Abstract
TGF-β1 activity results in methylation of lysine 4 of histone H3 (H3K4) through SET domain–containing lysine methyltransferase 7/9 (SET7/9) induction, which is important for the transcriptional activation of fibrotic genes in vitro. However, in vivo studies utilizing an experimental model of renal fibrosis are required to develop therapeutic interventions that target SET7/9. In this study, we investigated the signaling pathway of TGF-β1-induced SET7/9 expression and whether inhibition of SET7/9 suppresses renal fibrosis in unilateral ureteral obstruction (UUO) mice and kidney cell lines. Among the SET family, SET7/9 was upregulated on days 3 and 7 in UUO mice, and the upregulation was suppressed by TGF-β1 neutralizing antibody. TGF-β1 induced SET7/9 expression via Smad3 in normal rat kidney (NRK)-52E cells. In human kidney biopsy specimens from patients diagnosed with IgA nephropathy and membranous nephropathy, SET7/9 expression was positively correlated with the degree of interstitial fibrosis (r=0.59, P=0.001 in patients with IgA nephropathy; and r=0.58, P<0.05 in patients with membranous nephropathy). In addition, small interfering RNA-mediated knockdown of SET7/9 expression significantly attenuated renal fibrosis in UUO mice. Sinefungin, an inhibitor of SET7/9, also suppressed the expression of mesenchymal markers and extracellular matrix proteins and inhibited H3K4 mono-methylation (H3K4me1) in kidneys of UUO mice. Moreover, sinefungin had an inhibitory effect on TGF-β1-induced α-smooth muscle actin expression and H3K4me1 in both NRK-52E and NRK-49F cells. In conclusion, sinefungin, a SET7/9 inhibitor, ameliorates renal fibrosis by inhibiting H3K4me1 and may be a candidate therapeutic agent.
Keywords: cell signaling, obstructive nephropathy, renal fibrosis, TGF-β
CKD is estimated to occur in 13%–15% of the population in developed countries1,2 and is thus recognized as a worldwide health problem.3 Importantly, CKD is responsible for progression to kidney failure4 and is linked to substantial disease burden, as evidenced by increased morbidity and mortality.5,6 In order to prevent the development of CKD, inhibitors of the renin-angiotensin-aldosterone system have been established as a pharmacological intervention for CKD patients in clinical settings.7 However, the beneficial effects are not drastic and many patients eventually require renal replacement therapy.
Regardless of initial causes, renal fibrosis is a common pathway to ESKD.8 Pathologically, renal fibrosis is characterized by an increase in interstitial fibroblasts, deposition of extracellular matrix (ECM) proteins, and loss of peritubular capillaries.9,10 Although it has been reported that several cytokines participate in the pathogenesis of renal fibrosis, TGF-β1 has been identified as the most important mediator, playing a central role in the development of fibrosis.11,12 In addition to potentiating fibrosis, TGF-β1 functions as an anti-inflammatory effector molecule,13 suggesting that inhibition of TGF-β1 could lead to severe adverse effects by enhancing systemic inflammation.14 Therefore, novel strategies that specifically inhibit the fibrotic action of TGF-β1 are required.
Epigenetics refers to gene regulatory mechanisms specifically not due to changes in the primary nucleotide sequence of genes.15 For example, the histone tails on the nucleosome surface are targets for histone post-translational modifications (HPTMs), including acetylation, methylation, phosphorylation, and ubiquitylation.16 HPTMs are implicated in the regulation of chromatin structure, which is essential for determining genomic DNA accessibility.17 Notably, HPTMs are regulated by specific enzymes and form a histone code that modulates patterns of gene expression.18 A recent study showed that TGF-β1 increases the expression of lysine 4 of histone H3 (H3K4) methyltransferase SET domain–containing lysine methyltransferase 7/9 (SET7/9), and that subsequent H3K4 methylation plays an important role in TGF-β1-induced promoter activation of fibrosis-associated genes.19 These findings led us to the hypothesis that inhibition of SET7/9 will suppress renal fibrosis through inhibition of H3K4 methylation in a mouse model of renal fibrosis.
In this study, we show that TGF-β1 induces SET7/9 via the Smad3 pathway. We also demonstrate that SET7/9 is responsible for H3K4 methylation as well as renal fibrosis in unilateral ureteral obstruction (UUO) mice. Furthermore, renal expression of SET7/9 is correlated with fibrotic areas in renal biopsy samples that were diagnosed with immunoglobulin A nephropathy (IgAN) and membranous nephropathy (MN). Finally, the small molecule inhibitor of SET7/9, sinefungin,20,21 inhibits H3K4 methylation and ameliorates renal fibrosis. These data suggest that sinefungin, also known as an antifungal agent, may be a candidate therapeutic agent for CKD patients.
Results
Increased Expression of the H3K4 Methyltransferase SET7/9 in a Mouse Model of Renal Fibrosis
SET7/9 has been described as an epigenetic modification enzyme that promotes ECM protein production in vitro;19 however, expression of SET7/9 and the other SET family proteins in a mouse model of renal fibrosis remain unclear. Therefore, we first examined the gene expression profiles of genes encoding epigenetic modification enzymes with SET domains in UUO mice by quantitative real-time RT-PCR (qRT-PCR). As shown in Figure 1A, we found that SET7/9 gene expression increased remarkably on both day 3 and day 7 in UUO mice compared with a non-operated normal control group. Notably, SET mRNAs apart from SET7/9 did not significantly increase on day 7. SETD8 increased modestly on day 1 and day 3; SETD4 increased on day 1, and SETD5 was slightly upregulated on day 3. In contrast, SETD3 was suppressed on day 1, day 3, and day 7, and SETD6 was downregulated on day 3 and day 7. Western blot analysis revealed that the protein levels of SET7/9 were elevated in UUO kidneys compared with sham-operated controls, which was consistent with our mRNA results (Figure 1B). Immunohistochemical staining for SET7/9 was performed in order to identify SET7/9 tissue expression patterns. In UUO kidneys, marked staining of SET7/9 was detected in the nuclei of tubular epithelial cells and interstitial cells (Figure 1C).
Figure 1.
SET7/9 is upregulated in the kidney after obstructive injury. (A) Expression profiles of genes encoding epigenetic modification enzymes containing the SET domain in UUO mice. On day 1 (gray bars), day 3 (hatched bars), and day 7 (black bars) compared with non-operated normal control (white bars), UUO-induced mRNA levels were determined by qRT-PCR. Data were analyzed by one-way ANOVA followed by Dunnett post hoc test based on non-operated normal controls for each SET primer (n=5 for each group). (B) Elevation of SET7/9 protein in whole kidney extracts at day 7 after UUO compared with sham-operated control. Typical results of western blot analysis are shown in the upper panel. Band intensity was normalized to glyceraldehyde 3-phosphate dehydrogenase . Relative levels of SET7/9 expression are shown in the lower panel (n=5 for each group). (C) Immunohistochemical staining for SET7/9 demonstrating the localization of SET7/9 protein in the kidneys. *P<0.05; **P<0.01; ††P<0.01. GAPDH, glyceraldehyde 3-phosphate dehydrogenase; non-immune, control non-immune serum; Non-op CTL, non-operated normal control; Sham CTL, sham-operated controls.
SET7/9 Expression is Positively Regulated by TGF-β1 in UUO Mice and in Renal Cells
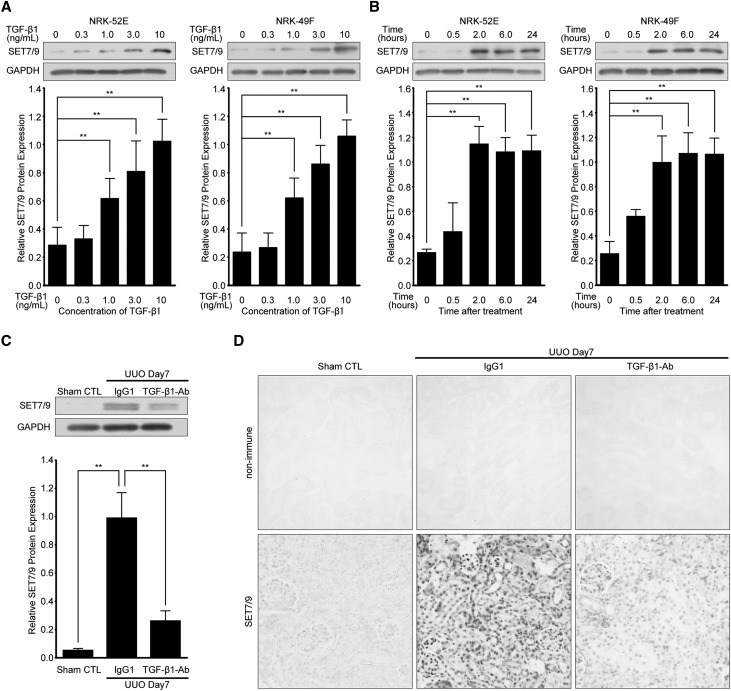
TGF-β1 expression induced by UUO22 is known to play an important role in the development of renal fibrosis. Therefore, we examined the role of TGF-β1 in SET7/9 expression in vitro and in vivo. Normal rat kidney (NRK)-52E cells, a rat kidney tubular epithelial cell line, and NRK-49F, a rat kidney interstitial fibroblast cell line, were used. TGF-β1-induced SET7/9 protein expression was upregulated in a dose-dependent manner, and SET7/9 expression significantly increased after 2 hours of stimulation in both cell lines compared with vehicle-treated controls (Figure 2, A and B). Furthermore, administration of neutralizing TGF-β1 antibody23 immediately after UUO attenuated UUO-induced SET7/9 expression, as detected by western blot analysis and immunohistochemistry (Figure 2, C and D).
Figure 2.
TGF-β1 induces SET7/9 expression in renal cells, and injection of neutralizing TGF-β1 antibody reduces SET7/9 expression after UUO. NRK-52E cells and NRK-49F cells were treated with TGF-β1. Representative western blot analysis showing the levels of SET7/9 protein expression in TGF-β1-stimulated NRK-52E cells and NRK-49F cells at (A) various dosages (time; 24 hours) and (B) time points (TGF-β1; 10 ng/mL). Expression levels were compared with vehicle-treated control. Quantification is shown in the lower panel. Data were analyzed by one-way ANOVA followed by Dunnett post hoc test based on vehicle-treated controls (n=5 for each group). (C) Representative western blot analysis with anti-SET7/9 antibody. Quantification is shown in the lower panel. Data were analyzed by one-way ANOVA followed by Dunnett post hoc test based on UUO mice with control IgG1 injection (n=5 for each group). (D) Images of SET7/9 staining demonstrating the levels of SET7/9 expression by intraperitoneal injection of neutralizing TGF-β1 antibody (TGF-β1-Ab) at a dose of 1.5 mg/kg/48 hours compared with control IgG1 at the same dose of TGF-β1-Ab. Original magnification, ×200. **P<0.01. Sham CTL, sham-operated controls; IgG1, UUO mice with control IgG1 injection; non-immune, control non-immune serum; TGF-β1-Ab, UUO mice with neutralizing TGF-β1 antibody injection.
SET7/9 Expression is Regulated by the TGF-β1–Smad3-dependent Pathway
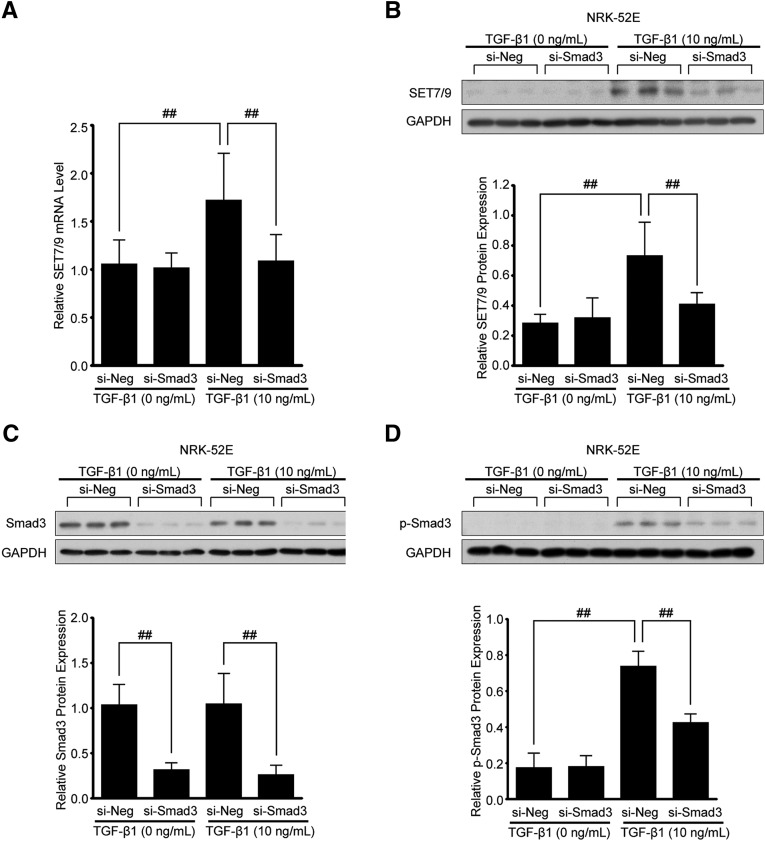
TGF-β1 is recognized to stimulate several pathways though activation of TGF-β receptors. Among those, we investigated whether TGF-β1-induced phosphorylation of Smad3 (p-Smad3), which is known as a canonical pathway for TGF-β1 signaling,24 is responsible for SET7/9 expression. In order to downregulate Smad3, NRK-52E cells were transfected with small interfering RNA (siRNA) oligonucleotides targeting Smad3 (si-Smad3) or negative control siRNA (si-Neg). After TGF-β1 stimulation (30 minutes for Smad3 or 24 hours for SET7/9), total protein from transfected cells was analyzed by western blots with SET7/9, Smad3, and p-Smad3 antibodies. Glyceraldehyde 3-phosphate dehydrogenase was used as an internal control. As shown in Figure 3, A and B, TGF-β1-induced SET7/9 mRNA and protein levels were significantly reduced in NRK-52E cells transfected with Smad3-siRNA. Likewise, Smad3-siRNA treatment significantly inhibited the expression of Smad3 and TGF-β1-induced p-Smad3 (Figure 3, C and D).
Figure 3.
Knockdown of Smad3 in NRK-52E cells inhibits TGF-β1-induced SET7/9 expression. NRK-52E cells were transfected with Smad3 siRNA (si-Smad3) or negative control (si-Neg) oligonucleotides. (A) SET7/9 mRNA levels determined by qRT-PCR in transfected NRK-52E cells with or without TGF-β1 (10 ng/mL, 24 hours). (B) Western blot analysis using SET7/9, (C) Smad3, and (D) phosphorylated Smad3 (p-Smad3) antibodies in transfected NRK-52E cells with or without TGF-β1 (10 ng/mL, 30 minutes or 24 hours). Total cell lysates were subjected to immunoblotting. Because p-Smad3 content peaked at 30 minutes after TGF-β1 stimulation, this time point was used only in p-Smad3 experiments. Quantification is shown in the lower panel (n=5 for each group). Data were analyzed by one-way ANOVA followed by the post hoc test using t test with Bonferroni correction. ##P<0.01. si-Neg, UUO mice with negative control oligonucleotides injection; si-Smad3, UUO mice with Smad3-siRNA injection.
Knockdown of SET7/9 in Vivo Attenuates TGF-β1-Induced Fibrogenesis in Obstructive Nephropathy
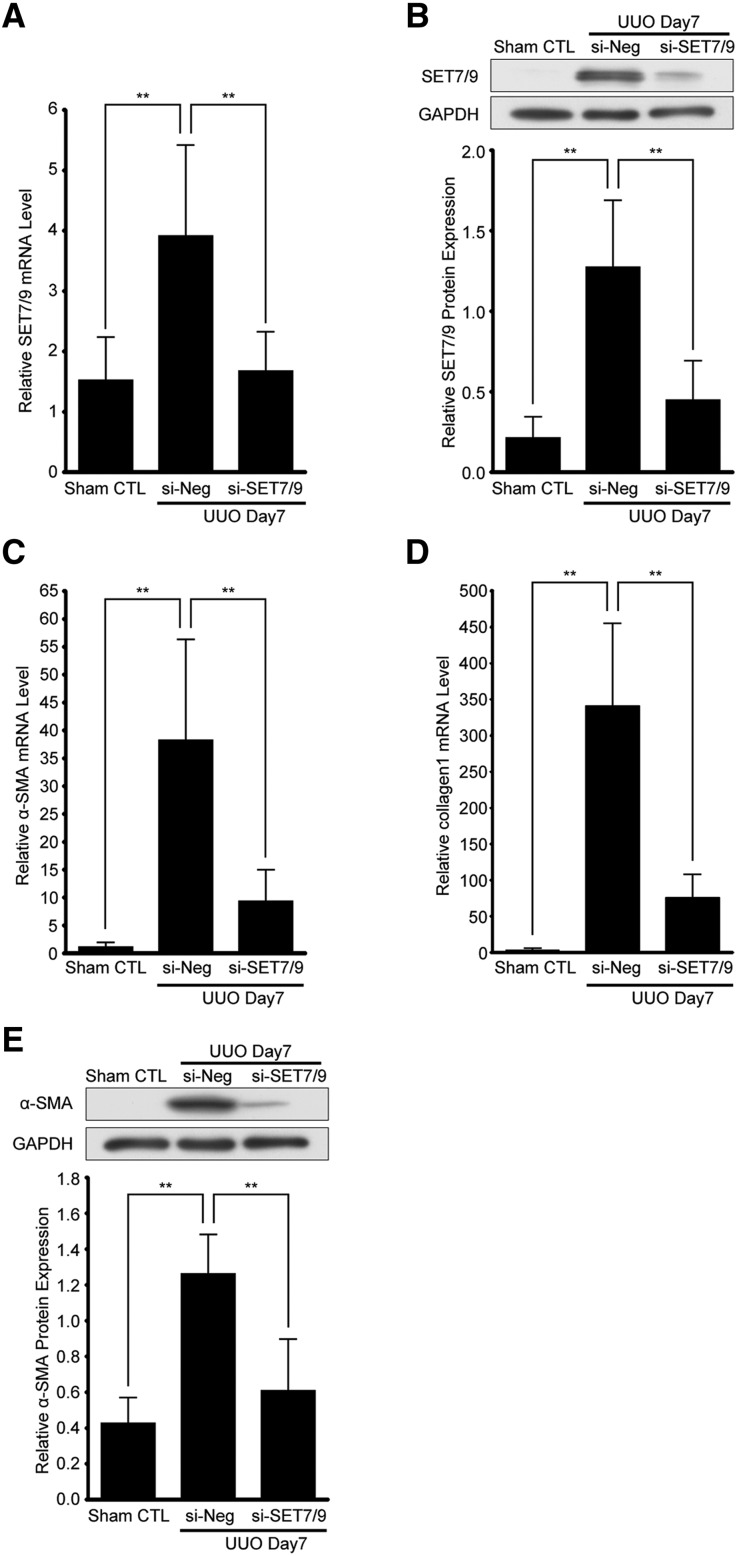
Next, we knocked down SET7/9 expression using siRNA in UUO mice to evaluate whether SET7/9 is responsible for fibrogenesis in vivo. First, we confirmed whether SET7/9 was sufficiently knocked down by SET7/9-siRNA injection. In the SET7/9-siRNA group, UUO-induced SET7/9 mRNA and protein expression was significantly decreased (Figure 4, A and B). Expression of α-smooth muscle actin (α-SMA) and collagen 1 were very low in the sham-operated control group, whereas they progressively increased in the negative control siRNA-injected UUO group. In contrast, α-SMA and collagen 1 were significantly decreased in the SET7/9-siRNA group (Figure 4, C–E).
Figure 4.
Knockdown of SET7/9 inhibits UUO-induced fibroblast activation in UUO mice. SET7/9-siRNA injection was performed at a dose of 7 mg/kg via the right ureter. (A) Expression level of SET7/9 mRNA determined by qRT-PCR. Gene expression was normalized to internal control 18S rRNA (n=5 for each group). (B) Representative western blot analysis showing the levels of SET7/9 expression. Quantification is shown in the lower panel (n=5 for each group). (C) Expression levels of α-SMA, and (D) collagen 1 mRNA determined by qRT-PCR. Gene expression was normalized to internal control 18S rRNA (n=5 for each group). (E) Typical results of western blot analysis showing the levels of α-SMA expression. Quantification is shown in the lower panel (n=5 for each group). Data were analyzed by one-way ANOVA followed by Dunnett post hoc test based on UUO mice with negative control oligonucleotides (si-Neg) injection. **P<0.01. Sham CTL, sham-operated controls; si-Neg, UUO mice with negative control oligonucleotides injection; si-SET7/9, UUO mice with SET7/9-siRNA injection.
SET7/9 Expression is Associated with the Degree of Fibrosis in Human Kidney
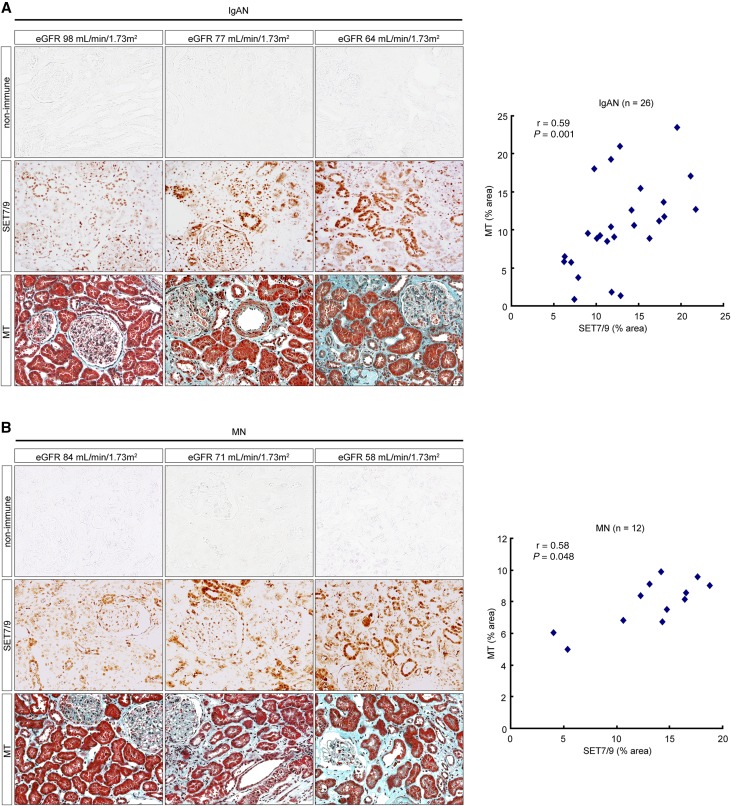
In order to assess the clinical significance of SET7/9 expression, we performed immunostaining for SET7/9 and correlated with Masson’s trichrome (MT) staining on biopsy samples. A total of 38 renal tissue specimens (24 men and 14 women) were collected from IgAN (n=26) and MN (n=12) patients who underwent renal biopsy for the first time at Hiroshima University Hospital from April, 2008 to December, 2010. In this patient population, the average clinical values were as follows: 47±18 years of age, body mass index was 22.51±3.22 kg/m2, creatinine clearance was 89.40±22.96 mL/minute, eGFR was 71.32±18.64 mL/minute/1.73 m2, and urinary protein excretion was 1.55±1.90 g/24 hours. The clinical characteristics of IgAN and MN patients in relation to renal function are shown in Supplemental Table 1 and Supplemental Table 2. The staining area for SET7/9 increased with the decline of renal function. SET7/9 was positively correlated with MT-positive fibrotic areas in a correlation diagram (Spearman correlation coefficient: r=0.59, P=0.001 in IgAN patients, and r=0.58, P<0.05 in MN patients) (Figure 5, A and B).
Figure 5.
Correlation between the expression of SET7/9 and the degree of fibrosis in IgAN and MN patients. (A) Representative images of SET7/9 staining and MT staining demonstrating strong SET7/9 staining with the decline of renal function in IgAN and (B) MN patients. Vertical columns show images from the same person. Scatter diagram of bivariate correlations in the right panel demonstrating a positive correlation between SET7/9 and MT staining. Spearman’s correlation coefficient test was used. r, Spearman correlation coefficient; r=0.59, P=0.001 in IgAN patients, and r=0.58, P<0.05 in MN patients. Non-immune, control non-immune serum. Original magnification, ×200. The Japanese GFR equation based on serum creatinine was used as an eGFR. eGFR (mL/minute/1.73 m2)=194×Scr−1.094×Age−0.287×0.739 (if female). Age, years old; Scr, serum creatinine (mg/dL); non-immune, control non-immune serum.
Sinefungin Ameliorates Renal Fibrosis in Obstructive Nephropathy
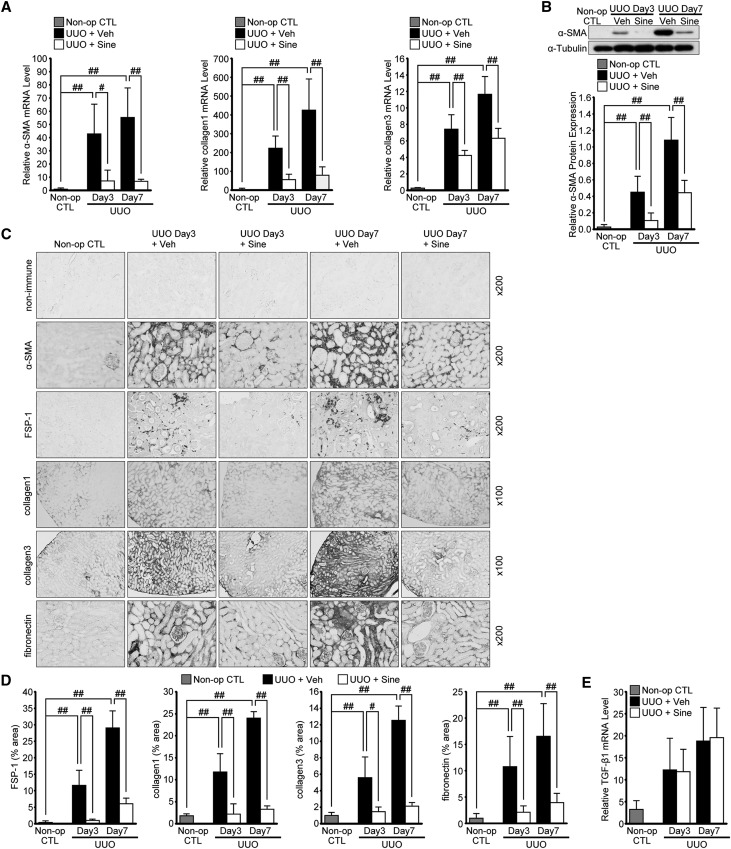
In view of the need for developing new therapeutic agents for the treatment of renal fibrosis, we assessed the effect of a small molecule inhibitor for SET7/9, sinefungin, on the expression of mesenchymal markers and ECM proteins in UUO mice. We examined α-SMA and fibroblast-specific protein-1 (FSP-1) as mesenchymal markers, and collagen 1, collagen 3, and fibronectin as ECM proteins. Following the injection of sinefungin, UUO-induced mRNA of α-SMA, collagen 1 and collagen 3 were markedly suppressed in the kidney, both at 3 days and 7 days after UUO (Figure 6A). Western blot analysis also showed that sinefungin inhibited α-SMA protein expression (Figure 6B). Our immunohistochemical analysis revealed that staining for α-SMA, FSP-1, collagen 1, collagen 3, and fibronectin in the kidney tissues increased at 3 days, with a further increase at 7 days, after UUO. In contrast, sinefungin injection ameliorated those both at 3 and 7 days after UUO (Figure 6, C and D). However, injection of sinefungin did not affect UUO-induced TGF-β1 mRNA expression levels (Figure 6E).
Figure 6.
Sinefungin ameliorates the UUO-induced increase in mesenchymal markers and deposition of ECM proteins. Mice were treated daily with sinefungin at a dose of 10 mg/kg by intraperitoneal injection. We examined α-SMA and FSP-1 as mesenchymal markers, and collagen 1, collagen 3, and fibronectin as ECM proteins. (A) α-SMA, collagen 1, and collagen 3 mRNA levels determined by qRT-PCR in UUO mice with or without sinefungin injection (n=5 for each group). (B) Representative western blot analysis demonstrating the levels of α-SMA protein expression in UUO mice with or without sinefungin injection. Quantification is shown in the lower panel (n=5 for each group). (C) Typical immnohistochemistry of α-SMA, FSP-1, collagen 1, collagen 3, and fibronectin in UUO mice with or without sinefungin injection. (D) Quantification of FSP-1, collagen 1, collagen 3, and fibronectin expression by immunohistochemical staining. (n=5 for each group). (E) TGF-β1 mRNA levels in UUO mice with or without sinefungin injection quantified by qRT-PCR (n=5 for each group). Data were analyzed by one-way ANOVA followed by the post hoc test using t test with Bonferroni correction. #P<0.05; ##P<0.01. Non-immune, control non-immune serum; Non-op CTL, non-operated normal controls; Sine, sinefungin; Veh, vehicle.
Sinefungin Inhibits H3K4me1 Simultaneously with the Amelioration of Renal Fibrosis in Obstructive Nephropathy
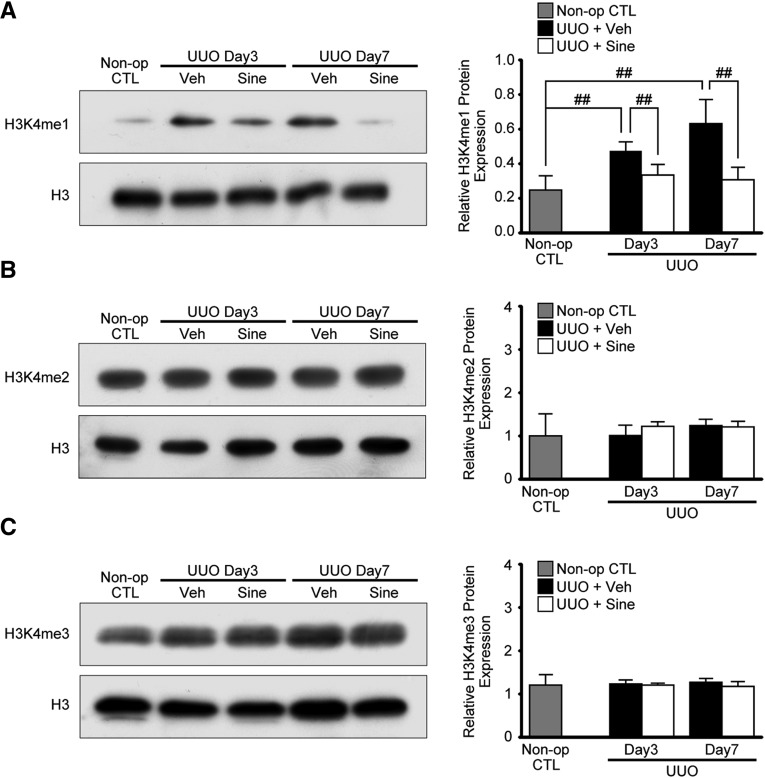
It has previously been reported that increased levels of H3K4 methylation promote transcriptional activation of TGF-β1-induced fibrotic gene expression.19 To identify the effect of sinefungin on SET7/9 during the development of renal fibrosis, we tested whether sinefungin inhibits H3K4 methylation. Injection of sinefungin significantly inhibited UUO-induced H3K4 mono-methylation (H3K4me1) in mouse kidney (Figure 7A). In contrast, H3K4 di-methylation (H3K4me2) and H3K4 tri-methylation (H3K4me3) in the kidneys of UUO mice did not show a significant change after the injection of sinefungin (Figure 7, B and C).
Figure 7.
Sinefungin inhibits UUO-induced upregulation of H3K4me1 in UUO mice concomitantly with the amelioration of renal fibrosis in obstructive nephropathy. The same protein lysates as in Figure 6 were used. (A) Representative western blot analysis showing the levels of H3K4me1, (B) H3K4me2, and (C) H3K4me3 protein expression in UUO mice with or without sinefungin injection (10 mg/kg). Quantification is shown in the right panel (n=5 for each group). Data were analyzed by one-way ANOVA followed by the post hoc test using t test with Bonferroni correction. ##P<0.01. Non-op CTL, non-operated normal controls; Sine, sinefungin; Veh, vehicle.
Sinefungin Inhibits TGF-β1-Induced α-SMA-positive Myofibroblast Expression and TGF-β1-induced H3K4me1 in Renal Cells
As shown in Figures 6 and 7, sinefungin inhibited H3K4me1 and ameliorated renal fibrosis induced by UUO. However, these results were from whole kidney lysates in UUO mice. To clarify the direct effect of sinefungin on TGF-β1-induced H3K4 methylation, we performed immunoblotting for H3K4 methylation in NRK-52E and NRK-49F cells. Pretreatment with sinefungin significantly reduced TGF-β1-induced α-SMA protein expression and inhibited H3K4me1 in a dose-dependent manner in both NRK-52E (Figure 8, A and B) and NRK-49F (Figure 9, A and B) cells. In contrast, sinefungin had no significant effect on H3K4me2 and H3K4me3 either in vivo in UUO kidneys or in vitro in epithelial cells (Figure 8, C and D) and fibroblasts (Figure 9, C and D). Finally, we examined whether TGF-β1 alters access located at H3K4me1-regulated sites using chromatin immunoprecipitation (ChIP) assays. We found that TGF-β1 increased H3K4me1 levels at collagen 1 (Col1a1), connective tissue growth factor (CTGF) and plasminogen activator inhibitor-1 (PAI-1) promoters in NRK-52E cells, and that sinefungin inhibited H3K4me1 levels (Figure 8E).
Figure 8.
Sinefungin ameliorates the TGF-β1-induced increase of α-SMA and inhibits the upregulation of histone H3K4 mono-methylation in renal epithelial cells. Pretreatment of sinefungin (0.5 or 1.0 μg/mL) was conducted 60 minutes before TGF-β1 (10 ng/mL) stimulation. (A) A typical western blot for α-SMA in NRK-52E cells. Quantification is shown in the lower panel (n=5 for each group). (B) Representative western blot analysis of the expression of histone H3K4me1, (C) H3K4me2, and (D) H3K4me3 expression in NRK-52E cells. Quantification is shown in the lower panel (n=5 for each group). (E) Representative ChIP assay analysis of the expression of binding of H3K4me1 protein to Col1a1, CTGF, and PAI-1 promoters in NRK-52E cells. ChIP assays were performed with H3K4me1 antibody. Immunoprecipitated DNA and input DNA were subjected to qRT-PCR. Results were normalized to input DNA (n=5 for each group). Data were analyzed by one-way ANOVA followed by the post hoc test using t test with Bonferroni correction. #P<0.05; ##P<0.01. Sine, sinefungin.
Figure 9.
Sinefungin ameliorates the TGF-β1-induced increase of α-SMA and inhibits the upregulation of H3K4me1 in renal fibroblast cells. Pretreatment of sinefungin (0.5 or 1.0 μg/mL) was carried out 60 minutes before TGF-β1 (10 ng/mL) stimulation. (A) A typical western blot for α-SMA expression in NRK-49F cells. Quantification is shown in the lower panel (n=5 for each group). (B) Representative western blot analysis of the expression of H3K4me1, (C) H3K4me2, and (D) H3K4me3 expression in NRK-49F cells. Quantification is shown in the lower panel (n=5 for each group). Data were analyzed by one-way ANOVA followed by the post hoc test using t test with Bonferroni correction. ##P<0.01. Sine, sinefungin.
Discussion
In this study, we have demonstrated five major findings. First, SET7/9 plays an important role in renal fibrogenesis in UUO mice. Second, TGF-β1 induces SET7/9 expression via the Smad3 pathway. Third, inhibition of SET7/9 suppresses TGF-β1-induced fibrogenesis. Fourth, SET7/9 expression is associated with the degree of fibrosis in the human kidney. Finally, sinefungin ameliorates renal fibrosis in UUO mice and inhibits TGF-β1-induced α-SMA expression as well as H3K4me1 in renal cells. Results from this study illustrate that sinefungin, known as an antifungal agent, may be a candidate therapeutic agent for CKD patients. Our studies also provide insights into the mechanism by which SET7/9 induces renal fibrogenesis in a mouse model of renal fibrosis.
Among a number of epigenetic modifications, the inhibitory effects of DNA methylation25 and micro RNA26 on renal fibrosis have been investigated so far. In this study, we first confirmed that histone methyltransferase SET7/9 was increased during the development of renal fibrosis in UUO mice and patients with IgAN and MN. We also showed that knockdown of SET7/9 with siRNA ameliorated renal fibrosis in UUO mice. These results suggest that increased SET7/9 contributes to renal fibrosis, and that SET7/9 is a novel and attractive target for the treatment of CKD. Moreover, we have demonstrated that sinefungin ameliorates renal fibrosis in UUO mice. Sinefungin functions as an S-adenosylmethionine (SAM) analog and competes for SAM binding, thereby inhibiting the SAM-dependent methyltransferase activity of SET7/9.20,21,27 Previous studies have described that sinefungin administrated to mice at a dose of 0.5–25 mg/kg inhibited the development of various fungi, viruses, and parasites.28,29 In this study, we administrated sinefungin at a dose of 10 mg/kg/day, which resulted in the suppression of renal fibrosis as well as H4K4me1. Taken together, SET7/9 upregulation is involved in the progression of renal fibrosis, and sinefungin suppresses renal fibrosis through SET7/9 inhibition.
We have demonstrated for the first time that inhibition of SET7/9 activity ameliorates renal fibrosis as well as decreasing H3K4me1 levels both in vivo and in vitro. Renal fibrosis is due to transcriptional activation of fibrotic genes. Recent studies have shown that methylated H3K4 correlated with transcriptionally competent chromatin, resulting in increased gene expression.30,31 Importantly, a previous study has reported that transcriptional activation of fibrosis-associated genes is positively regulated by H3K4me1/2/3 levels.19 In this study, we identified that UUO increases H3K4me1 levels, but not H3K4me2 and H3K4me3, indicating that H3K4me1 mainly contributes to the progression of renal fibrosis in vivo. In renal cell lines, we found that TGF-β1 induces H3K4me1, but not H3K4me2 and H3K4me3. Conversely, one recent study showed that SET7/9-induced methylation of H3K4 enhanced TGF-β1 expression in the liver of bile duct ligation rats.32 However, we did not observe any inhibitory effect of sinefungin on TGF-β1 production in UUO mice. These findings suggest that sinefungin suppresses renal fibrosis through inhibiting H3K4me1, but does not reduce TGF-β1 production.
We have described that UUO-induced SET7/9 expression is inhibited by TGF-β1-neutralizing antibody, indicating that TGF-β1 leads to SET7/9 expression in a mouse model of renal fibrosis. We also showed that TGF-β1-induced phosphorylation of Smad3 is responsible for SET7/9 expression. TGF-β1–Smad3 signaling is a canonical pathway, and p-Smad3 serves as a component of transcriptional factors that promote renal fibrosis.24 Notably, our results show that TGF-β1 also increases H3K4me1 levels at fibrotic gene promoters, such as Col1a1, CTGF, and PAI-1.19 Taken together, TGF-β1–Smad3 signaling plays an important role in the increased transcriptional activity of fibrosis-associated genes through SET7/9 production and subsequent H3K4me1.
TGF-β1 is not only known as a major mediator in the progression of renal fibrosis, but also functions as an anti-inflammatory cytokine, suggesting that systemic inhibition of TGF-β1 signaling may lead to systemic inflammation.14 In fact, Tgfb1-knockout mice exhibited a lethal postnatal inflammatory phenotype, whereas the systematic effect of long-term inhibition of TGF-β1 signaling in humans remains unclear.33 Among the histone modifications, H3K4me3 is reported to participate in an increased expression of forkhead box p3, resulting in regulatory T cell generation.34 In this study, we did not observe any inhibitory effect of sinefungin on H3K4me3, implying that sinefungin does not disturb the immunosuppressive function of regulatory T cells. In addition, a recent study has reported that TGF-β1 suppresses IL-2 production from T cells through H3K9me3, but not H3K4me1.35 These findings raise the possibility that inhibition of H3K4me1 is a novel and attractive therapeutic strategy for treating renal fibrosis.
In summary, SET7/9 expression is regulated by the TGF-β1–Smad3 pathway, leading to transcriptional activation of fibrotic genes through increased H3K4me1. We confirmed the actual expression of SET7/9 in renal biopsy samples from patients who were diagnosed with IgAN and MN, and showed that SET7/9 expression correlated with fibrotic areas. Inhibition of SET7/9 not only suppressed H3K4me1 levels but also ameliorated renal fibrosis in a mouse model of renal fibrosis. Furthermore, a small molecular inhibitor of SET7/9, sinefungin, also showed decreased H3K4me1 levels as well as suppressed fibrogenesis in vivo and in vitro. In conclusion, we identified inhibition of SET7/9 as a therapeutic target for kidney fibrosis, and suggest that sinefungin may be a candidate therapeutic agent for CKD patients.
Concise Methods
Animals
Male C57BL/6J mice (8 weeks of age) were purchased from Charles River Laboratories Japan (Yokohama, Japan). All animal experiments were approved by the Institutional Animal Care and Use Committee at Hiroshima University (Hiroshima, Japan) and were performed in accordance with the National Institutes of Health Guidelines on the Use of Laboratory Animals. UUO, a well characterized mouse model of renal fibrosis, was performed under general anesthesia as previously described.36 Mice were killed on day1, day 3, or day 7 after UUO and renal tissues were harvested.
Drug and siRNA Administration In Vivo
Mice were administered with neutralizing anti-TGF-β1 antibody (1D11, 1.5 mg/kg; R&D Systems, Minneapolis, MN), or normal mouse IgG1 (11711, 1.5 mg/kg; R&D Systems) immediately after UUO by intraperitoneal injection. The same treatments were repeated every 48 hours until mice were killed as previously described.37 siRNA (In Vivo Pre-designed SET7/9-siRNA and In Vivo Negative Control #1 siRNA; Ambion, Carlsbad, CA) and Invivofectamine 2.0 reagent (Invitrogen, Carlsbad, CA) complex (0.7 mg/mL) was prepared according to the manufacturer’s instructions. Immediately after right ureteral obstruction, 50 μL of SET7/9-siRNA solution (7 mg/kg) was injected retrogradely once into the right kidney via the ureter.38,39 Sinefungin (Sigma-Aldrich, St Louis, MO) was prepared as a suspension in distilled water and 0.9% NaCl solution, and administered intraperitoneally (0.1 mL per mouse) at a dose of 10 mg/kg per day immediately after UUO. The control group was administered an equal volume of vehicle (0.1 mL of distilled water and 0.9% NaCl solution) intraperitoneally. The same treatments were repeated every 24 hours until mice were killed. We selected the dose of sinefungin based on described studies.28,29
Cell Culture
NRK-52E and NRK-49F cells were obtained from the American Type Culture Collection (Manassas, VA). These cells were maintained in DMEM containing 5% FBS and penicillin/streptomycin. All cells were washed and growth was arrested for 24 hours in DMEM containing 0% FBS prior to each stimulation. Preincubation of sinefungin was carried out 60 minutes before TGF-β1 (R&D Systems) stimulation. NRK-52E and NRK-49F cells were treated with TGF-β1 at the indicated dosage levels and times.
siRNA Transfection In Vitro
NRK-52E cells were plated in six-well culture dishes and were transfected 6 hours later (30% confluent) with Smad3 Silencer Select siRNA (si-Smad3, 12.5 nM; Invitrogen) or Silencer Select Negative Control #1 siRNA (si-Neg; Invitrogen) using lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. After 6 hours, the transfected cells were washed, and fresh medium containing 0% FBS was added. The next day, cells were treated with or without TGF-β1, and processed for mRNA or protein extraction at the indicated time periods.
RNA Extraction and Quantitative Real-time RT-PCR
RNA extraction and qRT-PCR were performed as previously described.40 qRT-PCR was performed using an ABI 7500 fast real-time PCR system (Applied Biosystems, Foster City, CA), and expression of genes related to epigenetic modification of UUO mice was compared between the groups. Specific oligonucleotide primers and probes for α-SMA (assay ID: Mm00725412_s1), collagen 1 (assay ID: Mm00801666_g1), collagen 3 (assay ID: Mm01254476_m1), SETDB1 (assay ID: Mm00616964_m1), SETD2 (assay ID: Mm01250225_m1), SETD3 (assay ID: Mm01730314_gH), SETD4 (assay ID: Mm00520991_m1), SETD5 (assay ID: Mm00712606_m1), SETD6 (assay ID: Mm01243947_g1), SET7/9 (assay ID: Mm00499823_m1), SETD8 (assay ID: Mm03031474_g1), and 18S rRNA (endogenous control) were obtained as TaqMan gene expression assays (Applied Biosystems). The mRNA levels were normalized for the level of 18S rRNA.
Western Blot Analysis
Renal tissues or cells grown in six-well dishes were lysed in 2% SDS sample buffer and sonicated for 10–30 seconds using Taitec ultrasonic homogenizer VP-050 at 20% power. Immunoblotting was performed as previously described.41 Primary antibodies used in this study were anti-SET7/9 (Cell Signaling Technology, Danvers, MA), anti-p-Smad3 (Cell Signaling Technology), anti-Smad3 (Cell Signaling Technology), anti-H3K4me1 (Cell Signaling Technology), anti-H3K4me2 (Cell Signaling Technology), anti-H3K4me3 (Cell Signaling Technology), anti-histone H3 (Cell Signaling Technology), anti-α-SMA antibody (Sigma-Aldrich), anti-glyceraldehyde 3-phosphate dehydrogenase (Sigma-Aldrich), anti-β-actin (Sigma-Aldrich), and anti-α-tubulin (Sigma-Aldrich). Secondary antibodies used in this study were horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G antibody (Dako, Glostrup, Denmark) or goat anti-mouse immunoglobulin G antibody (Dako). Signals were detected using the SuperSignal West Dura or Pico system (Thermo Fisher, Rockford, IL). The intensity of each band was determined using ImageJ software (version 1.46r; National Institutes of Health, Bethesda, MD).
Histology and Immunohistochemistry
Histology and immunohistochemical staining were performed as previously described.42 The following primary antibodies were used: mouse monoclonal anti-α-SMA antibody (Sigma-Aldrich), rabbit polyclonal anti-S100A4 (FSP-1) antibody (Abcam, Inc., Cambridge, UK), rabbit polyclonal anti-collagen1 antibody (Abcam, Inc.), rabbit polyclonal anti-collagen3 antibody (Abcam, Inc.), mouse monoclonal anti-fibronectin antibody (Sigma-Aldrich), and rabbit polyclonal anti-SET7/9 antibody (Abcam, Inc.). The signal intensity of FSP-1, collagen1, collagen3, fibronectin, SET7/9, and MT staining were quantified using ImageJ software by examination of predetermined high (×200) or low (×100) power fields of the cortex (five fields) as previously described.37 In the case of human kidney, the study was approved by the Ethics Committee of Hiroshima University (H-895). Information on the patient samples is shown in Supplemental Table 1 and Supplemental Table 2.
ChIP assays
ChIP assays of Col1a1, CTGF, and PAI-1 were performed using a ChIP assay kit (EMD Millipore, Temecula, CA, USA) as previously described.43 DNA samples recovered from NRK-52E cells were subjected to qRT-PCR using gene-specific primers as follows:44 forward 5′-GGCTGGAGAAAGGTGGGTCT-3′ and reverse 5′-CCCAGGTATGCAGGGTAGGA-3′ were used for Col1a1, forward 5′-ATCAGGAAGGGTGCGAAGAG-3′ and reverse 5′-TCCACATTCCTCCGTCTGAA-3′ were used for CTGF, and forward 5′-gacaatATGTGCCCTGTGATTGtC-3′ and reverse 5′-AGGCTGCTCTACTGGTCCTTGC-3′ were used for PAI-1 as described previously.19
Statistical Analyses
Results are expressed as the mean±SD. Statistical analysis was performed using SPSS statistical software (version 22.0; IBM Corporation, Armonk, NY). Comparisons between two groups were analyzed by the t test. For multiple group comparisons, one-way ANOVA followed by Dunnett test or t test with Bonferroni correction were applied. For correlation analysis, we used the Spearman correlation coefficient. P<0.05 was considered to be statistically significant.
Disclosure
None.
Supplementary Material
Acknowledgments
This work was supported by the Ryokufukai Research Support Foundation. This work was partially carried out at the Analysis Center of Life Science, Natural Science Center for Basic Research and Development, Hiroshima University.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014090850/-/DCSupplemental
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Pani A, Bragg-Gresham J, Masala M, Piras D, Atzeni A, Pilia MG, Ferreli L, Balaci L, Curreli N, Delitala A, Loi F, Abecasis GR, Schlessinger D, Cucca F: Prevalence of CKD and its relationship to eGFR-related genetic loci and clinical risk factors in the SardiNIA study cohort. J Am Soc Nephrol 25: 1533–1544, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levey AS, Andreoli SP, DuBose T, Provenzano R, Collins AJ: Chronic kidney disease: common, harmful, and treatable – World Kidney Day 2007. J Am Soc Nephrol 18: 374–378, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Norris KC, Greene T, Kopple J, Lea J, Lewis J, Lipkowitz M, Miller P, Richardson A, Rostand S, Wang X, Appel LJ: Baseline predictors of renal disease progression in the African American Study of Hypertension and Kidney Disease. J Am Soc Nephrol 17: 2928–2936, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Mahmoodi BK, Matsushita K, Woodward M, Blankestijn PJ, Cirillo M, Ohkubo T, Rossing P, Sarnak MJ, Stengel B, Yamagishi K, Yamashita K, Zhang L, Coresh J, de Jong PE, Astor BC, Chronic Kidney Disease Prognosis Consortium : Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without hypertension: a meta-analysis. Lancet 380: 1649–1661, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S, RENAAL Study Investigators : Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Tampe D, Zeisberg M: Potential approaches to reverse or repair renal fibrosis. Nat Rev Nephrol 10: 226–237, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Zeisberg M, Neilson EG: Mechanisms of tubulointerstitial fibrosis. J Am Soc Nephrol 21: 1819–1834, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Liu Y: Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol 7: 684–696, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qi W, Chen X, Poronnik P, Pollock CA: Transforming growth factor-beta/connective tissue growth factor axis in the kidney. Int J Biochem Cell Biol 40: 9–13, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Liu Y: Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int 69: 213–217, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Wynn TA, Ramalingam TR: Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med 18: 1028–1040, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yanagita M: Inhibitors/antagonists of TGF-β system in kidney fibrosis. Nephrol Dial Transplant 27: 3686–3691, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Bonasio R, Tu S, Reinberg D: Molecular signals of epigenetic states. Science 330: 612–616, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Margueron R, Reinberg D: Chromatin structure and the inheritance of epigenetic information. Nat Rev Genet 11: 285–296, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Portela A, Esteller M: Epigenetic modifications and human disease. Nat Biotechnol 28: 1057–1068, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Jenuwein T, Allis CD: Translating the histone code. Science 293: 1074–1080, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Sun G, Reddy MA, Yuan H, Lanting L, Kato M, Natarajan R: Epigenetic histone methylation modulates fibrotic gene expression. J Am Soc Nephrol 21: 2069–2080, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benarroch D, Qiu ZR, Schwer B, Shuman S: Characterization of a mimivirus RNA cap guanine-N2 methyltransferase. RNA 15: 666–674, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonham K, Hemmers S, Lim YH, Hill DM, Finn MG, Mowen KA: Effects of a novel arginine methyltransferase inhibitor on T-helper cell cytokine production. FEBS J 277: 2096–2108, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chevalier RL: Growth factors and apoptosis in neonatal ureteral obstruction. J Am Soc Nephrol 7: 1098–1105, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Miyajima A, Chen J, Lawrence C, Ledbetter S, Soslow RA, Stern J, Jha S, Pigato J, Lemer ML, Poppas DP, Vaughan ED, Jr, Felsen D: Antibody to transforming growth factor-beta ameliorates tubular apoptosis in unilateral ureteral obstruction. Kidney Int 58: 2301–2313, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Shi Y, Massagué J: Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113: 685–700, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Bechtel W, McGoohan S, Zeisberg EM, Müller GA, Kalbacher H, Salant DJ, Müller CA, Kalluri R, Zeisberg M: Methylation determines fibroblast activation and fibrogenesis in the kidney. Nat Med 16: 544–550, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhong X, Chung AC, Chen HY, Meng XM, Lan HY: Smad3-mediated upregulation of miR-21 promotes renal fibrosis. J Am Soc Nephrol 22: 1668–1681, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng D, Yadav N, King RW, Swanson MS, Weinstein EJ, Bedford MT: Small molecule regulators of protein arginine methyltransferases. J Biol Chem 279: 23892–23899, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Bacchi CJ, Berens RL, Nathan HC, Klein RS, Elegbe IA, Rao KV, McCann PP, Marr JJ: Synergism between 9-deazainosine and DL-alpha-difluoromethylornithine in treatment of experimental African trypanosomiasis. Antimicrob Agents Chemother 31: 1406–1413, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nolan LL: Molecular target of the antileishmanial action of sinefungin. Antimicrob Agents Chemother 31: 1542–1548, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shilatifard A: Chromatin modifications by methylation and ubiquitination: implications in the regulation of gene expression. Annu Rev Biochem 75: 243–269, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Hampsey M, Reinberg D: Tails of intrigue: phosphorylation of RNA polymerase II mediates histone methylation. Cell 113: 429–432, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Sheen-Chen SM, Lin CR, Chen KH, Yang CH, Lee CT, Huang HW, Huang CY: Epigenetic histone methylation regulates transforming growth factor β-1 expression following bile duct ligation in rats. J Gastroenterol 49: 1285–1297, 2014 [DOI] [PubMed] [Google Scholar]
- 33.Yaswen L, Kulkarni AB, Fredrickson T, Mittleman B, Schiffman R, Payne S, Longenecker G, Mozes E, Karlsson S: Autoimmune manifestations in the transforming growth factor-beta 1 knockout mouse. Blood 87: 1439–1445, 1996 [PubMed] [Google Scholar]
- 34.Wei G, Wei L, Zhu J, Zang C, Hu-Li J, Yao Z, Cui K, Kanno Y, Roh TY, Watford WT, Schones DE, Peng W, Sun HW, Paul WE, O’Shea JJ, Zhao K: Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity 30: 155–167, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wakabayashi Y, Tamiya T, Takada I, Fukaya T, Sugiyama Y, Inoue N, Kimura A, Morita R, Kashiwagi I, Takimoto T, Nomura M, Yoshimura A: Histone 3 lysine 9 (H3K9) methyltransferase recruitment to the interleukin-2 (IL-2) promoter is a mechanism of suppression of IL-2 transcription by the transforming growth factor-β-Smad pathway. J Biol Chem 286: 35456–35465, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masaki T, Foti R, Hill PA, Ikezumi Y, Atkins RC, Nikolic-Paterson DJ: Activation of the ERK pathway precedes tubular proliferation in the obstructed rat kidney. Kidney Int 63: 1256–1264, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Doi S, Zou Y, Togao O, Pastor JV, John GB, Wang L, Shiizaki K, Gotschall R, Schiavi S, Yorioka N, Takahashi M, Boothman DA, Kuro-o M: Klotho inhibits transforming growth factor-β1 (TGF-β1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem 286: 8655–8665, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kushibiki T, Nagata-Nakajima N, Sugai M, Shimizu A, Tabata Y: Enhanced anti-fibrotic activity of plasmid DNA expressing small interference RNA for TGF-beta type II receptor for a mouse model of obstructive nephropathy by cationized gelatin prepared from different amine compounds. J Control Release 110: 610–617, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Xia Z, Abe K, Furusu A, Miyazaki M, Obata Y, Tabata Y, Koji T, Kohno S: Suppression of renal tubulointerstitial fibrosis by small interfering RNA targeting heat shock protein 47. Am J Nephrol 28: 34–46, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Doi T, Doi S, Nakashima A, Ueno T, Yokoyama Y, Kohno N, Masaki T: Mizoribine ameliorates renal injury and hypertension along with the attenuation of renal caspase-1 expression in aldosterone-salt-treated rats. PLoS ONE 9: e93513, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doi S, Masaki T, Arakawa T, Takahashi S, Kawai T, Nakashima A, Naito T, Kohno N, Yorioka N: Protective effects of peroxisome proliferator-activated receptor γ ligand on apoptosis and hepatocyte growth factor induction in renal ischemia-reperfusion injury. Transplantation 84: 207–213, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Ueno T, Nakashima A, Doi S, Kawamoto T, Honda K, Yokoyama Y, Doi T, Higashi Y, Yorioka N, Kato Y, Kohno N, Masaki T: Mesenchymal stem cells ameliorate experimental peritoneal fibrosis by suppressing inflammation and inhibiting TGF-β1 signaling. Kidney Int 84: 297–307, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakashima A, Kawamoto T, Honda KK, Ueshima T, Noshiro M, Iwata T, Fujimoto K, Kubo H, Honma S, Yorioka N, Kohno N, Kato Y: DEC1 modulates the circadian phase of clock gene expression. Mol Cell Biol 28: 4080–4092, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ohno H, Shinoda K, Ohyama K, Sharp LZ, Kajimura S: EHMT1 controls brown adipose cell fate and thermogenesis through the PRDM16 complex. Nature 504: 163–167, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.