Abstract
Dense deposit disease is caused by fluid-phase dysregulation of the alternative complement pathway and frequently deviates from the classic membranoproliferative pattern of injury on light microscopy. Other patterns of injury described for dense deposit disease include mesangioproliferative, acute proliferative/exudative, and crescentic GN. Regardless of the histologic pattern, C3 glomerulopathy, which includes dense deposit disease and C3 GN, is defined by immunofluorescence intensity of C3c two or more orders of magnitude greater than any other immune reactant (on a 0–3 scale). Ultrastructural appearances distinguish dense deposit disease and C3 GN. Focal and segmental necrotizing glomerular lesions with crescents, mimicking a small vessel vasculitis such as ANCA-associated GN, are a very rare manifestation of dense deposit disease. We describe our experience with this unusual histologic presentation and distinct clinical course of dense deposit disease, discuss the pitfalls in diagnosis, examine differential diagnoses, and review the relevant literature.
Keywords: C3 glomerulopathy, dense deposit disease, C3GN, necrotizing crescentic GN
Dense deposit disease (DDD) was classically described as a type of membranoproliferative GN with intramembranous electron dense deposits on ultrastructural examination.1 The membranoproliferative GN pattern is a constellation composed of thickened capillary walls with double contours, glomerular hypercellularity, and increased mesangial matrix. Light microscopy (LM) findings in DDD often deviate from this classic description,2 and the entity is now grouped under the umbrella term C3 glomerulopathy.3
We recently saw a patient with DDD presenting as a focal and segmental necrotizing GN with crescents, reminiscent of a small vessel vasculitis with an unusual clinical course. The diagnostic dilemma posed by this rare histologic pattern and pitfalls in interpretation of the immunofluorescence (IF) are discussed.
Case Report
A 10-year-old boy was admitted with complaints of generalized body swelling for 15 days and hematuria for 10 days. In addition, the child had a history of nonpruritic generalized skin rash, right hip pain (subsided with analgesics), fever (high-grade, intermittent), and vomiting at the onset. There was no preceding history of pharyngitis, sinusitis, pneumonia, or skin infection. On day 3 of admission, he developed oliguria followed by anuria. The patient was normotensive. His serum creatinine rose to 7 mg/dl; thus, hemodialysis was initiated. Urine microscopy showed active sediment with full-field dysmorphic red blood cells. Serum C3 was 85 mg/dl (normal 70–240) and antinuclear antibody, ANCA, and viral markers (serologies for HIV and hepatitis B and C) were negative.
The renal biopsy showed a total of 22 glomeruli, 10 of which showed segmental necrotizing lesions with overlying cellular crescents, fibrin in Bowman’s space, and Bowman’s capsule rupture (Figure 1, A and B). The glomerular capillary lumina were suffused by neutrophils; however, no mesangial infiltration was noted. There was no endocapillary/mesangial proliferation, capillary wall thickening, or splitting in any of the glomeruli. The tubulointerstitium displayed mild interstitial edema and focal acute tubular injury, along with numerous red blood cell casts. No vasculitis of arteries or arterioles or granulomatous inflammation was noted. There was no chronicity.
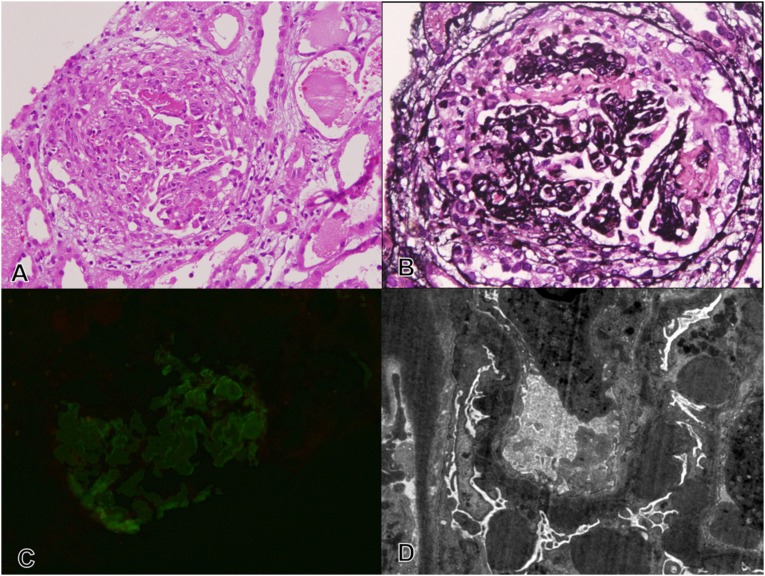
Figure 1.
A case of DDD presenting as a crescentic necrotizing GN with isolated C3c deposition and intramembranous and subepithelial dense deposits. Renal biopsy at first presentation. (A) A representative glomerulus with a segmental necrotizing lesion and overlying cellular crescent (hematoxylin and eosin staining). (B) The discontinuities in the tuft (arrow) are highlighted (silver methenamine staining) with surrounding fibrin deposition. (C) There is focal C3c deposition (1+, 0–3+ scale) on IF (FITC-C3c staining). (D) Ultrastructural examination shows intramembranous dense deposits in the GBM and Bowman’s capsule, with large irregular subepithelial deposits and foot process effacement (transmission EM, uranyl acetate-lead citrate). Original magnification, ×20 in A–C; ×1600 in D.
In view of the concomitant skin rash, the differential diagnoses considered were ANCA-negative pauci-immune crescentic GN and Henoch–Schönlein nephritis (HSN). IF revealed focal chunky capillary wall and mesangial deposition of C3 (1+, 0–3+ scale; Figure 1C) and fibrin (2+, 0–3+ scale) and was negative for all Igs. The IF profile excluded HSN and anti–glomerular basement membrane (GBM) disease. The focal C3 deposition was initially considered nonspecific; thus, on the basis of LM and IF findings, a diagnosis of ANCA-negative pauci-immune crescentic GN was suggested.
The child was given six pulses of methylprednisolone and 10 days of plasma exchange. Pulse cyclophosphamide was started at 500 mg/m2 per dose after the completion of six methyl prednisolone pulses and was continued at the same dose at 3-weekly intervals for a total of five doses. The urine output improved and the patient was discharged with a serum creatinine of 2 mg/dl; he was taking oral prednisolone (2 mg/kg per day), which was tapered over 2 weeks, followed by maintenance with oral mycophenolate mofetil (750 mg/d).
A year later, the child was again admitted with similar complaints of fever and rash, although no focus of infection could be identified. Investigations revealed a rapid rise in serum creatinine to 4.4 mg/dl. The serum C3 level was again low normal (77.7 mg/dl) and antinuclear antibody, ANCA, and viral markers were negative. A repeat renal biopsy was performed with the clinical suspicion of a relapse. The biopsy showed six glomeruli, three of which showed segmental necrotizing lesions with cellular crescents similar to the previous biopsy (Figure 2, A and B) along with acute tubular injury. There was no increase in tubulointerstitial chronicity. The tissue sent for IF contained renal medullary tissue. IF performed after proteinase K digestion of formalin-fixed, paraffin-embedded tissue showed coarse predominantly capillary wall granular deposition of C3 (3+, 0–3+ scale) (Figure 2C) with no staining for Igs.
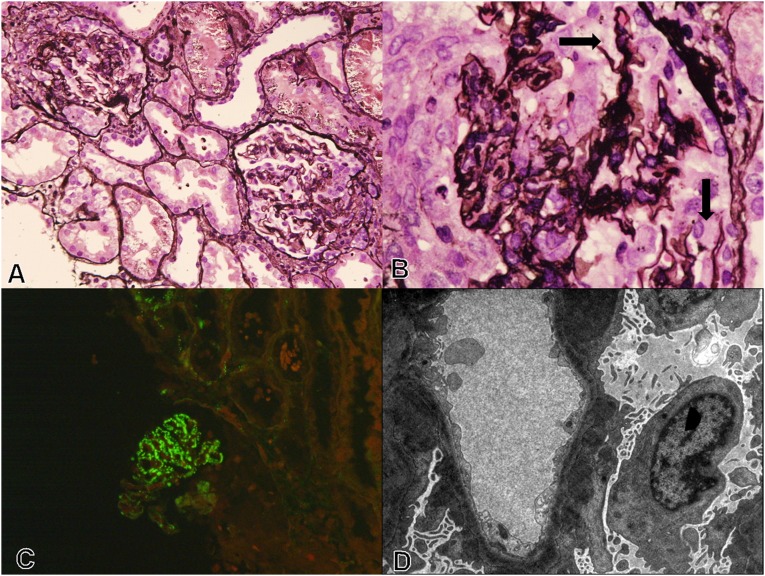
Figure 2.
Renal biopsy at the time of relapse. (A) Representative glomeruli with cellular crescents. Note the pliable capillary walls with absence of remodeling and lack of proliferative activity (silver methenamine staining). (B) Segmental GBM discontinuities are noted in few of the glomeruli (arrow) (silver methenamine staining). (C) IF shows coarse granular C3c deposition (3+, 0–3+ scale) in the capillary walls (FITC-C3c staining). (D) Ultrastructural examination shows intramembranous dense deposits with irregular subepithelial and mesangial deposits, along with secondary foot process effacement (transmission EM, uranyl acetate-lead citrate). Original magnification, ×10 in A and C; ×60 in B; ×1000 in D.
The IF profile of “isolated C3” staining prompted electron microscopy (EM), which to our surprise showed discontinuous intramembranous dense deposits along with numerous irregularly placed subepithelial and mesangial electron dense deposits, classic for DDD. Secondary podocyte damage with foot process effacement was also noted (Figure 2D).
Retrospectively, the biopsy from the first presentation was processed for EM and it too showed massive subepithelial and mesangial electron dense deposits along with intramembranous deposits (Figure 1D). Thus, it was clear that both biopsies showed DDD with histologic appearance of a focal and segmental necrotizing crescentic GN, mimicking a small vessel vasculitis.
The child was again started on pulse methyl prednisolone (six doses) and plasma exchanges (six alternate-day exchanges). He responded to treatment, remained dialysis free, and was discharged on prednisolone (60 mg orally, once daily) and mycophenolate (750 mg). He was also given two doses of rituximab (500 mg/dose) at weekly intervals. The child remains in remission 10 months later on the above regimen, with serum creatinine of 0.5 mg/dl, C3 of 80 mg/dl, and a spot urine protein/creatinine ratio of 0.02. ELISA for anti–factor H antibody was negative.
Discussion
GN with dominant C3 deposition, defined as C3c intensity two or more orders of magnitude greater than any other immune reactant (0–3 scale), has been recently termed C3 glomerulopathy. This new classification recognizes the fact that the glomerular morphology associated with a dominant C3 IF profile is heterogenous (i.e., not always a membranoproliferative pattern of injury) and incorporates the entities of DDD and C3 GN. Correct identification of this group of GN is important because it suggests alternative complement pathway dysfunction and prompts investigation of the complement system.3
The cause of underlying alternative pathway abnormality is also frequently heterogenous and may be genetic or acquired. Genetic abnormalities include homozygous loss of function or heterozygous mutations of complement proteins (C3), complement regulatory proteins (complement factor H [CFH], complement factor I [CFI], membrane cofactor protein, or CFH-related proteins 1–5), or presence of their allelic variants. Particular variants of CFH (exon 2 I62V, IVS 2 −18insTT, exon 9 Y402H, and exon 10 A473A) and of CFHR5 (exon 2P46S, −249T>C and −20T>C) may preferentially be associated with DDD.4 Acquired abnormalities may occur because of the presence of autoantibodies (e.g., C3 nephritic factor, anti–complement factor B, anti-CFI, anti-CFH, or anti-C3b) or due to paraproteins, which may act as autoantibodies to these complement regulatory proteins.
It is important to note that C3 glomerulopathy does not occur in all individuals harboring risk alleles or even mutations, possibly because of underlying control mechanisms. However, an infectious trigger may overwhelm this compensatory mechanism and lead to deposition of alternate complement components in the glomeruli, inciting various glomerular patterns of injury.5
Other differential diagnoses of a C3 dominant IF profile include postinfectious GN and juvenile acute nonproliferative GN, discussed below. A small subset of cases of postinfectious GN (C3 dominant immunostaining profile, subepithelial humps on EM) have an atypical clinical course with persistent hypocomplementemia, hematuria, or proteinuria and progression to ESRD. Sethi et al.6 demonstrated alternate pathway abnormality in the form of CFH mutation, polymorphisms, and/or C3 nephritic factor in 10 such patients.
In our patient, the C3 dominant IF profile with intramembranous dense deposits by EM was consistent with the diagnosis of DDD; however, the histologic presentation was confounding. A focal and segmental necrotizing pattern of GN has conventionally been attributed to a small vessel vasculitis–type injury affecting the GBM. Pathogenetically, this may be associated with ANCA vasculitis, lupus nephritis, or HSN, all of which may present with systemic features of a small vessel vasculitis.
Walker et al.2 identified four distinct histologic patterns of DDD, including mesangial proliferative (45%), membranoproliferative (25%), acute proliferative/exudative (12%) mimicking a postinfectious GN, and crescentic overlying a mesangioproliferative, membranoproliferative, focal, or diffuse endocapillary proliferative GN (18%). Two cases were deemed unclassified with intermediately electron dense transformation of the GBMs and mesangial region. None of the 81 cases in this series showed necrotizing glomerular lesions.
Similar to our case, Fervenza et al.7 described a 23-year-old man who presented with fever, renal dysfunction, hematuria, and low normal serum C3 and was diagnosed with C3 GN with necrotizing crescentic GN without evidence of mesangial proliferation or a membranoproliferative pattern of injury. The authors demonstrated a novel heterozygous factor H mutation (c.3350A>G in short consensus repeat 19) along with risk alleles (presence of two copies of the H402Y allele of CFH as well as two copies of the G102 and one copy of the L314 alleles of C3). This factor H mutation was highly unusual, because mutations in short consensus repeat 19 are usually associated with atypical hemolytic uremic syndrome. The patient responded to intravenous pulse steroids and was in clinical remission on maintenance therapy with low-dose oral steroids 1 year after initial presentation.
As discussed by Fervenza et al.,7 interpretation of IF in such cases is a potential diagnostic pitfall and absence of EM evaluation can result in a misdiagnosis. Even in ANCA-associated GN, various authors have noted up to 2+ staining (on a 0–4+ scale) of Igs or complement components. Thus, the term used for this group of lesions is “pauci-immune,” rather than “nonimmune.”8 The absence of ANCA in our patient, with the initial LM and IF findings suggestive of a pauci-immune crescentic GN, did not raise concerns because approximately 20% of patients with pauci-immune GN are ANCA negative.9
On review of the literature, both of these cases are reminiscent of the novel GN described by West et al.,9 originally in 1978 and updated in 2000, termed juvenile acute nonproliferative GN. In their series of 13 children (age at onset was consistently ≤12 years), the disease was characterized by sudden onset of gross hematuria, rapid renal dysfunction, and slightly depressed or low normal serum C3. No hypertension or nephrotic syndrome was noted. History suggestive of preceding infection including pharyngitis (n=3 cases), raised antistreptolysin titers or culture evidence of streptococcal infection (n=7 cases), or fever (n=11 cases) was seen along with a rash in four cases. On LM, crescents were present in 80% of cases, with exudative response in the underlying tuft, no or minimal mesangial matrix expansion, and occasional foci of fibrinoid necrosis (although less frequent than expected in an ANCA-associated GN). IF showed isolated C3 deposition. EM was characterized by subepithelial deposits on the paramesangial portion of the GBM, along with dense transformation of the GBM in three cases. Treatment with high-dose corticosteroids was highly successful in this series. Relapses associated with infection or fever were noted in five cases and one child had a spontaneous remission. Follow-up biopsies at the time of a relapse had similar histologic findings without progression of tubulointerstitial chronicity. The authors opined that rupture of the paramesangial portion of the GBM where large subepithelial deposits were seen was responsible for florid crescent formation and renal failure. Although this entity seemed similar to DDD on EM, the authors felt that the vastly different clinical course in these patients made a case for a separate designation for this disease.
With our newfound understanding of complement abnormality, we hypothesize that these cases may represent a milder form of C3 glomerulopathy (DDD/C3 GN) not associated with severe complementemia or GBM remodeling. In the event of an infectious trigger, the complement abnormality results in deposition of large subepithelial complement-rich deposits, which weaken the paramesangial GBM, causing rupture, crescent formation (mimicking a small vessel vasculitis), and presentation with renal failure. Treatment with steroids causes resolution of these deposits; however, relapses are frequent with subsequent infections. These patients do well on low-dose maintenance immunosuppression.
Therefore, the significance of this case is that it adds to the histologic spectrum of DDD and provides an important reminder that an isolated C3 IF profile should always prompt further investigation, including EM and complement studies. We also propose that this case may be part of a cohort within C3 glomerulopathies with a distinct clinicopathologic profile. These distinct elements include presentation with renal failure and hematuria, possibly after infectious trigger; absence of hypertension, nephrotic syndrome, or severe hypocomplementemia, with a biopsy typically showing necrotizing and crescentic GN without proliferation or evidence of remodeling; C3 dominant IF; and large subepithelial deposits with or without dense transformation of the GBM. Most importantly, patients with these features have a favorable clinical outcome and lack progression to ESRD.
Recognition of this entity with detailed study of the underlying complement abnormality is important to further our understanding of the spectrum of complement-related glomerular diseases.
Disclosures
None.
Acknowledgments
We acknowledge Dr. Sanjeev Sethi for his valuable opinion and Ram Singh and the EM facility at All India Institute of Medical Sciences for technical support.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Appel GB, Cook HT, Hageman G, Jennette JC, Kashgarian M, Kirschfink M, Lambris JD, Lanning L, Lutz HU, Meri S, Rose NR, Salant DJ, Sethi S, Smith RJ, Smoyer W, Tully HF, Tully SP, Walker P, Welsh M, Würzner R, Zipfel PF: Membranoproliferative glomerulonephritis type II (dense deposit disease): An update. J Am Soc Nephrol 16: 1392–1403, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Walker PD, Ferrario F, Joh K, Bonsib SM: Dense deposit disease is not a membranoproliferative glomerulonephritis. Mod Pathol 20: 605–616, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Pickering MC, D’Agati VD, Nester CM, Smith RJ, Haas M, Appel GB, Alpers CE, Bajema IM, Bedrosian C, Braun M, Doyle M, Fakhouri F, Fervenza FC, Fogo AB, Frémeaux-Bacchi V, Gale DP, Goicoechea de Jorge E, Griffin G, Harris CL, Holers VM, Johnson S, Lavin PJ, Medjeral-Thomas N, Paul Morgan B, Nast CC, Noel LH, Peters DK, Rodríguez de Córdoba S, Servais A, Sethi S, Song WC, Tamburini P, Thurman JM, Zavros M, Cook HT: C3 glomerulopathy: Consensus report. Kidney Int 84: 1079–1089, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abrera-Abeleda MA, Nishimura C, Smith JL, Sethi S, McRae JL, Murphy BF, Silvestri G, Skerka C, Józsi M, Zipfel PF, Hageman GS, Smith RJ: Variations in the complement regulatory genes factor H (CFH) and factor H related 5 (CFHR5) are associated with membranoproliferative glomerulonephritis type II (dense deposit disease). J Med Genet 43: 582–589, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sethi S, Fervenza FC: Membranoproliferative glomerulonephritis--a new look at an old entity. N Engl J Med 366: 1119–1131, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Sethi S, Fervenza FC, Zhang Y, Zand L, Meyer NC, Borsa N, Nasr SH, Smith RJ: Atypical postinfectious glomerulonephritis is associated with abnormalities in the alternative pathway of complement. Kidney Int 83: 293–299, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fervenza FC, Smith RJ, Sethi S: Association of a novel complement factor H mutation with severe crescentic and necrotizing glomerulonephritis. Am J Kidney Dis 60: 126–132, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haas M, Eustace JA: Immune complex deposits in ANCA-associated crescentic glomerulonephritis: A study of 126 cases. Kidney Int 65: 2145–2152, 2004 [DOI] [PubMed] [Google Scholar]
- 9.West CD, McAdams AJ, Witte DP: Acute non-proliferative glomerulitis: A cause of renal failure unique to children. Pediatr Nephrol 14: 786–793, 2000 [DOI] [PubMed] [Google Scholar]