Abstract
This study aims to establish a mouse global cerebral ischemia model in which the physiological parameter measurements and neuronal injury evaluations are conducted in the same group of animals and to identify the effect of post-ischemic core temperature (CT) on the outcome of neuronal injury. Global ischemia was induced by 12-min bilateral common carotid artery occlusion followed by 7 days of reperfusion in C57BL/6 mice. Immediately after occlusion, mice were randomly assigned to be kept in environments of different temperatures [25 °C (room temperature, group 1), 33–34 °C for 2 h (group 2), and 33–34 °C for 24 h (group 3)] before being returned to their home cages. We found that in group 1, CT declined to ~32 °C after ischemia and then recovered at 24 h post-ischemia; in group 2, CT remained at the pre-ischemia level during the first 2 h, declined after the mice were returned to room temperature, and recovered at 24 h post-ischemia; and in group 3, CT remained constant at the pre-ischemia level throughout the reperfusion period. The number of surviving neurons in a sector of the hippocampal CA1 region was significantly lower in all ischemic groups than in the sham controls, but the number was significantly higher in group 1 than that in groups 2 or 3 (P < 0.05). We observed that CT declines initially but recovers spontaneously at 24 h post-ischemia. Early post-ischemic hypothermia impacts the delayed neuronal injury, suggesting that tight temperature control immediately following ischemia is important to obtain the most reproducible neuronal damage in mouse models of cerebral global ischemia.
Keywords: Hypothermia, Hippocampus, Neuroprotection, Stroke, Temperature
1. Introduction
Ischemic stroke remains the leading cause of death and disability in the United States. Investigators have explored theoretic approaches for the treatment of cerebral ischemic injury (Aliev et al., 2002), yet no neuroprotective agents have been approved for clinical treatment of this disease. Agents such as free radical scavengers, excitatory amino acid antagonists, and calcium channel blockers have shown potential as effective therapies in animal models of stroke (Jin et al., 2002; Smith, 2004; Suk et al., 2002), but these have mostly failed in clinical practice (Gladstone et al., 2002). Many factors may contribute to these failed attempts, for example, clinical trial design, windows of opportunity, and drug toxicities (Vink et al., 2001; Vink and Van Den Heuvel, 2004). However, one major reason for the inability of pharmaceutical compounds to demonstrate clinical effect is now being recognized as the poor monitoring of physiologic parameters such as blood pressure and temperature in animal models (Fisher, 1999). Precise modulation of physiological parameters, particularly those such as blood pressure, blood gases, and temperature, are critical to the outcome of ischemic neuronal injury. Clinical data indicate that high blood pressure is a risk factor and prognostic predictor of acute ischemic stroke (Bath, 2004; Castillo et al., 2004) and that abnormities of blood pressure correlate with poor outcome after ischemic injury (Bath, 2004; Castillo et al., 2004; Huang and McNamara, 2004).
Transient global cerebral ischemia usually causes selective neuronal injury in vulnerable regions of the hippocampus in patients who survive cardiac arrest, but the mechanism of such injury remains unclear (Kim et al., 1999; Sheng et al., 1999b). Recently, technologic progress in gene manipulation has provided a powerful tool in the research of global cerebral ischemia-induced brain injury that has led to an increased use of the global cerebral ischemia model in mouse (Olsson et al., 2004a; Sheng et al., 1999a). The mouse model was developed by several groups, but physiologic parameter measurements/regulation, e.g., blood pressure, cerebral blood flow (CBF), and blood gas analysis, and the neuronal injury evaluation have always been carried out in separate cohorts of animals (Olsson et al., 2003; Yonekura et al., 2004). This practice may affect outcome of neuronal injury because parameters such as blood pressure that are regulated in one animal cohort may not represent the changes of parameters in another unregulated cohort of animals. More importantly, although the effect of temperature on the outcomes of ischemic injury has been widely recognized by researchers, and the temperature of head and core is strictly controlled during the ischemic and early reperfusion periods in the transient global ischemia model (Olsson et al., 2003; Wellons et al., 2000), the effect of post-ischemic core temperature changes and their impact on neuronal injury has not been characterized in this model.
The aims of this study were (1) to establish a mouse global cerebral ischemia model in which physiologic parameters and neuronal injury are measured in the same experimental group of animals and (2) to characterize changes in post-ischemic core temperature and determine their effect on the outcome of delayed neuronal injury in a sector of the CA1 region of hippocampus in the mouse global cerebral ischemia model.
2. Materials and methods
2.1. Animals
Experiments and procedures were carried out in accordance with the National Institutes of Health guidelines for the use of experimental animals. Protocols were approved by the Institutional Animal Care and Use Committee at Johns Hopkins University. Male C57BL/6 mice (20–30 g) were purchased from Charles River (Wilmington, MA).
2.2. Transient global cerebral ischemia
Global cerebral ischemia was initiated by occluding the bilateral common carotid arteries. Briefly, mice were anesthetized with halothane (1–5%). The trachea was intubated through the intact neck with a 22-gauge intravenous catheter, which was connected to a small-animal respirator (type 845, Harvard Apparatus, Holliston, MA). The respiratory rate and stroke volume were adjusted according to body weight. The skull was exposed on each side between the ear and eye to place laser-Doppler probes. A temperature probe was inserted under the scalp to the region of parietal bone for brain temperature measurement. The right femoral artery was exposed and cannulated with a PE-10 tube for the measurements of arterial blood pressure and arterial blood gases. An anterior midline incision was made in the neck, and both common carotid arteries were exposed and encircled loosely with a 4–0 silk thread to enable later occlusion with a non-traumatic aneurysm clip. Rectal and head temperature were controlled between 36.5 and 37.5 °C with a warm water-circulating blanket and a small heating lamp and recorded continuously with a Mon-a-therm temperature monitor (Model 6510, Mallinckrodt, Hazelwood, MO). After 10 min stabilization, global ischemia was induced by occluding the bilateral common carotid arteries for 12 min with aneurysm clips; reperfusion was induced by release of the clips.
2.3. Measurement of cerebral blood flow
Cerebral blood flow (CBF) in both hemispheres was measured transcranially by laser-Doppler flowmetry via probes placed on each temple. CBF was recorded continuously during 10 min of baseline recording, 12 min of ischemia, and 15 min of reperfusion. Reduction of CBF in each hemisphere was calculated as percentage of baseline.
2.4. Temperature measurements
Mouse body core temperature was measured by temperature-sensitive transponders [IPTT-200, IPTT-300 Bio Medic Data Systems, Inc. (BMDS), Seaford, DE, USA] implanted in the abdomen 1 week prior to ischemia. Core temperature was sampled every 30 s via receivers (BMDS, DAS Model 5002). This method minimizes stress and allows temperature to be monitored in the freely moving animal. Core temperature was measured pre-ischemia, during the early period (hours) of reperfusion, and then daily until mice were sacrificed on day 7 post-ischemia. The head and rectal temperatures were monitored by placing the appropriate probes on the surface of the cranial bone under the scalp or inserted in the rectum, respectively.
2.5. Blood pressure and arterial blood gas analysis
The right femoral artery was cannulated and connected to a pressure transducer. Mean arterial blood pressure (MABP) was monitored continuously through a pressure transducer coupled to a digital acquisition system for computerized recording (Polyview, Grass Inc. Div., Astro-Med Inc., West Warwick, RI). MABP was recorded before cerebral global ischemia, during the 12-min ischemia, and for the first 15 min of the reperfusion period. Baseline MABP was modulated between 60 and 80 mmHg by slight adjustments of halothane flow. Two samples of arterial blood (0.1 mL each) were taken before baseline recording and 15 min post-global ischemia to determine arterial PaO2, PaCO2, and pH.
2.6. Histology
Neuronal injury was evaluated as previously described (Wei et al., 2008). In brief, 7 days after ischemia, animals were euthanized with sodium pentobarbital (35–50 mg/kg intraperitoneally) and transcardially perfused with 0.9% saline, followed by 4% paraformaldehyde. The brains were removed, embedded in paraffin, cut into 10-µm sections, and stained with hematoxylin and eosin. Ischemic neuronal damage in a delineated sector of CA1 region of the hippocampus was evaluated at the coronal level corresponding to −1.94 ± 0.01, −2.42 ± 0.01, and −2.90 ± 0.01 mm from the bregma (Kitagawa et al., 1998a; Olsson et al., 2003). We used the most linear section of the CA1 region, as seen under low magnification and aligned with the parallel lines of the microscope grid. The diameter (D) of the observed field (100×) was obtained by the standard grid-lined slide provided by the microscope manufacturer. Numbers of surviving neurons in the CA1 regions were counted in each hemisphere under light microscopy (100× oil lens) and expressed as the average number mm−2 (Kitagawa et al., 1998a; Olsson et al., 2003).
2.7. Experimental groups
Four cohorts were included in the study. Mice in three of the cohorts were subjected to the 12-min global cerebral ischemia procedure and then randomly assigned to three groups as follows: (1) mice were maintained at room temperature (25 °C), and the core temperature was allowed to regulate spontaneously; (2) mice were kept in a warm chamber (33–34 °C) for 2 h after the procedure and then returned to a cage at room temperature; (3) mice were kept in a warm chamber (33–34 °C) for 24 h after the procedure and then returned to a cage at room temperature. The fourth cohort was composed of sham-operated mice that were treated exactly as the mice in group 3, except that the common carotid arteries were not occluded.
2.8. Statistics
One-way ANOVA followed by a Newman–Keul’s multiple comparison test was used to compare control and treated groups. For tests involving two groups, unpaired Student’s t-test was used. Data are presented as mean ± SD; a value of P < 0.05 was considered to be statistically significant.
3. Results
3.1. Induction of global cerebral ischemia in mouse
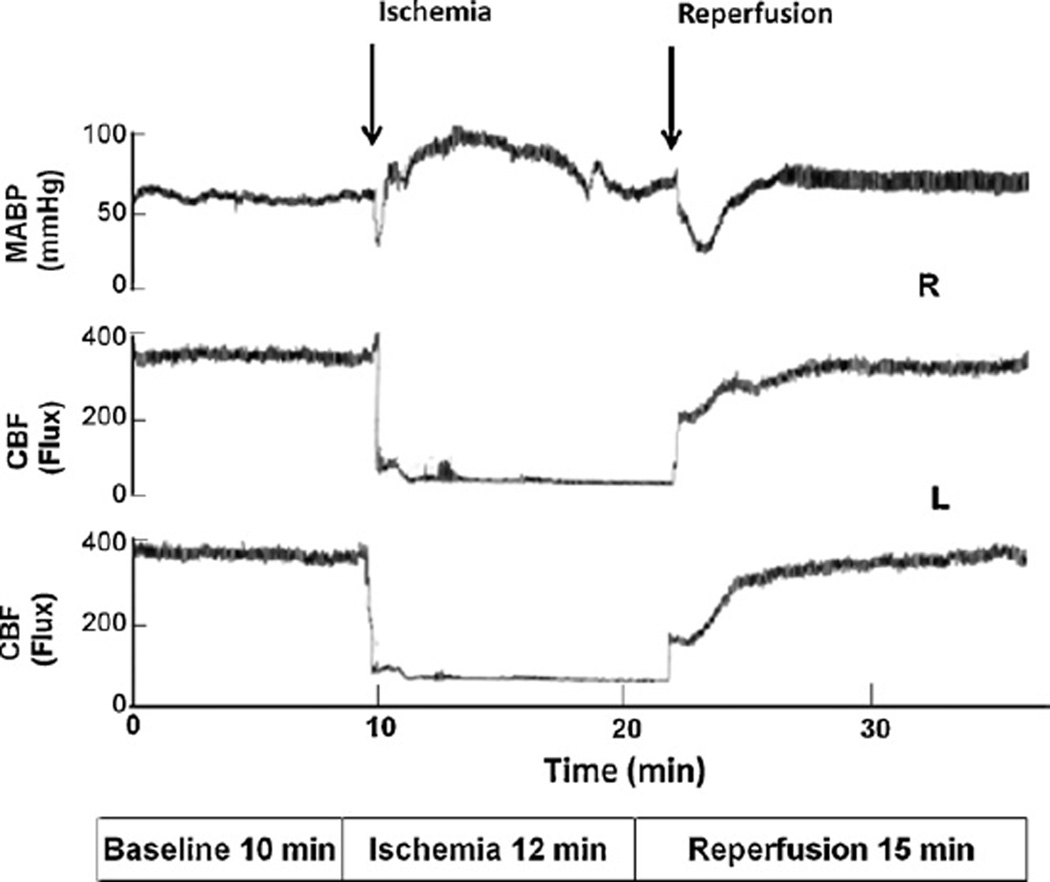
Typical changes in MABP and CBF that occurred during the course of bilateral common carotid artery occlusion and reperfusion are shown in Fig. 1. We observed that MABP increased rapidly at the initiation of occlusion and then slowly decreased but remained higher than the base-line level. At the start of ischemia, CBF decreased rapidly at first, increased slightly as a result of the increase in MABP, and then slowly decreased to a stable, but low level. Upon reperfusion, MABP decreased to below baseline initially and then slowly increased to the baseline level. The CBF rapidly increased during the early period of reperfusion and then slowly reached a level approximately equal to baseline.
Fig. 1.
Changes in mean arterial blood pressure (MABP) and relative cerebral blood flow (CBF) in each hemisphere in mice subjected to 12-min global cerebral ischemia followed by reperfusion. MABP increased and CBF decreased during bilateral common carotid artery occlusion.
3.2. Physiologic parameters
In each of the four groups of mice, the head and rectal temperature were strictly controlled between 36.5 and 37.5 °C (Fig. 2A and B) during early ischemia and the initial 15 min of reperfusion. Therefore, no significant temperature differences were observed between the groups at these time points. During the 10 min of equilibration, pre-ischemia MABP was controlled to between 60 and 80 mmHg. The baseline values of MABP were 74.6 ± 9.6, 72.9 ± 8.9, 72.1 ± 6.2, and 76.3 ± 7.7 mmHg for the room-temperature group, 2-h temperature-controlled group, 24-h temperature-controlled group, and sham-operated group, respectively. MABP increased to a peak within 3 min after occlusion reaching values of 93.5 ± 13.6, 98.7 ± 14.4, 101.7 ± 19.2, and 72.6 ± 7.7 mmHg for the four groups. Upon reperfusion, the MABP in the three ischemic groups declined to 59.9 ± 17.7, 57.1 ± 14.9, 57.7 ± 15.4 mmHg, while that of the sham group remained at 69.7 ± 7.0 mmHg.
Fig. 2.
Head and core temperature, MABP, and CBF in each hemisphere were recorded in mice subjected to 12-min global cerebral ischemia followed by reperfusion. The respective head (A) and core (B) temperatures were not different among groups during or early after ischemia. (C) The MABP was significantly higher in animals subjected to global cerebral ischemia than in sham-operated animals. (D) Upon initiation of ischemia, CBF decreased to a level significantly lower than that of sham-operated animals and then returned to near baseline after reperfusion. *P < 0.01 compared to sham-operated animals.
The CBF in both hemispheres dropped significantly after occlusion, but no significant difference in CBF reduction was observed between the left and right hemisphere in any of the three ischemic groups. CBF decreased to 11.8 ± 11.5%, 15.9 ± 22.0%, and 9.6 ± 16.7% of baseline in the room-temperature group, 2-h temperature-controlled group, and 24-h temperature-controlled group, respectively at 8–9 min post-ischemia. As expected, CBF of the sham group remained fairly constant at 100.6 ± 14.1% of baseline. CBF reduction was not significantly different among the three ischemic groups but was significantly lower in the ischemic groups than in the sham-operated group during the occlusion periods (P < 0.01). Analysis of the blood gas data is summarized in Table 1.
Table 1.
Blood gas analysis.
| Groupa | n | pH | PaCO2 | PaO2 | |||
|---|---|---|---|---|---|---|---|
| 10 min pre- ischemia |
15 min post- ischemia |
10 min pre- ischemia |
15 min post- ischemia |
10 min pre- ischemia |
15 min post- ischemia |
||
| Sham | 4 | 7.32 ± 0.02 | 7.31 ± 0.06 | 38.9 ± 3.5 | 42.2 ± 3.3 | 131.8 ± 20.0 | 123.4 ± 11.9 |
| RT | 12 | 7.32 ± 0.05 | 7.31 ± 0.03 | 40.6 ± 3.2 | 42.8 ± 3.6 | 134.1 ± 15.0 | 119.0 ± 12.4 |
| 2 h | 18 | 7.34 ± 0.04 | 7.31 ± 0.04 | 38.4 ± 2.6 | 43.2 ± 5.9 | 137.3 ± 13.7 | 134.7 ± 13.2 |
| 24 h | 11 | 7.34 ± 0.04 | 7.32 ± 0.05 | 39.1 ± 1.9 | 39.8 ± 4.9 | 141.0 ± 11.8 | 125.2 ± 18.9 |
Mice underwent bilateral cerebral artery occlusion and then were exposed directly to room temperature air (RT) or to a 33–34 °C environment for 2 or 24 h before being returned to room temperature. Sham-operated animals were exposed to the 33–34 °C environments for 24 h.
3.3. Changes in post-ischemic core temperature
The pre-ischemic core temperature ranged between 36.5 and 37.5 °C in all cohorts. At the onset of reperfusion, core temperature rapidly declined to approximately 32 °C in the animals that were kept at room temperature but slowly recovered to pre-ischemic levels by 24 h post-ischemia. This finding suggests a 24-h dysfunction of core temperature regulation in animals after global forebrain ischemia (Fig. 3A). In the second group of mice, core body temperature remained well controlled at 35.5–37.5 °C during the 2 h that they were in the temperature-controlled chamber, but it rapidly declined to approximately 32 °C once the animals were transferred to room-temperature cages. Once again, the core temperature slowly recovered to normal at about 24 h after ischemia (Fig. 3B). Core body temperature of the mice kept in the temperature-controlled chamber stayed consistently between 36.0 and 37.5 °C while they were in the chamber and remained at this level after they were transferred to room temperature until they were sacrificed (Fig. 3C).
Fig. 3.
Characterization of core body temperature in mice subjected to 12-min global cerebral ischemia and then exposed to a warm temperature-controlled chamber for different lengths of time. (A) Core temperature decreased to about 32 °C but spontaneously recovered 24 h post-ischemia in mice that were kept at room temperature after ischemia. (B) In mice exposed to a 33–34 °C chamber for 2 h, core temperature remained constant for 2 h, decreased to about 32 °C upon exposure to room-temperature air, and then spontaneously recovered 24 h post-ischemia. (C) Core temperature remained consistently at 37 °C in mice that were kept in a temperature-controlled chamber for 24 h before being returned to room temperature.
3.4. Effect of temperature regulation on the outcome of delayed neuronal injury in a sector of CA1 region
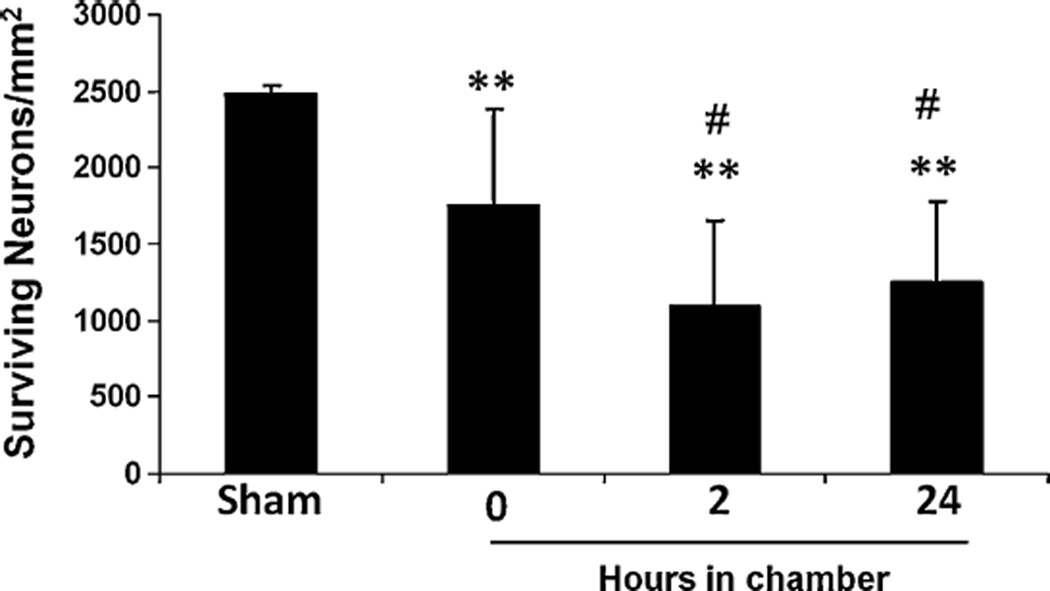
All sham mice survived after surgery and no neuronal injury was seen in the CA1 region of those animals. For animals subjected to 12-min global cerebral ischemia, the mortality rate was approximately 30% in each of the groups, and neuronal damage was observed in several brain regions, particularly in hippocampus (CA1, CA3), striatum, and cortex. Neuronal injury was more reproducible in the CA1 region of hippocampus than in other areas of brain; consequently, we focused all further analysis there. The density of surviving neurons in the CA1 region of the sham-operated mice (2481 ± 52 mm−2; n = 4) was significantly higher than that of animals subjected to 12-min cerebral global ischemia followed by 7 days of reperfusion and kept respectively at room temperature (1747 ± 635 mm−2; n = 12), a temperature-controlled warm chamber for 2 h (1098 ± 557 mm−2; n = 18), or a temperature-controlled warm chamber for 24 h (1249 ± 536 mm−2; n = 11). The number of surviving neurons in the CA1 region of animals kept at room temperature was approximately 75% that of the sham-operated animals, whereas significant differences were observed in the neuronal survival in animals kept in the temperature-controlled chamber for 2 or 24 h (approximately 50% that of the sham controls), suggesting that short-term post-ischemic hypothermia is neuroprotective. The data also indicate that the initial 2 h post-ischemia might be critical for hypothermic protection because neuronal survival was not significantly different between animals kept in the temperature-controlled chamber for 2 h and those kept for 24 h after cerebral global ischemia (Fig. 4).
Fig. 4.
Neuronal viability in the hippocampal CA1 region of mice subjected to 12-min global cerebral ischemia and then exposed to a warm temperature-controlled chamber for different lengths of time. Neuronal survival in the CA1 region was significantly decreased in mice subjected to 12-min global cerebral ischemia followed by 7 days of reperfusion compared to sham-operated mice. However, neuronal survival was significantly higher in animals kept at room temperature (25 °C) than in animals that were kept in a 33–34 °C chamber for 2 or 24 h after ischemia. Results are shown as mean ± SD. **P < 0.01 vs. sham-operated mice, #P < 0.05 vs. animals kept at room temperature.
4. Discussion
In this study, we optimized a global forebrain ischemia model in mouse in which we can monitor physiological parameters and evaluate neuronal injury in the same cohort of animals. We characterized the change in post-ischemic core temperature and its effect on the outcome of neuronal injury in this model. We observed that core temperature decreased after global cerebral ischemia and then spontaneously recovered after approximately 24 h. In addition, the early post-ischemic hypothermia impacted the delayed neuronal injury in hippocampus, suggesting that normothermic control immediately following ischemia is essential for obtaining a consistent and reproducible neuronal damage in a mouse model of cerebral global ischemia.
Patients who survive cardiac arrest by cardiopulmonary resuscitation usually suffer from delayed neuronal injury in the vulnerable regions of brain, particularly the hippocampal CA1 region. Such injury causes impairment in learning and memory (Madl and Holzer, 2004). The technology of genetically engineered mice has provided a unique opportunity in medical research to study the impact of neurological and cardiovascular events on learning and memory (Olsson et al., 2004a; Sheng et al., 1999a). C57BL/6 and 129 mouse substrains are commonly used for the creation of transgenic mice. Studies have shown that C57BL/6 mice are particularly susceptible to global ischemic injury (Fujii et al., 1997), and many laboratories are maintaining their transgenic mice on this background. Therefore, we chose the C57BL/6 mouse strain for our current study.
Several groups have attempted to establish mouse global ischemia models that result in uniform injury in the CA1 region (Gillingwater et al., 2004; Ohtaki et al., 2003; Olsson et al., 2004b). However, the variability of neuronal injury induced by global cerebral ischemia remains problematic. Anatomic variability in the posterior communicating arteries, reduction in CBF upon occlusion of the bilateral common carotid arteries, and duration of ischemia can all influence the neuronal loss in the CA1 region (Kitagawa et al., 1998b; Olsson et al., 2004b; Yonekura et al., 2004). Monitoring of physiological parameters and core temperature control are likely to be critical and affect the outcomes of neuronal injury in this model.
Temperature is another important factor thought to influence the CA1 injury induced by global cerebral ischemia (Preston and Webster, 2004). Hypothermia during the periods of ischemia and post-ischemia has been shown to be protective in stroke and traumatic brain injury (Henderson et al., 2003; Olsen et al., 2003). Mild post-ischemic hypothermia conveys substantial and lasting protection of hippocampal CA1 neurons in gerbils and rats (Colbourne and Corbett, 1994; Wiedau-Pazos et al., 1996). Strict control of temperature in animals subjected to global ischemia may also minimize the variability. Indeed, in previous studies, other investigators have strictly controlled temperature of the head and core to around 37 °C during the ischemic period, but temperature control in the post-ischemic period has varied. For example, Yonekura et al. (2004) returned animals to room-temperature cages immediately after ischemia, whereas Olsson et al. (2003) kept animals at 33.5 °C for 24 h before returning them to room temperature. In contrast, Gillingwater et al. (2004) kept animals at 28 °C for 2 h. Our preliminary study suggests that mice subjected to 12-min global ischemia did not maintain core temperature at a normal level (36–37 °C). As shown here, this impairment in temperature control returned to normal about 24 h after ischemia. Therefore, to maintain the normothermic core temperature consistently throughout the experiment, one must keep animals in a 33–34 °C environment for at least 24 h. This is the optimal post-ischemic care temperature and duration to minimize the core temperature variation for animals subjected to 12-min cerebral global ischemia to obtain uniform neuronal injury. In our study, blood gases remained generally within normal ranges in the mice following cerebral global ischemia (Table 1). Low pH, which occurs during ischemic acidification of tissue appears to affect brain injury via acid-sensing ion channels (ASICs) when pH dips below 6 (Huang and McNamara, 2004; Xiong et al., 2008). Following brain injury, PO2 and pH are significantly decreased, while PaCO2 is increased (Zauner et al., 2002). Low PaO2 or high PaCO2 is associated with poor outcomes in patients after brain injury (Carmona Suazo et al., 2000; van den Brink et al., 2000), whereas maintaining blood gases at a more physiologic level following brain injury has been shown to result in better outcomes (Kaneda et al., 2001; Zauner et al., 2002). Therefore, we believe that monitoring of blood gases is important. Our data indicated that blood gases did not significantly change after ischemia as compared to those in the sham-operated groups, suggesting that the blood gases in this experimental model may have stayed within normal ranges.
In summary, we have established a cerebral global ischemia model in mouse in which measurements of physiological parameters and neuronal injury are made in the same animal. In this model, the post-ischemic core temperature impacts the outcome of delayed neuronal injury, and the initial 2 h after ischemia are critical for hypothermic neuroprotection. This result indicates that precise temperature control is critical for obtaining a uniform neuronal injury in cerebral global ischemia models of mouse.
Acknowledgements
This work was supported in part by grants from the NIH NS046400 and AG022971 (SD). The author would like to thank Claire Levine for her assistance in preparing this manuscript and Drs. Raymond Koehler and Adam Sapirstein for their helpful contributions.
References
- Aliev G, Smith MA, Seyidov D, Neal ML, Lamb BT, Nunomura A, et al. The role of oxidative stress in the pathophysiology of cerebrovascular lesions in Alzheimer’s disease. Brain Pathol. 2002;12:21–35. doi: 10.1111/j.1750-3639.2002.tb00419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath P. High blood pressure as risk factor and prognostic predictor in acute ischaemic stroke: when and how to treat it? Cerebrovasc Dis. 2004;17(Suppl 1):51–57. doi: 10.1159/000074795. [DOI] [PubMed] [Google Scholar]
- Carmona Suazo JA, Maas AI, van den Brink WA, van Santbrink H, Steyerberg EW, Avezaat CJ. CO2 reactivity and brain oxygen pressure monitoring in severe head injury. Crit Care Med. 2000;28:3268–3274. doi: 10.1097/00003246-200009000-00024. [DOI] [PubMed] [Google Scholar]
- Castillo J, Leira R, Garcia MM, Serena J, Blanco M, Davalos A. Blood pressure decrease during the acute phase of ischemic stroke is associated with brain injury and poor stroke outcome. Stroke. 2004;35:520–526. doi: 10.1161/01.STR.0000109769.22917.B0. [DOI] [PubMed] [Google Scholar]
- Colbourne F, Corbett D. Delayed and prolonged post-ischemic hypothermia is neuroprotective in the gerbil. Brain Res. 1994;654:265–272. doi: 10.1016/0006-8993(94)90488-x. [DOI] [PubMed] [Google Scholar]
- Fisher M. Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30:2752–2758. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]
- Fujii M, Hara H, Meng W, Vonsattel JP, Huang Z, Moskowitz MA. Strain-related differences in susceptibility to transient forebrain ischemia in SV-129 and C57black/6 mice. Stroke. 1997;28:1805–1810. doi: 10.1161/01.str.28.9.1805. [discussion 11]. [DOI] [PubMed] [Google Scholar]
- Gillingwater TH, Haley JE, Ribchester RR, Horsburgh K. Neuroprotection after transient global cerebral ischemia in Wld(s) mutant mice. J Cereb Blood Flow Metab. 2004;24:62–66. doi: 10.1097/01.WCB.0000095798.98378.34. [DOI] [PubMed] [Google Scholar]
- Gladstone DJ, Black SE, Hakim AM. Toward wisdom from failure: lessons from neuroprotective stroke trials and new therapeutic directions. Stroke. 2002;33:2123–2136. doi: 10.1161/01.str.0000025518.34157.51. [DOI] [PubMed] [Google Scholar]
- Henderson WR, Dhingra VK, Chittock DR, Fenwick JC, Ronco JJ. Hypothermia in the management of traumatic brain injury. A systematic review and meta-analysis. Intensive Care Med. 2003;29:1637–1644. doi: 10.1007/s00134-003-1848-2. [DOI] [PubMed] [Google Scholar]
- Huang Y, McNamara JO. Ischemic stroke: “acidotoxicity” is a perpetrator. Cell. 2004;118:665–666. doi: 10.1016/j.cell.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Jin K, Nagayama T, Mao X, Kawaguchi K, Hickey RW, Greenberg DA, et al. Two caspase-2 transcripts are expressed in rat hippocampus after global cerebral ischemia. J Neurochem. 2002;81:25–35. doi: 10.1046/j.1471-4159.2002.00781.x. [DOI] [PubMed] [Google Scholar]
- Kaneda T, Ku K, Inoue T, Onoe M, Oku H. Postischemic reperfusion injury can be attenuated by oxygen tension control. Jpn Circ J. 2001;65:213–218. doi: 10.1253/jcj.65.213. [DOI] [PubMed] [Google Scholar]
- Kim YH, Kim EY, Gwag BJ, Sohn S, Koh JY. Zinc-induced cortical neuronal death with features of apoptosis and necrosis: mediation by free radicals. Neuroscience. 1999;89:175–182. doi: 10.1016/s0306-4522(98)00313-3. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Matsumoto M, Tsujimoto Y, Ohtsuki T, Kuwabara K, Matsushita K, et al. Amelioration of hippocampal neuronal damage after global ischemia by neuronal overexpression of BCL-2 in transgenic mice. Stroke. 1998a;29:2616–2621. doi: 10.1161/01.str.29.12.2616. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Matsumoto M, Yang G, Mabuchi T, Yagita Y, Hori M, et al. Cerebral ischemia after bilateral carotid artery occlusion and intraluminal suture occlusion in mice: evaluation of the patency of the posterior communicating artery. J Cereb Blood Flow Metab. 1998b;18:570–579. doi: 10.1097/00004647-199805000-00012. [DOI] [PubMed] [Google Scholar]
- Madl C, Holzer M. Brain function after resuscitation from cardiac arrest. Curr Opin Crit Care. 2004;10:213–217. doi: 10.1097/01.ccx.0000127542.32890.fa. [DOI] [PubMed] [Google Scholar]
- Ohtaki H, Mori S, Nakamachi T, Dohi K, Yin L, Endo S, et al. Evaluation of neuronal cell death after a new global ischemia model in infant mice. Acta Neurochir Suppl. 2003;86:97–100. doi: 10.1007/978-3-7091-0651-8_22. [DOI] [PubMed] [Google Scholar]
- Olsen TS, Weber UJ, Kammersgaard LP. Therapeutic hypothermia for acute stroke. Lancet Neurol. 2003;2:410–416. doi: 10.1016/s1474-4422(03)00436-8. [DOI] [PubMed] [Google Scholar]
- Olsson T, Hansson O, Nylandsted J, Jaattela M, Smith ML, Wieloch T. Lack of neuroprotection by heat shock protein 70 overexpression in a mouse model of global cerebral ischemia. Exp Brain Res. 2004a;154:442–449. doi: 10.1007/s00221-003-1683-2. [DOI] [PubMed] [Google Scholar]
- Olsson T, Nygren J, Hakansson K, Lundblad C, Grubb A, Smith ML, et al. Gene deletion of cystatin C aggravates brain damage following focal ischemia but mitigates the neuronal injury after global ischemia in the mouse. Neuroscience. 2004b;128:65–71. doi: 10.1016/j.neuroscience.2004.06.024. [DOI] [PubMed] [Google Scholar]
- Olsson T, Wieloch T, Smith ML. Brain damage in a mouse model of global cerebral ischemia. Effect of NMDA receptor blockade. Brain Res. 2003;982:260–269. doi: 10.1016/s0006-8993(03)03014-2. [DOI] [PubMed] [Google Scholar]
- Preston E, Webster J. A two-hour window for hypothermic modulation of early events that impact delayed opening of the rat blood-brain barrier after ischemia. Acta Neuropathol (Berl) 2004;108:406–412. doi: 10.1007/s00401-004-0905-4. [DOI] [PubMed] [Google Scholar]
- Sheng H, Laskowitz DT, Mackensen GB, Kudo M, Pearlstein RD, Warner DS. Apolipoprotein E deficiency worsens outcome from global cerebral ischemia in the mouse. Stroke. 1999a;30:1118–1124. doi: 10.1161/01.str.30.5.1118. [DOI] [PubMed] [Google Scholar]
- Sheng H, Laskowitz DT, Pearlstein RD, Warner DS. Characterization of a recovery global cerebral ischemia model in the mouse. J Neurosci Methods. 1999b;88:103–109. doi: 10.1016/s0165-0270(99)00018-7. [DOI] [PubMed] [Google Scholar]
- Smith WS. Pathophysiology of focal cerebral ischemia: a therapeutic perspective. J Vasc Interv Radiol. 2004;15:S3–S12. doi: 10.1097/01.rvi.0000108687.75691.0c. [DOI] [PubMed] [Google Scholar]
- Suk K, Kim SY, Leem K, Kim YO, Park SY, Hur J, et al. Neuroprotection by methanol extract of Uncaria rhynchophylla against global cerebral ischemia in rats. Life Sci. 2002;70:2467–2480. doi: 10.1016/s0024-3205(02)01534-5. [DOI] [PubMed] [Google Scholar]
- van den Brink WA, van Santbrink H, Steyerberg EW, Avezaat CJ, Suazo JA, Hogesteeger C, et al. Brain oxygen tension in severe head injury. Neurosurgery. 2000;46:868–876. doi: 10.1097/00006123-200004000-00018. [discussion 76-8]. [DOI] [PubMed] [Google Scholar]
- Vink R, Nimmo AJ, Cernak I. An overview of new and novel pharmacotherapies for use in traumatic brain injury. Clin Exp Pharmacol Physiol. 2001;28:919–921. doi: 10.1046/j.1440-1681.2001.03548.x. [DOI] [PubMed] [Google Scholar]
- Vink R, Van Den Heuvel C. Recent advances in the development of multifactorial therapies for the treatment of traumatic brain injury. Expert Opin Investig Drugs. 2004;13:1263–1274. doi: 10.1517/13543784.13.10.1263. [DOI] [PubMed] [Google Scholar]
- Wei G, Kibler KK, Koehler RC, Maruyama T, Narumiya S, Dore S. Prostacyclin receptor deletion aggravates hippocampal neuronal loss after bilateral common carotid artery occlusion in mouse. Neuroscience. 2008;156:1111–1117. doi: 10.1016/j.neuroscience.2008.07.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellons JC, 3rd, Sheng H, Laskowitz DT, Burkhard Mackensen G, Pearlstein RD, Warner DS. A comparison of strain-related susceptibility in two murine recovery models of global cerebral ischemia. Brain Res. 2000;868:14–21. doi: 10.1016/s0006-8993(00)02216-2. [DOI] [PubMed] [Google Scholar]
- Wiedau-Pazos M, Goto JJ, Rabizadeh S, Gralla EB, Roe JA, Lee MK, et al. Altered reactivity of superoxide dismutase in familial amyotrophic lateral sclerosis. Science. 1996;271:515–518. doi: 10.1126/science.271.5248.515. [DOI] [PubMed] [Google Scholar]
- Xiong ZG, Pignataro G, Li M, Chang SY, Simon RP. Acid-sensing ion channels (ASICs) as pharmacological targets for neurodegenerative diseases. Curr Opin Pharmacol. 2008;8:25–32. doi: 10.1016/j.coph.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonekura I, Kawahara N, Nakatomi H, Furuya K, Kirino T. A model of global cerebral ischemia in C57 BL/6 mice. J Cereb Blood Flow Metab. 2004;24:151–158. doi: 10.1097/01.WCB.0000096063.84070.C1. [DOI] [PubMed] [Google Scholar]
- Zauner A, Clausen T, Alves OL, Rice A, Levasseur J, Young HF, et al. Cerebral metabolism after fluid-percussion injury and hypoxia in a feline model. J Neurosurg. 2002;97:643–649. doi: 10.3171/jns.2002.97.3.0643. [DOI] [PubMed] [Google Scholar]