Abstract
The prevalence of cefotaxime-resistant Salmonella enterica serotype Virchow has dramatically increased in South Korea since the first isolation in 2011. Of 68 isolates collected over 10 years, 28 cefotaxime-resistant isolates harbored the blaCTX-M-15 extended-spectrum β-lactamase gene and were closely related genetically, demonstrating the clonal dissemination of CTX-M-15–producing Salmonella Virchow in South Korea.
Keywords: extended-spectrum β-lactamases, ESBL, Salmonella enterica, CTX-M-15, Salmonella enterica serotype Virchow, IncHI2, emergence, clonal dissemination, S. enterica, South Korea, bacteria, prevalence, nontyphoidal salmonella, antimicrobial resistance, surveillance, salmonella
Nontyphoidal salmonella, a foodborne pathogen, causes human gastroenteritis worldwide. Among >2,500 different Salmonella enterica serotypes, Salmonella Enteritidis and Salmonella Typhimurium are the most common serotypes responsible for human salmonellosis (1). In Europe, Salmonella Virchow has recently increased in prevalence, and a high proportion of isolated strains are resistant to multiple antimicrobial drugs (2–4).
Third-generation cephalosporins are widely used to treat major bacterial infections in humans and animals (5). However, the emergence and rapid spread of drug-resistant bacteria has become a serious public health concern. Extended-spectrum β-lactamases (ESBLs) are known to confer antimicrobial drug resistance by hydrolyzing most β-lactam antimicrobial drugs, including third-generation cephalosporins (5). Since the first report from Spain in 2000 of strains producing CTX-M-9 (6), which confers resistance to cefotaxime, various CTX-M–type ESBLs have been identified in Salmonella Virchow. In Spain, Belgium, and France, CTX-M-9 and CTX-M-2 producers spread clonally in humans and poultry (7,8). In addition, the blaCTX-M-15 gene was identified in porcine isolates in South Korea (9). These reports demonstrate that CTX-M–producing Salmonella Virchow clones can be easily transmitted to humans through food products of animal origin. In South Korea, the incidence of Salmonella Virchow infections in humans has increased over the years, necessitating a nationwide survey of antimicrobial drug resistance in Salmonella Virchow isolates.
The Study
During 2005–2014 in South Korea, local public health laboratories participating in the national surveillance network isolated 68 Salmonella Virchow strains from feces samples from patients with acute diarrhea. Until 2010, <5 Salmonella Virchow strains were isolated per year, but this number gradually increased to 17 in 2014 (Figure). Salmonella Virchow consistently ranked among the top 10 serotypes in prevalence during each study year in South Korea, accounting for ≈1.5%–2% of salmonellosis cases.
Figure.
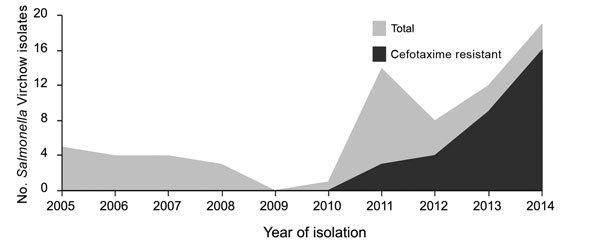
Temporal distribution of Salmonella enterica serotype Virchow isolates in South Korea, 2005–2014.
We used the broth microdilution method (10) to perform antimicrobial susceptibility testing of Salmonella Virchow; results showed that 54 (79.4%) of the 68 isolates were resistant to >1 of the 15 antimicrobial agents tested (Table; Technical Appendix Figure). The highest resistance rates were noted for nalidixic acid (77.9%), followed by ampicillin (44.1%), cefotaxime (44.1%), tetracycline (42.6%), and gentamicin (23.5%). Only 2 (2.9%) isolates were resistant to ciprofloxacin, but 50 (73.5%) had intermediate resistance. All of the isolates were susceptible to chloramphenicol, amikacin, or imipenem. Multidrug resistance, defined as resistance to at least 3 different classes of antimicrobial agents, was found in 30 (44.1%) isolates (Table).
Table. Antimicrobial resistance profiles of Salmonella enterica serotype Virchow isolates in South Korea, 2005–2014*.
| Isolate no. | Antimicrobial drug | No. resistant isolates |
|---|---|---|
| 1 | – | 14 |
| 2 | NAL | 18 |
| 3 | TCY | 2 |
| 4 | NAL, SXT | 2 |
| 5 | TCY, NAL | 2 |
| 6 | AMP, CEF, CTX, NAL | 5 |
| 7 | AMP, CEF, CTX, NAL, TCY | 7 |
| 8 | AMP, CEF, CTX, GEN, NAL | 1 |
| 9 | AMP, CEF, CTX, GEN, NAL, TCY | 15 |
| 10 | AMC, AMP, CEF, CTX, FOX, SAM, NAL | 1 |
| 11 |
AMC, AMP, CEF, CTX, FOX, SAM, NAL, TCY |
1 |
| Total | 68 |
*AMC, amoxicillin/clavulanic acid; AMP, ampicillin; CEF, cephalothin; CTX, cefotaxime; FOX, cefoxitin; GEN, gentamicin; NAL, nalidixic acid; SAM, ampicillin/sulbactam; SXT, trimethoprim/sulfamethoxazole; TCY, tetracycline; –, pansusceptible.
All multidrug-resistant isolates showed resistance to third-generation cephalosporins. In South Korea, cefotaxime-resistant strains were first isolated in 2011. Since then, 4, 9, and 14 isolates were collected in 2012, 2013, and 2014, respectively (Figure). The rates of cefotaxime resistance in Salmonella Virchow have increased markedly, from 21.4% in 2011 to 82.3% in 2014. This annual trend of increasing cefotaxime resistance in South Korea is of interest because the rates were substantially higher than those reported in Spain during 2002–2006 (15%) (11) and Belgium during 2009–2013 (<10%) (12). Moreover, even in Israel and Switzerland, where the incidence of Salmonella Virchow was relatively higher than that in South Korea, antimicrobial drug resistance to third-generation cephalosporins was rare (3,4).
Among the 30 cefotaxime-resistant Salmonella Virchow isolates, 28 were confirmed to be ESBL-producers. PCR and sequencing of β-lactamase genes (13) confirmed that these 28 isolates harbored a blaCTX-M-15 gene, whereas the other 2 contained a blaCMY-2 gene. Cefotaxime resistance was transferred by conjugation from 9 Salmonella Virchow isolates to Escherichia coli J53 recipients, and the blaCTX-M-15 gene was identified in transconjugants. Southern blotting and PCR-based replicon typing (14) showed that all plasmids in transconjugants were ≈215 kb in size and possessed an IncHI2 plasmid, which was further assigned to sequence type (ST) 2 by plasmid double locus sequence typing (15). The analysis of the genetic environment surrounding the blaCTX-M-15 gene (13) in transconjugants showed that insertion sequences ISEcp1 and orf477 were detected 48 bp upstream and downstream of the blaCTX-M-15 gene, respectively. This ISEcp1-blaCTX-M-15–open reading frame 477 transposable unit was also identified in other incompatibility groups of the plasmids in Enterobacteriaceae. Furthermore, it was identical to that of the ST2-IncHI2 plasmid of Salmonella Enteritidis isolated from humans and poultry meat in South Korea (J. Kim, unpub. data), suggesting that the blaCTX-M-15 gene in Salmonella Virchow might have originated from ISEcp1-mediated transposition followed by interspecies spread through the IncHI2-type plasmid studied here.
All of the CTX-M-15–producing strains had reduced ciprofloxacin susceptibility (MICs of 0.25–0.5 µg/mL). All 10 randomly selected isolates harbored a single substitution within the quinolone resistance–determining region of GyrA at codon 83 (Ser→Phe), which is the major mutation described in Salmonella species (8). Because fluoroquinolones and third-generation cephalosporins are the drugs of choice for treating severe salmonella infections in humans, the reduced ciprofloxacin susceptibility in cefotaxime-resistant Salmonella Virchow is considered a critical risk factor for infections with these strains.
The genetic relationship of the 68 Salmonella Virchow isolates was determined by using multilocus sequence typing (http://mlst.warwick.ac.uk/mlst/dbs/Senterica) and pulsed-field gel electrophoresis (PFGE) according to a standardized protocol. Seven multilocus sequence typing loci displayed 4 different profiles, and most isolates belonged to sequence type (ST) 16 (n = 59), followed by ST197 (n = 6), ST359 (n = 2), and ST426 (n = 1). All of the 28 CTX-M-15–producing strains were typed as ST16, but the cefotaxime-susceptible isolates were also assigned to this type. The PFGE analysis yielded sufficient discriminatory power in typing Salmonella Virchow isolates; 22 XbaI and 21 BlnI PFGE patterns were generated. Although the isolates shared >70% similarity, CTX-M-15–producing strains clustered on the basis of a similarity value of 90% (Technical Appendix Figure), indicating the clonality of cefotaxime-resistant strains.
For humans, the main route of Salmonella infection is the consumption of contaminated food of animal origin, and Salmonella Virchow is one of the most prevalent serotypes identified in poultry and poultry products. The use of cephalosporins in animal production has led to emergence of antimicrobial drug–resistant Salmonella Virchow clones among food animals (8), posing a threat to public health because of the possible transmission of these bacteria through the food chain. In fact, the XbaI PFGE pattern identified among human isolates in this study was identical to that in 2 cefotaxime-resistant Salmonella Virchow strains isolated in 2012 from poultry meat in our collection (Technical Appendix Figure). Furthermore, this pattern appeared similar to the patterns of Salmonella Virchow harboring the blaCTX-M-15 gene on an ST2-IncHI2 plasmid that was isolated from organically raised pigs in South Korea (9). Resistant clinical strains were collected from 13 of 15 local public health laboratories in South Korea during 2011–2014; thus, although the mode of the spread of Salmonella Virchow in humans is not established, it has been postulated that CTX-M-15–producing Salmonella Virchow might have disseminated clonally through the nationwide distribution of contaminated food products rather than through independent emergence.
Conclusions
We analyzed the antimicrobial drug susceptibility and genetic relatedness of Salmonella Virchow isolates from patients with diarrhea in South Korea. Of 68 isolates obtained during 2005–2014, a total of 30 were resistant to third-generation cephalosporins. The prevalence rate of the resistant strains has dramatically increased since the isolation of cefotaxime-resistant strains in 2011. These findings suggest that the cefotaxime-resistant isolates are genetically closely related and harbor a plasmid carrying the blaCTX-M-15 gene of the same compatibility group (ST2-IncHI2), representing clonal dissemination of CTX-M-15–producing Salmonella Virchow in South Korea and posing an urgent threat to public health. Therefore, more comprehensive surveillance is required to prevent further spread of resistant clonal strains.
Composite dendrogram of the genetic relatedness among Salmonella enterica serotype Virchow isolates in Republic of Korea.
Acknowledgment
Members of the PulseNet Korea Working Group follow (listed in alphabetical order by the location, in South Korea, of their local institute for public health and environment): Sunhee Park (Busan), Kwisung Park (Chungcheongnam-do), Eunjin Lee (Daegu), Mijung Kim (Daejeon), Woonho Kim and Minjung Park (Gyeonggi-do), Minji Kim (Gwangju), Yeong-Hee Yeo (Gyeongsangnam-do), Sungyoung Son (Gangwon-do), Junghee Kim (Incheon), Kiho Nam (Incheon airport), Jinsuk Lim (Jeju), Cheonhyeon Kim (Jeollabuk-do), Byeong-Jun Song (Jeollanam-do), Hyunjung Seung (Seoul), and Gwiae Lee (Ulsan).
We thank the members of the PulseNet Korea working group for collecting isolates of the Salmonella Virchow strains used in this study.
This work was supported by a grant from the Korea Centers for Disease Control and Prevention (4847-311-210).
Biography
Dr. J.S. Kim is a senior researcher at Center for Infectious Disease in the Korea National Institute of Health. His primary research interests include antimicrobial drug susceptibility and molecular epidemiology of enteric bacteria.
Footnotes
Suggested citation for this article: Kim JS, Yun YS, Kim SJ, Jeon SE, Lee DY, Chung GT, et al. Rapid emergence and clonal dissemination of CTX-M-15–producing Salmonella enterica serotype Virchow, South Korea. Emerg Infect Dis. 2016 Jan [date cited]. http://dx.doi.org/10.3201/eid2201.151220
These co–first authors contributed equally to this article.
Members of the PulseNet Korea Working Group are listed at the end of the article.
References
- 1.Hendriksen RS, Vieira AR, Karlsmose S, Lo Fo Wong DM, Jensen AB, Wegener HC, et al. Global monitoring of Salmonella serovar distribution from the World Health Organization Global Foodborne Infections Network Country Data Bank: results of quality assured laboratories from 2001 to 2007. Foodborne Pathog Dis. 2011;8:887–900. 10.1089/fpd.2010.0787 [DOI] [PubMed] [Google Scholar]
- 2.Matheson N, Kingsley RA, Sturgess K, Aliyu SH, Wain J, Dougan G, et al. Ten years experience of Salmonella infections in Cambridge, UK. J Infect. 2010;60:21–5. 10.1016/j.jinf.2009.09.016 [DOI] [PubMed] [Google Scholar]
- 3.Bonalli M, Stephan R, Kappeli U, Cernela N, Adank L, Hachler H. Salmonella enterica serotype Virchow associated with human infections in Switzerland: 2004–2009. BMC Infect Dis. 2011;11:49. 10.1186/1471-2334-11-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bassal R, Reisfeld A, Andorn N, Yishai R, Nissan I, Agmon V, et al. Recent trends in the epidemiology of non-typhoidal Salmonella in Israel, 1999–2009. Epidemiol Infect. 2012;140:1446–53. 10.1017/S095026881100197X [DOI] [PubMed] [Google Scholar]
- 5.Miriagou V, Tassios PT, Legakis NJ, Tzouvelekis LS. Expanded-spectrum cephalosporin resistance in non-typhoid Salmonella. Int J Antimicrob Agents. 2004;23:547–55. 10.1016/j.ijantimicag.2004.03.006 [DOI] [PubMed] [Google Scholar]
- 6.Simarro E, Navarro F, Ruiz J, Miro E, Gomez J, Mirelis B. Salmonella enterica serovar Virchow with CTX-M–like β-lactamase in Spain. J Clin Microbiol. 2000;38:4676–8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riaño I, García-Campello M, Sáenz Y, Alvarez P, Vinué L, Lantero M, et al. Occurrence of extended-spectrum β-lactamase–producing Salmonella enterica in northern Spain with evidence of CTX-M-9 clonal spread among animals and humans. Clin Microbiol Infect. 2009;15:292–5. 10.1111/j.1469-0691.2008.02673.x [DOI] [PubMed] [Google Scholar]
- 8.Bertrand S, Weill FX, Cloeckaert A, Vrints M, Mairiaux E, Praud K, et al. Clonal emergence of extended-spectrum β-lactamase (CTX-M-2)–producing Salmonella enterica serovar Virchow isolates with reduced susceptibilities to ciprofloxacin among poultry and humans in Belgium and France (2000 to 2003). J Clin Microbiol. 2006;44:2897–903. 10.1128/JCM.02549-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tamang MD, Gurung M, Nam HM, Moon DC, Kim SR, Jang GC, et al. Prevalence and characterization of Salmonella in pigs from conventional and organic farms and first report of S. serovar 1,4,[5],12:i:- from Korea. Vet Microbiol. 2015;178:119–24. 10.1016/j.vetmic.2015.05.005 [DOI] [PubMed] [Google Scholar]
- 10.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; twenty-second informational supplement. Document M100-S22. 2012. [cited 2015 Jan 1]. http://antimicrobianos.com.ar/ATB/wp-content/uploads/2012/11/M100S22E.pdf
- 11.Herrera-León S, González-Sanz R, Rodriguez I, Rodicio MR, Echeita MA. Spread of a multiresistant CTX-M-9–producing Salmonella enterica serotype Virchow phage type 19 in Spain. Eur J Clin Microbiol Infect Dis. 2010;29:901–5. 10.1007/s10096-010-0939-6 [DOI] [PubMed] [Google Scholar]
- 12.Ceyssens PJ, Mattheus W, Vanhoof R, Bertrand S. Trends in serotype distribution and antimicrobial susceptibility in Salmonella enterica isolates from humans in Belgium, 2009 to 2013. Antimicrob Agents Chemother. 2015;59:544–52. 10.1128/AAC.04203-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JS, Kim J, Jeon SE, Kim SJ, Kim NO, Hong S, et al. Complete nucleotide sequence of the IncI1 plasmid pSH4469 encoding CTX-M-15 extended–spectrum β-lactamase in a clinical isolate of Shigella sonnei from an outbreak in the Republic of Korea. Int J Antimicrob Agents. 2014;44:533–7. 10.1016/j.ijantimicag.2014.08.007 [DOI] [PubMed] [Google Scholar]
- 14.Carattoli A, Bertini A, Villa L, Falbo V, Hopkins KL, Threlfall EJ. Identification of plasmids by PCR-based replicon typing. J Microbiol Methods. 2005;63:219–28. 10.1016/j.mimet.2005.03.018 [DOI] [PubMed] [Google Scholar]
- 15.García-Fernández A, Carattoli A. Plasmid double locus sequence typing for IncHI2 plasmids, a subtyping scheme for the characterization of IncHI2 plasmids carrying extended-spectrum β-lactamase and quinolone resistance genes. J Antimicrob Chemother. 2010;65:1155–61. 10.1093/jac/dkq101 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Composite dendrogram of the genetic relatedness among Salmonella enterica serotype Virchow isolates in Republic of Korea.


