Abstract
Macrophages are a heterogeneous cell type implicated in injury, repair, and fibrosis after AKI, but the macrophage population associated with each phase is unclear. In this study, we used a renal bilateral ischemia-reperfusion injury mouse model to identify unique monocyte/macrophage populations by differential expression of Ly6C in CD11b+ cells and to define the function of these cells in the pathophysiology of disease on the basis of microarray gene signatures and reduction strategies. Macrophage populations were isolated from kidney homogenates by fluorescence-activated cell sorting for whole genome microarray analysis. The CD11b+/Ly6Chigh population associated with the onset of renal injury and increase in proinflammatory cytokines, whereas the CD11b+/Ly6Cintermediate population peaked during kidney repair. The CD11b+/Ly6Clow population emerged with developing renal fibrosis. Principal component and hierarchical cluster analyses identified gene signatures unique to each population. The CD11b+/Ly6Cintermediate population had a distinct phenotype of wound healing, confirmed by results of studies inhibiting the macrophage colony-stimulating factor 1 receptor,whereas the CD11b+/Ly6Clow population had a profibrotic phenotype. All populations, including the CD11b+/Ly6Chigh population, carried differential inflammatory signatures. The expression of M2-specific markers was detected in both the CD11b+/Ly6Cintermediate and CD11b+/Ly6Clow populations, suggesting these in vivo populations do not fit into the traditional classifications defined by in vitro systems. Results of this study in a renal ischemia-reperfusion injury model allow phenotype and function to be assigned to CD11b+/Ly6C+ monocyte/macrophage populations in the pathophysiology of disease after AKI.
Keywords: pathophysiology of renal disease and progression, ischemia-reperfusion, macrophages, gene expression, fibrosis, transcriptional profiling
Renal ischemia-reperfusion injury (IRI) is one of the main clinical causes of AKI,1,2 which is defined as an abrupt reduction in kidney function. Repair of the damaged tubular epithelium will result in full recovery in a subset of patients3,4; however, numerous recent clinical studies have identified associations between AKI, CKD, and progression to ESRD.5–8 Maladaptive repair leads to permanent structural and functional changes which culminate in tubulointerstitial fibrosis and chronic inflammation, central to CKD.1,9–11 Macrophages have been implicated in the injury, repair, and fibrosis associated with various renal diseases including AKI.12,13 In fact, CKD patients with high levels of interstitial macrophage infiltration have the poorest renal outcome.14
Macrophages are a heterogeneous cell population that can switch from one phenotype to another in response to extracellular cues.15 In vitro studies have defined the classically activated macrophages as proinflammatory, M1 macrophages, whereas the alternatively activated, M2 macrophages typically modulate the immune response to promote repair and fibrosis.15,16 The M2 classification has been further subdivided into M2a–c.13,17 However, it is unclear how these populations defined in the in vitro setting with limited cytokine exposure will translate in vivo with more diverse cytokine signaling. Therefore, we have focused our efforts on defining macrophage populations based on their function during the pathophysiology of disease.18
In this study, we use a murine model of renal IRI characterized by three different phases postreperfusion: injury, repair, and fibrosis, to identify the macrophage population associated with each phase. The populations are distinguished by differential Ly6C expression, which has been widely used to identify functionally discrete monocyte/macrophage populations.18–25 To establish their role in the pathophysiology of AKI, a small molecule c-fms inhibitor was used to inhibit macrophage proliferation and activation at different stages of disease. Furthermore, the newly identified populations of monocytes/macrophages were isolated and sorted from the kidney for microarray analysis. Our study is the first to show in the renal IRI model that each of the three populations carries a unique gene signature that provides insight into their functional role in the pathophysiology of acute and chronic disease following AKI.
Results
In Vivo Model of AKI Shows Distinct Phases of Injury, Repair, and Developing Fibrosis
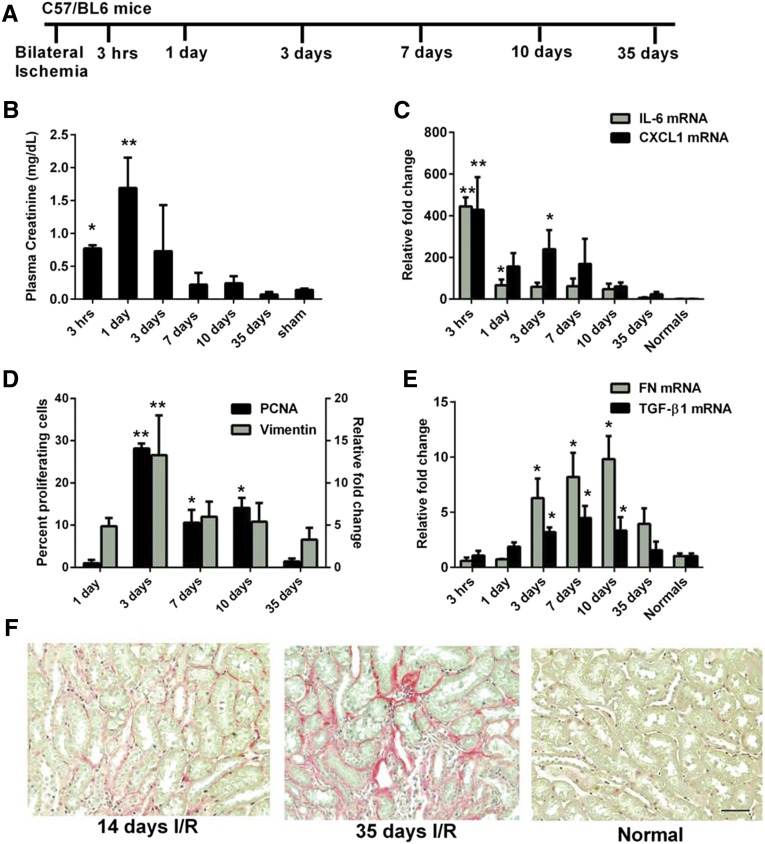
Samples were harvested for up to 35 days postreperfusion to define the timeframe of the various phases of the AKI model (Figure 1A). Plasma creatinine levels show that the loss of renal function is greatest 1 day postreperfusion, returning to baseline by 7 days (Figure 1B). A proinflammatory burst is measured in the kidney at 3 hours postreperfusion with peaks in mRNA expression of IL-6 and CXCL1 (Figure 1C). The return of plasma creatinine to normal levels is due to the surviving tubular epithelium dedifferentiating, proliferating and repolarizing to restore tubular structure and function.4 Repair of the tubular epithelium is measured by proliferating cell nuclear antigen (PCNA) staining and vimentin mRNA expression with peaks in proliferation and dedifferentiation, respectively, quantitated 3 days postreperfusion (Figure 1D). Significant increases in extracellular matrix genes, fibronectin, collagen Iα1, and IIIα1 as well as the profibrotic gene, TGF-β1, are measured between 3 and 10 days postreperfusion (Figure 1E and data not shown). This translates into histologic evidence of tubulointerstitial fibrosis measured by picrosirius red at 14 and 35 days (Figure 1F), demonstrating the long-term effects of renal IRI. Thus, based on the time points examined in this model, injury predominates in the acute phase for 1 day postreperfusion followed by repair that peaks at 3 days postreperfusion and declines by day 7 postreperfusion in agreement with published studies.10,26 Importantly, our study highlights that fibrosis develops from 7 to 35 days postreperfusion following bilateral renal IRI.
Figure 1.
Renal IRI model shows distinct phases of injury, repair, and developing fibrosis. (A) Schematic representation of time course experiments in which C57/BL6 mice underwent bilateral ischemia followed by sacrifice at different reperfusion times. (B) Plasma creatinine indicates peak loss in renal function at 1 day postreperfusion with a return to baseline levels by 7 days. *P≤0.05 indicates a significant difference from the sham. Sham plasma collected at 24 hours postreperfusion. **P≤0.05 indicates a significant difference from all other groups. (C) RT-PCR of whole-kidney lysates indicates an inflammatory burst at 3 hours postreperfusion measured by peak levels of IL-6 and CXCL1. *P≤0.05 indicates a significant difference from normal and 35 days. **P≤0.05 indicates a significant difference from all other groups. (D) PCNA staining in the outer medulla and vimentin mRNA expression show peak proliferation and dedifferentiation, respectively, at 3 days postreperfusion during repair of the tubular epithelium when kidney function returns. *P≤0.05 indicates a significant difference from 35 days and 1 day. **P≤0.05 indicates a significant difference from all other groups. (E) RT-PCR of whole-kidney lysates shows significant elevations from 3 to 10 days postreperfusion of fibrosis associated genes, fibronectin and TGF-β1, suggesting developing fibrosis *P≤0.05 indicates a significant difference from normal. (F) Picrosirius red staining of kidney sections at 14 and 35 days postreperfusion highlights fibrosis in the outer medulla in contrast to normal kidney which shows minimal staining. Scale bars, 100 µm. n=4 for all groups except 10 days which has n=6.
Changes in Macrophage Ly6C expression and Function are Measured During the Different Phases Following Renal IRI
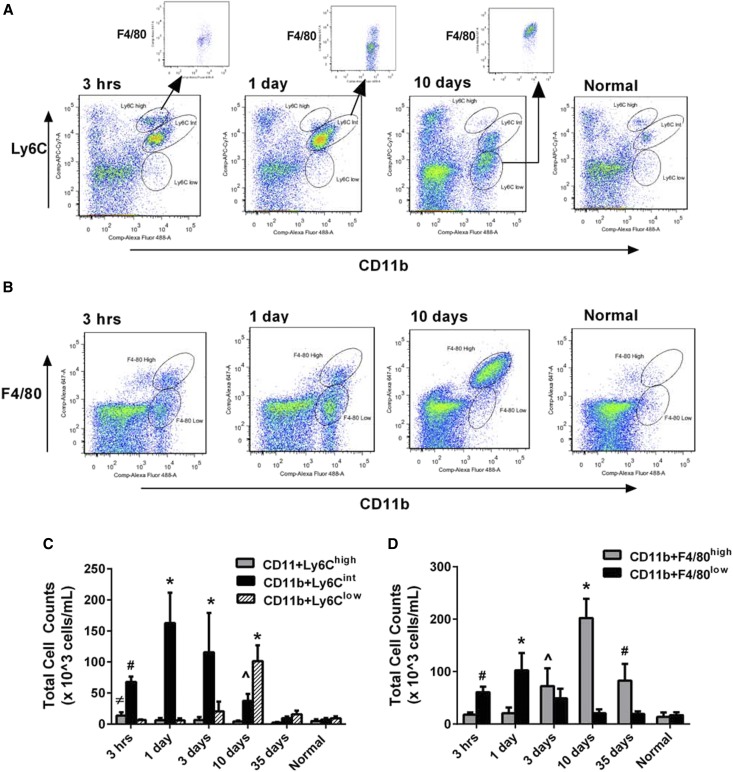
Because differential Ly6C expression identifies functionally discrete monocyte/macrophage populations in various tissues,18–25 flow cytometry analysis of the cell surface marker Ly6C was carried out to identify distinct monocyte/macrophage subsets in kidney homogenates following renal IRI. Populations of monocytes/macrophages are defined as CD11b+ and Ly6Chigh, Ly6Cintermediate(int) or Ly6Clow (Figure 2A). All three populations are present in the normal kidney at low levels. Correlating with the proinflammatory burst at 3 hours postreperfusion (Figure 1C), elevated numbers of the Ly6Chigh population are measured, which are F4/80low (Figure 2, A+inset, C). The Ly6Cint population appears at 3 hours postreperfusion and persists as the dominant population at 3 days with varying levels of F4/80 expression (Figure 2, A+inset, C). PCNA staining and vimentin expression peak at this time (Figure 1D), suggesting this population may be involved in repair. Finally, the Ly6Clow population emerges at 3 days postreperfusion and peaks at 10 days with F4/80high expression (Figure 2, A+inset, C). Elevations in extracellular matrix and profibrotic genes are measured (Figure 1E), suggesting this population may play a role in the fibrosis that develops in this in vivo model.
Figure 2.
Changes in Ly6C expression are measured in the kidney following IRI. (A) Flow-cytometric analysis of CD11b+ cells identifies three distinct populations based on Ly6Chigh, int or low, which vary in prevalence postreperfusion. Gating of the CD11b+/Ly6Chigh population at 3 hours postreperfusion shows that these cells are also F4/80low (inset), whereas the CD11b+/Ly6Cint population at 24 hours is primarily F4/80low with some cells F4/80high (inset). Ten days post reperfusion the CD11b+/Ly6Clow population is only F4/80high (inset). (B) Flow-cytometric analysis of CD11b+/F4/80high or low cells indicates changes in F4/80 over time. The CD11b+/F4/80low population is the major population from 3 hours to 1 day postreperfusion, whereas the CD11b+/F4/80high population predominates by 10 days. (C) Quantitation of total cell number indicates the Ly6Chigh population is present at 3 hours postreperfusion during the inflammatory burst, the Ly6Cint population is dominant during the repair phase, while the Ly6Clow population emerges during the fibrotic phase. *P≤0.05 indicates a significant difference from all other time points of respective Ly6C population. #P≤0.05 indicates a significant difference from all other time points of respective Ly6C population except 10 days. ^P≤0.05 indicates a significant difference from all other time points of respective Ly6C population except 3 hours and 35 days. ≠P≤0.05 indicates a significant difference of respective Ly6C population compared with 10 days, 35 days, and normal. (D) Quantitation of total cell number indicates that CD11b+/F4/80high cells increase over time, peaking at 10 days, whereas CD11b+/F4/80low cells peak at 1 day postreperfusion, declining thereafter. *P≤0.05 indicates a significant difference from all time points of the respective population. #P≤0.05 indicates a significant difference from all time points of the respective population except 3 days. ^P≤0.05 indicates a significant difference from all time points of the respective population except 35 days. n=4 for all groups except 10 days which has n=6.
Further, flow-cytometric analysis of F4/80 expression in CD11b+ cells indicates that as early as 3 hours postreperfusion and extending to 3 days, the majority of cells are F4/80low (Figure 2, B and D). However, 10 days postreperfusion, the number of F4/80high cells has peaked and persists at 35 days (Figure 2, B and D), suggesting this population represents mature macrophages.27
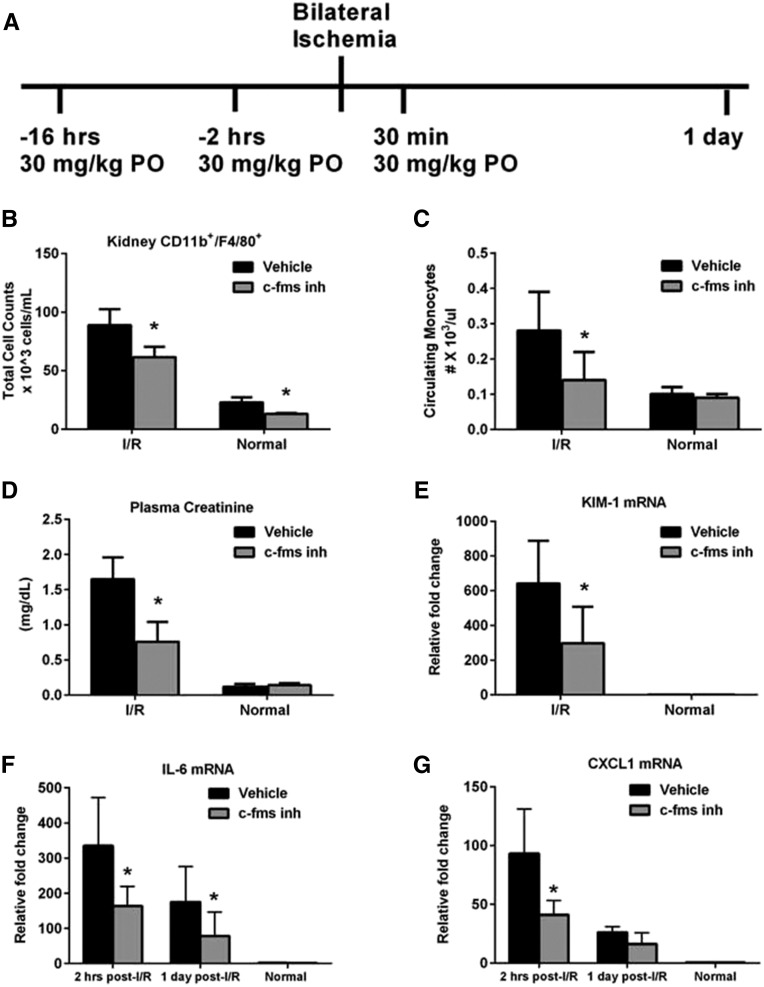
Moreover, inhibition of the c-fms receptor which mediates proliferation, differentiation and survival of monocytes/macrophages28 when bound to colony-stimulating factor demonstrates that inhibition of monocytes/macrophages prior to IRI (Figure 3A) dampens the proinflammatory milieu leading to renoprotection. An approximately 50% decrease is measured 2 hours postreperfusion in kidney mRNA expression of IL-6 (Figure 3F) and CXCL1 (Figure 3G), with significant reductions in IL-6 at 1 day. Significant reductions in plasma creatinine (Figure 3D), BUN (data not shown), kidney injury molecule-1 (KIM-1; Figure 3E), and neutrophil gelatinase-associated lipocalin (NGAL; data not shown) are measured and a decrease in kidney CD11b+/F4/80 cells (Figure 3B) and circulating monocytes (Figure 3C) persists.
Figure 3.
Pretreatment with a c-fms inhibitor reduces the number of kidney CD11b+/F4/80 cells, inflammation and kidney injury. (A) Schematic representation of preventative treatment with a c-fms inhibitor (c-fms inh) to deplete monocyte/macrophages during the injury phase of disease. Animals were dosed at 30 mg/kg, PO 16 hours and 2 hours prior to surgery and 30 min postreperfusion. (B) Flow-cytometric analysis of total kidney CD11b+/F4/80+ cells at 24 hours postreperfusion indicates that treatment results in reduced numbers in both injured and normal mice. (C) Reduced circulating levels of monocytes persists at 24 hours. (D) Plasma creatinine indicates that there is reduced loss of kidney function with treatment. Similarly, (E) qRT-PCR of markers of kidney structural injury KIM-1 and NGAL (data not shown) are significantly reduced. (F) qRT-PCR of IL-6 and (G) CXCL1 indicate that the c-fms inhibitor dampens the expression of both cytokines at 2 hours postreperfusion while IL-6 remains reduced through 1 day postreperfusion. *P≤0.05 indicates a significant difference from the respective vehicle group. n=9 for vehicle, I/R, n=10 for c-fms inhibitor, I/R, n=4 for normals.
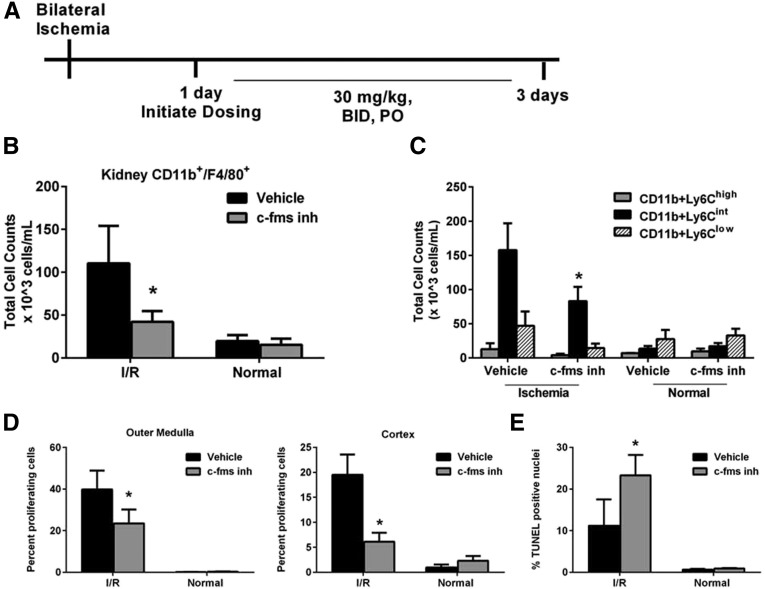
However, when the c-fms inhibitor is administered 1–3 days postreperfusion (Figure 4A) when tubular epithelial proliferation and vimentin expression are at peak levels (Figure 1D), there is a significant decline in kidney repair. Cellular proliferation measured by PCNA staining of the tubular epithelium in the cortex and outer medulla is significantly decreased (Figure 4D) concomitant with a significant increase in cell death measured by terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL; Figure 4E). There are trends of increased KIM-1 and NGAL mRNA expression (P=0.19 and 0.10, respectively; data not shown) yet no difference in plasma creatinine is measured (data not shown). Total kidney CD11b+/F4/80+ cells (Figure 4B) are significantly decreased with c-fms inhibitor treatment. Importantly, further analysis of CD11b+/Ly6C+ cells demonstrates that the Ly6Cint population is significantly reduced by approximately 50% (P<0.05), whereas the Ly6Chigh and Ly6Clow populations remain equivalent (Figure 4C). Thus, the cell proliferation and death data jointly suggest a role of the Ly6Cint population in kidney repair.
Figure 4.
c-fms inhibitor treatment from 1 to 3 days postreperfusion reduces the number of kidney CD11b+/F4/80 cells and kidney repair. (A) Schematic representation of depletion regimen during the repair phase by treating with the c-fms inhibitor twice daily at 30 mg/kg, PO for a total of 4 doses from 24 to 72 hours postreperfusion. (B) Flow-cytometric analysis of total kidney CD11b+/F4/80+ cells indicates c-fms inhibitor treatment results in reduced numbers postreperfusion. (C) Importantly, the Ly6Cint population is significantly reduced while no significant changes are observed in Ly6Chigh or Ly6Clow. (D) PCNA staining to quantitate cell proliferation as a marker of kidney repair shows significantly reduced levels in the tubular epithelium in both the cortex and outer medulla. (E) Quantitation of cell death by TUNEL staining indicates increased cell death during the repair phase of tubular epithelia in the outer medulla with c-fms inhibitor treatment. Less than 3% of TUNEL-positive cells are found in the cortex (data not shown). *P≤0.05 indicates a significant difference from respective vehicle group. n=4 for vehicle, I/R, n=7 for c-fms inhibitor, I/R, n=3 for normals.
Genomic Analysis Identifies a Unique Gene Signature for Each Macrophage Population
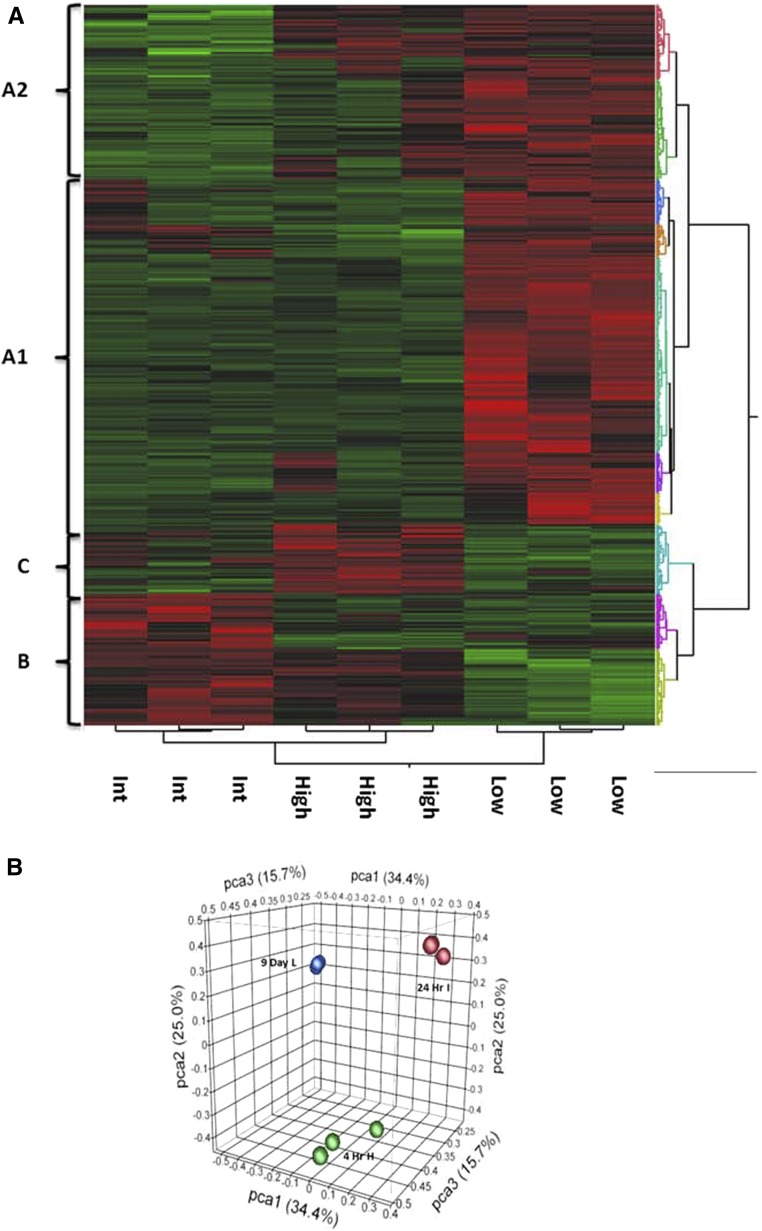
As there are conflicting reports on the role of macrophages in fibrosis based on studies with liposome clodronate29 and the c-fms inhibitor,30 a genomic approach was taken. Single-cell suspensions of kidney homogenates were flow-sorted based on CD11b/Ly6C expression to isolate each population followed by whole genome array. Based on hierarchical cluster analysis, a unique gene signature is identified for each population (Figure 5A). Principal component analysis confirms the differential gene expression in each population (Figure 5B).
Figure 5.
Genomic analysis identifies a unique gene signature for each of the populations of macrophages. Cells were sorted based on CD11b+/Ly6C expression at time points enriched for a particular population: CD11b+/Ly6Chigh at 4 hours postreperfusion, CD11b+/Ly6Cint at 1 day postreperfusion, and CD11b+/Ly6Clow at 9 days postreperfusion. Next, the sorted populations underwent whole-genome mRNA profiling with Affymetrix mouse gene microarray. (A) Hierarchical clustering is carried out to identify differentially expressed genes between macrophage populations. The heat map indicates that each population has a unique gene signature. There are 72 genes upregulated in the Ly6Chigh population, represented as cluster C, 102 genes upregulated in the Ly6Cint population represented as cluster B, and 740 genes upregulated in the Ly6Clow population, represented as cluster A1. Cluster A2 represents 237 genes that are primarily upregulated in the Ly6Clow population with some overlapping upregulation in the Ly6Chigh population. (B) Principal component analysis confirms that the populations are different. n=3.
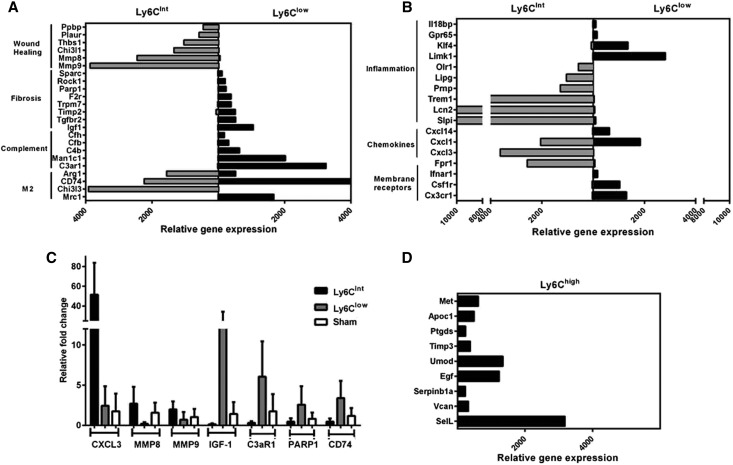
A number of differentially expressed genes highlight the distinct phenotypes of the populations. The CD11b+/Ly6Chigh population carries a gene signature associated with the monocyte/macrophage immune response and monocyte/macrophage differentiation (Figure 6D). In contrast, the CD11b+/Ly6Cint population expresses numerous genes associated with wound healing (Figure 6A) and the CD11b+/Ly6Clow population expresses genes associated with fibrosis (Figure 6A). Interestingly, both macrophage populations express a variety of chemokines, membrane receptors, and inflammation-associated genes, suggesting both carry an inflammatory signature (Figure 6B); however, complement-associated genes are exclusively expressed by the Ly6Clow population (Figure 6A). The expression of M2-specific macrophage markers such as Arg1, CD74, Chi3l3, and Mrc1 suggest that both populations may be considered M2 macrophages; however, only Chi3l3 and Mrc1 would be useful to distinguish between them (Figure 6A). A subset of genes was confirmed by quantitative RT-PCR (qRT-PCR) and the results verify the accuracy of the microarray analysis (Figure 6C).
Figure 6.
The gene signature indicates a unique phenotype and function for each CD11b/Ly6C population. (A) There is a switch from a reparative Ly6Cint population to a profibrotic Ly6Clow population. Genes that are associated with wound healing are uniquely upregulated in the Ly6Cint population, while genes associated with fibrosis are uniquely expressed in the Ly6Clow. The Ly6Clow population is enriched for complement genes. However, markers of M2 macrophages are present in both populations, with some markers differentially expressed. (B) Both the CD11b+/Ly6Cint and CD11b+/Ly6Clow populations carry an inflammatory signature with various membrane receptors, chemokines and other genes associated with inflammation expressed in both populations. (C) qRT-PCR on sorted macrophage populations confirms the microarray data by showing that the representative genes in (A) and (B) are uniquely upregulated in one population. (D) Genes associated with monocyte/macrophage immune responses are highly expressed in the CD11b+/Ly6Chigh subset. n=3.
The top 15 cell processes associated with the genes uniquely upregulated in the Ly6Cint population were identified using Pathway Studio software. The majority of the cell processes identified is associated with inflammation and wound healing, including: immune response, innate immune response, inflammatory response, cell differentiation, tissue remodeling, phagocytosis, and vascularization (Table 1). Importantly, these data further support our hypothesis that the Ly6Cint population plays a role in repair following AKI. Querying published microarray data sets using NextBio identified statistically significant strong correlations between the genes upregulated in the Ly6Cint population and several studies of macrophages activated in culture systems with various stimuli, indicating that the Ly6Cint population represents activated macrophages (Table 2).
Table 1.
Top 15 cell processes regulated by genes upregulated in the Ly6Cint population
| Cell Process | P Value |
|---|---|
| Immune response | 6.64E-12 |
| Cell adhesion | 1.42E-08 |
| Innate immune response | 8.25E-08 |
| Neutrophil chemotaxis | 7.91E-07 |
| Inflammatory response | 1.29E-06 |
| Epithelial to mesenchymal transition | 2.50E-06 |
| Cell differentiation | 4.00E-06 |
| Neutrophil activation | 6.44E-06 |
| Apoptosis of neutrophils | 6.87E-06 |
| Tissue remodeling | 7.57E-06 |
| Phagocytosis | 1.14E-05 |
| Cell-cell adhesion | 1.24E-05 |
| Leukocyte migration | 1.43E-05 |
| Immunity | 2.01E-05 |
| Vascularization | 3.55E-05 |
Table 2.
Studies of stimulated macrophages that have significant overlap with genes uniquely upregulated in the Ly6Cint population
| Study Name (Reference) | P Value | Species |
|---|---|---|
| Bone marrow-derived macrophages infected 48 hr with M. bovis BCG wild-type versus MyD88 KO (J.E. Qualis, GSE22935) | 7.9E-78 | Mouse |
| J774.A1 macrophages infected 48 hr with Brucella suis VTRS1 versus uninfected controls (Y. He, GSE21117) | 4.3E-56 | Mouse |
| Macrophages from STAT6 KO mice cultured 10d with 10 uM rosiglitazone + 20 ng/mL IL-4 versus RSG only (A. Szanto, GSE25088) | 6.5E-52 | Mouse |
| Bone marrow-derived macrophages stimulated with Th2 cytokine IL-4 (F.O. Martinez, GSE35435) | 1.9E-41 | Mouse |
| RAW 264.7 mouse macrophages + 3.5 mM HOCl 6 hr versus untreated (C.G. Woods, GSE15457) | 2.4E-40 | Mouse |
Statistically significant, strong correlations also were identified between the genes upregulated in the Ly6Clow population and several studies analyzing fibrotic tissues, including mouse lung tissue treated with bleomycin to induce fibrosis, and cirrhotic liver and kidney biopsies from human patients with interstitial fibrosis at 1 year post-transplant (Table 3). Importantly, these data support our hypothesis that the Ly6Clow population is involved with the fibrosis that develops following renal IRI.
Table 3.
Studies associated with fibrosis that have significant overlap with genes uniquely upregulated in the Ly6Clow population
| Study Name (Reference) | P Value | Species | Organ |
|---|---|---|---|
| Mouse lung wild-type 7d after intratracheal bleomycin 1 mg/kg versus no bleomycin (T. Oga60) | 7.10E-27 | Mouse | Lung |
| Lungs from wild-type mice 21d after endotracheal injection of bleomycin versus vehicle (T. Liu61) | 1.10E-26 | Mouse | Lung |
| Renal allografts: subclinical interstitial fibrosis 12mo post-transplant versus time zero (W.D. Park48) | 4.20E-24 | Human | Kidney |
| Cirrhotic hepatic inflammatory cells - adipose stem cells treatment 2×every 2wk versus PBS control (Y. Sakai, GSE40395) | 2.80E-23 | Mouse | Liver |
| Kidney biopsy 1yr post-Tx with histology interstitial fibrosis + cellular infiltration versus normal (W.D. Park47) | 1.00E-19 | Human | Kidney |
Discussion
Macrophage infiltration into the kidney correlates with decline in kidney function of patients with CKD.14 These data suggest that macrophages play a critical role in the progression of kidney disease; however, the mechanism remains unclear. Our study is the first to phenotype and define a functional role of monocytes/macrophages based on differential Ly6C expression over time in the pathophysiology of renal IRI. Three unique subsets of monocytes/macrophages that correlate with injury, repair, or fibrosis were identified. Whole-genome analysis of the three different subsets identified a distinct gene signature for each that provided insight into the role of the Ly6Chigh in injury, the Ly6Cint population in repair, and the Ly6Clow population in fibrosis. It also highlighted that these populations do not fit into the canonical M1/M2 classifications defined by in vitro assays. Finally, these data provide insight into mechanisms by which monocytes/macrophages contribute to acute and chronic disease following renal IRI.
Differential Ly6C expression has been widely used to identify functionally discrete monocyte/macrophage populations.18–25 In a murine model of kidney fibrosis by unilateral ureteral obstruction (UUO), three different populations of macrophages were identified by differential Ly6C expression.19 Our data confirm and extend these findings. Similarly, we show that one of the first populations to appear in the renal IRI model is the CD11b+/Ly6Chigh population, which correlates with the proinflammatory phase characteristic of this model and which carries an inflammatory signature unique from the CD11b+/Ly6int and CD11b+/Ly6Clow. However, as the renal IRI model is a reversible model that undergoes repair, our c-fms inhibition study, which was confirmed by the microarray data, demonstrates the CD11b+/Ly6Cint population mediates kidney recovery. Duffield19 also identified a CD11b+/Ly6Cint population, but based on mRNA expression of a subset of monocyte/macrophage genes in the UUO model, characterized this population in transition from the proinflammatory CD11b+/Ly6Chigh to profibrotic CD11b+/Ly6Clow. In contrast, our microarray data identify a unique gene signature of wound healing for the CD11b+/Ly6Cint population, which is distinct from that of the Ly6Chigh and Ly6Clow. Thus, in aggregate, the data suggest that the functional definition and phenotype of the macrophage populations reflects the pathophysiology of the animal model from which they are isolated. Thus, it is not surprising that the CD11b+/Ly6Cint population in the UUO model which does not have a repair phase carries a gene signature reflective of a transition population, in contrast to the CD11b+/Ly6Cint population in the renal IRI model which undergoes recovery.
Moreover, similar to Duffield’s findings in the UUO model,19 our microarray studies in the renal IRI model implicate the CD11b+/Ly6Clow population as profibrotic. We identified similar (Igf1) yet novel genes (Parp1, TRPM7, SPARC, ROCK1), confirming a role in kidney fibrosis of the CD11b+/Ly6Clow population. However, in other organs, such as the liver in a model of reversible fibrosis, the Ly6Clow population is involved in the resolution of fibrosis while the Ly6Chigh population drives fibrosis.18 In a model of myocardial infarction, the Ly6Chigh population scavenged necrotic debris while the Ly6Clow population was implicated in reparative processes.25 Thus, the phenotype of a particular Ly6C population may differ based on organ and pathophysiology of disease. Therefore, as suggested by Murray et al.,31 studies should focus on the function of the macrophage rather than definition by cell surface marker expression.
Additional rationale to focus on macrophage function comes from our data in which we show that the Ly6Cint and Ly6Clow populations do not fit into the canonical M1/M2 classifications defined by in vitro assays. The M2 markers, Arg1 and CD74, are expressed in both populations; however, the M2 markers, Chi3l3 and Mrc1, are expressed only in the Ly6Cint and Ly6Clow populations, respectively. No M1 markers are detected in any population. The nomenclature of M1 and M2 macrophages was based on in vitro studies in which cells were exposed to one cytokine; however, in the disease setting there will be a milieu of various cytokines so the classification of macrophages based on cell surface marker expression becomes complex.31 Thus, in light of current efforts to harmonize nomenclature for macrophage studies,31 we have focused our studies on defining macrophage populations based on function.
The origin of the macrophage subsets may also vary based on organ and disease. Recruitment and differentiation of Ly6Chigh monocytes were identified as the primary source of profibrotic macrophages in a UUO model.19 In a study focused on reparative macrophages following renal IRI, recruitment of monocytes as well as in situ proliferation of resident macrophages were shown to contribute to increases in the reparative population.32 In a liver fibrosis model, Ly6Chigh monocytes differentiated into two different macrophage subsets: profibrotic and proresolution.18 In a myocardial infarction model, two different subsets of monocytes were recruited to differentiate into two different subsets of macrophages.25 Our data show that Ly6C expression decreases while F4/80 expression increases over time, suggesting that the Ly6Chigh subset may be more monocytic while the Ly6Clow subset is more mature. This is supported by the expression of genes in the low population associated with macrophage differentiation from monocytes including: Mcm633 and Nrp1 (neuropilin-1).34 Also, chemokines and membrane receptors expressed in both the Ly6Cint and low populations, including Cxcl14, Cxcl3, Csf1r, and Cx3cr1, have been linked to the recruitment of monocytes.35,36 Collectively, these data suggest that monocyte recruitment and resident cell proliferation are likely to be essential for the expansion of the macrophage populations in our studies, but further experiments would be required to confirm.
Several groups have examined the deleterious role of macrophages in renal IRI by demonstrating renoprotection after reducing macrophage number by genetic deletion of Ccr2 and Cx3cr137 or treatment with liposome clodronate,26,38 a widely accepted means of inducing macrophage apoptosis.39 Macrophages have also been linked to kidney repair following AKI by using liposome clodronate during the recovery phase to deplete macrophages, leading to reduced proliferation of the tubular epithelium.26,32 In our studies, we use a small molecule inhibitor of c-fms to prevent the differentiation and activation of macrophages and confirm published reports showing that macrophages are involved in both the injury and repair phases of renal IRI.26,32,40 Moreover, we extend this observation by gene expression analysis. Further studies are needed to determine whether the Ly6Cint population arises from the Ly6Chigh population or whether the gene expression profile of the Ly6Cint population changes to one of repair from 3 hours to 1 day postreperfusion.
To understand potential mechanisms by which macrophages regulate injury and repair, our strategy was to focus only on genes uniquely expressed in each population without consideration of genes that are up- or downregulated in each respective phenotype over time. A limitation of the analysis is that we are also comparing cell populations harvested at different time points when sufficient cells were present to harvest RNA. Moreover, we cannot rule out that gene expression profiles could be altered through the tissue-processing and cell-sorting procedure. Because CD11b and Ly6C can be expressed on dendritic cells and neutrophils, we must also consider that these cells could be present in our sorted populations.
Several genes upregulated in the Ly6Chigh population are linked with the monocyte/macrophage immune response. Apoc1 and Met associate with monocyte-macrophage differentiation,41,42 whereas Egf mediates monocyte/macrophage chemotaxis.43 Sell (L-selectin) facilitates cell adhesion and rolling. Importantly, analysis of the cell processes linked to the Ly6Cint subset indicate a role in cell differentiation, tissue remodeling, phagocytosis and vascularization, which are all key processes in repair of the tubular epithelium. Mmp8 contributes to the resolution of inflammation,44 while Mmp9 regulates re-epithelialization and collagen fibril formation during wound repair.45 Chi3l1 encodes a protein secreted by macrophages that mediates kidney repair by limiting tubular cell death,46 which may explain the increased cell death with c-fms inhibitor treatment. Collectively, these data clearly indicate the Ly6Cint subset plays a paracrine role in repair of the tubular epithelium.
Due to the inconsistent reports about depleting macrophages to establish a role in kidney fibrosis,29,30 we investigated the gene signature of the Ly6Clow population. One key piece of evidence supporting a role in fibrosis is the common gene signature identified between the Ly6Clow population and numerous genomic studies linked to fibrotic diseases including kidney transplant patients that have interstitial fibrosis.47,48 Additionally, several genes are directly linked to fibrosis. SPARC regulates the expression of secreted extracellular matrix proteins to mediate fibrosis49,50 while Timp2 inhibits extracellular matrix turnover by matrix metalloproteinases (MMPs) leading to matrix accumulation.51 Igf1 released by macrophages may attenuate myofibroblast apoptosis52 or stimulate type I collagen release.53 Activation of complement is involved in progressive tubulointerstitial disease.54 Clearly, the genetic signature of the Ly6Clow population indicates that it contributes to kidney fibrosis.
Incomplete repair of the tubular epithelium following AKI leads to progressive kidney disease driven by chronic inflammation, tubulointerstitial fibrosis, and vascular dropout.9,10,55 Our data indicate that one subset of macrophages has a beneficial role in repair of the tubular epithelium, while another has a detrimental role in the progression to chronic disease following AKI. Interestingly, both populations have an inflammatory signature and we propose, based on our data and clinical studies showing that CKD is a proinflammatory state,56 that it is the chronic inflammation which develops following acute injury that drives the progression to CKD. Because it is unknown whether there is a dynamic relationship between the different subsets of macrophages, the best therapeutic approach may be to target macrophage paracrine signaling. Our microarray analyses identified several genes which may be key contributors to repair of the kidney or progressive fibrosis. Future studies should aim to determine whether enhancing repair or minimizing fibrosis and inflammation would improve patient outcome.
Concise Methods
Please refer to the Detailed Methods in the online Supplemental Material for additional information.
Mouse Model of Bilateral Renal IRI
In vivo studies were approved by the Genzyme Institutional Animal Care and Use Committee. C57BL/6 male mice (8–10 weeks, Taconic, Germantown, NY) underwent bilateral renal IRI for 28 min or sham surgery followed by reperfusion for various times as previously described.11 Surgery was carried out through bilateral flank incisions with animals on homeothermic operating tables to maintain body temperature at 37°C through rectal probe during the ischemia and reperfusion episodes. After surgery, animals were hydrated with intraperitoneal injection of 1 ml sterile saline. Pain medication (buprenorphine, 0.05 mg/kg, SC) was administered before surgery and then again at 12-hr intervals for 2 days starting 24 hours later. Sham surgeries were identical without the bilateral clamping. At sacrifice, retro-orbital blood was collected followed by whole-body perfusion. Harvested kidneys were snap-frozen for molecular analysis, minced for flow-cytometric analysis or fixed for paraffin embedding and processing as previously described.11
Plasma creatinine and BUN values were measured on a Roche Integra 400 Bioanalyzer (Roche Diagnostics, Indianapolis, IN). Whole blood was analyzed for circulating monocytes (Sysmex XT-V Automated Hematology Analyzer; Sysmex, Kobe, Japan). A small molecule inhibitor of c-fms kinase activity (30 mg/kg, PO) was used to reduce monocyte/macrophage proliferation, differentiation and survival, based on published reports.30,57
Morphologic Analysis
Picrosirius red staining and immunostaining of proliferating cell nuclear antigen (PCNA; Abcam, Inc.) and TUNEL (ApopTag Red Apoptosis Determination Kit; EMD Millipore) were carried out as previously detailed.11,58,59 To quantify reactivity in kidney tubules, four to six random fields of the outer medulla or cortex per section per animal were photographed at 20× followed by Metamorph analysis (Molecular Devices, Sunnyvale, CA). Data are expressed as mean±SEM.
Quantitative RT-PCR
For qRT-PCR, total RNA was extracted from snap-frozen tissues with the RNeasy Kit (Qiagen, Valencia, CA) and reverse-transcribed to cDNA (Clontech, Mountain View, CA), as previously described.11 Probes and primers specific for mouse (Applied Biosystems, Foster City, CA) included IL-6 (Mm00446190_m1), CXCL1 (Mm04207460_m1), fibronectin (Mm01256744_m1), TGFβ1 (Mm01178820_m1), KIM-1 (Mm00506686_m1), NGAL (Mm01324470_m1), CXCL3 (Mm01701838_m1), IGF1 (Mm00439560_m1), C3AR1 (Mm02620006_s1), PARP1 (Mm01321084_m1), CD74 (Mm00658576_m1), MMP8 (Mm00439509_m1), and MMP9 (Mm00442991_m1). Data were normalized to 18S (Hs99999901_s1) and expressed as fold change compared with normal.
Flow Cytometry
For flow cytometry, single-cell suspensions of the kidney were made by digesting the tissue in 1 mg/ml collagenase B, 1.2 U/mL dispase, 5 U/mL DNase II followed by a series of filtering and washing as described.58 Fc receptors on single cells (1×106) were blocked with RPMI containing 10% normal mouse serum/0.05% NaAzide followed by incubation of cells with an antibody cocktail containing Alexa Fluor 488 rat anti-mouse CD11b (BD Biosciences, Franklin Lakes, NJ), APC rat anti-mouse F4/80 (eBioscience, San Diego, CA) and APC Cy7 rat anti-mouse Ly6C (BD Biosciences), each at a 1:20 dilution. After washing, cells were incubated with UV-fixable live-dead dye (Invitrogen, Carlsbad, CA) followed by analysis on a BD LSRII flow cytometer (BD Biosciences) with FlowJo 7.6 software (TreeStar, Ashland, OR). For gating, only live cells were included in the analysis followed by dot plots to identify cell populations of Ly6C versus CD11b and F4.80 versus CD11b. Once the CD11b+Ly6Chigh, int and low populations were gated, each population was then plotted for CD11b versus F4/80 to determine the expression levels of F4/80 of each individual population. To accurately measure cell number, counting beads (Invitrogen) were used following the manufacturer’s recommendation.
Microarray Analysis
Time points for microarray analysis were selected when the Ly6C population of interest dominated to optimize mRNA isolation and amplification. Cell populations were sorted on a BD FACSARIA II cell sorter (BD Bioscience) based on CD11b+/Ly6C high, intermediate or low for RNA isolation (Qiagen) and amplification (NuGen Ovation Pico WTA v2, NuGen Technologies, San Carlos, CA) for cDNA production. The amplified cDNA library was further fragmented and labeled with NuGen Encore Biotin Module (NuGen Technologies) followed by hybridization to Affymetrix GeneChip Mouse Genome 430 2.0 array. Raw CEL files were robust multiarray average preprocessed in Affymetrix Expression Console and imported into Array Studio v6.1 (OmicSoft, Cary, NC) for further downstream statistical analysis. The expression data set was filtered to reduce background noise and median normalized across filtered probes. Principal Component Analysis separated ischemia and sham kidneys. Inference reports (t tests) were generated for IRI and sham group separated by population and time course as well as overall IRI versus sham. Similarly, hierarchical clustering was performed to show differentially expressed genes between IRI versus Sham. Statistical significance was set at P<0.01 with no multiple testing correction. To understand potential mechanisms by which macrophages regulate injury, repair and fibrosis, our strategy was to focus only on genes uniquely expressed in each population without consideration of genes that are up- or downregulated in each respective phenotype over time. Differentially expressed gene lists (FC±1.5, P<0.01) from comparative analysis were correlated to public gene expression repository with commercial software NextBio (NextBio, Cupertino, CA). Links between genes and cell processes/phenotype were identified with Pathway Studio (Ariadne Genomics, Rockville, MD).
One-way ANOVA followed by the Newman–Keuls multiple comparison test and two-way ANOVA followed by Sidak’s multiple comparison test or t tests were used when appropriate to determine statistical differences using GraphPad Prism v6.0 software (GraphPad Software, Inc., La Jolla, CA). Data are expressed as mean±SD, unless otherwise indicated.
Disclosure
None.
Supplementary Material
Acknowledgments
M. Gershenovich, J. Campos-Rivera, M. Zhang, S. Ledbetter and A. Zuk are employees of Genzyme, a Sanofi Company. M. Clements and P. Du are employees of Biogen Idec and C. Chaber is an employee of Roche Diagnostics.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014111138/-/DCSupplemental.
References
- 1.Bonventre JV, Yang L: Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121: 4210–4221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharfuddin AA, Molitoris BA: Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol 7: 189–200, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Cerdá J, Lameire N, Eggers P, Pannu N, Uchino S, Wang H, Bagga A, Levin A: Epidemiology of acute kidney injury. Clin J Am Soc Nephrol 3: 881–886, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV: Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2: 284–291, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR: Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis 53: 961–973, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsu CY, Ordoñez JD, Chertow GM, Fan D, McCulloch CE, Go AS: The risk of acute renal failure in patients with chronic kidney disease. Kidney Int 74: 101–107, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, Collins AJ: Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol 20: 223–228, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lo LJ, Go AS, Chertow GM, McCulloch CE, Fan D, Ordoñez JD, Hsu CY: Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int 76: 893–899, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Venkatachalam MA, Griffin KA, Lan R, Geng H, Saikumar P, Bidani AK: Acute kidney injury: a springboard for progression in chronic kidney disease. Am J Physiol Renal Physiol 298: F1078–F1094, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV: Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med 16: 535–543, 1p, 143, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clements ME, Chaber CJ, Ledbetter SR, Zuk A: Increased cellular senescence and vascular rarefaction exacerbate the progression of kidney fibrosis in aged mice following transient ischemic injury. PLoS ONE 8: e70464, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duffield JS: Macrophages and immunologic inflammation of the kidney. Semin Nephrol 30: 234–254, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Harris DC: Macrophages in renal disease. J Am Soc Nephrol 22: 21–27, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Eardley KS, Zehnder D, Quinkler M, Lepenies J, Bates RL, Savage CO, Howie AJ, Adu D, Cockwell P: The relationship between albuminuria, MCP-1/CCL2, and interstitial macrophages in chronic kidney disease. Kidney Int 69: 1189–1197, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Sica A, Mantovani A: Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122: 787–795, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murray PJ, Wynn TA: Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 11: 723–737, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anders HJ, Ryu M: Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney Int 80: 915–925, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Ramachandran P, Pellicoro A, Vernon MA, Boulter L, Aucott RL, Ali A, Hartland SN, Snowdon VK, Cappon A, Gordon-Walker TT, Williams MJ, Dunbar DR, Manning JR, van Rooijen N, Fallowfield JA, Forbes SJ, Iredale JP: Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci U S A 109: E3186–E3195, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin SL, Castaño AP, Nowlin BT, Lupher ML, Jr, Duffield JS: Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. J Immunol 183: 6733–6743, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Gordon S, Taylor PR: Monocyte and macrophage heterogeneity. Nat Rev Immunol 5: 953–964, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B: Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med 204: 1057–1069, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ingersoll MA, Spanbroek R, Lottaz C, Gautier EL, Frankenberger M, Hoffmann R, Lang R, Haniffa M, Collin M, Tacke F, Habenicht AJ, Ziegler-Heitbrock L, Randolph GJ: Comparison of gene expression profiles between human and mouse monocyte subsets. Blood 115: e10–e19, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karlmark KR, Weiskirchen R, Zimmermann HW, Gassler N, Ginhoux F, Weber C, Merad M, Luedde T, Trautwein C, Tacke F: Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology 50: 261–274, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Movahedi K, Laoui D, Gysemans C, Baeten M, Stangé G, Van den Bossche J, Mack M, Pipeleers D, In’t Veld P, De Baetselier P, Van Ginderachter JA: Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res 70: 5728–5739, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ: The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 204: 3037–3047, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, Ruhrberg C, Cantley LG: Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol 22: 317–326, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirsch S, Austyn JM, Gordon S: Expression of the macrophage-specific antigen F4/80 during differentiation of mouse bone marrow cells in culture. J Exp Med 154: 713–725, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pixley FJ, Stanley ER: CSF-1 regulation of the wandering macrophage: complexity in action. Trends Cell Biol 14: 628–638, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Ko GJ, Boo CS, Jo SK, Cho WY, Kim HK: Macrophages contribute to the development of renal fibrosis following ischaemia/reperfusion-induced acute kidney injury. Nephrol Dial Transplant 23: 842–852, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Ma FY, Liu J, Kitching AR, Manthey CL, Nikolic-Paterson DJ: Targeting renal macrophage accumulation via c-fms kinase reduces tubular apoptosis but fails to modify progressive fibrosis in the obstructed rat kidney. Am J Physiol Renal Physiol 296: F177–F185, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA: Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41: 14–20, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang MZ, Yao B, Yang S, Jiang L, Wang S, Fan X, Yin H, Wong K, Miyazawa T, Chen J, Chang I, Singh A, Harris RC: CSF-1 signaling mediates recovery from acute kidney injury. J Clin Invest 122: 4519–4532, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lehtonen A, Ahlfors H, Veckman V, Miettinen M, Lahesmaa R, Julkunen I: Gene expression profiling during differentiation of human monocytes to macrophages or dendritic cells. J Leukoc Biol 82: 710–720, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Ji JD, Park-Min KH, Ivashkiv LB: Expression and function of semaphorin 3A and its receptors in human monocyte-derived macrophages. Hum Immunol 70: 211–217, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M: The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25: 677–686, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Manthey CL, Johnson DL, Illig CR, Tuman RW, Zhou Z, Baker JF, Chaikin MA, Donatelli RR, Franks CF, Zeng L, Crysler C, Chen Y, Yurkow EJ, Boczon L, Meegalla SK, Wilson KJ, Wall MJ, Chen J, Ballentine SK, Ott H, Baumann C, Lawrence D, Tomczuk BE, Molloy CJ: JNJ-28312141, a novel orally active colony-stimulating factor-1 receptor/FMS-related receptor tyrosine kinase-3 receptor tyrosine kinase inhibitor with potential utility in solid tumors, bone metastases, and acute myeloid leukemia. Mol Cancer Ther 8: 3151–3161, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Li L, Huang L, Sung SS, Vergis AL, Rosin DL, Rose CE, Jr, Lobo PI, Okusa MD: The chemokine receptors CCR2 and CX3CR1 mediate monocyte/macrophage trafficking in kidney ischemia-reperfusion injury. Kidney Int 74: 1526–1537, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Day YJ, Huang L, Ye H, Linden J, Okusa MD: Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: role of macrophages. Am J Physiol Renal Physiol 288: F722–F731, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Van Rooijen N, Sanders A: Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods 174: 83–93, 1994 [DOI] [PubMed] [Google Scholar]
- 40.Menke J, Iwata Y, Rabacal WA, Basu R, Yeung YG, Humphreys BD, Wada T, Schwarting A, Stanley ER, Kelley VR: CSF-1 signals directly to renal tubular epithelial cells to mediate repair in mice. J Clin Invest 119: 2330–2342, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Q, DeFrances MC, Zarnegar R: Induction of met proto-oncogene (hepatocyte growth factor receptor) expression during human monocyte-macrophage differentiation. Cell Growth Differ 7: 821–832, 1996 [PubMed] [Google Scholar]
- 42.Liu H, Shi B, Huang C-C, Eksarko P, Pope RM: Transcriptional diversity during monocyte to macrophage differentiation. Immunol Lett 117: 70–80, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lamb DJ, Modjtahedi H, Plant NJ, Ferns GAA: EGF mediates monocyte chemotaxis and macrophage proliferation and EGF receptor is expressed in atherosclerotic plaques. Atherosclerosis 176: 21–26, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Gutiérrez-Fernández A, Inada M, Balbín M, Fueyo A, Pitiot AS, Astudillo A, Hirose K, Hirata M, Shapiro SD, Noël A, Werb Z, Krane SM, López-Otín C, Puente XS: Increased inflammation delays wound healing in mice deficient in collagenase-2 (MMP-8). FASEB J 21: 2580–2591, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kyriakides TR, Wulsin D, Skokos EA, Fleckman P, Pirrone A, Shipley JM, Senior RM, Bornstein P: Mice that lack matrix metalloproteinase-9 display delayed wound healing associated with delayed reepithelization and disordered collagen fibrillogenesis. Matrix Biol 28: 65–73, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmidt IM, Hall IE, Kale S, Lee S, He CH, Lee Y, Chupp GL, Moeckel GW, Lee CG, Elias JA, Parikh CR, Cantley LG: Chitinase-like protein Brp-39/YKL-40 modulates the renal response to ischemic injury and predicts delayed allograft function. J Am Soc Nephrol 24: 309–319, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park WD, Griffin MD, Cornell LD, Cosio FG, Stegall MD: Fibrosis with inflammation at one year predicts transplant functional decline. J Am Soc Nephrol 21: 1987–1997, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park WD, Stegall MD: A meta-analysis of kidney microarray datasets: investigation of cytokine gene detection and correlation with rt-PCR and detection thresholds. BMC Genomics 8: 88, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Socha MJ, Manhiani M, Said N, Imig JD, Motamed K: Secreted protein acidic and rich in cysteine deficiency ameliorates renal inflammation and fibrosis in angiotensin hypertension. Am J Pathol 171: 1104–1112, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang JC, Lai S, Guo X, Zhang X, de Crombrugghe B, Sonnylal S, Arnett FC, Zhou X: Attenuation of fibrosis in vitro and in vivo with SPARC siRNA. Arthritis Res Ther 12: R60, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fan D, Takawale A, Basu R, Patel V, Lee J, Kandalam V, Wang X, Oudit GY, Kassiri Z: Differential role of TIMP2 and TIMP3 in cardiac hypertrophy, fibrosis, and diastolic dysfunction. Cardiovasc Res 103: 268–280, 2014 [DOI] [PubMed] [Google Scholar]
- 52.Wynes MW, Frankel SK, Riches DW: IL-4-induced macrophage-derived IGF-I protects myofibroblasts from apoptosis following growth factor withdrawal. J Leukoc Biol 76: 1019–1027, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Butt RP, Laurent GJ, Bishop JE: Mechanical load and polypeptide growth factors stimulate cardiac fibroblast activity. Ann N Y Acad Sci 752: 387–393, 1995 [DOI] [PubMed] [Google Scholar]
- 54.Brown KM, Sacks SH, Sheerin NS: Mechanisms of disease: the complement system in renal injury—new ways of looking at an old foe. Nat Clin Pract Nephrol 3: 277–286, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Chawla LS, Eggers PW, Star RA, Kimmel PL: Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med 371: 58–66, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gupta J, Mitra N, Kanetsky PA, Devaney J, Wing MR, Reilly M, Shah VO, Balakrishnan VS, Guzman NJ, Girndt M, Periera BG, Feldman HI, Kusek JW, Joffe MM, Raj DS, CRIC Study Investigators : Association between albuminuria, kidney function, and inflammatory biomarker profile in CKD in CRIC. Clin J Am Soc Nephrol 7: 1938–1946, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Illig CR, Chen J, Wall MJ, Wilson KJ, Ballentine SK, Rudolph MJ, DesJarlais RL, Chen Y, Schubert C, Petrounia I, Crysler CS, Molloy CJ, Chaikin MA, Manthey CL, Player MR, Tomczuk BE, Meegalla SK: Discovery of novel FMS kinase inhibitors as anti-inflammatory agents. Bioorg Med Chem Lett 18: 1642–1648, 2008 [DOI] [PubMed] [Google Scholar]
- 58.Zuk A, Gershenovich M, Ivanova Y, MacFarland RT, Fricker SP, Ledbetter S: CXCR4 antagonism as a therapeutic approach to prevent acute kidney injury. Am J Physiol Renal Physiol 307: F783–F797, 2014 [DOI] [PubMed] [Google Scholar]
- 59.Zuk A, Bonventre JV, Brown D, Matlin KS: Polarity, integrin, and extracellular matrix dynamics in the postischemic rat kidney. Am J Physiol 275: C711–C731, 1998 [DOI] [PubMed] [Google Scholar]
- 60.Oga T, Matsuoka T, Yao C, Nonomura K, Kitaoka S, Sakata D, Kita Y, Tanizawa K, Taguchi Y, Chin K, Mishima M, Shimizu T, Narumiya S: Prostaglandin F(2alpha) receptor signaling facilitates bleomycin-induced pulmonary fibrosis independently of transforming growth factor-beta. Nat Med 15: 1426–1430, 2009 [DOI] [PubMed] [Google Scholar]
- 61.Liu T, Baek HA, Yu H, Lee HJ, Park BH, Ullenbruch M, Liu J, Nakashima T, Choi YY, Wu GD, Chung MJ, Phan SH: FIZZ2/RELM-β induction and role in pulmonary fibrosis. J Immunol 187: 450–461, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.