Abstract
αKlotho is a multifunctional protein highly expressed in the kidney. Soluble αKlotho is released through cleavage of the extracellular domain from membrane αKlotho by secretases to function as an endocrine/paracrine substance. The role of the kidney in circulating αKlotho production and handling is incompletely understood, however. Here, we found higher αKlotho concentration in suprarenal compared with infrarenal inferior vena cava in both rats and humans. In rats, serum αKlotho concentration dropped precipitously after bilateral nephrectomy or upon treatment with inhibitors of αKlotho extracellular domain shedding. Furthermore, the serum half-life of exogenous αKlotho in anephric rats was four- to five-fold longer than that in normal rats, and exogenously injected labeled recombinant αKlotho was detected in the kidney and in urine of rats. Both in vivo (micropuncture) and in vitro (proximal tubule cell line) studies showed that αKlotho traffics from the basal to the apical side of the proximal tubule via transcytosis. Thus, we conclude that the kidney has dual roles in αKlotho homeostasis, producing and releasing αKlotho into the circulation and clearing αKlotho from the blood into the urinary lumen.
Keywords: [REMOVED HYPERLINK FIELD]Klotho, Transcytosis, Nephrectomy, Cell & Transport Physiology, renal proximal tubule cell, distal tubule
αKlotho was originally identified as an anti-aging gene as its disruption confers a premature aging-like syndrome.1 Overexpression of αKlotho reverses defects resulting from αKlotho deficiency and extends lifespan in mice.1,2 αKlotho has extremely pleiotropic effects on multiple organs and is involved in many physiologic processes.3,4 Two other paralogs that share homology to αKlotho, are βKlotho,5 and Klotho/lactase-phlorizin hydrolase-related protein (Klph); also called γKlotho.6 Both α- and β-Klotho function as co-receptors of different fibroblast growth factor (FGF) isoforms and serve distinct biologic actions.4,7–9
αKlotho is a type-I single-pass transmembrane protein with a long extracellular region harboring β-glucosidase-like motifs called Kl domains.1,5,6,10 αKlotho expression is restricted to a few organs including the kidney,1 parathyroid glands,11 and sinoatrial node12 where it forms tetrameric complexes with FGFR1c, 3c, or 4 (2Klotho:2FGFR), and serves as the high-affinity receptor for circulating FGF23 to regulate mineral metabolism.4,13–15 βKlotho is expressed in the liver and fat and forms complexes with FGFR1c or 4 to support FGF15/19 and FGF21 signaling. βKlotho is involved in regulation of caloric homeostasis and bile secretion.3,16,17 γKlotho is expressed in the skin and kidney, complexes with FGFR1b, 1c, 2c, or 4 in vitro but its physiologic function is not known.4,6
Of the three Klotho paralogs, αKlotho is the only one known to date to function both as a membrane-bound protein (co-receptor for FGF23) and a circulating endocrine substance. The extracellular portion of αKlotho is released from the cell by proteolytic cleavage18,19 and is present in the blood,20,21 urine,21 and cerebrospinal fluid.20 Theoretically, soluble αKlotho can form a complex with FGF and FGF receptor (FGFR) thus fulfilling its role as a co-receptor. However, as a soluble protein, it has much weaker action than transmembrane αKlotho thus seriously questioning whether soluble αKlotho can serve as a “deliverable co-receptor” for FGF23.14,15,22 It is more likely that soluble αKlotho circulates to exert its effects independent of FGF23 and FGFR.
In the kidney, soluble αKlotho acts from the urinary luminal side as an autocrine or paracrine enzyme to regulate transporters and ion channels. αKlotho modifies the Na+-phosphate cotransporter NaPi-2a as a glucuronidase21 and the renal outer medullary K+ and TRPV5 calcium channel as a sialidase.23,24 In addition, αKlotho functions as a glucuronidase on the basolateral membrane modifying organic cation transport.25 These enzymatic effects are clearly not due to αKlotho’s function as a co-receptor for FGF23 and are FGF23-independent. The phosphaturic effect of injected soluble αKlotho is fully intact in the FGF23−/− mouse and in cultured cells in the complete absence of FGF23.21 Further support of the endocrine action of circulating αKlotho is the fact that the systemic administration of recombinant Klotho protein26,27 or viral delivery of αKlotho28 can rescue a multitude of the phenotypic features of genetic αKlotho deficiency in different organ systems.26,29
The kidney has the highest level of expression of αKlotho, but it is important to know whether αKlotho circulating in serum is derived from the kidney. Serum αKlotho levels in patients with CKD are extremely variable30–39 probably due to assay-related variance. In contrast, renal αKlotho levels are uniformly reduced in CKD.37,40 Animal studies provide more consistent data supporting CKD as a state of global αKlotho deficiency,10,41–43 which was recently confirmed in humans.44 This finding supports but does not prove the model that the kidney is the source of endocrine αKlotho. The strongest evidence to date comes from renal tubule-specific partial deletion of αKlotho, which caused reduced serum αKlotho levels and systemic features closely resembling the phenotype in global αKlotho deletion mice; indicating that the kidney may be the principal source of endocrine αKlotho.45
Even less studied than its source, the route of elimination of circulating αKlotho is completely unknown. There is no information on how this 130 kD protein is cleared from the circulation. We previously demonstrated the presence of intravenously injected exogenous extracellular domain of recombinant αKlotho protein in animal urine42 but how αKlotho gets into the urine is not known.
The present study addresses several fundamental questions of αKlotho biology. Understanding the source and clearance of Klotho is critical because restoration of αKlotho in deficient states reverses the phenotype,2,26,43 which has great therapeutic potential.2,26,43 We show that the kidney is the principal contributor to circulating αKlotho and simultaneously, is also the major organ where circulating αKlotho is taken up from the circulation. Thus kidney disease is expected to affect both production and clearance of αKlotho in a complex fashion. Finally, circulating αKlotho is transported into the urine through transepithelial transcytosis, which provides an avenue for circulating αKlotho to function as luminal enzyme to modulate target proteins. These fundamental concepts are important for understanding the pathophysiologic mechanisms of αKlotho deficiency in kidney disease and any contemplation in replacement therapy.
Results
The Kidney as a Source of Circulating αKlotho
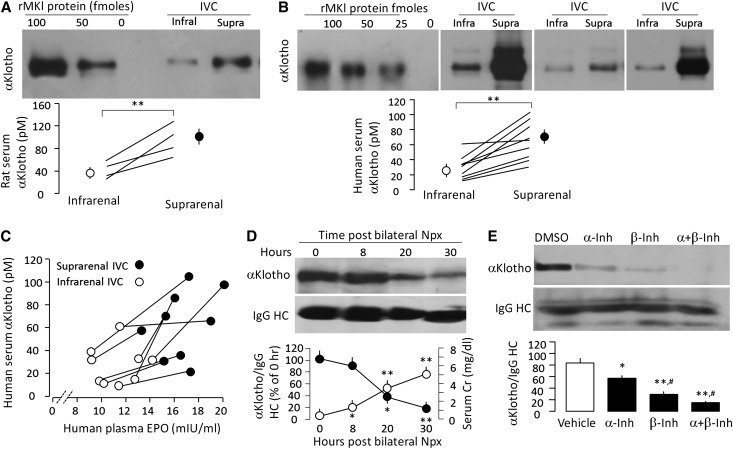
While αKlotho mRNA (RT-PCR, in situ hybridization, and RNA blot) is expressed in multiple tissues including heart, aorta, colon, pituitary gland, thyroid gland, pancreas, and gonads, the strongest expression by far is in the kidney.1,12,21 αKlotho protein expression is detected in renal tubules,21,46 choroid plexus,46 islet cells in pancreas,47 and the parathyroid gland.11,48,49 However, the high expression in the kidney does not necessarily indicate that circulating αKlotho is of renal origin. To test whether the kidney is a major source of endocrine αKlotho in mammals, we measured serum αKlotho protein in suprarenal and infrarenal vena cava of normal rats by direct puncture and human subjects who underwent right heart catheterization. All patients had eGFR≥60 ml/min/1.73 m2. The medical conditions of the patients are shown in Supplementary Table 1. Similar infrarenal-to-suprarenal increment in caval αKlotho level was observed in both rat and human serum samples (Figure 1, A and B). We plotted serum αKlotho against serum erythropoietin, a well-known renal-derived hormone, and found that as serum erythropoietin rose (Figure 1C), and serum creatinine (SCr) decreased from infrarenal-to-suprarenal inferior vena cava (Supplementary Figure 1), αKlotho increased indicating that the kidney secretes αKlotho into the circulation.
Figure 1.
The kidney contributes to circulating blood αKlotho. (A) αKlotho protein levels in inferior vena cava (IVC) in normal SD rats. Upper panel shows immunoblot of αKlotho protein immunoprecipitated with anti-αKlotho antibody from 0.1 ml serum from infrarenal and suprarenal IVC. Different amounts of recombinant full-length murine αKlotho protein (rMKl) in left three lanes. Bottom panel show four independent animals with each one connected by lines. Means±SD are shown as circles and error bars. Statistical significance was assessed by paired t test, and accepted when **P<0.01. (B) αKlotho protein levels in human IVC. Upper panel shows immunoblot of αKlotho proteins immunoprecipitated with anti-αKlotho antibody from 0.1 ml serum from infrarenal and suprarenal IVC from three subjects. Bottom panel is a summary from nine patients and significant difference between infrarenal and suprarenal IVC was assessed by paired t test, and significant differences were accepted when **P<0.01. (C) Serum αKlotho levels (y axis) were plotted against serum erythropoietin (EPO) levels (x axis) measured in the same sample. The infrarenal and suprarenal samples from the same individuals are connected by lines. (D) Change in endogenous serum αKlotho in anephric rats subjected to bilateral nephrectomy (Npx). Blood was drawn at the specified time points post-surgery. Serum αKlotho was determined by immunoprecipitation-immunoblot. Upper panel is a representative blot. Bottom panel is a summary of serum αKlotho and serum Cr from three independent experiments. Data are expressed as means±SD. Statistical significance was assessed by one-way ANOVA followed by Student–Newman–Keuls test, and significant differences were accepted when *P<0.05; **P<0.01* versus hour 0. (E) WT mice were injected intraperitoneally with α- or/and β-secretase inhibitors (α-Inh, β-Inh) for 2 days and serum αKlotho was determined by immunoprecipitation-immunoblot. Upper panel is one set of representative data. Bottom panel is a summary of arbitrary unit of serum αKlotho over IgG HC from three independent experiments. Statistical significance was assessed by one-way ANOVA followed by post hoc Student–Newman–Keuls test, and was considered significant when *P<0.05 or **P<0.01 versus vehicle (DMSO); #P<0.05 versus α-secretase inhibitor.
We next performed the classic organ ablation experiment to test the nephrogenic origin of αKlotho. When we removed both kidneys, serum αKlotho level dropped significantly to about half the normal level in one day. The fall in serum αKlotho parallels the rise in SCr (Figure 1D) and BUN (Supplemental Figure 2). The anephric state did not permit studies to continue for longer than 40–50 hours.
Type-I transmembrane proteins can be cleaved and released by A disintegrin and metalloproteases (ADAM) which is a metalloprotease that functions as “sheddase” for membrane-anchored growth factors, cytokines, and receptors.50 ADAM plays an important role in modulation of cell signaling in physiology and pathophysiology.50–52 Studies on cultured cell and kidney slices indicated that α-secretase (ADAM 10/17) and β-secretase modulate αKlotho ectodomain shedding and becoming soluble αKlotho protein,18,19 by acting on two cleavage sites: one close to the juxtamembrane region (aa 950 approximately981) and another between the KL1 and KL2 domains.53 To examine if secretases modulate circulating αKlotho in vivo, we injected α-secretase inhibitor doxycline hyclate and/or β-secretase inhibitor III into the intraperitoneal cavity of normal mice for 2 days and determined serum αKlotho in 48 hours. Serum αKlotho levels were decreased with either one or both α- and β-secretase inhibitors (Figure 1E) supporting the notion that αKlotho release depended on secretases.
The Kidney as a Portal of Clearance of Circulating αKlotho from Blood
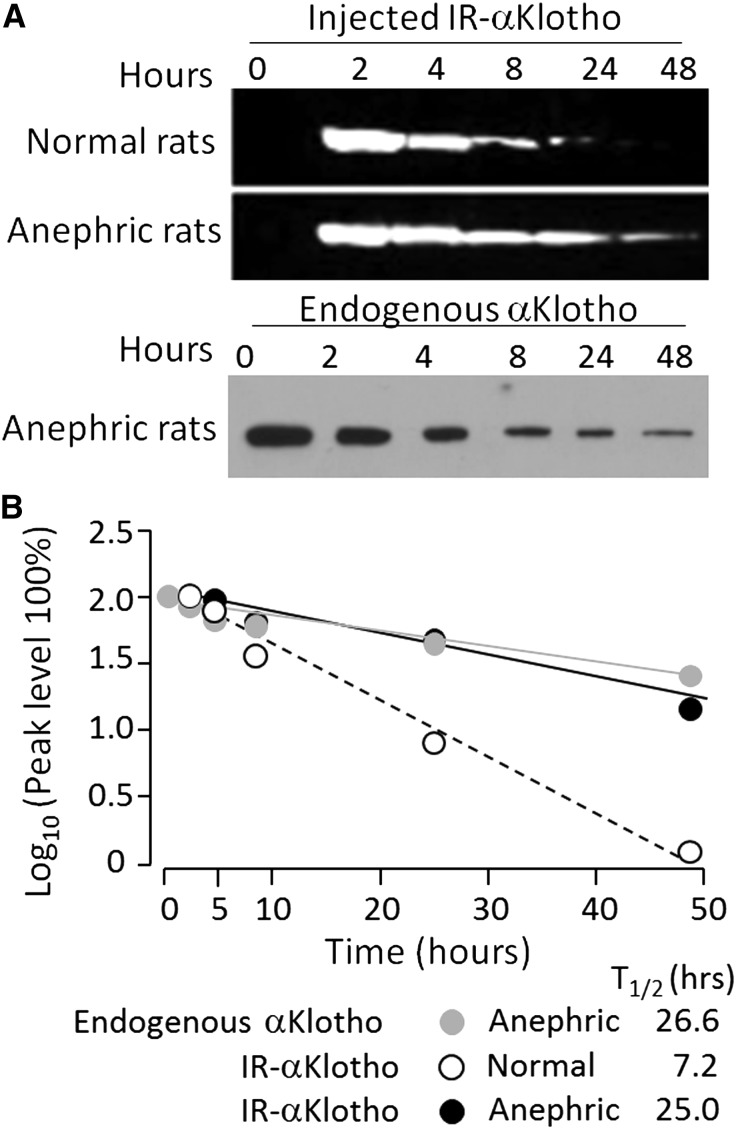
It is not known how αKlotho is cleared from the circulation. To examine this, we injected fluorescent exogenous recombinant αKlotho protein into the intraperitoneal cavity of normal and anephric rats. The levels of circulating exogenous αKlotho protein in anephric rats were similar to those in normal rats immediately after injection (Figure 2A), but the half-life of exogenous αKlotho protein in normal rats was much shorter than that in anephric rats (Figure 2, B) and the half-life of endogenous αKlotho upon nephrectomy closely approximates that of exogenous αKlotho in the anephric rats (Figure 2B, gray and black closed circles).
Figure 2.
The kidney clears circulating αKlotho. (A) Anephric rats were prepared by bilateral nephrectomy (Npx). Exogenous recombinant αKlotho protein labeled with infrared dye (IR-αKlotho) was injected and blood was drawn at the specified time points post-surgery and labeled αKlotho in the blood is shown in the upper panel. Endogenous αKlotho in serum of rats after Npx is shown in the bottom panel. Three independent experiments were performed showing similar results. (B) Half-life (T1/2) of exogenous αKlotho in normal and anephric rats and of endogenous αKlotho protein in anephric rats.
Distribution of Exogenous αKlotho Protein in Rodent Organs
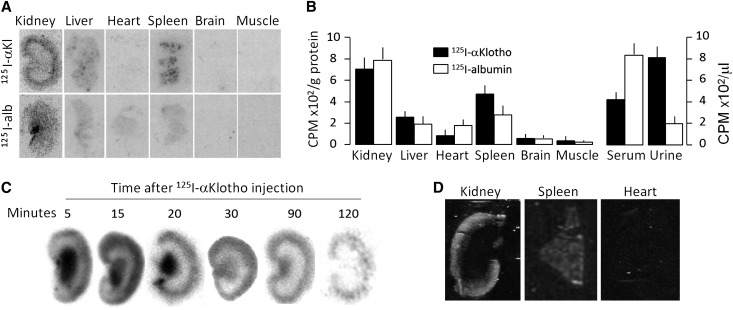
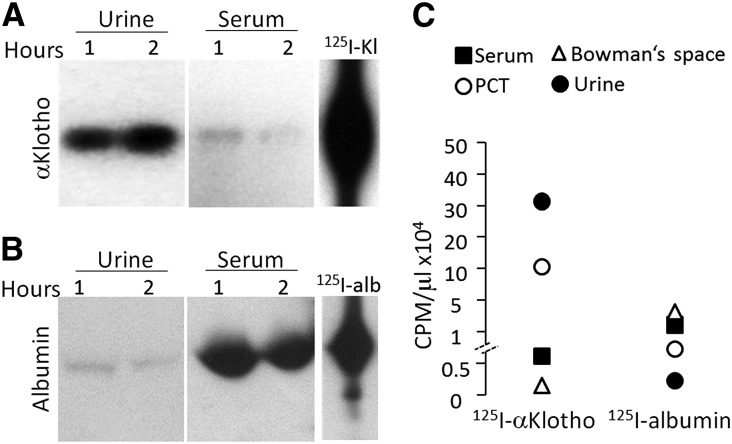
The lengthening of the half-life of exogenous αKlotho with nephrectomy implies that the kidney may be clearing αKlotho. Alternatively, the anephric state can alter αKlotho clearance by other organs. To distinguish these possibilities, we examined the anatomic fate of exogenous labeled αKlotho protein in the kidney. After an intravenous injection of 125I-labeled αKlotho protein (125I-αKlotho) into normal rats, we found that the kidney is a major organ for exogenous αKlotho protein uptake, with much lower signals in other organs (Figure 3A). The signal of the 125I-albumin control is unquestionably different from that of 125I-labeled αKlotho (Figure 3A), indicating that the signal of αKlotho in the kidney is not just due to trapping of αKlotho in renal vascular beds. Quantitative scintillation counting in tissue homogenates showed high signal of 125I-labeled αKlotho in the kidney and in urine compared with that in the blood (Figure 3B), supporting that the kidney may be a major organ of αKlotho uptake as well as its excretion.
Figure 3.
Tissue distribution of exogenous recombinant mouse αKlotho in normal rats. 125I-αKlotho and 125I-albumin were intravenously injected into normal Sprague-Dawley rats and blood, urine, and organs were collected. (A) Representative autoradiographs of organs harvested from rats injected with 125I-αKlotho and 125I-albumin. (B) Radioactivity of 125I-αKlotho and 125I-albumin in organs normalized to protein concentration (left panel), and of 125I-αKlotho and 125I-albumin in serum and urine (right panel). Data summarized from four independent experiments. (C) 125I-labeled αKlotho protein was intravenously injected into normal rats and kidneys were harvested at specific time points and cryosections were made for autoradiography. (D) Infrared-labeled αKlotho was intraperitoneally injected into normal rats. Two hours later, kidney, spleen, and heart were harvested, sectioned, and scanned with IR imager system.
We found a high signal in the renal medulla and papilla immediately after injection of 125I–αKlotho, which diminished by 30 min and disappeared by 120 min (Figure 3C). This signal likely came from free 125I released from 125I-αKlotho which we detected (data not shown) and is largely unavoidable. To independently confirm the high signal in the kidney, we covalently labeled recombinant αKlotho with infrared dye42 and examined αKlotho distribution in several organs in normal rats. Similar to the radioactive labeling, exogenous infrared labeled αKlotho protein was prominently distributed in the kidney and spleen, sparsely in the heart (Figure 3D), and not detectable in aorta, brain, and muscle (data not shown).
To characterize the clearance profile of αKlotho from the circulation into urine, we intravenously injected 125I-αKlotho into normal rats and simultaneously measured the radioactivity in the urine and serum over 2 hours. The serum αKlotho signal quickly decreased by half over approximately 4 min (due to redistribution) followed by a slower steady decline (Supplemental Figure 3A). The 125I-αKlotho clearance pattern was clearly different from that of 125I-albumin, which was mainly retained in the circulation (Supplemental Figure 3B). As serum 125I-αKlotho radioactivity decreased, urinary radioactivity rose simultaneously (Supplemental Figure 3C). In comparison, radioactivity of albumin in the urine remained low (Supplemental Figure 3D). These findings support the notion that αKlotho protein is cleared from blood through the kidney into the urine.
Distribution of Exogenous αKlotho Protein in the Kidney
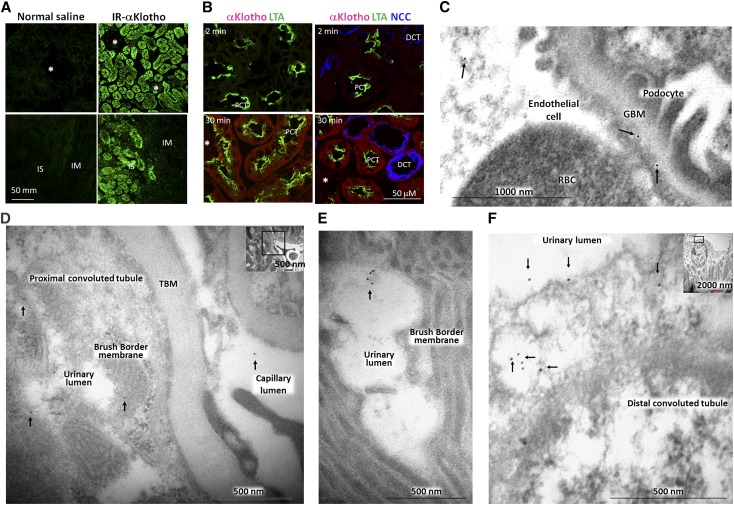
As discussed previously, upon injection of 125I-αKlotho to rats, autoradiography showed that the signal in the renal cortex appeared first and gradually increased followed by a signal in the inner medulla later. The signal in the cortical rim persisted up to 120 minutes (Figure 3C). The infrared (IR) labeled αKlotho imaging clearly demonstrates that exogenous αKlotho was not in glomeruli but exclusively present in renal tubules in the cortex and the outer strip of the medulla (Figure 4A).
Figure 4.
The distribution of exogenous recombinant mouse αKlotho in the kidney. (A) Infrared dye labeled αKlotho (IR-αKlotho) or normal saline was intraperitoneally injected into normal rats. Two hours later, kidney was harvested, sectioned, and imaged with IR microscope. (B) αKlotho labeled with two types of fluorescent dye: Alexa 555 C2 Maleimide (λex 555 nm, λem 565 nm) and TAMRA-SE (λex 546 nm, λex 579 nm) was intravenously injected into normal rats, and kidneys were collected at specific time points and subjected to immunofluorescent staining for αKlotho, NaCl cotransporter (NCC), and Lotus-Tetragonolobus lectins (LTA). Asterisks depict glomerulus. DCT: distal convoluted tubule; IM: inner medulla; IS: inner strip of outer medulla; PCT: proximal convoluted tubule. (C–F). αKlotho protein was intraperitoneally injected into homozygous αKlotho-deficient mice and the kidneys were harvested for immunogold electron microscopy. Representative electron micrographs of exogenous αKlotho in (C) glomeruli, (D, E) proximal tubules, (F) distal tubules. Arrows indicate gold particle (labeled αKlotho protein).
To further localize the segment of renal tubules in the cortex where exogenous αKlotho is taken up, we intravenously injected doubled-labeled fluorescent αKlotho into normal mice. There was no detectable signal of labeled IgG in the kidney (negative control, data not shown), whereas a clear αKlotho signal is present in the cortex starting 2 minutes after injection and a stronger signal was observed in 30 minutes. Labeled-αKlotho was present in both proximal and distal tubules (Figure 4B) but not in the glomeruli (not shown).
To delineate the locale of exogenous αKlotho further, we injected recombinant extracellular domain of mouse αKlotho into homozygous αKlotho hypomorphic (kl/kl) mice.1 As expected, immunoelectron microscopy (EM) did not detect endogenous αKlotho protein in the kidney of kl/kl mice (data not shown). Exogenous αKlotho was clearly found inside the glomerular capillary, the glomerular basement membrane (GBM), but not in Bowman’s space despite extensive search over many images from many glomeruli (Figure 4C). However, exogenous αKlotho was present in the lumen, apical and basolateral membranes, and cytoplasm in both distal and proximal tubules, and in peri-tubular capillary lumen (Figure 4, D–F, Supplemental Figure 4).
High radioactive counts were present both in the serum and urine (Supplemental Figure 3, A and C). To exclude the possibility that radioactivity in urine and serum was merely free 125I released from 125I-αKlotho, we resolved the urine proteins by SDS-PAGE followed by autoradiography. There was indeed some free 125I that ran off the gel (data not shown) but full-length αKlotho was clearly detectable in urine (Figure 5A). Small amounts of albumin were also detected in urine but very limited (Figure 5B).
Figure 5.
Intact exogenous αKlotho is present in blood and urine. 125I-αKlotho and 125I-albumin were intravenously injected into normal rats and blood and urine were collected 1 and 2 hours after injection, respectively. (A) 125I-αKlotho and (B) 125I-albumin in urine and serum were visualized on SDS-PAGE. (C) Urine from free-flow micropuncture originating from PCT, Bowman’s space, bladder urine, and serum of rats injected with 125I-αKlotho or 125I-albumin, were collected at 1 hour after injection and the same volumes were subjected to scintillation counting.
The fact that αKlotho is not filtered may be intuitively expected for a 130 kDa protein. Since αKlotho functions as an enzyme,13,21,54 it can theoretically modify the glycans and hence reduce the negative charge barrier of the GBM with resultant leak of αKlotho into urine. To exclude that, we treated fresh normal kidney slices with αKlotho, glucuronidase, or sialidase, and examined their effects on negative charge. αKlotho did not affect glomerular negative charge rendering glomerular leak unlikely (Supplemental Figure 5).
To further explore how αKlotho gets into the urine, we measured radioactivity in the proximal lumen urine using free-flow micropuncture.21 Radioactivity was highest in the urine but undetectable in Bowman’s space (Figure 5C), which is consistent with immunoelectron micrographs (Figure 4C) showing no visible αKlotho antigen in Bowman’s space. 125I-αKlotho was detected in high quantity in the lumen which is remarkably different from that of the control protein 125I-albumin (Figures 3B and 5C).
In light of the fact that injected IR-labeled full length αKlotho is present in urine,42 we conclude that exogenous αKlotho protein is cleared from blood by the kidney and excreted into urine at the level of the renal tubule. We do not exclude other possibilities such as hepatic and splenic clearance as exogenous αKlotho was also found in the liver and spleen (Figure 3A).
Transcytosis of αKlotho by Renal Proximal Tubules
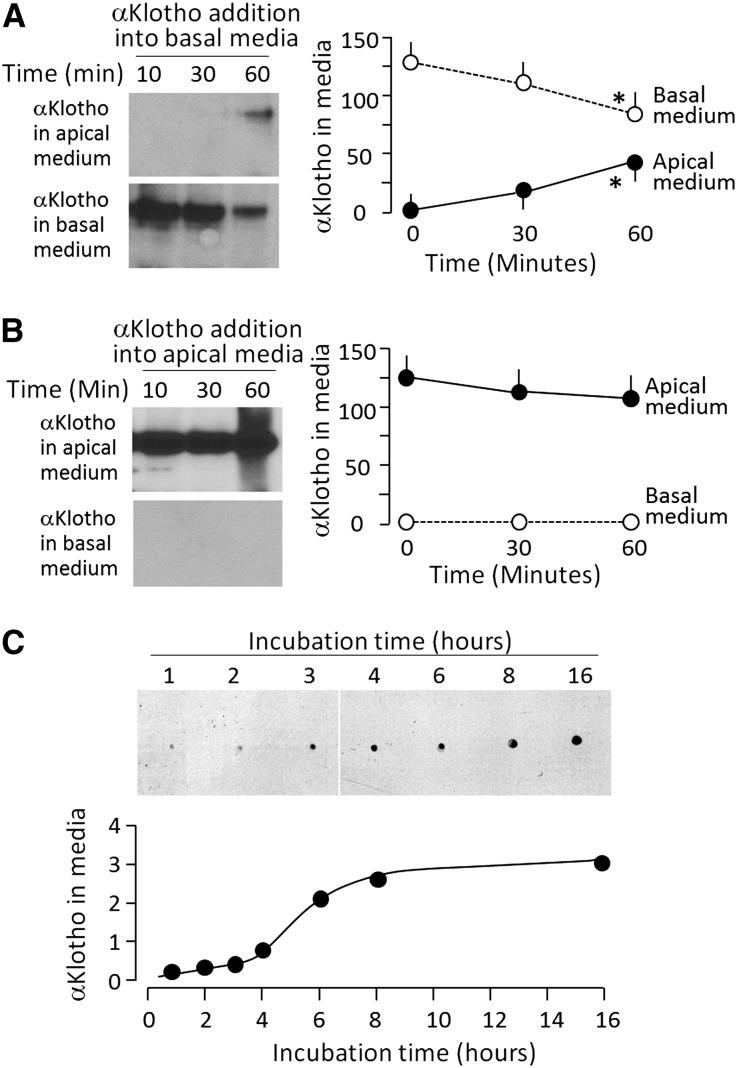
The absence and presence of αKlotho in Bowman’s space and proximal lumen, respectively, suggests that αKlotho traffics across renal tubules from basolateral membrane to luminal membrane. We next used OK cells, an opossum kidney cell line with renal proximal tubular features55 as an in vitro model to examine αKlotho transcytosis. αKlotho was taken up by OK cells from both apical and basal media, but only αKlotho internalized from the basal side was secreted into apical media (Figure 6A); internalized αKlotho from apical media was not released into basal media (Figure 6B) suggesting unidirectional trafficking. There was no transcytosis of albumin or IgG across OK cells either from basal to apical or from apical to basal side (data not shown). Taken together with the dot blot data shown in Figure 6C, one can say that αKlotho is taken up by OK cells from the basal side, then crosses the cell to the apical side which occurs as early as 30 minutes and plateaus by 8 hours.
Figure 6.
αKlotho transcytosis from basal to apical side in OK cells. OK cells were seeded on Transwell plates and grown to confluence to separate apical and basolateral side. (A) αKlotho with C-terminal 6His tag was added to the basal medium. At given time points after incubation, media were collected from both apical and basal side, and subjected to immunoblot to quantify exogenous αKlotho protein with anti-His antibody (left panel). Right panel is a summary of total amount of αKlotho at each time point in each compartment. Data are expressed as means±SD from three independent experiments. Statistical significance was assessed by one-way ANOVA followed by Student–Newman–Keuls test, and significant differences were accepted when *P<0.05 versus 0 min. (B) αKlotho with C-terminal 6His tag was added to the apical medium. At given time points after incubation, media were collected from apical and basal side and subjected to immunoblot for quantitation of αKlotho protein with anti-His antibody (left panel). Right panel is a summary of total amount of αKlotho at each time point in each compartment. Data are expressed as means±SD from three independent experiments. Statistical significance was assessed by one-way ANOVA followed by Student–Newman–Keuls test. None of the changes were statistically significant. (C). Time course of transcytosis. Media were collected from the apical side when αKlotho with C-terminal 6His tag was added to the basal side, and subjected to dot blot αKlotho protein with anti-His antibody (upper panel). Bottom panel shows arbitrary densitometric units of αKlotho protein. Three independent experiments showed identical results.
Discussion
The main findings in this study are: (1) the kidney produces and releases soluble αKlotho into the systemic circulation by secretases-mediated shedding of the ectodomain of αKlotho, (2) the kidney is an important organ to clear soluble αKlotho from the circulation, (3) αKlotho traffics across renal tubules from basolateral to intracellular location and is then secreted across the apical membrane into the urinary lumen.
αKlotho is found in urine and serum of rodents and humans2,20,42,56; and the kidney has the highest abundance of αKlotho compared with other organs. Clinical and experimental animal studies showed low renal tubular αKlotho expression in both acute kidney injury and CKD10,40,42,43,56–62 and low circulating αKlotho levels in kidney diseases of a variety of etiologies in animals42,43,56,62 and humans.32,44,61,63 These data suggest that the kidney contributes to circulating αKlotho. Now we provide direct experimental evidence that the kidney is the source of circulating αKlotho by demonstrating step-up of αKlotho concentration from infrarenal to suprarenal vena cava in both mice and humans. Further evidence for a renal source of circulating αKlotho is that serum αKlotho falls rapidly upon bilateral nephrectomy although one cannot rule out the possibility that the acute anephric state has suppressive effect on extrarenal source of αKlotho. We cannot extend our bilateral nephrectomy experiment beyond 2–3 days to test whether circulating αKlotho levels disappear entirely. Both proximal convoluted tubules and distal convoluted tubules express αKlotho protein and transcripts,21 but we do not know whether both segments release αKlotho into the circulation. It is unclear whether other organs contribute to circulating soluble αKlotho in physiologic11,12,45,48,64 or pathologic states.65 Lindberg and colleagues generated a conditional partial αKlotho deletion in renal tubules45 with a similar phenotype as global αKlotho deletion. In addition, serum αKlotho level was reduced by approximately 80% compared with wild-type and αKlotho shedding from kidney explants was undetectable,45 suggesting that kidney is a principal contributor of circulating αKlotho.
A second role of the kidney in αKlotho homeostasis is clearance from the circulation. The kidney likely serves to remove αKlotho, and acts as a conduit to deliver it to the tubular lumen. Endogenous αKlotho protein is still present in the circulation 2 days after nephrectomy. The post-nephrectomy endogenous αKlotho half-life approaches that of exogenous αKlotho in the anephric state (Figure 2), indicating that the kidney is a major organ to clear circulating αKlotho. In CKD vitamin D receptor agonist increased serum and urine αKlotho levels, but did not increase αKlotho expression in kidney, aorta, brain, and parathyroid.65 In the absence of kidneys, one does not know which are the predominant organs of production and clearance. Extra-renal αKlotho production might be low or undetectable under normal αKlotho status and in acute loss of renal function, but could possibly be stimulated in chronic αKlotho deficiency akin to increased hepatic production of erythropoietin in renal failure.66
The renal actions of αKlotho include, but are not limited to, suppression of NaPi activity in proximal,21 inhibition of renal outer medullary K+ in the thick ascending limb and the cortical collecting duct,24 stimulation of TRPV5 in renal distal tubules,23,67,68 and protection of kidney from oxidative injury.42,57–59,69,70 To exert its action on transporters, entrance to the tubular lumen is a prerequisite. We definitively show that exogenously administered αKlotho protein is present in proximal and distal tubular lumen (Figures 4 and 5), which can explain the effect on tubular transport with αKlotho injection.21,24
Soluble αKlotho protein is the extracellular domain of membrane Klotho (approximately 130 kDa)18–20 which is not filtered across the glomerular barrier.71 Furthermore, we did not see decreased negative charge in the GBM when kidney sections were directly incubated with αKlotho (Supplemental Figure 5) and in vivo studies did not show increased urinary protein excretion in normal rats acutely perfused with αKlotho and Tg-Kl mice with chronic higher levels of αKlotho.21,43 Free-flow micropuncture from Bowman’s space showed low radioactivity after injection with 125I-αKlotho (Figure 5C). Thus, the exogenous αKlotho that ends up in the urine is tubular in origin probably through transcytosis across proximal renal tubules. This was further tested by adding αKlotho to the basolateral media of OK cells and confirmed by the presence of αKlotho in the apical media (Figure 6). In contrast, there is no traffic of αKlotho from apical media to basolateral media. Transcytosis may serve as a way to clear circulating αKlotho, or as a mean to channel αKlotho to the lumen to modulate transporters or channels on the apical membrane of renal tubules. Two points are noteworthy. Firstly, both proximal and distal tubules take up circulating αKlotho (Figure 4B) but we cannot discern at present the relative contribution of each of these segments to trans-tubular αKlotho transport. Secondly, αKlotho in the urine can be hematogenously derived via transcytosis but it can also originate from the renal tubules as well. Currently, we do not know the relative contribution from circulation versus renal tubules; and from proximal tubules versus distal tubules to urinary αKlotho.
In conclusion, the kidney is the principal organ in the maintenance of αKlotho homeostasis by contributing to circulating αKlotho. The clearance of circulating αKlotho is also partially mediated by the kidney. Hematogenously derived αKlotho is present in renal tubular lumen which is a key site of physiologic action of αKlotho. Therefore, the kidney assumes several functions in αKlotho homeostasis: contribution to systemic endocrine αKlotho, clearance of circulating αKlotho, and delivery of αKlotho to the tubular lumen. With the renal source of circulating αKlotho established, the next step would be to delineate why and how αKlotho is drastically reduced with both acute and CKD which is of immense clinical significance. In addition, establishing extrarenal sources of αKlotho and testing whether there is upregulation of αKlotho production from extrarenal sources when renal production fails will also be of paramount importance.
Concise Methods
Human Studies
Nine human subjects (49.0±6.2 years) who underwent right heart catheterization were enrolled for this study (Supplementary Table 1), which was approved by the Institutional Review Board at the University of Texas Southwestern Medical Center, and all subjects gave informed consent. During right heart catheterization, suprarenal and infrarenal vena caval blood samples were obtained and sera were immediately separated after centrifugation at 4°C and stored at −80°C for future study.
Serum αKlotho was determined by immunoprecipitation-immunoblot assay described previously.21,42,63 Briefly, 0.1 ml serum was immunoprecipitated with a synthetic anti-αKlotho Fab (sb106)63 and immune complex was eluted with Laemmli sample buffer, and subject to immunoblot with KM2076 antibody.21,42,63 The specific signals on the autoradiograms based on 130 kD mobility were quantified with ImageJ Program (National Institutes of Health (NIH), Bethesda, Maryland).
Animal Studies
αKlotho hypomorphic (kl/kl) mice were described previously.1 kl/kl mice and their wild-type (WT) littermates were maintained at the Animal Research Center at the University of Texas Southwestern Medical Center. Currently all mice are 129 S1/SVlmJ (129 SV) background age from 6 to 8 weeks. Normal Sprague-Dawley (SD) rats (220–250 g body weight) were purchased from Charles River Laboratories (Wilmington, MA). For αKlotho clearance study, rats underwent bilateral nephrectomy (anephric rats) or laparotomy with manual manipulation of the kidneys (sham rats). Rats or mice were intravenously or intraperitoneally injected once with labeled full extracellular domain of recombinant mouse αKlotho protein (rMKl) (R&D Systems, Minneapolis, MN) at a dose of 0.1 mg/kg body weight.
To examine if secretases modulate blood αKlotho, doxycline hyclate (Sigma-Aldrich, St. Louis, MO), an α-secretase inhibitor at 25 mg/kg/day, and/or β-secretase inhibitor III (Calbiochem, Billerica, MA) at 2.5 mg/kg/day were intraperitoneally injected into normal WT mice daily for 2 days, blood and kidneys were harvested at 48 hours to determine serum and renal αKlotho. All animal studies were approved by the Institutional Animal Care and Use Committee at the University of Texas Southwestern Medical Center, Dallas, TX.
Antibodies and Other Key Reagents
Rat monoclonal anti-human Klotho antibody, KM20761,2 was used for immunoblotting and immunoelectron microscopy; and the synthetic anti-αKlotho antibody sb10663 was used for immunoprecipitation of serum Klotho. Anti-His antibody was purchased from Invitrogen (Carlsbad, CA), NaCl cotransporter (NCC) from Chemicon, (EMD Millipore, Billerica, MA), Lotus-Tetragonolobus lectins (LTA) from Vector laboratories (Burlingame, CA), and other chemicals were obtained from Sigma-Aldrich, except otherwise noted. Culture media were purchased from Invitrogen; penicillin and streptomycin from Cambrex (East Rutherford, NJ); infrared dye 800 CW from LI-COR Biosciences (Lincoln, NE), carboxy-tetramethyl-rhodamine succinimidyl ester from G-Biosciences (St. Louis, MO); Transwell plates from Corning Inc. (Corning, NY); enhanced chemiluminescence detection kit from GE Healthcare (Piscataway, NJ); nitrocellulose and polyvinylidene difluoride (PVDF) membranes from EMD Millipore; soluble murine Klotho protein containing the extracellular domain (amino acid number 35–982) from R&D Systems; 125I material from GE Healthcare.
αKlotho Labeling
Different methods were used to label C-terminal 6-His tagged αKlotho protein (R&D Systems, Minneapolis, MN) or albumin (Sigma-Aldrich, St. Louis, MO) with 125I, infrared (IR)-dye or fluorescein. Iodine labeling of purified αKlotho protein was performed using IODO-GEN (Pierce, Rockford, IL) and 125I according to the protocols provided by the manufacturers. Specific activity of 125II-αKlotho was determined by trichloroacetic acid precipitation. For IR dye labeling, recombinant mouse αKlotho protein was reacted with the IR dye 800 CW according to the kit’s instructions (LI-COR Biosciences). Labeled protein was confirmed by observation of the correct size band of labeled αKlotho on 7.5% SDS-PAGE with Odyssey Infrared Imaging System (LI-COR Biosciences). αKlotho was labeled with two types of fluorescent dye: Alexa 555 C2 Maleimide (λex 555 nm, λem 565 nm) (Invitrogen) and TAMRA-SE (λex 546 nm, λex 579 nm) using Hook-Dye labeling kit (G Biosciences) at sulfhydryl, arginine, and lysine residues to enhance fluorescent signal. For in vitro cell culture experiments, 1 μg/ml of labeled αKlotho was added in apical or basal media of insert of 6-well Transwell plate (Corning Inc.). For in vivo animal experiments, mice were intravenously injected with 0.1 mg/kg BW of labeled αKlotho.
Clearance of Labeled αKlotho in Rats and Mice
Normal Munich Wistar rats (220–250 g BW) were anesthetized with Inactin (100 mg/kg BW), and a bonus of labeled αKlotho was injected through the jugular vein (0.1 mg/kg BW). For the experiment of injection of 125I-labeled αKlotho or 125I-labeled albumin, fluid collection by free-flow micropuncture of Bowman’s space, and proximal convoluted tubules was performed using our published methods.21 In brief, the left kidney was exposed, and the left ureter was catheterized for urine collection. Proximal tubules were identified by their characteristic configuration after lissamine green dye injection and punctured with glass capillaries. The volume of fluid was measured in a calibrated constant-bore glass capillary. The radioactivity of fluids was determined by scintillation accounting and normalized to fluid volume. At specified time points, blood was drawn from retro-orbital venous sinus, and spot urine was collected. 125I-labeled αKlotho or 125I-labeled albumin in collected urine and serum was quantified by scintillation counting. Homogenates of different organs were made and radioactivity in organ homogenates was measured by scintillation counting, and normalized to protein in organ homogenates. Organ sections (10 μm) were subjected to autoradiography.
Distribution and Location of Labeled αKlotho in the Kidney
For the experiment of injection of IR-labeled αKlotho, normal SD rats were used. Four μl of serum or fresh spot urine were solubilized in Laemmli sample buffer, fractionated by 7.5% SDS-PAGE, and scanned with Odyssey Infrared Imaging System (LI-COR Biosciences). Kidneys were snap-frozen in liquid N2 and stored in −80°C. Four μm cryosections were made and scanned directly without fixation using Odyssey Infrared Imaging System or Storm™ 860 imager (GE Healthcare). For the experiment of injection of fluorescent labeled αKlotho, kidney sections were imaged by immunofluorescent microscopy, and immunohistologically stained with αKlotho, NaCl cotransporter (NCC), and Lotus tetragonolobus lectins (LTA).
Opossum Kidney (OK) Cells
OK cells were cultured and maintained in high-glucose (450 mg/dl) Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 g/ml) as described.21 For transcytosis study, OK cells were seeded on Transwell, and grown to complete confluence for 2 days (typical transepithelial resistance approximately 1–5 kΩ).72 For uptake study, fluorescein-labeled αKlotho protein with C-terminal 6His tag was added into either apical or basal compartment and incubated for 4 hours. Apical or basal media was then collected for immunoblot for αKlotho with anti-His antibody. For determination of radioactivity, 10 μl of media from different compartments and cell lysate was subject to scintillation counting and also subjected to SDS-PAGL followed by autoradiography.
Immunoblot
Immunoblotting was performed as described.21,42 Briefly, total lysates were extracted from kidney tissues and OK cells in radioimmunoprecipitation (RIPA) buffer (150 mM NaCl, 50 mM Tris–HCl, pH 7.4, 5 mM EDTA, 1% Triton X-100, 0.5% deoxycholate, and 0.1% SDS) containing fresh phosphatase inhibitors and protease inhibitors. Protein content in samples was determined by the method of Bradford.21,42 Thirty μg of protein were fractionated by SDS-PAGE, and electrophoretically transferred to polyvinylidene difluoride (PVDF) membranes. After blocking in nonfat milk, membranes were probed with anti-Klotho monoclonal antibody (KM2076)42 (overnight 4°C) and followed by secondary antibodies conjugated with horseradish peroxidase (GE Healthcare). Antibody against β-actin (Sigma-Aldrich) was used for loading control. Signal was visualized by enhanced chemiluminescence (GE Healthcare), and quantified densitometrically by the Scion/NIH ImageJ software (Scion, Frederick, MD).
For assay of serum αKlotho, 100 μl of serum from rodents was subjected to immunoprecipitated enrichment by sb106 αKlotho44 and was immunoblotted by rat anti-human αKlotho (KM2076).
Immunoelectron Microscopy
Mouse recombinant Klotho protein (0.1 mg/kg BW) was intraperitoneally injected once into kl/kl mice and mice were sacrificed 24 hours after injection. Kidneys were harvested and fixed with 2.5% paraformaldehyde via aortic perfusion, removed, and post-fixed in 4% paraformaldehyde (4°C for 4 hours). Immunogold labeling of ultrathin frozen tissue sections was performed as described.21 Kidney cortex was infiltrated with 2.3 M sucrose overnight, frozen in liquid nitrogen, and 70–80-nm-thick sections were made (Ultramicrotome Reichert Ultracut E; Leica Microsystems, Wetzlar, Germany) and mounted on Formvar-coated nickel grids. The sections were incubated with KM2076 antibody and followed by incubation with gold-conjugated protein A (10-nm gold particles, Sigma-Aldrich) for 60 minutes. After staining with uranyl acetate, sections were visualized with Jeol 1200 EX transmission electron microscope (Jeol Ltd., Akishima, Japan).
Statistical Analyses
Data are expressed as the means±SD. Statistical analysis was performed using unpaired t test, or paired t test, or ANOVA followed by Student–Newman–Keuls test whenever appropriate. A P-value of≤0.05 was considered statistically significant. Unless otherwise stated, representative figures reflect the results in a minimum of three independent experiments.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was in part supported by the National Institutes of Health (R01-DK092461, R01-091392, and R01-DK13686), O’Brien Kidney Research Center at the University of Texas Southwestern Medical Center (P30-DK07938), American Heart Foundation Western Affiliate Beginning-Grant-in-Aid (0865235F), the Simmons Family Foundation, the Charles and Jane Pak Foundation, the Pak Center Innovative Research Support, and the Canadian Institutes of Health Research (MOP-93725).
The authors would like to thank Dr. Philipp Scherer for assistance with infrared dye labeling and for providing infrared scan system, and Drs. Michel Baum, Jyothsna Gattineni and Xiao Yan for helpful discussions during the preparation of the manuscript.
Authors are grateful to Ms. Jean Paek for technical support, and to Ms. Carolyn Griffith for assistance in enrollment of research subjects.
A.B. was in part supported by Visiting Scholar Award from National Natural Science Foundation of China (81170660H0509, 81270408H0220), and Provincial Natural Science Foundation of Jiangsu, China (BK2011849).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014101030/-/DCSupplemental.
References
- 1.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI: Mutation of the mouse Klotho gene leads to a syndrome resembling ageing. Nature 390: 45–51, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M: Suppression of aging in mice by the hormone Klotho. Science 309: 1829–1833, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu MC, Kuro-o M, Moe OW: Renal and extrarenal actions of Klotho. Semin Nephrol 33: 118–129, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu MC, Shiizaki K, Kuro-o M, Moe OW: Fibroblast growth factor 23 and Klotho: physiology and pathophysiology of an endocrine network of mineral metabolism. Annu Rev Physiol 75: 503–533, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ito S, Kinoshita S, Shiraishi N, Nakagawa S, Sekine S, Fujimori T, Nabeshima YI: Molecular cloning and expression analyses of mouse βKlotho, which encodes a novel Klotho family protein. Mech Dev 98: 115–119, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Ito S, Fujimori T, Hayashizaki Y, Nabeshima Y: Identification of a novel mouse membrane-bound family 1 glycosidase-like protein, which carries an atypical active site structure. Biochim Biophys Acta 1576: 341–345, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Kurosu H, Kuro-o M: The Klotho gene family and the endocrine fibroblast growth factors. Curr Opin Nephrol Hypertens 17: 368–372, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Wu X, Weiszmann J, Ge H, Baribault H, Stevens J, Hawkins N, Vonderfecht S, Gardner J, Gupte J, Sheng J, Wang M, Li Y: A unique FGF23 with the ability to activate FGFR signaling through both αKlotho and βKlotho. J Mol Biol 418: 82–89, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Kurosu H, Choi M, Ogawa Y, Dickson AS, Goetz R, Eliseenkova AV, Mohammadi M, Rosenblatt KP, Kliewer SA, Kuro-o M: Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J Biol Chem 282: 26687–26695, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohyama Y, Kurabayashi M, Masuda H, Nakamura T, Aihara Y, Kaname T, Suga T, Arai M, Aizawa H, Matsumura Y, Kuro-o M, Nabeshima Y, Nagail R: Molecular cloning of rat Klotho cDNA: markedly decreased expression of Klotho by acute inflammatory stress. Biochem Biophys Res Commun 251: 920–925, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, Mohammadi M, Sirkis R, Naveh-Many T, Silver J: The parathyroid is a target organ for FGF23 in rats. J Clin Invest 117: 4003–4008, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takeshita K, Fujimori T, Kurotaki Y, Honjo H, Tsujikawa H, Yasui K, Lee JK, Kamiya K, Kitaichi K, Yamamoto K, Ito M, Kondo T, Iino S, Inden Y, Hirai M, Murohara T, Kodama I, Nabeshima Y: Sinoatrial node dysfunction and early unexpected death of mice with a defect of Klotho gene expression. Circulation 109: 1776–1782, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Huang CL, Moe OW: Klotho: a novel regulator of calcium and phosphorus homeostasis. Pflugers Arch 462: 185–193, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T: Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444: 770–774, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro-o M: Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem 281: 6120–6123, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zweers SJ, Booij KA, Komuta M, Roskams T, Gouma DJ, Jansen PL, Schaap FG: The human gallbladder secretes fibroblast growth factor 19 into bile: towards defining the role of fibroblast growth factor 19 in the enterobiliary tract. Hepatology 55: 575–583, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Ito S, Fujimori T, Furuya A, Satoh J, Nabeshima Y, Nabeshima Y: Impaired negative feedback suppression of bile acid synthesis in mice lacking betaKlotho. J Clin Invest 115: 2202–2208, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen CD, Podvin S, Gillespie E, Leeman SE, Abraham CR: Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci U S A 104: 19796–19801, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bloch L, Sineshchekova O, Reichenbach D, Reiss K, Saftig P, Kuro-o M, Kaether C: Klotho is a substrate for alpha-, beta- and gamma-secretase. FEBS Lett 583: 3221–3224, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, Hashimoto N, Fujimori T, Nabeshima Y: Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett 565: 143–147, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Hu MC, Shi M, Zhang J, Pastor J, Nakatani T, Lanske B, Razzaque MS, Rosenblatt KP, Baum MG, Kuro-o M, Moe OW: Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J 24: 3438–3450, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goetz R, Nakada Y, Hu MC, Kurosu H, Wang L, Nakatani T, Shi M, Eliseenkova AV, Razzaque MS, Moe OW, Kuro-o M, Mohammadi M: Isolated C-terminal tail of FGF23 alleviates hypophosphatemia by inhibiting FGF23–FGFR–Klotho complex formation. Proc Natl Acad Sci U S A 107: 407–412, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cha SK, Ortega B, Kurosu H, Rosenblatt KP, Kuro-O M, Huang CL: Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc Natl Acad Sci U S A 105: 9805–9810, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cha SK, Hu MC, Kurosu H, Kuro-o M, Moe O, Huang CL: Regulation of renal outer medullary potassium channel and renal K+ excretion by Klotho. Mol Pharmacol 76: 38–46, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panesso MC, Shi M, Cho HJ, Paek J, Ye J, Moe OW, Hu MC: Klotho has dual protective effects on cisplatin-induced acute kidney injury. Kidney Int 85: 855–870, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen TH, Kuro-O M, Chen CH, Sue YM, Chen YC, Wu HH, Cheng CY: The secreted Klotho protein restores phosphate retention and suppresses accelerated aging in Klotho mutant mice. Eur J Pharmacol 698: 67–73, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Ravikumar P, Ye J, Zhang J, Pinch SN, Hu MC, Kuro-o M, Hsia CC, Moe OW: α-Klotho protects against oxidative damage in pulmonary epithelia. Am J Physiol Lung Cell Mol Physiol 307: L566–L575, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith RC, O’Bryan LM, Farrow EG, Summers LJ, Clinkenbeard EL, Roberts JL, Cass TA, Saha J, Broderick C, Ma YL, Zeng QQ, Kharitonenkov A, Wilson JM, Guo Q, Sun H, Allen MR, Burr DB, Breyer MD, White KE: Circulating αKlotho influences phosphate handling by controlling FGF23 production. J Clin Invest 122: 4710–4715, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiraki-Iida T, Iida A, Nabeshima Y, Anazawa H, Nishikawa S, Noda M, Kuro-o M, Nabeshima Y: Improvement of multiple pathophysiological phenotypes of klotho (kl/kl) mice by adenovirus-mediated expression of the klotho gene. J Gene Med 2: 233–242, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Wan M, Smith C, Shah V, Gullet A, Wells D, Rees L, Shroff R: Fibroblast growth factor 23 and soluble klotho in children with chronic kidney disease. Nephrol Dial Transplant 28: 153–161, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Seiler S, Wen M, Roth HJ, Fehrenz M, Flügge F, Herath E, Weihrauch A, Fliser D, Heine GH: Plasma Klotho is not related to kidney function and does not predict adverse outcome in patients with chronic kidney disease. Kidney Int 83: 121–128, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Pavik I, Jaeger P, Ebner L, Wagner CA, Petzold K, Spichtig D, Poster D, Wüthrich RP, Russmann S, Serra AL: Secreted Klotho and FGF23 in chronic kidney disease Stage 1 to 5: a sequence suggested from a cross-sectional study. Nephrol Dial Transplant 28: 352–359, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Drüeke TB, Massy ZA: Circulating Klotho levels: clinical relevance and relationship with tissue Klotho expression. Kidney Int 83: 13–15, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Yokoyama K, Imura A, Ohkido I, Maruyama Y, Yamazaki Y, Hasegawa H, Urae J, Sekino H, Nabeshima Y, Hosoya T: Serum soluble α-klotho in hemodialysis patients. Clin Nephrol 77: 347–351, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Shimamura Y, Hamada K, Inoue K, Ogata K, Ishihara M, Kagawa T, Inoue M, Fujimoto S, Ikebe M, Yuasa K, Yamanaka S, Sugiura T, Terada Y: Serum levels of soluble secreted α-Klotho are decreased in the early stages of chronic kidney disease, making it a probable novel biomarker for early diagnosis. Clin Exp Nephrol 16: 722–729, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Pavik I, Jaeger P, Ebner L, Poster D, Krauer F, Kistler AD, Rentsch K, Andreisek G, Wagner CA, Devuyst O, Wüthrich RP, Schmid C, Serra AL: Soluble Klotho and autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 7: 248–257, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Asai O, Nakatani K, Tanaka T, Sakan H, Imura A, Yoshimoto S, Samejima K, Yamaguchi Y, Matsui M, Akai Y, Konishi N, Iwano M, Nabeshima Y, Saito Y: Decreased renal α-Klotho expression in early diabetic nephropathy in humans and mice and its possible role in urinary calcium excretion. Kidney Int 81: 539–547, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Akimoto T, Yoshizawa H, Watanabe Y, Numata A, Yamazaki T, Takeshima E, Iwazu K, Komada T, Otani N, Morishita Y, Ito C, Shiizaki K, Ando Y, Muto S, Kuro-o M, Kusano E: Characteristics of urinary and serum soluble Klotho protein in patients with different degrees of chronic kidney disease. BMC Nephrol 13: 155, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akimoto T, Shiizaki K, Sugase T, Watanabe Y, Yoshizawa H, Otani N, Numata A, Takeshima E, Yamazaki T, Miki T, Ito C, Pastor JV, Iwazu Y, Saito O, Muto S, Kuro-o M, Kusano E: The relationship between the soluble Klotho protein and the residual renal function among peritoneal dialysis patients. Clin Exp Nephrol 16: 442–447, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Koh N, Fujimori T, Nishiguchi S, Tamori A, Shiomi S, Nakatani T, Sugimura K, Kishimoto T, Kinoshita S, Kuroki T, Nabeshima Y: Severely reduced production of Klotho in human chronic renal failure kidney. Biochem Biophys Res Commun 280: 1015–1020, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Sugiura H, Yoshida T, Shiohira S, Kohei J, Mitobe M, Kurosu H, Kuro-o M, Nitta K, Tsuchiya K: Reduced Klotho expression level in kidney aggravates renal interstitial fibrosis. Am J Physiol Renal Physiol 302: F1252–F1264, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Hu MC, Shi M, Zhang J, Quiñones H, Kuro-o M, Moe OW: Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int 78: 1240–1251, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu MC, Shi M, Zhang J, Quiñones H, Griffith C, Kuro-o M, Moe OW: Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol 22: 124–136, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barker SL, Pastor J, Carranza D, Quiñones H, Griffith C, Goetz R, Mohammadi M, Ye J, Zhang J, Hu MC, Kuro-o M, Moe OW, Sidhu SS: The demonstration of αKlotho deficiency in human chronic kidney disease with a novel synthetic antibody. Nephrol Dial Transplant 30: 223–233, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lindberg K, Amin R, Moe OW, Hu MC, Erben RG, Östman Wernerson A, Lanske B, Olauson H, Larsson TE: The kidney is the principal organ mediating klotho effects. J Am Soc Nephrol 25: 2169–2175, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li SA, Watanabe M, Yamada H, Nagai A, Kinuta M, Takei K: Immunohistochemical localization of Klotho protein in brain, kidney, and reproductive organs of mice. Cell Struct Funct 29: 91–99, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Abramovitz L, Rubinek T, Ligumsky H, Bose S, Barshack I, Avivi C, Kaufman B, Wolf I: KL1 internal repeat mediates Klotho tumor suppressor activities and inhibits bFGF and IGF-I signaling in pancreatic cancer. Clin Cancer Res 17: 4254–4266, 2011 [DOI] [PubMed] [Google Scholar]
- 48.Galitzer H, Ben-Dov IZ, Silver J, Naveh-Many T: Parathyroid cell resistance to fibroblast growth factor 23 in secondary hyperparathyroidism of chronic kidney disease. Kidney Int 77: 211–218, 2010 [DOI] [PubMed] [Google Scholar]
- 49.Hofman-Bang J, Martuseviciene G, Santini MA, Olgaard K, Lewin E: Increased parathyroid expression of klotho in uremic rats. Kidney Int 78: 1119–1127, 2010 [DOI] [PubMed] [Google Scholar]
- 50.Melenhorst WB, Visser L, Timmer A, van den Heuvel MC, Stegeman CA, van Goor H: ADAM17 upregulation in human renal disease: a role in modulating TGF-alpha availability? Am J Physiol Renal Physiol 297: F781–F790, 2009 [DOI] [PubMed] [Google Scholar]
- 51.Melenhorst WB, van den Heuvel MC, Timmer A, Huitema S, Bulthuis M, Timens W, van Goor H: ADAM19 expression in human nephrogenesis and renal disease: associations with clinical and structural deterioration. Kidney Int 70: 1269–1278, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Cong R, Li Y, Biemesderfer D: A disintegrin and metalloprotease 10 activity sheds the ectodomain of the amyloid precursor-like protein 2 and regulates protein expression in proximal tubule cells. Am J Physiol Cell Physiol 300: C1366–C1374, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen CD, Tung TY, Liang J, Zeldich E, Tucker Zhou TB, Turk BE, Abraham CR: Identification of cleavage sites leading to the shed form of the anti-aging protein klotho. Biochemistry 53: 5579–5587, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tohyama O, Imura A, Iwano A, Freund JN, Henrissat B, Fujimori T, Nabeshima Y: Klotho is a novel beta-glucuronidase capable of hydrolyzing steroid beta-glucuronides. J Biol Chem 279: 9777–9784, 2004 [DOI] [PubMed] [Google Scholar]
- 55.Hu MC, Fan L, Crowder LA, Karim-Jimenez Z, Murer H, Moe OW: Dopamine acutely stimulates Na+/H+ exchanger (NHE3) endocytosis via clathrin-coated vesicles: dependence on protein kinase A-mediated NHE3 phosphorylation. J Biol Chem 276: 26906–26915, 2001 [DOI] [PubMed] [Google Scholar]
- 56.Yu J, Deng M, Zhao J, Huang L: Decreased expression of klotho gene in uremic atherosclerosis in apolipoprotein E-deficient mice. Biochem Biophys Res Commun 391: 261–266, 2010 [DOI] [PubMed] [Google Scholar]
- 57.Aizawa H, Saito Y, Nakamura T, Inoue M, Imanari T, Ohyama Y, Matsumura Y, Masuda H, Oba S, Mise N, Kimura K, Hasegawa A, Kurabayashi M, Kuro-o M, Nabeshima Y, Nagai R: Downregulation of the Klotho gene in the kidney under sustained circulatory stress in rats. Biochem Biophys Res Commun 249: 865–871, 1998 [DOI] [PubMed] [Google Scholar]
- 58.Mitobe M, Yoshida T, Sugiura H, Shirota S, Tsuchiya K, Nihei H: Oxidative stress decreases klotho expression in a mouse kidney cell line. Nephron, Exp Nephrol 101: e67–e74, 2005 [DOI] [PubMed] [Google Scholar]
- 59.Sugiura H, Yoshida T, Tsuchiya K, Mitobe M, Nishimura S, Shirota S, Akiba T, Nihei H: Klotho reduces apoptosis in experimental ischaemic acute renal failure. Nephrol Dial Transplant 20: 2636–2645, 2005 [DOI] [PubMed] [Google Scholar]
- 60.Haruna Y, Kashihara N, Satoh M, Tomita N, Namikoshi T, Sasaki T, Fujimori T, Xie P, Kanwar YS: Amelioration of progressive renal injury by genetic manipulation of Klotho gene. Proc Natl Acad Sci U S A 104: 2331–2336, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sakan H, Nakatani K, Asai O, Imura A, Tanaka T, Yoshimoto S, Iwamoto N, Kurumatani N, Iwano M, Nabeshima Y, Konishi N, Saito Y: Reduced renal α-Klotho expression in CKD patients and its effect on renal phosphate handling and vitamin D metabolism. PLoS ONE 9: e86301, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hu MC, Shi M, Cho HJ, Adams-Huet B, Paek J, Hill K, Shelton J, Amaral A, Faul C, Taniguchi M, Wolf M, Brand M, Takahashi M, Kuro-o M, Hill J, Moe OW: Klotho and phosphate are modulators of pathologic uremic cardiac remodeling [published online ahead of print October 17, 2014]. J Am Soc Nephrol 10.1681/ASN2014050465 [DOI] [PMC free article] [PubMed]
- 63.Scholze A, Liu Y, Pedersen L, Xia S, Roth HJ, Hocher B, Rasmussen LM, Tepel M: Soluble α-klotho and its relation to kidney function and fibroblast growth factor-23. J Clin Endocrinol Metab 99: E855–E861, 2014 [DOI] [PubMed] [Google Scholar]
- 64.Avin KG, Coen PM, Huang W, Stolz DB, Sowa GA, Dubé JJ, Goodpaster BH, O’Doherty RM, Ambrosio F: Skeletal muscle as a regulator of the longevity protein, Klotho. Front Physiol 5: 1–9, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lau WL, Leaf EM, Hu MC, Takeno MM, Kuro-o M, Moe OW, Giachelli CM: Vitamin D receptor agonists increase Klotho and osteopontin while decreasing aortic calcification in mice with chronic kidney disease fed a high phosphate diet. Kidney Int 82: 1261–1270, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fandrey J, Bunn HF: In vivo and in vitro regulation of erythropoietin mRNA: measurement by competitive polymerase chain reaction. Blood 81: 617–623, 1993 [PubMed] [Google Scholar]
- 67.Chang Q, Hoefs S, van der Kemp AW, Topala CN, Bindels RJ, Hoenderop JG: The beta-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science 310: 490–493, 2005 [DOI] [PubMed] [Google Scholar]
- 68.Lu P, Boros S, Chang Q, Bindels RJ, Hoenderop JG: The beta-glucuronidase klotho exclusively activates the epithelial Ca2+ channels TRPV5 and TRPV6. Nephrol Dial Transplant 23: 3397–3402, 2008 [DOI] [PubMed] [Google Scholar]
- 69.Sugiura H, Yoshida T, Mitobe M, Yoshida S, Shiohira S, Nitta K, Tsuchiya K: Klotho reduces apoptosis in experimental ischaemic acute kidney injury via HSP-70. Nephrol Dial Transplant 25: 60–68, 2010 [DOI] [PubMed] [Google Scholar]
- 70.Yamamoto M, Clark JD, Pastor JV, Gurnani P, Nandi A, Kurosu H, Miyoshi M, Ogawa Y, Castrillon DH, Rosenblatt KP, Kuro-o M: Regulation of oxidative stress by the anti-aging hormone klotho. J Biol Chem 280: 38029–38034, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vehaskari VM, Root ER, Germuth FG, Jr, Robson AM: Glomerular charge and urinary protein excretion: effects of systemic and intrarenal polycation infusion in the rat. Kidney Int 22: 127–135, 1982 [DOI] [PubMed] [Google Scholar]
- 72.Schwegler JS, Heuner A, Silbernagl S: Electrical properties of cultured renal tubular cells (OK) grown in confluent monolayers. Pflugers Arch 415: 183–190, 1989 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.