Abstract
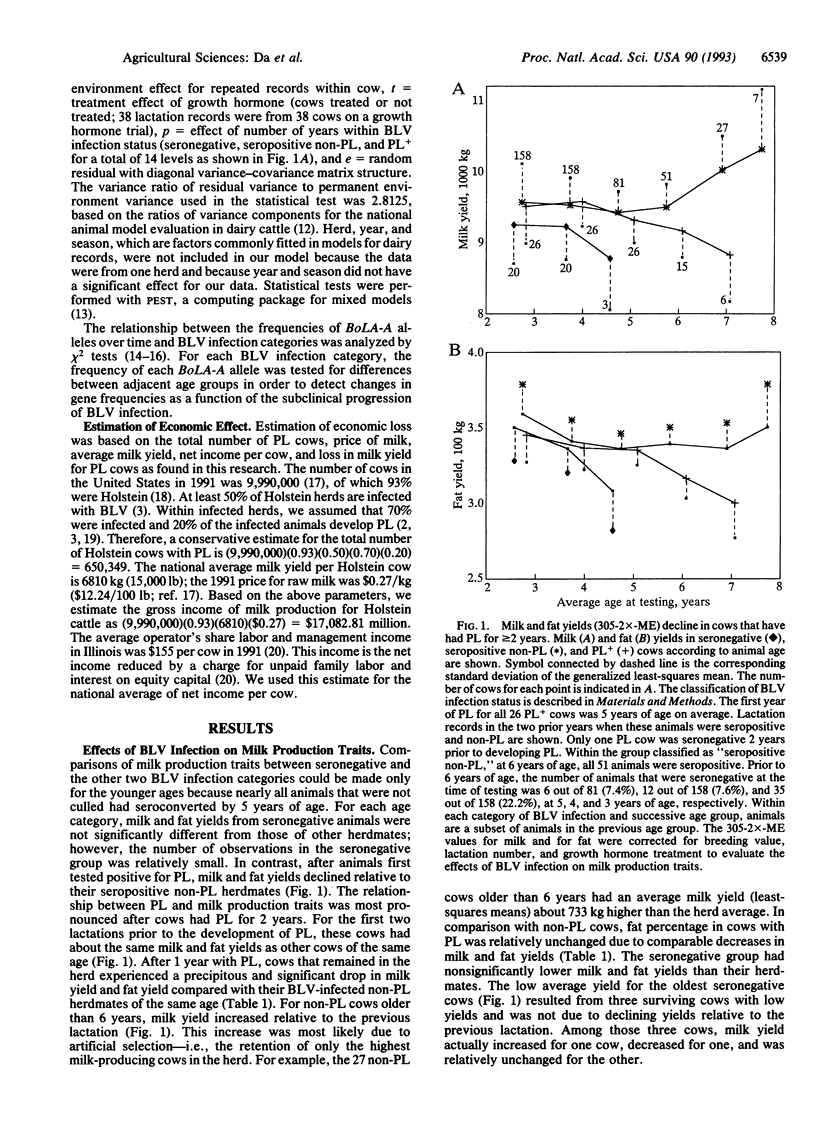
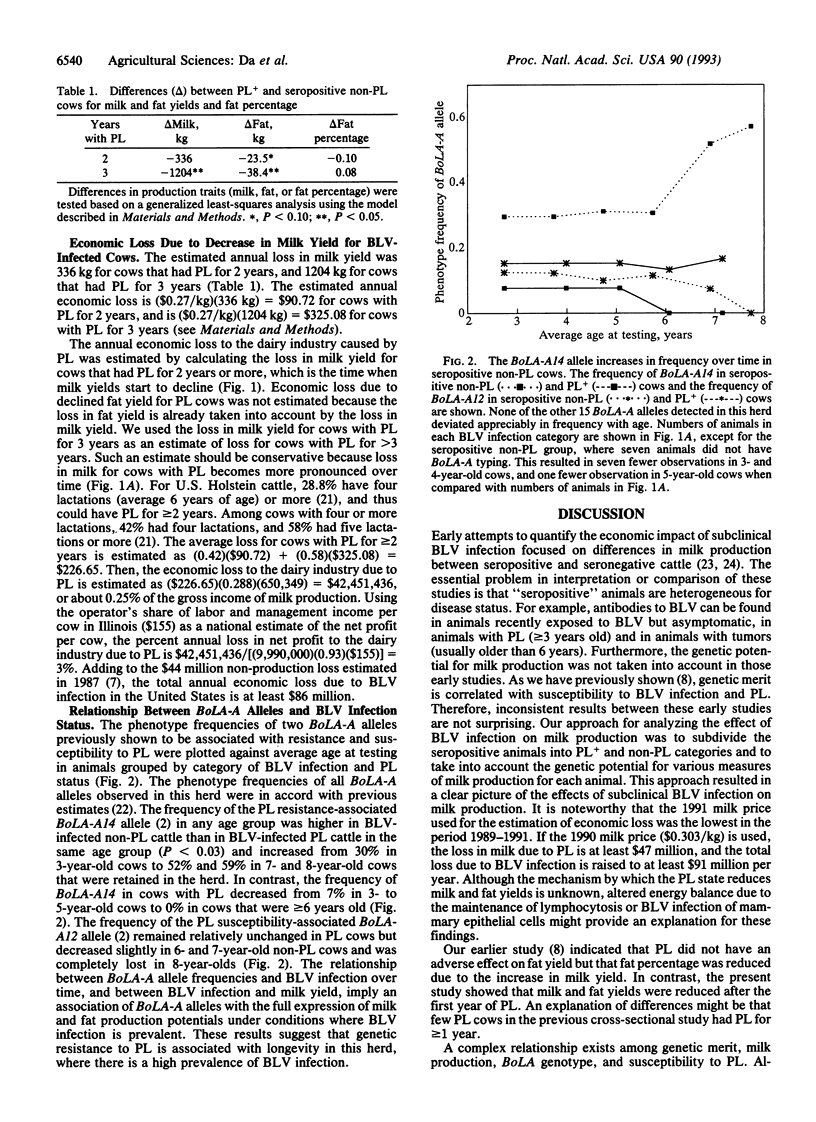
Effects of bovine leukemia virus (BLV) infection on milk and fat yields were studied by using data collected from Holstein cows over a 6-year period. Milk and fat yields in BLV-infected cows with persistent lymphocytosis (PL) declined significantly relative to their BLV-infected non-PL herdmates. Declines were most pronounced in cows older than 6 years. The estimated loss to the dairy industry due to PL is more than $42 million annually. A major histocompatibility complex class I (BoLA-A) allele that has been previously associated with resistance to PL was associated with longevity and realization of milk production potentials, indicating that genetic resistance to PL will have an economic benefit in herds where BLV is endemic.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abt D. A., Marshak R. R., Kulp H. W., Pollock R. J., Jr Studies on the relationship between lymphocytosis and bovine leukosis. Bibl Haematol. 1970;(36):527–536. doi: 10.1159/000391747. [DOI] [PubMed] [Google Scholar]
- Bernoco D., Lewin H. A., Andersson L., Arriens M. A., Byrns G., Cwik S., Davies C. J., Hines H. C., Leibold W., Lie O. Joint Report of the Fourth International Bovine Lymphocyte Antigen (BoLA) Workshop, East Lansing, Michigan, USA, 25 August 1990. Anim Genet. 1991;22(6):477–496. doi: 10.1111/j.1365-2052.1991.tb00719.x. [DOI] [PubMed] [Google Scholar]
- Brenner J., Van-Haam M., Savir D., Trainin Z. The implication of BLV infection in the productivity, reproductive capacity and survival rate of a dairy cow. Vet Immunol Immunopathol. 1989 Oct;22(3):299–305. doi: 10.1016/0165-2427(89)90017-2. [DOI] [PubMed] [Google Scholar]
- Bull R. W., Lewin H. A., Wu M. C., Peterbaugh K., Antczak D., Bernoco D., Cwik S., Dam L., Davies C., Dawkins R. L. Joint report of the Third International Bovine Lymphocyte Antigen (BoLA) Workshop, Helsinki, Finland, 27 July 1986. Anim Genet. 1989;20(1):109–132. doi: 10.1111/j.1365-2052.1989.tb00849.x. [DOI] [PubMed] [Google Scholar]
- Davies C. J., Antczak D. F. Production and characterization of alloantisera specific for bovine class II major histocompatibility complex antigens. Anim Genet. 1991;22(5):417–434. doi: 10.1111/j.1365-2052.1991.tb00701.x. [DOI] [PubMed] [Google Scholar]
- Ferrer J. F., Marshak R. R., Abt D. A., Kenyon S. J. Persistent lymphocytosis in cattle: its cause, nature and relation to lymphosarcoma. Ann Rech Vet. 1978;9(4):851–857. [PubMed] [Google Scholar]
- HALDANE J. B. The estimation and significance of the logarithm of a ratio of frequencies. Ann Hum Genet. 1956 May;20(4):309–311. doi: 10.1111/j.1469-1809.1955.tb01285.x. [DOI] [PubMed] [Google Scholar]
- Huber N. L., DiGiacomo R. F., Evermann J. F., Studer E. Bovine leukemia virus infection in a large Holstein herd: prospective comparison of production and reproductive performance in antibody-negative and antibody-positive cows. Am J Vet Res. 1981 Sep;42(9):1477–1481. [PubMed] [Google Scholar]
- Langston A., Ferdinand G. A., Ruppanner R., Theilen G. H., Drlica S., Behymer D. Comparison of production variables of bovine leukemia virus antibody-negative and antibody-positive cows in two California dairy herds. Am J Vet Res. 1978 Jul;39(7):1093–1098. [PubMed] [Google Scholar]
- Lewin H. A., Schmitt K., Hubert R., van Eijk M. J., Arnheim N. Close linkage between bovine prolactin and BoLA-DRB3 genes: genetic mapping in cattle by single sperm typing. Genomics. 1992 May;13(1):44–48. doi: 10.1016/0888-7543(92)90200-c. [DOI] [PubMed] [Google Scholar]
- Lewin H. A., Wu M. C., Stewart J. A., Nolan T. J. Association between BoLA and subclinical bovine leukemia virus infection in a herd of Holstein-Friesian cows. Immunogenetics. 1988;27(5):338–344. doi: 10.1007/BF00395129. [DOI] [PubMed] [Google Scholar]
- Mammerickx M., Lorenz R. J., Straub O. C., Donnelly W. J., Flensburg J. C., Gentile G., Markson L. M., Ressang A. A., Taylor S. M. Bovine hematology. III. Comparative breed studies on the leukocyte parameters of several European cattle breeds as determined in the common reference laboratory. Zentralbl Veterinarmed B. 1978 May;25(4):257–267. [PubMed] [Google Scholar]
- Sagata N., Yasunaga T., Tsuzuku-Kawamura J., Ohishi K., Ogawa Y., Ikawa Y. Complete nucleotide sequence of the genome of bovine leukemia virus: its evolutionary relationship to other retroviruses. Proc Natl Acad Sci U S A. 1985 Feb;82(3):677–681. doi: 10.1073/pnas.82.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOLF B. On estimating the relation between blood group and disease. Ann Hum Genet. 1955 Jun;19(4):251–253. doi: 10.1111/j.1469-1809.1955.tb01348.x. [DOI] [PubMed] [Google Scholar]
- Wu M. C., Shanks R. D., Lewin H. A. Milk and fat production in dairy cattle influenced by advanced subclinical bovine leukemia virus infection. Proc Natl Acad Sci U S A. 1989 Feb;86(3):993–996. doi: 10.1073/pnas.86.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eijk M. J., Stewart-Haynes J. A., Beever J. E., Fernando R. L., Lewin H. A. Development of persistent lymphocytosis in cattle is closely associated with DRB2. Immunogenetics. 1992;37(1):64–68. doi: 10.1007/BF00223546. [DOI] [PubMed] [Google Scholar]



