Abstract
FSGS is the most common primary glomerular disease underlying ESRD in the United States and is increasing in incidence globally. FSGS results from podocyte injury, yet the mechanistic details of disease pathogenesis remain unclear. This has resulted in an unmet clinical need for cell-specific therapy in the treatment of FSGS and other proteinuric kidney diseases. We previously identified Yes-associated protein (YAP) as a prosurvival signaling molecule, the in vitro silencing of which increases podocyte susceptibility to apoptotic stimulus. YAP is a potent oncogene that is a prominent target for chemotherapeutic drug development. In this study, we tested the hypothesis that podocyte-specific deletion of Yap leads to proteinuric kidney disease through increased podocyte apoptosis. Yap was selectively silenced in podocytes using Cre-mediated recombination controlled by the podocin promoter. Yap silencing in podocytes resulted in podocyte apoptosis, podocyte depletion, proteinuria, and an increase in serum creatinine. Histologically, features characteristic of FSGS, including mesangial sclerosis, podocyte foot process effacement, tubular atrophy, interstitial fibrosis, and casts, were observed. In human primary FSGS, we noted reduced glomerular expression of YAP. Taken together, these results suggest a role for YAP as a physiologic antagonist of podocyte apoptosis, the signaling of which is essential for maintaining the integrity of the glomerular filtration barrier. These data suggest potential nephrotoxicity with strategies directed toward inhibition of YAP function. Further studies should evaluate the role of YAP in proteinuric glomerular disease pathogenesis and its potential utility as a therapeutic target.
Keywords: focal segmental glomerulosclerosis, glomerular disease, podocyte
FSGS is increasing in incidence globally for unclear reasons and is now the most common primary glomerular disease in the United States.1–8 This heterogeneous disorder results directly from podocyte depletion below a critical threshold number. Podocytes do not regenerate in response to injury and loss. In experimental models, a reduction in podocyte number by 20% causes FSGS and low-level sustained proteinuria, while a 40% reduction leads to high-level sustained proteinuria and a decline in renal function.9 Besides FSGS, podocyte depletion has been associated with diabetic and IgA nephropathy.10–13 Unfortunately, significant gaps remain despite advances in podocyte cell biology and it is unclear how podocytes undergo injury and loss at the molecular level. Cell-specific therapy is unavailable, resulting in an unmet clinical need. This is particularly concerning because proteinuria is an independent risk factor for cardiovascular disease.
Yes-associated protein (YAP) is a key effector target of the Hippo signaling pathway, a highly conserved kinase cascade that regulates organ size and survival.14 Tissue-specific overexpression of YAP in hepatocytes results in hepatomegaly and Yap deletion impairs intestinal regeneration.15,16 YAP is a potent oncogene whose expression and activation is associated with several solid malignancies,17 making it an attractive target for chemotherapeutic drug development.18 YAP is a proline-rich, SH3 and 14–3-3 binding domain–containing transcriptional coactivator.19 It has conserved serine-phosphorylation domains that mediate its subcellular shuttling from the cytoplasm to the cell nucleus, where it acts as a transcriptional coactivator to promote cell survival and differentiation.20 When YAP is phosphorylated at the Ser127 site by Hippo kinases acting downstream of kidney and brain expressed protein, it is inactively sequestered in the cytoplasm in a complex with 14–3-3.21 Conversely, dephosphorylated YAP relocates to the nucleus where it becomes functionally active, interacting with a number of transcription factors (e.g., RUNX, Pax3, PPARγ, Smad2/3/4) that mediate cellular development, differentiation and survival.20
We previously identified YAP as a prosurvival signaling molecule in podocytes that inhibits dendrin-mediated apoptosis in vitro.22 Under normal physiologic conditions, YAP is highly expressed in podocyte nuclei and the silencing of Yap in podocytes makes them more susceptible to staurosporine- and adriamycin-induced apoptosis. Specifically, Yap knockdown is associated with enhanced caspase 3/7 activity.22 In our current study, we explored the physiologic role of YAP in podocytes in vivo. We generated podocyte-specific Yap knockout mice that developed proteinuria and glomerular lesions consistent with FSGS including podocyte apoptosis, depletion, and progressive renal failure. Our findings implicate YAP as a physiologic antagonist of podocyte apoptosis whose function is essential to maintaining the integrity of the kidney filter and suggest a potential role for YAP activation as a therapeutic strategy in proteinuric kidney disease.
Results
Generation of Podocyte-Specific Yap Knockout Mice
We previously demonstrated that silencing of the Yap gene in vitro increases podocyte susceptibility to apoptotic stimulus.22 We sought to explore whether Yap deletion would make podocytes more susceptible to injury in vivo. Because constitutive Yap deletion is embryonic lethal,23 we generated podocyte-specific Yap-knockout (Yap-KO) mice by conditional targeting of exon 2 of the Yap allele, as previously described.24 This strategy targets both YAP1–1 and YAP1–2 splice isoforms of the Yap gene. Yap was deleted by Cre recombinase under the control of the podocyte-specific podocin promoter (Supplemental Figure 1A). PodCre+Yapfl/fl mice were born in the same Mendelian ratio as PodCre-Yapfl/fl littermate controls with genotyping confirmed by PCR on tail DNA extracts (Supplemental Figure 1B).
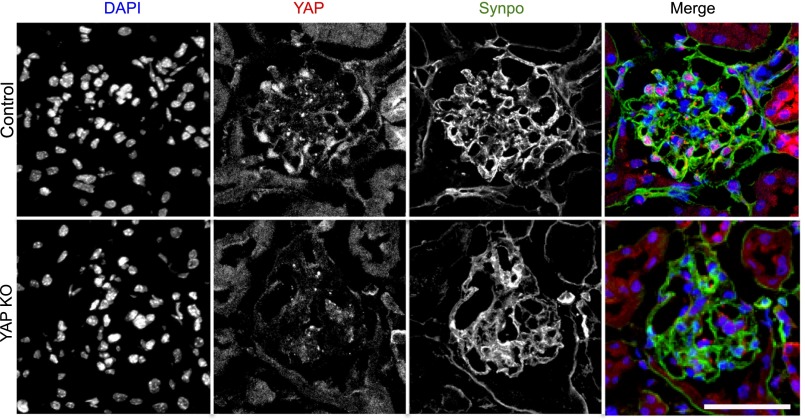
Yap silencing was confirmed by immunofluorescence labeling of paraformaldehyde (PFA)-perfused kidneys (Figure 1). Consistent with our previously published results,22 we detected YAP expression in both the nucleus and cytoplasm of podocytes in control (PodCre-Yapfl/fl) mice. In the cytoplasm, YAP colocalized with the podocyte marker synaptopodin (Figure 1A, upper panels). YAP expression was not detected in the glomeruli of Yap-KO (PodCre+Yapfl/fl) mice, although synaptopodin was still detected, albeit with reduced expression (Figure 1A, lower panels).
Figure 1.
Immunofluorescence labeling of YAP in glomeruli. Upper panels: YAP (red) colocalizes with the podocyte-specific marker synaptopodin (green) with additional nuclear expression colocalizing with DAPI (blue) detected. Lower panels: confirmation of reduction in YAP protein expression with podocyte-specific gene silencing. Scale bar, 50 µm.
Podocyte-Specific Yap Deletion Leads to Proteinuria and Renal Dysfunction
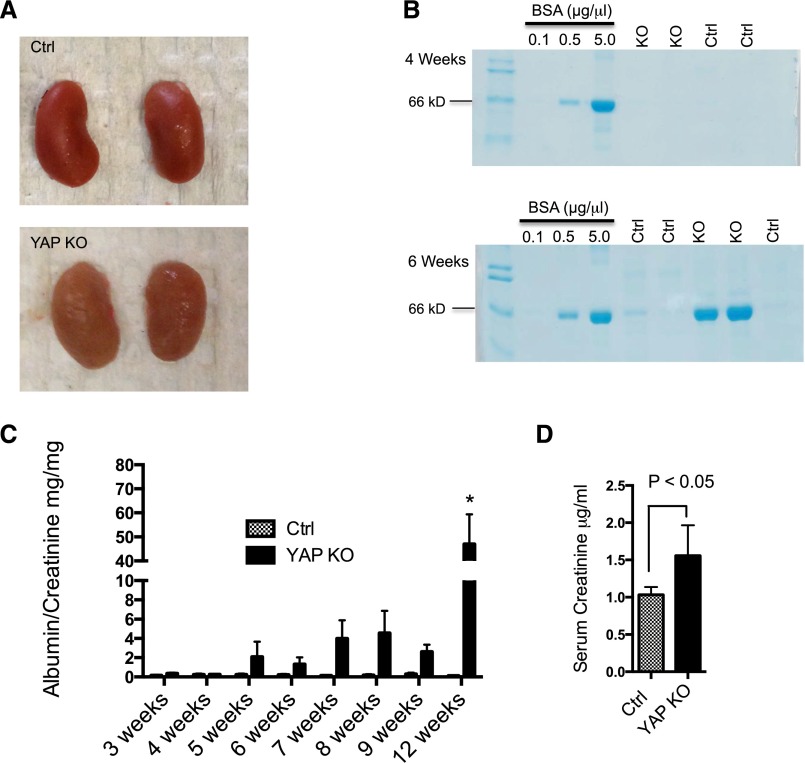
We examined the gross appearance of control and experimental kidneys and found that kidneys from Yap-KO mice had a corrugated capsule at 12 weeks (Figure 2A). There was no significant difference in kidney size or kidney weight between experimental and control mice. We collected spot urine from control and experimental mice weekly beginning at age 3 weeks after initial qualitative screening for proteinuria with Coomassie-blue–stained SDS-PAGE gels. No proteinuria was detected in either group up to 4 weeks, but Yap-KO mice developed proteinuria between 5 and 6 weeks of age (Figure 2B). Urine albumin-creatinine ratio (ACR) was performed on spot urine samples to quantify proteinuria up to 12 weeks. Between weeks 5 and 9, Yap-KO mice had more proteinuria than the controls, but due to variability in the magnitude, the difference was not statistically significant until week 12 (mean ACR in Yap-KO mice 47.04±12.35 versus 0.11±0.01 mg/mg for control mice, P<0.01; Figure 2C). Renal function was determined by measuring serum creatinine by liquid chromatography–tandem mass spectrometry. At 12 weeks, Yap-KO mice had a significantly greater mean serum creatinine (1.56±0.4 μg/ml) than their control littermates (1.03±0.25 μg/ml, P<0.02; Figure 2D). Taken together, these findings suggest that Yap deletion in podocytes results in impairment in renal function associated with the development of proteinuria.
Figure 2.
Kidney appearance and function with Yap gene silencing. (A) Yap-KO kidneys are of comparable size to controls (Ctrl), but exhibit a corrugated capsule at 12 weeks of age. (B) SDS-PAGE Coomassie-blue staining of standard BSA (numbers indicate μg/μl) and urine from Yap-KO and control mice. Proteinuria first appears between 5 and 6 weeks of age in Yap-KO mice. In each respective lane, 5 μl of urine and 1 μl of BSA standard were loaded. (C) Albumin-creatinine ratios demonstrating weekly progression of proteinuria in Yap-KO and control mice. At week 12, P<0.01. (D) Serum creatinine measurements showing a decrease in kidney function in Yap-KO mice. Ctrl, Pod-Cre-Yapflox/flox.
Podocyte-Specific Yap Deletion Leads to FSGS
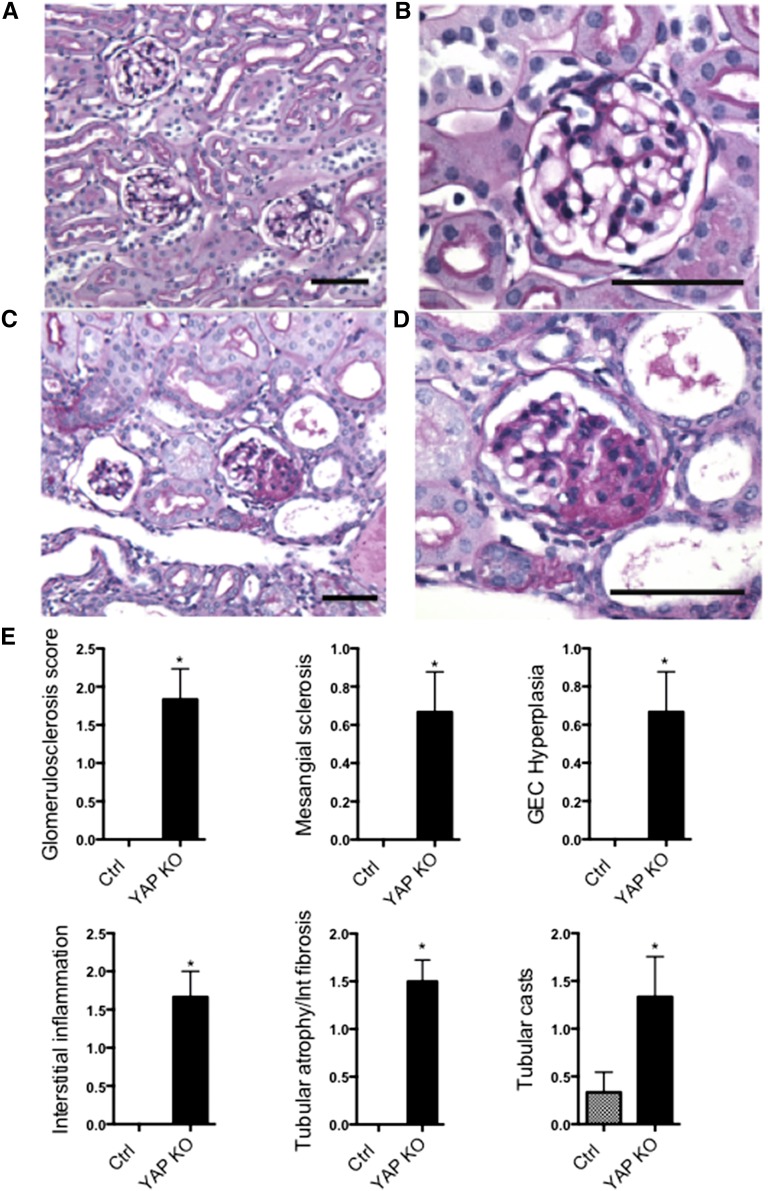
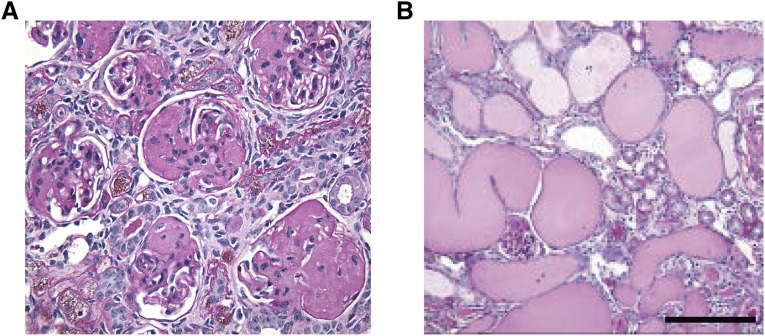
Yap-KO mice did not have glomerular or tubulointerstitial abnormalities at birth (Supplemental Figure 2), but developed progressive disease correlating with the development of proteinuria. These changes were best illustrated and quantified at the 12-week time point (Figure 3), when there was no evidence of glomerular scarring or tubular injury in control mice (Figure 3, A and B). Conversely, Yap-KO mice displayed FSGS and glomerular epithelial cell hyperplasia (Figure 3, C and D). Associated pathologic abnormalities in Yap-KO mice included tubular atrophy and interstitial fibrosis, interstitial inflammation and the development of tubular casts. Pathologic scoring performed by a blinded independent renal pathologist (V.D.) confirmed that these changes were significant compared to control mice (Figure 3E). Yap-KO mice had a median survival of 25.9 weeks and at 32 weeks showed characteristics of ESRD including global glomerulosclerosis (Figure 4A) and extensive proteinaceous casts (Figure 4B).
Figure 3.
PAS stainings of Yap-KO and control (Ctrl) mice at 12 weeks. (A,B) Ctrl kidney sections at low and high power. (C) Yap-KO mice with focal glomerulosclerosis and tubular dilatation and casts. (D) Yap-KO mouse with a lesion of focal segmental glomerulosclerosis. (E) Quantification of glomerular and tubular injury in 12-week-old mice. Scale bars, (A) and (C) 70 µm; (B) and (D) 50 µm.
Figure 4.
Severe kidney disease in aged Yap-KO mice. (A) 32-week-old Yap-KO mouse PAS section with segmentally and globally sclerosed glomeruli. (B) 32-week-old Yap-KO mouse PAS section with diffuse tubular dilatation and proteinaceous casts. Scale bars, 50 µm.
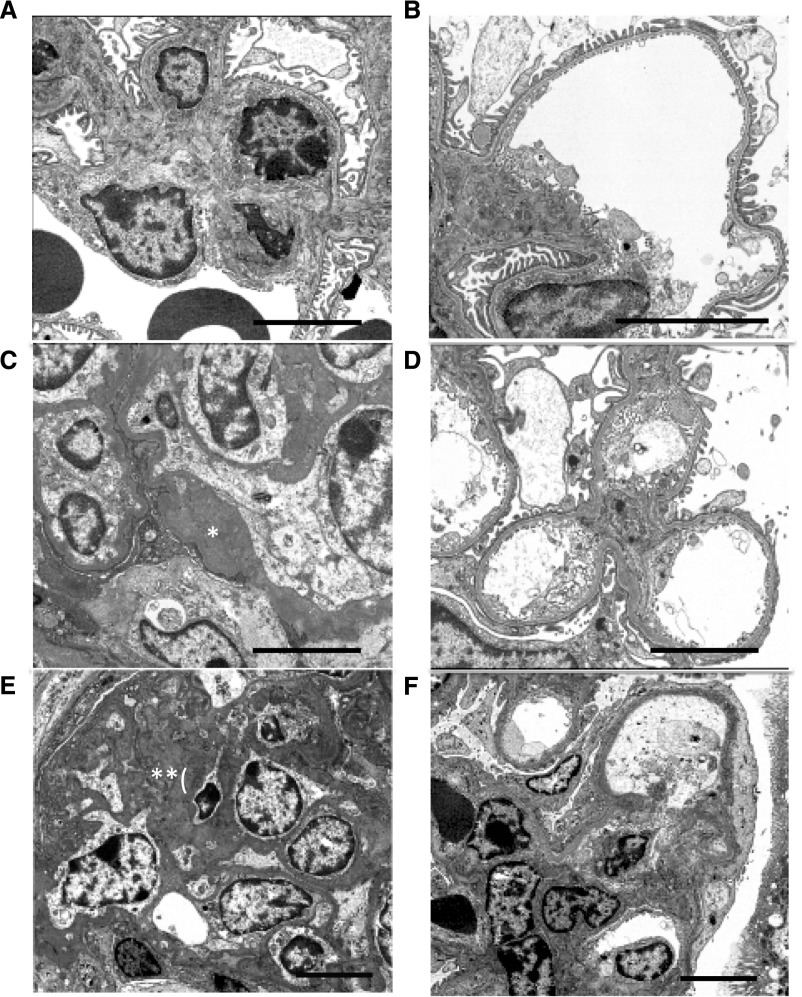
By electron microscopy we observed normal-appearing mesangial regions and intact podocyte foot processes in control mice (Figure 5, A and B). Yap-KO mice displayed variable severity of FSGS at the same 12-week time point. Representative early changes included moderate mesangial matrix accumulation, FSGS and patchy podocyte foot process effacement (Figure 5, C and D). Some animals in this group had more severe disease including severe mesangial matrix expansion, more extensive FSGS, and diffuse podocyte foot process effacement (Figures 5, E and F). Taken together, these findings confirmed that Yap silencing in podocytes was sufficient for the development of FSGS.
Figure 5.
Electron microscopy images of Yap-KO mice. (A) Control with normal-appearing mesangial matrix. (B) Control with intact podocyte foot processes. (C) Yap-KO with moderate mesangial matrix accumulation (*). (D) Yap-KO with patchy foot process effacement. (E) Yap-KO with extensive FSGS with severe mesangial matrix expansion (**). (F) Yap-KO with diffuse podocyte foot process effacement. Scale bars, 3 µm.
YAP is a Physiologic Inhibitor of Podocyte Apoptosis
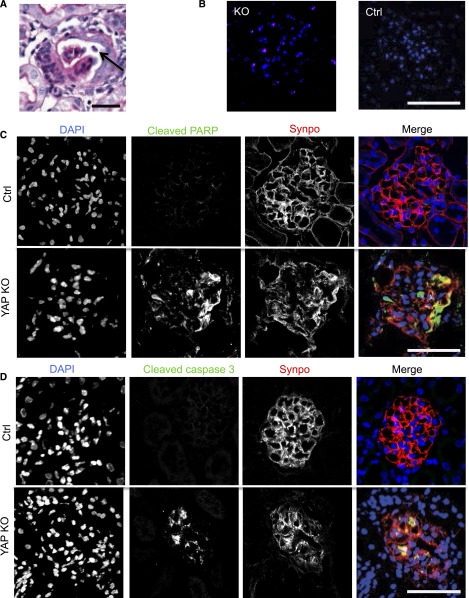
Because YAP expression suppresses apoptosis in hepatocytes15 and makes podocytes less susceptible to apoptosis,22 we hypothesized that podocytes in Yap-KO mice were undergoing apoptosis. At 12 weeks, by light microscopy of periodic acid–Schiff (PAS)-stained glomeruli, we detected focally detached cells with karyorrhectic nuclei shed into the urinary space in the podocyte-specific Yap-KO mice (Figure 6A). These findings were not detected in the control mice. Terminal deoxynucleotidyl transferase–mediated 2′-deoxyuridine 5′-triphosphate nick-end labeling–positive cells were also detected in the glomeruli of Yap-KO but not control mice (Figure 6B). We identified the cells undergoing apoptosis as podocytes by the expression and partial colocalization of cleaved poly adenosine diphosphate ribose polymerase with synaptopodin (Figure 6C). Earlier in the course of disease, at 7.5 weeks, we also detected cleaved caspase 3 colocalizing with synaptopodin in Yap-KO but not control mice (Figure 6D). These findings demonstrate that podocyte-specific Yap-KO mice develop podocyte apoptosis.
Figure 6.
Podocyte apoptosis in Yap-KO mice. (A) PAS staining with apoptotic cell nucleus (arrow) in Yap-KO mouse at 12 weeks. (B) Terminal deoxynucleotidyl transferase–mediated 2′-deoxyuridine 5′-triphosphate nick-end labeling–positive nuclei in Yap-KO but not control glomeruli at 12 weeks. (C) Anti-cleaved poly adenosine diphosphate ribose polymerase (PARP) immunofluorescence staining in control and Yap-KO mice at 12 weeks. (D) Cleaved caspase 3 staining in control and Yap-KO mice at 7.5 weeks. Scale bars, (A) 20 µm; (B-D) 50 µm.
Yap Deletion Causes Podocyte Depletion
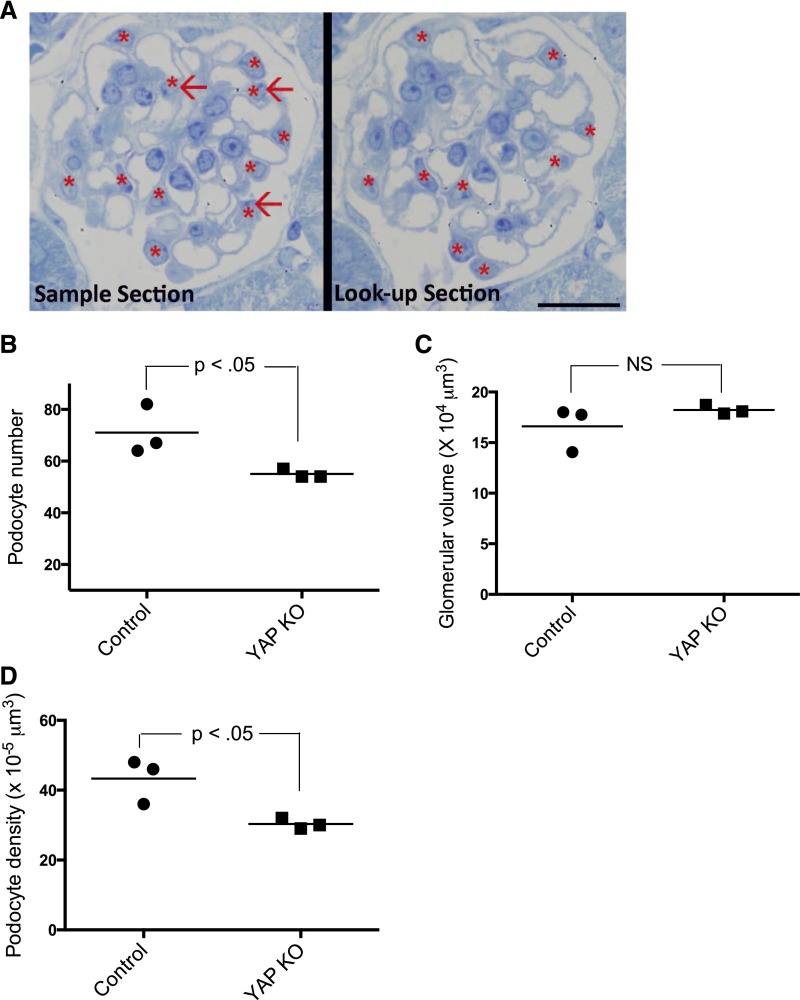
Because we observed podocyte apoptosis in Yap-KO mice at 12 weeks (Figure 6), we performed a design-based quantification of podocyte number and podocyte density in our experimental animals. Podocyte number was determined using the disector/fractionator principle where nuclei present in the first section of each disector pair but not present in the second section were counted (Figure 7A).25,26 The number of podocytes per glomerulus in each mouse (n=3) was significantly reduced in Yap-KO mice compared with control littermates (55±1.7 versus 71±9.6, P<0.05) (Figure 7B). Glomerular volume was not different between Yap-KO and control mice (16.6±2.2 versus 18.2±0.4×104 μm3, P=0.28) (Figure 7C). A reduction in podocyte density is an accurate predictor of glomerular disease progression.27 The reduction in podocyte number led to the podocyte density in Yap-KO mice being significantly lower than that of control littermates (43±6.4 versus 30±1.5×10−5 µm3, P<0.05) (Figure 7D). Taken together, these findings demonstrate that podocyte-specific deletion of YAP directly results in podocyte depletion.
Figure 7.
Morphometric measurements. (A) Toluidine-blue staining showing quantification of podocyte number in adjacent epon sections 1-µm thick. Only podocyte nuclei (asterisk) present in the sample section but not present in the look-up section (red arrows) were counted. Scale bar: 20 µm. (B) Podocyte number was significantly reduced in Yap-KO mice at 12 weeks compared with the control. (C) Glomerular volumes were not significantly increased in Yap-KO compared with control (P=NS). (D) Podocyte density was reduced in Yap-KO mice compared with controls (n=3 mice, 30 total glomeruli per group).
YAP Expression in Human Glomeruli
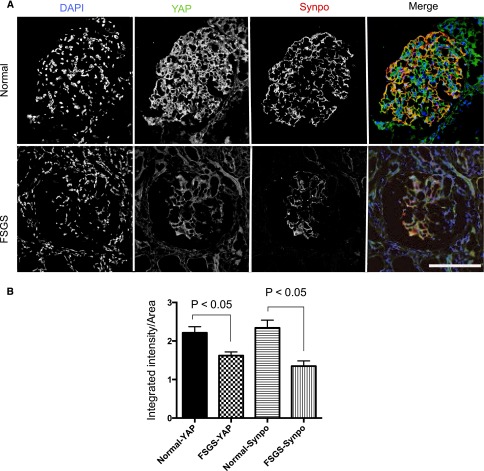
Because the histologic features in our model of Yap silencing in podocytes were similar to the phenotype in human FSGS, we determined the expression of YAP in human glomeruli under normal and FSGS conditions. In human subjects (n=6) with normal glomeruli, YAP displayed a similar expression pattern to that seen in mice with cytoplasmic colocalization with synaptopodin and additional nuclear expression consistent with its canonical dual compartment properties (Figure 8A). In human primary FSGS (n=7 patients), YAP expression was reduced in both sclerotic and nonsclerotic areas, again in a distribution similar to that of synaptopodin (Figure 8A). Quantification of fluorescence intensity using Metamorph software as previously described28 confirmed the reduction of both YAP (1.36 fold, P<0.004) and synaptopodin expression (1.73-fold, P<0.004) in human FSGS (Figure 8B).
Figure 8.
Expression of YAP in human glomeruli. (A) YAP expression in normal glomeruli compared with a patient with FSGS. Scale bar, 50 µm. (B) Quantification of fluorescence intensity.
Discussion
In this study, we demonstrate the essential role of YAP as a physiologic antagonist of podocyte apoptosis. We show that podocyte-specific deletion of Yap is sufficient to cause proteinuria, podocyte foot process effacement, podocyte depletion, and ultimately ESRD. Histologically, Yap deletion resulted in classic FSGS lesions on light and electron microscopy. These results are consistent with our previously reported characterization of YAP as an antiapoptotic signaling molecule whose silencing in vitro makes cultured podocytes more susceptible to apoptotic stimulus.22 Our current findings are the first demonstration of the in vivo relevance of YAP signaling to chronic glomerular disease and serve as the basis for further clinical exploration of the Hippo-YAP signaling axis.
YAP signaling has previously been explored in kidney tubules, particularly related to cyst formation.29 In autosomal polycystic kidney disease, YAP dephosphorylation and nuclear localization have been detected in cystic epithelium and dilated tubules of conditional PKD1 null mice.29 This suggests a possible role for YAP in pathogenic cyst growth and underscores the role of YAP as a progrowth molecule. Interestingly, deletion of Taz, a close homolog of Yap, results in renal cystic disease.30,31 The phenotype associated with tubular specific deletion of Yap has not been described, but available data thus far suggest a complex interplay between Taz and Yap to maintain cellular homeostasis. The distinct functions of YAP and TAZ during development was illustrated recently by our group (A.R. and H.M.).24 It was shown that deletion of Taz in Six2 progenitor cells leads to tubular cysts, while Six2-Cre–mediated Yap deletion results in impaired nephron development.24 The latter finding stands in contrast to our results where podocin-Cre–mediated Yap deletion, occurring later in development, did not impair nephrogenesis but resulted in chronic proteinuric kidney disease in adult mice. These findings are not contradictory because, unlike podocin, the Six2 promoter drives expression in all cells that are derived from the cap mesenchyme. Future studies will also be necessary to determine the expression level and role of TAZ in podocytes.
Canonically, YAP is the target effector of Hippo kinases Mst and Lats that promote its phosphorylation and cytoplasmic sequestration.21 YAP becomes active in the dephosphoryated form where it translocates to the nucleus and activates transcription factors that promote cell survival. We recently demonstrated that YAP is highly expressed in podocyte nuclei under baseline conditions.22 That suggested a potential role for YAP as a physiologic antagonist of podocyte apoptosis, a theory further supported by our current results. Further studies will be needed to determine the correlation between YAP phosphorylation, its nuclear-cytoplasmic localization and antiapoptotic function in podocytes. Additionally, because YAP promotes podocyte survival in vitro by inhibiting the pro-apoptotic function of dendrin,22 another dual compartment shuttling molecule,32,33 the mechanical basis for interaction between the two molecules under normal and disease conditions will need to be determined.
YAP transcriptional activity is enhanced in a number of solid malignancies, making it an attractive target for drug development. Our findings suggest potential nephrotoxicity specifically related to podocyte injury with potential agents that inhibit YAP function. Glomerular injury associated with chemotherapy has been demonstrated with bevacizumab, where targeting vascular endothelial growth factor production from podocytes leads to endothelial injury and thrombotic microangiopathy.34,35 Caution from a renal perspective will therefore be needed in the development of agents that target the YAP oncogene.
Podocyte apoptosis has been previously described during the course of glomerular disease progression,36–39 but to our knowledge this is the first demonstration that podocyte-specific silencing of a gene encoding an antiapoptotic protein results in FSGS and ESRD. Podocytes undergoing apoptosis had reduced synaptopodin expression, indicating possible dedifferentiation during injury and detachment into the urinary space. Our quantification of podocyte number with the unbiased disector/fractionator method in 12-week-old mice showed that a 22.5% reduction in podocyte number correlated with the phenotype of proteinuria, an elevated creatinine and the histologic appearance of FSGS. This is consistent with previous reports of a 20% podocyte loss threshold being sufficient to cause FSGS,9 and further highlights the essential role of podocyte homeostasis in preventing progressive glomerulosclerosis.
We have described the expression pattern of YAP in human glomeruli where it colocalizes with the podocyte marker synaptopodin. While the expression of synaptopodin is understandably absent in the sclerotic areas of glomeruli of patients with FSGS, it is also reduced in nonsclerotic areas, with lower levels associated with reduced steroid-responsiveness.40,41 Here we show that like synaptopodin, YAP expression is also reduced in primary FSGS, although to a slightly smaller degree. These findings do not prove that a reduction in YAP expression causes podocyte apoptosis and depletion in humans. The reduction in overall 4′,6-diamidino-2-phenylindole (DAPI)-positive cell number by alternate mechanisms of podocyte loss in FSGS (as shown in Figure 8A) could in theory result indirectly in reduced YAP and synaptopodin (synpo) expression. Further studies will be needed to determine the role of YAP in pathogenesis, clinical outcomes, and response to therapy in human FSGS and other proteinuric kidney disorders.
In summary, our study has shown that YAP serves as a physiologic antagonist of podocyte apoptosis and is essential for the maintenance of an intact glomerular filter. Future studies will be required to fully elucidate the role of YAP signaling in the pathogenesis of glomerular disease and its utility as a therapeutic target. These findings also raise the concern for nephrotoxicity with current strategies in development for YAP inhibition in cancer treatment.
Concise Methods
Creation and Genotyping of Podocyte-Specific Yap-KO Mice
For selective deletion of Yap in glomerular podocytes, Yapflox/flox mice24 were crossed with Podocin-Cre mice (The Jackson laboratory) to generate podocyte-specific Yap-KO mice. Tail or ear genotyping was performed by PCR according to previously described protocols. PCR primers included: cre Forward: CCATCTGCCACCAGCCAG, cre Reverse: TCGCCATCTTCCAGCAGG, Yap Forward: CACCCAGCTATTATAAGTGTGTG, Yap Reverse: TGTAAACAAATGTGATAAGTCA-GCC. Podocin-positive Yap-KO (homozygous for the floxed Yap allele with Podocin-Cre) and Podocin-negative Yap-KO controls (homozygous for the floxed Yap allele without Podocin-Cre) were used in these experiments.
Mice Perfusion, Kidney Histology, Immunofluorescence Staining of Kidney Tissues and Electron Microscopic Imaging of Kidney Tissues
To harvest the kidneys, mice were anesthetized first with isoflurane, and then injected with ketamine/xylazine. Kidneys were fixed by perfusion through the heart with 50 ml of a filtered 3% PFA solution in PBS at room temperature. Kidneys were harvested following perfusion, sectioned transversely into thirds and stored in solutions of 18% sucrose, 2.5% glutaraldehyde or 3% PFA for further processing for immunofluorescence, electron microscopy, and light microscopy, respectively. Fixed sections stored in sucrose overnight were frozen in a liquid nitrogen bath with an O.C.T. Compound (Tissue-Tek). All other sections were stored until they became saturated with solution. Sections from the 3% PFA solution were sent to the Histo-Immunology Core Laboratory at Icahn School of Medicine at Mount Sinai for PAS staining. Glutaraldehyde-fixed tissues were sent to the Morphometry and Stereology Laboratory at Charles R. Drew University for morphometric analysis. Electron microscopic grids were prepared and electron microscopy was performed at the Columbia Renal Pathology Laboratory using a JEOL 1011 electron microscope.
Pathologic Scoring
For quantitative analysis of kidney pathology, 200 glomeruli per sample were assessed on PAS-stained sections with semiquantitative scoring for glomerulosclerosis (segmental and global) performed as follows: 0, no sclerosis; 1, 1–10% of glomeruli sclerosed; 2, 11–25% of glomeruli sclerosed; 3, 26–50% of glomeruli sclerosed; 4, >50% of glomeruli sclerosed. A similar system was used to quantify percent cortical parenchyma with interstitial inflammation, percent cortical parenchyma with tubular atrophy/interstitial fibrosis, and percent cortical parenchyma with tubular casts. The degree of glomerular epithelial cell hyperplasia and mesangial sclerosis were also quantified blindly on a 0–3+ scale.
Proteinuria and Renal Function Measurements
Albuminuria was qualitatively screened using a 10% SDS-PAGE gel followed by Coomassie-blue staining. BSA standards of 0.1, 0.5, 5 μg were used and 5 μl urine was used from each sample to quantify urine albumin in duplicate using commercial kits, according to the manufacturer’s protocol (mouse albumin ELISA, Bethyl Laboratories). Urine creatinine was measured in duplicate using commercial kits according to the manufacturer’s protocol (Creatinine Urinary Colorimetric Assay Kit, Cayman Chemicals). Serum creatinine measurements were performed by liquid chromatography-tandem mass spectrometry at the UAB-UCSD O’Brien Core in Alabama.
Antibodies
Antibodies used in this study were as follows: mouse anti-synaptopodin (courtesy Dr. P. Mundel), Rabbit anti-YAP (Novus Nb11058358), cleaved poly adenosine diphosphate ribose polymerase p25 (Abcam, Inc.), cleaved caspase 3 (Abcam, Inc.), Alexa Fluor 488 goat anti-rabbit IgG (Life Technologies), Alexa Fluor 594 goat anti-mouse IgG (Life Technologies).
Terminal Deoxynucleotidyl Transferase–Mediated 2′-Deoxyuridine 5′-Triphosphate Nick-End Labeling Measurement of Apoptosis
For immunofluorescent labeling, mouse kidney sections were washed once with PBS and incubated with blocking solution (2% FCS, 2% BSA, 0.2% fish gelatin) for 30 min at room temperature before incubation with a mouse monoclonal anti-synaptopodin for 1 h at room temperature. After three washes with PBS for 5 min each, antigen-antibody complexes were visualized with an Alexa 488 species-specific secondary antibody (Life Technologies). Subsequently, detection of DNA fragmentation was done using the In Situ Cell Death detection kit, TMR red (Roche Diagnostics) according to the manufacturer’s protocol. Sections were also stained with DAPI (MP Biomedicals) and Sudan Black B (Sigma-Aldrich).
Morphometry
Some glutaraldehyde-fixed tissues were shipped at room temperature to the Morphometry and Stereology Laboratory at Charles R. Drew University, Los Angeles, where 1 mm cubes of cortex were embedded in epoxy. Sections 1 µm thick were cut using an ultramicrotome fitted with a Histo Jumbo diamond knife (Diatome US, Hatfield, PA). Serial sections were cut and every tenth section and the adjacent section were saved to a microscope slide and stained with toluidine blue.42 A total of 20 pairs of sections were saved per kidney. Images were obtained using the ×100 objective lens (NA=1.40) on an Olympus BX51 microscope fitted with a DP71 digital camera and DP Controller software (Olympus America, Cypress, CA). Images were observed using Photoshop (Adobe Systems, Inc., San Jose, CA) on a 24-inch monitor at a window magnification of 100%. Ten glomeruli per kidney were analyzed for each morphometric parameter. Podocytes were counted using the disector/fractionator principle. Briefly, images of adjacent sections from throughout a glomerulus were observed and the number of profiles from podocyte nuclei present in the first section of a disector pair but not present in the second section was counted. The number of podocyte nuclei was used as a surrogate for the number of podocytes. Glomerular volume was measured using the Cavalieri principle.43 Podocyte numerical density was calculated by dividing podocyte number by glomerular volume and expressed as number per cubic micrometer.
Fluorescence Intensity Measurements
A fixed exposure time for each channel was set based on the most strongly fluorescent glomerulus prior to image acquisition. Using Metamorph software, after subtracting background, intensity values were calculated by dividing the sum of all intensity values for all pixels in the selected glomerular region (integrated) by the area of the region with pixel intensities within the thresholding region. All glomeruli visualized were included in the analysis.
Statistical Analysis
Results are presented as mean±SD. Unpaired t tests were used for comparison between groups where P<0.05 was considered statistically significant.
Study Approval
All mouse studies were approved by IACUC at the Icahn School of Medicine at Mount Sinai. Frozen sections from archived human biopsy material were obtained from Columbia University College of Physicians and Surgeons under an Institutional Review Board approved protocol entitled “Research with archival human renal biopsy tissues”. All biopsies were clinically indicated, and only extra tissue not required for diagnostic purposes was permitted for research use; therefore, no patient-informed consent was required.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by NIH grant DK081617 and an American Society of Nephrology Gottschalk Award to K.N.C., and an American Society of Nephrology Student Scholar Grant to M.S. Morphometric studies were supported by a grant from the NIMHD (Grant# U54-MD007598) to J.M.B. and S.B.N. Confocal imaging and Metamorph analysis was performed at the Microscopy Shared Resource Facility at the Icahn School of Medicine at Mount Sinai.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014090916/-/DCSupplemental.
References
- 1.Alhassan A, Mohamed WZ, Alhaymed M: Patterns of childhood nephrotic syndrome in Aljouf region, Saudi Arabia. Saudi J Kidney Dis Transpl 24: 1050–1054, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Bonilla-Felix M, Parra C, Dajani T, Ferris M, Swinford RD, Portman RJ, Verani R: Changing patterns in the histopathology of idiopathic nephrotic syndrome in children. Kidney Int 55: 1885–1890, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Braden GL, Mulhern JG, O’Shea MH, Nash SV, Ucci AA, Jr, Germain MJ: Changing incidence of glomerular diseases in adults. Am J Kidney Dis 35: 878–883, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Gulati S, Sharma AP, Sharma RK, Gupta A: Changing trends of histopathology in childhood nephrotic syndrome. Am J Kidney Dis 34: 646–650, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Gulati S, Sural S, Sharma RK, Gupta A, Gupta RK: Spectrum of adolescent-onset nephrotic syndrome in Indian children. Pediatr Nephrol 16: 1045–1048, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Haas M, Spargo BH, Coventry S: Increasing incidence of focal-segmental glomerulosclerosis among adult nephropathies: a 20-year renal biopsy study. Am J Kidney Dis 26: 740–750, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Hoseini R, Otukesh H, Fereshtehnejad SM, Tahoori A, Hooman N, Rahimzadeh N, Sadr S: Prevalence and outcome of focal segmental glomerulosclerosis in Iranian children with nephrotic syndrome. Iran J Kidney Dis 6: 18–24, 2012 [PubMed] [Google Scholar]
- 8.Kitiyakara C, Kopp JB, Eggers P: Trends in the epidemiology of focal segmental glomerulosclerosis. Semin Nephrol 23: 172–182, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, Saunders TL, Dysko RC, Kohno K, Holzman LB, Wiggins RC: Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol 16: 2941–2952, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, Coplon NS, Sun L, Meyer TW: Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest 99: 342–348, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steffes MW, Schmidt D, McCrery R, Basgen JM, International Diabetic Nephropathy Study Group : Glomerular cell number in normal subjects and in type 1 diabetic patients. Kidney Int 59: 2104–2113, 2001 [DOI] [PubMed] [Google Scholar]
- 12.White KE, Bilous RW, Marshall SM, El Nahas M, Remuzzi G, Piras G, De Cosmo S, Viberti G: Podocyte number in normotensive type 1 diabetic patients with albuminuria. Diabetes 51: 3083–3089, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Lemley KV, Lafayette RA, Safai M, Derby G, Blouch K, Squarer A, Myers BD: Podocytopenia and disease severity in IgA nephropathy. Kidney Int 61: 1475–1485, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Huang J, Wu S, Barrera J, Matthews K, Pan D: The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell 122: 421–434, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, Anders RA, Maitra A, Pan D: Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 130: 1120–1133, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai J, Zhang N, Zheng Y, de Wilde RF, Maitra A, Pan D: The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes Dev 24: 2383–2388, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson R, Halder G: The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat Rev Drug Discov 13: 63–79, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sudol M, Shields DC, Farooq A: Structures of YAP protein domains reveal promising targets for development of new cancer drugs. Semin Cell Dev Biol 23: 827–833, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basu S, Totty NF, Irwin MS, Sudol M, Downward J: Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Mol Cell 11: 11–23, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Wang K, Degerny C, Xu M, Yang XJ: YAP, TAZ, and Yorkie: a conserved family of signal-responsive transcriptional coregulators in animal development and human disease. Biochem Cell Biol 87: 77–91, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Oka T, Mazack V, Sudol M: Mst2 and Lats kinases regulate apoptotic function of Yes kinase-associated protein (YAP). J Biol Chem 283: 27534–27546, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Campbell KN, Wong JS, Gupta R, Asanuma K, Sudol M, He JC, Mundel P: Yes-associated protein (YAP) promotes cell survival by inhibiting proapoptotic dendrin signaling. J Biol Chem 288: 17057–17062, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morin-Kensicki EM, Boone BN, Howell M, Stonebraker JR, Teed J, Alb JG, Magnuson TR, O’Neal W, Milgram SL: Defects in yolk sac vasculogenesis, chorioallantoic fusion, and embryonic axis elongation in mice with targeted disruption of Yap65. Mol Cell Biol 26: 77–87, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reginensi A, Scott RP, Gregorieff A, Bagherie-Lachidan M, Chung C, Lim DS, Pawson T, Wrana J, McNeill H: Yap- and Cdc42-dependent nephrogenesis and morphogenesis during mouse kidney development. PLoS Genet 9: e1003380, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemley KV, Bertram JF, Nicholas SB, White K: Estimation of glomerular podocyte number: a selection of valid methods. J Am Soc Nephrol 24: 1193–1202, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basgen JM, Sobin C: Early chronic low-level lead exposure produces glomerular hypertrophy in young C57BL/6J mice. Toxicol Lett 225: 48–56, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teiken JM, Audettey JL, Laturnus DI, Zheng S, Epstein PN, Carlson EC: Podocyte loss in aging OVE26 diabetic mice. Anat Rec (Hoboken) 291: 114–121, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Potla U, Ni J, Vadaparampil J, Yang G, Leventhal JS, Campbell KN, Chuang PY, Morozov A, He JC, D’Agati VD, Klotman PE, Kaufman L: Podocyte-specific RAP1GAP expression contributes to focal segmental glomerulosclerosis-associated glomerular injury. J Clin Invest 124: 1757–1769, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Happé H, van der Wal AM, Leonhard WN, Kunnen SJ, Breuning MH, de Heer E, Peters DJ: Altered Hippo signalling in polycystic kidney disease. J Pathol 224: 133–142, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Hossain Z, Ali SM, Ko HL, Xu J, Ng CP, Guo K, Qi Z, Ponniah S, Hong W, Hunziker W: Glomerulocystic kidney disease in mice with a targeted inactivation of Wwtr1. Proc Natl Acad Sci U S A 104: 1631–1636, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Makita R, Uchijima Y, Nishiyama K, Amano T, Chen Q, Takeuchi T, Mitani A, Nagase T, Yatomi Y, Aburatani H, Nakagawa O, Small EV, Cobo-Stark P, Igarashi P, Murakami M, Tominaga J, Sato T, Asano T, Kurihara Y, Kurihara H: Multiple renal cysts, urinary concentration defects, and pulmonary emphysematous changes in mice lacking TAZ. Am J Physiol Renal Physiol 294: F542–F553, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Asanuma K, Akiba-Takagi M, Kodama F, Asao R, Nagai Y, Lydia A, Fukuda H, Tanaka E, Shibata T, Takahara H, Hidaka T, Asanuma E, Kominami E, Ueno T, Tomino Y: Dendrin location in podocytes is associated with disease progression in animal and human glomerulopathy. Am J Nephrol 33: 537–549, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Asanuma K, Campbell KN, Kim K, Faul C, Mundel P: Nuclear relocation of the nephrin and CD2AP-binding protein dendrin promotes apoptosis of podocytes. Proc Natl Acad Sci U S A 104: 10134–10139, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu S, Kim C, Baer L, Zhu X: Bevacizumab increases risk for severe proteinuria in cancer patients. J Am Soc Nephrol 21: 1381–1389, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, Richardson C, Kopp JB, Kabir MG, Backx PH, Gerber HP, Ferrara N, Barisoni L, Alpers CE, Quaggin SE: VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 358: 1129–1136, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ziyadeh FN, Wolf G: Pathogenesis of the podocytopathy and proteinuria in diabetic glomerulopathy. Curr Diabetes Rev 4: 39–45, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Harvey SJ, Jarad G, Cunningham J, Goldberg S, Schermer B, Harfe BD, McManus MT, Benzing T, Miner JH: Podocyte-specific deletion of dicer alters cytoskeletal dynamics and causes glomerular disease. J Am Soc Nephrol 19: 2150–2158, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shankland SJ: The podocyte’s response to injury: role in proteinuria and glomerulosclerosis. Kidney Int 69: 2131–2147, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Schiffer M, Mundel P, Shaw AS, Böttinger EP: A novel role for the adaptor molecule CD2-associated protein in transforming growth factor-beta-induced apoptosis. J Biol Chem 279: 37004–37012, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Wagrowska-Danilewicz M, Danilewicz M: [Synaptopodin immunoexpression in steroid-responsive and steroid-resistant minimal change disease and focal segmental glomerulosclerosis]. Nefrologia 27: 710–715, 2007 [PubMed] [Google Scholar]
- 41.Srivastava T, Garola RE, Whiting JM, Alon US: Synaptopodin expression in idiopathic nephrotic syndrome of childhood. Kidney Int 59: 118–125, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Bai XY, Basgen JM: Podocyte number in the maturing rat kidney. Am J Nephrol 33: 91–96, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gundersen HJ, Jensen EB: The efficiency of systematic sampling in stereology and its prediction. J Microsc 147: 229–263, 1987 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.