Abstract
Tobacco-free electronic cigarettes (e-cigarettes), which are currently not regulated by the FDA, have become widespread as a “safe” form of smoking. One approach to evaluate the potential toxicity of e-cigarettes and other types of potentially “reduced-harm” cigarettes is to compare their emissions of volatile organic compounds (VOCs), including reactive organic electrophillic compounds such as acrolein, and particulate matter to those of conventional and reference cigarettes. Our newly designed fast-flow tube system enabled us to analyze VOC composition and particle number concentration in real-time by promptly diluting puffs of mainstream smoke obtained from different brands of combustion cigarettes and e-cigarettes. A proton transfer reaction time-of-flight mass spectrometer (PTRMS) was used to analyze real-time cigarette VOC emissions with a 1 s time resolution. Particles were detected with a condensation particle counter (CPC). This technique offers real-time analysis of VOCs and particles in each puff without sample aging and does not require any sample pretreatment or extra handling. Several important determining factors in VOC and particle concentration were investigated: (1) puff frequency; (2) puff number; (3) tar content; (4) filter type. Results indicate that electronic cigarettes are not free from acrolein and acetaldehyde emissions and produce comparable particle number concentrations to those of combustion cigarettes, more specifically to the 1R5F reference cigarette. Unlike conventional cigarettes, which emit different amounts of particles and VOCs each puff, there was no significant puff dependence in the e-cigarette emissions. Charcoal filter cigarettes did not fully prevent the emission of acrolein and other VOCs.
Keywords: cigarette, e-cigarette, acrolein, particle size measurement, mass spectrometry (MS), volatile organic compounds (VOC)
1. INTRODUCTION
Mainstream smoke of conventional cigarettes has been well studied (Piade et al. 2013) and characterized over the past several decades. It is known to cause or contribute to the development of lung, liver, colorectal, prostate, and breast cancer, diseases of nearly all of the organs in the body, and other outcomes such as inflammation, impaired immune system, congenital malformations, and erectile dysfunction, etc. (US Department of Health and Human Services 2014). As for morbidity, more than 20 million premature deaths in the U.S. can be attributed to smoking over a time span of 50 years, 1964–2014 (US Department of Health and Human Services 2014). Since the first Surgeon General report in 1964, conventional cigarettes have been modified in several different ways to design potentially “reduced-harm cigarettes”, in efforts to lessen the harmful health effects (Pankow et al. 2007). For example, the modifications have included the use of porous paper, processed cellulose-acetate filters, charcoal filters, and ventilation holes in filters (Pauly et al. 2009). The most recent development in the search for a potentially reduced harm cigarette has been the electronic cigarette (e-cigarette). Its design differs greatly from any previous cigarette in that it does not contain tobacco; puffing on the device leads to volatilization of nicotine at elevated temperatures but in the absence of any combustion. This mode of cigarette use is often referred to as “vaping” instead of “smoking”. In general, there are two types of e-cigarettes: type A that uses an atomizer and type B that uses a cartomizer (Geiss et al. 2015). Type A consists of three parts: the refill liquid reservoir, an atomizer, and a battery. E-cigarettes of type B have a liquid cartridge with a heating element and a battery as second piece (Brown and Cheng, 2014). The liquid cartridge consists of a mixture of water, propylene glycol and/or vegetable glycerin, and differing amounts of dissolved nicotine and flavoring additives. E-cigarettes are still a new emerging product and they have an impressively large variety of available flavored cartridges (Tierney et al. 2015). A recent review by Chapman and Wu (2014) found that in 2011, adolescents aged 11–19 in grades 6–12 attributed to up to 3.3% of e-cigarette ever-use (meaning tried at least once) in the U.S., and their number increased to 6.8% in 2012.
In most studies, VOC measurements of mainstream smoke or vapor were limited to multi-step chemical analysis and low time resolution. High performance liquid chromatography (HPLC) and gas chromatography mass spectrometry (GCMS) analysis of VOCs and tar composition commonly require sample pretreatment such as extraction and/or derivatization (Geiss et al. 2015; Papousek et al. 2014; Goniewicz et al. 2014; Schripp et al. 2013; Roemer et al. 2012; Intorp et al. 2012; Uchiyama et al. 2010; Ohta et al. 2011). A recent puff-by-puff cigarette study by Sampson et al. (2014) used solid-phase microextraction-GCMS, which required less sample handling. Several other studies have analyzed cigarette smoke on a puff-per-puff basis using a variety of techniques such as two-dimensional characterization with fast GC combined with single-photon ionization mass spectrometry (Eschner et al. 2011), GC ultraviolet-diode array detection (Hatzinikolaou et al. 2006), and thermal desorption multidimensional GC-MS (Takanami et al. 2003). Cigarette VOCs have also been analyzed in high resolution real-time studies including vacuum ultraviolet single-photon ionization TOF MS (Tan et al. 2011), ion-molecule reaction MS (Liu et al. 2010), and tunable diode laser absorption spectroscopy (Harward et al. 2006; Thweatt et al. 2007). To the authors’ best of knowledge, there are currently no real-time VOC e-cigarette studies.
Mainstream smoke particles emitted by numerous types of cigarettes have been analyzed using various techniques such as a differential mobility analyzer and a centrifugal particle mass analyzer (Johnson et al. 2014), an optical aerosol spectrometer (van Dijk et al. 2012), a differential mobility spectrometer (Adam et al. 2009; Alderman and Ingebrethsen, 2011), and an electrical low-pressure impactor (ELPI) (Kane et al. 2010). Although e-cigarette particles have been studied in the last several years, using methods such as spectral transmission and an electrical mobility analyzer (Ingebrethsen et al. 2012), ELPI (Bertholon et al. 2013), a scanning mobility particle sizer (SMPS) (Williams et al. 2013; Zhang et al. 2013), a fast mobility particle sizer (FMPS) (Schripp et al. 2013; Fuoco et al. 2014; Manigrasso et al. 2015), and an aerosol spectrometer and ultrafine particle counter (Geiss et al. 2015), online particle concentration data represent an interesting complement to VOC data.
Tobacco smoke has been found to contain ~4800 substances (Baker 2006). As indicated above, these include highly electrophilic compounds such as acrolein. In contrast to other carcinogens (PAHs, N-nitrosamines, and dioxins) reactive organic electrophilic compounds detected in cigarette smoke (CS) do not require metabolic activation, but can react readily with proteins or bind covalently to nucleic acids (Fujioka and Shibamoto, 2006; Staimer et al. 2012). Moreover, mainstream cigarette smoke contains high concentrations of small particles. These particles are efficiently deposited in the smallest airways of the lung and the condensed organic material (such as nicotine) can diffuse deep into the respiratory tract (Fuoco et al. 2014). While e-cigarettes have not been fully studied, their vapor has also been found to contain several reactive carbonyls such as formaldehyde, acetaldehyde, and acrolein, and to also contain acetone (Grana et al. 2014). There are still many important questions left unanswered about the impact of e-cigarettes. (1) What are the potential risks? (2) Are there potentially harmful chemicals emitted? (3) Are there benefits associated with use (Piade et al. 2013; Grana et al. 2014)? This paper investigated question (2), more broadly, with a comparative study of the VOCs and particles in electronic, potentially reduced-harm, conventional, and Kentucky reference (University of Kentucky, Lexington, KY) cigarette smoke using a real-time fast flow tube setup. Kentucky reference cigarettes are made to be sufficiently homogeneous and to have well established measurement values such that they can be used for calibration as internal lab controls and be easily compared between laboratories (TJI Report 2013). To answer this question, we carried out chamber experiments to find the optimal dilution of cigarette smoke, and did measurements on a number of different cigarette types.
2. EXPERIMENTAL SETUP AND METHODOLOGY
2.1. Chamber Experiments
Although this study focused on real-time analysis of cigarette and e-cigarette emissions using a fast-flow tube, initial measurements were conducted in inflatable Teflon™ FEP coated bags, made in house, in order to optimize the experimental conditions. A large dilution (~103) of the initial cigarette mainstream smoke was necessary to analyze the VOC and particle content with an Ionicon Analytik Proton Transfer Reaction Time-of-Flight Mass Spectrometer (PTRMS) and a Scanning Mobility Particle Sizer (SMPS, TSI 3080 Electrostatic Classifier and TSI 3775 Condensation Particle Counter, CPC). The PTRMS settings for the drift voltage, temperature, and pressure were 600 V, 60 °C, and 2.26 mbar, respectively; the time resolution was 18 s. Two reference cigarettes, 1R5F and 3R4F, and one e-cigarette, e-cigarette-1, (18 mg nicotine/cartridge, propylene glycol, 3.6 V) were analyzed. Reference cigarettes 1R5F and 3R4F have filter ventilations of 70% and 29% (Sampson et al. 2014). To the author’s best of knowledge, filter ventilation data were not available for the conventional name brand cigarettes. Before use, each conventional cigarette was conditioned to a relative humidity of 60 ± 3% with exposure to headspace air above a ~75 wt% aqueous glycerol solution for 48 hrs in a closed container. A puff pump (Brailsford & Co. Inc. TD-2NA(7)), operated at a flow of 1.10 L/min, was connected to a solenoid air control valve (Ingersoll Rand, P251SS-012-D) that was timed by a control board (Teague Enterprises, TE-2) to provide a 2 s puff for a total mainstream smoke puff volume of ~37 ml at a frequency of 4 puffs/min. The 5th puff was sent into a Teflon™ bag prefilled with 150 L of zero air supplied with an FTIR purge gas generator (Parker model 75–62). The bag content was allowed to mix for 15 min before analysis. An additional experiment of a collection of 4 successive puffs under the conditions previously described looked at the particle behavior over time. A single experiment took more than an hour due to bag cleaning via flushing with zero air several times and preparation between samples.
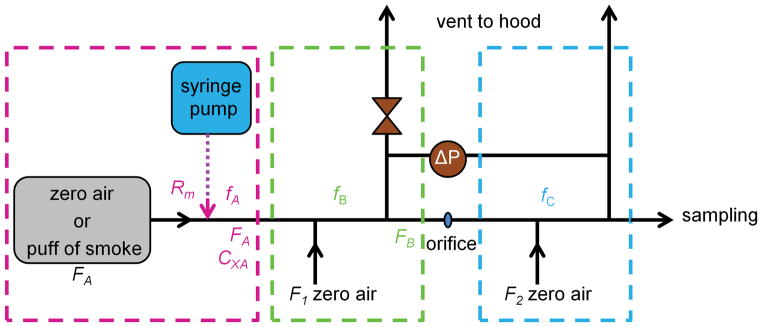
2.2. Fast-Flow Tube
To allow many cigarette samples to be analyzed in triplicate with minimal cleaning demands, we designed a stainless steel flow tube shown in Figure 1. The equations that approximately describe the concentrations of injected VOCs and particles in different sections of the flow tube setup are provided in the online supplemental information. This flow tube was capable of a fast dilution of the cigarette smoke to levels that the PTRMS and CPC instruments can comfortably measure. In short, a puff of mainstream smoke entered the flow tube and was diluted by (1) an addition of zero air flow (labeled F1 in Fig. 1), (2) a passage of a small fraction of the flow through an orifice, and (3) a second addition of zero air flow (labeled F2 in Fig. 1). The diluted mainstream smoke was sampled at the end of the flow tube with the PTRMS and CPC instruments; any excess smoke was vented to a hood. The conventional cigarettes were conditioned before the measurements as described in the previous section. The PTRMS settings differed from the previous by a higher time resolution (1 s) that allowed nearly real-time measurements for a range of puff frequencies. The flow tube was typically operated under standard temperature and pressure conditions with Reynolds numbers ranging from ~200 to ~500 suggesting a laminar flow regime inside the flow tube.
Figure 1.
Diagram of the fast-flow tube setup, where FA was the flow rate of either zero air or a puff of smoke delivered at 1.30 L/min; fA, fB, and fC were the volume fractions of a VOC in each region; Rm was the rate (μg/s) of a VOC entering the flow tube; Cx was the initial concentration (molecules/cm3) of a VOC in the flow tube; F1=F2 were dilution flows of zero air (e-cigarette: 5 L/min, cigarette: 10 L/min); and ΔP (e-cigarette: 52 torr, cigarette: 2.8 torr) was the pressure difference between sections B and C separated by a 1.25 mm orifice; the pressure difference was precisely set with a valve in section B.
2.3. Sampling
Various cigarette types listed in Table 1 were chosen to study the tar and filter type dependence of the VOC and particle emissions. Experiments for each cigarette were done in triplicate at each puff frequency of 1, 2, 3, or 4 puffs/min. The unfiltered-6 cigarette typically extinguished at 1 puff/min and we only provide data for this cigarette at frequencies of 2, 3, and 4 puffs/min. The puff pump operated at a flow of 1.30 L/min with a 2 s puff duration to provide for a total mainstream smoke puff volume of ~43 ml. The flow was set at this level in order to overcome any back pressure from the dilution flow. Although this study was not intended to mimic human smoking behavior, the smoking conditions used in these experiments can be considered to be similar to that of a more intense tobacco cigarette smoker and/or a hybrid e-cigarette smoker with a flow rate used by slow average e-cigarette users, but with half the volume being vaped (Talih et al. 2015). Larger flows may be required for e-cigarette puffing than conventional cigarettes and is variable between brands (Evans and Hoffman, 2014; Behar et al. 2015).
Table 1.
Tar and nicotine content of e-cigarettes and conventional cigarettes. Cigarettes were numbered to differentiate between brands. E-cigarette-1 contained propylene glycol with a voltage of 3.6 V and e-cigarette-2 contained vegetable glycerin with a voltage of 3.7 V. Charcoal-3 and charcoal-4 cigarettes have a charcoal mass loadinge of 37 mg and 50 mg. All cigarettes were contained in hard pack boxes except for the unfiltered-6 cigarette.
| Cigarette Type | Tar (mg/cig) | Nicotine (mg/cig) | Length (mm) | Circumference (mm) |
|---|---|---|---|---|
| e-cigarette-1 | 0 | 0.58 | 115e | 29e |
| e-cigarette-2 | 0 | 0.54 | 87e | 27e |
| 1R5F*a | 1.67 | 0.16 | 84 | 25 |
| 3R4F*a | 9.40 | 0.73 | 84 | 25 |
| charcoal-3*b | 8.00 | 0.70 | 84 | 25 |
| charcoal-4*c | 10.0 | 0.90 | 84 | 25 |
| menthol light-5*c | 6.00 | 0.50 | 84 | 25 |
| light-6*d | 10.0 | 0.80 | 84 | 25 |
| original-5*c | 12.0 | 0.80 | 84 | 25 |
| original-6*d | 16.0 | 1.20 | 84 | 25 |
| unfiltered-6d | 25.0 | 1.70 | 84 | 25 |
Denotes the presence of a cellulose acetate filter.
Obtained from the University of Kentucky (2015); tar and nicotine content was measured by FTC method.
International Organization for Standards (ISO) tar and nicotine yields from the Government of the Hong Kong Special Administrative Region (2014).
Tar and nicotine content obtained from advertisements.
Federal Trade Commission (FTC) yields from the Federal Trade Commission (1998).
Measured in lab.
The conventional cigarettes and e-cigarette, e-cigarette-2 (16 mg nicotine/cartridge, propylene glycol, 3.7 V), had different dilution flows of zero air (F1=F2) and pressure drops (ΔP) in the flow tube to make the measured signal consistent with dynamic ranges of the PTRMS and CPC instruments. The conventional cigarettes and e-cigarette were diluted by a factor of (~103) and (~102), respectively, with the exact dilution factor determined from a calibration. The conventional cigarettes (F1=10 L/min, ΔP=2.8 torr) were calculated to be diluted 24 times more than the e-cigarette (F1=5 L/min and ΔP=52 torr) by comparing the PTRMS signals between the two flow settings (with the linearity of PTRMS verified in a separate experiment). A conventional cigarette was lit upon the first puff whereas the e-cigarette would generate smoke only during the puffing mechanism. The battery of the e-cigarette was fully charged before each puff frequency experiment. A new cartridge was used for each different set (1, 2, 3, or 4 puffs/min) of puff frequency experiments. A separate experiment that looked at the puff number dependence of VOC and particle emissions in an e-cigarette was performed. The battery was recharged 4 times throughout the experiment. The variation in the VOC and particle emissions in the e-cigarette from sampling at different puff numbers of the cartridge lifetime were included in the values’ uncertainties as the triplicate experiments were performed in both increasing and decreasing order of puff frequency.
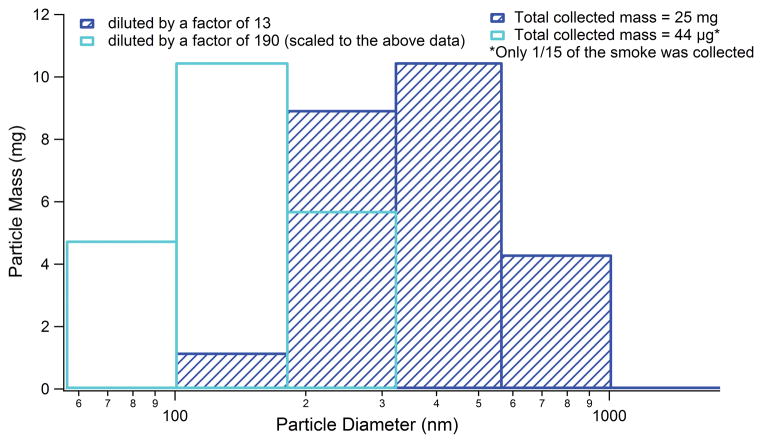
In a separate set of experiments particles of e-cigarette-2 were gravimetrically analyzed by collection on clean foil substrates using a multi-orifice uniform-deposit impactor (MOUDI, MSP model 110-R) sampling at 30 SLM under two different dilution settings: (1) dilution by a factor of 13 with all the smoke being sent in the MOUDI; (2) dilution by a factor of 190 similar to the fast flow tube, but with a fraction of the smoke being sent in the MOUDI.
2.4. Emissions
The PTRMS data were analyzed for the largest changes in the m/z peak intensities that represented a protonated parent species, [M+H]+, between a puff of mainstream smoke and background. From there, select m/z peaks that were considered reasonably free from any influence of possible fragmentation of larger VOCs (Buhr et al. 2002) were chosen for calibration experiments. In order to calibrate the PTRMS, a syringe pump was loaded with a 50 μL syringe filled with acetaldehyde (>95 %), acetone, acetonitrile, acrolein (>95 %), or methanol and delivered at varying rates on a μL/hr scale. All chemicals were purchased from Sigma-Aldrich. Calibration plots were created for PTRMS parent m/z signals as a function of the mass delivery rate (see equations in the supporting information section), Rm(μg/s), for methanol (m/z 33), acetonitrile (m/z 42), acetaldehyde (m/z 45), acrolein (m/z 57), and acetone (m/z 59). As the PTRMS cannot distinguish structural isomers, the m/z 59 peak represents the combined acetone and propanal signal, where the former has the larger contribution in conventional cigarettes (Chen and Moldoveanu, 2003; de Gouw et al. 2003; Uchiyama et al. 2013). An example of mass spectra near m/z 45 and m/z and 57 is shown in Figure S1. The PTRMS signal for the protonated carbon dioxide peak ([CO2+H]+ 44.998 m/z) was not detected in this study, as its proton affinity is too low relative to that of water and major VOCs of the cigarette smoke. However, if this peak was present, it would still be well separated from the acetaldehyde peak (m/z 45.034). Similarly, the acrolein peak (m/z 57.034) was well separated from that of the butenes peak (57.070 m/z). The flow that passed through the orifice (labeled FB in the equations in the supporting information section) was unknown and following the equations that describe the flow tube the dilution factor, DF, was necessary to determine the particle concentration before dilution. A separate acetone calibration was performed in a Teflon™ bag which involved flowing zero air past an injection port where acetone was added. This calibration was applied to an acetone syringe pump experiment to get the diluted fraction of acetone at the end of the flow tube, fc, to calculate DF, in equation 6 (see equations in the supporting information section), where the initial fraction, fA, of acetone was calculated using equations S1–S4.
The e-cigarette data were compared to the conventional cigarette data by taking into account the difference in dilutions in order to express all measurements in easily interpretable units of “overall amount emitted per puff”. The PTRMS raw data were converted to a mass delivery rate, summed over the 2 s puff, and dilution-corrected to give the amount (μg) of each VOC of interest per puff. The CPC data were dilution-corrected to give particle concentration, #/cm3 in the puff and that was further multiplied by the puff volume to give the total amount of particles per puff. The lifetime of the conventional cigarettes was assumed to be 9 puffs in order to compare total VOC and particle emissions between each type of cigarette. We wrote a MATLAB code that would take in extracted PTRMS VOC signal files and calculate areas of individual puffs for each sample.
3. RESULTS AND DISCUSSION
3.1. Chamber Experiments
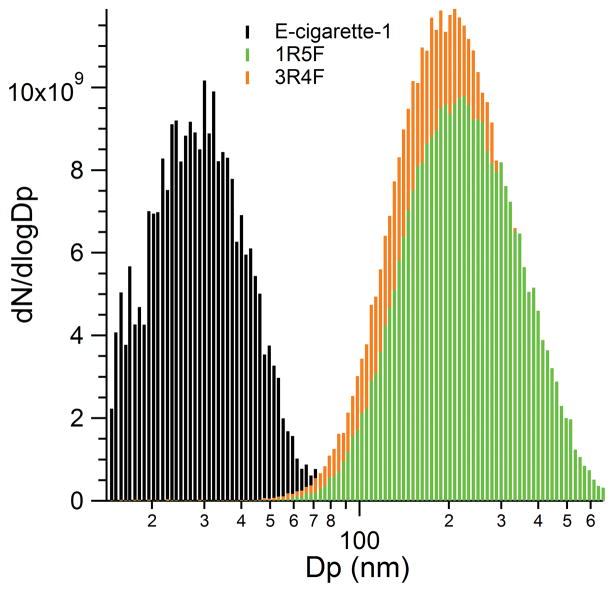
A representative plot of the particle size distributions of the diluted 5th puff of cigarettes e-cigarette-1, 1R5F, and 3R4F is shown in Figure 2. The dilution corrected particle number concentrations (also normalized by the total particle number concentrations measured through the CPC) in the puffs for the e-cigarette-1, 1R5F, and 3R4F samples were 4.0·109, 4.8·109, and 5.7·109 #/cm3, respectively. Conventional cigarettes have particle diameters ranging from 140–340 nm and number concentrations on the order of 109 #/cm3 (Bernstein 2004; Adam et al. 2009; Kane et al. 2010; Alderman and Ingebrethsen, 2011; Johnson al. 2014). E-cigarette particle number concentrations have been found to be of the same order of magnitude (Ingebrethsen et al. 2012; Fuoco et al. 2014; Geiss et al. 2015; Manigrasso et al. 2015). The small particle diameter, 30 nm, of e-cigarette-1 was most likely due to the high dilution of the smoke where most of the water and volatile components have evaporated before sampling (Schripp et al. 2013; Geiss et al. 2015). Ingebrethsen et al. (2012) found particle diameters with an electrical mobility analyzer of 2 s puffs of two cartomizer electronic cigarettes to be of diameters 14 nm and 21 nm, but the same samples characterized with a spectral extinction approach were found to have diameters of 300 nm and 240 nm, respectively. Therefore, it was reasonable to expect that the e-cigarette particle diameters should have significantly decreased upon dilution. The e-cigarette-1 particles of this study that were diluted by a factor of 103 and measured with the SMPS instrument had similar particle size diameters as the e-cigarette particles in the study by Ingebrethsen et al. (2012) that relied on an electrical mobility analyzer and also diluted the puffs by a factor of 103.
Figure 2.
Particle size distribution observed after injecting a single puff in a Teflon™ chamber filled with zero air. This data set was normalized to the total particle number concentrations data measured directly with the CPC and was then multiplied by the dilution factor.
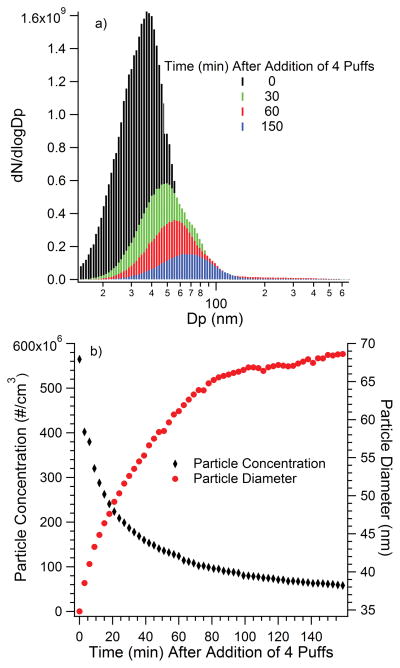
A bimodal distribution of e-cigarette particles from a single 4.3 s puff centered around 50 nm and 250 nm (estimated from their Figure 4B) was observed by Williams et al. (2013) using an SMPS. Schripp et al. (2013) also saw a bimodal distribution at particle diameters of 30 nm and 100 nm for a 3 s puff of a tank system e-cigarette. Much larger particle diameters, 600 nm from a cartridge without nicotine and 650 nm from a cartridge with nicotine, were found by Bertholon et al. (2013) using an ELPI that analyzed ten 2s successive puffs of a cartomizer e-cigarette. A study by Zhang et al. (2013) on a cartomizer e-cigarette found that for a single puff the particle diameters were 117 nm and 180 nm for cartridges with propylene glycol and vegetable glycerin. When the e-cigarette was sampled at a steady state such that the concentrated aerosol aged via condensation of vapors and coagulation, the particles size distribution was found to be bimodal; a small peak with a diameter near that of a single puff, and a larger peak more than twice the diameter of the smaller one was observed. This steady state aging may also explain the larger diameter particles observed by Bertholon et al. (2013) since 10 puffs were combined. Fuoco et al. (2014) found that the aerosol from a 2s puff of a tank system e-cigarette with varying nicotine and flavoring content had particle diameters of 120–165 nm; a smaller mode of 10 nm was only seen with an FMPS and was considered to be an artifact. Particle diameters of 107–165 nm for a variety of e-cigarette cartridges of differing nicotine levels and flavorings was found by Manigrasso et al. (2015) The absence of a larger mean particle diameter in the present e-cigarette-1 measurements may either be from evaporation or wall loss as the aerosol aged with time for 15 min before analysis. Geiss et al. (2015) found that particles larger than 300 nm would immediately drop in number concentration after puffing, owing to their higher vapor pressure. The particle size distribution and concentration for the combined 4 puff experiment is shown in Figure 3a,b. The particle diameter was twice as large as the single puff diameter as the smoke reached a steady state, a behavior similar as that seen by Zhang et al. (2013), although the particles were not as large and were not bimodal.
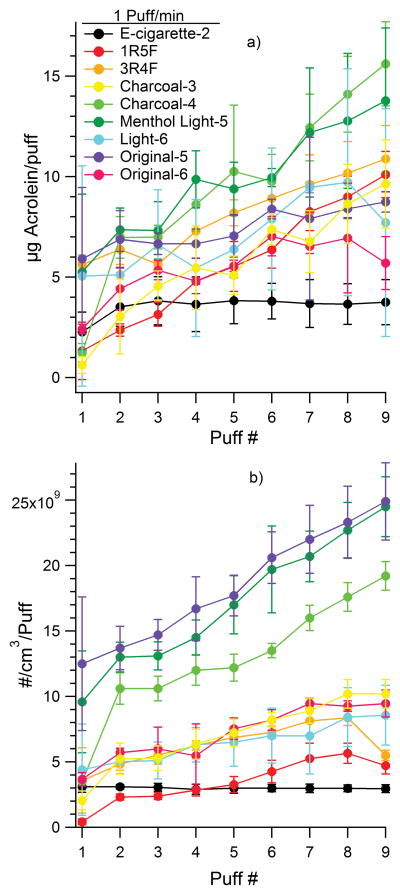
Figure 4.
Amount of acrolein (a) and particles (b) per puff for a puff frequency of 1 puff/min measured in fast flow tube experiments.
Figure 3.
Particle size distribution (a), particle concentration, and particle diameter (b), of e-cigarette-1 observed after injecting 4 successive puffs in a Teflon™ chamber filled with zero air. The data were corrected for dilution to reflect the concentrations in the puff volume.
The acrolein data from the PTRMS chamber experiments are listed in Table 2. Thweatt et al. (2007) analyzed 1R5F cigarettes and found that the 5th puff contained 1.90 μg of acrolein (estimated from their Figure 6) and that of the 1R5F cigarette was 15 μg (also observed by Uchiyama et al. (2013)) where the lifetime of the cigarette was 9 puffs (this was used to scale the 5th puff data in this study to per cigarette quantities). The values in our chamber study agree within uncertainty with previous literature values. Both studies by Roemer et al. (2012) and Uchiyama et al. (2013) found acrolein values for a 3R4F puff to be 56 μg/cigarette, whereas the acrolein content found in the chamber study was about half that amount (27 μg/cigarette), but the flow tube experiment result was similar (66 μg/cigarette). Although acrolein was not detected in e-cigarettes by Kosmider et al. (2014), it was mentioned that it may be a lower bound due to experimental limitations. Both Goniewicz et al. (2014) (upper limit value scaled down from the amount/150 puffs) and Tayyarah and Long (2014) found ~0.2 μg/puff of acrolein in e-cigarettes studied similar to that observed in our chamber study, but Geiss et al. (2015) found smaller amounts ranging from 0.5–13.5 ng/puff.
Table 2.
Emitted masses of acrolein measured in chamber experiments.
| Cigarette Type | acrolein (μg/5th puff) | * acrolein (μg/cigarette) |
|---|---|---|
| 1R5F | 2.43 ± 0.56 | 21.87 ± 5.04 |
| 3R4F | 2.99 ± 1.13 | 26.91 ± 10.17 |
| e-cigarette-1 | 0.290 ± 0.018 | 2.61 ± 0.16 |
values based on assuming a cigarette lifetime of 9 puffs
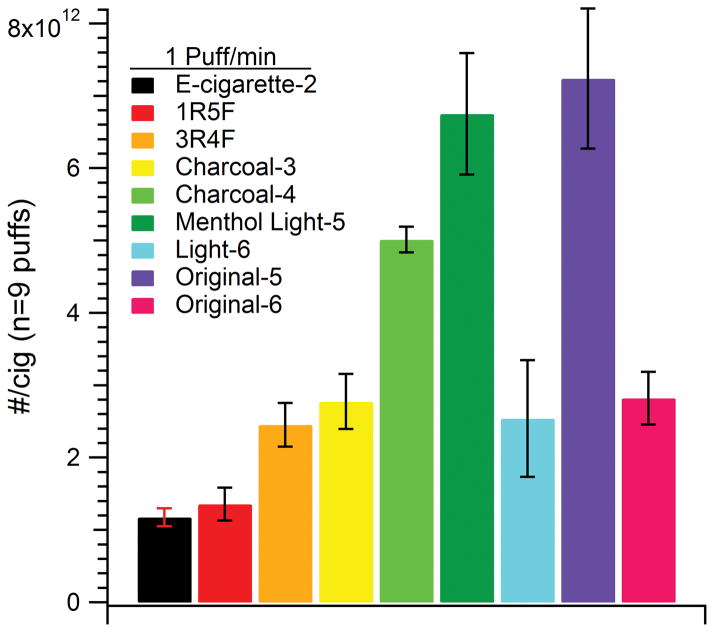
Figure 6.
The total number of particles emitted per puff by e-cigarettes and conventional cigarettes for 1 puff/min frequency. The values were based on assuming a cigarette lifetime of 9 puffs.
3.2. Real-Time Fast-Flow Tube Data
An example PTRMS acrolein time profile for 1R5F puffing at 1 puff/min frequency is shown in Figure S2 and a corresponding time profile of particle concentration is shown in Figure S3. Frequencies other than 1 puff/min for e-cigarette-2 did not allow enough time for the PTRMS signals to reach baseline before the next puff; an example of this convolution of peaks for acrolein is seen in Figure S4 (the particle time profile did not exhibit this problem as can be seen in Figure S5). The amount of VOCs and particle concentration increases with puff number for conventional cigarettes tested here, but not for the e-cigarette, e-cigarette-2, which had no puff number dependence. Kane et al. (2010) also observed an increase in particle concentration with puff number in several Kentucky reference cigarettes. A time profile of acrolein and particle concentration for all samples is seen in Figure 4a,b. There was no quantifiable difference in the total amount of VOCs in the cigarettes for different puff frequencies as can be seen in Figure S6. The main contributing factor to the uncertainties in this study was from the variability between cigarettes of the same brand and type. The particle counts of the cigarettes have no significant puff frequency dependence, except for menthol light-5 and original-5 where the 1 puff/min frequency had somewhat larger particle emissions than the other frequencies as seen in Figure S7.
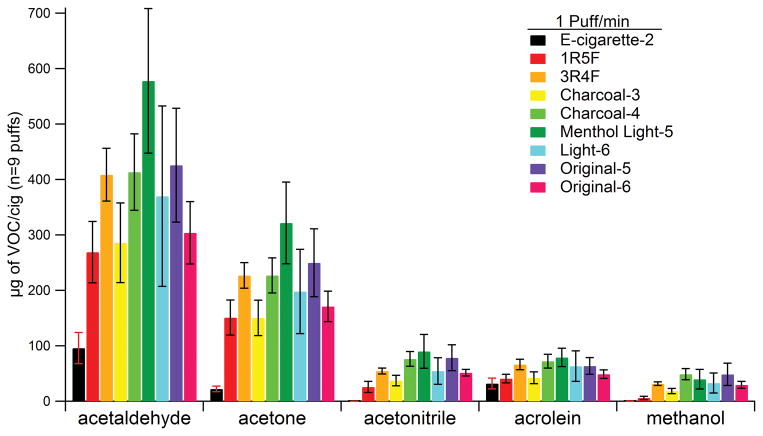
Acetaldehyde, acetone, and acrolein can form from thermal decomposition of sugars, cellulose, pectin, triglycerides, and glycerol (Rodgman and Perfetti, 2009; Piade et al. 2013). Acetonitrile may be formed by nitrogen sources that form ammonia as an intermediate, such as tobacco pigments and proteins. Tobacco leaves can produce methanol from cell signaling, but it can also be formed from other pyrolysis processes of pectin during smoking. The amounts of acetaldehyde, acetone, acetonitrile, acrolein, and methanol for samples are shown in Figure 5 (values listed in Table 3) for the puff frequency of 1 puff/min. Similar data for puff frequencies of 2, 3, and 4 puffs/min are seen in Figure S8–S10 and Table S1–S3. Of the selected VOCs in the cigarettes, acetaldehyde and acetone were the most abundant. The reference cigarettes had ~3 times more acrolein in the flow tube experiments than in the chamber experiments and they agreed with previous literature (Uchiyama et al. 2013; Intorp et al. 2012; Roemer et al. 2012; Thweatt et al. 2007) for acetaldehyde, but the 1R5F values for acetone and acrolein were higher, whereas the corresponding 3R4F values were similar. E-cigarette-2 in the flow tube experiments had ~10 times more acrolein than e-cigarette-1 measured in the chamber experiments. E-cigarette-1 and e-cigarette-2 and their cartridges were from different brands which might have contributed to this difference. Another possible contributor to this difference may include acrolein loss to the Teflon™ walls in the chamber experiments as carbonyl groups and double bonds increase a compound’s affinity to Teflon™ walls (Matsunaga and Ziemann, 2010).
Figure 5.
Amount of selected VOCs in an e-cigarette and conventional cigarettes for a puff frequency of 1 puff/min in fast flow tube experiments. The values were based on assuming a cigarette lifetime of 9 puffs.
Table 3.
Amount (μg in 9 puffs) of selected VOCs emitted by an e-cigarette and conventional cigarettes for a puff frequency of 1 puff/min in fast flow tube experiments. Numbers in parentheses represent standard deviations for n=3 samples.
| VOC | Acetaldehyde | Acetone | Acetonitrile | Acrolein | Methanol |
|---|---|---|---|---|---|
| e-cigarette-2 | 95.9 (28.3) | 22.0 (5.0) | 8.85 (2.14)·10−2 | 32.0 (9.9) | 0.292 (0.025) |
| 1R5F | 269 (55) | 151 (32) | 25.7 (10.1) | 40.9 (7.8) | 5.80 (2.84) |
| 3R4F | 409 (48) | 227 (23) | 54.6 (5.5) | 66.4 (9.4) | 31.7 (3.3) |
| charcoal-3 | 413 (69) | 227 (32) | 76.2 (13.4) | 72.3 (12.5) | 48.9 (10.0) |
| charcoal-4 | 286 (72) | 150 (32) | 37.2 (9.3) | 42.3 (10.8) | 18.4 (4.7) |
| menthol light-5 | 578 (130) | 322 (74) | 90.0 (30.6) | 78.9 (16.4) | 39.6 (17.7) |
| light-6 | 370 (163) | 198 (76) | 54.5 (24.0) | 63.4 (27.6) | 32.8 (18.1) |
| original-5 | 426 (103) | 250 (61) | 78.3 (23.5) | 63.6 (15.2) | 48.5 (20.2) |
| original-6 | 304 (56) | 171 (28) | 51.6 (5.9) | 48.8 (7.6) | 29.6 (6.2) |
Previous studies have found that almost all constituents in cigarette smoke have a positive correlation to tar content (Gregg et al. 2004). In this study e-cigarette-2, 1R5F, 3R4F, and original-5 follow the trend of increasing VOC emissions with increasing tar content. The charcoal cigarettes do not align with this trend, as the addition of a charcoal filter can decrease the amount of VOCs in smoke (Petraru et al. 2013). The charcoal-3 cigarette appeared to filter VOCs more efficiently than charcoal-4, which had 30% less charcoal loading. This can be seen by comparing VOC emissions of charcoal-3 and the 1R5F cigarette which has less than 25% the tar content of charcoal-3; VOC emissions were similar even though the tar content was different. The acetonitrile values also agreed with literature (Hoffmann et al. 2001; Purkis et al. 2014). There was no significant difference in the VOC emissions of charcoal-4 and the 3R4F cigarette which had similar tar content. The VOC content for brand 5 (menthol light-5 and original-5) and brand 6 (light-6 and original-6) cigarettes were more variable, but on average showed the opposite trend in that the lowest tar containing cigarette, menthol light-5, had the largest VOC emissions. Greg et al. (2004) observed that filter ventilation had a greater correlation to tar content than filter type. The cigarettes that deviated from the positive tar/VOC correlation other than the previously explained charcoal cigarettes were those that all had cellulose acetate filters. The variability in the VOC correlation to tar content was most likely due to filter ventilation which can have a larger degree in the reduction of VOCs than particles (Adam et al. 2010), although we point out again that filter ventilation data was not available for the conventional cigarettes in this study, to the best of the authors’ knowledge.
Although it was not possible to run particle size distributions and mass concentration measurements of cigarette smoke with the SMPS under experimental conditions of the flow tube, particle number concentrations were analyzed. For the 1 puff/min data, e-cigarette-2 was more similar to particle counts than VOC emissions of conventional cigarettes, especially the 1R5F cigarette. After these, in increasing order of particle count, were 3R4F, light-6, charcoal-3, original-6, charcoal-4, menthol light-5, and then original-5 cigarettes. The relative ratios of VOCs between cigarettes that changed for particle counts between cigarettes include e-cigarette-2, charcoal-3, charcoal-4, original-5, light-6 which have all appeared to increase except for that of the latter which decreased relative to the other cigarettes. One cannot expect the relative ratios of charcoal cigarette VOCs to other cigarettes to be the same for particle counts, as charcoal filters mainly reduce VOC levels. It was surprising to see that the largest tar containing cigarettes, unfiltered-6 and original-6, did not have the largest particle counts. One cannot further evaluate particle count results with the tar content in mind without further mass information of particles of cigarettes in this study.
In conventional cigarettes, the pyrolytic generation of acrolein from glycerol would contribute 30% more by weight than just bulk tobacco which contributes 5% to the total acrolein (Piade et al. 2013). Although e-cigarettes don’t involve combustion, cartridge solutions containing mainly vegetable glycerin or propylene glycol may be oxidized electrochemistry. Ohta et al. (2011) found that carbonyls increased at a battery output over 3V and Kosmider et al. (2014) saw an increase in carbonyls as the voltage increased from 3.2–4.8 V. These products of vegetable glycerin are seen from e-cigarette-2 in Figure 5.
The particle count observed at 1 puff/min rate is seen in Figure 6. The particle emissions were on the same order of magnitude for all samples, with least particle emitting samples being 1R5F and e-cigarette-2. We note that CPC data just provided total particle counts with no size information. To understand the extent of particle evaporation and resulting size perturbations of e-cigarette-2 under settings close to those of the fast flow tube, gravimetric analysis via MOUDI impaction at two different dilutions were compared (see Figure 7). The least diluted and aged e-cigarette-2 particles were centered at about ~350 nm, but when diluted to the same extent as the fast flow tube experiments, the center diameter shifted to ~150 nm. Although the particle sizes decreased, they were still larger than those observed in the chamber experiments (Figure 2–3). This particle shrinkage may be due to evaporation of water and other volatile components with dilution of the e-cigarette emission, as mentioned previously. To reflect this particle evaporation and size change of e-cigarette-2 particles upon dilution, the VOC content measured in this study should be taken as the total amount of VOCs for this specific dilution.
Figure 7.
Gravimetrically determined particle size distributions of e-cigarette-2 at two different dilutions: 1) dilution by 13 and 2) dilution by 190. The latter was close to the dilution used in the fast flow tube experiments.
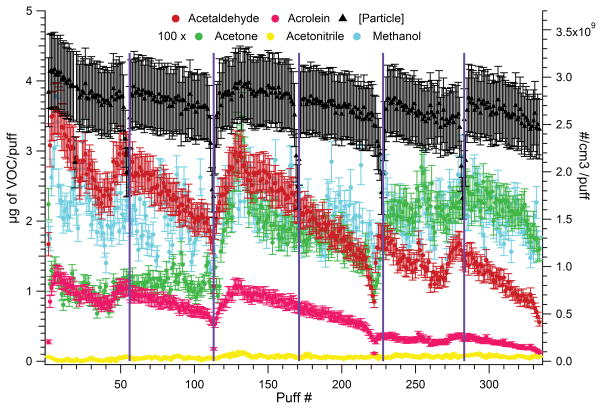
E-cigarette Emissions in Continuous Mode
An e-cigarette was operated until the entire cartridge was consumed, with VOC and particle concentration being recorded as a function of puff number. The measurement taken during consumption (about 250 puffs) of a single e-cigarette-2 cartridge showed that volatiles were not emitted with a consistent delivery rate as seen in Figure 8. The battery got depleted faster than the cartridge was consumed, and had to be recharged several times during the experiment (at points indicated by lines in Figure 8). The VOCs emissions seemed to generally decrease as either the battery or the cartridge got depleted, but in some instances, after initially decreasing, an increase occurred near the battery depletion. Acetaldehyde and acrolein had the largest decrease in delivery over battery depletion and the cartridge lifetime. Acetone delivery was quite variable and reached its highest concentration near the 130th puff. Methanol did not show a trend with the puff number and acetonitrile was not present. The particle concentration did not decrease within the measurement uncertainty.
Figure 8.
VOC and particle content of e-cigarette-2 as a function of puff number during continuous use with a single cartridge (the battery was fully recharged at the beginning of vaping and at each interval (indicated by a line).
4. CONCLUSIONS
We developed a fast-flow diluter for real-time observations of cigarette puffs. A cigarette injected the puff into the diluter and real-time sampling instruments were attached to the setup to perform smoke analysis without requiring pretreatment or extra sample handling. The e-cigarette particle emissions were similar to the low tar 1R5F reference cigarette and on the same order of magnitude as the rest of the conventional cigarettes. Acetaldehyde, acrolein, and acetone were found in the e-cigarette studied, supporting the evidence of oxidation of vegetable glycerin during vaping. Between different brands, flavoring, nicotine content, and battery voltage, e-cigarette emissions were highly variable which made it difficult to generalize their possible health effects. The difference in the increased particle volatility of e-cigarettes from cigarettes required similar dilution and analysis methods between different laboratory studies to allow faithful comparison. Although a limited number of substances were measured, this study suggests that e-cigarettes generate potentially harmful VOCs and sufficiently high particle number concentrations, hence further studies are warranted to evaluate the toxicological effects of e-cigarette emissions in comparison to conventional combustion cigarettes.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number R21CA164540 and the Family Smoking Prevention and Tobacco Control Act. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration. SAE acknowledges support from the National Science Foundation grant CHE-0909227.
References
- Adam T, McAughey J, McGrath C, Mocker C, Zimmermann R. Simultaneous on-line size and chemical analysis of gas phase and particulate phase of cigarette mainstream smoke. Anal Bioanal Chem. 2009;394:1193–1203. doi: 10.1007/s00216-009-2784-y. [DOI] [PubMed] [Google Scholar]
- Adam T, McAughey J, Mocker C, McGrath C, Zimmerman R. Influence of filter ventilation on the chemical composition of cigarette mainstream smoke. Anal Chim Acta. 2010;657:36–44. doi: 10.1016/j.aca.2009.10.015. [DOI] [PubMed] [Google Scholar]
- Alderman SL, Ingebrethsen BJ. Characterization of Mainstream Cigarette Smoke Particle Size Distributions from Commercial Cigarettes Using a DMS500 Fast Particulate Spectrometer and Smoking Cycle Simulator. Aerosol Sci Technol. 2011;45:1409–1421. [Google Scholar]
- Baker RR. Smoke generation inside a burning cigarette: modifying combustion to develop cigarettes that may be less hazardous to health. Prog Energy Combust Sci. 2006;32:373–385. [Google Scholar]
- Behar RZ, Hua M, Talbot P. Puffing topography and nicotine intake of electronic cigarette users. PLoS One. 2015;10:e0117222. doi: 10.1371/journal.pone.0117222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein DM. A review of the influence of particle size, puff volume, and inhalation pattern on the deposition of cigarette smoke particles in the respiratory tract. Inhalation Toxicol. 2004;16:675–689. doi: 10.1080/08958370490476587. [DOI] [PubMed] [Google Scholar]
- Bertholon JF, Becquemin MH, Roy M, Roy F, Ledur D, Annesi MI, Dautzenberg B. Comparison of the aerosol produced by electronic cigarettes with conventional cigarettes and the shisha. Rev Mal Respir. 2013;30:752–7. doi: 10.1016/j.rmr.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Brown CJ, Cheng JM. Electronic cigarettes: product characterisation and design considerations. Tob Control. 2014;23(Suppl 2):ii4–10. doi: 10.1136/tobaccocontrol-2013-051476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhr K, van Ruth S, Delahunty C. Analysis of volatile flavor compounds by Proton Transfer Reaction-Mass Spectrometry: fragmentation patterns and discrimination between isobaric and isomeric compounds. Int J Mass Spectrom. 2002;221:1–7. [Google Scholar]
- Chapman Carroll SL, Wu LT. E-cigarette prevalence and correlates of use among adolescents versus adults: a review and comparison. J Psychiatr Res. 2014;54:43–54. doi: 10.1016/j.jpsychires.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PX, Moldoveanu SC. Mainstream smoke chemical analyses for 2R4F Kentucky reference cigarette. Beitr Tabakforsch Int. 2003;20:448–458. [Google Scholar]
- de Gouw J, Warneke C, Karl T, Eerdekens G, van der Veen C, Fall R. Sensitivity and specificity of atmospheric trace gas detection by proton-transfer-reaction mass spectrometry. Int J Mass Spectrom. 2003;223–224:365–382. [Google Scholar]
- Eschner MS, Selmani I, Groger TM, Zimmermann R. Online Comprehensive Two-Dimensional Characterization of Puff-by-Puff Resolved Cigarette Smoke by Hyphenation of Fast Gas Chromatography to Single-Photon Ionization Time-of-Flight Mass Spectrometry: Quantification of Hazardous Volatile Organic Compounds. Anal Chem. 2011;83:6619–6627. doi: 10.1021/ac201070j. [DOI] [PubMed] [Google Scholar]
- Evans SE, Hoffman AC. Electronic cigarettes: abuse liability, topography and subjective effects. Tob Control. 2014;23(Suppl 2):ii23–9. doi: 10.1136/tobaccocontrol-2013-051489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federal Trade Commission. Tar, nicotine and carbon monoxide of the smoke of 1294 varieties of domestic cigarettes for the year 1998 1998 [Google Scholar]
- Fujioka K, Shibamoto T. Determination of toxic carbonyl compounds in cigarette smoke. Environ Toxicol. 2006;21:47–54. doi: 10.1002/tox.20153. [DOI] [PubMed] [Google Scholar]
- Fuoco FC, Buonanno G, Stabile L, Vigo P. Influential parameters on particle concentration and size distribution in the mainstream of e-cigarettes. Environ Pollut. 2014;184:523–529. doi: 10.1016/j.envpol.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Geiss O, Bianchi I, Barahona F, Barrero-Moreno J. Characterisation of mainstream and passive vapours emitted by selected electronic cigarettes. Int J Hyg Environ Health. 2015;218:169–180. doi: 10.1016/j.ijheh.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, Prokopowicz A, Jablonska-Czapla M, Rosik-Dulewska C, Havel C, Jacob P, 3rd, Benowitz N. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23:133–9. doi: 10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg E, Hill C, Hollywood M, Kearny M, McAdam K, McLaughlin D, Purkis S, Williams M. The UK smoke constituents testing study. Summary of results and comparison with other studies. Beitr Tabakforsch Int. 2004;21:117–138. [Google Scholar]
- Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation. 2014;129:1972–86. doi: 10.1161/CIRCULATIONAHA.114.007667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Government of the Hong Kong Special Administrative Region. Government Laboratory Tar and Nicotine Report. 2014 ( http://www.govtlab.gov.hk/english/pub_tnrpt.htm). (retreived 06.24.15.)
- Harward CN, Thweatt WD, Baren RE, Parrish ME. Determination of molecular line parameters for acrolein (C3H4O) using infrared tunable diode laser absorption spectroscopy. Spectrochim Acta, Part A. 2006;63A:970–980. doi: 10.1016/j.saa.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Hatzinikolaou DG, Lagesson V, Stavridou AJ, Pouli AE, Lagesson-Andrasko L, Stavrides JC. Analysis of the Gas Phase of Cigarette Smoke by Gas Chromatography Coupled with UV-Diode Array Detection. Anal Chem. 2006;78:4509–4516. doi: 10.1021/ac052004y. [DOI] [PubMed] [Google Scholar]
- Hoffmann D, Hoffmann I, El-Bayoumy K. The Less Harmful Cigarette: A Controversial Issue. A Tribute to Ernst L Wynder. Chem Res Toxicol. 2001;14:767–790. doi: 10.1021/tx000260u. [DOI] [PubMed] [Google Scholar]
- Ingebrethsen BJ, Cole SK, Alderman SL. Electronic cigarette aerosol particle size distribution measurements. Inhalation Toxicol. 2012;24:976–984. doi: 10.3109/08958378.2012.744781. [DOI] [PubMed] [Google Scholar]
- Intorp M, Purkis S, Wagstaff W. Determination of carbonyl compounds in cigarette mainstream smoke. The CORESTA 2010 collaborative study and recommended method. Beitr Tabakforsch Int. 2012;25:361–374. [Google Scholar]
- Johnson TJ, Olfert JS, Cabot R, Treacy C, Yurteri CU, Dickens C, McAughey J, Symonds JPR. Steady-state measurement of the effective particle density of cigarette smoke. J Aerosol Sci. 2014;75:9–16. [Google Scholar]
- Kane DB, Asgharian B, Price OT, Rostami A, Oldham MJ. Effect of smoking parameters on the particle size distribution and predicted airway deposition of mainstream cigarette smoke. Inhalation Toxicol. 2010;22:199–209. doi: 10.3109/08958370903161224. [DOI] [PubMed] [Google Scholar]
- Kosmider L, Sobczak A, Fik M, Knysak J, Zaciera M, Kurek J, Goniewicz ML. Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage. Nicotine Tob Res. 2014;16:1319–26. doi: 10.1093/ntr/ntu078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Feng S, van Heemst J, McAdam KG. New insights into the formation of volatile compounds in mainstream cigarette smoke. Anal Bioanal Chem. 2010;396:1817–1830. doi: 10.1007/s00216-010-3457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manigrasso M, Buonanno G, Fuoco FC, Stabile L, Avino P. Aerosol deposition doses in the human respiratory tree of electronic cigarette smokers. Environ Pollut. 2015;196:257–267. doi: 10.1016/j.envpol.2014.10.013. [DOI] [PubMed] [Google Scholar]
- Matsunaga A, Ziemann P. Gas-Wall Partitioning of Organic Compounds in a Teflon Film Chamber and Potential Effects on Reaction Product and Aerosol Yield Measurements. Aerosol Sci Technol. 2010;44:881–892. [Google Scholar]
- Ohta K, Uchiyama S, Inaba Y, Nakagome H, Kunugita N. Determination of Carbonyl Compounds Generated from the Electronic Cigarette Using Coupled Silica Cartridges Impregnated with Hydroquinone and 2,4-Dinitrophenylhydrazine. Bunseki Kagaku. 2011;60:791–797. [Google Scholar]
- Pankow JF, Watanabe KH, Toccalino PL, Luo W, Austin DF. Calculated cancer risks for conventional and potentially reduced exposure product cigarettes. Cancer Epidemiol, Biomarkers Prev. 2007;16:584–592. doi: 10.1158/1055-9965.EPI-06-0762. [DOI] [PubMed] [Google Scholar]
- Papousek R, Pataj Z, Novakova P, Lemr K, Bartak P. Determination of Acrylamide and Acrolein in Smoke from Tobacco and E-Cigarettes. Chromatographia. 2014;77:1145–1151. [Google Scholar]
- Pauly JL, O’Connor RJ, Paszkiewicz GM, Cummings KM, Djordjevic MV, Shields PG. Cigarette Filter-based Assays as Proxies for Toxicant Exposure and Smoking Behavior - A Literature Review. Cancer Epidemiol, Biomarkers Prev. 2009;18:3321–3333. doi: 10.1158/1055-9965.EPI-09-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petraru C, Balalau D, Ilie M, Balalau C. Evaluation of different kind of cigarette filters ability of to retain the toxic compounds of the vapor phase. A comparative graphical study. Farmacia (Bucharest, Rom) 2013;61:736–741. [Google Scholar]
- Piade JJ, Wajrock S, Jaccard G, Janeke G. Formation of mainstream cigarette smoke constituents prioritized by the World Health Organization - Yield patterns observed in market surveys, clustering and inverse correlations. Food Chem Toxicol. 2013;55:329–347. doi: 10.1016/j.fct.2013.01.016. [DOI] [PubMed] [Google Scholar]
- Purkis W, Mueller C, Intorp M, Seidel H. The Influence of Cigarette Designs and Smoking Regimes on Vapour Phase Yields. Beiträge zur Tabakforschung / Contributions to Tobacco Research. 2014;24:34. [Google Scholar]
- Rodgman A, Perfetti TA. The chemical components of tobacco and tobacco smoke. Boca Raton: CRC Press; 2009. pp. 249–250. [Google Scholar]
- Roemer E, Schramke H, Weiler H, Buettner A, Kausche S, Weber S, Berges A, Stueber M, Muench M, Trelles-Sticken E, Pype J, Kohlgrueber K, Voelkel H, Wittke S. Mainstream smoke chemistry and in vitro and in vivo toxicity of the reference cigarettes 3R4F and 2R4F. Beitr Tabakforsch Int. 2012;25:316–335. [Google Scholar]
- Sampson MM, Chambers DM, Pazo DY, Moliere F, Blount BC, Watson CH. Simultaneous Analysis of 22 Volatile Organic Compounds in Cigarette Smoke Using Gas Sampling Bags for High-Throughput Solid-Phase Microextraction. Anal Chem. 2014;86:7088–7095. doi: 10.1021/ac5015518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schripp T, Markewitz D, Uhde E, Salthammer T. Does e-cigarette consumption cause passive vaping? Indoor Air. 2013;23:25–31. doi: 10.1111/j.1600-0668.2012.00792.x. [DOI] [PubMed] [Google Scholar]
- Staimer N, Nguyen TB, Nizkorodov SA, Delfino RJ. Glutathione peroxidase inhibitory assay for electrophilic pollutants in diesel exhaust and tobacco smoke. Anal Bioanal Chem. 2012;403:431–441. doi: 10.1007/s00216-012-5823-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TJI Report. Health & Scientific Affairs. TOBACCO JOURNAL INTERNATIONAL; 2013. Feb, Reference products used in tobacco and smoke analysis. ( http://www2.ca.uky.edu/refcig/Reference%20and%20monitor%20products.pdf) [Google Scholar]
- Takanami Y, Chida M, Hasebe H, Sone Y, Suhara S. Analysis of cigarette smoke by an online thermal desorption system and multidimensional GC-MS. J Chromatogr Sci. 2003;41:317–322. doi: 10.1093/chromsci/41.6.317. [DOI] [PubMed] [Google Scholar]
- Talih S, Balhas Z, El HA, Baalbaki R. Effects of user puff topography, device voltage, and liquid nicotine concentration on electronic cigarette nicotine yield: measurements and model predictions. Nicotine Tob Res. 2015;17:150–7. doi: 10.1093/ntr/ntu174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan GB, Gao W, Huang ZX, Hong Y, Fu Z, Dong JG, Cheng P, Zhou Z. Vacuum ultraviolet single photon ionization time-of-flight mass spectrometer. Fenxi Huaxue. 2011;39:1470–1475. [Google Scholar]
- Tayyarah R, Long GA. Comparison of select analytes in aerosol from e-cigarettes with smoke from conventional cigarettes and with ambient air. Regul Toxicol Pharmacol. 2014;70:704–710. doi: 10.1016/j.yrtph.2014.10.010. [DOI] [PubMed] [Google Scholar]
- Thweatt WD, Harward CN, Parrish ME. Measurement of acrolein and 1,3-butadiene in a single puff of cigarette smoke using lead-salt tunable diode laser infrared spectroscopy. Spectrochim Acta, Part A. 2007;67A:16–24. doi: 10.1016/j.saa.2006.10.034. [DOI] [PubMed] [Google Scholar]
- Tierney PA, Brown JE, Karpinski CD, Luo W, Pankow JF. Flavour chemicals in electronic cigarette fluids. Tob Control. 2015 Apr 15; doi: 10.1136/tobaccocontrol-2014-052175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama S, Tomizawa T, Inaba Y, Kunugita N. Simultaneous determination of volatile organic compounds and carbonyls in mainstream cigarette smoke using a sorbent cartridge followed by two-step elution. J Chromatogr A. 2013;1314:31–37. doi: 10.1016/j.chroma.2013.09.019. [DOI] [PubMed] [Google Scholar]
- Uchiyama S, Inaba Y, Kunugita N. Determination of acrolein and other carbonyls in cigarette smoke using coupled silica cartridges impregnated with hydroquinone and 2,4-dinitrophenylhydrazine. J Chromatogr A. 2010;1217:4383–4388. doi: 10.1016/j.chroma.2010.04.056. [DOI] [PubMed] [Google Scholar]
- University of Kentucky. Reference Cigarette Program. 2013 from ( http://www2.ca.uky.edu/refcig/) (retrieved 06.24.15.)
- US Department of Health and Human Services. The Health Consequences of Smoking–50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [PubMed] [Google Scholar]
- van Dijk WD, Cremers R, Klerx W, Schermer TRJ, Scheepers PTJ. Application of cigarette smoke characterisation based on optical aerosol spectrometry. Dynamics and comparisons with tar values. Curr Anal Chem. 2012;8:344–350. [Google Scholar]
- Williams M, Villarreal A, Bozhilov K, Lin S, Talbot P. Metal and silicate particles including nanoparticles are present in electronic cigarette cartomizer fluid and aerosol. PLoS One. 2013;8:e57987. doi: 10.1371/journal.pone.0057987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Sumner W, Chen DR. In Vitro Particle Size Distributions in Electronic and Conventional Cigarette Aerosols Suggest Comparable Deposition Patterns. Nicotine Tob Res. 2013;15:501–508. doi: 10.1093/ntr/nts165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.