Abstract
Trimethlyamine-N-oxide (TMAO) was recently identified as a promoter of atherosclerosis. Patients with CKD exhibit accelerated development of atherosclerosis; however, no studies have explored the relationship between TMAO and atherosclerosis formation in this group. This study measured serum concentrations and urinary excretion of TMAO in a CKD cohort (n=104), identified the effect of renal transplant on serum TMAO concentration in a subset of these patients (n=6), and explored the cross-sectional relationship between serum TMAO and coronary atherosclerosis burden in a separate CKD cohort (n=220) undergoing coronary angiography. Additional exploratory analyses examined the relationship between baseline serum TMAO and long-term survival after coronary angiography. Serum TMAO concentrations demonstrated a strong inverse association with eGFR (r2=0.31, P<0.001). TMAO concentrations were markedly higher in patients receiving dialysis (median [interquartile range], 94.4 μM [54.8–133.0 μM] for dialysis-dependent patients versus 3.3 μM [3.1–6.0 μM] for healthy controls; P<0.001); whereas renal transplantation resulted in substantial reductions in TMAO concentrations (median [min–max] 71.2 μM [29.2–189.7 μM] pretransplant versus 11.4 μM [8.9–20.2 μM] post-transplant; P=0.03). TMAO concentration was an independent predictor for coronary atherosclerosis burden (P=0.02) and predicted long-term mortality independent of traditional cardiac risk factors (hazard ratio, 1.26 per 10 μM increment in TMAO concentration; 95% confidence interval, 1.13 to 1.40; P<0.001). In conclusion, serum TMAO concentrations substantially increase with decrements in kidney function, and this effect is reversed by renal transplantation. Increased TMAO concentrations correlate with coronary atherosclerosis burden and may associate with long-term mortality in patients with CKD undergoing coronary angiography.
Keywords: cardiovascular disease, CKD, coronary artery disease, ESRD, atherosclerosis, mortality
Patients with CKD have a high prevalence of cardiovascular comorbidities, which primarily contributes to the exceedingly high mortality in this group.1,2 For example, the 5-year survival for ESRD patients receiving dialysis is approximately 35%, with >50% of the mortality in this group resulting directly from cardiovascular causes.1 It is well established that CKD patients exhibit a disproportionate burden of atherosclerosis as compared with individuals having normal kidney function.2–5 Furthermore, a higher prevalence of traditional risk factors for the development of atherosclerosis, such as hypertension, diabetes and hyperlipidemia, only partially accounts for the accelerated atherosclerosis in CKD patients, leading to the hypothesis that unique risk factors must be present in this population.6,7
Trimethylamine-N-oxide (TMAO) is a circulating organic compound produced by the metabolism of dietary l-carnitine and choline,8,9 which was recently found to directly induce atherosclerosis in rodents. Moreover, high serum TMAO concentrations have been observed to strongly associate with incident cardiovascular events in humans with preserved kidney function,8–11 providing solid clinical evidence to support a link between TMAO and cardiovascular pathology. Both l-carnitine and choline are metabolized by intestinal bacteria to trimethylamine, a metabolite which is absorbed from the intestine and subsequently oxidized via hepatic flavin monooxygenase enzymes to form TMAO.12 Under normal physiologic conditions, circulating TMAO is rapidly cleared from the bloodstream, almost exclusively by urinary excretion.13,14
To date, there are very few published reports on TMAO metabolism in patients with CKD,15–17 and the association between TMAO concentrations and atherosclerosis burden in this group has not been investigated. Given that TMAO clearance is largely dependent on the renal excretion of this compound, and that prior studies have demonstrated TMAO to induce atherosclerotic plaque formation in rodents, we postulated that TMAO concentrations would be elevated in patients with CKD and be associated with the accelerated atherosclerosis observed in this population. Thus, in the current study we gathered several independent cohorts to accomplish the following goals: (1) to determine the relationship between serum TMAO concentrations and eGFR in a population of patients with CKD; (2) to examine changes in serum TMAO after renal transplantation to define the modifiability of TMAO concentrations following the restoration of renal function; and (3) to investigate the association of TMAO concentrations with atherosclerosis burden among CKD patients undergoing coronary angiography. Additionally, as an exploratory aim, we examined the association of baseline TMAO concentrations with long-term mortality in patients undergoing coronary angiography. Collectively, these studies could delineate the association of TMAO with kidney function and establish a foundation for TMAO as a novel promoter and modifiable risk factor for atherosclerosis formation in patients with CKD.
Results
Cross-Sectional Assessment of Serum TMAO Concentrations in CKD
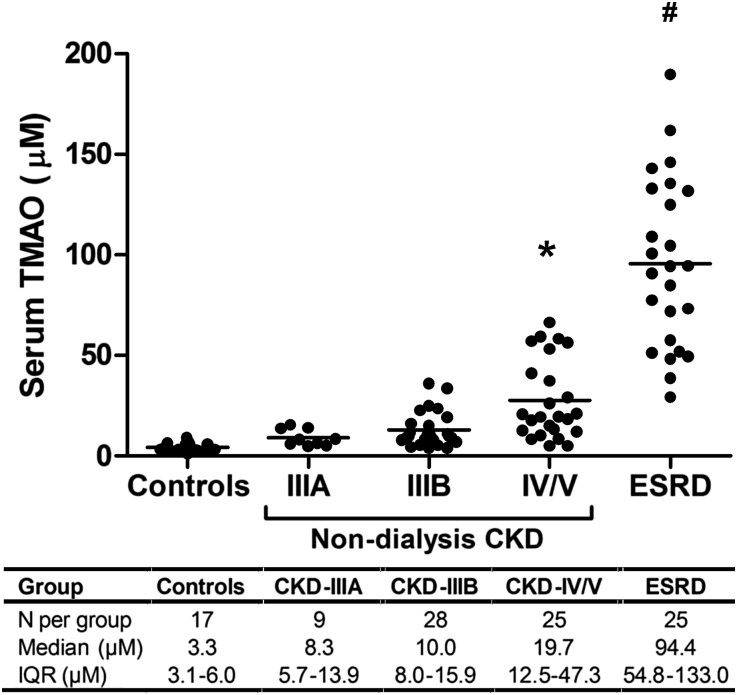
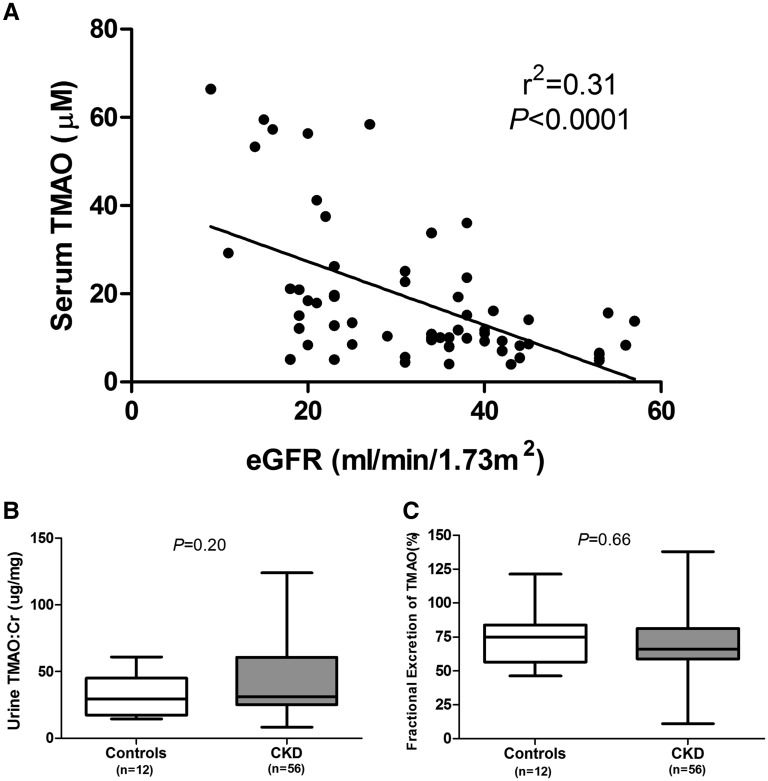
To evaluate whether circulating TMAO concentrations are elevated in CKD, we performed a cross-sectional study of serum TMAO concentrations in a group of patients (n=104) with variable degrees of kidney function, ranging from normal function to end-stage disease (Figure 1). We observed a graded elevation of serum TMAO concentrations with advancing CKD stage, with median concentrations in dialysis-dependent patients with ESRD being roughly 30-fold higher than in controls (94.4 μM for dialysis-dependent patients versus 3.3 μM for controls, P<0.001). Simple linear regression analysis of CKD patients with residual kidney function revealed a strong inverse relationship between eGFR and serum TMAO concentrations (Figure 2A, r2=0.31, P<0.001). The urine TMAO:Cr ratio and the fractional urinary excretion of TMAO (FETMAO) were equivalent in CKD and control patients (Figures 2, B and C, respectively; TMAO:Cr=43.2±29.7 for CKD group versus 31.6±15.8 for controls, P=0.20; FETMAO=70.3±20.7% for CKD group versus 73.2±20.6% for control group, P=0.66). Similarly, an analysis of FETMAO and TMAO:Cr when stratified by CKD stage revealed no apparent difference in the median values for these urinary parameters compared with control patients (Supplemental Figure 1). Demographic characteristics of this cohort are summarized in Supplemental Table 1.
Figure 1.
Cross-sectional analysis of serum TMAO concentrations by CKD stage. Serum TMAO concentrations were measured in a cohort of patients (n=104) with varying severity of kidney disease, ranging from normal kidney function (controls) to dialysis-dependent ESRD. Patients were grouped according to CKD staging: controls (eGFR≥60 ml/min per 1.73 m2), stage IIIA (eGFR 45–59 ml/min per 1.73 m2), stage IIIB (eGFR 30–44 ml/min per 1.73 m2), stage IV/V (eGFR<30 and not dialysis-dependent), and ESRD (receiving chronic hemodialysis therapy) *P<0.05; #P<0.001 compared with control group.
Figure 2.
Relationship between serum and urine TMAO concentrations in CKD. (A) Linear regression analysis depicting the relationship between serum TMAO and eGFR in CKD patients from a cross-sectional cohort (excluding patients with ESRD). (B) Comparison of the spot urine TMAO to creatinine ratio (TMAO:Cr) in controls and CKD patients with residual kidney function. (C) Comparison of the spot fractional excretion of TMAO (FETMAO) in controls and patients with CKD with residual kidney function (box plots depict median + interquartile range and whiskers illustrate minimum-maximum values).
Impact of Renal Transplantation on Serum TMAO Concentrations
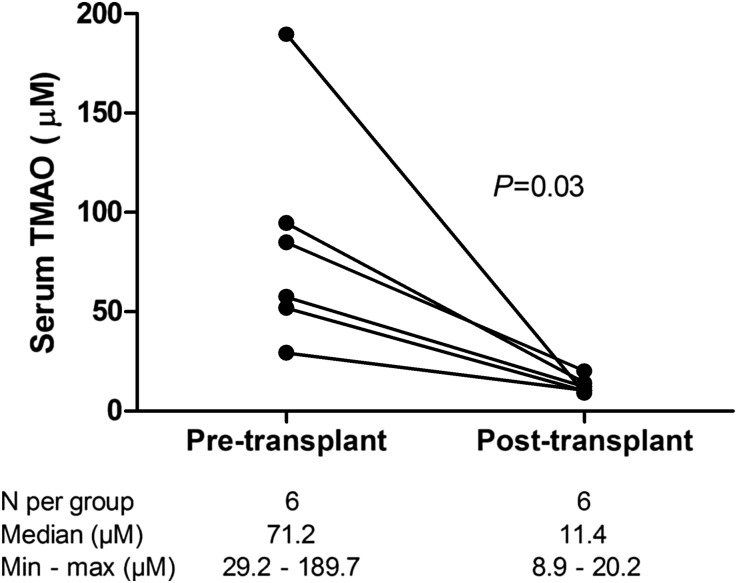
To investigate whether extreme elevations of serum TMAO observed in advanced CKD were reversible with restoration of kidney function, we evaluated serum TMAO concentrations prior to and 3 months after successful renal transplantation in six patients with advanced-stage kidney disease (one patient with eGFR of 11 ml/min per 1.73 m2 and five dialysis-dependent patients with ESRD). All patients demonstrated a marked reduction in serum TMAO following transplantation, with a median pretransplant TMAO concentration of 71.3 μM and post-transplant TMAO concentration of 11.4 μM for the group (Figure 3, P=0.03).
Figure 3.
Characterization of serum TMAO changes following renal transplantation. Serum TMAO concentrations in six patients with ESRD prior to and three months following successful renal transplantation.
Correlation between Serum TMAO Concentration and Coronary Atherosclerosis in CKD
To investigate the correlation of elevated serum TMAO and atherosclerosis in a population with CKD, we measured serum TMAO concentrations in 220 study participants with impaired kidney function (ranging from stage IIIA CKD to dialysis-dependent ESRD) who underwent coronary angiography and long-term mortality follow-up as part of the Diabetes Genome Project (DGP).18,19 Table 1 summarizes the baseline characteristics of these study participants, stratified by tertiles of TMAO concentration. Similar to the findings in our cross-sectional cohort, individuals with higher serum TMAO concentrations in the DGP cohort exhibited lower eGFR. The highest TMAO tertile contained a greater proportion of males, a higher prevalence of diabetes, and a higher prevalence of peripheral vascular disease. There was no significant association of TMAO tertiles with age, race, tobacco use, hypertension, history of myocardial infarction, prior coronary stenting or bypass grafting, congestive heart failure, cholesterol, triglycerides, C-reactive protein, albumin, or medication use (statins, angiotensin converting enzyme-inhibitors, angiotensin receptor blockers, or β-blockers).
Table 1.
Baseline demographic characteristics stratified by TMAO tertile for CKD cohort undergoing atherosclerosis assessment by coronary angiography
| TMAO Tertiles | P Value | ||||
|---|---|---|---|---|---|
| Total Cohort (n=220) | Tertile 1 | Tertile 2 | Tertile 3 | ||
| 0.63 to <5.58 μM (n=73) | 5.58 to <9.26 μM (n=73) | 9.26–163.03 μM (n=74) | |||
| Demographics | |||||
| Age (yr) | 69.7±10.3 | 70.3±8.9 | 71.2±8.6 | 67.7±12.8 | 0.11 |
| Gender | 0.05 | ||||
| Male | 94 (42.7%) | 23 (31.5%) | 33 (45.2%) | 38 (51.4%) | |
| Female | 126 (57.3%) | 50 (68.5%) | 40 (54.8%) | 36 (48.6%) | |
| Race | 0.19 | ||||
| Caucasian | 200 (90.9%) | 70 (95.9%) | 67 (91.8%) | 63 (85.1%) | |
| African American | 16 (7.3%) | 3 (4.1%) | 5 (6.8%) | 8 (10.8%) | |
| Other | 4 (1.8%) | 0 (0.0%) | 1 (1.4%) | 3 (4.1%) | |
| Hypertension | 214 (97.3%) | 71 (97.3%) | 70 (95.9%) | 73 (98.6%) | 0.54 |
| Current smoker | 15 (6.8%) | 5 (6.8%) | 4 (5.5%) | 6 (8.1%) | 0.94 |
| Body mass index | 30.6±7.0 | 30.4±7.1 | 30.3±7.5 | 31.2±6.5 | 0.71 |
| Diabetes | 141 (64.1%) | 39 (53.4%) | 46 (63.0%) | 56 (75.7%) | 0.02 |
| History of CVD | 55 (25.0%) | 17 (23.3%) | 18 (24.7%) | 20 (27.0%) | 0.87 |
| History of PVD | 73 (33.2%) | 17 (23.3%) | 22 (30.1%) | 34 (45.9%) | 0.01 |
| History of CHF | 69 (31.4%) | 16 (21.9%) | 28 (38.4%) | 25 (33.8%) | 0.09 |
| History of MI | 81 (36.8%) | 27 (37.0%) | 25 (34.2%) | 29 (39.2%) | 0.82 |
| History of PCI | 107 (48.9%) | 28 (38.9%) | 38 (52.1%) | 41 (55.4%) | 0.11 |
| History of CABG | 57 (25.9%) | 19 (26.0%) | 19 (26.0%) | 19 (25.7%) | 0.99 |
| Laboratory parameters | |||||
| TMAO (μM) | 6.9 (4.8–10.9) | 4.0 (3.2–4.8) | 6.9 (6.0–7.7) | 17.6 (10.9–31.3) | |
| eGFR (ml/min per 1.73 m2) | 40.3±13.9 | 45.2±9.8 | 44.3±9.6 | 31.4±16.7 | <0.001 |
| CKD stage | <0.001 | ||||
| IIIA | 89 (40.5%) | 41 (56.2%) | 33 (45.2%) | 15 (20.3%) | |
| IIIB | 89 (40.5%) | 26 (35.6%) | 35 (47.9%) | 28 (37.8%) | |
| IV | 20 (9.1%) | 4 (5.5%) | 5 (6.8%) | 11 (14.9%) | |
| V | 2 (0.9%) | 1 (1.4%) | 0 (0.0%) | 1 (1.4%) | |
| ESRD | 20 (9.1%) | 1 (1.4%) | 0 (0.0%) | 19 (25.7%) | |
| Cholesterol (mg/dl) | 161.2±42.2 | 168.0±40.8 | 162.9±40.5 | 152.8±44.4 | 0.09 |
| Triglycerides (mg/dl) | 153.2±94.9 | 150.3±109.6 | 158.2±92.1 | 151.3±82.4 | 0.86 |
| Albumin (mg/dl) | 3.6±0.6 | 3.6±0.4 | 3.6±0.5 | 3.5±0.8 | 0.25 |
| C-reactive protein (mg/dl) | 12.4±25.2 | 10.3±16.7 | 13.8±31.3 | 13.1±25.5 | 0.67 |
| Medication use | |||||
| Statins | 163 (74.1%) | 61 (83.6%) | 50 (68.5%) | 52 (70.3%) | 0.08 |
| Beta-blockers | 173 (78.6%) | 61 (83.6%) | 52 (71.2%) | 60 (81.1%) | 0.16 |
| ACE-inhibitors | 107 (48.6%) | 36 (49.3%) | 38 (52.1%) | 33 (44.6%) | 0.66 |
| ARB | 43 (19.5%) | 14 (19.2%) | 19 (26.0%) | 10 (13.5%) | 0.16 |
| Atherosclerosis quantification | |||||
| Diseased vessels >70% | 0.03 | ||||
| 0 | 35 (15.9%) | 12 (16.4%) | 15 (20.5%) | 8 (10.8%) | |
| 1 | 95 (43.2%) | 36 (49.3%) | 27 (37.0%) | 32 (43.2%) | |
| 2 | 39 (17.7%) | 17 (23.3%) | 13 (17.8%) | 9 (12.2%) | |
| 3 | 51 (23.2%) | 8 (11.0%) | 18 (24.7%) | 25 (33.8%) | |
| Multivessel disease >70% | 90 (40.9%) | 25 (34.2%) | 31 (42.5%) | 34 (45.9%) | 0.33 |
| Modified Gensini score | 6.7±4.6 | 5.9±4.0 | 6.8±4.4 | 7.4±5.1 | 0.11 |
Continuous variables presented as median (interquartile range) or mean±SD. CVD, cerebrovascular disease; PVD, peripheral vascular disease; MI, myocardial infarction; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; ACE, angiotensin converting enzyme; ARB, angiotensin receptor blockers.
Individuals in the highest TMAO tertile demonstrated a greater coronary atherosclerotic burden, as defined by a higher mean modified Gensini score, compared with participants in the lower TMAO tertiles (7.4±5.1 versus 5.9±4.0 for tertile 1, P=0.11 for intertertile trend). When serum TMAO was treated as a continuous variable, a statistically significant correlation with the modified Gensini score was observed (r=0.17, P=0.02). Similarly, after developing a predictive model including traditional atherosclerotic risk factors, TMAO remained a significant predictor of Gensini score (P=0.02).
Because only a subset of this cohort had urine samples collected (n=98), we were unable to fully assess the effect of proteinuria on the relationship between TMAO and atherosclerosis burden. However, in an additional subanalysis to determine the correlation between the urine protein-to-creatinine ratio and Gensini scoring, there was no evidence of a correlation between these parameters (r=0.10, P=0.33).
Association between TMAO and Long-term Mortality following Coronary Angiography
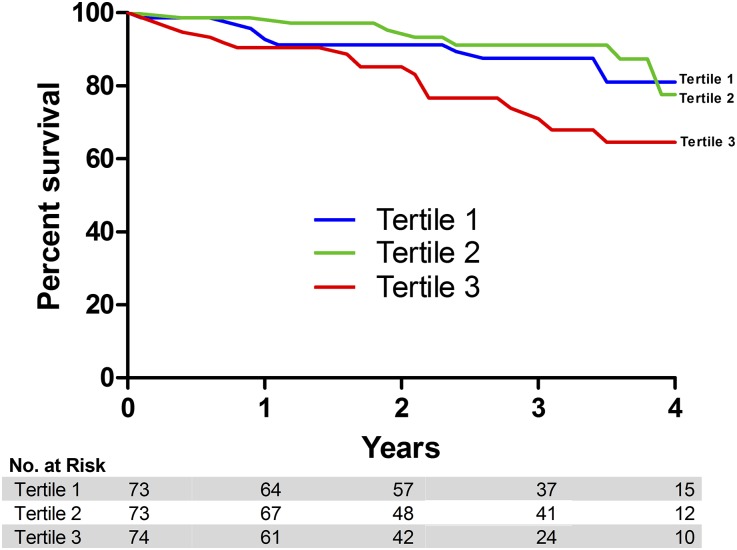
Figure 4 illustrates the survival curves for study participants divided by TMAO tertile. The 4-year survival for individuals with the highest TMAO concentrations (tertile 3) was 64.6%, compared with 77.6% for tertile 2 and 81.1% for tertile 1 (Figure 4). When comparing survival across TMAO tertiles, individuals in the highest tertile exhibited a greater 4-year mortality compared with individuals in lower tertiles; however, this association was not statistically significant (unadjusted hazard ratio of 1.95 [95% confidence interval (95% CI), 0.91 to 4.17] compared with tertile 1, P>0.05, and 2.08 [95% CI, 0.95 to 4.57] compared with tertile 2, P>0.05, respectively). Conversely, when this relationship was examined considering TMAO as a continuous variable, TMAO was independently associated with 4-year mortality. There was a 19% increase in mortality for every 10 μM increment in serum TMAO (HR 1.19, 95% CI, 1.10 to 1.29, P<0.001) in the unadjusted model. Similarly, in the multivariate prediction model TMAO remained a predictor of mortality (Table 2; HR 1.26, 95% CI, 1.13 to 1.40, P<0.001).
Figure 4.
Survival curve for TMAO tertiles in a longitudinal cohort. Kaplan–Meier survival analysis based on TMAO tertiles for 220 patients with CKD enrolled in the Diabetes Genome Project study.
Table 2.
Predictors of all-cause mortality
| Predictor Variable | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| TMAO (per 10 μM increase) | 1.26 (1.13–1.40) | <0.001 |
| Age (per 10 year increase) | 1.76 (1.17–2.64) | <0.01 |
| C-reactive protein (per 1 mg/dL increase) | 1.014 (1.01–1.02) | <0.001 |
| History of congestive heart failure | 2.68 (1.35–5.29) | <0.01 |
| History of peripheral vascular disease | 2.56 (1.23–5.32) | 0.01 |
Covariates available to the model were: TMAO, eGFR, diabetes, hypertension, race, gender, age, body mass index, CKD stage, history of percutaneous intervention, triglycerides, cholesterol, history of coronary artery bypass grafting, history of myocardial infarction, history of cerebrovascular accident, history of peripheral vascular disease, history of congestive heart failure, and smoking status.
Discussion
To our knowledge, the current study is the first investigation to comprehensively define the association between serum TMAO concentrations and residual kidney function across a broad spectrum of GFR estimates and to demonstrate an independent association between TMAO and coronary atherosclerotic burden in a CKD cohort. In conjunction with a recent publication by Wang et al. suggesting TMAO to be a direct inducer of atherosclerosis in rodents,9 and additional studies in non-CKD human cohorts demonstrating an association with TMAO and adverse cardiovascular outcomes,8,10,11 we believe our current observations provide strong preliminary evidence to support TMAO as a nontraditional risk factor for atherosclerosis formation in patients with CKD.
Our observation of a direct inverse relationship between TMAO and kidney function supports and extends prior studies suggesting that TMAO clearance from the circulation is largely dependent on urinary excretion. However, the extent of TMAO elevation in advanced CKD stages was far greater than originally anticipated. The median serum TMAO concentration in ESRD individuals was 94.4 μM, roughly 30-fold higher than in our control group with normal kidney function (Figure 1). This observation is particularly striking when compared with the non-CKD cohort reported by Tang et al.10 The median serum TMAO concentration in their cohort was 3.7 µM with an interquartile range of 2.4–6.2 µM. Even with this modest variation in serum TMAO, they observed a 2.54-fold increased risk of myocardial infarction, stroke, or death in the highest TMAO quartile compared with the lowest quartile. In a more recent publication by this same group, serum TMAO concentrations were observed both to independently predict all-cause mortality in a nondialysis CKD cohort and to contribute directly to renal fibrosis in animal models.17 Of note, exploratory survival analyses in our cohort confirmed TMAO as a predictor of mortality by demonstrating a 26% increased risk of long-term mortality per 10 μM increase in serum TMAO, even after adjusting for other traditional cardiovascular risk factors (Table 2). Based on this estimate, it could be postulated that an ESRD population would be at substantial risk for cardiovascular mortality given the considerably elevated serum TMAO concentrations observed in this setting. However, because the DGP cohort contained very few patients with ESRD receiving dialysis, it is unclear if TMAO would remain an independent predictor of mortality endpoints if similar analyses were repeated in larger populations comprised solely of dialysis patients. Thus, future large-scale studies which are specifically designed to examine the predictive value of TMAO for cardiovascular and mortality outcomes in both CKD and ESRD populations will be necessary to determine the generalizability of our initial observations to these separate groups.
The generation of trimethylamine from dietary precursors occurs within the intestinal lumen and appears to be exclusively dependent on the presence of intestinal bacteria, as interventions to alter the composition of the gut microbiome have led to marked reductions in circulating TMAO concentrations in both rodents and humans with intact kidney function;9,10 however, it remains unclear which intestinal microbes are primarily responsible for this process. The role of the intestinal microbiome in the development of CKD comorbidities has been the topic of extensive biomedical research over the last decade, with numerous studies attempting to describe a pathophysiologic link between alterations in gut flora and various clinical observations in CKD, including cardiovascular disease.20 Several previous studies have suggested a shift in the gut microbiome in CKD,21,22 as well as altered intestinal barrier function in this setting.23–26 Thus, it is plausible that an increased intestinal absorption of trimethylamine or hepatic synthesis of TMAO could contribute to the elevated TMAO concentrations in CKD.
It is noteworthy that we observed comparable urinary TMAO:Cr values between CKD and control patients (Figure 2). The urine TMAO concentration, when normalized to that of creatinine, is a measure of the TMAO excretion rate and, under steady-state conditions, this should be equal to the rate of TMAO generation. Our results therefore suggest that the intestinal absorption of trimethylamine and hepatic production of TMAO are largely unchanged in CKD, so the increments in serum TMAO in earlier CKD stages are likely driven by reductions in renal excretion. Furthermore, our observation that FETMAO is largely unchanged suggests that the major contributor to the reduction in TMAO excretion is reduced glomerular filtration of TMAO, while tubular reabsorption is largely unaffected. Our finding of a dramatic reduction in serum TMAO following renal transplantation (Figure 3) provides strong evidence for reversibility of TMAO elevations in advanced CKD, and may partially explain the significant survival benefit associated with the restoration of kidney function in patients with renal transplants.27,28 Nonetheless, it is clear that further studies exploring the physiology of TMAO generation and metabolism are warranted to more thoroughly define the etiology of TMAO elevations in CKD.
While the observed association between serum TMAO concentrations and coronary atherosclerosis in our CKD cohort is consistent with prior observations in animals and non-CKD human populations, the mechanism whereby TMAO potentiates atherosclerosis development remains poorly defined. The prevailing hypothesis in the literature is that TMAO promotes an imbalance in cholesterol uptake and efflux from macrophages, leading to an abundance of pro-atherogenic foam cells migrating to the arterial wall.8,9 Current evidence suggests that this could occur by either promoting macrophage expression of cholesterol scavenger receptors, CD36 and SR-A1, or by restricting reverse cholesterol transport out of these cells. Interestingly, numerous studies have suggested enhanced scavenger receptor expression on cells of the monocyte/macrophage lineage, along with defects in macrophage cholesterol efflux, in humans with CKD.29–33 Furthermore, studies in ApoE−/− mice, a rodent model of atherosclerosis, demonstrate that induction of kidney disease in this model enhances macrophage cholesterol accumulation independent of alterations in plasma cholesterol concentrations.34
This investigation has both important strengths and limitations. The strengths of this study include: (1) the use of several unrelated CKD cohorts exhibiting a broad range of eGFR measurements for the exploration and validation of the relationship between serum TMAO and eGFR; (2) the extensive characterization of the coronary atherosclerosis burden in all study participants in the DGP cohort using a standardized method of atherosclerosis quantification; (3) the long (4-year) follow-up for mortality endpoints; and (4) the demonstration of short-term reversibility of high serum TMAO concentrations following renal transplantation. Potential limitations of this work include: (1) a lack of ethnic diversity (90% Caucasian) which may affect the generalizability of our findings to other populations with CKD; (2) limited inclusion of outcomes data from patients with ESRD, which are the subset of patients with CKD with the highest concentrations of TMAO and at the greatest risk for adverse cardiovascular outcomes; (3) the relatively small number of mortality endpoints and lack of data on cause of death in the DGP cohort preventing us from obtaining a more nuanced understanding of the relationship between TMAO and cardiovascular mortality; and (4) the potential for confounding by unmeasured risk factors, such as proteinuria.
The current investigation advances our understanding of cardiovascular disease in CKD by providing a novel mechanistic link to explain the presence of accelerated atherosclerosis in patients with reduced kidney function. As such, our study demonstrates a strong inverse relationship between circulating concentrations of TMAO, a novel atherogenic compound, and residual kidney function in patients with CKD, and defines TMAO as an independent predictor of coronary atherosclerosis in this population. Based on evidence that TMAO production is dependent on dietary precursors and the intestinal microbiome, TMAO may represent a novel modifiable risk factor and therapeutic target for reducing cardiovascular mortality in patients with CKD.
Concise Methods
Study Design
We assembled data from five cohorts to accomplish our goals in this investigation. A cross-sectional analysis of TMAO serum and urine concentrations across CKD stages was performed using stored patient samples from three separate prospective studies: (1) Impact of Vitamin D Therapies on Monocyte Function in CKD (clinicaltrials.gov ID: NCT01222234); (2) Differential Effects of Ergocalciferol and Cholecalciferol Therapies in CKD (clinicaltrials.gov no. NCT01835691); and (3) Impact of Exercise on Cognitive Function in ESRD (clinicaltrials.gov no. NCT02145702). Only samples from the baseline study visits (prior to study interventions) were used for these cross-sectional analyses. To assess changes in TMAO following successful treatment of ESRD with renal transplantation, we used stored serum samples from the Cognitive Impairment and Imaging Correlates in ESRD study (clinicaltrials.gov no. NCT01883349). TMAO was measured in samples collected from the baseline and 3-month post-transplant visits.
The cross-sectional association of TMAO concentrations with the burden of coronary atherosclerosis and its association with subsequent survival were conducted using stored serum samples and clinical data collected from individuals with CKD (eGFR<60 ml/min per 1.73 m2) who participated in the Diabetes Genome Project (clinicaltrials.gov no. NCT00428961).18,19 Briefly, consecutive patients undergoing coronary angiography were invited, prior to their procedure, to participate in a prospective registry of genetic factors and biomarkers associated with atherosclerosis and prognosis. For the current study, patients were stratified by CKD stage and a subset of patients from each stage (IIIA–V, or ESRD) was randomly selected for analysis of TMAO concentrations. Glomerular filtration rate was estimated via the CKD-EPI equation as previously described,35 and CKD stages were defined according to the Kidney Disease Outcomes Quality Initiative guidelines.36 Study participants underwent quantitative coronary angiography at Saint Luke’s Hospital Imaging Core Lab,37 and atherosclerotic burden was quantified using the modified Gensini score.38 Four-year survival was assessed through the Social Security Death Master file. All of these studies were approved by the Institutional Review Boards at either the University of Kansas Medical Center (KUMC) or St. Luke’s Health System, and complied with the Declaration of Helsinki. All participants in these studies provided written informed consent for study participation. Because all patient data were de-identified for the post-hoc analyses of TMAO in stored biologic samples, a human subjects research exemption was granted by the KUMC Institutional Review Board for this work.
Laboratory Analyses
Serum and urine TMAO concentrations were measured by ultra-high performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) using heated electrospray ionization (positive mode) and selected reaction monitoring as previously described.39 Briefly, the UHPLC-MS/MS system consisted of an Accela autosampler, Accela UHPLC binary pump, and a TSQ Quantum Ultra triple quadruple mass spectrometer (Thermo Fisher Scientific, San Jose, CA). Serum or urine (50 µL) was combined with an internal standard (200 µL of d9-TMAO at 0.5 µg/mL) in methanol to precipitate protein. After centrifugation 20 µL of supernatant was diluted with 100 µL of 75:25 acetonitrile:methanol and 10 µL of this mixture was injected onto the UHPLC-MS/MS system. The mobile phases used for the analysis were 10 mM ammonium formate pH 3.5 (mobile phase A) and acetonitrile (mobile phase B), delivered in a gradient at a flow rate of 400 µL/min. The analytical column was a Waters Acquity BEH amide 50×2.1, 1.7 µm held at 10°C. The selected reaction monitoring scheme followed transitions of the [M+H]+ precursor to selected product ions with the following values: m/z 76.1 → 58.1 for TMAO, m/z 85.1 → 66.2 for d9-TMAO. The standard curve used for plasma samples ranged from 0.010 to 5.00 µg/mL (0.13–66.6 µM), while the standard curve used for urine samples ranged from 1.00 to 150 µg/mL (13.3–1,997 µM). Samples that were above the upper limit of quantification were diluted in phosphate-buffered saline to within the assay range. The within-run and between-run precision (percent coefficient of variation) was <7.5%. Recovery of TMAO spiked into human plasma and urine ranged from 93.0% to 103% at three different concentrations in both matrices.
For the studies based at KUMC, serum creatinine was measured on an AU5800 autoanalyzer (Beckman Coulter, Brea, CA) and urine creatinine was measured using a colorimetric urine detection kit (Arbor Assays, Ann Arbor, MI). For the DGP cohort, serum creatinine, albumin, hs-CRP and lipid measurements were performed on a Vitros 5600 autoanalyzer (Ortho Clinical Diagnostics, Raritan, NJ). The urinary TMAO:creatinine ratio was presented as micrograms of TMAO per mg of creatinine, and urinary FETMAO was calculated by the following formula: [(urine TMAO in μg/ml/ serum TMAO in μg/ml) / (urine creatinine in mg/dl / serum creatinine in mg/dl)]×100.
Statistical Analyses
Between-group comparisons for the cross-sectional evaluation of TMAO concentrations across CKD stages was performed using one-way ANOVA. The relationship between TMAO and eGFR was determined by simple linear regression analysis. Comparison of urine TMAO parameters between CKD patients and controls was performed utilizing unpaired two-sided Student’s t test, while the change in TMAO following renal transplantation was evaluated by paired two-sided Student’s t test. The population from the Diabetes Genome Project was divided into tertiles of TMAO and baseline characteristics displayed. Continuous variables were compared using one-way ANOVA and discrete variables compared using chi-squared test or Fisher’s exact test, as appropriate. The modified Gensini score demonstrated a non-normal distribution; thus, log transformation was performed and all goodness-of-fit tests (Kolmogorov–Smirnov, Cramer–van Mises and Anderson–Darling) supported the lognormal distribution as a good model. Correlations between the log Gensini Score and TMAO were tested using a Pearson correlation coefficient. A regression model was developed to predict the log Gensini score using a stepwise selection method with 0.20 to enter the model and 0.05 to stay in the model. The variables that were allowed to enter the model included TMAO, eGFR, diabetes, hypertension, race, gender, age, body mass index, CKD stage, history of percutaneous intervention, triglycerides, cholesterol, history of coronary artery bypass grafting, history of myocardial infarction, history of cerebrovascular accident, history of peripheral vascular disease, history of congestive heart failure, and smoking status. To determine the effect of TMAO on survival, an unadjusted Cox proportional hazards model was developed with TMAO as a continuous variable. A model to predict survival was developed using Cox proportional hazards and having an entry criteria of 0.20 and a stay criteria of 0.05. The same covariates utilized in the Gensini score model were available to enter the survival model. Unadjusted Kaplan–Meier curves were used to depict survival for the TMAO tertiles.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by a Clinical Pilot Grant and Trail Blazer Award from Frontiers: The Heartland Institute for Clinical and Translational Research as part of a CTSA grant to KUMC from the National Institutes of Health National Center for Advancing Translational Science (National Center for Advancing Translational Sciences, grant UL1-TR000001) (to J.R.S.), a Mentored Clinical Scientist Grant from the KUMC Department of Internal Medicine (to J.R.S.), and a KUMC Kidney Institute Pilot Research Grant (to A.G.). The Diabetes Genome Project was funded by the St. Luke’s Hospital Foundation. The funding sources had no role in the design or analysis of the study, or the preparation of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Trimethylamine N-Oxide as a Novel Therapeutic Target in CKD,” on pages 8–10.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014111063/-/DCSupplemental.
References
- 1.US Renal Data System : USRDS 2013 Annual Report, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2013 [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Foley RN, Parfrey PS, Sarnak MJ: Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol 9[Suppl]: S16–S23, 1998 [PubMed] [Google Scholar]
- 4.Jungers P, Massy ZA, Nguyen Khoa T, Fumeron C, Labrunie M, Lacour B, Descamps-Latscha B, Man NK: Incidence and risk factors of atherosclerotic cardiovascular accidents in predialysis chronic renal failure patients: a prospective study. Nephrol Dial Transplant 12: 2597–2602, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Olechnowicz-Tietz S, Gluba A, Paradowska A, Banach M, Rysz J: The risk of atherosclerosis in patients with chronic kidney disease. Int Urol Nephrol 45: 1605–1612, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menon V, Gul A, Sarnak MJ: Cardiovascular risk factors in chronic kidney disease. Kidney Int 68: 1413–1418, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW, American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention : Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 108: 2154–2169, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL: Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 19: 576–585, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL: Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472: 57–63, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL: Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 368: 1575–1584, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z, Tang WH, Buffa JA, Fu X, Britt EB, Koeth RA, Levison BS, Fan Y, Wu Y, Hazen SL: Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur Heart J 35: 904–910, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lang DH, Yeung CK, Peter RM, Ibarra C, Gasser R, Itagaki K, Philpot RM, Rettie AE: Isoform specificity of trimethylamine N-oxygenation by human flavin-containing monooxygenase (FMO) and P450 enzymes: selective catalysis by FMO3. Biochem Pharmacol 56: 1005–1012, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Al-Waiz M, Mitchell SC, Idle JR, Smith RL: The metabolism of 14C-labelled trimethylamine and its N-oxide in man. Xenobiotica 17: 551–558, 1987 [DOI] [PubMed] [Google Scholar]
- 14.Mitchell SC, Zhang AQ, Noblet JM, Gillespie S, Jones N, Smith RL: Metabolic disposition of [14C]-trimethylamine N-oxide in rat: variation with dose and route of administration. Xenobiotica 27: 1187–1197, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Bain MA, Faull R, Fornasini G, Milne RW, Evans AM: Accumulation of trimethylamine and trimethylamine-N-oxide in end-stage renal disease patients undergoing haemodialysis. Nephrol Dial Transplant 21: 1300–1304, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Rhee EP, Souza A, Farrell L, Pollak MR, Lewis GD, Steele DJ, Thadhani R, Clish CB, Greka A, Gerszten RE: Metabolite profiling identifies markers of uremia. J Am Soc Nephrol 21: 1041–1051, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang WH, Wang Z, Kennedy DJ, Wu Y, Buffa JA, Agatisa-Boyle B, Li XS, Levison BS, Hazen SL: Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res 116: 448–455, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marso SP, Mehta SK, Frutkin A, House JA, McCrary JR, Kulkarni KR: Low adiponectin levels are associated with atherogenic dyslipidemia and lipid-rich plaque in nondiabetic coronary arteries. Diabetes Care 31: 989–994, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Lindsey JB, House JA, Kennedy KF, Marso SP: Diabetes duration is associated with increased thin-cap fibroatheroma detected by intravascular ultrasound with virtual histology. Circ Cardiovasc Interv 2: 543–548, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Ramezani A, Raj DS: The gut microbiome, kidney disease, and targeted interventions. J Am Soc Nephrol 25: 657–670, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaziri ND, Wong J, Pahl M, Piceno YM, Yuan J, DeSantis TZ, Ni Z, Nguyen TH, Andersen GL: Chronic kidney disease alters intestinal microbial flora. Kidney Int 83: 308–315, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Hida M, Aiba Y, Sawamura S, Suzuki N, Satoh T, Koga Y: Inhibition of the accumulation of uremic toxins in the blood and their precursors in the feces after oral administration of Lebenin, a lactic acid bacteria preparation, to uremic patients undergoing hemodialysis. Nephron 74: 349–355, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Vaziri ND, Yuan J, Nazertehrani S, Ni Z, Liu S: Chronic kidney disease causes disruption of gastric and small intestinal epithelial tight junction. Am J Nephrol 38: 99–103, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Magnusson M, Magnusson KE, Sundqvist T, Denneberg T: Increased intestinal permeability to differently sized polyethylene glycols in uremic rats: effects of low- and high-protein diets. Nephron 56: 306–311, 1990 [DOI] [PubMed] [Google Scholar]
- 25.Magnusson M, Magnusson KE, Sundqvist T, Denneberg T: Impaired intestinal barrier function measured by differently sized polyethylene glycols in patients with chronic renal failure. Gut 32: 754–759, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Almeida Duarte JB, de Aguilar-Nascimento JE, Nascimento M, Nochi RJ, Jr: Bacterial translocation in experimental uremia. Urol Res 32: 266–270, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK: Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341: 1725–1730, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Port FK, Wolfe RA, Mauger EA, Berling DP, Jiang K: Comparison of survival probabilities for dialysis patients vs cadaveric renal transplant recipients. JAMA 270: 1339–1343, 1993 [PubMed] [Google Scholar]
- 29.Chmielewski M, Bryl E, Marzec L, Aleksandrowicz E, Witkowski JM, Rutkowski B: Expression of scavenger receptor CD36 in chronic renal failure patients. Artif Organs 29: 608–614, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Wu CC, Chen JS, Lin SH, Chu P, Lin YF, Lin SM, Liao TN: Aberrant activation of the TNF-alpha system and production of Fas and scavenger receptors on monocytes in patients with end-stage renal disease. Artif Organs 29: 701–707, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Ando M, Lundkvist I, Bergström J, Lindholm B: Enhanced scavenger receptor expression in monocyte-macrophages in dialysis patients. Kidney Int 49: 773–780, 1996 [DOI] [PubMed] [Google Scholar]
- 32.Ando M, Gåfvels M, Bergström J, Lindholm B, Lundkvist I: Uremic serum enhances scavenger receptor expression and activity in the human monocytic cell line U937. Kidney Int 51: 785–792, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Konishi Y, Okamura M, Konishi M, Negoro N, Yoshida T, Inoue T, Kanayama Y, Yoshikawa J: Enhanced gene expression of scavenger receptor in peripheral blood monocytes from patients on cuprophane haemodialysis. Nephrol Dial Transplant 12: 1167–1172, 1997 [DOI] [PubMed] [Google Scholar]
- 34.Zuo Y, Yancey P, Castro I, Khan WN, Motojima M, Ichikawa I, Fogo AB, Linton MF, Fazio S, Kon V: Renal dysfunction potentiates foam cell formation by repressing ABCA1. Arterioscler Thromb Vasc Biol 29: 1277–1282, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, Kurella Tamura M, Feldman HI: KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis 63: 713–735, 2014 [DOI] [PubMed] [Google Scholar]
- 37.St. Luke's Mid America Heart Institute Imaging Core Laboratory website. http://www.saintlukeshealthsystem.org/imaging-core-lab-research-studies. Accessed September 2nd, 2014
- 38.Gensini GG: A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol 51: 606, 1983 [DOI] [PubMed] [Google Scholar]
- 39.Ocque AJ, Stubbs JR, Nolin TD: Development and validation of a simple UHPLC-MS/MS method for the simultaneous determination of trimethylamine N-oxide, choline, and betaine in human plasma and urine. J Pharm Biomed Anal 109: 128–135, 2015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.