Abstract
RATIONALE
Melatonin modifies physiological and behavioral responses to psychostimulants, with the MT1 and MT2 melatonin receptors specifically implicated in facilitating methamphetamine-induced sensitization in melatonin-proficient mice.
OBJECTIVE
To assess differences in locomotor sensitization after a single dose of methamphetamine in low melatonin-expressing C57BL/6 wild-type and MT1KO mice, and comparing with melatonin-expressing C3H/HeN mice.
METHODS
Mice received a vehicle or methamphetamine (1.2 mg/kg, i.p.) pretreatment (Day 1) during the light (ZT5–9) or dark (ZT 19–21) periods in novel test arenas. Locomotor sensitization was assessed by methamphetamine challenge after an eight-day (Day 9) abstinence. TH protein expression was evaluated by immunofluorescence and Western blot analysis.
RESULTS
Methamphetamine pretreatment induced statistically significant locomotor sensitization upon challenge after eight-day abstinence in C3H and C57 wild-type mice during the light period. The magnitude of sensitization in C57 mice was diminished in the dark period and completely abrogated in MT1 receptor knockout (MT1KO) mice. No differences were observed in tyrosine hydroxylase immunoreactivity in the mesolimbic dopamine system. Additional exposures to the test arenas after methamphetamine pretreatment (Nights 2–6) enhanced sensitization.
CONCLUSIONS
Deletion of the MT1 melatonin receptor abolishes sensitization induced by a single METH pretreatment. The magnitude of sensitization is also altered by time of day and contextual cues. We conclude that the MT1 melatonin receptor is emerging as a novel target of therapeutic intervention for drug abuse disorders.
Keywords: melatonin, MT1 melatonin receptor, methamphetamine, sensitization, C57BL/6 mouse
INTRODUCTION
The MT1 and MT2 melatonin receptors mediate a myriad of physiological actions of the pineal hormone melatonin (Dubocovich et al. 2010). Melatonin is synthesized and released according to a circadian rhythm with peak pineal and plasma levels at night and, as such it is thought to be the principal agent signaling the duration of the dark phase of the 24h day to peripheral tissues (Gillette and Mitchell 2002; Reiter 1991). Melatonin signaling has been implicated in various responses triggered by drugs of abuse. Melatonin attenuates indicators of neurotoxicity in cell culture (Kongsuphol et al. 2009; Suwanjang et al. 2010) and animal model systems (Hirata et al. 1998; Itzhak et al. 1998; Kaewsuk et al. 2009). At the behavioral level, responses to amphetamine and cocaine vary by time of day (Akhisaroglu et al. 2004; Gaytan et al. 1999). Diurnal variations in cocaine-induced locomotor sensitization (Uz et al. 2003) and reward-seeking behavior (Kurtuncu et al. 2004) are lost in melatonin-deficient pinealectomized mice. Further, work in our laboratory shows that genetic deletion of both MT1 and MT2 receptors prevents the development and expression of methamphetamine (METH)-induced locomotor sensitization in melatonin-proficient C3H/HeN mice (Hutchinson et al. 2012). Altogether, these data indicate that melatonin and its receptors play important roles in the modulation of behavioral responses by drugs of abuse, providing a new therapeutic target for drug addiction-related disorders.
Locomotor sensitization is a form of behavioral plasticity in which early exposures to drugs of abuse increase the magnitude of later drug-stimulated locomotor responses (Hirabayashi and Alam 1981; Pierce and Kalivas 1997). This phenomenon has been employed in rodents as a model for studying the neuroadaptations that occur during repeated drug exposures, because drug-stimulated locomotor responses are thought to be mediated by the same central dopaminergic pathways involved with motivation and incentive attribution (Wise and Bozarth 1987). Locomotor sensitization is correlated with neurochemical sensitization of the dopaminergic pathways and with facilitated self-administration in rodent models (Addy et al. 2010; Vezina 2004). Neuroadaptations in the dopaminergic pathway have been proposed to increase the “incentive salience” of the drug and drug-associated contextual cues, which is possibly associated with craving and compulsive drug seeking emblematic of addiction (Pierce and Kalivas 1997; Robinson and Berridge 2008). Studies using this model have revealed modulation of sensitization by genetic alterations (Shen et al. 2010; Takino et al. 2009), time of day (Gaytan et al. 1999; Uz et al. 2002), environmental context (Badiani et al. 1995a) and adjunct drug treatments targeting a variety of receptors, channels and transporters (Chiu et al. 2005; Futamura et al. 2010; Kurokawa et al. 2011).
Reports on drug-induced sensitization have generally relied on experimental models wherein the effect of a drug is tested during and/or after a sequence of multiple intermittent administrations (Stewart and Badiani 1993). There are, however, reports showing statistically significant increases in locomotor sensitization following a single pretreatment with cocaine (Kozanian et al. 2012; Valjent et al. 2010), amphetamine (Paz et al. 2011) and METH (Kozanian et al. 2012; Nikaido et al. 2001). As with sensitization paradigms based on repeated pretreatments, single-pretreatment locomotor sensitization is modified by contextual cues (Valjent et al. 2010) and pharmacological agents (Paz et al. 2011). The use of a single pretreatment is potentially advantageous due to its circumvention of dependence-related phenomena that could confound the interpretation of behavioral assessments (Stewart and Badiani 1993; Valjent et al. 2010). Thus a single psychostimulant treatment is sufficient to induce a state of sensitization that can be modified by experimental conditions and additional drug treatment.
In this study we employed a single METH pretreatment to assess the functional role of the MT1 melatonin receptor in locomotor sensitization expressed by C57BL/6 wild-type and MT1 receptor knockout (MT1KO) mice. C57 mice were chosen for these experiments based on their genetic predisposition for null or greatly reduced pineal melatonin synthesis (Kasahara et al. 2010; Roseboom et al. 1998; von Gall et al. 2000). C57 mice are endowed with mutations that markedly compromise melatonin synthesis: a point mutation/frameshift in the arylalkylamine N-acetyltransferase that converts serotonin to the intermediate N-acetylserotonin (Roseboom et al. 1888), and a truncation of the hydroxyindole-O-methyltransferase, which generates melatonin from N-acetylserotonin (Kasahara et al. 2010). Focusing on these phenomena in a model system that combines a low/null-melatonin milieu with a targeted disruption of the MT1 melatonin receptor allows for the assessment of ligand-independent MT1 receptor function in locomotor sensitization, as this receptor exhibits constitutive activity (Browning et al. 2000; Ersahin et al. 2002; Roka et al. 1999; Soares et al. 2003). This study demonstrated statistically significant MT1 melatonin receptor-dependent locomotor sensitization in METH-pretreated C57 mice during the light period with diminished magnitude during the dark period. We also found a diurnal variation in the magnitude of sensitization in C3H/HeN mice induced by a single METH- pretreatment. The data suggest the MT1 melatonin receptor in the C57 mice as the mediator of the sensitizing properties of METH after a single pretreatment.
MATERIALS AND METHODS
Animals
Male wild-type C3H/HeN (n=42) and C57BL/6 mice having wild-type or MT1KO genotypes (N=118) (Dubocovich et al. 2005) were bred in colonies maintained at the Laboratory Animal Facility of the University at Buffalo. Mice were bred under a 14h:10h light/dark cycle (150 to 200 lux during the daytime at the level of the cage), in a temperature (22 ± 1°C) and humidity-controlled environment with food and water provided ad libitum. Mice were switched to a 12/12 light/dark cycle at 6–12 weeks of age within ventilated and light-tight cabinets 10–14 days prior to experiments. The 12:12 light dark cycle is traditionally used in rodent research paradigms (Faith and Huerkamp 2009), and departures toward longer or shorter day lengths was found to increase neophobic behaviors in freely-exploring C3H mice (Kopp et al. 1999). Genotype was confirmed by PCR analysis of genomic DNA prepared from tail biopsies collected at weaning (3 weeks of age). All animal procedures were performed in accordance with the guidelines set forth by the National Institutes of Health and the Institutional Animal Care and Use Committee of the University at Buffalo.
Drugs
(+)-Methamphetamine hydrochloride was obtained from Sigma. Mice were randomly assigned to groups receiving either vehicle (saline, i.p.) or METH (1.2 mg/kg or 2.4 mg/kg in saline, i.p.) during the pretreatment tests (Day 1). METH (1.2 mg/kg) was administered to all mice during the challenge tests (Days 9, 17 and 27). Saline was injected during habituation trials on Days −3 to −1. METH doses were calculated in terms of the salt form of METH. All treatments were administered via intraperitoneal injections to deliver 0.01 ml solution per gram of mouse weight.
METH-Induced Sensitization Test Apparatus and Protocol
Sensitization tests were conducted in 16 cylindrical (20 cm diameter × 20 cm height) Plexiglas arenas, the shape and size of which were shown to facilitate optimal expression of sensitization (Hirabayashi et al. 1991). To assess sensitization after a single METH pretreatment, mice were first habituated to the arenas in three daily 2h trials wherein three saline injections were administered at the start (−60 min), midpoint (0 min) and end (60 min) of each test. During the pretreatment trial (Day 1), VEH was given at the start (−60 min) and end (60 min) of the trial, with VEH or METH given to half the mice at the midpoint (0 min). We used multiple injections during each test to prevent timing- or cue-based learning associations that could confound data interpretation (Valjent et al. 2010). The mice were minimally disturbed for eight days following the pretreatment test. The challenge test on Day/Night 9 was conducted by subjecting all mice to a sequence of VEH at the test start, METH at the midpoint, and VEH at the test end. Challenge tests on Days 17 and 27 followed the same protocol. Sensitization after repeated pretreatments (Supplemental Figure 1) was assessed as described in Hutchinson et al. (2012). For all locomotor tests, data were acquired by overhead camera imagery and digital video analysis by the LocoScan system (CleverSys, Reston VA). Light phase tests were conducted at Zeitgeber Time (ZT) 5–7 (lights on at ZT 0) with illumination of approximately 50 lux at the floor of the locomotor arena, and dark phase tests were conducted at ZT 19–21 in complete darkness with the aid of night-vision goggles and infrared light for camera imaging.
Tyrosine Hydroxylase Immunofluorescence
Mice were anesthetized with 0.24 ml/kg Avertin (1.4%) and perfused transcardially at 5 mg/ml for 10 min with 0.9% saline followed by 20 min with 4% paraformaldehyde in phosphate-buffered saline (PBS; 0.01M, pH 7.4). Brains were dissected, post-fixed in 4% paraformaldehyde for 4h, and transferred to 30% sucrose cryoprotectant for 2 days. Brains were embedded in Optimal Cutting Temperature compound (Sakura Finetek, Torrance, CA USA), frozen in 2-methylbutane on dry ice, and stored at −80°C. Sections (30 µm) containing caudate-putamen (CPu) and nucleus accumbens (NAc) were taken from bregma 1.70 mm to 0.62 mm and every sixth section (250 µm apart) was selected for analysis. Ventral tegmental area (VTA) sections were taken from bregma −2.92 mm to −3.88 mm and every fourth section was selected for analysis.
Sections were washed and incubated with 1:1000 mouse anti-tyrosine hydroxylase (TH) primary antibody (Millipore, Billerica, MA) in PBS containing 0.5% Triton X-100 and 1% normal goat serum overnight at 4°C. Sections were stained with 1:1000 Alexa Fluor 488 goat anti-mouse secondary antibody (Invitrogen, Carlesbad, CA) for 90 min and coverslipped. Eight-bit images of CPu and NAc were acquired using a 20× objective under a Nikon Eclipse fluorescence microscope (Nikon, Melville, NY). VTA images were taken as mosaics (20× objective) with a Zeiss Axio Observer Microscope equipped with automatic stage (Carl Zeiss AG, Oberkochen, Germany). ImageJ software (National Institute of Health) was used to evaluate the mean gray value of TH immunoreactivity in the CPu, NAc core and NAc shell, where TH protein in axon terminals produce a diffuse staining pattern. Background fluorescence was sampled in an unstained region and subtracted from CPu and NAc mean gray values. Labeled cell bodies were counted in VTA regions delineated by the investigator based on atlas coordinates (Paxinos and Franklin 2001) and TH fluorescence margins. All image analysis and quantification was done by an investigator blind to genotype.
TH Western Blot Analysis
Brains were harvested from C57 WT (n=8) and MT1KO mice (n=8) and 1 mm sections were prepared on a slicer matrix (Zivic, Pittsburgh, PA). CPu, NAc, and dopaminergic midbrain nuclei including VTA were collected as 1 mm punches, frozen on dry ice and stored at −80°C until analysis. The tissues were homogenized in lysis buffer containing 50 mM Tris-Cl pH 7.4, 150 mM NaCl, 1% Triton X-100, and Complete Protease Inhibitor Cocktail (Roche Diagnostics, Atlanta, GA). Samples containing 15 µg protein (determined using Bradford assay; Bio-Rad, Hercules, CA) were subjected to SDS-PAGE (8% acrylamide) and transferred to polyvinylidene difluoride membranes. Blots were incubated overnight at 4°C in either mouse anti-TH (1:1000; Millipore, Temecula, CA) or rabbit monoclonal anti-GAPDH (1:1000; Cell Signaling, Boston, MA) in TBST (20 mM Tris, 136mM NaCl, 0.1% Tween-20) containing 5% bovine serum albumin (BioRad, Hercules, CA). Proteins were detected with 1:10000 HRP-conjugated goat anti-mouse (Santa Cruz Biotechnology, Santa Cruz, CA) or 1:10000 HRP-conjugated goat anti-rabbit (Jackson ImmunoResearch Laboratories, West Grove, PA) followed by enhanced chemiluminescence (Thermo Scientific, Rockford, IL). Bands at approximately 59 kDA (TH) and 37 kDa (GAPDH) were quantitated using Bio-Rad Quantity 1-D software.
Statistical Analysis
Locomotor activity was assessed by distance traveled in 5 min and 1h intervals. Time course data was analyzed by two-way mixed design analysis of variance (ANOVA) having the factors of pretreatment drug (VEH or METH) and time, followed by Bonferroni post hoc comparisons. Total activity before (−55 to 0 min) and after (5 to 60 min) the pretreatment/challenge injection at 0 min was also calculated and analyzed by t-tests. Statistical results (F and p values) for locomotor time course analyses report the main effect of the drug pretreatment. Data from immunofluorescence and Western blot studies were analyzed by t-tests. Quantitative PCR data was analyzed by two-way ANOVA. All analyses were computed using GraphPad Prism v. 5.02 (GraphPad Software, Inc., LaJolla, CA). For all analyses p values < 0.05 were considered statistically significant.
RESULTS
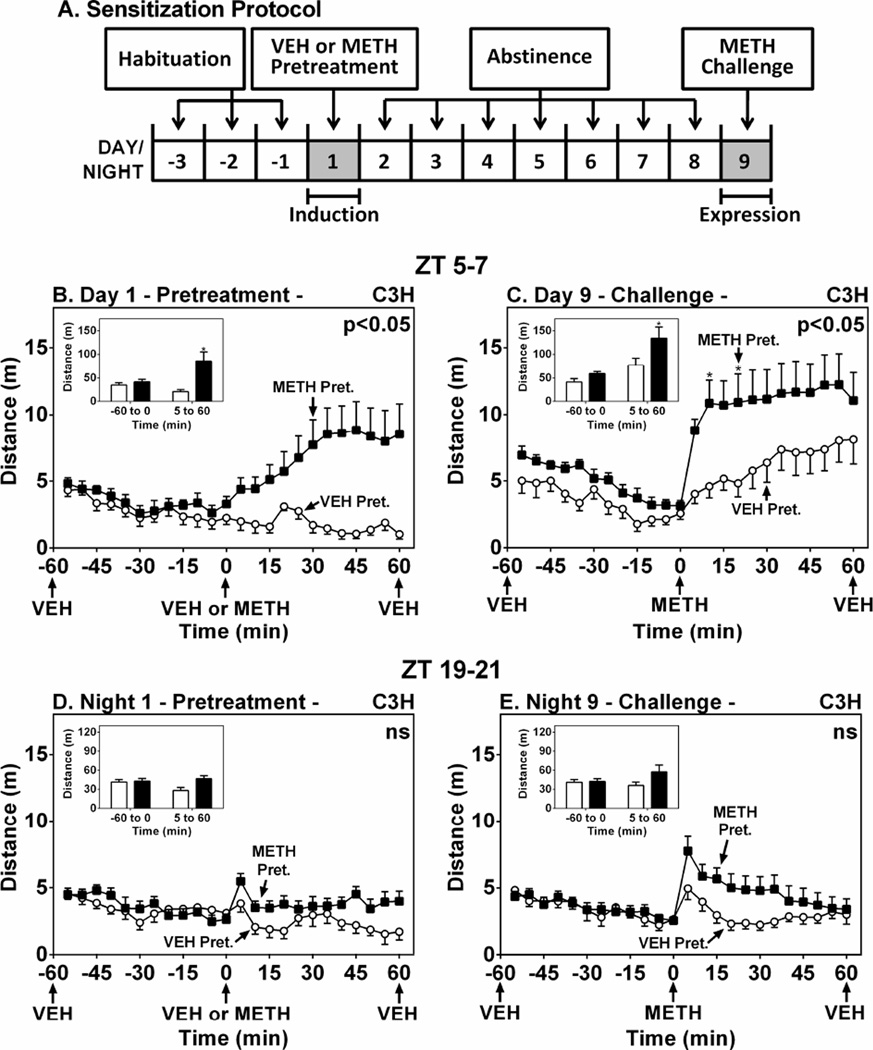
The goal of these studies was to assess the role of the MT1 melatonin receptor in METH-induced sensitization in C57 mice with low/null melatonin levels. Sensitization was measured by challenge with a METH dose in C57 mice, after six daily METH pretreatments and five-day abstinence period. This protocol induced sensitization in the C3H/HeN mouse (Hutchinson et al. 2012), however it did not develop or express locomotor sensitization in C57 WT mice (Supplemental Fig. 1). We then employed a single METH pretreatment protocol in a novel environment and challenged C3H mice after 8 days (Fig. 1A), which was previously used to induce cocaine-mediated sensitization in mice (Valjent et al. 2010). During the light period at ZT 5–7, a single METH (1 mg/kg, i.p.) treatment on Day 1 significantly increased locomotor activity (Fig. 1B) (F(1,14)=7.92, p < 0.05) and METH challenge on Day 9 significantly expressed locomotor sensitization (Fig. 1C) (F(1,14)=4.95, p < 0.05). In contrast, the effect of METH pretreatment at ZT 19–21 was not significant on Night 1 (Fig. 1D) (F(1,14)=5.62, p > 0.05) and locomotor sensitization was not expressed on Night 9 (Fig. 1E) (F(1,14)=2.31, p > 0.05).
Figure 1.
METH-Induced Locomotor Sensitization in C3H/HeN Mice during the Light and Dark Phases. Panel A shows the protocol for assessment of locomotor sensitization via a single METH pretreatment. Mice were pretreated at either ZT 5–7 (B and C) or ZT 19–21 (D and E) with VEH (○) or METH (1.2 mg/kg, i.p.) (■) on Day/Night 1, then challenged with METH on Day/Night 9. Ordinates represent locomotor activity as distance traveled in 5-min intervals as a function of time (abscissae). Insets show total distance traveled (m) from −55 to 0 min and from 5–60 min. Treatments were administered as denoted by arrows under the abscissae. * p < 0.05 compared to VEH.
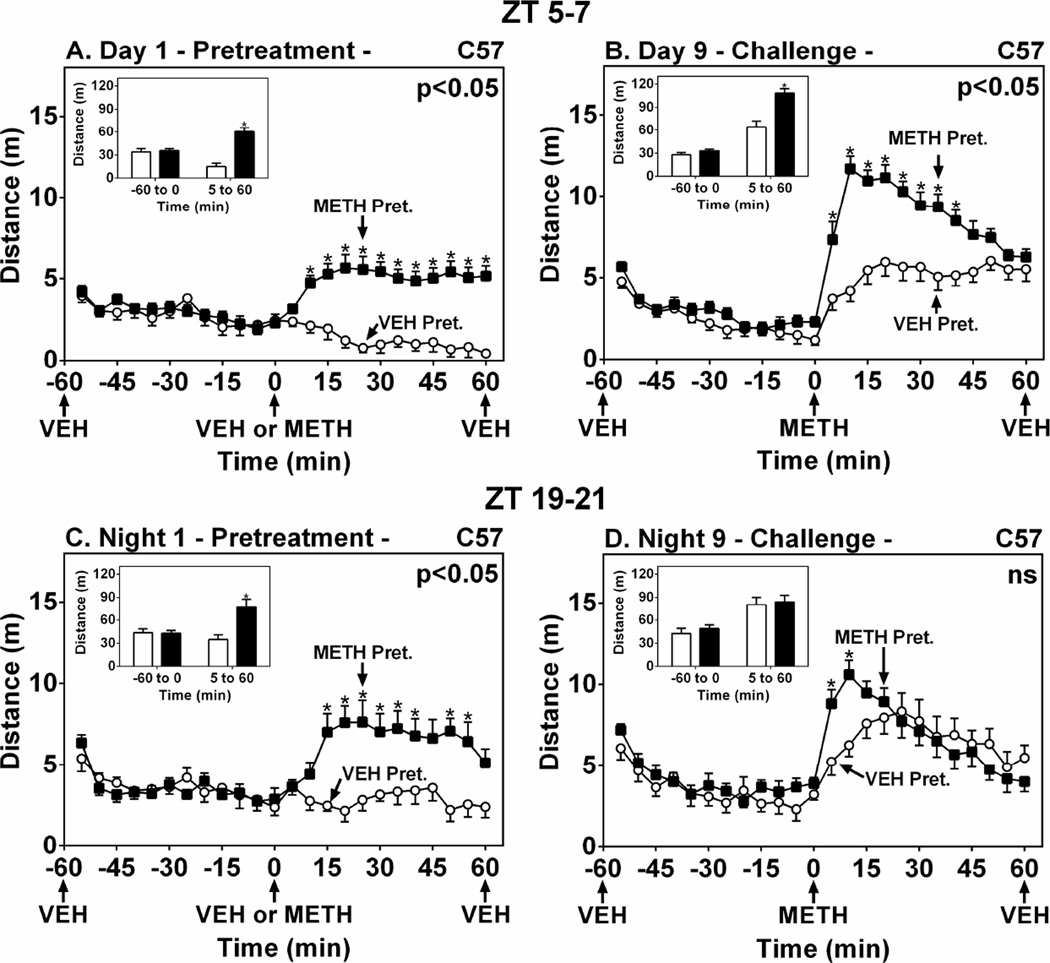
We then proceeded to assess METH-induced sensitization in melatonin-deficient C57 mice using the single METH pretreatment protocol developed in the C3H mouse (Fig. 1). METH pretreatment of C57 WT mice at ZT 5–7 significantly increased locomotor activity compared to VEH on Day 1 (Fig. 2A) (F(1,22)=18.74, p < 0.05) and induced locomotor sensitization on Day 9 (Fig. 2B) (F(1,22)=17.55, p < 0.05). During the dark period at ZT 19–21, METH pretreatment again triggered an enhanced locomotor response on Night 1 (Fig. 2C) (F(1,14)=5.28, p < 0.05). METH challenge elicited a larger locomotor response in METH-pretreated mice at only the 5 and 10 min time points after METH challenge (Fig. 2D) (Bonferroni post-hoc comparisons), indicating an overall reduction in the magnitude of sensitization compared to ZT 5–7.
Figure 2.
METH-Induced Locomotor Sensitization in C57BL/6 WT Mice during the Light and Dark Phases. Ordinates represent locomotor activity as distance traveled in 5-min intervals as a function of time (abscissae). Mice were pretreated at either ZT 5–7 (A and B) or ZT 19–21 (C and D) with VEH (○) or METH (1.2 mg/kg, i.p.) (■) on Day/Night 1, then challenged with METH on Day/Night 9. Insets show total distance traveled (m) from −55 to 0 min and from 5–60 min. Treatments were administered as denoted by arrows under the abscissae. * p < 0.05 compared to VEH.
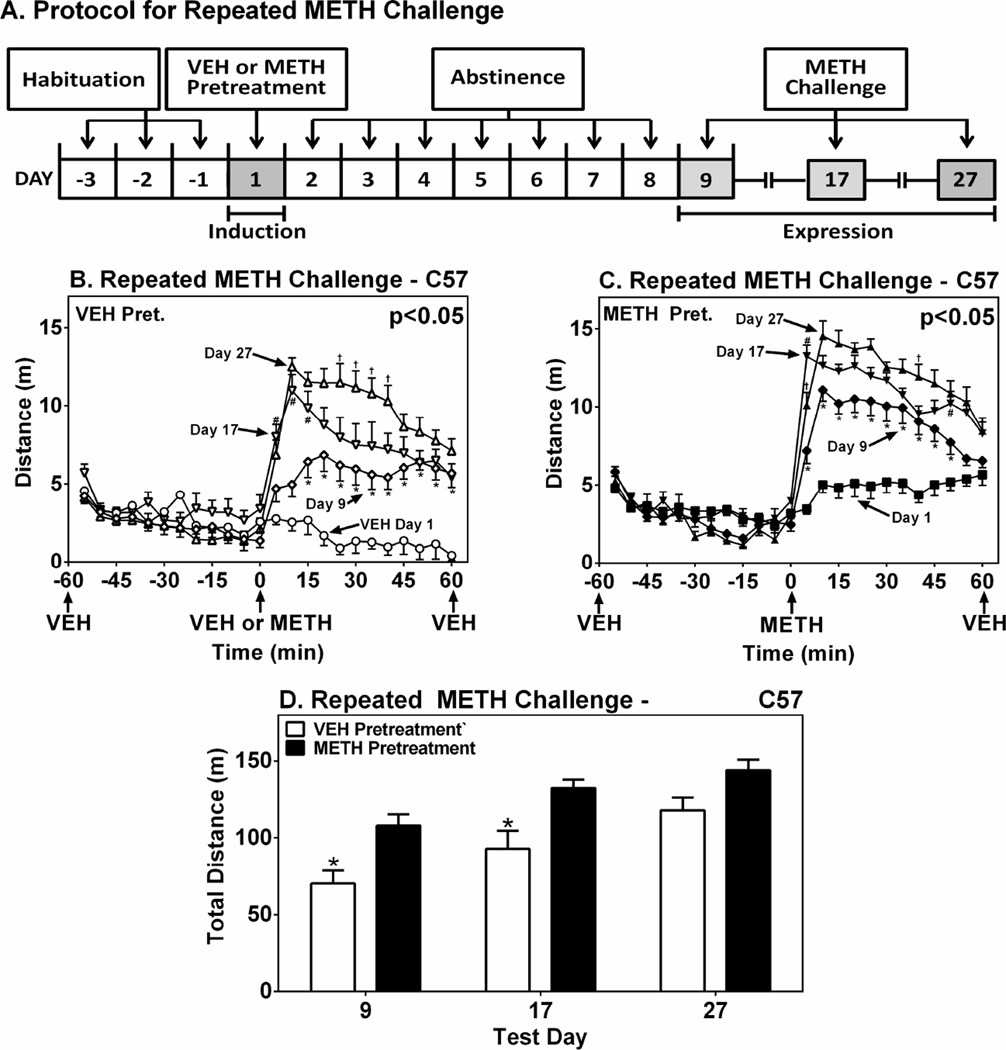
To examine the temporal course of sensitization beyond Day 9, C57 WT mice pretreated (Day 1) and challenged (Day 9) during the light phase both groups were challenged again on Days 17 and 27 (Fig. 3A) with METH. These studies were performed during the light phase based on the larger response observed at that time of day compared to the dark phase (see Fig. 2B and D). Time course plots of the 2h locomotor tests conducted during the pretreatment and challenge trials are shown in Figs. 3B and 3C. These data allow for within-groups comparisons evaluating the effect of successive METH challenges in mice first pretreated with VEH- or METH-. VEH pretreated mice expressed larger locomotor responses on Day 17 compared to Day 9, and again on Day 27 compared to Day 17 (Bonferroni tests), revealing a sensitization effect induced by repeated METH challenges (Fig. 3B). The METH pretreated mice expressed a greater sensitization response on Day 17 compared to Day 9, but the response on Day 27 was not statistically significantly larger than that on Day 17 (p > 0.05) (Fig. 3C). Total locomotor responses in 1h test periods on Days 9, 17 and 27 are shown in Fig. 3D to allow between-groups analysis of the effect of VEH and METH pretreatment at each sensitization test. Compared to VEH-pretreated controls METH-pretreated mice expressed a statistically significantly larger locomotor response to METH challenges on Days 9 and 17, but not Day 27 (Fig. 3D). These results show that METH-induced locomotor sensitization progressively increases after multiple challenges, each separated by 8-–10 days. Furthermore, a saturation point is evident in the METH pretreated mice by virtue of the statistically equivalent responses expressed after the second (Day 17) and third (Day 27) METH challenges.
Figure 3.
Duration of Locomotor Sensitization in WT Mice after Repeated METH Challenges. Panel A shows the protocol for assessing sensitization after a single pretreatment and multiple repeated challenges. Ordinates in B and C show mean±SEM distance traveled in 5-min intervals as a function of time (abscissae). Graphs show serial time course traces for a group of C57 WT mice pretreated with VEH on Day 1 and challenged with METH on Days 9, 17 and 27; and a group of mice pretreated with METH on Day 1 and challenged with METH on Days 9, 17 and 27. Statistical significance of the difference among test days is shown in the upper right of graphs. * p < 0.05 compared to Day 1. # p < 0.05 compared to Day 9. † p < 0.05 compared to Day 17. Panel D shows direct comparisons between VEH and METH-pretreated mice on each challenge test day. Ordinate represents mean±SEM distance traveled in from 0 to 60 min. * p<0.05 compared to VEH pretreatment.
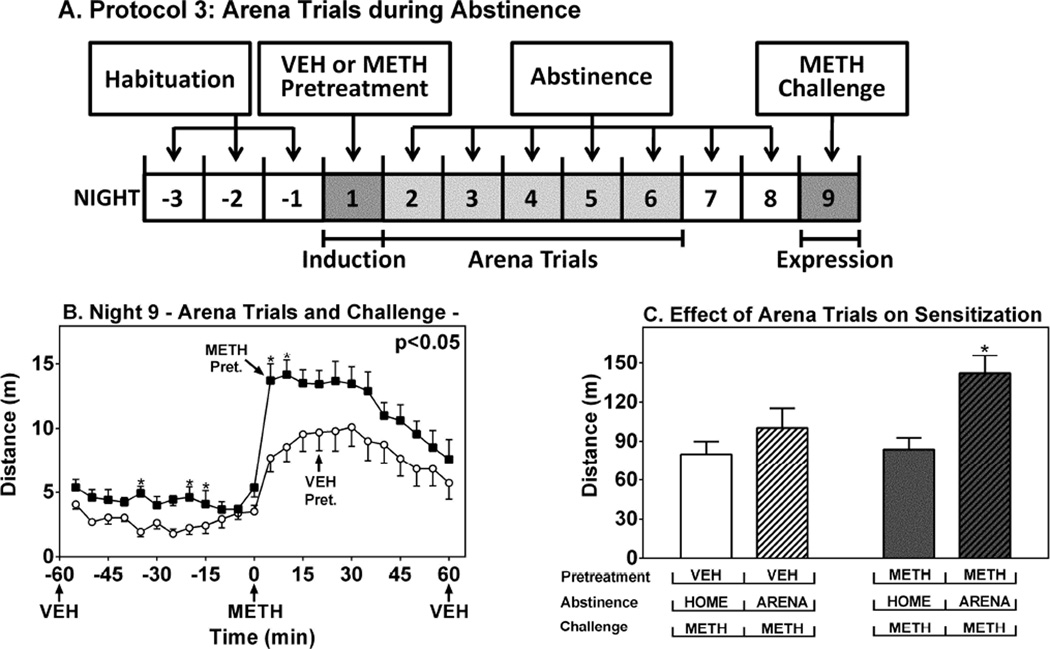
We next addressed the effect of environmental context on the expression of locomotor sensitization during the dark phase. C57 WT mice were habituated and pretreated with VEH or METH at ZT 19–21 on Night 1, then placed in the testing arenas for 2h periods for the dark phases of the next five days (Nights 2–6) with no treatments (Fig. 4A). METH-pretreated mice subjected to these arena trials expressed higher locomotor activity after the METH challenge compared to VEH-pretreated controls, indicating expression of sensitization (5–60 min) (Fig. 4B) (F(1,14)=7.71, p < 0.05). Interestingly, METH pretreated mice subjected to the arena trials expressed higher locomotor activity (−55 to 0 min) before the METH challenge compared to VEH pretreated controls, suggesting a conditioned response to the arenas (Fig. 4B). The arena trials increased the magnitude of METH-induced locomotor sensitization compared to mice that remained in the home cage for the entirety of the abstinence period (Fig. 4C).
Figure 4.
Effect of Novelty on METH-Induced Locomotor Sensitization in WT Mice at ZT 19–21. Ordinates represent distance traveled in intervals of 5 min as a function of time (A) or 1h as a function of treatment conditions (B) (abscissae). White bars, VEH pretreatment; gray bars, METH pretreatment; plain bars, home cage during abstinence; hatched bars, arena trials during abstinence. *p < 0.05 compared to corresponding home cage conditions.
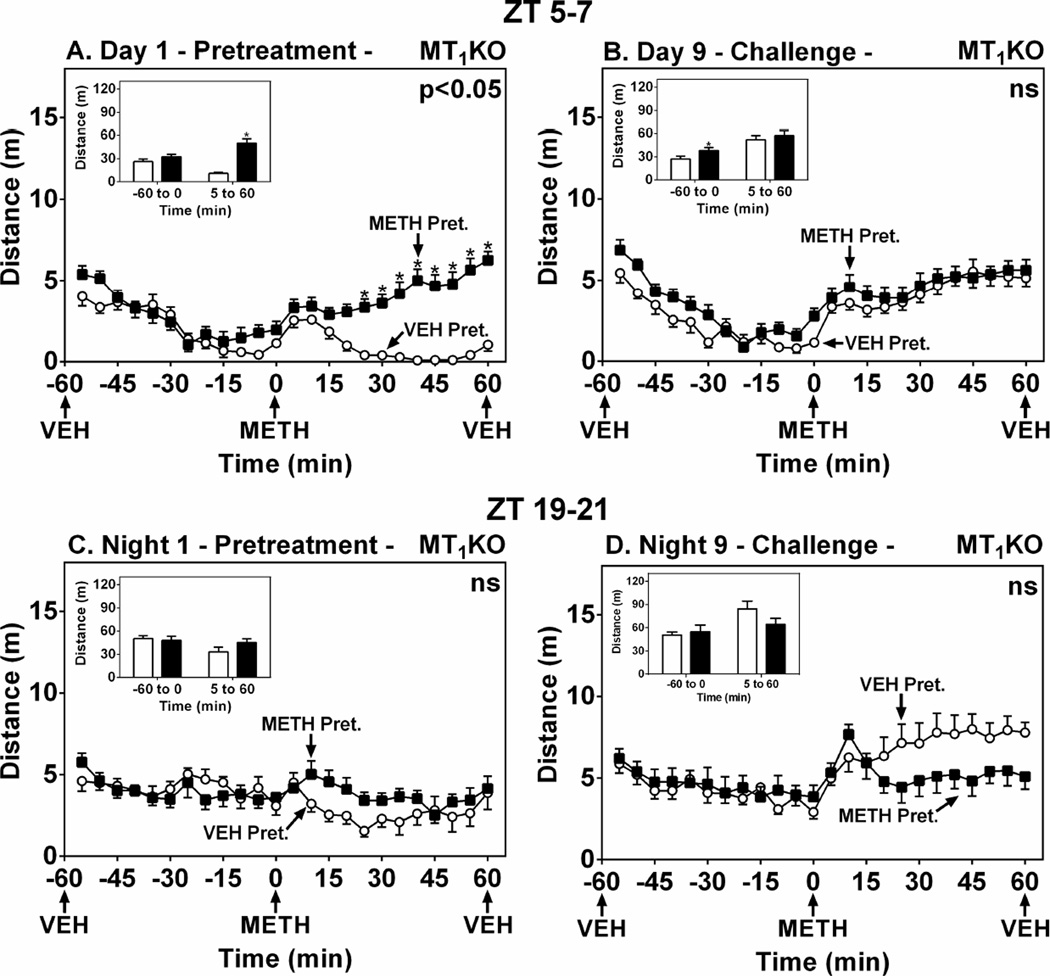
In MT1KO mice, METH pretreatment on Day 1 triggered a significant locomotor effect compared to VEH at ZT 5–7 (Fig. 5A) (F(1,21)=21.18, p<0.05). On Day 9, the METH-pretreated mice expressed greater locomotor activity prior to METH challenge, indicating a modest conditioned response induced by METH pretreatment (Fig. 5B, inset) (t=2.16, df=21, p < 0.05). Responses to METH challenge were not significantly different between the pretreatment groups, indicating no expression of sensitization induced by METH pretreatment (Fig. 5B) (F(1,21)=1.70, p > 0.05). At ZT 19–21, METH pretreatment did not enhance the acute locomotor response in MT1KO mice on Night 1 (Fig. 5C) (F(1,13)=0.65, p > 0.05), nor did it induce sensitization on Day 9 (Fig 5D) (F(1,13)=0.75, p > 0.05). Similarly, sensitization was not observed in MT1KO mice even when the pretreatment dose was raised to 2.4 mg/kg (data not shown).
Figure 5.
MT1 Melatonin Receptor Deletion Abrogates METH-Induced Locomotor Sensitization in C57 Mice. Ordinates represent locomotor activity as distance traveled in 5-min intervals as a function of time (abscissae). Mice were pretreated at either ZT 5–7 (A and B) or ZT 19–21 (C and D) with VEH (○) or METH (1.2 mg/kg, i.p.) (■) on Day/Night 1, then challenged with METH on Day/Night 9. Insets show total distance traveled (m) from −55 to 0 min and from 5–60 min. Treatments were administered as denoted by arrows under the abscissae. * p < 0.05 compared to VEH.
The differential expression of METH-induced locomotor sensitization between C57 WT and MT1KO mice could be attributed to a developmental defect in the mesolimbic dopamine pathways of the MT1KO mice. To address this question, we immunostained WT and MT1KO brain sections for TH, a marker of dopaminergic nerve terminals in the CPu and NAc, and of neuronal cell bodies in the VTA. Fluorescence imaging of the stained sections revealed a diffuse staining pattern consistent with TH expression in nerve terminals in the CPU and NAc. No significant genotype-associated differences in TH fluorescence intensity were found in the CPu, NAc core and NAc shell (Supplemental Fig. 2A–C and E–G). Likewise no significant differences were found between WT and MT1KO in the TH-positive cell density or overall size of the VTA (Supplemental Fig. 2D and H). TH protein levels determined by Western blot analysis were also unchanged between WT and MT1KO in the CPu (Supplemental Fig. 3A), NAc (Supplemental Fig. 3B), and midbrain nuclei including VTA and substantia nigra (Supplemental Fig. 3C).
DISCUSSION
Locomotor sensitization has been widely used to evaluate the capacity of various treatments and genetic manipulations to alter the effects of psychostimulants. In this study we employed locomotor sensitization to assess the role of the MT1 melatonin receptor in a low/null melatonin-expressing C57 WT and MT1KO mice. We demonstrated a statistically significant locomotor sensitization to a METH challenge after 8 days of withdrawal following a single METH pretreatment in both C3H and C57 mice. This is distinct from our observations using a repeated METH pretreatment sensitization protocol, wherein C57 mice express suppressed locomotor sensitization (Supplemental Fig. 1) compared to C3H WT mice (Hutchinson et al. 2012). The expression of sensitization after a single pretreatment was reduced during the dark phase in both C57 and C3H WT mice, which aligns with a report of suppressed cocaine-induced sensitization during the dark phase in C3H mice (Uz et al. 2003). Locomotor sensitization was observed in both VEH- and METH-pretreated mice after repeated METH challenges, with the magnitude of the effect being significantly larger in METH-pretreated mice than in VEH-pretreated controls up to Day 17, and with a ceiling effect encountered on Day 27 in the METH-pretreated group after a total of four METH treatments. Sensitization was completely absent in C57 MT1KO mice during both light and dark phases even when tested at a higher pretreatment dose, which to our knowledge provides first evidence of an obligate role for the MT1 receptor in this process. The locomotor differences between the WT and MT1KO mice were probably not due to a developmental defect in the mesolimbic dopamine system, as TH immunoreactivity expression was not different in the CPu, NAc, and VTA between the C57 WT and MT1KO mice. Exposure to the locomotor arenas in the dark phase during abstinence enhanced the magnitude of sensitization in METH-pretreated C57 WT mice, supporting a role for contextual cues in modulating the sensitized response. In showing a crucial role for the MT1 melatonin receptor in METH-induced locomotor sensitization, our findings expand upon prior studies which report alterations in psychostimulant-induced effects by exogenous and/or pineal gland function (Hirata et al. 1998; Kaewsuk et al. 2009; Kongsuphol et al. 2009; Kurtuncu et al. 2004).
Deletion of the MT1 receptor alone blocked sensitization during the light phase in C57 mice but not in C3H mice (Hutchinson et al. 2012). The basal locomotor responses of VEH-pretreated WT and MT1KO mice on Day 1 during both light and dark phases are not significantly different, suggesting that the locomotor output systems of the two genotypes are equivalent and not affected by nonspecific developmental compensation (Crusio et al. 2009). With the caveat that the C3H and C57 mice have numerous genetic differences, the complete loss of sensitization in C57 MT1KO mice as compared to the WT may be related to the levels of endogenous melatonin, as C57 mice have been regarded as a mouse model of melatonin deficiency (Torres-Farfan et al. 2006; Uz et al. 2002). The MT1 receptor expresses considerable ligand-independent activity in human recombinant cell culture models (Browning et al. 2000; Roka et al. 1999), in rat caudal artery (Ersahin et al. 2002) and in rat ovary (Soares et al. 2003). The presence of constitutively active MT1 melatonin receptors may facilitate METH-induced sensitization in WT mice, suggesting that MT1 receptor activity, rather than expression, is required for the induction of sensitization in C57 mice. Alternatively, very low levels of endogenous melatonin expressing high affinity binding for the MT1 receptor may maintain persistent receptor signaling and mediate locomotor sensitization in WT mice (Dubocovich et al. 2010). Von Gall et al. (2000) showed that C57BL mice may actually produce extremely low levels of endogenous melatonin in the pineal gland. Rhythmicity of melatonin synthesis was not found, but may be difficult to determine in C57 mice on account of the melatonin levels being very near the limit of detection of the assay used (von Gall et al. 2000). Even considering the plethora of genetic differences between C3H and C57 mice, the relative contributions of constitutive receptor activity and/or persistent activation of the MT1 melatonin receptor by endogenous melatonin may facilitate METH-induced sensitization.
The optimizing effect of context and environmental novelty on the expression of locomotor sensitization is well-established in the literature (Badiani et al. 1995a; Badiani et al. 1995b). In attempting to address the effects of context and novelty, we found that the additional arena trials augmented the expression of sensitization in METH-pretreated C57 mice during the dark phase, which had been suppressed in mice that remained in the home cage for the abstinence period (See Fig. 1). It is noteworthy that the mice expressed increased locomotor activity during the hour prior to METH challenge after repeated arena trials, an effect not observed in mice maintained in home cages during abstinence. This observation suggests an effect of conditioned hyperactivity, wherein contexts paired with a drug take on the ability to increase locomotor activity in a drug free-state. Conditioned hyperactivity has been reported to arise from METH treatment in Sprague-Dawley rats (Bevins and Peterson 2004), in Swiss Webster mice (Rauhut and Bialecki 2011), and in C57 mice (Orsini et al. 2004). In the present study, the arena trials during abstinence may have repeatedly reactivated and reconsolidated the memory of METH treatment and strengthened the Pavlovian pairing between drug and context (Dudai 2006). We postulate that the positive effect of the arena trials on the expression of sensitization after METH challenge during the dark phase may arise from the conditioned hyperactivity effect bolstering the sensitization effect.
Psychostimulant treatments modulate the expression of the clock gene period 1 (Per1), a clock gene whose expression oscillates with a 24h period in tissues throughout the brain and periphery (Falcon and McClung 2009). Per1 protein levels are highest in the NAc and CPu during the light phase compared to dark (Uz et al. 2003), and this pattern correlates directly with diurnal variations in locomotor sensitization induced by cocaine (Abarca et al. 2002) and by METH (Uz et al. 2003). METH increases Per1 mRNA expression 1h after a 5 mg/kg treatment (Nikaido et al. 2001), and the same dose of METH sensitizes Per1 mRNA induction triggered by a 0.5 mg/kg challenge seven days later (Nikaido et al. 2001). Rhythmic diurnal expression of Per1 is lost in mice lacking the MT1 receptors, indicating a requirement of this receptor for normal Per1gene function (Imbesi et al. 2009). In the present studies, METH-induced sensitization after a single pretreatment was stronger during the light phase compared the dark phase in WT mice and was lost completely at both times of day in MT1KO mice. Thus it is likely that the magnitude of METH-induced locomotor sensitization during the light and dark phases is correlated with rhythmic Per1 expression in our C3H and C57 mice. A hypothesis for further investigation would propose the induction of sensitization facilitated by high Per1 levels through constitutively active MT1 receptors during the light phase and attenuated sensitization responses during the dark phase when Per1 protein levels are relatively low.
Our studies exploit the effect of locomotor sensitization to show variations in METH responsiveness caused by removal of the MT1 melatonin receptor, by testing at different times of day, and by additional exposures to the drug-paired environment. Perhaps most importantly, our results illustrate the ability of the MT1 receptor to modulate the development and expression of METH-induced locomotor sensitization. Blockade of the MT1 receptor by selective competitive antagonists would be expected to attenuate the sensitizing effects of METH, which are associated with neurobiological changes leading to addiction (Pierce and Kalivas 1997; Robinson and Berridge 2008). The discovery and use of novel MT1 receptor antagonists could open the possibility of new therapeutics capable of remediating symptoms arising from METH abuse.
Supplementary Material
Supplemental Figure 1. Locomotor Responses in C57 Mice Subjected to Six Daily VEH or METH Pretreatments. Experimental protocol is shown in Panel A. Ordinates on the line graphs represent distance traveled (mean±SEM) in 2h tests as a function of Pretreatment Day (B) and in 5 min intervals as a function of time during the challenge test on Day 11 (C). * p < 0.05 when compared to VEH; ns denotes not significant.
Supplemental Figure 2. TH Immunofluorescence in the Mesolimbic Dopamine System of C57 WT and MT1KO Mice. Representative images show CPu, NAc core, NAc shell and VTA for C57 WT (A–D) and MT1KO mice (E–H). TH protein expression was evaluated by fluorescence intensity (mean gray value, mean±SEM) in the CPu, NAc core and NAc shell (I–K). Density of TH-positive cell bodies (mean±SEM) was assessed in the VTA (L). Scale bar in Panel A corresponds to all CPu and NAc images. No significant (ns) differences were found between WT (n=4) and MT1KO mice (n=4).
Supplemental Figure 3. TH Protein Levels in the Mesolimbic Dopamine System. Western blot analysis of brain homogenates from C57 WT (n=8) and MT1KO mice (n=8). Representative blot images are shown for CPu (A), NAc (B) and VTA (C) with corresponding semiquantitative densitometry analyses. Ordinates represent relative mean ± SEM of TH band intensity normalized to corresponding GAPDH values. No significant (ns) differences were observed between genotypes.
Acknowledgements
The authors thank Iwona Stepien, Kathleen McGowan and Peter Crombe for their capable assistance with animal management and genotyping, and equipment maintenance.
Conflict of Interest
This work was funded by R01DA021870 to MLD. The authors declare that over the last three years MLD was a consultant for and received compensation from Takeda Pharmaceutical North America Inc.
Footnotes
Author Contributions
All authors contributed to the conception, design, planning, data acquisition, and/or analysis of these studies. AJH drafted the manuscript which was revised and edited with substantial feedback from all authors. All authors approved the final version of the manuscript before submission.
REFERENCES
- Abarca C, Albrecht U, Spanagel R. Cocaine sensitization and reward are under the influence of circadian genes and rhythm. Proc Natl Acad Sci U S A. 2002;99:9026–9030. doi: 10.1073/pnas.142039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addy NA, Daberkow DP, Ford JN, Garris PA, Wightman RM. Sensitization of rapid dopamine signaling in the nucleus accumbens core and shell after repeated cocaine in rats. J Neurophysiol. 2010;104:922–931. doi: 10.1152/jn.00413.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhisaroglu M, Ahmed R, Kurtuncu M, Manev H, Uz T. Diurnal rhythms in cocaine sensitization and in Period1 levels are common across rodent species. Pharmacol Biochem Behav. 2004;79:37–42. doi: 10.1016/j.pbb.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Badiani A, Anagnostaras SG, Robinson TE. The development of sensitization to the psychomotor stimulant effects of amphetamine is enhanced in a novel environment. Psychopharmacology (Berl) 1995a;117:443–452. doi: 10.1007/BF02246217. [DOI] [PubMed] [Google Scholar]
- Badiani A, Browman KE, Robinson TE. Influence of novel versus home environments on sensitization to the psychomotor stimulant effects of cocaine and amphetamine. Brain Res. 1995b;674:291–298. doi: 10.1016/0006-8993(95)00028-o. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Peterson JL. Individual differences in rats' reactivity to novelty and the unconditioned and conditioned locomotor effects of methamphetamine. Pharmacol Biochem Behav. 2004;79:65–74. doi: 10.1016/j.pbb.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Browning C, Beresford I, Fraser N, Giles H. Pharmacological characterization of human recombinant melatonin mt(1) and MT(2) receptors. Br J Pharmacol. 2000;129:877–886. doi: 10.1038/sj.bjp.0703130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CT, Ma T, Ho IK. Attenuation of methamphetamine-induced behavioral sensitization in mice by systemic administration of naltrexone. Brain Res Bull. 2005;67:100–109. doi: 10.1016/j.brainresbull.2005.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crusio WE, Goldowitz D, Holmes A, Wolfer D. Standards for the publication of mouse mutant studies. Genes Brain Behav. 2009;8:1–4. doi: 10.1111/j.1601-183X.2008.00438.x. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML, Delagrange P, Krause DN, Sugden D, Cardinali DP, Olcese J. International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol Rev. 2010;62:343–380. doi: 10.1124/pr.110.002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubocovich ML, Hudson RL, Sumaya IC, Masana MI, Manna E. Effect of MT1 melatonin receptor deletion on melatonin-mediated phase shift of circadian rhythms in the C57BL/6 mouse. J Pineal Res. 2005;39:113–120. doi: 10.1111/j.1600-079X.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- Dudai Y. Reconsolidation: the advantage of being refocused. Curr Opin Neurobiol. 2006;16:174–178. doi: 10.1016/j.conb.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Ersahin C, Masana MI, Dubocovich ML. Constitutively active melatonin MT(1) receptors in male rat caudal arteries. Eur J Pharmacol. 2002;439:171–172. doi: 10.1016/s0014-2999(02)01407-3. [DOI] [PubMed] [Google Scholar]
- Faith RE, Huerkamp MJ. Environmental Considerations for Research Animals. In: Hessler J, Lehner N, editors. Planning and Designing Research Animal Facilities. London: Elsevier; 2009. pp. 59–83. [Google Scholar]
- Falcon E, McClung CA. A role for the circadian genes in drug addiction. Neuropharmacology. 2009;56(Suppl 1):91–96. doi: 10.1016/j.neuropharm.2008.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futamura T, Akiyama S, Sugino H, Forbes A, McQuade RD, Kikuchi T. Aripiprazole attenuates established behavioral sensitization induced by methamphetamine. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1115–1119. doi: 10.1016/j.pnpbp.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Gaytan O, Lewis C, Swann A, Dafny N. Diurnal differences in amphetamine sensitization. Eur J Pharmacol. 1999;374:1–9. doi: 10.1016/s0014-2999(99)00243-5. [DOI] [PubMed] [Google Scholar]
- Gillette MU, Mitchell JW. Signaling in the suprachiasmatic nucleus: selectively responsive and integrative. Cell Tissue Res. 2002;309:99–107. doi: 10.1007/s00441-002-0576-1. [DOI] [PubMed] [Google Scholar]
- Hirabayashi M, Alam MR. Enhancing effect of methamphetamine on ambulatory activity produced by repeated administration in mice. Pharmacol Biochem Behav. 1981;15:925–932. doi: 10.1016/0091-3057(81)90056-3. [DOI] [PubMed] [Google Scholar]
- Hirabayashi M, Okada S, Tadokoro S. Comparison of sensitization to ambulation-increasing effects of cocaine and methamphetamine after repeated administration in mice. J Pharm Pharmacol. 1991;43:827–830. doi: 10.1111/j.2042-7158.1991.tb03188.x. [DOI] [PubMed] [Google Scholar]
- Hirata H, Asanuma M, Cadet JL. Melatonin attenuates methamphetamine-induced toxic effects on dopamine and serotonin terminals in mouse brain. Synapse. 1998;30:150–155. doi: 10.1002/(SICI)1098-2396(199810)30:2<150::AID-SYN4>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Hutchinson AJ, Hudson RL, Dubocovich ML. Genetic deletion of MT(1) and MT(2) melatonin receptors differentially abrogates the development and expression of methamphetamine-induced locomotor sensitization during the day and the night in C3H/HeN mice. J Pineal Res. 2012;53:399–409. doi: 10.1111/j.1600-079X.2012.01010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbesi M, Arslan AD, Yildiz S, Sharma R, Gavin D, Tun N, Manev H, Uz T. The melatonin receptor MT1 is required for the differential regulatory actions of melatonin on neuronal 'clock' gene expression in striatal neurons in vitro. J Pineal Res. 2009;46:87–94. doi: 10.1111/j.1600-079X.2008.00634.x. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Martin JL, Black MD, Ali SF. Effect of melatonin on methamphetamine- and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced dopaminergic neurotoxicity and methamphetamine-induced behavioral sensitization. Neuropharmacology. 1998;37:781–791. doi: 10.1016/s0028-3908(98)00067-7. [DOI] [PubMed] [Google Scholar]
- Kaewsuk S, Sae-ung K, Phansuwan-Pujito P, Govitrapong P. Melatonin attenuates methamphetamine-induced reduction of tyrosine hydroxylase, synaptophysin and growth-associated protein-43 levels in the neonatal rat brain. Neurochem Int. 2009;55:397–405. doi: 10.1016/j.neuint.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Kasahara T, Abe K, Mekada K, Yoshiki A, Kato T. Genetic variation of melatonin productivity in laboratory mice under domestication. Proc Natl Acad Sci U S A. 2010;107:6412–6417. doi: 10.1073/pnas.0914399107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongsuphol P, Mukda S, Nopparat C, Villarroel A, Govitrapong P. Melatonin attenuates methamphetamine-induced deactivation of the mammalian target of rapamycin signaling to induce autophagy in SK-N-SH cells. J Pineal Res. 2009;46:199–206. doi: 10.1111/j.1600-079X.2008.00648.x. [DOI] [PubMed] [Google Scholar]
- Kopp C, Vogel E, Rettori MC, Delagrange P, Renard P, Lesieur D, Misslin R. Regulation of emotional behaviour by day length in mice: implication of melatonin. Behav Pharmacol. 1999;10:747–752. doi: 10.1097/00008877-199912000-00006. [DOI] [PubMed] [Google Scholar]
- Kozanian OO, Gutierrez A, Mohd-Yusof A, McDougall SA. Ontogeny of methamphetamine-induced and cocaine-induced one-trial behavioral sensitization in preweanling and adolescent rats. Behav Pharmacol. 2012;23:367–379. doi: 10.1097/FBP.0b013e32835651c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa K, Shibasaki M, Mizuno K, Ohkuma S. Gabapentin blocks methamphetamine-induced sensitization and conditioned place preference via inhibition of alpha(2)/delta-1 subunits of the voltage-gated calcium channels. Neuroscience. 2011;176:328–335. doi: 10.1016/j.neuroscience.2010.11.062. [DOI] [PubMed] [Google Scholar]
- Kurtuncu M, Arslan AD, Akhisaroglu M, Manev H, Uz T. Involvement of the pineal gland in diurnal cocaine reward in mice. Eur J Pharmacol. 2004;489:203–205. doi: 10.1016/j.ejphar.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Nikaido T, Akiyama M, Moriya T, Shibata S. Sensitized increase of period gene expression in the mouse caudate/putamen caused by repeated injection of methamphetamine. Mol Pharmacol. 2001;59:894–900. doi: 10.1124/mol.59.4.894. [DOI] [PubMed] [Google Scholar]
- Orsini C, Buchini F, Piazza PV, Puglisi-Allegra S, Cabib S. Susceptibility to amphetamine-induced place preference is predicted by locomotor response to novelty and amphetamine in the mouse. Psychopharmacology (Berl) 2004;172:264–270. doi: 10.1007/s00213-003-1647-z. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KB. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic Press; 2001. [Google Scholar]
- Paz MC, Assis MA, Cabrera RJ, Cancela LM, Bregonzio C. The AT(1) angiotensin II receptor blockade attenuates the development of amphetamine-induced behavioral sensitization in a two-injection protocol. Synapse. 2011;65:505–512. doi: 10.1002/syn.20868. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Rauhut AS, Bialecki V. Development and persistence of methamphetamine-conditioned hyperactivity in Swiss-Webster mice. Behav Pharmacol. 2011;22:228–238. doi: 10.1097/FBP.0b013e328345f741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter RJ. Melatonin: the chemical expression of darkness. Mol Cell Endocrinol. 1991;79:C153–C158. doi: 10.1016/0303-7207(91)90087-9. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roka F, Brydon L, Waldhoer M, Strosberg AD, Freissmuth M, Jockers R, Nanoff C. Tight association of the human Mel(1a)-melatonin receptor and G(i): precoupling and constitutive activity. Mol Pharmacol. 1999;56:1014–1024. doi: 10.1124/mol.56.5.1014. [DOI] [PubMed] [Google Scholar]
- Roseboom PH, Namboodiri MA, Zimonjic DB, Popescu NC, Rodriguez IR, Gastel JA, Klein DC. Natural melatonin 'knockdown' in C57BL/6J mice: rare mechanism truncates serotonin N-acetyltransferase. Brain Res Mol Brain Res. 1998;63:189–197. doi: 10.1016/s0169-328x(98)00273-3. [DOI] [PubMed] [Google Scholar]
- Shen X, Purser C, Tien LT, Chiu CT, Paul IA, Baker R, Loh HH, Ho IK, Ma T. mu-Opioid receptor knockout mice are insensitive to methamphetamine-induced behavioral sensitization. J Neurosci Res. 2010;88:2294–2302. doi: 10.1002/jnr.22386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares JM, Jr, Masana MI, Ersahin C, Dubocovich ML. Functional melatonin receptors in rat ovaries at various stages of the estrous cycle. J Pharmacol Exp Ther. 2003;306:694–702. doi: 10.1124/jpet.103.049916. [DOI] [PubMed] [Google Scholar]
- Stewart J, Badiani A. Tolerance and sensitization to the behavioral effects of drugs. Behav Pharmacol. 1993;4:289–312. [PubMed] [Google Scholar]
- Suwanjang W, Phansuwan-Pujito P, Govitrapong P, Chetsawang B. The protective effect of melatonin on methamphetamine-induced calpain-dependent death pathway in human neuroblastoma SH-SY5Y cultured cells. J Pineal Res. 2010;48:94–101. doi: 10.1111/j.1600-079X.2009.00731.x. [DOI] [PubMed] [Google Scholar]
- Takino N, Sakurai E, Kuramasu A, Okamura N, Yanai K. Roles of the histaminergic neurotransmission on methamphetamine-induced locomotor sensitization and reward: a study of receptors gene knockout mice. Int Rev Neurobiol. 2009;85:109–116. doi: 10.1016/S0074-7742(09)85008-3. [DOI] [PubMed] [Google Scholar]
- Torres-Farfan C, Seron-Ferre M, Dinet V, Korf HW. Immunocytochemical demonstration of day/night changes of clock gene protein levels in the murine adrenal gland: differences between melatonin-proficient (C3H) and melatonin-deficient (C57BL) mice. J Pineal Res. 2006;40:64–70. doi: 10.1111/j.1600-079X.2005.00279.x. [DOI] [PubMed] [Google Scholar]
- Uz T, Akhisaroglu M, Ahmed R, Manev H. The pineal gland is critical for circadian Period1 expression in the striatum and for circadian cocaine sensitization in mice. Neuropsychopharmacology. 2003;28:2117–2123. doi: 10.1038/sj.npp.1300254. [DOI] [PubMed] [Google Scholar]
- Uz T, Javaid JI, Manev H. Circadian differences in behavioral sensitization to cocaine: putative role of arylalkylamine N-acetyltransferase. Life Sci. 2002;70:3069–3075. doi: 10.1016/s0024-3205(02)01559-x. [DOI] [PubMed] [Google Scholar]
- Valjent E, Bertran-Gonzalez J, Aubier B, Greengard P, Herve D, Girault JA. Mechanisms of locomotor sensitization to drugs of abuse in a two-injection protocol. Neuropsychopharmacology. 2010;35:401–415. doi: 10.1038/npp.2009.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev. 2004;27:827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- von Gall C, Lewy A, Schomerus C, Vivien-Roels B, Pevet P, Korf HW, Stehle JH. Transcription factor dynamics and neuroendocrine signalling in the mouse pineal gland: a comparative analysis of melatonin-deficient C57BL mice and melatonin-proficient C3H mice. Eur J Neurosci. 2000;12:964–972. doi: 10.1046/j.1460-9568.2000.00990.x. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Locomotor Responses in C57 Mice Subjected to Six Daily VEH or METH Pretreatments. Experimental protocol is shown in Panel A. Ordinates on the line graphs represent distance traveled (mean±SEM) in 2h tests as a function of Pretreatment Day (B) and in 5 min intervals as a function of time during the challenge test on Day 11 (C). * p < 0.05 when compared to VEH; ns denotes not significant.
Supplemental Figure 2. TH Immunofluorescence in the Mesolimbic Dopamine System of C57 WT and MT1KO Mice. Representative images show CPu, NAc core, NAc shell and VTA for C57 WT (A–D) and MT1KO mice (E–H). TH protein expression was evaluated by fluorescence intensity (mean gray value, mean±SEM) in the CPu, NAc core and NAc shell (I–K). Density of TH-positive cell bodies (mean±SEM) was assessed in the VTA (L). Scale bar in Panel A corresponds to all CPu and NAc images. No significant (ns) differences were found between WT (n=4) and MT1KO mice (n=4).
Supplemental Figure 3. TH Protein Levels in the Mesolimbic Dopamine System. Western blot analysis of brain homogenates from C57 WT (n=8) and MT1KO mice (n=8). Representative blot images are shown for CPu (A), NAc (B) and VTA (C) with corresponding semiquantitative densitometry analyses. Ordinates represent relative mean ± SEM of TH band intensity normalized to corresponding GAPDH values. No significant (ns) differences were observed between genotypes.