Abstract
Angiotensin-converting enzyme inhibitors (ACEi) for renin-angiotensin-aldosterone system (RAAS) blockade are routinely used to slow CKD progression. However, vitamin D may also promote renoprotection by suppressing renin transcription through cross-talk between RAAS and vitamin D-fibroblast growth factor-23 (FGF-23)-Klotho pathways. To determine whether vitamin D levels influence proteinuria and CKD progression in children, we performed a post hoc analysis of the Effect of Strict Blood Pressure Control and ACE Inhibition on Progression of CKD in Pediatric Patients (ESCAPE) cohort. In 167 children (median eGFR 51 ml/min per 1.73 m2), serum 25-hydroxyvitamin D (25(OH)D), FGF-23, and Klotho levels were measured at baseline and after a median 8 months on ACEi. Children with lower 25(OH)D levels had higher urinary protein/creatinine ratios at baseline (P=0.03) and at follow-up (P=0.006). Levels of 25(OH)D and serum vitamin D-binding protein were not associated, but 25(OH)D ≤50 nmol/L associated with higher diastolic BP (P=0.004). ACEi therapy also associated with increased Klotho levels (P<0.001). The annualized loss of eGFR was inversely associated with baseline 25(OH)D level (P<0.001, r=0.32). Five-year renal survival was 75% in patients with baseline 25(OH)D ≥50 nmol/L and 50% in those with lower 25(OH)D levels (P<0.001). This renoprotective effect remained significant but attenuated with ACEi therapy (P=0.05). Renal survival increased 8.2% per 10 nmol/L increase in 25(OH)D (P=0.03), independent of eGFR; proteinuria, BP, and FGF-23 levels; and underlying renal diagnosis. In children with CKD, 25(OH)D ≥50 nmol/L was associated with greater preservation of renal function. This effect was present but attenuated with concomitant ACEi therapy.
Keywords: vitamin D, chronic kidney disease, proteinuria, children, glomerular filtration rate, ACE inhibitors
Proteinuria and hypertension are major determinants of CKD progression and contribute to glomerulosclerosis, interstitial inflammation and progressive renal scarring, which are mediated, in part, through activation of the renin-angiotensin-aldosterone system (RAAS).1 Decreasing proteinuria, regardless of its cause, is beneficial in slowing progressive loss of renal function.2,3 Clinical trials of proteinuric chronic nephropathies indicate that RAAS inhibition with angiotensin-converting enzyme inhibitors (ACEi) and angiotensin II receptor blockers (ARB) can attenuate CKD progression,2–4 yet there are patients who only partially benefit from ACEi/ARB treatment.4,5 The combination of an ACEi and ARB,6 increased doses of each,7 or the addition of renin blockade with aliskiren8 have had little effect on renal preservation. Recent studies have suggested that vitamin D can suppress renin gene transcription,9 and that angiotensin II decreases renal Klotho expression.10 This “cross-talk” between the RAAS and the vitamin D-fibroblast growth factor 23 (FGF23)-Klotho pathways suggests that modulation of one system can have positive effects on the other.
Low vitamin D levels have been associated with proteinuria in animal models and patients with proteinuric renal failure. In preclinical models, paricalcitol, a selective activator of the vitamin D receptor, reduced albuminuria and slowed the progression of kidney injury.11 Knockout of the vitamin D receptor in diabetic mice was associated with severe albuminuria and glomerulosclerosis.12 In models of diabetic nephropathy, combined treatment with paricalcitol and an ARB blocked the development of albuminuria, reduced renal expression of renin, maintained the structure of the glomerular filtration barrier, and reduced glomerulosclerosis.13 In a randomized controlled trial the addition of paricalcitol to ACEi or ARB therapy safely reduced residual albuminuria in patients with diabetic nephropathy.14 In addition, vitamin D may have a blood pressure lowering effect.15
There is a high prevalence of vitamin D deficiency in children, starting from early stages of CKD.16 This may, in part, account for proteinuria that is seen with advancing renal failure and possibly explain reduced the response to ACEi/ARB treatment. Previous studies have not looked at an association between vitamin D levels and proteinuria in patients without a primary proteinuric renal disease. Also, it is not known at what level, if any, vitamin D is renoprotective.
We hypothesize that normal 25(OH)D levels are associated with reduced proteinuria and attenuate CKD progression in children. We performed a post-hoc analysis of the ESCAPE trial (Effect of Strict Blood Pressure Control and ACE Inhibition on the Progression of Renal Failure in Pediatric Patients) to examine an association between 25(OH)D levels and proteinuria, hypertension, and renal survival and to study a mechanism for 25(OH)D effects on RAAS blockade.
Results
One hundred sixty-seven children from the original ESCAPE trial were included in this post-hoc analysis. At baseline, the median age of the study cohort was 11.4 (8.0–13.8) years and median eGFR 50.9 (35–63) ml/min per 1.73 m2. There were 98 boys (59%). Underlying diagnoses were congenital anomalies of the kidneys and urinary tract (CAKUT) in 129 (77.3%), glomerulopathies in 15 (9%), and other congenital or hereditary nephropathies in 23 (13.7%) patients. The median urinary PCR at baseline was 0.74 (0.23–1.84) mg/mg (=83.6 [26–208] mg/mmol). None of the patients had nephrotic syndrome.
Clinical details of the study population are described in Table 1. The number of patients on vitamin D supplements (cholecalciferol) and active vitamin D analogs (all on calcitriol) were comparable at baseline and follow-up (Table 1). There was no difference in 25(OH)D levels between patients who received cholecalciferol supplementation versus those not on any vitamin D therapy at any time point (P=0.09).
Table 1.
Clinical and biochemical characteristics of the study population
| Characteristics | Baseline (n=167) | Follow-up (n=167) | P Valuea | Median Change (IQR)b |
|---|---|---|---|---|
| Clinical features | ||||
| Systolic BP | ||||
| mmHg | 115 (105–125) | 105 (100–118) | <0.001 | −7 (–20–5) |
| SDS | 0.46 (–0.18–1.2) | −0.23 (–0.86–0.47) | <0.001 | −0.67 (–1.64–0.18) |
| Diastolic BP | ||||
| mmHg | 70 (64–86) | 64 (57–73) | <0.001 | −7 (–18–3) |
| SDS | 1.13 (0.44–1.86) | 0.22 (–0.38–1.13) | <0.001 | −0.8 (–1.7–0.18) |
| 24-hour mean arterial pressure | ||||
| mmHg | 87 (82–93) | 80 (75.9–85) | <0.001 | −6 (–16–1) |
| SDS | 1.03 (0.29–2.1) | −0.15 (–0.85–0.57) | <0.001 | −1.26 (–2.16 to –0.37) |
| Biochemical measures | ||||
| Estimated GFR (ml/min per 1.73 m2) | 50.9 (35.1–63.3) | 45.8 (28.8–58.9) | 0.08 | −1.74 (–4.1 to –0.25) |
| Urine PCR (mg/mg) | 0.74 (0.23–1.84) | 0.39 (0.15–1.11) | 0.003 | −0.77 (–1.9 to –0.2) |
| 25(OH)D (nmol/L) | 72.3 (46–95.3) | 69 (50.8–99.0) | 0.98 | 2.4 (–24.7–23.3) |
| Calcium (mmol/L) | 2.4 (2.3–2.5) | 2.4 (2.3–2.5) | 0.91 | 0.0 (–0.01–0.15) |
| Phosphate (mmol/L) | 1.5 (1.3–1.6) | 1.5 (1.3–1.7) | 0.59 | 0 (-0.3–0.13) |
| Parathyroid hormone (pmol/L) | 5.2 (3.4–8.3) | 8.0 (4.2–16.1) | 0.04 | 2.2 (–1.4–4.9) |
| FGF-23 (RU/ml) | 182 (118–285) | 308 (184–445) | <0.001 | 94 (–96–256) |
| Soluble-Klotho (pg/mL) | 379 (337–445) | 537 (449–569) | <0.001 | 148 (55–243) |
| VDBP (µmol/L) | 6.8 (3.9–8.2) | 7.2 (4.2–6.9) | 0.75 | 3.9 (–1.7–4.6) |
| (n=107) | (n=91) | |||
| Urinary TGF-β1/creatinine ratio (ng/g) | 25.8 (14.4–42.8) | 13.5 (10.1–41.8) | 0.003 | −7.3 (–4.7–11.1) |
| (n=142) | (n=138) | |||
| Medications | ||||
| Cholecalciferol (n [%]) | 10 [5.9] | 12 [7.1] | 0.98 | — |
| Calcitriol (n [%]) | 72 [43] | 75 [44.9] | 0.96 | — |
All values are described as median and interquartile range.
P values describe baseline versus follow-up levels using paired t test.
IQR, interquartile range.
Lower 25-Hydroxyvitamin D Levels Are Associated with Greater Proteinuria
At baseline, children with the lowest 25(OH)D levels had the highest levels of proteinuria (P=0.03, r=–0.17; Figure 1A). After ACEi treatment for a median of 8 months, children with higher 25(OH)D levels continued to have lower levels of proteinuria (P<0.01, r=–0.21; Figure 1B).
Figure 1.
Correlation between 24-hour urinary PCR and serum 25(OH)D levels. (A) Baseline. (B) Follow-up. To convert mg/mg to mg/mmol, multiply by 113.
Serum 25(OH)D levels were not influenced by the underlying renal diagnosis; children with glomerulopathies had comparable levels to those with CAKUT and other nephropathies (P=0.48; Supplemental Figure 1A). Patients with glomerulopathies and other hereditary nephropathies did not have more proteinuria than those with CAKUT (P=0.14; Supplemental Figure 1B). Serum 25(OH)D was not associated with vitamin D binding protein (VDBP) levels (Table 1; P=0.81 and 0.7 at baseline and follow-up, respectively), nor with serum albumin (P=0.21 and 0.82 at baseline and follow-up, respectively). Only 10 (5.9%) and 12 (7.1%) patients received cholecalciferol at baseline and follow-up; there was no difference in their 25(OH)D levels compared with patients who did not receive cholecalciferol, and there was no difference in their eGFR or urinary PCR compared with the rest of the cohort. Patients who were on calcitriol (n=72 at baseline and 75 at follow-up) did not have any difference in proteinuria or eGFR levels compared with those who did not receive an active vitamin D analog (P=0.33 at baseline and P=0.85 at follow-up). As expected, a seasonal variation in 25(OH)D levels was seen (Supplemental Figure 2). None of the patients developed hypercalcemia from vitamin D treatment.
Lower 25-Hydroxyvitamin D Levels Are Associated with Higher Diastolic BP
There was an inverse association between the diastolic BP before the start of ACEi and baseline serum 25(OH)D levels (P=0.014, r=–0.19 and P=0.038, r=–0.16 for diastolic BP in mmHg and diastolic BP standard deviation score, respectively). Patients with 25(OH)D levels <50 nmol/L had higher diastolic BP than those with levels ≥50 nmol/L (ANOVA P=0.004; Figure 2). The association between diastolic BP and 25(OH)D persisted even on ACEi treatment (P<0.004, r=–0.22). The systolic BP and 24-hour mean arterial BP at baseline and follow-up did not show any association with 25(OH)D levels at any time point.
Figure 2.
Diastolic BP is inversely associated with serum 25(OH)D at baseline. Expressed in three ranges of 25-(OH)D levels (<50, 50–75, >75 nmol/L).
Lower 25-Hydroxyvitamin D Levels Are Positively Associated with Loss of eGFR
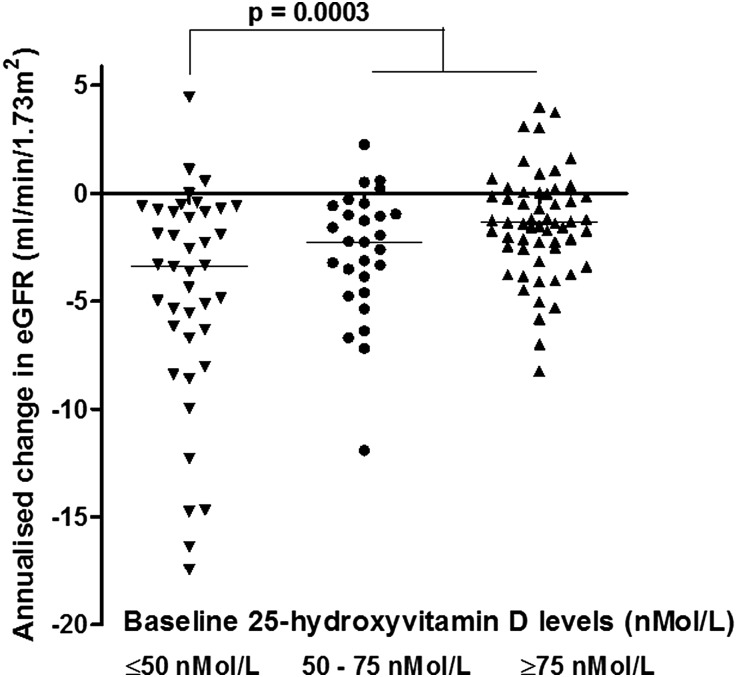
25(OH)D levels showed an association with eGFR at baseline (P=0.002; r=0.24) but not on ACEi treatment (P=0.16; Supplemental Figure 3, A and B). The median annualized loss of eGFR was –1.74 (–4.1 to –0.25) ml/min per 1.73 m2 across the study cohort; children with glomerulopathies and other hereditary nephropathies had a greater annualized decline in eGFR compared with those with CAKUT (P<0.06, ANOVA). The annualized loss of eGFR was greater in patients with baseline 25(OH)D levels <50 nmol/L compared with those with 25(OH)D levels ≥50 nmol/L (P=0.0003, r=0.32; Figure 3). The association between eGFR loss and baseline 25(OH)D levels was seen across all diagnostic groups, including those with CAKUT (P=0.0009, ANOVA).
Figure 3.
Annualized change in eGFR expressed across three ranges of 25(OH)D levels (<50, 50–75, >75 nmol/L) in patients with CAKUT (n=129).
The effect of 25(OH)D levels on a predetermined composite end point of renal survival (defined as an annualized loss of eGFR≥50% or progression to ESRD (eGFR<10 ml/min per 1.73m2) or need for renal replacement therapy) was examined. Overall, 44 patients reached the composite renal end point. The 5-year renal survival was 75% in patients with baseline 25(OH)D ≥50 nmol/L compared with 50% in patients with lower 25(OH)D (P<0.001; Figure 4A). After starting ACEi the beneficial effect of 25(OH)D on renal disease progression was attenuated, but remained significant: the 5-year renal survival was 73% in patients with 25(OH)D ≥50 nmol/L as compared with 57% in patients with lower 25(OH)D (P=0.046; Figure 4B). Using the mean 25(OH)D level between baseline and follow-up as a measure of 25(OH)D exposure, the 5-year renal survival was 75% in patients with 25(OH)D ≥50 nmol/L as compared with 48.2% in patients with lower 25(OH)D (P=0.0008; Figure 4C).
Figure 4.
25(OH)D levels predict 5-year renal survival. (A) Baseline. (B) Follow-up. (C) Mean 25(OH)D levels predict renal survival.
In Cox proportional hazard analysis adjusting for all factors potentially influencing renal disease progression (i.e., baseline eGFR, proteinuria, BP, age, gender, and renal diagnosis), 25(OH)D was an independent predictor of renal disease progression (Table 2). Baseline eGFR, proteinuria and underlying renal diagnoses, but not BP, also showed an independent association with renal survival. The effect of serum 25(OH)D levels on renal disease progression was independent of serum FGF-23 (Table 2, Model 2). The risk of attaining the composite renal end point was reduced by 8.2% for each 10 nmol/L increase in baseline 25(OH)D: P=0.03; HR 0.92 (95% CI, 0.85 to 0.99; Table 2, Model 2).
Table 2.
Cox proportional hazard analysis for renal survival (adjusting for all factors potentially influencing renal disease progression)
| Model 1 | Model 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Parameter Estimate | Hazard Ratio | 95% Hazard Ratio Confidence Limits | Pr-ChiSq | Parameter Estimate | Hazard Ratio | 95% Hazard Ratio Confidence Limits | Pr-ChiSq | ||
| eGFR | −0.06796 | 0.934 | 0.914 | 0.955 | <0.0001 | −0.05600 | 0.946 | 0.921 | 0.971 | <0.0001 |
| Urine PCR | 0.55429 | 1.741 | 1.289 | 2.352 | 0.0003 | 0.62668 | 1.871 | 1.337 | 2.619 | 0.0003 |
| Mean arterial BP SDS | −0.01346 | 0.987 | 0.817 | 1.192 | 0.8888 | 0.05218 | 1.054 | 0.862 | 1.287 | 0.6098 |
| 25(OH)D (per 10 nmol/L) | −0.07940 | 0.924 | 0.862 | 0.990 | 0.0254 | −0.08562 | 0.918 | 0.849 | 0.993 | 0.0324 |
| Renal diagnosis (non-CAKUT) | −1.46553 | 0.231 | 0.123 | 0.433 | <0.0001 | −1.59705 | 0.202 | 0.100 | 0.409 | <0.0001 |
| Male gender | −0.59751 | 0.550 | 0.300 | 1.008 | 0.0530 | −0.58872 | 0.555 | 0.286 | 1.077 | 0.0817 |
| Age (year) | 0.06019 | 1.062 | 0.986 | 1.143 | 0.1101 | 0.08872 | 1.093 | 1.009 | 1.184 | 0.0296 |
| FGF-23 (10 RU/ml) | 0.0304 | 1.031 | 1.005 | 1.057 | 0.0185 | |||||
Pr-ChiSq, probability by chi-squared test.
RAAS Blockade Increases s-Klotho Levels
After a median of 8 months on ACEi treatment, serum FGF-23 and s-Klotho levels significantly increased (P<0.001 for both; Table 1; Figure 5A and B) without any associated changes in serum calcium or phosphate (Table 1). FGF-23 levels showed a strong inverse correlation with eGFR both at baseline (P<0.001, r=–0.70) and after ACEi treatment (P<0.001, r=–0.77). s-Klotho levels did not correlate with eGFR (P=0.82, r=0.02 at baseline and P=0.45, r=0.06 at follow-up) or the annualized change in eGFR (P=0.55, r=0.002). There was no significant difference in FGF23 or s-Klotho levels based on the underlying renal diagnoses.
Figure 5.
(A) Serum FGF-23 levels at baseline and after ACEi treatment. (B) Soluble-Klotho levels at baseline and after ACEi treatment.
The urinary TGF-β1/creatinine ratio was significantly reduced after a median of 8 months on ACEi treatment (P=0.003; Table 1). There was no association between s-Klotho and 25(OH)D or change in urinary TGF-β)1/creatinine ratio levels at any time point.
Discussion
In this post-hoc analysis of the ESCAPE cohort we have shown that in children with CKD, 25(OH)D levels ≥50 nmol/L were associated with greater preservation of renal function, possibly as a result of reduction in proteinuria and diastolic BP. This effect was present but attenuated on ACEi treatment, suggesting that 25(OH)D may have an additive effect to RAAS blockade. 25(OH)D levels did not correlate with serum vitamin D binding protein (VDBP) or albumin, implying that lower 25(OH)D levels were not due to urinary losses of 25(OH)D.
Pharmacological blockade of the RAAS is the cornerstone of renoprotective therapy in CKD. Although ACEi and ARBs are shown to retard the progression of renal disease, largely through their capacity to reduce hypertension and proteinuria, progression to ESRD cannot be prevented in many patients with CKD.4,5,17 The amount of residual proteinuria under RAAS blockade, and in particular an absent or blunted response to RAAS blockers, is a strong predictor of long-term CKD progression.18 In the ESCAPE trial, although there was an approximately 50% reduction in proteinuria in the first 6 months of ACEi treatment, proteinuria gradually increased with ongoing ACEi therapy, returning to baseline levels by 3 years.4 Because an antiproteinuric effect is closely associated with preservation of renal function, alternative strategies are required to treat residual proteinuria or breakthrough proteinuria that develops on ACEi treatment. Intensification of RAAS blockade is often limited by side effects such as hyperkalemia and hypotension,7,8 necessitating the use of adjunctive therapies that operate through alternative pathways.
Converging evidence from experimental studies and clinical trials suggest that vitamin D receptor (VDR) activation may have antiproteinuric effects through modulation of the RAAS system.11,13,19 Activation of the VDR can suppress the renin gene by interaction with a major transcription factor binding site: vitamin D analogs bind to the VDR and blocks formation of the cyclic adenosine monophosphate response element-cAMP response element-binding protein complexes in the promoter region of the renin gene,9 thereby reducing renin expression. VDR null mice have increased renin gene expression in their kidneys, accompanied by increased plasma angiotensin II levels, hypertension, and cardiac hypertrophy.12 Conversely, when wild-type mice are treated with calcitriol, renal renin production was decreased.20
Clinical trials in adults with CKD have shown that vitamin D may augment RAAS blockade.14,21–25 In a meta-analysis of six studies using active vitamin D analogs, a significant reduction in proteinuria was achieved in patients on active vitamin D therapy (paricalcitol in four studies14,21,23,25 and calcitriol22,24 in two). This was an additive effect to ongoing RAAS blockade as 84% of patients received an ACEi or ARB for the duration of their study. Both the number of patients who achieved proteinuria reduction (odds ratio 2.72, P<0.001) as well as the level of proteinuria reduction (mean difference –16% versus +6%; P<0.001) were greater with vitamin D analogs compared with controls.17 Importantly, a dose-dependent effect of vitamin D on albuminuria was not consistently observed in these trials, whereas the retrospective nature of our study allowed us to determine a threshold effect of vitamin D treatment on renal survival.
Nutritional vitamin D supplements such as cholecalciferol have a wide therapeutic window, and have been studied in one randomized study: in 100 adults in predialysis CKD who were followed up for 6 months, cholecalciferol treatment achieved mean 25(OH)D levels ≥60 nmol/L and reduced the urinary protein excretion by 53%.26 In our study, normal levels of 25(OH)D provided similar renoprotective benefits, and patients who received calcitriol did not have any further reduction in their proteinuria, BP or change in eGFR compared with those who were not on calcitriol. We do not have data on the dose of calcitriol prescribed, and due to a limited availability of serum, we were unable to check 1,25(OH)2D levels. The absence of any effect of calcitriol treatment on proteinuria or eGFR may be due to variable levels achieved, and possibly also the short half-life of calcitriol compared with cholecalciferol. Importantly, all of these studies have been conducted in adults, with 50–100% of study participants having diabetes mellitus.14,21–26 There are no studies in children, who are usually free of diabetes and in whom the underlying renal disease is rarely proteinuric renal failure. Thus, clinical trials with cholecalciferol, a safe and effective vitamin D supplement with minimal need for monitoring, are recommended in children with CKD. However, care must be taken in using a safe and effective vitamin D dosing schedule because a recent study has suggested that a high loading dose of ergocalciferol can lead to high FGF-23 levels.27 Also, long-term studies are needed to determine if there is a breakthrough from the antiproteinuric effect of vitamin D, as seen with RAAS blockade.4
We found an association between mean diastolic BP and serum 25(OH)D levels at baseline, but no correlation with systolic BP. Recent studies have shown an association between BP circulating levels of 25(OH)D28 as well as with genetic variations in CYP1A1 and CYP1B1.29 While both studies have shown an association of 25(OH)D with systolic and diastolic BP, a stronger correlation was seen between 25(OH)D and diastolic BP. These findings need to be further explored in a prospective longitudinal study.
We found a significant increase in s-Klotho levels after RAAS blockade. CKD is known to be a state of Klotho deficiency, even in children with CKD.30 In animal models of kidney disease, angiotensin II decreases renal Klotho expression and this downregulation is prevented by RAAS blockade.10,31,32 S-klotho is an anti-aging phosphaturic protein that is shown to confer cardiorenal protection in different experimental models of metabolic and kidney diseases by enhancing antioxidant, antisenescence, and antiapoptotic mechanisms.33,34 In a recent study, serum s-Klotho levels were inversely associated with proteinuria in adults with CKD stages 1–2,35 suggesting a possible association between proteinuria-induced interstitial inflammation and downregulation of Klotho synthesis. There are few clinical studies, but in adults with diabetic kidney disease treatment with valsartan was associated with an increase in s-Klotho, although this did not associate with a reduction in albuminuria.36 Cross-talk between the vitamin D and RAAS pathways may also confer additional anti-inflammatory effects of vitamin D therapy to RAAS blockade.16 In animal models, VDR activation is associated with inhibition of TGF-β11–13,19 and reduced expression of IL-6 and IL-8 in podocytes and tubular cells, suggesting reduced intrarenal inflammation and fibrosis.37,38 However, in this retrospective study we were not able to find an association between urinary TGF-β1 expression and Klotho levels at baseline or after RAAS blockade.
The retrospective nature of our study is a clear limitation; however, this novel association between 25(OH)D levels and proteinuria as well as preservation of eGFR in childhood CKD generates hypotheses for future randomized controlled studies of the renoprotective effects of vitamin D supplementation. While serum 25(OH)D levels are lower in patients with greater proteinuria due to urinary loss of VDBP in patients with nephrotic range proteinuria, we39 and others40 have shown that urinary VDBP loss is not associated with plasma VDBP or 25(OH)D levels in children and adults with chronic kidney disease, where urinary loss of VDBP is not sufficient to affect vitamin D status. Although we were not able to comment on the antiproteinuric and renoprotective effect of different vitamin D analogs, patients who were on calcitriol (approximately 45%) did not have less proteinuria or higher eGFRs as compared with those who did not receive any vitamin D analog. This suggests a possible threshold effect of VDR stimulation, but requires further prospective studies comparing the effects of colecalciferol and active vitamin D analogs on renal preservation. The soluble Klotho assay used in our study measures the larger 130 kD cleaved protein. A smaller fragment of 68–70 kD, as a result of alternative mRNA splicing and currently of unknown significance, may be present in the circulation, but is not detected by this assay.
In conclusion, in children with CKD, 25(OH)D levels >50 nmol/L were associated with better preservation of renal function, even in the presence of concomitant ACEi therapy. Vitamin D is an effective, easily available, safe, and cheap nutritional supplement that may be a useful adjunctive treatment to RAAS blockade to retard progressive renal function decline. Randomized controlled studies on the renoprotective effects of vitamin D in childhood CKD are required.
Concise Methods
Study Population
This study is a post-hoc analysis of the ESCAPE trial, a randomized controlled study showing that strict BP control with a fixed dose of ACE inhibition slows the progression of renal disease. Briefly, the ESCAPE trial included 468 children from 33 European centers of age 3–18 years with an eGFR of 15–80 ml/min per 1.73 m2 with hypertension who received a fixed dose of the ACEi ramipril (6 mg/m2 per day) and were randomly assigned to either a conventional BP target (50th to 90th percentile of 24-hour mean arterial BP) or an intensified BP target (below the 50th percentile). Children were included in this study based on the availability of paired blood samples at baseline and after a follow-up period of at least 6 months. All measures were taken at baseline (prior to ACEi treatment or after a wash-out phase of 4 (2–4) months in those who were previously on an ACEi) and after a median follow-up of 8 (8–10) months on ACEi therapy.
Outcome Measures
The effect of 25(OH)D levels on change in 24-hour urinary protein excretion, BP, eGFR and renal survival (defined as a predetermined composite end point of annualized loss of eGFR > 50% or progression to ESRD (eGFR<10 ml/min per 1.73 m2) or need for renal replacement therapy) were studied. Because an acute decrease in eGFR (<25% decrease) is expected after the start of ACEi therapy, the eGFR recorded 2 months after the initiation of ramipril was used as a baseline for the analysis of the reduction in eGFR over time.
In order to examine a potential mechanism of the 25(OH)D effect on reduction of proteinuria, FGF23, s-Klotho and TGF-β1 were measured in a subgroup of patients (based on availability of serum samplea) at baseline and follow-up. To exclude a confounding effect of VDBP loss on serum 25(OH)D levels, VDBP levels were measured in a subgroup of children, based on availability of serum samples. The effect of serum 25(OH)D levels on mineral dysregulation (serum calcium, phosphate, parathyroid hormone, FGF23 and s-Klotho) was examined.
Blood Pressure Monitoring
Ambulatory BP monitoring was performed using Spacelabs 90207 oscillometric devices (Spacelabs Healthcare, Snoqualmie, WA) at baseline and every 6 months throughout the study period. All BP readings were normalized to standard deviation scores using European reference data sets.41
Laboratory Assessments
All biochemical measurements were made in a central laboratory at baseline and final follow-up. Measurements of serum and urine creatinine levels and urine protein concentration were performed as part of the ESCAPE trial as previously described.4 25(OH)D was analyzed by isotope-dilution liquid chromatography-tandem mass spectrometry [expressed as a sum of 25(OH)D2 and 25(OH)D3]. The interassay coefficient of variation was 2.8%. Plasma FGF-23 concentrations were determined using a second-generation human FGF-23 (C-terminal) ELISA (Immutopics International, San Clemente, CA). The intra- and interassay coefficients of variation were 3.8% and 6.3%, respectively. s-Klotho concentrations were measured by a solid-phase sandwich ELISA (Immuno-Biologic Laboratories Co. Ltd., Gunma, Japan). The intra- and interassay coefficients of variation were 2.4% and 6.2%, respectively. VDBP assay was performed using a noncompetitive (sandwich) ELISA (K2314, Immun Diagnostik, Germany). The intra- and interassay coefficients of variability were 4.4% and 6.0%, respectively. Urinary excretion of TGF-β1 was assayed using ELISA (DRG Instruments GmbH, Marburg, Germany) as previously described.42
Statistical Analysis
Results are expressed as median and interquartile range (IQR) unless otherwise stated. Univariate comparisons of continuous variables between the groups were performed using an unpaired t test for normally distributed data, or the nonparametric Mann–Whitney U or Kruskal–Wallis test for non-normally distributed variables. Comparisons of continuous variables between baseline and final follow-up were performed using a paired t test or the non-parametric Wilcoxon test as appropriate. For multiple comparisons of several groups, repeated measures ANOVA with Bonferroni correction or Kruskal–Wallis test was performed as appropriate. Spearman correlation tests were used for correlation analyses. The time to development of the composite end point was determined by Kaplan–Meier analysis, with the use of log-rank statistics to test for differences in the rates of the end points and by Cox proportional-hazard modeling to assess the effects of potential risk factors.
In 159 children in whom full data were available, Cox hazard analysis was performed to include variables that are well known to predict renal disease progression or influence 25(OH)D levels provided that they were significant on univariate analysis at P<0.15. BMI Standard deviation core did not show any correlation with 25(OH)D levels (r=0.02, P=0.79) nor annualized renal disease progression (r=–0.04, P=0.57) on univariate analysis and was not included in the Cox model. Although, as expected, the season of blood sampling influenced 25(OH)D levels (Supplemental Figure 3), the influence of a constantly changing process such as season per se on a long-term outcome of renal progression is difficult to justify and season was excluded from the model. In a second model we also included FGF-23 (Table 2, Model 2), but complete data were available for only 139 patients. Klotho, calcium and phosphate were not significant on univariate analysis and were excluded from the Cox regression analysis in order to limit the number of variables and avoid potential overadjustment.
All statistical analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, NC). For all analyses, P<0.05 was considered to be statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
The following are members of the ESCAPE Study Group – Local investigators (in alphabetical order of center):
A. Anarat (Cukurova University School of Medicine, Balcali, Adana, Turkey); A. Bakkaloglu and F. Ozaltin (Hacettepe University Faculty of Medicine, Sihhiye, Ankara, Turkey); A. Peco-Antic (University Children’s Hospital, Belgrade, Serbia); U. Querfeld and J. Gellermann (Charité Children’s Hospital, Berlin, Germany); P. Sallay (Semmelweis University Budapest, First Department of Pediatrics, Budapest, Hungary); D. Drozdz (Polish-American Children’s Hospital, Jagiellonian University Collegium Medicum, Krakow, Poland); K.-E. Bonzel and A.-M. Wingen (University Hospital Essen, Essen, Germany); A. Zurowska and I. Balasz (Department of Pediatric and Adolescent Nephrology and Hypertension, Medical University of Gdansk, Gdansk, Poland); A. Trivelli and F. Perfumo (G. Gaslini Institute, Genova, Italy); D.E. Müller-Wiefel and K. Möller (University Children’s Hospital Hamburg-Eppendorf, Hamburg, Germany); G. Offner and B. Enke (Hannover Medical School, Children’s Hospital, Hannover, Germany); E. Wühl, C. Gimpel, O.Mehls, and F. Schaefer (Center for Pediatric and Adolescent Medicine, Heidelberg, Germany); S. Emre (University of Istanbul, Istanbul Medical Faculty, Capa, Istanbul, Turkey); S. Caliskan (Istanbul University, Cerrahpasa Medical Faculty, Istanbul, Turkey); S. Mir (Ege University Medical Faculty, Department of Pediatrics Bornova, Izmir, Turkey); S. Wygoda (Urban Hospital St. Georg, Leipzig, Germany); K. Hohbach-Hohenfellner (University Children’s Hospital, Mainz, Germany); N. Jeck and G. Klaus (Department of Pediatrics, Philipps University Marburg, Marburg, Germany); G. Ardissino and S. Testa (IRCCS Ospedale Maggiore, Policlinico-Mangiagalli, Milano, Italy); G. Montini (Azienda Ospedaliera-Università di Padova, Padova, Italy); M. Charbit and P. Niaudet (Hospital Necker, Paris, France); A. Caldas-Afonso and A. Fernandes-Teixeira (Hospital S. Joao, Porto, Portugal); J. Dušek (Department of Pediatrics, University Hospital Motol, Prague, Czech Republic); M.C. Matteucci, S. Picca, and A. Mastrostefano (Ospedale Pediatrico Bambino Gesù, Rome, Italy); M. Wigger (University Children’s Hospital, Rostock, Germany); U.B. Berg and G. Celsi (Karolinska Institute, Huddinge University Hospital, Stockholm, Sweden); M. Fischbach and J. Terzic (Hopitaux Universitaires de Strasbourg, Pediatrie 1, Strasbourg, France); J. Fydryk and T. Urasinski (Pomeranian Academy of Medicine, Szczecin, Poland); R. Coppo and Licia Peruzzi (Ospedale Infantile Regina Margherita, Torino, Italy); K. Arbeiter (University Children’s Hospital, Vienna, Austria); A. Jankauskiené (Vilnius University Pediatric Center, Vilnius, Lithuania); R. Grenda, M. Litwin, and R. Janas (Children’s Memorial Health Hospital, Warsaw, Poland); G. Laube and T.J. Neuhaus (University Children’s Hospital, Zürich, Switzerland).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014090947/-/DCSupplemental.
References
- 1.Remuzzi G, Cattaneo D, Perico N: The aggravating mechanisms of aldosterone on kidney fibrosis. J Am Soc Nephrol 19: 1459–1462, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Ruggenenti P, Schieppati A, Remuzzi G: Progression, remission, regression of chronic renal diseases. Lancet 357: 1601–1608, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Taal MW, Brenner BM: Renoprotective benefits of RAS inhibition: from ACEI to angiotensin II antagonists. Kidney Int 57: 1803–1817, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Wühl E, Trivelli A, Picca S, Litwin M, Peco-Antic A, Zurowska A, Testa S, Jankauskiene A, Emre S, Caldas-Afonso A, Anarat A, Niaudet P, Mir S, Bakkaloglu A, Enke B, Montini G, Wingen AM, Sallay P, Jeck N, Berg U, Caliskan S, Wygoda S, Hohbach-Hohenfellner K, Dusek J, Urasinski T, Arbeiter K, Neuhaus T, Gellermann J, Drozdz D, Fischbach M, Möller K, Wigger M, Peruzzi L, Mehls O, Schaefer F, ESCAPE Trial Group : Strict blood-pressure control and progression of renal failure in children. N Engl J Med 361: 1639–1650, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Ruggenenti P, Perna A, Remuzzi G, GISEN Group Investigators : Retarding progression of chronic renal disease: the neglected issue of residual proteinuria. Kidney Int 63: 2254–2261, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Mann JF, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, Wang X, Maggioni A, Budaj A, Chaithiraphan S, Dickstein K, Keltai M, Metsärinne K, Oto A, Parkhomenko A, Piegas LS, Svendsen TL, Teo KK, Yusuf S, ONTARGET investigators : Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 372: 547–553, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Vogt L, Navis G, de Zeeuw D: Individual titration for maximal blockade of the renin-angiotensin system in proteinuric patients: a feasible strategy? J Am Soc Nephrol 16[Suppl 1]: S53–S57, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Harel Z, Gilbert C, Wald R, Bell C, Perl J, Juurlink D, Beyene J, Shah PS: The effect of combination treatment with aliskiren and blockers of the renin-angiotensin system on hyperkalaemia and acute kidney injury: systematic review and meta-analysis. BMJ 344: e42, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan W, Pan W, Kong J, Zheng W, Szeto FL, Wong KE, Cohen R, Klopot A, Zhang Z, Li YC: 1,25-dihydroxyvitamin D3 suppresses renin gene transcription by blocking the activity of the cyclic AMP response element in the renin gene promoter. J Biol Chem 282: 29821–29830, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Yoon HE, Ghee JY, Piao S, Song JH, Han DH, Kim S, Ohashi N, Kobori H, Kuro-o M, Yang CW: Angiotensin II blockade upregulates the expression of Klotho, the anti-ageing gene, in an experimental model of chronic cyclosporine nephropathy. Nephrol Dial Transplant 26: 800–813, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mizobuchi M, Morrissey J, Finch JL, Martin DR, Liapis H, Akizawa T, Slatopolsky E: Combination therapy with an angiotensin-converting enzyme inhibitor and a vitamin D analog suppresses the progression of renal insufficiency in uremic rats. J Am Soc Nephrol 18: 1796–1806, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP: 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest 110: 229–238, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Z, Zhang Y, Ning G, Deb DK, Kong J, Li YC: Combination therapy with AT1 blocker and vitamin D analog markedly ameliorates diabetic nephropathy: blockade of compensatory renin increase. Proc Natl Acad Sci U S A 105: 15896–15901, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Zeeuw D, Agarwal R, Amdahl M, Audhya P, Coyne D, Garimella T, Parving HH, Pritchett Y, Remuzzi G, Ritz E, Andress D, de ZD : Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet 376: 1543–1551, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Pilz S, Tomaschitz A, Ritz E, Pieber TR: Vitamin D status and arterial hypertension: a systematic review. Nat Rev Cardiol 6: 621–630, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Shroff R, Wan M, Rees L: Can vitamin D slow down the progression of chronic kidney disease? Pediatr Nephrol 27: 2167–2173, 2012 [DOI] [PubMed] [Google Scholar]
- 17.de Borst MH, Hajhosseiny R, Tamez H, Wenger J, Thadhani R, Goldsmith DJ: Active vitamin D treatment for reduction of residual proteinuria: a systematic review. J Am Soc Nephrol 24: 1863–1871, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmieder RE, Mann JF, Schumacher H, Gao P, Mancia G, Weber MA, McQueen M, Koon T, Yusuf S, ONTARGET Investigators : Changes in albuminuria predict mortality and morbidity in patients with vascular disease. J Am Soc Nephrol 22: 1353–1364, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan X, Li Y, Liu Y: Paricalcitol attenuates renal interstitial fibrosis in obstructive nephropathy. J Am Soc Nephrol 17: 3382–3393, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Kong J, Qiao G, Zhang Z, Liu SQ, Li YC: Targeted vitamin D receptor expression in juxtaglomerular cells suppresses renin expression independent of parathyroid hormone and calcium. Kidney Int 74: 1577–1581, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Agarwal R, Acharya M, Tian J, Hippensteel RL, Melnick JZ, Qiu P, Williams L, Batlle D: Antiproteinuric effect of oral paricalcitol in chronic kidney disease. Kidney Int 68: 2823–2828, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Krairittichai U, Mahannopkul R, Bunnag S: An open label, randomized controlled study of oral calcitriol for the treatment of proteinuria in patients with diabetic kidney disease. J Med Assoc Thai 95[Suppl 3]: S41–S47, 2012 [PubMed] [Google Scholar]
- 23.Fishbane S, Chittineni H, Packman M, Dutka P, Ali N, Durie N: Oral paricalcitol in the treatment of patients with CKD and proteinuria: a randomized trial. Am J Kidney Dis 54: 647–652, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Liu LJ, Lv JC, Shi SF, Chen YQ, Zhang H, Wang HY: Oral calcitriol for reduction of proteinuria in patients with IgA nephropathy: a randomized controlled trial. Am J Kidney Dis 59: 67–74, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Thadhani R, Appelbaum E, Pritchett Y, Chang Y, Wenger J, Tamez H, Bhan I, Agarwal R, Zoccali C, Wanner C, Lloyd-Jones D, Cannata J, Thompson BT, Andress D, Zhang W, Packham D, Singh B, Zehnder D, Shah A, Pachika A, Manning WJ, Solomon SD: Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA 307: 674–684, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Molina P, Górriz JL, Molina MD, Peris A, Beltrán S, Kanter J, Escudero V, Romero R, Pallardó LM: The effect of cholecalciferol for lowering albuminuria in chronic kidney disease: a prospective controlled study. Nephrol Dial Transplant 29: 97–109, 2014 [DOI] [PubMed] [Google Scholar]
- 27.Turner C, Dalton N, Inaoui R, Fogelman I, Fraser WD, Hampson G: Effect of a 300 000-IU loading dose of ergocalciferol (Vitamin D2) on circulating 1,25(OH)2-vitamin D and fibroblast growth factor-23 (FGF-23) in vitamin D insufficiency. J Clin Endocrinol Metab 98: 550–556, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Vimaleswaran KS, Cavadino A, Berry DJ, Jorde R, Dieffenbach AK, Lu C, Alves AC, Heerspink HJ, Tikkanen E, Eriksson J, Wong A, Mangino M, Jablonski KA, Nolte IM, Houston DK, Ahluwalia TS, van der Most PJ, Pasko D, Zgaga L, Thiering E, Vitart V, Fraser RM, Huffman JE, de Boer RA, Schöttker B, Saum KU, McCarthy MI, Dupuis J, Herzig KH, Sebert S, Pouta A, Laitinen J, Kleber ME, Navis G, Lorentzon M, Jameson K, Arden N, Cooper JA, Acharya J, Hardy R, Raitakari O, Ripatti S, Billings LK, Lahti J, Osmond C, Penninx BW, Rejnmark L, Lohman KK, Paternoster L, Stolk RP, Hernandez DG, Byberg L, Hagström E, Melhus H, Ingelsson E, Mellström D, Ljunggren O, Tzoulaki I, McLachlan S, Theodoratou E, Tiesler CM, Jula A, Navarro P, Wright AF, Polasek O, Wilson JF, Rudan I, Salomaa V, Heinrich J, Campbell H, Price JF, Karlsson M, Lind L, Michaëlsson K, Bandinelli S, Frayling TM, Hartman CA, Sørensen TI, Kritchevsky SB, Langdahl BL, Eriksson JG, Florez JC, Spector TD, Lehtimäki T, Kuh D, Humphries SE, Cooper C, Ohlsson C, März W, de Borst MH, Kumari M, Kivimaki M, Wang TJ, Power C, Brenner H, Grimnes G, van der Harst P, Snieder H, Hingorani AD, Pilz S, Whittaker JC, Järvelin MR, Hyppönen E, LifeLines Cohort Study investigators. International Consortium for Blood Pressure (ICBP) Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) consortium. Global Blood Pressure Genetics (Global BPGen) consortium. Caroline Hayward : Association of vitamin D status with arterial blood pressure and hypertension risk: a mendelian randomisation study. Lancet Diabetes Endocrinol 2: 719–729, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park HY, Kim JH, Bae S, Choi YY, Park JY, Hong YC: Interaction effect of serum 25-hydroxyvitamin D levels and CYP1A1, CYP1B1 polymorphisms on blood pressure in an elderly population. J Hypertens 33: 69–76, 2015 [DOI] [PubMed] [Google Scholar]
- 30.Wan M, Smith C, Shah V, Gullet A, Wells D, Rees L, Shroff R: Fibroblast growth factor 23 and soluble klotho in children with chronic kidney disease. Nephrol Dial Transplant 28: 153–161, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Mitani H, Ishizaka N, Aizawa T, Ohno M, Usui S, Suzuki T, Amaki T, Mori I, Nakamura Y, Sato M, Nangaku M, Hirata Y, Nagai R: In vivo klotho gene transfer ameliorates angiotensin II-induced renal damage. Hypertension 39: 838–843, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Zhou Q, Lin S, Tang R, Veeraragoo P, Peng W, Wu R: Role of Fosinopril and Valsartan on Klotho Gene Expression Induced by Angiotensin II in Rat Renal Tubular Epithelial Cells. Kidney Blood Press Res 33: 186–192, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Kuro-o M: Klotho in health and disease. Curr Opin Nephrol Hypertens 21: 362–368, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Shroff R, Shanahan CM: Klotho: an elixir of youth for the vasculature? J Am Soc Nephrol 22: 5–7, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Hage V, Pelletier S, Dubourg L, Drai J, Cuerq C, Lemoine S, Hadj-Aissa A, Laville M, Fouque D: In Chronic Kidney Disease, Serum alpha-Klotho is Related to Serum Bicarbonate and Proteinuria, J Ren Nutr, 24: 390–394, 2014 [DOI] [PubMed] [Google Scholar]
- 36.Karalliedde J, Maltese G, Hill B, Viberti G, Gnudi L: Effect of renin-angiotensin system blockade on soluble Klotho in patients with type 2 diabetes, systolic hypertension, and albuminuria. Clin J Am Soc Nephrol 8: 1899–1905, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanchez-Niño MD, Bozic M, Córdoba-Lanús E, Valcheva P, Gracia O, Ibarz M, Fernandez E, Navarro-Gonzalez JF, Ortiz A, Valdivielso JM: Beyond proteinuria: VDR activation reduces renal inflammation in experimental diabetic nephropathy. Am J Physiol Renal Physiol 302: F647–F657, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Isakova T, Gutiérrez OM, Patel NM, Andress DL, Wolf M, Levin A: Vitamin D deficiency, inflammation, and albuminuria in chronic kidney disease: complex interactions. J Ren Nutr 21: 295–302, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Prytuła A, Wells D, McLean T, Balona F, Gullett A, Knott C, Cantwell M, Hassen K, Ledermann S, Rees L, Shroff R: Urinary and dialysate losses of vitamin D-binding protein in children on chronic peritoneal dialysis. Pediatr Nephrol 27: 643–649, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Doorenbos CR, de Cuba MM, Vogt L, Kema IP, van den Born J, Gans RO, Navis G, de Borst MH: Antiproteinuric treatment reduces urinary loss of vitamin D-binding protein but does not affect vitamin D status in patients with chronic kidney disease. J Steroid Biochem Mol Biol 128: 56–61, 2012 [DOI] [PubMed] [Google Scholar]
- 41.Wühl E, Witte K, Soergel M, Mehls O, Schaefer F, German Working Group on Pediatric Hypertension : Distribution of 24-h ambulatory blood pressure in children: normalized reference values and role of body dimensions. J Hypertens 20: 1995–2007, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Grenda R, Wühl E, Litwin M, Janas R, Sladowska J, Arbeiter K, Berg U, Caldas-Afonso A, Fischbach M, Mehls O, Sallay P, Schaefer F, ESCAPE Trial group : Urinary excretion of endothelin-1 (ET-1), transforming growth factor-beta1 (TGF-beta1) and vascular endothelial growth factor (VEGF165) in paediatric chronic kidney diseases: results of the ESCAPE trial. Nephrol Dial Transplant 22: 3487–3494, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.