −6.8, 0.0) for children in the highest tertile compared to those in the middle tertile and a −0.8 point decrease (95% CI: −4.1, 2.6) in the lowest compared to the middle tertile (Supplemental Figure 1). There were no linear or non-linear associations between Mnpost and MDI at 24-months or PDI at 12 or 24-months.
Table 3.
Adjusteda coefficients (β) and (95% CI) in points on the MDI and PDI of the Bayley Scales of Infant Development per two-fold increase in manganese levels (AUC 55Mn:43Ca), CHAMACOS study.
| Outcome | Prenatal Dentine |
Postnatal Dentine |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| N | β | (95% CI) | p-value | N | β | (95% CI) | p-value | ||
| MDI 6-months | 177 | −1.0 | (−2.7, 0.6) | 0.21 | 176 | −0.8 | (−1.4, −0.2) | 0.01 | # |
| PDI 6-months | 180 | −0.4 | (−2.9, 2.1) | 0.75 | 177 | −0.7 | (−1.6, 0.1) | 0.10 | # |
| MDI 12-months | 187 | −1.1 | (−3.3, 1.1) | 0.32 | 185 | −0.9 | (−1.8, −0.1) | 0.03 | |
| PDI 12-months | 187 | 2.1 | (−1.3, 5.5) | 0.22 | 185 | −0.4 | (−1.7, 0.9) | 0.57 | |
| MDI 24-months | 185 | 1.1 | (−1.8, 4.0) | 0.47 | 181 | 0.6 | (−0.6, 1.7) | 0.33 | |
| PDI 24-months | 186 | 0.1 | (−2.5, 2.6) | 0.96 | 182 | −0.4 | (−1.4, 0.6) | 0.44 | |
Adjusted for child's age, sex, maternal education, maternal intelligence, psychometrician, location of assessment, household poverty and HOME score at the time of the assessment. Postnatal models also adjusted for prenatal Mn. Excluding outliers with studentized residuals > |3|.
Non-linear (pGAM<0.05).
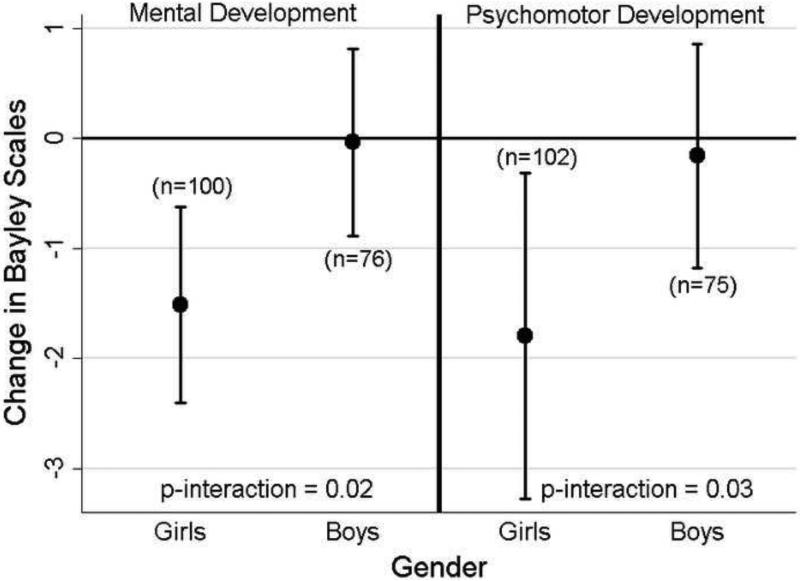
We observed a significant interaction between Mnpost and sex for both MDI (p=0.02) and PDI (p=0.03) at 6-months (Figure 1). There was a significant inverse and linear relationship of Mnpost with both MDI (−1.5 points; 95% CI: −2.4, −0.6) and PDI (−1.8 points; 95% CI: −3.3, −0.3) among girls, but no relationship among boys. We also observed a significant interaction between Mnpost and sex for MDI at 24-months (p=0.02) with better scores among boys (1.7 point increase; 95% CI: 0.2, 3.2) and no significant relationship among girls (−0.6 points; 95% CI: −2.3, 1.1).
Figure 1.
Adjusteda coefficients (β) and (95% CI) in points on the MDI and PDI of the Bayley Scales of Infant Development at 6-months per two-fold increase in postnatal manganese levels (AUC 55Mn:43Ca) stratified by sex.
a Adjusted for child's age, maternal education, maternal intelligence, psychometrician, location of assessment, household poverty, HOME score at 6-months and prenatal Mn dentin. Excluding outliers with studentized residuals > |3|.
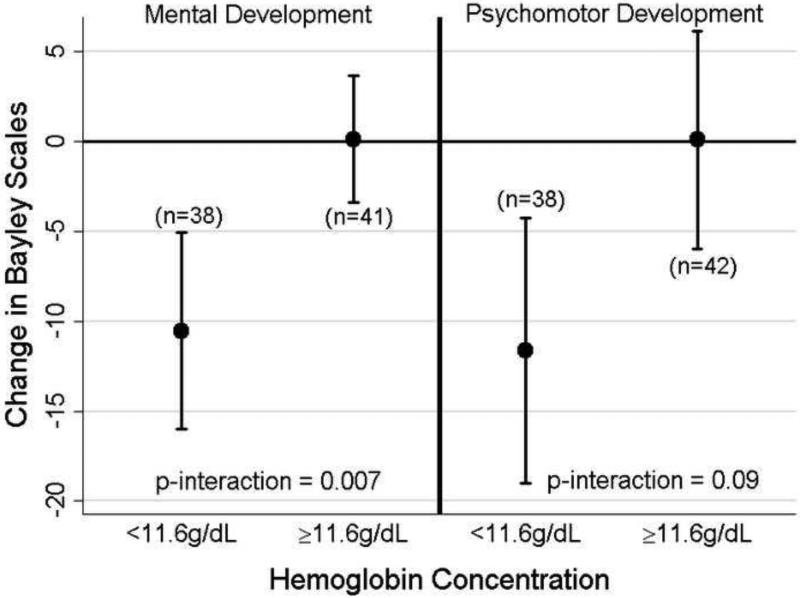
Among girls but not boys, we observed significant interactions between Mnpre levels and prenatal maternal hemoglobin levels for MDI (p=0.007) and PDI (p=0.09) at 6-months (Supplemental Table 1). Girls whose mothers had lower hemoglobin levels (< 11.6 g/dL; n=38) experienced a decrease of 10.5 points (95% CI: −16.2, −4.8) for MDI and 11.6 points (95% CI: −19.3, −3.9) for PDI per two-fold increase in Mnpre, while girls whose mothers had higher hemoglobin levels showed no association between Mnpre and either outcome (Figure 2). There were no significant interactions (p<0.1) between prenatal iron status and Mnpre at 12 and 24-months for girls or boys (Supplemental Table 1). The only direct relationship between prenatal maternal hemoglobin concentrations and neurodevelopment was among girls for MDI at 12-months with a one g/dL increase in hemoglobin associated with an increase of 3.0 points (95% CI: 0.4, 5.5) (Supplemental Table 2).
Figure 2.
Adjusteda coefficients (β) and (95% CI) in points on the MDI and PDI of the Bayley Scales of Infant Development among girls at 6-months for a two-fold increase in prenatal manganese levels (AUC 55Mn:43Ca) stratified by maternal hemoglobin concentrations during pregnancy.
a Adjusted for child's age, maternal education, maternal intelligence, psychometrician, location of assessment, household poverty, and HOME score at 6-months. Excluding outliers with studentized residuals > |3|.
Among the relatively few participants (n=28) with prenatal blood lead ≥ 2 μg/dL, there was a borderline inverse association (p=0.09) between Mnpre levels and MDI at 6-months with a decrease of −4.5 points (95% CI: −9.7, 0.7) compared to −0.9 points (95% CI: −2.8, 1.1) for those with blood lead concentrations < 2 μg/dL (Supplemental Figure 2). The interaction between Mnpre levels and prenatal blood lead was not significant for MDI at 6-months (p=0.84) and there were no associations between Mnpre or Mnpost and MDI or PDI at any other time point when stratifying by blood lead level or sex (Supplemental Table 3).
Results were similar after excluding children born preterm, when adjusting for prenatal organophosphate metabolites in urine and DDT or DDE in blood, and when weighting regression models for inverse probability of inclusion (Supplemental Tables 4 – 8). In adjusted longitudinal models using GEE, there was a significant inverse relationship between Mnpost and MDI (−1.0 points; 95% CI: −1.8, −0.1) for girls only; no other significant associations were seen for girls, boys, or all children combined (Supplemental Table 9).
4. Discussion
In this study, we observed modest but statistically significant decrements in girls’ mental and physical development during the first year of life in association with Mn exposures occurring in the first 2.5 months of life (i.e. those accumulated during the postnatal period of tooth dentin development; Mnpost). In our cohort, a doubling of Mnpost represented a shift from the 25th percentile to the 75th percentile; an increase from the 10th percentile to the 90th percentile would result in an estimated decrease of approximately three points in the MDI and PDI among girls. Associations for Mnpost levels remained significant when controlling for Mnpre suggesting that early postnatal levels were more relevant to first-year neurodevelopment in our cohort, despite higher exposure levels in the prenatal than postnatal period. The early postnatal period is a time of rapid brain development (Nowakowski and Hayes 1999), especially for the cortex and cognitive development (Meredith et al. 2012), and therefore might be a more sensitive time window for Mn exposure (Roels et al. 2012).
Previous studies have also observed stronger effects in girls than boys for associations of Mn with cognition (Riojas-Rodriguez et al. 2010; Roels et al. 2012), long-term memory (Torres-Agustin et al. 2011), and externalizing behaviors and attention problems (Menezes-Filho et al. 2014). Longer-lasting neuronal morphological changes have been observed in female than male mice that were not due to differential Mn accumulation between genders but due to differences in sensitivity to Mn exposure (Madison et al. 2011). A recent rodent study found that developmental exposure to Mn alters dopamine and corticosterone levels in the brain as a function of age, stress and sex with higher neostriatal dopamine and norepinephrine levels in Mn treated females (Vorhees et al. 2014). Possible mechanisms by which exposure to Mn and other metals could result in gender differences in neurotoxicity include differential uptake and excretion, interactions with hormones and neurotransmitters, and differential regulation of oxidative stress (Llop et al. 2013). Future epidemiological studies should conduct sex-stratified analyses when evaluating the relationship between Mn exposure and neurodevelopment.
For PDI at 6-months of age, we observed a decrease of 3.4 points in the highest compared to the middle tertile of Mnpost. As an essential nutrient, Mn associations with neurodevelopment might follow an inverted U-shaped function with detrimental associations at high and low exposure levels compared to the middle tertile, which would likely represent healthy levels of Mn. A previous study of Mn exposure and infant neurodevelopment found an inverted U-shaped association between children's blood Mn at 12-months and concurrent MDI with a decrease of approximately three points in both the lowest and highest quintiles compared with the middle three quintiles; results attenuated at 18- and 24-months (Claus Henn et al. 2010). That study observed no association with Mn blood levels at 24-months and MDI and, unlike the present study, found no relationships with PDI at either 12- or 24-months. We observed significant linear associations with Mnpost dentin levels and MDI at 6 and 12-months of age. These findings suggest associations between infant neurodevelopment and concurrent Mn exposure from birth through 12-months that are potentially non-linear, but that effects of early postnatal Mn exposure on neurodevelopment do not persist as children grow older.
We observed a substantial inverse relationship between Mnpre levels and both mental and psychomotor development at 6-months of age among girls whose mothers had lower hemoglobin concentrations (<11.6 g/dL), and therefore lower iron stores, during pregnancy. Relationships between Mn levels in teeth and neurodevelopment may be related to iron deficiency because Mn uptake increases with low iron stores (Park et al. 2013; Smith et al. 2013; Kim et al. 2014). Unlike previous studies (Claus Henn et al. 2012; Lin et al. 2013), we found no significant interaction effects between Mn and blood lead levels in our cohort. CHAMACOS children had lower mean blood lead concentrations at 12-months (1.7 μg/dL) and 24-months (1.9 μg/dL) than children from the Mexico City cohort (mean concentrations = 5.1 and 5.0 μg/dL, respectively) (Claus Henn et al. 2012) and lower median cord blood lead concentrations (0.7 μg/dL) than children in the Taiwan cohort (1.3 μg/dL) in which previous interactions were found (Lin et al. 2013).
The fact that there was no relationship between Mnpost levels and neurodevelopment at 24-months of age may be related to the development of Mn regulatory mechanisms after birth (Yoon et al. 2011), as evidenced by lower blood Mn concentrations at 24-months compared to birth (Gunier et al. 2014) and 12-months (Claus Henn et al. 2010). Our Mnpost levels only reflect exposure through the first few months of life and may not capture the relevant exposure period for neurodevelopment at 24-months of age as most previous studies have observed a relationship between neurodevelopment and concurrent Mn exposure levels. Alternatively, the fact that the relationship between Mn levels and neurodevelopment are no longer present at an older age when assessments should be more reliable suggests plasticity of function and that our findings are of less importance for long-term neurodevelopment.
The only previous study that evaluated Mn levels in children's teeth (n=27) measured Mn in tooth enamel instead of dentin and reported a correlation between prenatal tooth Mn levels and behavioral problems at 3-years of age (Ericson et al. 2007). We measured Mn in dentin because it can be linked more reliably to developmental timing of exposure since dentin, unlike enamel, is completely mineralized from earliest development (Smith 1998), and our own validation study of Mn in teeth showed associations for Mn in dentin but not enamel with Mn in cord blood and house dust (Arora et al. 2012).
One limitation of this study is that our relatively small sample size limited our ability to evaluate for interactions. Another limitation is that we used hemoglobin concentrations to assess iron deficiency because serum ferritin measurements, a better indicator of iron status (Radlowski and Johnson 2013), were not available, and children's iron status was not available to evaluate potential interactions with Mnpost exposure. A limitation of our novel use of dentin Mn levels is that it precludes direct comparison with other studies, which have assessed Mn in different media. In a subset of our participants (n=59), the median cord blood Mn concentration was similar to other studies that observed associations with neurodevelopmental outcomes in young children (Takser et al. 2003; Lin et al. 2013; Yu et al. 2014).
To our knowledge, this was the first study to evaluate the relationship between infant neurodevelopment and both prenatal and postnatal Mn levels. The main strength of this study was the use of Mn measured in dentin, which reflected accumulated exposure over a longer period of time during critical periods for neurodevelopment than would have been achieved through use of blood or hair Mn measures. A major advantage of using Mn levels measured in dentin is that prenatal and postnatal levels can be distinguished and measured retrospectively in shed teeth, allowing us to evaluate Mnpre levels as a confounder of Mnpost levels. In all models, the inverse relationship with neurodevelopment was stronger for Mnpost levels. Levels of Mnpre may also provide a more accurate measure of fetal Mn levels since Mn concentrations measured in maternal blood were not correlated with Mnpre levels (r = −0.16) and trended in the opposite direction during pregnancy (Gunier et al. 2014). Another strength is that neurodevelopment was assessed at several time points using Bayley Scales in a prospective cohort of children with extensive data on potential confounders.
5. Conclusions
We observed effect modification by sex with inverse relationships among girls between Mnpost levels and early mental and psychomotor development. We found evidence of deficits in mental and motor development at 6-months of age in association with Mnpre levels for girls whose mothers had lower prenatal hemoglobin concentrations, indicative of iron deficiency. These deficits were no longer present when the children reached 24-months, suggesting that the effects of perinatal Mn levels do not persist.
Supplementary Material
Highlights.
Manganese is an essential nutrient but neurotoxic at high exposure levels.
We measured manganese levels in prenatal and postnatal dentin of shed teeth.
We examined the relationship between manganese levels and early neurodevelopment.
We observed an inverse association between manganese and neurodevelopment in girls.
We found an interaction between prenatal manganese and hemoglobin levels in girls.
Acknowledgments
We would like to thank the CHAMACOS families, staff and community partners. This publication was made possible by research supported by grant numbers: PO1 ES009605 (NIEHS) and R82670901 and RD83451301 (USEPA).
Abbreviations
- AUC
area under the curve
- CES-D
Center for Epidemiologic Studies Depression Scale
- CHAMACOS
Center for the Health Assessment of Mothers and Children of Salinas
- CI
confidence interval
- DAP
dialkyl phosphate
- DDE
p, p’-dichlorodiphenyldichloroethylene
- DDT
p, p’-dichlorodiphenyltrichloroethylene
- GEE
generalized estimating equations
- HOME
Home Observation for Measurement of the Environment
- LOD
limit of detection
- MDI
mental development index
- Mn
manganese
- Mnpre
manganese in prenatal dentin
- Mnpost
manganese in prenatal dentin
- PDI
psychomotor development index
- PPVT
Peabody Picture Vocabulary Test
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Information on the approval by the human subjects committee: The study protocol was approved by the University of California, Berkeley Institutional Review Board.
Statement of financial interest: Dr. Asa Bradman discloses a potential competing financial interest as a member of the Science Advisory Board for The Organic Center. None of the other authors declares any actual or potential competing financial interests.
References
- Abbassi-Ghanavati M, Greer LG, Cunningham FG. Pregnancy and laboratory studies: a reference table for clinicians. Obstet Gynecol. 2009;114(6):1326–31. doi: 10.1097/AOG.0b013e3181c2bde8. [DOI] [PubMed] [Google Scholar]
- Arora M, Hare D, Austin C, Smith DR, Doble P. Spatial distribution of manganese in enamel and coronal dentine of human primary teeth. Sci Total Environ. 2011;409:1315–9. doi: 10.1016/j.scitotenv.2010.12.018. [DOI] [PubMed] [Google Scholar]
- Arora M, Bradman A, Austin C, Vedar M, Holland N, Eskenazi B, et al. Determining fetal manganese exposure from mantle dentine of deciduous teeth. Environ Sci Technol. 2012;46:5118–25. doi: 10.1021/es203569f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash M, Nelson S. Wheeler's dental anatomy, physiology, and occlusion. W.B. Saunders; Philadelphia, PA: 2003. [Google Scholar]
- Aschner JL, Aschner M. Nutritional aspects of manganese homeostasis. Molecular Aspects of Medicine. 2005;26(4–5):353–62. doi: 10.1016/j.mam.2005.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr JR, Maggio VL, Barr DB, Turner WE, Sjödin A, Sandau CD, et al. New high-resolution mass spectrometric approach for the measurement of polychlorinated biphenyls and organochlorine pesticides in human serum. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;794:137–48. doi: 10.1016/s1570-0232(03)00451-3. [DOI] [PubMed] [Google Scholar]
- Bayley M. The Bayley Scales of Infant Development. 2nd e. Psychological Corporation; San Antonio, TX: 1993. [Google Scholar]
- Bouchard MF, Sauve S, Barbeau B, Legrand M, Brodeur ME, Bouffard T, et al. Intellectual Impairment in School-Age Children Exposed to Manganese from Drinking Water. Environ Health Perspect. 2011;119:138–143. doi: 10.1289/ehp.1002321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell B, Bradley R. Home Observation for Measurement of the Environment. University of Arkansas; Little Rock, AR: 1984. [Google Scholar]
- Chang S, Zeng L, Brouwer I, Kok F, Yan H. Effect of iron deficiency anemia in pregnancy on child mental development in rural China. Pediatrics. 2013;131:e755–63. doi: 10.1542/peds.2011-3513. [DOI] [PubMed] [Google Scholar]
- Chung SE, Cheong HK, Ha EH, Kim BN, Ha M, Kim Y, et al. Maternal Blood Manganese and Early Neurodevelopment: The Mothers and Children's Environmental Health (MOCEH) Study. Environ Health Perspect. 2015 doi: 10.1289/ehp.1307865. DOI: 10.1289/ehp.1307865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus Henn B, Ettinger AS, Schwartz J, Tellez-Rojo MM, Lamadrid-Figueroa H, Hernandez-Avila M, et al. Early Postnatal Blood Manganese Levels and Children's Neurodevelopment. Epidemiol. 2010;21:433–439. doi: 10.1097/ede.0b013e3181df8e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus Henn B, Kim J, Wessling-Resnick M, Tellez-Rojo MM, Jayawardene I, Ettinger AS, et al. Associations of iron metabolism genes with blood manganese levels: a population-based study with validation data from animal models. Environ Health. 2011;10 doi: 10.1186/1476-069X-10-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus Henn B, Schnaas L, Ettinger AS, Schwartz J, Lamadrid-Figueroa H, Hernandez-Avila M, et al. Associations of early childhood manganese and lead coexposure with neurodevelopment. Environ Health Perspect. 2012;120:126–31. doi: 10.1289/ehp.1003300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn L, Dunn L. Peabody Picture Vocabulary Test, Revised. American Guidance Service; Circle Pines, MN: 1981. [Google Scholar]
- Eastman RR, Jursa TP, Benedetti C, Lucchini RG, Smith DR. Hair as a biomarker of environmental manganese exposure. Environ Sci Technol. 2013;47:1629–37. doi: 10.1021/es3035297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson JE, Crinella FM, Clarke-Stewart KA, Allhusen VD, Chan T, Robertson RT. Prenatal manganese levels linked to childhood behavioral disinhibition. Neurotoxicol Teratol. 2007;29:181–7. doi: 10.1016/j.ntt.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Marks AR, Bradman A, Fenster L, Johnson C, Barr DB, et al. In utero exposure to dichlorodiphenyltrichloroethane (DDT) and dichlorodiphenyldichloroethylene (DDE) and neurodevelopment among young Mexican American children. Pediatrics. 2006;118:233–41. doi: 10.1542/peds.2005-3117. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Marks AR, Bradman A, Harley K, Barr DB, Johnson C, Morga N, et al. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ Health Perspect. 2007;115:792–8. doi: 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunier RB, Bradman A, Jerrett M, Smith DR, Harley KG, Austin C, et al. Determinants of manganese in prenatal dentin of shed teeth from CHAMACOS children living in an agricultural community. Environ Sci Technol. 2013;47:11249–57. doi: 10.1021/es4018688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunier RB, Mora AM, Smith D, Arora M, Austin C, Eskenazi B, Bradman A. Biomarkers of manganese exposure in pregnant women and children living in an agricultural community in California. Environ Sci Technol. 2014;48(24):14695–702. doi: 10.1021/es503866a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan JW, Lancaster T. Instrumental variables and inverse probability weighting for causal inference from longitudinal observational studies. Stat Methods Med Res. 2004;13:17–48. doi: 10.1191/0962280204sm351ra. [DOI] [PubMed] [Google Scholar]
- Kim Y, Kim BN, Hong YC, Shin MS, Yoo HJ, Kim JW, et al. Co-exposure to environmental lead and manganese affects the intelligence of school-aged children. Neurotoxicology. 2009;30:564–71. doi: 10.1016/j.neuro.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Kim Y, Park S. Iron deficiency increases blood concentrations of neurotoxic metals in children. Korean J Pediatr. 2014;57(8):345–50. doi: 10.3345/kjp.2014.57.8.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llop S, Lopez-Espinosa MJ, Rebagliato M, Ballester F. Gender differences in the neurotoxicity of metals in children. Toxicology. 2013;311:3–12. doi: 10.1016/j.tox.2013.04.015. [DOI] [PubMed] [Google Scholar]
- Lin CC, Chen YC, Su FC, Lin CM, Liao HF, Hwang YH, et al. In utero exposure to environmental lead and manganese and neurodevelopment at 2 years of age. Environ Res. 2013;123:52–7. doi: 10.1016/j.envres.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Lucchini RG, Guazzetti S, Zoni S, Donna F, Peter S, Zacco A, et al. Tremor, olfactory and motor changes in Italian adolescents exposed to historical ferro-manganese emission. Neurotoxicology. 2012;33(4):687–96. doi: 10.1016/j.neuro.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison JL, Wegrzynowicz M, Aschner M, Bowman AB. Gender and manganese exposure interactions on mouse striatal neuron morphology. Neurotoxicology. 2011;32:896–906. doi: 10.1016/j.neuro.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes-Filho JA, Bouchard M, Sarcinelli Pde N, Moreira JC. Manganese exposure and the neuropsychological effect on children and adolescents: a review. Rev Panam Salud Publica. 2009;26:541–8. doi: 10.1590/s1020-49892009001200010. [DOI] [PubMed] [Google Scholar]
- Menezes-Filho JA, Novaes Cde O, Moreira JC, Sarcinelli PN, Mergler D. Elevated manganese and cognitive performance in school-aged children and their mothers. Environ Res. 2011;111:156–163. doi: 10.1016/j.envres.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes-Filho JA, de Carvalho-Vivas CF, Viana GF, Ferreira JR, Nunes LS, Mergler D, et al. Elevated manganese exposure and school-aged children's behavior: A gender-stratified analysis. Neurotoxicology. 2014;45:293–300. doi: 10.1016/j.neuro.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Meredith RM, Dawitz J, Kramvis I. Sensitive time-windows for susceptibility in neurodevelopmental disorders. Trends Neurosci. 2012;35:335–44. doi: 10.1016/j.tins.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Nowakowski RS, Hayes NL. CNS development: an overview. Dev Psychopathol. 1999;11:395–417. doi: 10.1017/s0954579499002126. [DOI] [PubMed] [Google Scholar]
- Olsson AO, Nguyen JV, Sadowski MA, Barr DB. A liquid chromatography/electrospray ionization-tandem mass spectrometry method for quantification of specific organophosphorus pesticide biomarkers in human urine. Anal Bioanal Chem. 2003;376:808–815. doi: 10.1007/s00216-003-1978-y. [DOI] [PubMed] [Google Scholar]
- Park S, Sim CS, Lee H, Kim Y. Blood manganese concentration is elevated in infants with iron deficiency. Biol Trace Elem Res. 2013;155:184–9. doi: 10.1007/s12011-013-9782-9. [DOI] [PubMed] [Google Scholar]
- Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Radlowski EC, Johnson RW. Perinatal iron deficiency and neurocognitive development. Front Hum Neurosci. 2013;7:585. doi: 10.3389/fnhum.2013.00585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riojas-Rodriguez H, Solis-Vivanco R, Schilmann A, Montes S, Rodriguez S, Rios C, et al. Intellectual Function in Mexican Children Living in a Mining Area and Environmentally Exposed to Manganese. Environ Health Perspect. 2010;118:1465–1470. doi: 10.1289/ehp.0901229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Barranco M, Lacasaña M, Aguilar-Garduño C, Alguacil J, Gil F, González-Alzaga B. Association of arsenic, cadmium and manganese exposure with neurodevelopment and behavioural disorders in children: a systematic review and meta-analysis. Sci Total Environ. 2013:454–455. 562–77. doi: 10.1016/j.scitotenv.2013.03.047. [DOI] [PubMed] [Google Scholar]
- Roels H, Bowler R, Kim Y, Claus Henn B, Mergler D, Hoet P, et al. Manganese exposure and cognitive deficits: a growing concern for manganese neurotoxicity. Neurotoxicology. 2012;33:872–80. doi: 10.1016/j.neuro.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabel N, Johansson C, Kühnisch J, Robertson A, Steiniger F, Norén JG, et al. Neonatal lines in the enamel of primary teeth-A morphological and scanning electron microscopic investigation. Arch Oral Biol. 2008;53:954–63. doi: 10.1016/j.archoralbio.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Smith C. Cellular and chemical events during enamel maturation. Crit Rev. Oral Biol. Med. 1998;9:128–161. doi: 10.1177/10454411980090020101. [DOI] [PubMed] [Google Scholar]
- Smith D, Gwiazda R, Bowler R, Roels H, Park R, Taicher C, et al. Biomarkers of Mn exposure in humans. Am J Ind Med. 2007;50:801–811. doi: 10.1002/ajim.20506. [DOI] [PubMed] [Google Scholar]
- Smith EA, Newland P, Bestwick KG, Ahmed N. Increased whole blood manganese concentrations observed in children with iron deficiency anaemia. J Trace Elem Med Biol. 2013;27:65–9. doi: 10.1016/j.jtemb.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Takser L, Mergler D, Hellier G, Sahuquillo J, Huel G. Manganese, monoamine metabolite levels at birth, and child psychomotor development. Neurotoxicology. 2003;24:667–674. doi: 10.1016/S0161-813X(03)00058-5. [DOI] [PubMed] [Google Scholar]
- Takser L, Lafond J, Bouchard M, St-Amour G, Mergler D. Manganese levels during pregnancy and at birth: relation to environmental factors and smoking in a Southwest Quebec population. Environ Res. 2004;95:119–25. doi: 10.1016/j.envres.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Torres-Agustin R, Rodriguez-Agudelo Y, Schilmann A, Solis-Vivanco R, Montes S, Riojas-Rodriguez H, et al. Effect of environmental manganese exposure on verbal learning and memory in Mexican children. Environ Res. 2013;121:39–44. doi: 10.1016/j.envres.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Teeguarden JG, Gearhart J, Clewell HJ, 3rd, Covington TR, Nong A, Andersen ME. Pharmacokinetic Modeling of Manganese. III. Physiologic Approaches Accounting for Background and Tracer Kinetics. J Toxicol Environ Health A. 2007;70:1515–26. doi: 10.1080/15287390701384635. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Graham DL, Amos-Kroohs RM, Braun AA, Grace CE, Schaefer TL, et al. Effects of developmental manganese, stress, and the combination of both on monoamines, growth, and corticosterone. Toxicol Rep. 2014;1:1046–1061. doi: 10.1016/j.toxrep.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Parvez F, Factor-Litvak P, Ahsan H, Levy D, et al. Arsenic and manganese exposure and children's intellectual function. Neurotoxicology. 2011;32:450–7. doi: 10.1016/j.neuro.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon M, Schroeter JD, Nong A, Taylor MD, Dorman DC, Andersen ME, et al. Part III. Physiologically based pharmacokinetic modeling of fetal and neonatal manganese exposure in humans: Describing manganese homeostasis during development. Toxicol. Sci. 2011;122(2):297–316. doi: 10.1093/toxsci/kfr141. [DOI] [PubMed] [Google Scholar]
- Yu XD, Zhang J, Yan CH, Shen XM. Prenatal exposure to manganese at environment relevant level and neonatal neurobehavioral development. Environ Res. 2014;133:232–8. doi: 10.1016/j.envres.2014.04.012. [DOI] [PubMed] [Google Scholar]
- Zota AR, Ettinger AS, Bouchard M, Amarasiriwardena CJ, Schwartz J, Hu H, Wright RO. Maternal blood manganese levels and infant birth weight. Epidemiol. 2009;20:367–73. doi: 10.1097/EDE.0b013e31819b93c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.