Abstract
Longitudinal studies testing the relationship between repeated measures of vitamin D or fibroblast growth factor 23 (FGF23) and infectious and cardiac hospitalizations and death in hemodialysis patients have not been reported. We examined the association between yearly 25-hydroxyvitamin D (25(OH)D), 1,25-dihydroxyvitamin D (1,25(OH)2D), and FGF23 serum levels and various clinical outcomes using time-dependent Cox regression models with repeated yearly measures and fixed-covariate Cox models with only baseline values after controlling for important clinical covariates in the HEMO study. During a median follow-up of 3 years, 582 of the 1340 participants died, and 499 and 514 participants had a hospitalization or death attributed to infectious and cardiac causes, respectively. Patients in the highest 25(OH)D quartile had the lowest risk of infectious events (hazard ratio [HR] 0.66 versus the lowest quartile; 95% confidence interval [95% CI], 0.49–0.89), cardiac events (HR, 0.71; 95% CI, 0.53–0.96), and all-cause mortality (HR, 0.46; 95% CI, 0.34–0.62) in time-dependent analyses. No significant associations of 1,25(OH)2D with clinical outcomes were observed in time-dependent or fixed-covariate Cox models. In contrast, the highest FGF23 quartile was associated with a higher risk of infectious events (HR, 1.57 versus the lowest quartile; 95% CI, 1.13–2.18), cardiac events (HR, 1.49; 95% CI, 1.06–2.08), and all-cause mortality (HR, 1.50; 95% CI, 1.07–2.12) in fixed-covariate Cox models. The addition of inflammation markers into the statistical models did not attenuate these associations. Thus, disordered mineral metabolism may affect outcomes in chronic hemodialysis patients.
Keywords: chronic hemodialysis, chronic inflammation, epidemiology and outcomes, fibroblast, vitamin D
Cardiac events are the major cause of death in hemodialysis patients, accounting for approximately 40–50% of deaths.1,2 Similarly, infections remain a very common cause of morbidity and mortality.3–5 Several nontraditional risk factors may contribute to these two important clinical outcomes in this high-risk population, such as CKD-associated mineral and bone disorder6 which includes a wide range of abnormalities in vitamin D metabolism7–9 and phosphate homeostasis.10,11
Lower serum levels of both 25-hydrovitamin D (25(OH)D) and 1,25-dihydroxyvitamin D (1,25(OH)2D) have been associated with increased mortality within 90 days of initiating hemodialysis.8 However, no studies have evaluated annual repeated measures of vitamin D analytes in hemodialysis patients over time, which reflect long-term levels more accurately than single time points. There are also no longitudinal studies on the associations between vitamin D and cardiac outcomes in these patients. Hence, the combination of significant 25(OH)D deficiency, impaired conversion of 25(OH)D to 1,25(OH)2D, and high numbers of cardiac events render the dialysis population an ideal model to study the impact of vitamin D analytes on adjudicated cardiac outcomes.
Vitamin D has also important effects on the innate and adaptive immune systems, by modulating the production of endogenous antimicrobial peptides and regulating the inflammatory cascade.12,13 There is evidence that cathelicidin transcription, which are key bactericidal proteins, are particularly dependent of sufficient 25(OH)D circulating levels.14 Epidemiologic studies testing the association between vitamin D deficiency and risk of infection are, nonetheless, lacking in the hemodialysis population.
Bone-derived fibroblast growth factor 23 (FGF23) is a phosphaturic hormone. By inhibiting the expression of 25-hydroxyvitamin D-1α-hydroxylase (CYP27B1), FGF23 also acts as a counter-regulatory hormone to 1,25(OH)2D production.15,16 Epidemiologic studies have shown high circulating FGF23 levels to be an independent predictor of development of left ventricular hypertrophy and mortality in the pre-dialysis and incident hemodialysis population.17–20 However, its association with cardiac hospitalizations and deaths in chronic hemodialysis patients has not been clearly established. Furthermore, previous studies were limited by the lack of serial measurements of circulating FGF23 levels.
FGF23 has also been suggested to be a regulator of innate immunity.21 Recent studies reported that FGF23 treatment of mononuclear cells isolated from healthy human peripheral blood and peritoneal dialysis effluents from uremic patients decreased the mRNA expression of CYP27B1.21,22 Accordingly, it is reasonable to hypothesize that circulating FGF23 excess decreases the intracrine production of 1,25(OH)2D, which consequently decreases the transcription and the production of cathelicidins14 leading to an increase in infectious outcomes.
Substantial amounts of literature have shown that high serum markers of inflammation predict a high risk of death in hemodialysis patients and have suggested a role in the pathophysiology of adverse outcomes in hemodialysis.23,24 Given the high prevalence of inflammation, vitamin D deficiency, and FGF23 excess in hemodialysis, we also examined whether the relationships with cardiac and infectious events and all-cause mortality with lower vitamin D and higher FGF23 serum levels were attenuated with statistical adjustment for inflammatory markers.
The HEMO study was designed to examine if greater dialytic urea removal or the use of high-flux dialysis could improve outcomes in hemodialysis patients.25 A strength of the HEMO study was that the first cardiac and infectious hospitalizations of the participants were adjudicated by an Outcomes Committee.25 In addition, serum samples were collected annually from study participants that allowed us to examine the association between cardiac and infectious outcomes with the most recent annual measurements of serum vitamin D and FGF23. In the present study, we exploited the serum samples and data collected in the HEMO study to examine the relationships between serum levels of vitamin D and FGF23 with cardiac and infectious hospitalizations and deaths.
Results
Of the 1340 participants included in this analysis, the mean (±SD) age at the time of randomization was 57±14 years; 45% were male and 64% were Black. The median number of years on dialysis was 2.7 (interquartile range [IQR], 1.4–5.1). Of these participants, 499 (37%) and 514 (38%) had infectious and cardiac composite events, respectively and there were 582 (43%) deaths from all causes. The distributions of baseline serum 25(OH)D, 1,25(OH)2D, and FGF23 levels were skewed to the right with a median of 19.1 (IQR, 14.2–26.6) ng/ml, 6.3 (IQR, 2.9–14.5) pg/ml, and 3118 (IQR, 726–12928) pg/ml, respectively. The mean serum calcium and phosphorus levels were 9.3±0.9 mg/dl and 5.8±1.9 mg/dl, respectively. The median intact parathyroid hormone (iPTH) level was 190 (IQR, 83–426) pg/ml and 53.7% of the participants were receiving intravenous vitamin D analogs in the form of calcitriol.
Figure 1, A–C presents the median and IQR for 25(OH)D, 1,25(OH)2D, and FGF23 serum levels according to assignment to standard or high dose of dialysis and to a low-flux or high-flux dialyzer in the HEMO study. When calculating the coefficient of variation (CV) for each mineral bone disorder parameter from the yearly measurements, FGF23 (median CV=0.56 [IQR, 0.24–0.93]) showed the highest CV value followed by 1,25(OH)2D (median CV=0.53 [IQR, 0.23–0.90]), and 25(OH)D (median CV=0.16 [IQR, 0.10–0.30]).
Figure 1.
Box-and-whisker plots for distribution of 25-hydroxyvitamin D (A), 1,25-dihydroxyvitamin D (B), and FGF23 (C) serum levels during the course of the HEMO study assigned to standard or high dose of dialysis and to a low or high-flux dialyzer. std, standard.
Serum 25(OH)D levels correlated directly with serum calcium (r=0.14; P<0.001), serum 1,25(OH)2D (r= 0.32; P<0.001), serum FGF23 (r=0.12; P<0.001), and inversely with serum iPTH (r=−0.10; P<0.002), serum high-sensitivity C-reactive protein (hs-CRP; r=−0.11; P<0.001), and interleukin-6 (IL-6; r=−0.10; P<0.01) levels; but did not correlate with serum phosphorus. In addition to its correlation with 25(OH)D, serum FGF23 levels had a direct correlation with serum phosphorus (r=0.52; P<0.001), serum iPTH (r=0.10; P<0.003), and serum 1,25(OH)2D (r= 0.10; P=0.001). There was no correlation between serum FGF23 with serum hs-CRP and IL-6.
Table 1 and Supplemental Table 1 display demographics and cardiovascular and infectious risk factors by quartiles of serum 25(OH)D and 1,25(OH)2D, respectively. Participants with higher serum 25(OH)D levels were younger, more frequently Caucasian and male, were less likely to have diabetes, and more likely to have a higher serum albumin, calcium, and 1,25(OH)2D levels and lower serum iPTH and hs-CRP levels. Participants with higher 1,25(OH)2D serum levels were more likely to be males, and have a lower prevalence of diabetes and cardiovascular disease (CVD). They were more likely to have a higher serum calcium, 25(OH)D, iPTH, and FGF23 levels. Of note, they were also more likely to have lower serum phosphorus and receive more intravenous vitamin D analogs.
Table 1.
Baseline characteristics of study participants by quartiles of baseline serum 25(OH)D levels
| 25 (OH)D Levels (ng/ml) | Q1 | Q2 | Q3 | Q4 | P Value For Trend |
|---|---|---|---|---|---|
| <14 | 14–19 | 19–26 | >26 | ||
| n=335 | n=335 | n=335 | n=335 | ||
| Age; years | 58 (13) | 59 (13) | 58 (14) | 55 (16) | 0.01 |
| Female; N | 243 (72.5) | 204 (60.7) | 170 (50.9) | 121 (36.1) | <0.001 |
| Black; N | 252 (75.2) | 234 (69.6) | 214 (64.1) | 158 (47.2) | <0.001 |
| Dialysis duration; years | 2.6 [1.4–4.7] | 2.5 [1.5–4.8] | 2.6 [1.3–5.0] | 3.0 [1.4–5.7] | 0.56 |
| Diabetes; N | 184 (54.9) | 168 (50.0) | 132 (39.5) | 103 (30.8) | <0.001 |
| CVD; N | 276 (82.4) | 271 (80.6) | 260 (77.8) | 249 (74.3) | 0.06 |
| Catheter use; N | 23 (6.9) | 21 (6.2) | 13 (3.9) | 15 (4.5) | 0.27 |
| Current smoking; N | 155 (46.3) | 158 (47.2) | 180 (53.9) | 183 (54.6) | 0.05 |
| High-Kt/V assignment; N | 160 (47.8) | 175 (52.1) | 161(48.2) | 173(51.6) | 0.56 |
| High-flux assignment; N | 175(52.2) | 167(49.7) | 169(50.6) | 163(48.7) | 0.82 |
| Serum albumin; mg/dl | 3.5 (0.4) | 3.6 (0.4) | 3.6 (0.3) | 3.7 (0.3) | <0.001 |
| Serum calcium; mg/dl | 9.2 (0.9) | 9.3 (0.9) | 9.3 (0.9) | 9.5 (1.0) | <0.001 |
| Serum phosphorus; mg/dl | 5.7 (2.0) | 5.8 (1.8) | 5.8 (1.8) | 5.8 (2.0) | 0.92 |
| Serum 1,25(OH)2D; pg/ml | 3.3 [1.6–10.8] | 5.2 [2.3–14.2] | 6.0 [3.4–14.7] | 8.9 [5.4–17.8] | <0.001 |
| Serum iPTH; pg/ml | 249 [113–456] | 185 [75–411] | 185 [86–398] | 165 [66–418] | 0.001 |
| Serum FGF23; pg/ml | 2403 [582–9755] | 3357 [846–12247] | 2365 [609–11905] | 4458 [1039–19010] | <0.001 |
| Serum hs-CRP; mg/L | 7.8 [2.9–16.6] | 6.3 [2.9–15.8] | 6.0 [2.6–14.2] | 4.8 [2.2–12.4] | 0.01 |
| Serum IL-6; pg/ml | 3.3 [1.7–8.3] | 3.1 [1.7–6.6] | 3.2 [1.8–6.8] | 2.7 [1.5–5.7] | 0.07 |
| Serum TNF-α; pg/ml | 30.2 [19.4–46.7] | 27.4 [18.8–40.0] | 32.1 [21.8–44.8] | 30.1 [19.7–46.1] | 0.09 |
| Serum IFN-γ; pg/ml | 1.4 [1.1–2.0] | 1.4 [1.1–1.9] | 1.5 [1.2–2.1] | 1.4 [1.1–2.2] | 0.29 |
| Vitamin D analog administration; % | 179 (53.4) | 194 (57.7) | 178 (53.3) | 169 (50.4) | 0.30 |
Data are presented as N (%), mean (SD) or median [IQR].
Higher FGF23 levels were significantly associated with younger age, male sex, white race, more years on chronic hemodialysis, and higher serum albumin, calcium, phosphorus, 25(OH)D, 1,25(OH)2D, and iPTH levels. The prevalence of diabetes was lower in the higher FGF23 groups; however, vitamin D administration was more frequent (Table 2).
Table 2.
Baseline characteristics of study participants by quartiles of baseline serum FGF23 levels
| FGF23 Levels (pg/ml) | Q1 | Q2 | Q3 | Q4 | P Value for Trend |
|---|---|---|---|---|---|
| <724 | 728–3115 | 3122–12923 | >12932 | ||
| n=335 | n=335 | n=335 | n=335 | ||
| Age; years | 61 (13) | 60 (13) | 58(14) | 51 (15) | <0.001 |
| Female; N | 221 (66.0) | 183 (54.6) | 175 (52.2) | 159 (47.5) | <0.001 |
| Black; N | 243 (72.5) | 214 (63.9) | 213 (63.6) | 188 (56.1) | <0.001 |
| Dialysis duration; years | 2.1 [1.0–3.9] | 2.5 [1.4–4.5] | 2.6 [1.5–5.3] | 3.6 [2.0–6.6] | <0.001 |
| Diabetes; N | 180 (53.7) | 166 (49.6) | 146 (43.6) | 95 (28.4) | <0.001 |
| CVD; N | 265 (79.1) | 280 (83.6) | 268 (80.0) | 243 (72.5) | 0.005 |
| Catheter use; N | 22 (6.6) | 19 (5.7) | 21 (6.3) | 10 (3.0) | 0.15 |
| Current smoking; N | 160 (47.8) | 178 (53.1) | 163 (48.8) | 175 (52.2) | 0.44 |
| High-Kt/V assignment; N | 173 (51.6) | 171 (51.0) | 172 (51.3) | 153 (45.7) | 0.35 |
| High-Flux assignment; N | 167 (49.8) | 190 (56.7) | 158(47.2) | 159 (47.5) | 0.05 |
| Serum albumin; mg/dl | 3.5 (0.4) | 3.6 (0.3) | 3.6 (0.3) | 3.7 (0.4) | <0.001 |
| Serum calcium; mg/dl | 9.1 (0.8) | 9.1 (0.9) | 9.4 (0.9) | 9.6 (1.1) | <0.001 |
| Serum phosphorus; mg/dl | 4.4 (1.3) | 5.6 (1.5) | 6.0 (1.7) | 7.1 (1.9) | <0.001 |
| Serum 25(OH)D; ng/ml | 18 [13–24] | 19 [14–25] | 19 [14–28] | 21 [15–31] | 0.001 |
| Serum 1,25(OH)2D; pg/ml | 5.7 [3.0–13.1] | 5.3 [2.4–12.4] | 6.8 [2.8–15.0] | 8.0 [3.2–16.6] | 0.01 |
| Serum iPTH; pg/ml | 171 [73–327] | 188 [87–396] | 159 [75–411] | 284 [101–552] | <0.001 |
| Serum hs-CRP; mg/L | 6.4 [2.4–17.0] | 6.5[3.1–14.9] | 6.2 [2.8–12.8] | 5.1[2.2–13.9] | 0.48 |
| Serum IL-6; pg/ml | 2.8 [1.6–6.6] | 3.5 [1.7–7.9] | 2.9 [1.7–7.4] | 3.1 [1.6–5.7] | 0.25 |
| Serum TNF-α; pg/ml | 29.8 [18.1–42.7] | 31.4 [20.1–46.7] | 30.0 [20.6–42.2] | 29.0 [19.6–42.8] | 0.66 |
| Serum IFN-δ; pg/ml | 1.3 [1.1–1.8] | 1.5 [1.1–2.1] | 1.4 [1.1–2.1] | 1.5 [1.1–2.1] | 0.004 |
| Vitamin D analog administration; % | 159 (47.5) | 166 (49.6) | 185 (55.2) | 210 (62.7) | <0.001 |
Data are presented as N (%), mean (SD) or median [IQR].
Vitamin D and Risk of Infectious and Cardiac Events and All-Cause Mortality
In time-dependent analyses, higher cumulative time-averaged serum 25(OH)D levels were consistently associated with lower risk of the infectious composite outcome, cardiac composite outcome, and of all-cause mortality in all models. In the base covariate adjustment model (Model 1), the HRs for the infectious composite, the cardiac composite, and all-cause mortality outcomes were consistently reduced for each of the second, third, and fourth quartiles compared with the first quartile of 25(OH)D. Additional adjustment for the inflammatory markers, serum albumin, hs-CRP, IL-6, tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ), did not attenuate the association. Further adjustment for the administration of vitamin D analogs had no effect on the association (Table 3). These relationships were weaker when the baseline 25(OH)D levels were used as the exposure variable, with statistically significant associations persisting only for all-cause mortality in Models 1 and 3 (Table 3).
Table 3.
Associations of serum 25(OH)D levels with infectious composite events, cardiac composite events or all-cause mortality
| 25(OH)D ng/ml | Baseline 25(OH)D as Exposure | Time-Dependent 25(OH)D as Exposure | ||||||
|---|---|---|---|---|---|---|---|---|
| Composite of First Infectious Hospitalization or Infectious Death | ||||||||
| HR (95% CI), by Quartile | HR (95% CI), by Quartile | |||||||
| Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | |
| <14 | 14–19 | 19–26 | >26 | <14 | 14–19 | 19–26 | >26 | |
| # of Events | 132 | 131 | 123 | 113 | 132 | 131 | 123 | 113 |
| Model 1 | 1.0 | 1.00 (0.77–1.30) | 0.96 (0.72–1.26) | 0.86 (0.64–1.16) | 1.0 | 0.79 (0.61–1.01) | 0.72 (0.55–0.94) | 0.66 (0.49–0.89) |
| Model 2 | 1.0 | 1.02 (0.78–1.34) | 1.08 (0.81–1.43) | 0.94 (0.69–1.28) | 1.0 | 0.79 (0.61–1.02) | 0.75 (0.57–0.98) | 0.72 (0.53–0.98) |
| Model 3 | 1.0 | 1.00 (0.77–1.30) | 0.95 (0.72–1.25) | 0.84 (0.62–1.14) | 1.0 | 0.79 (0.61–1.01) | 0.72 (0.55–0.94) | 0.66 (0.49–0.89) |
| Composite of First Cardiac Hospitalization or Cardiac Death | ||||||||
| # of Events | 133 | 138 | 127 | 116 | 133 | 138 | 127 | 116 |
| Model 1 | 1.0 | 1.05 (0.80–1.37) | 1.00 (0.75–1.33) | 0.94 (0.69–1.28) | 1.0 | 0.77 (0.59–0.99) | 0.74 (0.57–0.98) | 0.71 (0.53–0.96) |
| Model 2 | 1.0 | 1.06 (0.81–1.39) | 1.02 (0.77–1.37) | 0.99 (0.72–1.35) | 1.0 | 0.75 (0.58–0.98) | 0.73 (0.55–0.95) | 0.71 (0.52–0.96) |
| Model 3 | 1.0 | 1.06 (0.81–1.37) | 0.98 (0.74–1.31) | 0.89 (0.65–1.21) | 1.0 | 0.77 (0.59–1.00) | 0.74 (0.57–0.97) | 0.70 (0.52–0.94) |
| All-Cause Mortality | ||||||||
| # of Events | 167 | 151 | 139 | 125 | 167 | 151 | 139 | 125 |
| Model 1 | 1.0 | 0.88 (0.68–1.14) | 0.80 (0.61–1.05) | 0.72 (0.53–0.97) | 1.0 | 0.57 (0.45–0.73) | 0.50 (0.38–0.65) | 0.46 (0.34–0.62) |
| Model 2 | 1.0 | 0.93 (0.71–1.21) | 0.92 (0.69–1.22) | 0.84 (0.61–1.14) | 1.0 | 0.60 (0.47–0.77) | 0.55 (0.42–0.72) | 0.54 (0.40–0.74) |
| Model 3 | 1.0 | 0.88 (0.68–1.14) | 0.79 (0.61–1.04) | 0.70 (0.51–0.94) | 1.0 | 0.57 (0.45–0.73) | 0.50 (0.38–0.65) | 0.46 (0.34–0.62) |
Model 1: Adjusted for age, gender, race, diabetic status, history of cardiac disease, years of dialysis, smoking status, urea Kt/V, and dialysis membrane flux assignments, central catheter use, serum calcium, serum phosphorus, serum intact parathyroid hormone, serum 1,25-dihydroxyvitamin D, and serum FGF23.
Model 2: Adjusted for covariates in Model 1 plus serum albumin, serum high-sensitivity C-reactive protein, interleukin-6, tumor necrosis factor-α, and interferon-γ.
Model 3: Adjusted for covariates in Model 2 plus vitamin D analog administration.
Quartile 1 is the reference group in all models.
No statistically significant associations were observed between baseline or time-dependent 1,25(OH)2D serum levels and clinical outcomes (Table 4), when modeled as quartile for the base model (Model 1), or after adjusting for markers of inflammation or administration of vitamin D analogies (Models 2 and 3).
Table 4.
Associations of serum 1,25(OH)2D levels with infectious composite events, cardiac composite events or all-cause mortality
| 1,25(OH)2D pg/ml | Baseline 1,25(OH)2D as Exposure | Time-Dependent 1,25(OH)2D as Exposure | ||||||
|---|---|---|---|---|---|---|---|---|
| Composite of First Infectious Hospitalization or Infectious Death | ||||||||
| HR (95% CI), by Quartile | HR (95% CI), by Quartile | |||||||
| Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | |
| < 2.8 | 2.8–6.3 | 6.3–14.4 | >14.4 | < 2.8 | 2.8–6.3 | 6.3–14.4 | >14.4 | |
| # of Events | 118 | 131 | 120 | 130 | 118 | 131 | 120 | 130 |
| Model 1 | 1.0 | 1.16 (0.88–1.53) | 0.96 (0.72–1.28) | 1.24 (0.93–1.65) | 1.0 | 1.29 (0.98–1.68) | 1.02 (0.77–1.35) | 1.17 (0.88–1.54) |
| Model 2 | 1.0 | 1.09 (0.82–1.45) | 0.84 (0.62–1.13) | 1.16 (0.87–1.54) | 1.0 | 1.26 (0.96–1.66) | 0.97 (0.72–1.29) | 1.17 (0.88–1.55) |
| Model 3 | 1.0 | 1.18 (0.89–1.56) | 1.00 (0.74–1.36) | 1.31 (0.96–1.80) | 1.0 | 1.29 (0.98–1.69) | 1.02 (0.77–1.36) | 1.17 (0.88–1.56) |
| Composite of First Cardiac Hospitalization or Cardiac Death | ||||||||
| # of Events | 129 | 127 | 120 | 138 | 129 | 127 | 120 | 138 |
| Model 1 | 1.0 | 0.81 (0.62–1.08) | 0.78 (0.58–1.04) | 0.96 (0.72–1.27) | 1.0 | 1.23 (0.94–1.62) | 1.00 (0.75–1.32) | 1.05 (0.79–1.39) |
| Model 2 | 1.0 | 0.81 (0.61–1.07) | 0.77 (0.57–1.03) | 0.98 (0.74–1.30) | 1.0 | 1.24 (0.95–1.63) | 1.00 (0.76–1.33) | 1.08 (0.81–1.43) |
| Model 3 | 1.0 | 0.84 (0.64–1.12) | 0.88 (0.65–1.20) | 1.14 (0.83–1.58) | 1.0 | 1.25 (0.95–1.64) | 1.05 (0.79–1.40) | 1.14 (0.85–1.52) |
| All-Cause Mortality | ||||||||
| # of Events | 149 | 148 | 132 | 153 | 149 | 148 | 132 | 153 |
| Model 1 | 1.0 | 0.85 (0.65–1.11) | 0.80 (0.60–1.07) | 1.03 (0.79–1.36) | 1.0 | 0.92 (0.71–1.18) | 0.73 (0.56–0.96) | 0.92 (0.70–1.20) |
| Model 2 | 1.0 | 0.84 (0.63–1.11) | 0.77 (0.58–1.04) | 1.02 (0.76–1.35) | 1.0 | 0.90 (0.70–1.17) | 0.74 (0.56–0.98) | 0.98 (0.74–1.30) |
| Model 3 | 1.0 | 0.87 (0.66–1.14) | 0.85 (0.63–1.15) | 1.13 (0.84–1.54) | 1.0 | 0.92 (0.71–1.18) | 0.73 (0.55–0.96) | 0.92 (0.70–1.21) |
Model 1: Adjusted for age, gender, race, diabetic status, history of cardiac disease, years of dialysis, smoking status, urea Kt/V, and dialysis membrane flux assignments, central catheter use, serum calcium, serum phosphorus, serum intact parathyroid hormone, serum 25-hydroxyvitamin D, and serum FGF23.
Model 2: Adjusted for covariates in Model 1 plus serum albumin, serum high-sensitivity C-reactive protein, interleukin-6, tumor necrosis factor-α, and interferon-γ.
Model 3: Adjusted for covariates in Model 2 plus vitamin D analog administration.
Quartile 1 is the reference group in all models.
FGF23 and The Risk of Infectious and Cardiac Events and All-Cause Mortality
Higher circulating levels of baseline FGF23 levels were associated with statistically significant increases in the HR of each of the three outcomes (infectious composite, cardiac composite, and all-cause mortality), after adjusting for the covariates in the base model (Model 1). HRs for the highest quartile of FGF23 were 1.57 (95% CI, 1.13–2.18), 1.49 (95% CI, 1.06–2.08), and 1.50 (95% CI, 1.07–2.12) for the infectious and cardiac composite outcome and for all-cause mortality, respectively (Model 1). Results were weaker when FGF23 was modeled as time-dependent exposure variable (Table 5). These relationships persisted after expanding the covariates to include markers of inflammation in Model 2 and after adjusting for administration of vitamin D (Model 3).
Table 5.
Associations of serum FGF23 levels with infectious composite event, cardiac composite event, and all-cause mortality
| FGF23 pg/ml | Baseline FGF23 as Exposure | Time-dependent FGF23 as Exposure | ||||||
|---|---|---|---|---|---|---|---|---|
| Composite of First Infectious Hospitalization or Infectious Death | ||||||||
| HR (95% CI), by Quartile | HR (95% CI), by Quartile | |||||||
| Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | |
| <724 | 728–3115 | 3122–12923 | >12923 | <724 | 728–3115 | 3122–12923 | >12923 | |
| # of Events | 133 | 112 | 134 | 120 | 133 | 112 | 134 | 120 |
| Model 1 | 1.0 | 0.95 (0.72–1.24) | 1.42 (1.06–1.89) | 1.57 (1.13–2.18) | 1.0 | 1.13 (0.86–1.49) | 1.34 (1.00–1.80) | 1.49 (1.06–2.11) |
| Model 2 | 1.0 | 0.91 (0.68–1.21) | 1.37 (1.02–1.84) | 1.41 (1.00–1.99) | 1.0 | 1.11 (0.84–1.47) | 1.30 (0.97–1.75) | 1.32 (0.93–1.88) |
| Model 3 | 1.0 | 0.95 (0.72–1.25) | 1.43 (1.07–1.91) | 1.59 (1.14–2.22) | 1.0 | 1.13 (0.86–1.49) | 1.35 (1.00–1.80) | 1.50 (1.06–2.12) |
| Composite of First Cardiac Hospitalization or Cardiac Death | ||||||||
| # of Events | 127 | 127 | 140 | 120 | 127 | 127 | 140 | 120 |
| Model 1 | 1.0 | 1.05 (0.80–1.40) | 1.28 (0.96–1.71) | 1.49 (1.06–2.08) | 1.0 | 0.98 (0.74–1.29) | 1.24 (0.90–1.72) | 1.33 (1.01–1.76) |
| Model 2 | 1.0 | 1.06 (0.79–1.42) | 1.29 (0.96–1.73) | 1.50 (1.06–2.11) | 1.0 | 0.95 (0.72–1.25) | 1.21 (0.87–1.68) | 1.33 (1.01–1.75) |
| Model 3 | 1.0 | 1.05 (0.79–1.39) | 1.30 (0.97–1.73) | 1.52 (1.08–2.13) | 1.0 | 0.98 (0.75–1.29) | 1.26 (0.91–1.74) | 1.36 (1.03–1.79) |
| All-Cause Mortality | ||||||||
| # of Events | 146 | 147 | 157 | 132 | 146 | 147 | 157 | 132 |
| Model 1 | 1.0 | 1.13 (0.85–1.48) | 1.32 (0.99–1.75) | 1.50 (1.07–2.12) | 1.0 | 1.13 (0.87–1.48) | 1.21 (0.91–1.61) | 1.55 (1.11–2.17) |
| Model 2 | 1.0 | 1.15 (0.86–1.53) | 1.36 (1.01–1.83) | 1.50 (1.06–2.14) | 1.0 | 1.05 (0.80–1.39) | 1.15 (0.86–1.53) | 1.27 (0.90–1.79) |
| Model 3 | 1.0 | 1.14 (0.86–1.50) | 1.32 (0.99–1.76) | 1.55 (1.09–2.18) | 1.0 | 1.13 (0.87–1.48) | 1.21 (0.91–1.61) | 1.56 (1.11–2.18) |
Model 1:Adjusted for age, gender, race, diabetic status, history of cardiac disease, years of dialysis, smoking status, urea Kt/V, and dialysis membrane flux assignments, central catheter use, serum calcium, serum phosphorus, serum intact parathyroid hormone, serum 25-hydroxyvitamin D, and serum 1,25-dihydroxyvitamin D.
Model 2: Adjusted for covariates in Model 1 plus serum albumin, serum high-sensitivity C-reactive protein, interleukin-6, tumor necrosis factor-α, and interferon-γ.
Model 3: Adjusted for covariates in Model 2 plus vitamin D analog administration.
Quartile 1 is the reference group in all models.
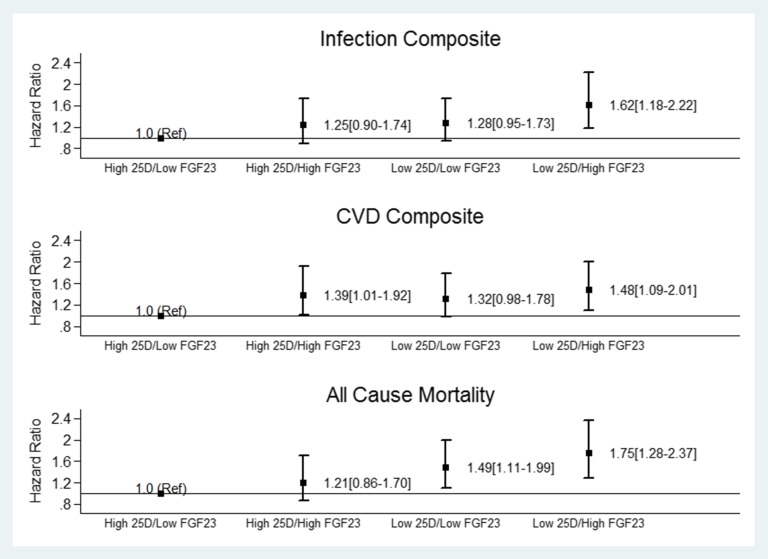
Given our findings above, subsequent analyses were performed to examine the association between serum levels of 25(OH)D and FGF23 with each of the three outcomes (infectious composite, cardiac composite, and all-cause mortality) in time-varying analyses. The entire cohort was divided into four groups on the basis of the median 25(OH)D and FGF23 serum levels. Cox proportional hazard models, adjusted for the base model (Model 1), show that the highest risk for the infectious and cardiac composite and all-cause mortality occurred in HEMO participants with a 25(OH)D serum level below the median and with an FGF23 serum level greater than the median value (Figure 2).
Figure 2.
The association of low serum levels of 25-hydroxyviatmin D (25(OH)D with the infectious and cardiac composite and all-cause mortality in the HEMO study are more pronounced with higher serum FGF23. The graph displays the HR and 95% CI for each of the outcomes in the HEMO study according to serum median levels of 25(OH)D and FGF23.
Discussion
In this large cohort of chronic hemodialysis patients with long-term follow-up, we observed that higher serum levels of 25(OH)D were associated with decreased risks of infectious events (composite of infectious hospitalization and infectious death), cardiac events (composite of cardiac hospitalization and cardiac deaths) and all-cause deaths. In contrast, while the median serum 1,25(OH)2D concentration was lower than the definition of 1,25(OH)2D deficiency (i.e., serum levels <22 pg/ml),26 there were no associations between 1,25(OH)2D and any of the clinical outcomes examined. In addition, high serum FGF23 levels were also associated with infectious and cardiac events. Thus, the novel aspects of this study include: (1) the longitudinal assessment of markers of mineral metabolism within individual chronic hemodialysis patients and its relationship with cardiac outcomes, (2) the association of low serum 25(OH)D and high FGF23 levels with infectious events, and (3) inflammation does not appear to be a potential mechanism underlying the strong association between vitamin D deficiency and FGF23 excess with outcomes in hemodialysis as these epidemiologic relationships were not significantly attenuated by the adjustment for important markers of inflammation. Our data suggest that disordered mineral metabolism is not only a consequence of chronic dialysis, but it may contribute to major adverse clinical outcomes in this population. Interventional studies are urgently needed to determine whether therapeutic strategies that delay or attenuate the severity of disordered mineral metabolism can improve these outcomes.
Higher circulating 25(OH)D levels, when modeled as a time-dependent covariate, showed a graded relationship with a decreased risk of infectious and cardiac events and all-cause mortality that was robust to all modeling strategies and independent of markers of inflammation. There are at least two hypotheses that potentially explain these strong associations when modeled as a time-dependent covariate when compared with a single time point. One hypothesis is that nutritional vitamin D, which is an important determinant of 25(OH)D levels, is actually directly or indirectly protective against these systemic events, by virtue of its biologic effects.6–9 A second hypothesis is that high serum 25(OH)D level is merely a biomarker of good general health. In this regard, physical inactivity, diets low in dairy and oily fish, adiposity, and other unhealthy habits have been reported to be associated with low circulating 25(OH)D levels.27
The stronger association observed with the time-dependent analyses when compared when using a single time point of 25(OH)D level is likely due to the fact that time-dependent Cox regression models relate infectious and cardiac events to the most recent 25(OH)D serum level within the prior year.
The discovery that many extrarenal tissues also possess both the 1-α-hydroxylase enzyme and the vitamin D receptors (VDR) has provided new insights into the important physiologic autocrine and paracrine roles of vitamin D in various organs.28–31 According to this paradigm, the availability of circulating 25(OH)D is essential for these organs to generate 1,25(OH)2D which subsequently acts in an autocrine or paracrine manner to serve their respective local needs. Thus, contrary to the kidney, the extrarenal production of 1,25(OH)2D does not contribute to the circulating pool of 1,25(OH)2D.28 This might explain the association between circulating levels of 25(OH)D and outcomes but the lack of association between circulating levels of 1,25(OH)2D with clinical events in the present analysis.
The effects of vitamin D on immunity represent a relatively new and exciting area of investigation. Both 1-α-hydroxylase and VDR are expressed in immune cells, suggesting that 25(OH)D has paracrine or autocrine function in these cells.7,28,32 When Toll-like receptors on macrophages bind bacterial wall lipopolysaccharide, 1-α-hydroxylase and VDR expression is upregulated, resulting in the local conversion of 25(OH)D to 1,25(OH)2D, which in turn increases the expression of bactericidal proteins, cathelicidin, and beta defensin.14 Cathelicidin transcription is particularly dependent on sufficient circulating levels of 25(OH)D.14,33 The findings of the present study are consistent with these biologic principles and other epidemiologic reports linking low serum 25(OH)D levels to increased risks of infection in the nondialysis population.34,35 The present study makes an important contribution to the literature by demonstrating this association in the chronic dialysis population and provides another plausible mechanism for the well known impaired immunity in these patients.
The exact mechanisms by which 25(OH)D may protect against CVD have not been fully delineated. It is, however, known that vitamin D can stimulate the secretion/action of insulin, regulate blood pressure through its modulation of renin production, inhibit cellular proliferation, and alter the inflammatory response associated with atherogenesis.36 All of these reported effects of vitamin D appear to be mediated by the active form 1,25(OH)2D3 although recent evidence suggests that 25(OH)D might also have a direct effect on the vitamin D receptor.29 In fact, London et al.37 found that serum 25(OH)D levels positively correlated with brachial artery distensibility and negatively correlated with aortic pulse wave velocity in a cross-sectional study of chronic hemodialysis patients. Wolf et al. showed that lower serum levels of 25(OH)D in incident chronic hemodialysis patients were associated with an increased risk of all-cause and CVD mortality during a short-term follow-up of 3 months.8 The present study confirmed and extended these findings and shows a long-term (median 3 years) inverse association between high serum 25(OH)D levels and cardiac events. Collectively, these data are supportive of a potential cardio-protective effect of vitamin D supplementation, although large long-term randomized clinical trials to confirm or refute this effect in chronic dialysis patients are lacking.
In the present study, we also found higher serum FGF23 levels to be a predictor of infectious events, cardiac events, and all-cause mortality, independent of traditional CVD risk factors and inflammatory markers. This association was particularly strong when baseline FGF23 values were used as the exposure, but was attenuated in the time-dependent models.
A novel finding of the present study was the relationship between higher serum FGF23 levels and infectious events. It is well established that FGF23 is an endocrine hormone acting in the kidney as a phosphaturic hormone and a suppressor of 1,25(OH)2D production, through an inhibition of the CYP27B1 enzyme.15,16 In addition, it is possible that FGF23 also regulates the innate immunity.21,22 A plausible hypothesis is that FGF23 inhibits CYP27B1 in monocytic cells, with subsequent effects on intracellular synthesis of 1,25(OH)2D.21,22,28 In fact, treatment of peripheral blood mononuclear cells isolated from peritoneal dialysate effluents of uremic patients decreased the mRNA expression of CYP27B1 and conversion of 25(OH)D to 1,25(OH)2D.21,22 These biologic effects of FGF23 on monocytic cells support the observation in the present study, which is the first clinical evidence of a relationship between higher serum FGF23 levels and infectious events.
Previous observational studies have found similar relationship of serum FGF23 concentration with all-cause mortality but less is known about the longitudinal relationship between serum FGF23 levels with cardiac events in hemodialysis patients. In a prospective cohort of incident dialysis patients, the risk of all-cause mortality increased with higher serum FGF23 levels20; however, specific cardiac hospitalization or causes of death were not examined. Increased FGF23 levels have also been shown to be associated with left ventricular hypertrophy in both pre-dialysis and dialysis patients,38,39 which appears to be a plausible mechanism underlying the relationship between higher serum levels of FGF23 and cardiac events in the present study.
Our study has several limitations. First, we were unable to determine whether decreased 25(OH)D or increased FGF23 serum levels have direct biologic effects. Second, there was no information on the use of nutritional vitamin D supplementation in the HEMO study database. However, given the time period in which the HEMO study was performed (mostly in the 1990s), nutritional vitamin D supplementation was not commonly encouraged in this patient population. The present study does not attempt to ascertain the sources of vitamin D, such as the food intake, sun exposure or vitamin D supplements; instead it examines the associations between vitamin D serum levels and clinical events regardless of the sources of vitamin D. Third, there was no assessment of important confounding variables like vitamin D-binding protein or cathelicidin levels. Finally, this study was comprised of participants undergoing chronic hemodialysis; caution should be exercised when extrapolating these results to people with milder forms of CKD.
These limitations notwithstanding, our study also has several strengths. First, to our knowledge, this study is the first evaluating the longitudinal association of components of the vitamin D axis and FGF23 with major adverse clinical events in chronic hemodialysis patients. Second, the HEMO study from which these data were obtained had a large number of patients with a median follow-up period of 3 years. Third, infectious and cardiac events, as used in the present analysis, were adjudicated by an outcome committee in the HEMO study. Fourth, the comprehensive dataset allowed for adjustment of important regulators of mineral metabolism, including serum calcium, phosphorus, and iPTH levels. Finally, serum levels of several important inflammatory markers, including hs-CRP, IL-6, TNF-α, and IFN-γ, were measured and used in statistical adjustments.
In conclusion, higher serum 25(OH)D levels were independently associated with lower risks of infectious and cardiac events in chronic hemodialysis patients. In contrast, higher serum FGF23 levels were a strong and independent predictor of the risk of infectious and cardiac events. Further studies are needed to further confirm the postulated mechanisms underlying these associations and whether interventions that increase serum 25(OH)D levels and/or block FGF23 might improve the clinical outcomes of chronic dialysis patients.
Concise Methods
Study Population
The HEMO study was a prospective, randomized, multicenter clinical trial with a 2×2 factorial design and equal allocation to each treatment arm.25 In total, 1846 hemodialysis patients were randomly assigned to either low-flux or high-flux membrane dialyzers and to either a standard dose of dialysis targeting an equilibrated dose (eKt/V of urea) of 1.05 or a high dose targeting an eKt/V of urea of 1.45. Among the eligibility criteria were (1) a minimum of 3 months on hemodialysis, and (2) residual kidney urea clearance of <1.5 ml/min per 35 l of urea distribution volume to limit the contribution from native kidneys and hence maximize the relative effect of dialysis on total body solute clearances. Of note, the primary results of that study showed no statistical difference in all-cause mortality between the high-dose and low-dose arms and between the high-flux and low-flux arms.
We restricted our analytical cohort to 1340 HEMO study participants because of lack of samples in some HEMO study participants (Supplemental Figure 1). The cohort included 706 HEMO participants who provided stored samples at their baseline visit, and 634 additional participants in whom 1-year samples, but not baseline samples, were available. In this report, we treat the 1-year visit and covariates at that visit, for this latter cohort as their “baseline” visit, and relate exposure variables at this visit to clinical outcomes. The respective institutional review board at the University of Colorado Denver and the University of Utah approved this ancillary study.
Exposure Variables
All serum samples collected during the course of the HEMO study (March 1995 to October 2000) were stored in a central repository at −80°C until they were shipped to the University of Washington for analyses. The stability of vitamin D metabolites and FGF23 in frozen samples has been previously well described.19,40 The primary exposure variables for the present analysis were serum levels of 25(OH)D, 1,25(OH)2D, and FGF23.
25(OH)D
Total 25(OH)D (sum of 25(OH)D2 and 25(OH)D3) was measured using immunoaffinity purification and liquid chromatography-tandem mass spectrometry. Calibration was confirmed with National Institute of Standards and Technology’s standard reference material 972.41 The lower limit of detection was 1.6 ng/ml and 2.0 ng/ml for 25(OH)D2 and 25(OH)D3, respectively. The between-assay imprecision (%CV) was 10.3% for 25(OH)D2 and 6.0% for 25(OH)D3.
1,25(OH)2D
Serum 1,25(OH)2D levels were also measured using high-performance liquid chromatography–tandem mass spectrometry following immunoaffinity purification of the samples. 1,25(OH)2D2 and 1,25(OH)2D3 levels were reported separately and total 1,25(OH)2D calculated from these values. Limits of detection with this method were 5.6 pg/ml and 6.8 pg/ml for 1,25(OH)2D2 and 1,25(OH)2D3. The interassay CVs were 11.4% and 12.3% for each analyte, respectively. This methodology does not detect and does not have interference from paricalcitol or the C-3 epimeric forms of 25(OH)D3 and 1,25(OH)2D3.
FGF23
Intact FGF23 was measured using the Kainos immunoassay, which detects the full-length, biologically intact FGF23 molecule via midmolecule and distal epitopes. The intra- and interassay CVs were 3.8% and 3.0%, respectively, for this assay. The Kainos immunoassay assay has been shown to be the most sensitive assay for FGF23.42
Outcomes
The planned duration of follow-up for the HEMO study ranged from 0.8 to 6.6 years (mean 4.24 years), depending on the time of randomization of the individual participants. Because of deaths and transplantation, however, the mean actual follow-up duration was 2.84 years. Reflecting termination of follow-up due to death and transplant, the mean (SD) follow-up duration for mortality in this subcohort was 2.80 (1.7) years. Classifications of outcomes were made at the clinical centers and then reviewed by an outcome committee composed of HEMO study investigators who were unaware of the treatment assignments.25 The outcomes for this analysis included: (1) time to infectious event (composite of first infectious hospitalization or infectious death), (2) time to cardiac event (composite of first cardiac hospitalization or cardiac death), and (3) time to death from any cause. Of note, all-cause mortality was the primary outcome of the HEMO study, while the infection and cardiac composites were prespecified secondary outcomes.
Covariates
Patient age, gender, race, duration of chronic dialysis, diabetic status (based on past or current use of hypoglycemic agents), comorbid medical conditions, and history of cigarette smoking were recorded during the baseline phase of the trial. Presence of central venous catheter as hemodialysis vascular access and use of medications including active vitamin D analogs were recorded every 6 months during follow-up. Serum albumin was measured using nephelometry, and serum calcium, phosphorus, and iPTH were measured at 6-month intervals by the sites’ local laboratories using standard techniques. From the stored serum samples, inflammatory markers were measured. These markers included hs-CRP, IL-6, TNF-α, and IFN-γ. Serum hs-CRP was measured using a UniCel DxC system from Beckman. The inter-assay CV for this assay was 4.9%. Serum IL-6, TNF-α, and IFN-γ were measured using the luminex platform using EMD Millipore’s reagents. The inter-assay CVs were 18.9%, 18.9%, and 19.7% for each analyte.
Statistical Analyses
Demographic, cardiovascular risk factors, and serum markers of inflammation were compared across quartiles of baseline serum 25(OH)D, 1,25(OH)2D, and FGF23 levels using the chi-squared test for categorical factors, and ANOVA for continuous variables, with log transformations applied to variables with heavy positive skewness. Pearson product-moment correlations were used to summarize the cross-sectional relationships among calcium, phosphorus, 25(OH)D, 1,25(OH)2D, iPTH, FGF23, and inflammatory marker concentrations at baseline, following logarithmic transformation when appropriate. CVs for 25(OH)D, 1,25(OH)2D, and FGF23 we calculated from the yearly measurements.
Separate analyses for 25(OH)D, 1,25(OH)2D, and FGF23 were performed. Each of these exposure variables was analyzed as a categorical variable split into four quartiles, with the lowest quartile serving as the reference category. The relative hazards between the higher quartiles and the lowest quartile were used to characterize the pattern of the relationship between each exposure and outcome, allowing for the possibility of nonlinear relationships.
We used time-dependent Cox regression to relate the clinical outcomes to the cumulative average serum 25(OH)D, 1,25(OH)2D, and FGF23 concentrations. Covariates which changed over time (age, prior years on dialysis, use of a central venous catheter, smoking, mineral metabolism markers, and inflammatory markers) were updated at each annual assessment corresponding to the exposure variable measurements. Fixed-covariate Cox regression was used to relate the same outcomes to the baseline values of these minerals after adjustment for the baseline covariates described below.
We used a combination of criteria based on the HEMO study design, subject-matter considerations, and biologic understanding to designate two nested sets of covariates for inclusion in the Cox regression models. The base model (labeled as Model 1) included age, gender, race, diabetic status, history of cardiac disease, number of years on dialysis, smoking status, Kt/V, and flux treatment assignments in the HEMO study, use of central catheter as vascular access, as well as serum calcium, phosphorus, iPTH, 25(OH)D, 1,25(OH)2D, and FGF23 concentrations. Serum 25(OH)D, 1,25(OH)2D, and FGF23 were included in the base model only when the variable was not modeled as an exposure. The first nine of the covariates listed above were among the basic set of prespecified baseline factors that have been included in most prior analyses of the HEMO study data. The final six covariates were added specifically as potential confounders for the relationship between the proposed exposure variables and clinical outcomes. In Model 2, the covariates included those used in the base model, plus serum concentrations of albumin and inflammatory markers, which are also possible confounders. The serum inflammatory markers included hs-CRP, IL-6, TNF-α, and IFN-γ. Model 2 was performed to test if inflammation might be in the causal pathway of low vitamin D or high FGF23 serum levels leading to clinical outcomes. Model 3 included the covariates included in Model 2 plus usage of vitamin D analogs. The results of Model 1 are given primary emphasis in our interpretation of the results.
Proportional hazards assumptions were evaluated using plots of Schoenfeld residuals and consideration of interaction terms between predictor variables and follow-up time. We considered models with cubic splines in quantitative variables and plots of martingale residuals to identify deviations from the linearity assumptions of the Cox regression models. Last value carried forward imputation was used to impute missing data in time-dependent covariates. Two-tailed values of P<0.05 were considered statistically significant without formal adjustment for multiple comparisons; however, results were not deemed as conclusive unless they were observed consistently for both the categorical and continuous models in the exposure variables. All statistical analyses were performed with Statistical Analysis System software, version 9.3 (SAS Institute, Cary, NC).
Disclosures
None.
Supplementary Material
Acknowledgments
The research reported in this study was supported by National Institute of Diabetes and Digestive and Kidney Diseases grant R01 DK081473, the HEMO Executive Committee and the HEMO site investigators and institutions.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014101009/-/DCSupplemental.
References
- 1.Cheung AK, Sarnak MJ, Yan G, Dwyer JT, Heyka RJ, Rocco MV, Teehan BP, Levey AS: Atherosclerotic cardiovascular disease risks in chronic hemodialysis patients. Kidney Int 58: 353–362, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Allon M: Evidence-based cardiology in hemodialysis patients. J Am Soc Nephrol 24: 1934–1943, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foley RN, Guo H, Snyder JJ, Gilbertson DT, Collins AJ: Septicemia in the United States dialysis population, 1991 to 1999. J Am Soc Nephrol 15: 1038–1045, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Allon M, Radeva M, Bailey J, Beddhu S, Butterly D, Coyne DW, Depner TA, Gassman JJ, Kaufman AM, Kaysen GA, Lewis JA, Schwab SJ, HEMO Study Group : The spectrum of infection-related morbidity in hospitalized haemodialysis patients. Nephrol Dial Transplant 20: 1180–1186, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Chavers BM, Solid CA, Gilbertson DT, Collins AJ: Infection-related hospitalization rates in pediatric versus adult patients with end-stage renal disease in the United States. J Am Soc Nephrol 18: 952–959, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Evenepoel P, Rodriguez M, Ketteler M: Laboratory abnormalities in CKD-MBD: markers, predictors, or mediators of disease? Semin Nephrol 34: 151–163, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Holick MF: Vitamin D deficiency. N Engl J Med 357: 266–281, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Wolf M, Shah A, Gutierrez O, Ankers E, Monroy M, Tamez H, Steele D, Chang Y, Camargo CA, Jr, Tonelli M, Thadhani R: Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int 72: 1004–1013, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Drechsler C, Verduijn M, Pilz S, Dekker FW, Krediet RT, Ritz E, Wanner C, Boeschoten EW, Brandenburg V, NECOSAD Study Group : Vitamin D status and clinical outcomes in incident dialysis patients: results from the NECOSAD study. Nephrol Dial Transplant 26: 1024–1032, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM: Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15: 2208–2218, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Plantinga LC, Fink NE, Melamed ML, Briggs WA, Powe NR, Jaar BG: Serum phosphate levels and risk of infection in incident dialysis patients. Clin J Am Soc Nephrol 3: 1398–1406, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chonchol M, Kendrick J, Targher G: Extra-skeletal effects of vitamin D deficiency in chronic kidney disease. Ann Med 43: 273–282, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gunville CF, Mourani PM, Ginde AA: The role of vitamin D in prevention and treatment of infection. Inflamm Allergy Drug Targets 12: 239–245, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zügel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL: Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311: 1770–1773, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Saito H, Maeda A, Ohtomo S, Hirata M, Kusano K, Kato S, Ogata E, Segawa H, Miyamoto K, Fukushima N: Circulating FGF-23 is regulated by 1alpha,25-dihydroxyvitamin D3 and phosphorus in vivo. J Biol Chem 280: 2543–2549, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Stubbs J, Liu S, Quarles LD: Role of fibroblast growth factor 23 in phosphate homeostasis and pathogenesis of disordered mineral metabolism in chronic kidney disease. Semin Dial 20: 302–308, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Gutiérrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, Sarwar A, Hoffmann U, Coglianese E, Christenson R, Wang TJ, deFilippi C, Wolf M: Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation 119: 2545–2552, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutiérrez OM, Steigerwalt S, He J, Schwartz S, Lo J, Ojo A, Sondheimer J, Hsu CY, Lash J, Leonard M, Kusek JW, Feldman HI, Wolf M, Chronic Renal Insufficiency Cohort (CRIC) Study Group : Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 305: 2432–2439, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kendrick J, Cheung AK, Kaufman JS, Greene T, Roberts WL, Smits G, Chonchol M, HOST Investigators : FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol 22: 1913–1922, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gutiérrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Jüppner H, Wolf M: Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 359: 584–592, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bacchetta J, Salusky IB, Hewison M: Beyond mineral metabolism, is there an interplay between FGF23 and vitamin D in innate immunity? Pediatr Nephrol 28: 577–582, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bacchetta J, Sea JL, Chun RF, Lisse TS, Wesseling-Perry K, Gales B, Adams JS, Salusky IB, Hewison M: Fibroblast growth factor 23 inhibits extrarenal synthesis of 1,25-dihydroxyvitamin D in human monocytes. J Bone Miner Res 28: 46–55, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qureshi AR, Alvestrand A, Divino-Filho JC, Gutierrez A, Heimbürger O, Lindholm B, Bergström J: Inflammation, malnutrition, and cardiac disease as predictors of mortality in hemodialysis patients. J Am Soc Nephrol 13[Suppl 1]: S28–S36, 2002 [PubMed] [Google Scholar]
- 24.van Tellingen A, Grooteman MP, Schoorl M, Bartels PC, Schoorl M, van der Ploeg T, ter Wee PM, Nubé MJ: Intercurrent clinical events are predictive of plasma C-reactive protein levels in hemodialysis patients. Kidney Int 62: 632–638, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Eknoyan G, Beck GJ, Cheung AK, Daugirdas JT, Greene T, Kusek JW, Allon M, Bailey J, Delmez JA, Depner TA, Dwyer JT, Levey AS, Levin NW, Milford E, Ornt DB, Rocco MV, Schulman G, Schwab SJ, Teehan BP, Toto R, Hemodialysis (HEMO) Study Group : Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med 347: 2010–2019, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, Andress DL: Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int 71: 31–38, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Chonchol M, Scragg R: 25-Hydroxyvitamin D, insulin resistance, and kidney function in the Third National Health and Nutrition Examination Survey. Kidney Int 71: 134–139, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Duso AS, Brown AJ, Slatopolsky E: Vitamin D. Am J Physiol Renal Physiol 289: F8–F28, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Ritter CS, Armbrecht HJ, Slatopolsky E, Brown AJ: 25-Hydroxyvitamin D(3) suppresses PTH synthesis and secretion by bovine parathyroid cells. Kidney Int 70: 654–659, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Holick MF: Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr 80[Suppl]: 1678S–1688S, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Stumpf WE, Sar M, Reid FA, Tanaka Y, DeLuca HF: Target cells for 1,25-dihydroxyvitamin D3 in intestinal tract, stomach, kidney, skin, pituitary, and parathyroid. Science 206: 1188–1190, 1979 [DOI] [PubMed] [Google Scholar]
- 32.van Etten E, Stoffels K, Gysemans C, Mathieu C, Overbergh L: Regulation of vitamin D homeostasis: implications for the immune system. Nutr Rev 66[Suppl 2]: S125–S134, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Jeng L, Yamshchikov AV, Judd SE, Blumberg HM, Martin GS, Ziegler TR, Tangpricha V: Alterations in vitamin D status and anti-microbial peptide levels in patients in the intensive care unit with sepsis. J Transl Med 7: 28–36, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ginde AA, Mansbach JM, Camargo CA, Jr: Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med 169: 384–390, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laaksi I, Ruohola JP, Tuohimaa P, Auvinen A, Haataja R, Pihlajamäki H, Ylikomi T: An association of serum vitamin D concentrations < 40 nmol/L with acute respiratory tract infection in young Finnish men. Am J Clin Nutr 86: 714–717, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Holick MF: Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr 79: 362–371, 2004 [DOI] [PubMed] [Google Scholar]
- 37.London GM, Guérin AP, Verbeke FH, Pannier B, Boutouyrie P, Marchais SJ, Mëtivier F: Mineral metabolism and arterial functions in end-stage renal disease: potential role of 25-hydroxyvitamin D deficiency. J Am Soc Nephrol 18: 613–620, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Hsu HJ, Wu MS: Fibroblast growth factor 23: a possible cause of left ventricular hypertrophy in hemodialysis patients. Am J Med Sci 337: 116–122, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutiérrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro-O M, Kusek JW, Keane MG, Wolf M: FGF23 induces left ventricular hypertrophy. J Clin Invest 121: 4393–4408, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lissner D, Mason RS, Posen S: Stability of vitamin D metabolites in human blood serum and plasma. Clin Chem 27: 773–774, 1981 [PubMed] [Google Scholar]
- 41.Phinney KW: Development of a standard reference material for vitamin D in serum. Am J Clin Nutr 88: 511S–512S, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Imel EA, Peacock M, Pitukcheewanont P, Heller HJ, Ward LM, Shulman D, Kassem M, Rackoff P, Zimering M, Dalkin A, Drobny E, Colussi G, Shaker JL, Hoogendoorn EH, Hui SL, Econs MJ: Sensitivity of fibroblast growth factor 23 measurements in tumor-induced osteomalacia. J Clin Endocrinol Metab 91: 2055–2061, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.