ABSTRACT
Objectives:
To describe the epidemiological characteristics and the clinical manifestations of the confirmed dengue cases over a ten-year period in Barbados, one of the English-speaking Caribbean countries.
Methods:
All the cases of confirmed dengue from 2000 to 2009 were retrospectively studied. Long-term trends in incidence rate, demographic characteristics such as age, gender and seasonal distribution; clinical manifestations, immunological characteristics, need for hospitalization and mortality were studied.
Results:
There were 3413 confirmed cases of dengue including 778 (22.8%) children and 2635 (77.2%) adults. The mean annual incidence rate of dengue was 1.36/1000 population. The median age of the persons with confirmed dengue was 27 years. The largest number of cases was seen in the 11 to 16-year age group. Hospitalization was required in 13.1% of dengue cases; 72.5% and 84% of all dengue were secondary infections among the children and adults, respectively. Dengue haemorrhagic fever accounted for 2.2% and 6% of all confirmed dengue among children and adults, respectively. The overall case fatality rate in this study was 0.35%.
Conclusions:
Dengue is a significant health problem primarily in adolescents and young adults. It is characterized by less severe cases and lower mortality rate.
Keywords: Barbados, dengue, epidemic
RESUMEN
Objetivos:
Describir las características epidemiológicas y manifestaciones clínicas de los casos de dengue confirmados durante un período de diez años en Barbados, uno de los países del Caribe anglófono.
Métodos:
Se estudiaron retrospectivamente todos los casos de dengue confirmados desde el año 2000 al 2009. Se estudiaron las tendencias a largo plazo en la tasa de incidencia, y las características demográficas como edad, género y distribución estacional. Asimismo, se estudiaron las manifestaciones clínicas, características inmunológicas, necesidad de hospitalización y mortalidad.
Resultados:
Hubo 3413 casos confirmados de dengue, incluyendo 778 (22.8%) niños y 2635 (77.2%) adultos. La tasa promedio de incidencia anual del dengue fue 1.36/1000 habitantes. El promedio de edad de las personas con dengue confirmado fue 27 años. El mayor número de casos se observó en el grupo de edad de 11 a 16 años. Se requirió hospitalización fue requerido en el 13.1% de los casos de dengue. En el 72.5% y el 84% de los casos de dengue hubo infecciones secundarias entre los niños y adultos, respectivamente. La fiebre hemorrágica del dengue hemorrágico representó el 2.2% y 6% de todos los casos de dengue confirmados entre niños y adultos, respectivamente. La tasa de letalidad general en el presente estudio fue de 0.35%.
Conclusiones:
El dengue es un importante problema de salud principalmente en adolescentes y adultos jóvenes. Se caracteriza por casos menos graves y tasa de mortalidad más baja.
INTRODUCTION
Dengue is the most common and is geographically the most rapidly spreading vector-borne viral disease that has established itself globally in both endemic and epidemic cycles (1– 2). It is an important emerging public health issue with increasing morbidity and mortality in the South and Central Americas including the Caribbean (3–7). Dengue is reported to be endemic in most of the English-speaking Caribbean, including Barbados, with an epidemic occurring every four to five years (7–8). All four serotypes have been circulating in the Caribbean and all of the serotypes have been concurrently isolated in some of the countries since 2001, including Barbados (7–8).
Although there is a perceived increase in disease burden, dengue epidemiology in the Americas including the Caribbean region has not been well documented, with a paucity of longterm epidemiological studies in published literature (7, 9, 10). Published seroprevalence studies from the English-speaking Caribbean have shown a high prevalence of DENV-specific antibodies among children (11–13). Very few studies from the English-speaking Caribbean have attempted to characterize the dengue epidemics in this region (14, 15). Available reports in the published literature are those from the passive surveillance data collected by the regional health agencies, based on the cases reported by the member countries, which are known to have underestimated the true extent of the disease burden (1, 16).
Barbados has a well-organized public healthcare infrastructure with free healthcare for its citizens at the point of delivery. There is an ongoing vector control programme and a dedicated dengue testing laboratory, making active surveillance possible. All these provide an excellent opportunity to study the trends in the epidemiology of dengue. In this report, the first population-based, long-term study of confirmed dengue cases among both children and adults in the English speaking Caribbean, we characterize dengue epidemiology during the last decade and describe the clinical manifestations and the disease outcomes in patients with dengue in Barbados.
SUBJECTS AND METHODS
This retrospective, population based study reports on dengue during the period January 2000 to December 2009. This report includes all the laboratory confirmed dengue cases (either positive IgM titre in acute serum sample and/or positive for NS1 (non-structural protein 1) antigen and/or positive dengue virus culture in blood) from among all the suspected cases of dengue in Barbados. Necessary ethical approval for this study was obtained from the Institutional Review Board on Ethics in Research on Human Subjects at The University of the West Indies and the Ethics Committee at the Queen Elizabeth Hospital (QEH).
Dengue, being endemic in Barbados, is suspected in any person presenting with fever of more than 24 hours duration and the cause of the fever is not obvious at presentation. Furthermore, dengue is a notifiable disease in this country. The Government of Barbados, which provides free healthcare to its citizens, has had an enhanced dengue surveillance system (EDSS) in place since 2000. While passive dengue surveillance system (PDSS) involves the collection of data from unsolicited reports of suspected cases, EDSS surveillance involves active case finding. It encourages and supports healthcare providers to report dengue (16). A blood sample is routinely obtained from all the suspected cases of dengue and is sent to the local Dengue Laboratory in Barbados for dengue testing. When a febrile person presents during the first five days of the illness, blood is tested for the NS1 antigen along with the IgM and IgG antibodies and if the NSI and IgM were both negative and the blood sample was drawn before day three of the illness then a blood test is repeated after day five of illness for the dengue IgM and IgG antibodies. When febrile persons present between days five and 15 of the illness, blood is tested for the dengue IgM and IgG antibodies. Non-structural protein 1 antigen detection has been available only since 2006 and in the period prior to 2006, samples taken during the first five days of the illness were sent to the regional reference laboratory at the Caribbean Epidemiology Centre (CAREC) in Trinidad and Tobago for viral culture. All persons suspected of having dengue are further investigated and treated appropriately based on their clinical presentations. If the persons need hospitalization, they are referred to the Queen Elizabeth Hospital, which is the only tertiary care hospital and which provides over 90% of all inpatient care in this country.
IgG and IgM antibody-capture enzyme-linked immunosorbent assay (ELISAs) were performed according to manufacturer’s instructions (Focus Diagnostics, Cypress, CA90630 USA). Platelia™ Dengue NS1 Ag-ELISA (Biorad Laboratories, Marnes-La-Coquette, France) was used for the NS1 antigen detection. The assay uses murine monoclonal antibody for capture and revelation.
Demographic and clinical data on all suspected febrile persons (both children and adults) who were tested for dengue were collected from the dengue register at the Department of Pathology at the QEH during the years prior to 2005 and from the Central Dengue Laboratory thereafter. Supplementary clinical information was gathered from the patient’s case records. The following case definitions were used:
Primary dengue case was where IgM was positive but IgG was negative while secondary dengue case was one where both IgM and IgG were positive in the acute serum sample (17).
The four World Health Organization (WHO) classification (17) and case definitions were used to classify the clinical symptoms in these children and adults as – undifferentiated fever (UF), dengue fever (DF) or dengue haemorrhagic fever (DHF) or expanded dengue syndrome (EDS). Diagnosis of DHF was assigned to confirmed cases of dengue who presented with the following four criteria: acute sudden onset of high fever for two to seven days, haemorrhagic manifestations with at least a positive tourniquet test, platelet count < 100 000 × 109/L, haemo-concentration (rising packed cell volume > 20%) or other evidence of plasma leakage, for example, ascites, pleural effusions or low level of serum protein/albumin. The diagnosis of EDS was assigned to patients with dengue who had unusual or atypical manifestation such as neurological, hepatic, renal or other isolated organ involvement.
Data were stored in a specially designed Microsoft®Access database and were analysed using SPSS® statistical software package version 11. Microsoft® Excel was used for the generation of all graphs and tables. Proportion and 95% confidence interval (CI) were calculated using binomial distribution and results were corrected for continuity. Associations between categorical variables were assessed for statistical significance by Chi-squared test. A probability (p) of ≤ 0.05 was considered statistically significant.
RESULTS
There was a total of 7878 persons suspected and investigated for dengue in Barbados during the 10-year study period. A total of 3413 persons including 778 children (22.8%; 95% CI: 21.4%, 24.3%) and 2635 adults (77.2%; 95% CI: 75.6%, 78.6%) were confirmed to have dengue. The overall annual incidence rate of dengue was 1.36/1000 population (range 0.59/1000 population – 2.31/1000 population). During the non-epidemic years, the annual incidence rate of dengue varied from 0.59 to 1.85/1000 population. The time trend in the annual number of dengue cases is shown in Fig. 1.
Fig. 1. Time trend in the number of suspected and confirmed cases of dengue in Barbados, 2000–2009.
While dengue was seen throughout the study period, the years 2001 and 2007 witnessed dengue in epidemic proportions (epidemic threshold was defined as case number two standard deviations above the previous year). Most cases of dengue (56.8%; 95% CI: 51.7%, 62.2%) were seen during the months of October to January. The basic demographics of the study population are shown in Table 1. The median age of the persons with confirmed dengue was 27 years (range < 1 year to 96 years). Most of the cases of dengue (81.0%; 95% CI: 78.4%, 83.1%) were seen in the ages six years to 50 years. Only 3.3% of confirmed dengue was seen in persons older than 60 years. The mean annual incidence rate of dengue among children under 16 years was 1.38/1000 children, while the mean annual incidence rate among the adult population was 1.17/1000 adults.
Table 1. Selected characteristics of the dengue epidemics in Barbados, 2000–2009.
| Number of dengue cases | Children | Adults | Total | |
|---|---|---|---|---|
| Suspected | 1860 (100%) | 6018 (100%) | 7878 (100%) | |
| Confirmed | 778 (41.8%) | 2635 (43.8%) | 3413 (43.3%) | |
| Gender (confirmed cases) | ||||
| Female | 395 (50.8%) | 1581 (60%) | 1976 (57.9%) | |
| Male | 383 (49.2%) | 1054 (40%) | 1437 (42.1%) | |
| Care pattern (confirmed cases) | ||||
| Ambulatory | 597 (76.7%) | 2370 (90%) | 2967 (86.9%) | |
| Hospitalized | 181 (23.3%) | 265 (10.1%) | 446 (13.1%) | |
| Immune status (confirmed cases) | ||||
| Primary infection | 126 (27.5%) | 236 (16%) | 362 (18.6%) | |
| Secondary infection | 331 (72.5%) | 1239 (84%) | 1570 (81.4%) | |
| Mortality | ||||
| (rate/100 confirmed cases) | 2(0.26) | 10(0.38) | 12(0.35) | |
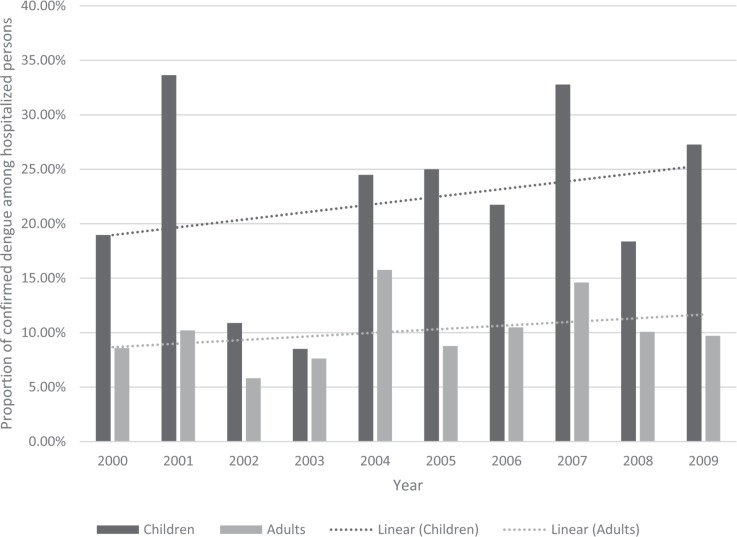
Of the 3413 cases of confirmed dengue, 446 cases of dengue (13.10%; 95% CI: 11.9%, 14.2%) occurred among hospitalized persons. The remainder of the cases were diagnosed in ambulatory patients presenting with symptoms and signs suggestive of dengue but not of the severe forms. Figure 2 shows the time trend in the proportion of the dengue cases that were diagnosed among hospitalized children and adults. Overall, 23.3% (95% CI: 20.3%, 26.4%) and 10.1% (95% CI: 9.2%, 11.6%) of dengue during the study period were diagnosed among the hospitalized children and adults, respectively (p = 0.002). Among the children, 39.6% (95% CI: 32.2%, 47.6%) and 18.1% (95% CI: 14.3%, 22.6%) of all dengue was diagnosed among the hospitalized children under the age of six years and those in the age group 11 to 15 years, respectively (p < 0.001). Of note, a higher proportion of dengue occurred in adults who were hospitalized in the years 2001 (15.3%; 95% CI: 12.3%, 18.9%) and 2007 (25.9%; 95% CI: 21.9%, 30.2%), both of which were epidemic years. The difference in the proportion of dengue cases among the hospitalized persons during the epidemic years (2001 and 2007) as compared to the nonepidemic years was not statistically significant (p = 0.057).
Fig. 2. Proportion of confirmed dengue among the hospitalized persons in Barbados, 2000–2009.
Both the IgM and the IgG results were available in 1932 cases of confirmed dengue. Analysis of cases where the results from both the IgM and IgG antibodies tests done at the time of presentation were available revealed that, overall, 81.4% (95% CI: 79.6%, 83.1%) of all cases of dengue were secondary infection with IgG being positive. Among the children, 72.5% (95% CI: 68.0%, 76.4%) of all dengue cases were secondary infection, while among the adults, 84% (95% CI: 82.0%, 85.9%) of all dengue cases were secondary infection. In children less than 12 months of age, 79.7% (95% CI: 69.2%, 88.9%) had secondary infection. Among the children, there was an increasing trend (p = 0.056) in the proportion of dengue that was secondary infection over the study period. However, among the adults, there was no trend in the proportion of dengue that was secondary infection over the study period. Overall, 78.4% (95% CI: 75.3%, 81.2%) of all dengue seen among the ambulatory persons was secondary infection, whereas 87.7% (95% CI: 81.2%, 93.1%) of dengue among the hospitalized persons was secondary cases (p = 0.068). The clinical manifestations of dengue among the children and adults are shown in Table 2.
Table 2. The clinical manifestation among children and adults with confirmed dengue in Barbados, 2000–2009.
| Clinical manifestations | Children n (%) |
Adults n (%) |
|---|---|---|
| Undifferentiated fever | 317(40.7%) | 821 (31.2%) |
| Classical dengue | 250(32.1%) | 1332 (50.6%) |
| Dengue haemorrhagic fever | 17 (2.2%) | 148 (5.6%) |
| Expanded dengue syndrome | 20 (2.6%) | 89 (3.4%) |
| Insufficient data | 164(21.1%) | 245 (9.3%) |
| Total confirmed dengue cases | 778 (100%) | 2635 (100%) |
Among the children, EDS included the diagnosis of hepatitis, meningo-encephalitis, myocarditis and shock with multi-organ failure. Expanded dengue syndrome in adults included the diagnosis of hepatitis, myocarditis, shock with multi-organ failure and meningo-encephalitis. While 68.9% (95% CI: 62.6%, 74.5%) of dengue manifesting as undifferentiated fever and classical dengue fever was secondary infection, 95.4% (95% CI: 90.3%, 99.1%) of the dengue presenting as EDS and all 100% of the dengue presenting as DHF were secondary cases (p = 0.01). All 12 deaths were among the patients with severe form of dengue manifesting as either the dengue haemorrhagic fever or expanded dengue syndrome with shock and multi-organ failure. The overall case fatality rate (number of deaths from confirmed dengue divided by the total number of confirmed cases of dengue and expressed as percentage) in this study was 0.35% (Table 1).
DISCUSSION
Dengue cases were seen during all of the years of study. There was an epidemic in 2001 and a second, larger epidemic in 2007 (Fig. 1). For the purpose of this study, epidemic threshold was defined as case numbers two standard deviations above the previous year (18). The annual incidence rate of dengue in this study varied during the 10-year period. Although there was an overall declining trend in the incidence of dengue seen during the study period, it was not statistically significant (p = 0.67). A report from Nicaragua in Central America presented a very similar picture of incidence trend to that seen in this study (19). As the number of years since re-emergence of dengue in a population increases, the number of susceptible population would decline with increasing monotypic and polytypic herd immunity (20). This would naturally lead to the declining and subsequent stabilizing of the overall incidence rate in that population.
Our average annual incidence rate (1.36/1000 population) of dengue compares well with those reported from Puerto Rico and Brazil, where dengue was re-introduced during the same time period as Barbados (21, 22). However, it was much higher when compared with the annual incidence rate (< 1/1000 population) reported in similar studies from Thailand where dengue was introduced or re-introduced much earlier than those in Barbados (23). It is noteworthy that a much lower incidence rate was reported for the non-Hispanic Caribbean (0.71/1000 population) for a similar study period in a study of dengue in the Americas (3). The North American region had a very low incidence rate. The lower incidence rate reported in that regional study (3) may be reflective of the under-reporting inherent in the existing passive surveillance system in most countries. This has been pointed out in some of the studies based on active surveillance and those of modelling studies (10, 21).
In Barbados, dengue is a disease of adolescents and young adults with less than a fourth of all confirmed dengue occurring in children (< 16 years). The number of cases of dengue is seen to peak among the 11–15 year age group (12.2% of all cases) and nearly half of all the cases occurred in the 10 to 30-year age group. This pattern is usually indicative of a shorter time period since the re-emergence of dengue in this population in the 1990s. Reports from other southeast Asian and Central and South American countries where dengue re-emerged earlier than the English Caribbean have shown that the majority of the cases occur in children (24–25). Rodriguez-Barraquer et al, in a simulation study from Brazil, have clearly demonstrated the age shift and severity of dengue with the passage of time since the re-emergence of dengue in that population (20). In epidemiological studies from the southeast Asian countries which are hyperendemic with all four serotypes in circulation, dengue is reported to be predominantly a childhood disease (26–27). In areas such as Nepal (28) and Hawaii (29) experiencing the first major outbreak of dengue fever, the majority of cases occur in the adult population. With the occurrence of infections from additional serotypes, hyperendemicity develops and the dengue will typically emerge as a disease of early childhood while clinical dengue in adults would be rare (26–27). This phenomenon has been observed in some of the southeast Asian countries, where all the serotypes (DENV-1-4) are circulating simultaneously (30).
Over four-fifths of all cases of dengue were secondary infection; 72.5% and 84% of all dengue in children and adults, respectively were secondary infection. Further analysis of age and IgG positivity revealed that there was a high positivity during the first year of life, falling to a level less than 20% by the second year of life followed by exponential rise in the positivity reaching a peak of over 80% by 20 years and then remaining at that level. This is a reflection of early age at exposure to the infection and high vector density which is reported to occur with maturing endemicity (30). In contrast to the situation observed in Barbados, a recent study from Puerto Rico reported a much lower proportion of secondary infection at 49.4% (21). In an endemic country like Thailand, it has been shown that vector abundance (ie more frequent cases of DF) drives mean age down, whereas lower numbers of vectors drive the age upward with less frequent cases of dengue (31). In our study, 10.1% of all dengue among adults and 23.3% of all dengue among children were seen in persons who needed to be hospitalized. This is in contrast with those reported from the neighbouring Hispanic Caribbean, with more recent emergence of dengue and lower proportion of secondary cases compared to that seen in this study, where less than 5% of all patients were hospitalized (21). Sequential infection of two serotypes leads to more severe type of disease (eg DHF). This phenomenon, termed “antibody-dependent enhancement”, increases the number of infected cells and is thought to lead to higher virus titres and a more pronounced inflammatory response (31). Hence the cases of DHF will be observed in the early adulthood group. Data from Trinidad and Tobago show that it was predominantly the 20–39-year age group that had DHF (32).
Overall, 5% of all cases of dengue in this study presented as DHF; 2.2% and 5.6% of children and adults, respectively with dengue presented as DHF. Likewise, a recent and similarly designed study from the neighbouring Hispanic Caribbean reported 4% of all dengue as DHF (21). This is in keeping with the pattern seen in populations with more recent re-emergence of dengue and lesser endemicity with fewer secondary cases and fewer hospitalization, in contrast to the greater endemicity with higher proportion of secondary cases and greater number of hospitalization. With the passage of time, both the number and the severity of the cases are expected to shift to the lower age group (20).
One of the major limitations of this study was the possible under estimation of the disease burden. Cases of dengue where the patient did not present for medical care may have been missed. Also, cases of dengue where the attending physician either did not consider dengue as a possible diagnosis or did not investigate for dengue might have been missed.
In summary, dengue cases peaked in the 11 to 25-year age group. It occurs throughout the year but most of the cases were seen during the rainy months of October to January. Over four-fifths of dengue in adults and nearly three-fourths of those in children were secondary infection. Five per cent of all dengue manifested as DHF. Less than one-fourth of children and one-tenth of adults with dengue required hospitalization. The overall case fatality rate from dengue was 0.35%.
ACKNOWLEDGEMENTS
We acknowledge the contribution made by the staff at the Public Health Laboratory for providing the necessary information. We thank Prerna Singh and Pranav Kumar Singh in assisting with review of the manuscript for English language.
REFERENCES
- 1.Gubler DJ. Dengue, urbanization and globalization: the unholy trinity of the 21(st) century. Trop Med Health. 2011;39:3–11. doi: 10.2149/tmh.2011-S05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guha-Sapir D, Schimmer B. Dengue fever: new paradigms for a changing epidemiology. Emerg Themes Epidemiol. 2005;2:10–15. doi: 10.1186/1742-7622-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.San Martin JL, Brathwaite O, Zambrano B, Solorzano JO, Bouckenooghe A, Dayan GH, et al. The epidemiology of dengue in the Americas over the last three decades: a worrisome reality. Am J Trop Med Hyg. 2010;82:128–135. doi: 10.4269/ajtmh.2010.09-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan American Health Organization (PAHO) Region of the Americas from 1995 through 2007. Washington, DC: PAHO; 2007. Number of reported cases of dengue and dengue hemorrhagic fever (DHF) [Google Scholar]
- 5.Díaz-Quijano FA, Waldman EA. Factors associated with dengue mortality in Latin America and the Caribbean: an ecological study. Am J Trop Med Hyg. 2012;86:328–334. doi: 10.4269/ajtmh.2012.11-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tapia-Conyer R, Méndez-Galván JF, Gallardo-Rincón H. The growing burden of dengue in Latin America. J Clin Virol. 2009;2:S3–S6. doi: 10.1016/S1386-6532(09)70286-0. [DOI] [PubMed] [Google Scholar]
- 7.Campione-Piccardo J, Ruben M, Vaughan H, Morrison-Glasgow V. Dengue viruses in the Caribbean. Twenty years of dengue virus isolates from the CAREC. West Indian Med J. 2003;52:191–198. [PubMed] [Google Scholar]
- 8.Gittens-St Hilaire M, Clarke-Greenidge N. An analysis of the subtypes of dengue fever infections in Barbados 2003–2007 by reverse transcriptase polymerase chain reaction. Virol J. 2008;5:152–152. doi: 10.1186/1743-422X-5-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray NE, Quam MB, Wilder-Smith A. Epidemiology of dengue: past, present and future prospects. Clin Epidemiol. 2013;5:299–309. doi: 10.2147/CLEP.S34440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;25:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown MG, Vickers IE, Salas RA, Smikle MF. Patterns of dengue virus IgM and IgG antibodies in suspected cases of dengue in Jamaica, 2003–2006. Hum Antibodies. 2009;18:29–34. doi: 10.3233/HAB-2009-0199. [DOI] [PubMed] [Google Scholar]
- 12.Campbell CA, George A, Salas RA, Williams SA, Doon R, Chadee DD, et al. Seroprevalence of dengue in Trinidad using rapid test kits: a cord blood survey. Acta Trop. 2007;101:153–158. doi: 10.1016/j.actatropica.2006.11.009. Epub 2007 Jan 24. [DOI] [PubMed] [Google Scholar]
- 13.Panagos A, Lacy ER, Gubler DJ, Macpherson CN. Dengue in Grenada. Rev Panam Salud Publica. 2005;17:225–229. [PubMed] [Google Scholar]
- 14.Amarakoon AMD, Chen AA, Rawlins SC, Taylor MA. Dengue epidemics—its association with precipitation and temperature, and its seasonality in some Caribbean countries. West Indian Med J. 2004;53(Suppl 2):60–60. [Google Scholar]
- 15.Teelucksingh S, Lutchman G, Udit A, Pooransingh S. Childhood dengue shock syndrome in Trinidad. West Indian Med J. 1999;48:115–117. [PubMed] [Google Scholar]
- 16.Silk BJ, Berkelman RL. A review of strategies for enhancing the completeness of notifiable disease reporting. J Public Health Man. 2005;11:191–200. doi: 10.1097/00124784-200505000-00003. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization . comprehensive guidelines. New Delhi: WHO Regional Office for South-East Asia; 1999. Prevention and control of dengue and dengue haemorrhagic fever; pp. 29–29. [Google Scholar]
- 18.Ranzinger S, Horstick O, Marx M, Kroeger A. What does dengue disease surveillance contribute to predicting and detecting outbreaks and describing trends? Trop Med Int Heal. 2008;3:1022–1041. doi: 10.1111/j.1365-3156.2008.02112.x. [DOI] [PubMed] [Google Scholar]
- 19.Sequeira M, Espinoza H, Amador JJ, Domingo G, Quintanilla M, de los Santos T. Dengue in Nicaragua. Seattle, Washington: PATH; 2010. [Google Scholar]
- 20.Rodriguez-Barraquer I, Cordeiro MT, Braga C, de Souza WV, Marques ET, Cummings DA, et al. From re-emergence to hyperendemicity: the natural history of the dengue epidemic in Brazil. PLoS Negl Trop Dis. 2011;5: doi: 10.1371/journal.pntd.0000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramos MM, Argüello DF, Luxemburger C. Epidemiological and clinical observations on patients with dengue in Puerto Rico: results from the first year of enhanced surveillance, June 2005–May 2006. Am J Trop Med Hyg. 2008;79:123–127. [PubMed] [Google Scholar]
- 22.Teixeira MG, Siqueira JB, Jr, Ferreira GLC, Bricks L, Joint G. Epidemiological trends of dengue disease in Brazil (2000–2010): a systematic literature search and analysis. PLoS Negl Trop Dis. 2013;7: doi: 10.1371/journal.pntd.0002520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cummings DA, Iamsirithaworn S, Lessler JT, McDermott A, Prasanthong R, Nisalak A, et al. The impact of the demographic transition on dengue in Thailand: insights from a statistical analysis and mathematical modeling. PLoS Med. 2009;6: doi: 10.1371/journal.pmed.1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hammond SN, Balmaseda A, Perez L, llez Y, Saborio SI, Mercado JC, et al. Differences in dengue severity in infants, children, and adults in a 3-year hospital-based study in Nicaragua. Am J Trop Med Hyg. 2005;73:1063–1070. [PubMed] [Google Scholar]
- 25.Kittigul L, Pitakarnjanakul P, Sujirarat D, Siripanichgon K. The differences of clinical manifestations and laboratory findings in children and adults with dengue virus infection. J Clin Virol. 2007;39:76–81. doi: 10.1016/j.jcv.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 26.Guzman MG, Alvarez M, Halstead SB. Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: an historical perspective and role of antibody-dependent enhancement of infection. Arch Virol. 2013;158:1445–1459. doi: 10.1007/s00705-013-1645-3. [DOI] [PubMed] [Google Scholar]
- 27.Bhatia R, Dash AP, Sunyoto T. Changing epidemiology of dengue in South-East Asia. WHO South-East Asia J Public Health. 2013;2:23–27. doi: 10.4103/2224-3151.115830. [DOI] [PubMed] [Google Scholar]
- 28.Sedhain A, Adhikari S, Bhattarai GR, Regmi S, Subedee LR, Chaudhary SK, et al. A clinicoradiological and laboratory analysis of dengue cases during an outbreak in central Nepal. Dengue Bulletin. 2012;36:134–148. [Google Scholar]
- 29.Effler PV, Pang L, Kitsutani P, Vorndam V, Nakata M, Ayers T, et al. Dengue fever, Hawaii, 2001–2002. Emerg Infect Dis. 2005;11:742–749. doi: 10.3201/eid1105.041063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagao Y, Tawatsin A, Thammapalo S, Thavara U. Geographical gradient of mean age of dengue haemorrhagic fever patients in northern Thailand. Epidemiol Infect. 2012;140:479–490. doi: 10.1017/S0950268811000653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt AC. Response to dengue fever—the good, the bad, and the ugly? N Eng J Med. 2010;363:484–487. doi: 10.1056/NEJMcibr1005904. [DOI] [PubMed] [Google Scholar]
- 32.Chadee DD, Williams FL, Kitron UD. Epidemiology of dengue fever in Trinidad, West Indies: the outbreak of 1998. Ann Trop Med Parasitol. 2004;98:305–312. doi: 10.1179/000349804225003307. [DOI] [PubMed] [Google Scholar]