Abstract
Lithium induces proliferation in the epithelium of renal collecting ducts. A recent small-scale cohort study reported a strong association between use of lithium and increased risk of renal neoplasia. We therefore conducted a large-scale pharmacoepidemiologic study of the association between long-term use of lithium and risk of upper urinary tract cancer, including renal cell cancer and cancers of the renal pelvis or ureter. We identified all histologically verified upper urinary tract cancer cases in Denmark between 2000 and 2012 from the Danish Cancer Registry. A total of 6477 cases were matched by age and sex to 259,080 cancer-free controls. Data on lithium use from 1995 to 2012 were obtained from the Danish Prescription Registry. We estimated the association between long-term use of lithium (≥5 years) and risk of upper urinary tract cancer using conditional logistic regression with adjustment for potential confounders. Long-term use of lithium was observed among 0.22% of cases and 0.17% of controls. This yielded an overall nonsignificant adjusted odds ratio (OR) of 1.3 (95% confidence interval [95% CI], 0.8–2.2) for upper urinary tract cancer associated with long-term use of lithium. Analyses stratified by stage and subtype of upper urinary tract cancer revealed slight but nonsignificant increases in the ORs for localized disease (OR, 1.6; 95% CI, 0.8–3.0) and for renal pelvis/ureter cancers (OR, 1.7; 95% CI, 0.5–5.4). In conclusion, in our nationwide case-control study, use of lithium was not associated with an increased risk of upper urinary tract cancer.
Keywords: kidney cancer, lithium, pharmacoepidemiology
Lithium therapy has been used for patients with bipolar affective disorders for more than 50 years and is still a major therapeutic option in bipolar disorders1,2 and as augmentation in severe unipolar depression.3,4 The established adverse effects of lithium therapy are primarily related to the urinary system and include polyuria, microcysts in the cortex and the cortical–outer medullary junction,5–8 and more infrequently, toxic interstitial nephritis, which may lead to impaired GFR and renal fibrosis.5,6,9–11 It has been suggested that the nephrotoxic effects of lithium may also lead to renal cell carcinoma of different subtypes.12,13 A putative mechanism could be through inhibition of glycogen synthase kinase-3β (GSK-3β)14 which is expressed in the adult kidney.15 GSK-3β promotes apoptosis and inhibits cell proliferation.16,17 GSK-3β-immunoreactive protein is associated with lithium-induced renal microcysts7 found consistently among patients in long-term lithium therapy.5,6,18,19 Microcysts derive from proliferation of epithelial cells in the distal nephron, likely both distal convoluted and connecting tubules, as well as cortical collecting ducts,7,8,18,20 and in some cases the microcysts contain papillary projections indicative of a pre-malignant stage.12 The distal nephron and collecting ducts express the amiloride-sensitive epithelial sodium channel, ENaC, which is the main cellular pathway for lithium uptake in the kidney.21–23 The ENaC is also expressed in the urothelium of the renal pelvis and ureter, although it is not clear if lithium enters these cells.24
The potential long-term inhibition of GSK-3β by lithium with concurrent inflammation5,6 raises the possibility that lithium therapy may be exerting a carcinogenic effect in the kidney.12,13 However, the lithium level required to inhibit GSK-3β is relatively high,16 and there is no laboratory evidence of neoplastic changes in the urinary tract epithelium associated with lithium therapy. Nevertheless, because lithium therapy is used chronically by many patients, often over decades, the hyperproliferation of cells in the renal epithelium/urinary tract induced by lithium may eventually result in malignant transformation. Very little data are available from epidemiologic studies. Two case reports showed co-incident renal cell carcinoma and chronic lithium intake7,12 and recently, a small cohort study suggested an up to ten-fold increased risk of renal cell cancer among long-term lithium users,13 however, the validity of these findings has been questioned.25
Given the nephrotoxic effects of long-term lithium therapy, the widespread use of lithium and the recent epidemiologic findings, it is of paramount importance to assess whether lithium use has a carcinogenic potential in the kidney. We undertook the present study to evaluate whether long-term chronic use of lithium is associated with an increased risk of kidney or other upper urinary tract cancers (UUTCs) in a nationwide population-based epidemiologic study.
Results
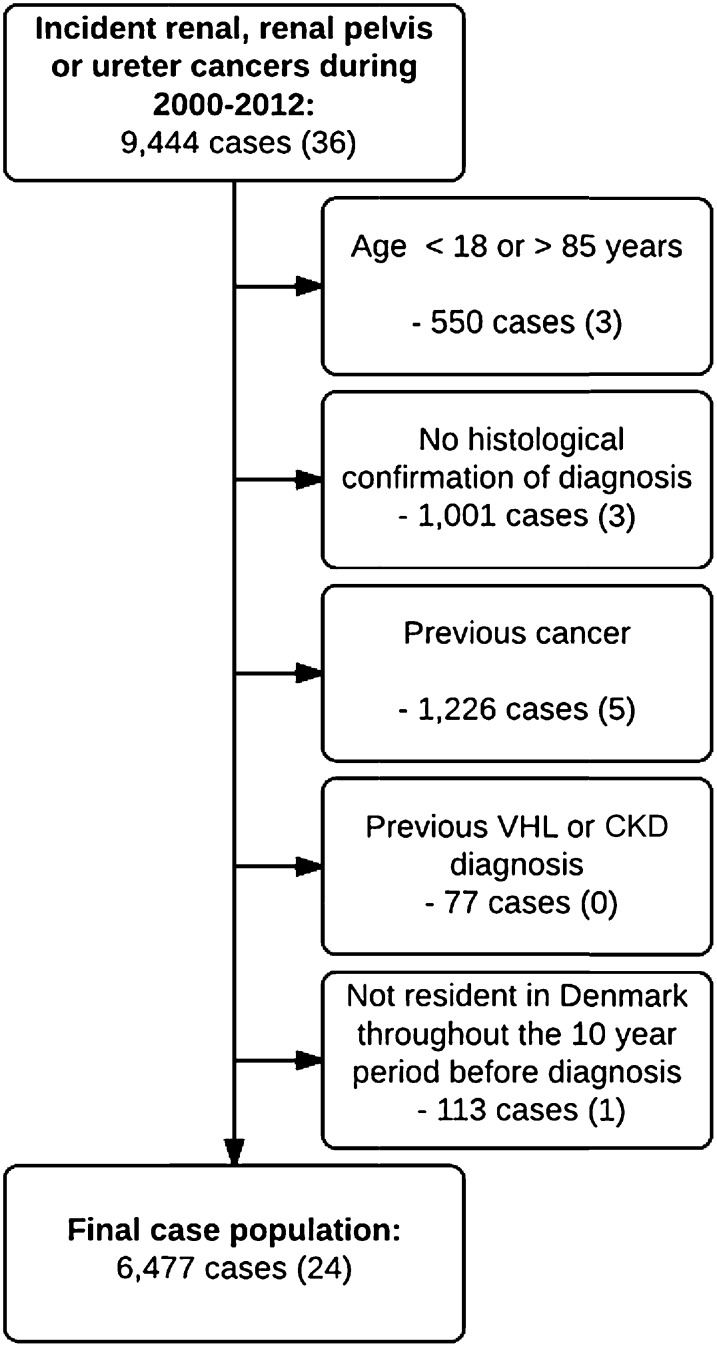
We identified 9444 incident UUTCs (cancers of the kidney, renal pelvis or ureter) between January 1, 2000, and December 31, 2012. After exclusions, the study population comprised 6477 cancer cases (Figure 1) that were matched to 259,080 controls.
Figure 1.
Flow chart of the selection of cases. The numbers of ever-users of lithium within each study or exclusion group are shown in parentheses. VHL, von-Hippel Lindau syndrome.
The use of lithium in the Danish population was stable during the study period (Figure 2). Among cases and controls, 0.37% and 0.43%, respectively, had used lithium (≥2 prescriptions), and 0.22% and 0.17% had used lithium for more than 5 years (Table 1). These exposure prevalences yielded overall adjusted odds ratios (ORs) for UUTCs associated with ever or long-term use of lithium, respectively, of 0.9 (95% CI, 0.6–1.3) and 1.3 (95% CI, 0.8–2.2) (Table 2). Adjustment for potential confounders had generally minimal impact on the ORs. The 14 cases associated with long-term lithium use comprised ten cases of renal cell carcinomas, three urethelial carcinomas and one case of uncertain histology.
Figure 2.
Annual prevalence of lithium use in the general Danish population. Data from medstat.dk46
Table 1.
Characteristics of upper urinary tract cancer cases and their age- and sex-matched controls
| Cases | Controls | |
|---|---|---|
| (n=6477) | (n=259,080) | |
| Age, median (IQR, years) | 65 (57–73) | 65 (57–73) |
| Male gender | 4184 (64.6%) | 167,360 (64.6%) |
| Renal cancer | 5648 (87.2%) | NA |
| Pelvis or ureter cancer | 829 (12.8%) | NA |
| Use of lithium prior to index date | ||
| Non-use | 6453 (99.6%) | 257,978 (99.6%) |
| Ever use | 24 (0.37%) | 1102 (0.43%) |
| Long-term use (≥5 years) | 14 (0.22%) | 447 (0.17%) |
| Drugs | ||
| Non-aspirin NSAID | 3542 (54.7%) | 127,821 (49.3%) |
| Aspirin | 1639 (25.3%) | 54,488 (21.0%) |
| Paracetamol | 1243 (19.2%) | 41,774 (16.1%) |
| Statins | 1394 (21.5%) | 47,111 (18.2%) |
| Loop diuretics | 858 (13.2%) | 26,513 (10.2%) |
| Thiazides | 1637 (25.3%) | 50,153 (19.4%) |
| β-Blockers | 1619 (25.0%) | 51,228 (19.8%) |
| Vascular calcium-channel blockers | 1424 (22.0%) | 39,955 (15.4%) |
| Inhibitors of RAS | 2108 (32.5%) | 61,302 (23.7%) |
| Diagnoses | ||
| Hypertension | 1022 (15.8%) | 27,275 (10.5%) |
| Diabetes, type 1 | 156 (2.4%) | 4297 (1.7%) |
| Diabetes, type 2 | 688 (10.6%) | 21,073 (8.1%) |
| COPD | 471 (7.3%) | 16,624 (6.4%) |
| Alcohol-related disease | 530 (8.2%) | 18,739 (7.2%) |
| Moderate/severe renal disease | 145 (2.2%) | 2603 (1.0%) |
| Highest achieved education | ||
| Short (7–10 years) | 2444 (37.7%) | 89,942 (34.7%) |
| Medium (11–13 years) | 2301 (35.5%) | 92,518 (35.7%) |
| Long (>13 years) | 974 (15.0%) | 46,928 (18.1%) |
IQR, interquartile range; RAS, renin-angiotensin system; COPD, chronic obstructive pulmonary disease.
Table 2.
Association between exposure to lithium and risk of upper urinary tract cancer, specified by exposure pattern within the entire follow-up period, excluding the last year prior to the index date
| Exposure group | Cases | Controls | Adjusted ORa | Adjusted ORb |
|---|---|---|---|---|
| Non-use | 6453 | 257,978 | 1.0 (ref.) | 1.0 (ref.) |
| Ever use | 24 | 1102 | 0.9 (0.6–1.3) | 0.9 (0.6–1.3) |
| Long-term use (≥5 years) | 14 | 447 | 1.3 (0.7–2.1) | 1.3 (0.8–2.2) |
| Duration of use: | ||||
| <1 year | 3 | 213 | 0.6 (0.2–1.8) | 0.5 (0.2–1.7) |
| 1–4.99 years | 7 | 442 | 0.6 (0.3–1.3) | 0.6 (0.3–1.4) |
| 5–9.99 years | 10 | 332 | 1.2 (0.6–2.3) | 1.2 (0.7–2.3) |
| ≥10 years | 4 | 115 | 1.4 (0.5–3.8) | 1.5 (0.5–4.0) |
Adjusted for age and gender (by design; risk-set matching).
Fully adjusted model, adjusted for (1) low-dose aspirin and non-aspirin NSAIDs, paracetamol, statins, thiazides, beta-blockers, vascular calcium-channel blockers, inhibitors of the renin-angiotensin system, and loop diuretics; (2) hypertension, type 1 or type 2 diabetes, chronic obstructive pulmonary disease, alcohol-related disease, and moderate to severe renal disease; and (3) highest achieved education.
No material variation in the association of lithium use and UUTC risk was seen in analyses defined according to duration of lithium use (Table 2). Individuals aged ≥70 years experienced a slightly higher OR (OR, 1.9; 95% CI, 0.9–3.9) than those of younger ages (Table 3). A similar difference in ORs was seen in separate analyses of females (OR, 1.6; 95% CI, 0.8–3.3) and males (OR, 1.0; 95% CI, 0.5–2.3) (Table 3). Long-term use of lithium was associated with slightly higher ORs for cancers of the pelvis/ureter (OR, 1.7; 95% CI, 0.5–5.4) compared with renal cancers (OR, 1.2; 95% CI, 0.7–2.2). Similarly, a slightly increased OR was observed for localized UUTCs (OR, 1.6; 95% CI, 0.8–3.0), whereas the OR was close to unity for non-localized UUTCs (OR, 0.8; 95% CI, 0.3–2.6) (Table 3). However, the statistical precision of the stratified analyses was limited and none of the associations was statistically significant.
Table 3.
Associations between long-term exposure to lithium (≥5 years) and risk of upper urinary tract cancer, specified by patient subgroups, type of cancer and stage
| Subgroup | Cases exposed/unexposed | Controls exposed/unexposed | Adjusted ORa | Adjusted ORb |
|---|---|---|---|---|
| All | 14/6477 | 447/259,080 | 1.3 (0.7–2.1) | 1.3 (0.8–2.2) |
| Males | 6/4184 | 240/167,360 | 1.0 (0.4–2.2) | 1.0 (0.5–2.3) |
| Females | 8/2293 | 207/91,720 | 1.5 (0.8–3.1) | 1.6 (0.8–3.3) |
| Age <50 years | 0/624 | 11/24,960 | — | — |
| Age 50–69 years | 6/3515 | 259/140,600 | 0.9 (0.4–2.1) | 1.0 (0.4–2.2) |
| Age 70+ years | 8/2338 | 177/93,520 | 1.8 (0.9–3.7) | 1.9 (0.9–3.9) |
| No history of renal disease | 14/6332 | 428/256,477 | 1.3 (0.8–2.2) | 1.4 (0.8–2.4) |
| No history of hypertension | 13/5455 | 399/231,805 | 1.4 (0.8–2.4) | 1.5 (0.8–2.5) |
| No history of diabetes | 12/5770 | 404/237,180 | 1.2 (0.7–2.2) | 1.2 (0.7–2.2) |
| Subtype | ||||
| Renal cancers | 11/5648 | 377/225,920 | 1.2 (0.6–2.1) | 1.2 (0.7–2.2) |
| Renal pelvis and ureter cancers | 3/829 | 70/33,160 | 1.7 (0.5–5.5) | 1.7 (0.5–5.4) |
| Stage | ||||
| Localized | 10/3561 | 254/142,440 | 1.6 (0.8–3.0) | 1.6 (0.8–3.0) |
| Non-localized | 3/2254 | 148/90,160 | 0.8 (0.3–2.5) | 0.8 (0.3–2.6) |
| Unknown | 1/662 | 45/26,480 | 0.9 (0.1–6.5) | 0.9 (0.1–6.8) |
Adjusted for age and gender (by design).
Fully adjusted model, adjusted for (1) low-dose aspirin and non-aspirin NSAIDs, paracetamol, statins, thiazides, beta-blockers, vascular calcium-channel blockers, inhibitors of the renin-angiotensin system, and loop diuretics; (2) hypertension, type 1 or type 2 diabetes, chronic obstructive pulmonary disease, alcohol-related disease, and moderate to severe renal disease; and (3) highest achieved education.
Analyses of lamotrigine or valproate as primary exposure yielded results similar to those of the main analyses, with ORs for UUTCs of 0.7 (95% CI, 0.4–1.3) and 0.9 (95% CI, 0.6–1.5) associated with long-term use of lamotrigine and valproate, respectively (for full results, see Supplemental Appendix C). Increasing the lag time to 24 months yielded a slightly increased OR for UUTCs associated with long-term use of lithium (OR, 1.5; 95% CI, 0.9–2.6), whereas the OR was close to unity in the analysis with no lag time (OR, 1.2; 95% CI, 0.7–2.0).
In the evaluation of misclassification of long-term exposure due to left truncation of prescription history in 1995, using the OPED database, we identified 1683 ever users (≥2 prescriptions) and 877 long-term users (≥5 years) of lithium during 1995–2012. Among the long-term users, 66.2% (n=581) had also used lithium during 1990–1994 (median 744 days; interquartile range, 318–1257 days). Among subjects with <5 years use of lithium within the study exposure period, the corresponding prevalence was 19.4% (n=326, median 705 days; interquartile range, 366–1130 days).
Finally, assuming a true OR of 1.3, we estimated that 12,364 person-years of long-term lithium use would be required to elicit one additional case of UUTC. The upper limit of the confidence interval for the OR of 1.3 (i.e., 2.2) would correspond to one additional UUTC for every 3091 person-years of lithium exposure.
Discussion
In our nationwide study of Danish UUTC cases during 2000–2012, we found no overall association between long-term use of lithium and risk of UUTC, and only slight variation of risk estimates in subgroup analyses. Even under the assumption of the “worst case scenario” with an OR of up to 2.2, the absolute effect sizes were small.
The main strengths of our study were the sample size and the nationwide approach. As almost all health care service in Denmark is undertaken by the public health system, we had almost complete population coverage. The UUTC cases were identified from the Danish Cancer Registry, which has accurate and virtually complete registration of incident cancer in Denmark,26 and the UUTC diagnoses were restricted to histologically verified cases, further enhancing case validity. The use of the Danish Prescription Registry also ensured complete and high-quality assessment of drug use,27 with up to 18 years of drug exposure history. In addition, analyses of lithium utilization from a regional database demonstrated that a considerable proportion of the long-term users of lithium had used lithium longer than we could account for in the present study. It is thus unlikely that exposure beyond 18 years would have any important additional impact on the UUTC risk profile of lithium.
Our study also had some limitations. We had only indirect information on heavy smoking, which is an established risk factor for both renal cancer and cancers of the pelvis or ureter.28 Individuals suffering from bipolar disorder and severe depression, including lithium users, smoke on average more than the general population,29,30 and thus smoking might have confounded the risk estimates in our study. However, any confounding by smoking would result in an overestimation of the association between lithium use and UUTC risk, and thus our finding of mainly neutral associations between lithium use and UUTC risk provides further reassurance of our null results. Lastly, our data material does not allow retrieval of additional information from the Pathology Registry. Thus, no information was available on subtypes of renal cell carcinomas; pathologic details that might have been of interest as to the hypotheses described in the studies by Rookmaaker et al.12 and Zaidan et al.13 As such, our study cannot exclude an excess risk of rare subtypes of renal cell carcinoma.
The suggested carcinogenic effect of lithium was derived from observations of lithium-induced proliferation of renal epithelial cells in animal and human kidney specimens, interstitial chronic inflammation and microcysts in human kidneys; and allegedly induced by inhibition of GSK3β, which has been established to suppress cell proliferation and stimulate apoptosis. However, to our knowledge, only one previous epidemiologic study has evaluated the association between lithium use and cancer risk; Zaidan et al.13 reported a ten-fold increased risk of renal cell cancer associated with lithium use in a small cohort study. The precise methodology of Zaidan et al.’s study is somewhat unclear, and the high relative risk estimate was based on only seven invasive cases of renal cell cancer. Moreover, the fact that the lithium users in the study population were referred for renal imaging, and that the underlying reason for such imaging per se is likely associated with an increased cancer risk, were not taken into account. Therefore, we do not believe that the findings by Zaidan et al. should be taken as any evidence that lithium is associated with an increased risk of developing renal neoplasia, as also commented by Licht et al.25
Our results might be affected by surveillance bias, as lithium users frequently undergo renal function tests and generally have more frequent contact with the health care system compared with individuals without bipolar disorder or severe depression, and no lithium use. This might cause overestimation of the association between lithium use and UUTC risk. However, because an existing renal cancer does not generally influence renal function tests except in the latest stages, this is probably not a major limitation. A surveillance effect could, however, be mediated if the threshold for referral to renal imaging is lower for individuals using lithium, resulting in earlier diagnosis of renal cancers (lead-time bias31). This was suggested by Licht et al.25 and might explain our finding of a slightly elevated OR for localized UUTCs. Again, it is worth mentioning that such an effect would bias the risk estimates upwards, again providing reassurance that our findings are compatible with a null association.
In conclusion, we can exclude any major effect of long-term lithium use on the risk of renal cancer or cancers of the renal pelvis or ureter. Although long-term lithium use may be associated with other renal adverse effects, it is reassuring that lithium use does not appear to induce renal cancer, and that lithium can be kept on the armamentarium for conditions as debilitating as bipolar disorder and severe unipolar depression.
Concise Methods
The study was conducted within a nationwide case-control population. We compared the use of lithium among persons diagnosed with UUTCs (cases) to that of cancer-free persons (controls) to estimate the OR for UUTCs associated with long-term use of lithium defined as at least 5 years of cumulative exposure.
Data sources
We used five Danish nationwide registries: the Danish Cancer Registry,26,32 the National Prescription Registry,27 the National Patient Register,33 Registers in Statistics Denmark on educational level and income,34,35 and the Civil Registration System.36 The data sources are described in detail in Supplemental Appendix A.
Virtually all medical care in Denmark is furnished by the national health authorities, allowing true population-based register linkage studies covering all inhabitants of Denmark. Data were linked by the personal identification number, a unique identifier assigned to all Danish residents since 1968.36 All linkages were performed within Statistics Denmark, a governmental institution that collects and processes information for a variety of statistical and scientific purposes.
Cases and controls
From the Danish Cancer Registry, we identified all individuals in Denmark with a first-time diagnosis of UUTCs, defined as invasive cancer of the kidney (ICD10, C64), renal pelvis (C65) or ureter (C66), between January 1, 2000, and December 31, 2012, using the date of cancer diagnosis as the index date. To ensure the validity of our case material, we restricted cases to histologically verified UUTCs. Exclusion criteria were age outside the range 18–85 years at index date and any residency outside Denmark within 10 years prior to index date, thus ensuring at least 10 years of follow-up for all study subjects. We further excluded individuals with a previous history of cancer (except non-melanoma skin cancer), von-Hippel Lindau syndrome (ICD8: 75982; ICD10: Q85.8–9), and cystic kidney disease (ICD8: 59324; ICD10: Q61).
Controls were selected by the use of risk set sampling. For each case, we selected 40 controls among all Danish citizens fulfilling the exclusion criteria for cases and of the same gender and birth year. Controls were assigned an index date identical to that of the corresponding case.
Subjects were eligible for sampling as controls before they became cases. Thereby, the calculated ORs are unbiased estimates of the incidence rate ratios that would have emerged from a cohort study in the source population.37
Exposure definition
Our primary exposure was use of lithium. Ever use of lithium was defined as having filled two or more prescriptions (ATC code38 N05AN01) of lithium prior to the index date. Long-term use of lithium was defined as ≥5 years of treatment prior to the index date.
The duration of each prescription required for estimation of cumulative exposure duration is not recorded in the Prescription Registry. To overcome this limitation, we used a method based on the waiting time distribution,39,40 providing an estimate of the average duration of each lithium prescription of 64 days. We thus considered an individual exposed from the date of filling a prescription for lithium and 64 days onward.
In all exposure calculations, we disregarded prescriptions redeemed within 12 months prior to the index date. Such recent exposure is unlikely to be associated with cancer development, and moreover, drug use has been shown to increase up to 12 months prior to a cancer diagnosis,41 raising the possibility of reverse causation bias.42
Main analysis
The analysis followed a conventional matched case-control approach using conditional logistic regression. In the main analysis, we considered UUTCs (i.e., cancers of the renal parenchyma, renal pelvis or ureter) as a composite end-point and estimated ORs for UUTCs associated with long-term use of lithium. In all analyses, use of lithium was compared with non-use (<2 prescriptions) of lithium.
Any confounding effect from age, gender and calendar time was handled via the matching procedure. Further, the following potential confounders were identified and incorporated in the logistic regression: (1) Use of drugs known or suspected to modify renal function or risk of UUTC, including low-dose aspirin and non-aspirin NSAIDs, paracetamol, statins, thiazides, beta-blockers, vascular calcium-channel blockers, inhibitors of the renin-angiotensin system, and loop diuretics. (2) Prior diagnoses of diseases known or suspected to modify renal function or risk of renal or other cancers, including hypertension, type 1 or type 2 diabetes, chronic obstructive pulmonary disease, alcohol-related disease, and moderate to severe renal disease. (3) Highest achieved education (as a crude measure of socioeconomic status). Details of the potential confounders, including codes, are presented in Supplemental Appendix B. As in the assessment of drug exposure, we disregarded the period 12 months prior to the index date in the identification of confounder status.
We performed a number of pre-planned sub-analyses and sensitivity analyses.
First, as an explorative analysis of a potential dose–response effect, we performed analyses stratified according to cumulative duration of lithium use.
Second, we examined associations for UUTCs with lithium use within subgroups defined by gender, age, or history of renal disease, diabetes or hypertension.
Third, we computed ORs for associations between long-term use of lithium and two subtypes of UUTCs, i.e., renal cancers (of which the majority are renal cell carcinomas) and cancers of the renal pelvis or ureter (which comprise almost exclusively urothelial carcinomas) as two separate outcomes.
Fourth, we stratified the analyses by clinical stage, i.e., localized or non-localized disease.
Fifth, we repeated all analyses using lamotrigine or valproate as primary exposure instead of lithium. The rationale behind these analyses was that if important unmeasured or unknown confounders were associated with use of lithium, these confounders would also be associated with other drug therapy used for the same indication. Lamotrigine and valproate are both used as ‘mood stabilizers’ in bipolar affective disorder.
Sixth, as drug use before 1995 (start of Prescription Registry) was unavailable, some long-term use of lithium was misclassified in our study. To evaluate the true exposure length among patients with a minimum of 5 years of lithium use, we performed an explorative analysis within the OPED database43 (initiated in 1990).
Finally, as a sensitivity analysis, we changed the 1 year lag time to 0 or 2 years, respectively.
Risk in individual users
For rare outcomes, even very high ORs may translate into a small excess risk for the individual user. In order to express the risk in absolute terms, we calculated the amount of exposure required to elicit one additional outcome (ENH), a measure equivalent to the number needed to treat used in clinical trials. The naturalistic ENH measure was used.44 In brief, this measure estimates the number of person-year-exposure exceeding the 5-year limit for long-term use that would be required to induce one additional case of UUTCs in the given background population. The prevalence of long-term lithium use in the background population was modeled from the exposure pattern among study controls.44
Other
All analyses were performed using Stata Release 13.0 (StataCorp., College Station, TX). The study was approved by the Danish Data Protection Agency. According to Danish law, studies based solely on register data do not require approval from an ethics review board.45
Disclosures
The authors have no conflicts of interest to declare, financial or otherwise.
Supplementary Material
Acknowledgments
The authors would like to acknowledge psychiatrist John Teilmann Larsen, consultant in nephrology Claus Bistrup, and scientific assistant Lotte Rasmussen for valuable review of the manuscript.
Anton Pottegård is funded by the Danish Council for Independent Research (grant 4004-00234B).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015010061/-/DCSupplemental.
References
- 1.Malhi GS, Berk M: Pharmacotherapy of bipolar disorder: the role of atypical antipsychotics and experimental strategies. Hum Psychopharmacol 17: 407–412, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Licht RW: Lithium: still a major option in the management of bipolar disorder. CNS Neurosci Ther 18: 219–226, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauer M, Adli M, Ricken R, Severus E, Pilhatsch M: Role of lithium augmentation in the management of major depressive disorder. CNS Drugs 28: 331–342, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Bschor T: Lithium in the treatment of major depressive disorder. Drugs 74: 855–862, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Hestbech J, Hansen HE, Amdisen A, Olsen S: Chronic renal lesions following long-term treatment with lithium. Kidney Int 12: 205–213, 1977 [DOI] [PubMed] [Google Scholar]
- 6.Hansen HE, Hestbech J, Olsen S, Amdisen A: Renal function and renal pathology in patients with lithium-induced impairment of renal concentrating ability. Proc Eur Dial Transplant Assoc 14: 518–527, 1977 [PubMed] [Google Scholar]
- 7.Kjaersgaard G, Madsen K, Marcussen N, Christensen S, Walter S, Jensen BL: Tissue injury after lithium treatment in human and rat postnatal kidney involves glycogen synthase kinase-3β-positive epithelium. Am J Physiol Renal Physiol 302: F455–F465, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Kjaersgaard G, Madsen K, Marcussen N, Jensen BL: Lithium induces microcysts and polyuria in adolescent rat kidney independent of cyclooxygenase-2. Physiol Rep 2: e00202, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ottosen PD, Sigh B, Kristensen J, Olsen S, Christensen S: Lithium induced interstitial nephropathy associated with chronic renal failure. Reversibility and correlation between functional and structural changes. Acta Pathol Microbiol Immunol Scand [A] 92: 447–454, 1984 [DOI] [PubMed] [Google Scholar]
- 10.Ottosen PD, Nyengård JR, Jacobsen NO, Christensen S: A morphometric and ultrastructural study of lithium-induced changes in the medullary collecting ducts of the rat kidney. Cell Tissue Res 249: 311–315, 1987 [DOI] [PubMed] [Google Scholar]
- 11.Ottosen PD, Jacobsen NO, Christensen S: Lithium-induced morphological changes in the rat kidney at different levels of urine flow. Pharmacol Toxicol 63: 108–113, 1988 [DOI] [PubMed] [Google Scholar]
- 12.Rookmaaker MB, van Gerven HAJM, Goldschmeding R, Boer WH: Solid renal tumours of collecting duct origin in patients on chronic lithium therapy. Clin Kidney J 5: 412–415, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaidan M, Stucker F, Stengel B, Vasiliu V, Hummel A, Landais P, Boffa J-J, Ronco P, Grünfeld J-P, Servais A: Increased risk of solid renal tumors in lithium-treated patients. Kidney Int 86: 184–190, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Stambolic V, Ruel L, Woodgett JR: Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol 6: 1664–1668, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Lau KF, Miller CC, Anderton BH, Shaw PC: Expression analysis of glycogen synthase kinase-3 in human tissues. J Pept Res 54: 85–91, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Klein PS, Melton DA: A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci U S A 93: 8455–8459, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pap M, Cooper GM: Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-Kinase/Akt cell survival pathway. J Biol Chem 273: 19929–19932, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Markowitz GS, Radhakrishnan J, Kambham N, Valeri AM, Hines WH, D’Agati VD: Lithium nephrotoxicity: a progressive combined glomerular and tubulointerstitial nephropathy. J Am Soc Nephrol 11: 1439–1448, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Farres MT, Ronco P, Saadoun D, Remy P, Vincent F, Khalil A, Le Blanche AF: Chronic lithium nephropathy: MR imaging for diagnosis. Radiology 229: 570–574, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Christensen BM, Kim Y-H, Kwon T-H, Nielsen S: Lithium treatment induces a marked proliferation of primarily principal cells in rat kidney inner medullary collecting duct. Am J Physiol Renal Physiol 291: F39–F48, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Walter SJ, Sampson B, Shirley DG: A micropuncture study of renal tubular lithium reabsorption in sodium-depleted rats. J Physiol 483: 473–479, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kortenoeven MLA, Li Y, Shaw S, Gaeggeler H-P, Rossier BC, Wetzels JFM, Deen PMT: Amiloride blocks lithium entry through the sodium channel thereby attenuating the resultant nephrogenic diabetes insipidus. Kidney Int 76: 44–53, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Christensen BM, Zuber AM, Loffing J, Stehle J-C, Deen PMT, Rossier BC, Hummler E: alphaENaC-mediated lithium absorption promotes nephrogenic diabetes insipidus. J Am Soc Nephrol 22: 253–261, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hager H, Kwon TH, Vinnikova AK, Masilamani S, Brooks HL, Frøkiaer J, Knepper MA, Nielsen S: Immunocytochemical and immunoelectron microscopic localization of alpha-, beta-, and gamma-ENaC in rat kidney. Am J Physiol Renal Physiol 280: F1093–F1106, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Licht RW, Grabenhenrich LB, Nielsen RE, Berghöfer A, International Group for the Study of Lithium (IGSLi) : Lithium and renal tumors: a critical comment to the report by Zaidan et al. Kidney Int 86: 857, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gjerstorff ML: The Danish Cancer Registry. Scand J Public Health 39[Suppl]: 42–45, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Kildemoes HW, Sørensen HT, Hallas J: The Danish National Prescription Registry. Scand J Public Health 39[Suppl]: 38–41, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Secretan B, Straif K, Baan R, Grosse Y, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V, WHO International Agency for Research on Cancer Monograph Working Group : A review of human carcinogens—Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol 10: 1033–1034, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH: Smoking and mental illness: A population-based prevalence study. JAMA 284: 2606–2610, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Heffner JL, Strawn JR, DelBello MP, Strakowski SM, Anthenelli RM: The co-occurrence of cigarette smoking and bipolar disorder: phenomenology and treatment considerations. Bipolar Disord 13: 439–453, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kramer BS: The science of early detection. Urol Oncol 22: 344–347, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Storm HH, Michelsen EV, Clemmensen IH, Pihl J: The Danish Cancer Registry—history, content, quality and use. Dan Med Bull 44: 535–539, 1997 [PubMed] [Google Scholar]
- 33.Lynge E, Sandegaard JL, Rebolj M: The Danish National Patient Register. Scand J Public Health 39[Suppl]: 30–33, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Jensen VM, Rasmussen AW: Danish Education Registers. Scand J Public Health 39[Suppl]: 91–94, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Baadsgaard M, Quitzau J: Danish registers on personal income and transfer payments. Scand J Public Health 39[Suppl]: 103–105, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Pedersen CB: The Danish Civil Registration System. Scand J Public Health 39[Suppl]: 22–25, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Rothman KJ, Greenland S, Lash TL: Modern Epidemiology, 3rd Ed., Philadelphia, PA, Lippincott, Williams & Wilkins, 2008 [Google Scholar]
- 38.WHO Collaborating Centre for Drug Statistics Methodology: Guidelines for ATC classification and DDD assignment, 2015. Available at: http://www.whocc.no/filearchive/publications/2015_guidelines.pdf. Accessed January 16, 2015
- 39.Hallas J, Gaist D, Bjerrum L: The waiting time distribution as a graphical approach to epidemiologic measures of drug utilization. Epidemiology 8: 666–670, 1997 [DOI] [PubMed] [Google Scholar]
- 40.Pottegård A, Hallas J: Assigning exposure duration to single prescriptions by use of the waiting time distribution. Pharmacoepidemiol Drug Saf 22: 803–809, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Jørgensen T, Herrstedt J, Friis S, Hallas J: Danish cancer patients during 1996 to 2006. J Geriatr Oncol 2012: 33–40
- 42.Csizmadl I, Collet J-P, Boivin J: Bias and confounding in pharmacoepidemiology. In: Pharmacoepidemiology, 4th Ed., edited by Strom BL, West Sussex, Wiley & Sons, 2007, pp 791–810 [Google Scholar]
- 43.Gaist D, Sørensen HT, Hallas J: The Danish prescription registries. Dan Med Bull 44: 445–448, 1997 [PubMed] [Google Scholar]
- 44.Hallas J, Christensen RD, Stürmer T, Pottegård A: Measures of ‘exposure needed for one additional patient to be harmed’ in population-based case-control studies. Pharmacoepidemiol Drug Saf 23: 868–874, 2014 [DOI] [PubMed] [Google Scholar]
- 45.Thygesen LC, Daasnes C, Thaulow I, Brønnum-Hansen H: Introduction to Danish (nationwide) registers on health and social issues: structure, access, legislation, and archiving. Scand J Public Health 39[Suppl]: 12–16, 2011 [DOI] [PubMed] [Google Scholar]
- 46.Statens Serum Institut: Medstat.dk [Internet]. Available at: www.medstat.dk/en. Accessed January 16, 2015
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.