Abstract
Proteinuria is routinely measured to assess renal allograft status, but the diagnostic and prognostic values of this measurement for renal transplant pathology and outcome remain unclear. We included 1518 renal allograft recipients in this prospective, observational cohort study. All renal allograft biopsy samples with concomitant data on 24-hour proteinuria were included in the analyses (n=2274). Patients were followed for ≥7 years post-transplantation. Compared with proteinuria <0.3 g/24 h, the hazard ratios for graft failure were 1.14 (95% confidence interval [95% CI], 0.81 to 1.60; P=0.50), for proteinuria 0.3–1.0 g/24 h, 2.17 (95% CI, 1.49 to 3.18; P<0.001), for proteinuria 1.0–3.0 g/24 h, and 3.01 (95% CI, 1.75 to 5.18; P<0.001), for proteinuria >3.0 g/24 h, independent of GFR and allograft histology. The predictive performance of proteinuria for graft failure was lower at 3 months after transplant (area under the receiver-operating characteristic curve [AUC] 0.64, P<0.001) than at 1, 2, and 5 years after transplant (AUC 0.73, 0.71, and 0.77, respectively, all P<0.001). Independent determinants of proteinuria were repeat transplantation, mean arterial pressure, transplant glomerulopathy, microcirculation inflammation, and de novo/recurrent glomerular disease. The discriminatory power of proteinuria for these intragraft injury processes was better in biopsy samples obtained >3 months after transplant (AUC 0.73, P<0.001) than in those obtained earlier (AUC 0.56, P<0.01), with 85% specificity but lower sensitivity (47.8%) for proteinuria >1.0 g/24 h. These data support current clinical guidelines to routinely measure proteinuria after transplant, but illustrate the need for more sensitive biomarkers of allograft injury and prognosis.
Keywords: proteinuria, kidney transplantation, survival, histopathology
The improvement in short-term graft attrition after kidney transplantation has not translated into proportionate ameliorations in long-term renal allograft survival.1 In recent years, specific diseases such as antibody-mediated rejection and de novo/recurrent glomerular disease have been identified as the primary causes of graft failure.2–4 The reason for the insufficient improvement of long-term renal allograft outcome is likely related to the inability to prevent or treat these specific diseases. In addition, one of the challenges for research in organ transplantation is to identify markers that are sufficiently diagnostic for these specific diseases, and that can be used as an end point in clinical studies.
In patients with CKD, there is increasing interest in using the degree of proteinuria as a surrogate end point for clinical trials, because proteinuria is directly related to the underlying glomerular disease process and strongly associates with progression to ESRD, with good specificity and sensitivity.5 Also, proteinuria is routinely measured in renal transplant recipients.6–8 Proteinuria, in the nephrotic range as well as low-grade, is associated with renal allograft outcome, but the sensitivity and specificity of proteinuria for graft failure have not been assessed,9–11 unless in a cross-sectional study with a low number of graft failures.12
Although proteinuria has been related to de novo/recurrent glomerulonephritis or with transplant glomerulopathy,7–9,13–15 the association between proteinuria and the strongly evolved diagnostic criteria for allograft pathology, in particular criteria for antibody-mediated rejection,16,17 has not been considered yet. Current clinical guidelines suggest that a kidney allograft biopsy be performed when there is new onset of proteinuria or unexplained proteinuria ≥3.0 g/g creatinine or ≥3.0 g/24 h. However, these international guidelines are not evidence-based (evidence level 2C).18
We thus aimed to assess the diagnostic and prognostic performance of measuring proteinuria after renal transplantation, both at the time of graft dysfunction (with clinically indicated biopsies) and during routine clinical follow-up (using protocol-specified biopsies). In view of the great effect of specific diseases such as antibody-mediated rejection and de novo/recurrent glomerulonephritis on outcome after kidney transplantation,2–4 with new therapeutic options available, insight into the diagnostic and prognostic value of proteinuria for these specific diseases is particularly useful. Moreover, as many research teams are evaluating novel noninvasive biomarkers for renal allograft injury processes, it is important to fully elucidate the diagnostic and prognostic value of proteinuria measurement, a simple, inexpensive, and noninvasive marker that is already universally available.
Results
Study Population Characteristics
In this prospective, observational cohort study, we enrolled all consecutive recipients of a renal allograft transplant at University Hospitals Leuven (Leuven, Belgium) between January 1991 and December 2001 (n=1197) in a test Cohort 1.4 All patients who underwent single kidney transplantation at the same institution between March 2004 and October 2007 were included in a validation Cohort 2.19 The patient population characteristics are provided in Table 1 and Supplemental Figure 1. No cross-match positive transplantations were performed in these patients. In Cohort 1 (n=1197 patients), 1443 post-transplant indication biopsies were performed in 752 individual kidney grafts. The mean time post-transplantation of the biopsies was 1.9±3.4 years (range 2 days–18.6 years). Only 78 biopsy samples (5.4%) were of insufficient quality to yield a diagnosis and to evaluate according to the Banff scheme,17 which left 1365 biopsies in 738 individual transplants for further analysis. Proteinuria measured in a 24-hour urine collection was available in 1335 cases (98%) within 1 week around the biopsy. Graft loss occurred in 313 of 1197 patients during follow-up. In Cohort 2 (n=321 patients), 922 post-transplant renal allograft biopsies were performed within the first 2 years after transplantation: 203 indication biopsies in 136 individual kidney grafts, in addition to 260, 257, and 202 good-quality protocol-specified biopsies at, respectively, 3, 12, and 24 months post-transplant.19 In 919 of 922 (99.8%; 200 indication biopsies and 719 protocol biopsies) of cases, concomitant 24-hour proteinuria data were available. Graft loss occurred in 45 of 321 patients during follow-up. The evolution of the histologic lesions over time after transplantation in indication biopsies performed in Cohorts 1 and 2 has been described previously.4,19
Table 1.
Patient demographics
| Cohort 1 (1991–2001) | Cohort 2 (2004–2007) | P Value | |
|---|---|---|---|
| Number of patients | 1197 | 321 | |
| Recipient characteristics | |||
| Recipient age | 49.1±13.3 | 53.9±13.6 | <0.001 |
| Recipient gender (male) | 698 (58.3%) | 197 (61.4%) | 0.32 |
| Recurrence potential of primary renal diseasea | 390 (32.6%) | 79 (24.6%) | <0.01 |
| Caucasian ethnicity | 1149 (96.0%) | 295 (91.9%) | 0.003 |
| Repeat transplantation | 148 (12.4%) | 45 (14.0%) | 0.43 |
| Pretransplant donor-specific antibodies | – | 17 (5.3%) | – |
| Number of HLA mismatches | 2.57±1.30 | 2.45±1.30 | 0.28 |
| Treatment with tacrolimus+MMF+steroids | 261 (21.8%) | 230 (71.7%) | <0.001 |
| Treatment with an mTOR inhibitor | – | 18 (5.6%) | – |
| Additional induction therapy | 168 (14.0%) | 135 (42.1%) | <0.001 |
| Donor characteristics | |||
| Donor age | 38.8±15.6 | 46.3±15.0 | <0.001 |
| Donor gender (male) | 747 (62.4%) | 184 (57.3%) | 0.10 |
| Deceased donor | 1186 (99.1%) | 302 (94.1%) | <0.001 |
| Cold ischemia time (hours) | 18.4±4.94 | 14.7±5.88 | <0.001 |
| Post-transplant data | |||
| Delayed graft function | 166 (13.9%) | 47 (14.6%) | 0.72 |
| Donor-specific antibodies at 1 year | – | 18/257 (7.0%) | – |
| Donor-specific antibodies at 2 years | – | 16/202 (7.9%) | – |
| eGFR at 1 year (mL/min per 1.73 m2) | 45.3±15.6 | 55.7±18.2 | <0.001 |
| eGFR at 2 years (mL/min per 1.73 m2) | 45.7±17.2 | 55.6±18.2 | <0.001 |
| eGFR at 10 years (mL/mi per 1.73 m2) | 46.5±20.2 | – | – |
| Proteinuria (g/24 h) at 1 year | 0.27±0.59 | 0.23±0.64 | 0.38 |
| Proteinuria (g/24 h) at 2 years | 0.27±0.60 | 0.20±0.35 | 0.11 |
| Proteinuria (g/24 h) at 10 years | 0.37±0.65 | – | – |
| 1-year dialysis-free patient survival | 90.4% | 96.3% | <0.001 |
| 5-year dialysis-free patient survival | 78.4% | 85.7% | 0.004 |
| 10-year dialysis-free patient survival | 59.9% | – | – |
| 1-year graft survivalb | 93.6% | 96.9% | 0.03 |
| 5-year graft survivalb | 86.2% | 91.4% | 0.02 |
| 10-year graft survivalb | 74.8% | – | – |
MMF, mycophenolate mofetil; mTOR, mammalian target of rapamycin.
Renal diseases with recurrence potential were primary FSGS, idiopathic membranous nephropathy, IgA nephropathy, membranoproliferative glomerulopathy, nephropathy associated with systemic lupus erythematosis, ANCA-associated vasculitis, anti-glomerular basement membrane disease, Henoch–Schönlein purpura nephropathy, hemolytic uremic syndrome, amyloidosis, monoclonal immunoglobulin deposition disease, and primary glomerulonephritis without clear diagnosis.
Graft survival was censored at time of recipient death.
Proteinuria and Kidney-Allograft Failure in the Overall Group
For Cohort 1, the mean follow-up time post-transplant of patients with a functioning graft at time of data extraction from the clinical database was 14.5±2.80 (range 10.4–20.4) years post-transplant. During follow-up after 1 year post-transplant, 116 of 619 (18.7%) patients with 24-hour proteinuria ≤0.3 g, 36 of 118 (30.5%) patients with 24-hour proteinuria >0.3–1.0 g, 14 of 36 (38.9%) patients with 24-hour proteinuria >1.0–3.0 g, and four of five (80%) patients with 24-hour proteinuria >3.0 g at 1 year lost graft function (P<0.001). In multivariate Cox proportional hazards analysis, the degree of proteinuria at 1, 2, and 5 years after transplantation was associated with kidney-allograft failure, adjusted for donor and recipient age and gender, baseline immunosuppression, repeat transplantation, delayed graft function, and T cell–mediated rejection, and independent of eGFR (Figure 1A, Supplemental Table 1). The striking association between proteinuria >3.0 g/24 h at 3 months and graft failure (Figure 1A) was due to the underlying pathology: recurrent FSGS in four cases, recurrent atypical hemolytic uremic syndrome in two patients, and transplant glomerulopathy in one patient. In the last patient, it is likely that the proteinuria at 3 months originated from the native kidneys affected by diabetes mellitus, as proteinuria disappeared completely and this allograft remains functional up to the day of data extraction (16 years).
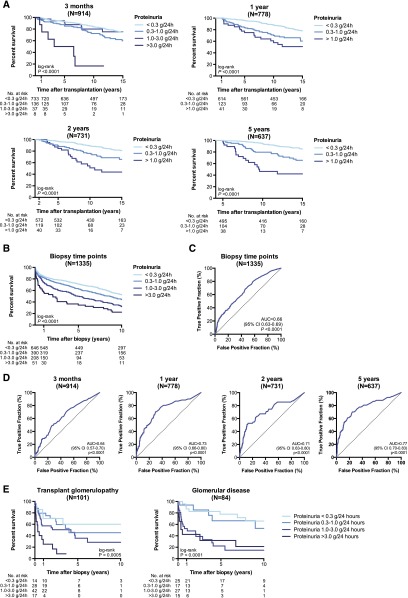
Figure 1.
The degree of proteinuria associates with graft survival. (A) Kaplan–Meier survival curve for the association between 24-hour proteinuria at different time points after transplantation and graft survival in the unselected cohort of 1197 kidney recipients. (B) Kaplan–Meier survival curve for the association between 24-hour proteinuria at time of an indication biopsy and postbiopsy graft failure. (C) ROC curve of the association between proteinuria and postbiopsy graft failure. (D) ROC curve of the association between proteinuria measured at different time points after transplantation and graft failure 5 years later. (E) Kaplan–Meier survival curves for graft survival according to the degree of proteinuria at time of biopsy with transplant glomerulopathy and at time of diagnosis of de novo/recurrent glomerular disease. cg, transplant glomerulopathy; glom.dis., de novo/recurrent glomerular disease; prot., proteinuria.
In Cohort 1, at the time of an indication biopsy for graft dysfunction (1335 data points), proteinuria was absent (<0.3 g/24 h) in 665 cases (49.8%), low-grade (0.3–1.0 g/24 h) in 401 cases (30.0%), moderate (1.0–3.0 g/24 h) in 217 cases (16.3%), and severe (>3.0 g/24 h) in 52 cases (3.9%). Univariate Cox analyses of the clinical and histologic determinants of graft failure are provided in Supplemental Table 2. In multivariate Cox regression analysis, adjusted for donor age and time after transplantation, the following variables were independently associated with graft failure (Table 2): low eGFR at time of the biopsy, transplant glomerulopathy, microcirculation inflammation, polyomavirus nephropathy, and interstitial fibrosis/tubular atrophy. When we added proteinuria to the multivariate model, proteinuria >1.0 g/24 h was highly significantly associated with graft failure. All other associations remained statistically significant, except de novo/recurrent glomerular disease, whose relation with graft loss was therefore fully dependent on its association with proteinuria.
Table 2.
Hazard ratio (multivariate models) for graft failure according to renal allograft histology, renal function, and proteinuria at time of biopsy, adjusted for donor age and time after transplantation in Cohort 1 (n=1335 indication biopsies)
| Parameter | Adjusted Hazard Ratio (95% CI) | P Value | |
|---|---|---|---|
| Model 1: Without proteinuria | |||
| eGFR at time of biopsy | 30–45 versus >45 mL/min per m2 | 1.48 (0.50–4.40) | 0.48 |
| 15–30 versus >45 mL/min per m2 | 4.21 (1.53–11.6) | <0.01 | |
| <15 versus >45 mL/min per m2 | 9.41 (3.37–26.3) | <0.001 | |
| Microcirculation inflammation | g+ptc ≥2 versus <2 | 1.45 (1.03–2.04) | 0.03 |
| IF/TA grade | Banff grade 1 versus 0 | 1.72 (1.19–2.50) | 0.004 |
| Banff grade 2–3 versus 0 | 3.44 (2.34–5.05) | <0.001 | |
| Transplant glomerulopathy | Banff grade 1 versus 0 | 1.24 (0.69–2.22) | 0.48 |
| Banff grade 2–3 versus 0 | 2.54 (1.54–4.18) | <0.001 | |
| De novo/recurrent glomerular disease | Present versus absent | 1.59 (0.99–2.56) | 0.06 |
| Polyomavirus associated nephropathy | Present versus absent | 5.84 (3.22–10.6) | <0.001 |
| Model 2: With proteinuria | |||
| Proteinuria at time of biopsy | 0.3–1.0 versus <0.3 g/24 h | 1.14 (0.81–1.60) | 0.50 |
| 1.0–3.0 versus <0.3 g/24 h | 2.17 (1.49–3.18) | <0.001 | |
| >3.0 versus <0.3 g/24 h | 3.01 (1.75–5.18) | <0.001 | |
| eGFR at time of biopsy | 30–45 versus >45 mL/min per m2 | 1.76 (0.59–5.30) | 0.31 |
| 15–30 versus >45 mL/min per m2 | 5.53 (1.99–15.4) | 0.001 | |
| <15 versus >45 mL/min per m2 | 11.7 (4.17–33.0) | <0.001 | |
| Microcirculation inflammation | g+ptc ≥2 versus <2 | 1.36 (0.97–1.91) | 0.07 |
| IF/TA grade | Banff grade 1 versus 0 | 1.82 (1.25–2.64) | 0.002 |
| Banff grade 2–3 versus 0 | 3.45 (2.34–5.07) | <0.001 | |
| Transplant glomerulopathy | Banff grade 1 versus 0 | 1.00 (0.55–1.82) | 0.99 |
| Banff grade 2–3 versus 0 | 1.83 (1.11–3.04) | 0.02 | |
| De novo/recurrent glomerular disease | Present versus absent | 1.35 (0.84–2.19) | 0.22 |
| Polyomavirus associated nephropathy | Present versus absent | 5.51 (3.06–9.92) | <0.001 |
g, glomerulitis; ptc, peritubular capillaritis; IF/TA, interstitial fibrosis/tubular atrophy.
The Kaplan–Meier curve for graft survival stratified according to the degree of proteinuria at time of an indication biopsy in Cohort 1 is shown in Figure 1B. Graft survival at 5 years postbiopsy was 71%, 63%, 50%, and 35% for concomitant 24 h proteinuria <0.3 g/24 h, 0.3–1.0 g/24 h, 1.0–3.0 g/24 h, and >3.0 g/24 h, respectively (P<0.001). Receiver-operating characteristic (ROC) analysis of 24-hour proteinuria for 5-year graft failure postbiopsy yielded an area under the receiver-operating characteristic curve (AUC) of 0.66 (95% CI 0.63–0.69; P<0.001). Specificity of proteinuria >1.0 g/24 h for 5-year postbiopsy failure was 86.1% (83.5–88.4%), but sensitivity was lower (31.7%; 27.0–36.7%) (Figure 1C, Table 3). Predictive accuracy of 24-hour proteinuria at 3 months after transplantation was weak (AUC 0.64 [95% confidence interval (95% CI) 0.57–0.70]; P<0.001) but increased at later time points (AUC 0.73 [95% CI, 0.66–0.80], 0.71 [95% CI, 0.63–0.80] and 0.77 [95% CI, 0.70–0.83] at, respectively, 1, 2, and 5 years after transplantation; all P<0.001). Negative predictive value of proteinuria <1.0 g/24 h was high, at 91.0–93.1%, while the positive predictive value increased over time after transplantation, from 19.5% at 3 months to 61.3% at 5 years post-transplantation (Figure 1D, Table 3).
Table 3.
Predictive performance of 24-hour proteinuria for graft failure in ROC analysis in Cohort 1
| AUC | P Value | Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|---|---|
| Graft failure at 5 years after proteinuria measurement | ||||||
| 3 months post-transplant (n=914) | ||||||
| Proteinuria >1.0 g/24 h | 0.64 (0.57–0.70) | <0.001 | 10.1% (4.47–19.0%) | 95.3% (93.7–96.7%) | 19.5% | 91.2% |
| Proteinuria >3.0 g/24 h | 5.06% (1.40–12.5%) | 99.5% (98.8–99.9%) | 50.0% | 91.1% | ||
| 1 year post-transplant (n=778) | ||||||
| Proteinuria >1.0 g/24 h | 0.73 (0.66–0.80) | <0.001 | 16.4% (8.15–28.1%) | 95.5% (93.8–96.9%) | 26.3% | 92.6% |
| Proteinuria >3.0 g/24 h | 4.92% (1.03–13.7%) | 99.6% (98.8–99.9%) | 60.0% | 91.9% | ||
| 2 years post-transplant (n=731) | ||||||
| Proteinuria >1.0 g/24 h | 0.71 (0.63–0.80) | <0.001 | 20.0% (10.4–33.0%) | 95.6% (93.7–97.0%) | 30.6% | 93.1% |
| Proteinuria >3.0 g/24 h | 5.45% (1.14–15.1%) | 99.4% (98.5–99.8%) | 50.0% | 92.2% | ||
| 5 years post-transplant (n=637) | ||||||
| Proteinuria >1.0 g/24 h | 0.77 (0.70–0.83) | <0.001 | 28.4% (18.0–40.7%) | 96.4% (94.5–97.7%) | 61.3% | 91.0% |
| Proteinuria >3.0 g/24 h | 4.48% (0.93–12.5%) | 99.8% (99.0–100%) | 100% | 88.6% | ||
| Graft failure at 5 years after indication biopsy | ||||||
| All biopsies (n=1335) | ||||||
| Proteinuria >1.0 g/24 h | 0.66 (0.63–0.69) | <0.001 | 31.7% (27.0–36.7%) | 86.1% (83.5–88.4%) | 51.5% | 73.2% |
| Proteinuria >3.0 g/24 h | 7.32% (4.88–10.5%) | 97.9% (96.6–98.8%) | 63.0% | 69.6% | ||
| Biopsies <3 months post-transplant (n=752) | ||||||
| Proteinuria >1.0 g/24 h | 0.62 (0.57–0.67) | <0.001 | 25.9% (19.4–33.3%) | 87.2% (84.0–89.9%) | 38.7% | 78.4% |
| Proteinuria >3.0 g/24 h | 2.41% (0.66–6.05%) | 98.3% (96.7–99.2%) | 35.7% | 75.8% | ||
| Biopsies > 3 months post-transplant (n=583) | ||||||
| Proteinuria >1.0 g/24 h | 0.71 (0.67–0.76) | <0.001 | 36.5% (29.8–43.5%) | 84.8% (80.0–88.7%) | 63.6% | 65.1% |
| Proteinuria >3.0 g/24 h | 10.8% (6.92–16.0%) | 97.2% (94.5–98.8%) | 75.0% | 60.5% | ||
PPV, positive predictive value; NPV, negative predictive value.
In Cohort 2, the mean follow-up time post-transplant of patients with a functioning graft at time of data extraction from the clinical database was 8.6±1.0 (range 6.9–10.5) years post-transplant. Despite a relatively shorter follow-up and better graft survival of the patients in Cohort 2, a similar association between proteinuria at 1 and 2 years after transplantation and graft failure was noted, but not at 3 months or at the time of an indication biopsy (Supplemental Figure 2, Supplemental Table 1). It should be noted that 88.0% (176 of 200) of indication biopsies in Cohort 2 were performed within the first 3 months post-transplant. Univariate Cox analysis of the clinical and histologic determinants of graft failure is provided in Supplemental Table 2. In multivariate analysis, the following parameters were independently associated with graft failure adjusted for donor age and time after transplantation: low eGFR, transplant glomerulopathy, and interstitial fibrosis/tubular atrophy. When proteinuria was introduced into the multivariate model, transplant glomerulopathy was omitted from the model, which illustrates the interdependence of proteinuria and transplant glomerulopathy (Supplemental Table 3). In both Cohorts 1 and 2, there was no association between the use of angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) and graft failure (Supplemental Table 2).
Sensitivity Analysis
In the sensitivity analysis, performed in Cohort 1, we assessed the robustness of our study results by investigating associations separately in subgroups of patients with specific histologic lesions. In patients with transplant glomerulopathy (n=101), the degree of moderate to severe proteinuria was significantly associated with postbiopsy graft loss: adjusted hazard ratio 1.28 (0.29–5.77; P=0.74) for 0.3–1.0 g/24 h compared with <0.3 g/24 h; 3.27 (0.87–11.9; P=0.08) for 1.0–3.0 g/24 h versus <0.3 g/24 h; and 6.59 (1.51–28.6; P=0.01) for >3.0 versus <0.3 g/24 h (Figure 1E). Graft survival at 5 years postdiagnosis of transplant glomerulopathy was 60%, 45%, 36%, and 8% for concomitant 24-hour proteinuria <0.3 g/24 h, 0.3–1.0 g/24 h, 1.0–3.0 g/24 h, and >3.0 g/24 h, respectively (P<0.01). The degree of proteinuria also associated significantly with the outcome after diagnosis of a de novo or recurrent glomerular disease (n=84) with an adjusted hazard ratio of 0.68 (0.02–19.3; P=0.82) for 0.3–1.0 g/24 h, 40.2 (3.66–440; P=0.003) for 1.0–3.0 g/24 h and 79.2 (5.82–1076; P<0.001) for >3.0 g/24 h, all in comparison with proteinuria <0.3 g/24 h (Figure 1E). Graft survival at 5 years postdiagnosis of de novo/recurrent glomerular disease was 78%, 66%, 22%, and 21% for concomitant 24-hour proteinuria <0.3 g/24 h, 0.3–1.0 g/24 h, 1.0–3.0 g/24 h, and >3.0 g/24 h, respectively (P<0.001).
Renal Allograft Histology and Proteinuria
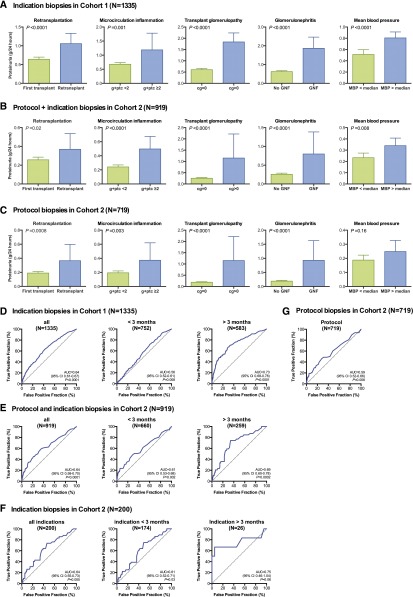
The univariate determinants of 24-hour proteinuria are provided in Supplemental Table 4. In multiple regression analysis (Figure 2, A–C, Table 4), the independent and consistent determinants of proteinuria (adjusted for donor age and time after transplantation) were repeat transplantation, transplant glomerulopathy, microcirculation inflammation, and de novo/recurrent glomerular disease. In addition, higher mean arterial pressure was also associated with higher degree of proteinuria. This was the case both in indication and protocol biopsies (Figure 2, A–C, Table 4). Despite the highly significant association between the presence of donor-specific HLA antibodies (DSA) and microcirculation inflammation (41% versus 11% microcirculation inflammation in patients with versus without DSA; P<0.001), DSA positivity in itself was not associated with proteinuria (Supplemental Table 4). Of note, the association between glomerular abnormalities and proteinuria was marked after the first 3 months post-transplant, but not in the first 3 months post-transplantation (Supplemental Table 5). In Cohort 1, there was a significant positive association between proteinuria and the use of ACE inhibitors or ARBs (Supplemental Table 4), which illustrates selection bias in the analyses on ACE inhibitor or ARB use.
Figure 2.
Proteinuria is a marker of renal allograft histology. (A–C) Association between clinical and histologic parameters and 24-hour proteinuria in Cohort 1 (A; n=1335 indication biopsies) and in Cohort 2 (B,C; n=919 biopsies, 200 indication biopsies and 719 protocol biopsies). These parameters were independently and consistently associated with 24-hour proteinuria in mixed-model repeated-measures analysis (Table 4). (D) ROC curve of the association between 24-hour proteinuria and the presence of transplant glomerulopathy, microcirculation inflammation, and/or de novo/recurrent glomerular disease in the concomitant renal allograft biopsy in Cohort 1 (n=1335). (E) ROC curve of the association between 24-hour proteinuria and the presence of transplant glomerulopathy, microcirculation inflammation, and or de novo/recurrent glomerular disease in the concomitant renal allograft biopsy (both protocol-specified and clinically indicated) in Cohort 2 (n=919). (F,G) ROC curves of the association between 24-hour proteinuria and the presence of transplant glomerulopathy, microcirculation inflammation, and or de novo/recurrent glomerular disease in the concomitant renal allograft biopsy, in the indication (F; n=200) and protocol (G; n=719) biopsies of Cohort 2.
Table 4.
Determinants of proteinuria in multivariate mixed model analysis, in Cohort 1 (n=1335 indication biopsies) and in Cohort 2 (n=919 indication and protocol biopsies)
| Cohort 1 (n=1335 indication biopsies) | Cohort 2 (n=919 indication and protocol biopsies) | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Estimate | Standard Error | t Value | P Value | Estimate | Standard Error | t Value | P Value |
| Mean arterial pressure at time of biopsy | ||||||||
| >median versus <mediana | 0.26 | 0.06 | 4.14 | <0.001 | 0.10 | 0.04 | 2.64 | <0.01 |
| Donor age | ||||||||
| 40–60 years versus <40 years | −0.17 | 0.08 | −2.27 | 0.02 | –b | –b | –b | –b |
| >60 years versus <40 years | −0.28 | 0.11 | −2.55 | 0.01 | –b | –b | –b | –b |
| Repeat transplantation | ||||||||
| Yes versus no | 0.41 | 0.10 | 4.06 | <0.001 | 0.13 | 0.07 | 1.97 | 0.050 |
| Microcirculation inflammation | ||||||||
| g+ptc ≥2 versus g+ptc <2 | 0.17 | 0.08 | 2.22 | 0.03 | 0.18 | 0.06 | 2.93 | 0.004 |
| Transplant glomerulopathy | ||||||||
| Banff grade >0 versus 0 | 0.80 | 0.18 | 4.50 | <0.001 | 0.88 | 0.15 | 5.94 | <0.001 |
| De novo/recurrent glomerular disease | ||||||||
| Present versus absent | 0.97 | 0.17 | 5.78 | <0.001 | 0.46 | 0.13 | 3.56 | <0.001 |
| Interstitial inflammation | ||||||||
| Banff grade 1 versus 0 | –b | –b | –b | –b | 0.16 | 0.06 | 2.67 | <0.01 |
| Banff grade 2–3 versus 0 | –b | –b | –b | –b | 0.15 | 0.06 | 2.48 | 0.01 |
| Vascular intimal thickening | ||||||||
| Banff grade 1 versus 0 | −0.05 | 0.10 | −0.55 | 0.59 | –b | –b | –b | –b |
| Banff grade 2–3 versus 0 | 0.37 | 0.12 | 3.12 | 0.002 | –b | –b | –b | –b |
Univariate analyses are provided in Supplemental Table 4. g, glomerulitis; ptc, peritubular capillaritis.
Median mean arterial pressure was 103 mmHg in Cohort 1 and 96 mmHg in Cohort 2.
Parameter not included in the final model after backward parameter selection.
Proteinuria as a Biomarker for Glomerular Disease
Next, we evaluated the diagnostic performance of proteinuria for glomerular disease in renal allograft biopsies. ROC curves are provided in Figure 2, D–G. Proteinuria later than 3 months post-transplant was highly significantly associated with the combined presence of either transplant glomerulopathy, microcirculation inflammation, or de novo/recurrent glomerular disease (Supplemental Table 5). The ROC AUC was 0.64 (0.61–0.67; P<0.001) in Cohort 1 and 0.64 (0.58–0.70; P<0.001) in Cohort 2. The specificity of 24-hour proteinuria at the time of an indication biopsy for these lesions was high (85.0–91.1% for proteinuria >1.0 g/24 h), but sensitivity was low (21.4–31.7%). Early after transplantation, the diagnostic accuracy was low (ROC AUC 0.56 [0.52–0.61] in Cohort 1). After the first 3 months post-transplantation in Cohort 1, the diagnostic accuracy of 24-hour proteinuria for either transplant glomerulopathy, microcirculation inflammation, or de novo/recurrent glomerular disease was better, with a ROC AUC of 0.73 (0.68–0.78; P<0.001), a sensitivity of proteinuria >1.0 g/24 h of 47.8% (40.2–55.4%), and a specificity of 85.2% (81.4–88.5%) (Table 5). The number of indication biopsies performed later than 3 months in Cohort 2 was too low (n=26) to allow for robust statistical analysis in this cohort. At protocol biopsy time points, per definition performed in clinically stable patients (with lower degrees of proteinuria), the association between 24-hour proteinuria and graft histology was also significant, but with lower sensitivity and specificity (Supplemental Figure 2G, Table 5).
Table 5.
Diagnostic performance of 24-hour proteinuria for the combination of transplant glomerulopathy, de novo/recurrent glomerular disease and/or microcirculation inflammation (g+ptc ≥ 2) in ROC analysis in Cohort 1 (n=1335) and Cohort 2 (n=919)
| Transplant Glomerulopathy, Glomerular Disease, and/or Microcirculation Inflammation | ||||||
|---|---|---|---|---|---|---|
| AUC | P Value | Sensitivity | Specificity | PPV | NPV | |
| Cohort 1 | ||||||
| All biopsies (n=1335) | ||||||
| Proteinuria >1.0 g/24 h | 0.64 (0.61–0.67) | <0.001 | 31.7% (27.1–36.5%) | 85.0% (82.6–87.2%) | 47.2% | 74.3% |
| Proteinuria >3.0 g/24 h | 7.98% (5.52–11.1%) | 98.1% (97.0–98.9%) | 65.4% | 71.4% | ||
| Biopsies <3 months post-transplant (n=752) | ||||||
| Proteinuria >1.0 g/24 h | 0.56 (0.52–0.61) | <0.01 | 18.8% (13.9–24.6%) | 84.9% (81.5–87.8%) | 34.1% | 71.2% |
| Proteinuria >3.0 g/24 h | 2.69 (0.99–2.76%) | 98.5% (97.0–99.3%) | 40.0% | 70.6% | ||
| Biopsies > 3 months post-transplant (n=583) | ||||||
| Proteinuria >1.0 g/24 h | 0.73 (0.68–0.78) | <0.001 | 47.8% (40.2–55.4%) | 85.2% (81.4–88.5%) | 58.2% | 78.7% |
| Proteinuria >3.0 g/24 h | 14.6% (9.77–20.7%) | 97.8% (95.8–98.9%) | 75.7% | 72.5% | ||
| Cohort 2 | ||||||
| All biopsies (n=919) | ||||||
| Proteinuria >1.0 g/24 h | 0.64 (0.58–0.70) | <0.001 | 12.5% (7.17–19.8%) | 97.1% (95.7–98.2%) | 39.5% | 88.1% |
| Proteinuria >3.0 g/24 h | 2.50% (0.52–7.13%) | 99.8% (99.1–100%) | 50.0% | 87.2% | ||
| Indication biopsies (n=200) | ||||||
| Proteinuria >1.0 g/24 h | 0.64 (0.55–0.73) | <0.01 | 21.4% (10.3–36.8%) | 91.1% (85.6–95.1%) | 39.1% | 81.4% |
| Proteinuria >3.0 g/24 h | 2.38% (0.06–12.6%) | 99.4% (96.5–100%) | 33.3% | 79.2% | ||
| Protocol-specified biopsies (n=719) | ||||||
| Proteinuria >1.0 g/24 h | 0.59 (0.52–0.66) | <0.01 | 7.69% (2.88–16.0%) | 98.6% (97.4–99.4%) | 40.0% | 89.8% |
| Proteinuria >3.0 g/24 h | 2.56% (0.31–8.96%) | 99.8% (99.1–100%) | 66.7% | 89.4% | ||
g, glomerulitis; ptc, peritubular capillaritis; PPV, positive predictive value; NPV, negative predictive value.
Discussion
For the first time, our analysis with integration of 24-hour proteinuria measurement, contemporary detailed histologic assessment, and long-term outcome data showed that proteinuria is a marker for graft outcome with reasonable predictive accuracy, especially after the first 3 months post-transplantation. Proteinuria was highly specific for transplant glomerulopathy, microcirculation inflammation, and de novo/recurrent glomerular disease, both in indication and in protocol biopsies. Moreover, the prognosis of these specific disease processes was primarily determined by the associated degree of proteinuria.
Although previous studies clearly illustrated that proteinuria at 3 and 12 months is associated with graft outcome,9,10,12 these studies did not provide insight into the value of proteinuria at time of clinical inquietude, when an indication biopsy is performed, or of proteinuria later after transplantation. Our study showed that the degree of proteinuria is a main determinant of graft failure at any time point after transplantation, independent of renal allograft function and independent of the underlying renal allograft histology. The prognosis of specific injury processes such as de novo/recurrent glomerular disease and transplant glomerulopathy largely depended on the degree of proteinuria. The prognostic accuracy of proteinuria for graft failure increased over time after transplantation, with good specificity and high negative predictive value. Proteinuria within the first months after transplantation is less predictive of graft outcome, which can explain why proteinuria at indication biopsy time points graft failure was not associated with graft failure in Cohort 2.
The high specificity of proteinuria for graft loss was counterbalanced by a relatively low sensitivity, which yielded a moderate overall predictive performance. The heterogeneity in the causes of graft failure,2–4 however, makes it unlikely that a single noninvasive biomarker will yield a high predictive performance for graft loss. It can be anticipated that only the combination of different markers that address this heterogeneity will yield both high sensitivity and high specificity for graft failure.
Next, proteinuria >1.0 g/24 h was a specific noninvasive marker for highly relevant intragraft injury processes such as microcirculation inflammation, transplant glomerulopathy, and de novo/glomerular disease in our study. The high specificity of proteinuria for these treatable diagnoses, both in indication and in protocol biopsies, provides the first evidence of current clinical guidelines that advocate the routine measurement of proteinuria.18 In addition, clinical guidelines suggest that a kidney allograft biopsy be performed when there is new onset of proteinuria or unexplained proteinuria ≥3.0 g/g creatinine or ≥3.0 g/24 h. Our data illustrate that this threshold is very conservative and that the detection of proteinuria >1.0 g/24 h could be a more sensitive threshold. The association between proteinuria and renal histology was weak in the first 3 months after transplantation, likely reflecting the contribution of residual renal function of the native kidneys in the first weeks.20,21
Despite the high specificity of proteinuria for transplant glomerulopathy, microcirculation inflammation, or glomerular disease, general diagnostic performance of 24-hour proteinuria for intragraft injury was only moderate, due to low sensitivity. Many patients with significant histologic injury did not have proteinuria >1.0 g/24 h, illustrating that proteinuria is a late phenomenon. In specific populations, for example, high-risk patients with cross-match–positive transplantation, with de novo DSA, or at high risk of glomerular disease recurrence, surveillance biopsies could thus be warranted in the absence of proteinuria or graft dysfunction, for the timely detection of subclinical injury.22 In this light, it seems surprising that the presence or occurrence of DSA did not independently associate with proteinuria in our study, which is in agreement with two recent studies.23,24 Although the number of patients positive for DSA was low in our cohort and the data should thus be interpreted cautiously, it could be hypothesized that the presence of DSA does not lead to proteinuria per se, but that proteinuria appears only if DSA cause glomerular injury.
Our study has several limitations. Whether our results also apply to cross-match–positive kidney transplants, and whether the association between the histology of antibody-mediated rejection, proteinuria and graft outcome would be more pronounced in this specific high-risk patient cohort, could not be inferred from our data. In addition, it should be envisaged that this study was performed in a European population with cross-match–negative transplants, and does not represent other cohorts at potentially higher (immunologic) risk (e.g., black recipients or positive cross-match transplants).25 Moreover, the absence of DSA testing in Cohort 1 obviates a thorough insight into the association of DSA on very long-term histology and graft outcome. To fully appreciate the diagnostic and prognostic importance of 24-hour proteinuria in renal transplantation, repeating these analyses and validation in a population at higher immunologic risk should be considered.
It has been shown that mammalian target of rapamycin (mTOR) inhibitor use can lead to proteinuria,26 but we did not observe this in our analysis, likely due to the very low number of patients treated with mTOR inhibitors in our cohorts. Furthermore, in Cohort 2, the association between proteinuria and graft failure was less significant than in Cohort 1, which likely relates to the overall better outcome in this more recent and smaller cohort, and to shorter follow-up time. Although proteinuria in 24-hour urine collections remains the reference method for quantification of proteinuria, it is cumbersome in clinical practice. Many centers use the proteinuria/creatinine ratio in spot urine samples to overcome this burden. Whether the diagnostic and prognostic importance of spot urine protein excretion is comparable to the value of 24-hour proteinuria remains to be evaluated. Protein/creatinine levels in spot urine samples were not available in this study. In addition, we have no data on albuminuria or proteinuria composition, which could discriminate between glomerular and tubular origin of the proteinuria, and could be a better marker in patients with total proteinuria <0.3 g/24 h.12,27,28 Finally, the potential benefit of renin-angiotensin system inhibitor use29 in kidney transplant patients could not be evaluated in our observational cohort study with inherent selection bias, as patients with proteinuria were more likely to receive renin-angiotensin system inhibitors compared with patients without proteinuria.
In conclusion, this analysis of the diagnostic performance of proteinuria for treatable disease processes (transplant glomerulopathy, microcirculation inflammation, and de novo or recurrent glomerular disease), both in indication and in protocol biopsies, provides the first scientific underpinning of the current clinical guidelines to routinely measure proteinuria after transplantation, and to pursue a histologic diagnosis when proteinuria >1.0 g/24 h is detected. The low sensitivity of 24-hour proteinuria for graft injury and failure illustrates the need for novel, more sensitive, noninvasive biomarkers of kidney graft injury and prognosis.
Concise Methods
Clinical Data and Proteinuria
All data were collected from the day of transplantation until the time of data extraction during the routine clinical follow-up of the transplant recipients. This study was approved by the Ethics Committee of the University Hospitals Leuven (S53364; ML7499). The clinical data in both cohorts were prospectively collected in electronic clinical patient charts, which were used for clinical patient management as well as being directly linked to the database used in this study. This clinical database was transferred to SAS data files (SAS Institute, Cary, NC) at the time of analysis. Proteinuria was assessed in 24-hour urine collections (total proteinuria/24 h) at 3 months, and at 1, 2, 5, and 10 years after transplantation, and at time of a renal allograft biopsy. Urinary 24-hour protein excretion remains the reference method for quantification of proteinuria in patients with glomerulonephritis.30 Proteinuria was graded as absent (≤0.3 g/24 h), mild (>0.3–1.0 g/24 h), moderate (>1.0–3.0 g/24 h), and severe (>3.0 g/24 h).7 Renal allograft function (eGFR) was estimated using the four-variable modification of diet in renal disease formula.31 Spot urine data on protein/creatinine levels were not available in these cohorts.
Biopsies and Histologic Scoring
All post-transplant renal allograft biopsies performed in this cohort, until time of data extraction, were included. In Cohort 1, post-transplant biopsies were performed for clinical indication only. No post-transplant surveillance biopsies were performed in this cohort. In Cohort 2, protocol-specified biopsies were performed systematically at 3, 12, and 24 months after transplantation, in addition to the clinically indicated biopsies (“indication biopsies”).
Slides were stained with hematoxylin eosin, periodic acid–Schiff, and silver methenamine (Jones). An immunohistochemical C4d stain (monoclonal antibody, dilution 1:500; Quidel Corporation, Santa Clara, CA) was performed on frozen tissue. One pathologist (E.L.) retrospectively reviewed all biopsies, blinded for the timing of the biopsy and for the clinical data. The severity of histologic lesions was semiquantitatively rescored according to the revised Banff criteria.17 In addition, the number of sclerosed glomeruli was scored separately (0=0%; 1=1–25%; 2=25–50%; 3≥50% glomerulosclerosis). The microcirculation inflammation score was defined as the sum of the Banff glomerulitis and peritubular capillaritis scores (range 0–6). Data on DSA were not available for Cohort 1. In Cohort 2, systematic follow-up HLA antibodies (ELISA HLA class I and class II Gen-Probe LifeCodes technique before 2008; since 2008 Luminex Gen-Probe LifeCodes LSA screening) and their donor-specificity in case of positive screening (using Luminex Gen-Probe LifeCodes LSA Single Antigen beads) were evaluated at time of protocol biopsies and also for clinical indication (in combination with indication biopsies).
Data Collection and Statistical Analysis
For variance analysis of continuous variables in different groups, nonparametric Wilcoxon–Mann–Whitney U, nonparametric ANOVA, and parametric one-way ANOVA were used. Dichotomous variables were compared using the chi-squared test. For assessing the clinical and histologic determinants of proteinuria, univariate and multivariate mixed-models repeated-measures analyses were used with an unstructured covariance matrix. ROC analysis was performed to evaluate diagnostic accuracy and to calculate specificity and sensitivity (with 95% confidence intervals). Univariate and multivariate Cox proportional hazards analysis, Kaplan–Meier analysis, and log-rank tests were used to examine the association between proteinuria and graft failure. In case of death with a functioning graft, we censored graft survival at time of death. To account for repeat biopsies within the same patient, the counting process model was used in the Cox proportional hazards models. The data for each subject with multiple events were described as data for multiple subjects where each has delayed entry and is followed until the next event. Survival analyses were confined to the first 10 years after the biopsy, with kidney-graft loss as the event of interest. Repeat transplants within the inclusion time frame were regarded as separate entities in statistical analysis. Log(−log(survival)) versus log(time) plots showed no nonproportionality of hazards. Patients with missing values were excluded from the analysis. All tests were two-sided and P values <0.05 were considered to indicate statistical significance. Factors with a P value <0.2 in univariate analyses were entered in hypothesis-generating multivariate analyses, with backward selection of the final model. We used SAS software (version 9.3; SAS Institute) for the statistical analyses and GraphPad Prism (version 6; GraphPad Software, San Diego, CA) for data presentation. This study had no external funding.
Disclosure
None.
Supplementary Material
Acknowledgments
The authors had full access to the data and take responsibility for its integrity. M.N., E.L., and M.P.E. designed the study. M.N., E.L., M.P.E., A.H., P.E., K.C., B.B., B.M., I.J., D.M., J.P., and D.H. collected data. M.N. analyzed the data and prepared figures.
All authors contributed to the report. All authors have read and agree with the manuscript as written.
M.N. and E.L. are supported by the Clinical Research Foundation of the University Hospitals Leuven, Belgium.
We thank the centers of the Leuven Collaborative Group for Renal Transplantation, the clinicians and surgeons, nursing staff and the patients.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015010062/-/DCSupplemental.
References
- 1.Lamb KE, Lodhi S, Meier-Kriesche HU: Long-term renal allograft survival in the United States: a critical reappraisal. Am J Transplant 11: 450–462, 2011 [DOI] [PubMed] [Google Scholar]
- 2.El-Zoghby ZM, Stegall MD, Lager DJ, Kremers WK, Amer H, Gloor JM, Cosio FG: Identifying specific causes of kidney allograft loss. Am J Transplant 9: 527–535, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Sellarés J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, Hidalgo LG, Famulski K, Matas A, Halloran PF: Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant 12: 388–399, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Naesens M, Kuypers DR, De Vusser K, Evenepoel P, Claes K, Bammens B, Meijers B, Sprangers B, Pirenne J, Monbaliu D, Jochmans I, Lerut E: The histology of kidney transplant failure: a long-term follow-up study. Transplantation 98: 427–435, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Cravedi P, Ruggenenti P, Remuzzi G: Proteinuria should be used as a surrogate in CKD. Nat Rev Nephrol 8: 301–306, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Nankivell BJ, Kuypers DR: Diagnosis and prevention of chronic kidney allograft loss. Lancet 378: 1428–1437, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Shamseddin MK, Knoll GA: Posttransplantation proteinuria: an approach to diagnosis and management. Clin J Am Soc Nephrol 6: 1786–1793, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Legendre C, Anglicheau D: Transplantation: Proteinuria in kidney transplantation: an ongoing story. Nat Rev Nephrol 9: 251–252, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Amer H, Fidler ME, Myslak M, Morales P, Kremers WK, Larson TS, Stegall MD, Cosio FG: Proteinuria after kidney transplantation, relationship to allograft histology and survival. Am J Transplant 7: 2748–2756, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Halimi JM, Buchler M, Al-Najjar A, Laouad I, Chatelet V, Marlière JF, Nivet H, Lebranchu Y: Urinary albumin excretion and the risk of graft loss and death in proteinuric and non-proteinuric renal transplant recipients. Am J Transplant 7: 618–625, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Cherukuri A, Welberry-Smith MP, Tattersall JE, Ahmad N, Newstead CG, Lewington AJ, Baker RJ: The clinical significance of early proteinuria after renal transplantation. Transplantation 89: 200–207, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Nauta FL, Bakker SJ, van Oeveren W, Navis G, van der Heide JJ, van Goor H, de Jong PE, Gansevoort RT: Albuminuria, proteinuria, and novel urine biomarkers as predictors of long-term allograft outcomes in kidney transplant recipients. Am J Kidney Dis 57: 733–743, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Peddi VR, Dean DE, Hariharan S, Cavallo T, Schroeder TJ, First MR: Proteinuria following renal transplantation: correlation with histopathology and outcome. Transplant Proc 29: 101–103, 1997 [DOI] [PubMed] [Google Scholar]
- 14.Chung J, Park SK, Park JS, Kim SC, Han DJ, Yu E: Glomerulonephritis is the major cause of proteinuria in renal transplant recipients: histopathologic findings of renal allografts with proteinuria. Clin Transplant 14: 499–504, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Serón D, Burgos D, Alonso A: Histology and proteinuria after renal transplantation. Transplant Rev (Orlando) 26: 20–26, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Loupy A, Hill GS, Jordan SC: The impact of donor-specific anti-HLA antibodies on late kidney allograft failure. Nat Rev Nephrol 8: 348–357, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, Castro MC, David DS, David-Neto E, Bagnasco SM, Cendales LC, Cornell LD, Demetris AJ, Drachenberg CB, Farver CF, Farris AB, 3rd, Gibson IW, Kraus E, Liapis H, Loupy A, Nickeleit V, Randhawa P, Rodriguez ER, Rush D, Smith RN, Tan CD, Wallace WD, Mengel M, Banff meeting report writing committee : Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant 14: 272–283, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group : KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 9[Suppl 3]: S1–S155, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Naesens M, Heylen L, Lerut E, Claes K, De Wever L, Claus F, Oyen R, Kuypers D, Evenepoel P, Bammens B, Sprangers B, Meijers B, Pirenne J, Monbaliu D, de Jonge H, Metalidis C, De Vusser K, Vanrenterghem Y: Intrarenal resistive index after renal transplantation. N Engl J Med 369: 1797–1806, 2013 [DOI] [PubMed] [Google Scholar]
- 20.D’Cunha PT, Parasuraman R, Venkat KK: Rapid resolution of proteinuria of native kidney origin following live donor renal transplantation. Am J Transplant 5: 351–355, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Myslak M, Amer H, Morales P, Fidler ME, Gloor JM, Larson TS, Stegall MD, Cosio FG: Interpreting post-transplant proteinuria in patients with proteinuria pre-transplant. Am J Transplant 6: 1660–1665, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Henderson LK, Nankivell BJ, Chapman JR: Surveillance protocol kidney transplant biopsies: their evolving role in clinical practice. Am J Transplant 11: 1570–1575, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Wiebe C, Gibson IW, Blydt-Hansen TD, Karpinski M, Ho J, Storsley LJ, Goldberg A, Birk PE, Rush DN, Nickerson PW: Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am J Transplant 12: 1157–1167, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Bachelet T, Couzi L, Lepreux S, Legeret M, Pariscoat G, Guidicelli G, Merville P, Taupin JL: Kidney intragraft donor-specific antibodies as determinant of antibody-mediated lesions and poor graft outcome. Am J Transplant 13: 2855–2864, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Gondos A, Döhler B, Brenner H, Opelz G: Kidney graft survival in Europe and the United States: strikingly different long-term outcomes. Transplantation 95: 267–274, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Letavernier E, Pe’raldi MN, Pariente A, Morelon E, Legendre C: Proteinuria following a switch from calcineurin inhibitors to sirolimus. Transplantation 80: 1198–1203, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Amer H, Lieske JC, Rule AD, Kremers WK, Larson TS, Franco Palacios CR, Stegall MD, Cosio FG: Urine high and low molecular weight proteins one-year post-kidney transplant: relationship to histology and graft survival. Am J Transplant 13: 676–684, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Jong PE, Bakker SJ, Gansevoort RT: What to measure-albuminuria or total proteinuria? Am J Kidney Dis 57: 1–2, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Soler MJ, Riera M, Gutierrez A, Pascual J: New options and perspectives for proteinuria management after kidney transplantation. Transplant Rev (Orlando) 26: 44–52, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Kidney Disease : Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group : KDIGO Clinical practice guideline for glomerulonephritis. Kidney Int Suppl 2: 139–274, 2012 [Google Scholar]
- 31.Levey AS, Greene T, Kusek J, Beck GJ, Group MS: A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol 11: A0828, 2000 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.