Abstract
Background
Hyperlipidemia and insulin resistance are risk factors for the development of metabolic syndrome and cardiovascular disease. We have previously observed that supplementation with essential amino acids (EAA) could lower plasma triglycerides, and may improve glucose metabolism.
Objective
We sought to determine whether EAA’s combined with whey protein and phytosterols would facilitate improvements in plasma lipids and insulin sensitivity in adults with mild hypertriglyceridemia.
Design
We enrolled nine subjects who were 50 years or older, had a documented plasma TG >150 mg/dl, and had not recently taken statin medications (within 6 weeks). Each subject served as his or her own control. These individuals underwent an oral glucose tolerance test (OGTT) before and after four weeks consumption of the oral nutritional supplement without dietary counseling or recommendations for physical activity.
Results
Plasma total cholesterol and LDL levels decreased in all nine volunteers (P<0.005 for cholesterol and P<0.02 for LDL). In six of these individuals, plasma triglycerides (TG) fell by 95±13 mg/dl (P=0.007); while the other three showed no TG reduction. Genotyping revealed that in two of the three individuals that did not have TG reduction in response to the nutritional supplementation. Insulin sensitivity (ISI) and the total AUCins/glucose were significantly reduced by leucine/EAAs and phytosterol supplementation (P=0.008).
Conclusions
These findings suggest that a dietary supplementation of EAAs and phytosterols may promote favorable reductions of blood lipids as well as insulin resistance in individuals with hypertriglyceridemia. Future larger studies of SNPs and TG response to dietary supplements will be of interest.
INTRODUCTION
Metabolic syndrome, a major risk factor for cardiovascular disease, is characterized by chronic elevation in plasma triglycerides, elevated blood pressure, and reduced insulin sensitivity [1]. Moreover, the chronic accumulation of low-density lipoprotein (LDL) and triglyceride-rich lipoproteins, including very low-density lipoproteins (VLDL), are also closely associated with metabolic syndrome and increased risk for cardiovascular disease [2]. As a result, hypertriglyceridemia may be utilized as a marker for an overall elevation in atherogenic lipids, although plasma triglycerides (TGs) may not always be measured on a routine basis [3]. Elevations in TGs are also considered a central component in the pathophysiology that leads to dyslipoproteinemia in insulin resistance, type 2 diabetes and the development of cardiovascular disease [4].
Conventional pharmacological therapy to reduce TGs includes nicotinic acid and peroxisome proliferator-activated receptor alpha (PPAR-α) agonist, fenofibrate. Nicotinic acid inhibits peripheral lipolysis, thereby reducing the delivery of FFA to the liver and decreasing hepatic triglyceride synthesis. However, side effects such as facial and peripheral flushing with diffuse itching are a major limitation in its use at adequate doses, and prolonged use has been reported to induce signs of liver damage in many (up to 50%) of the elderly patients [5]. PPAR-α agonists are thought to reduce circulating triglyceride levels by stimulating the oxidative capacity of mitochondria and peroxisomes [5]. As is the case with nicotinic acid, fibrates can also cause impaired liver function and pruritic rashes in a significant number of patients [6–8]. Various approaches have been used to limit triglyceride absorption, including dietary additives (e.g., Olestra). While these approaches may be effective in lowering triglyceride levels in the blood, their usefulness has been limited by the frequent induction of loose stools or diarrhea.
Isocaloric diets containing a relatively high proportion of protein have generally been shown to improve plasma triglycerides to the same extent as PPAR-α agonists and statins. For example, a diet consisting of 22% protein was observed to result in significantly lowered plasma triglycerides by 32% after 4 weeks, compared to a diet of 12% protein [9]. When patients with type 2 diabetes switched 15–30% of their calories from carbohydrates to protein, fasting TG decreased by 22% [10]. Plasma triglycerides were reduced by ~23% following a high protein diet in patients with pre-existing hypercholesterolemia [11,12]. The Omni Heart Trial study, in which the effect of increasing protein intake from 15 to 25% of total calories (with a corresponding reduction in carbohydrate calories), reported that total cholesterol and triglycerides were significantly reduced in the high protein group after six weeks [13]. Only the total triglycerides were significantly reduced when the high protein diet was compared with a diet in which the proportion of unsaturated fat, as opposed to protein, was increased. Importantly, substitution of protein for carbohydrate reduced atherogenic apo C-III-containing LDL and its precursor, apo C-III-containing VLDL [14].
The experimental design of most studies in which the protein content of the diet was altered may not distinguish between an effect of increasing protein content or decreasing intake of carbohydrate or fat. This is an important issue, since both carbohydrate and fat intake may influence plasma lipid profiles independently of protein. Thus, 3 weeks of a high carbohydrate diet promoted significant increases in plasma triglycerides due to increased hepatic de novo synthesis of triglycerides [12]. On the other hand, plasma triglycerides were decreased after either a high fat or a high protein diet, as compared to a high carbohydrate diet [12,15]. Numerous studies with a hypo-caloric high protein diet have demonstrated lowered plasma triglycerides, total cholesterol or VLDL cholesterol but it is not clear whether this is due to a caloric deficit or the increased proportion of protein intake [16,17].
Our group recently examined the influence of high leucine (40%)/essential amino acids (EAAs) on the plasma lipid profiles in healthy older subjects [18]. Total caloric intake was not significantly affected by the supplements, which were only 44 Kcal per dose and given twice daily. Circulating TG concentrations were reduced ~20% from the starting value after supplement ingestion for 16 weeks, with the greatest decreases observed in those subjects with the highest values. The magnitude of reduction of circulating TGs was comparable to that observed in our earlier study using fenofibrate in older subjects [19]. In addition to the lowering of TGs by EAAs, intrahepatic lipid was reduced by 50%.
In order to augment the lipid-lowering influence of our high Leucine/ EAA formulation, we have added ~ 0.7 grams of phytosterols/serving in the present study. It has been demonstrated that phytosterols may lower LDL cholesterol in a dose-dependent manner up to 2.5 gr/day [20]. Given that the average dietary intake of phytosterols in western civilization is <400 mg/day [21], it seems plausible that concomitant supplementation of phytosterols in a formulation that is also rich in EAA’s should be even more beneficial in lowering atherogenic blood lipids than utilizing EAA alone. We tested the hypothesis that dietary supplementation with high Leucine (Leu)/EAAs and phytosterols would lower triglycerides, total VLDL, and LDL-cholesterol in individuals with mild hypercholesterolemia. In addition, we proposed that dietary supplementation with (Leu)/EAAs and phytosterols would enhance insulin sensitivity as well. We also evaluated whether the improvements in blood lipids might be correlated with common single nucleotide polymorphisms of several genes that have been reported to be associated with elevated lipids, namely the zinc finger protein 259 (ZNF259), glucokinase (hexokinase 4) regulator (GCKR), lipoprotein lipase (LPL), and/or apolipoprotein (APOB) genes [22–25].
METHODS
We recruited and enrolled adults (males and females) with hypertriglyceridemia (>150 mg/dl, aged 50 years and older), of all races and ethnic backgrounds. None of the volunteers had utilized statins within the past 6 weeks. No subjects were enrolled who were currently taking corticosteroids, or participating in an active exercise program. In addition, history of mental illness, malignancy (within the past 6 months), liver dysfunction, diabetes (regardless of medication usage), or pregnancy were exclusion criteria for the study. Potential subjects were first asked to report for a screening visit, where they were then consented. Once informed consent was obtained, demographics, anthropometrics, vital signs measurements, and a fasting blood sample was collected for a baseline sample of plasma lipids.
Each subject served as his or her own control. All procedures followed in the described studies were performed in accordance with IRB guidelines administered by Schulman and Associates IRB and the ethical standards of the responsible institutional or regional committee on human experimentation or in accordance with the Helsinki Declaration of 1975 as revised in 1983.
Study One
Following the completion of the screening visit, plasma samples were analyzed and volunteers who met the inclusion criteria for participation in the study were asked to return for the second visit that included an oral glucose tolerance test (OGTT), body composition analysis by bioelectrical impedance, and an assessment of vital signs.
Supplementation Phase
Subjects returned to the study site every week (±2 days) to receive their week’s supply of Leu/EAA and phytosterol supplementation (Table 1), to have vital signs measured, to report the occurrence of any adverse events, to have compliance assessed, and to confirm their willingness to continue to participate in the study. Subjects reported on their consumption of the supplement and returned empty bottles from ingested supplement at each week’s visit. Seventy-five percent consumption of the Leu/EAA formulation throughout the 4-week period was considered to be adequate compliance.
Table 1.
Clinical characteristics.
| Pre- Supplementation |
Post- Supplementation |
|
|---|---|---|
| Age (years) | 59.8±3.4 | |
| Body Weight (kg) | 88.1±3.7 | 87.7±1.9 |
| BMI (kg/m2) | 28.7±0.8 | 28.4±0.7 |
| Fat Mass (kg) | 59.0±5.4 | 57.3±5.3 |
| Lean Mass (kg) | 135.1±8.0 | 135.7±9.0 |
| Percent Fat | 30.6±2.6 | 29.9±2.7 |
| Systolic Blood Pressure (mm/Hg) | 131±4 | 128±5 |
| Diastolic Blood Pressure (mm/Hg) | 76±3 | 75±2 |
| Plasma Insulin (uU/ml) | 9.7±0.9 | 7.1±1.1* |
Study Two
Subjects returned to the study site at the conclusion of the 4-week supplementation period for post-testing of body composition, vital signs, blood lipids and an OGTT. After completion of this visit, subjects were dismissed.
Calculations
Whole-body insulin sensitivity was estimated by the Insulin Sensitivity Index (ISI) = 10000/square root of ([fasting glucose × fasting insulin] × [mean glucose × mean insulin during OGTT]) [26]. We also evaluated the influence of supplementation on the total AUCins/gluc (an established method of assessing insulin secretion during an OGTT) [27]. We calculated this variable using the trapezoidal rule applied to the insulin and glucose responses during the OGTT. An alpha-level of 5% was used to determine statistical significance for all variables in this study.
Single Nucleotide Polymorphism Determination
DNA was extracted from blood samples using the QIAamp® DNA mini kit (Qiagen). The DNA concentration of each sample was determined using the Qubit (Invitrogen; Life Technologies Grand Island, NY). Genotyping was performed on the ViiA™ 7 Real-Time PCR System in 5 ul reaction in 384 well plates using the 2× genotyping master mix (Life Technologies) and 20× genotyping primer/probes synthesized by Life Technologies. The SNPs genotyped were rs964184 (ZNF259), rs1260326 (GCKR), rs12678919 (LPL) and rs1042034 (APOB) and 10 no template controls (NTC) were genotyped with the samples. Genotyping data were analyzed in the ViiA7 sequence detection software v2.0. Amplification plots were assessed for correct genotyping calls. There was > 97% genotype call rate.
Statistical Analysis
Variables were summarized using means and standard deviations. The influence of Leu/EAAs and phytosterols on the physiological variables was evaluated using Student’s two-tailed t-test. Bonferroni correction was applied for multiple comparisons.
RESULTS
Subjects
We enrolled 12 volunteers; three subjects dropped out due to their inability to comply with the protocol. Therefore, 9 volunteers (59.8±3.4 years/age) comprised of 4 women and 5 men completed all aspects of the study. For these individuals, the average weight and BMI was 88.2±1.9 kg, and 28.7±0.8 kg/m2, respectively.
Clinical characteristics
There was no significant change in body weight after 4 weeks of supplementation (Figure 1). In addition, there were no significant changes in fat mass (Δ=0.8±0.7 kg), lean body mass (Δ=−0.3±0.8 kg), or percent fat (Δ=0.1±0.9%) (Table 1). There was no significant change in systolic or diastolic blood pressure from pre- to post-supplementation (Table 2).
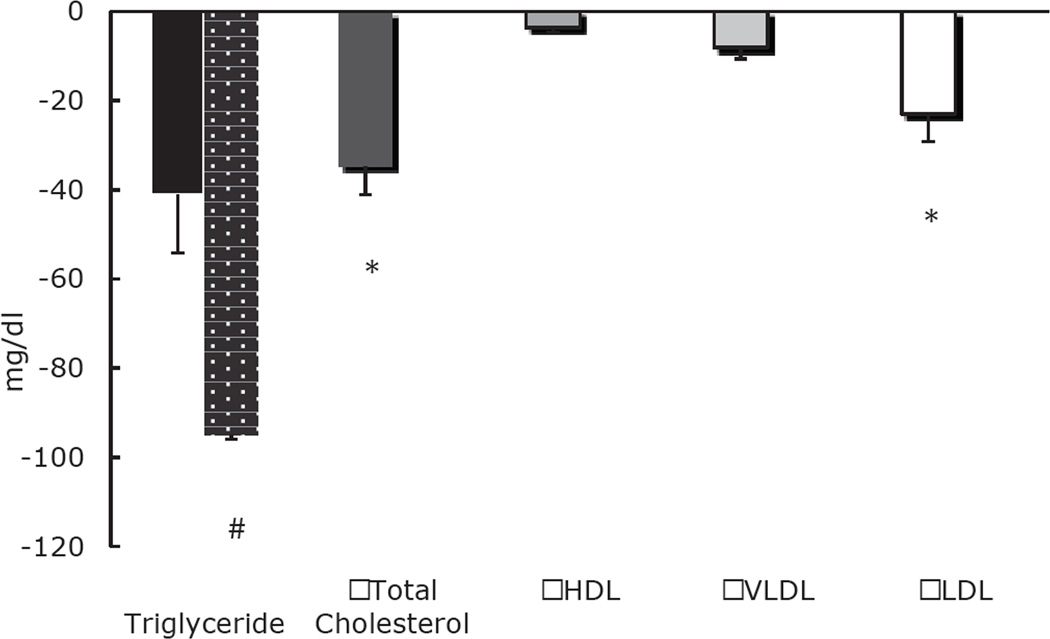
Figure 1.
EAA/Whey Protein/Phytosterol supplement-based changes in plasma triglyceride (TG), cholesterol, HDL, VLDL and LDL concentrations. * Indicates significant reduction from pre- to post-supplementation. # In six subjects that showed a response in TG levels after 4 weeks, the reduction was significant (95±13 mg/dl, P=0.007).
Table 2.
SNPs of genes that may contribute to hypertriglyceridemia.
| Subject # | ZNF259 | GCKR | LPL | APOB |
|---|---|---|---|---|
| rs964184 | rs1260326 | rs12678919 | rs1042034 | |
| 1 | CC | CC | AA | TT |
| 2 | CC | CC | AA | TT |
| 3 | CC | TT | AA | CT |
| 4 | CC | CC | AA | TT |
| 5 | CC | CC | AA | TT |
| 6 | CC | CT | AA | CT |
| 7 | CC | CT | AA | TT |
| 8 | CG | CC | AA | TT |
| 9 | CC | CT | AA | TT |
Plasma lipids
There was a slight trend towards a decrease in plasma triglycerides from pre- to post-supplementation in the volunteers (P =0.12) (Figure 1). There was an increase in triglycerides in three of the nine individuals; however, in the remaining six subjects, there was a significant and remarkable decrease in plasma triglycerides from 271±30 mg/dl to 176±28 mg/dl (P=0.007) (Figure 1).
In all subjects, there was a significant decrease in total cholesterol from 231±13 mg/dl at baseline to 197±15 mg/dl following 4 weeks of supplementation (P=0.005, Figure 1). In a similar fashion, LDL-cholesterol decreased significantly from 142±11 mg/dl at pre-supplementation to 119±14 mg/dl post-supplementation (P=0.02, Figure 1). While there was an absolute reduction in VLDL, it was not significant (P=0.12) (Figure 2). There was also a small, but not significant, decrease in HDL from 40±3 mg/dl at baseline to 36±3 mg/dl post-supplementation (P=0.06) (Figure 1).
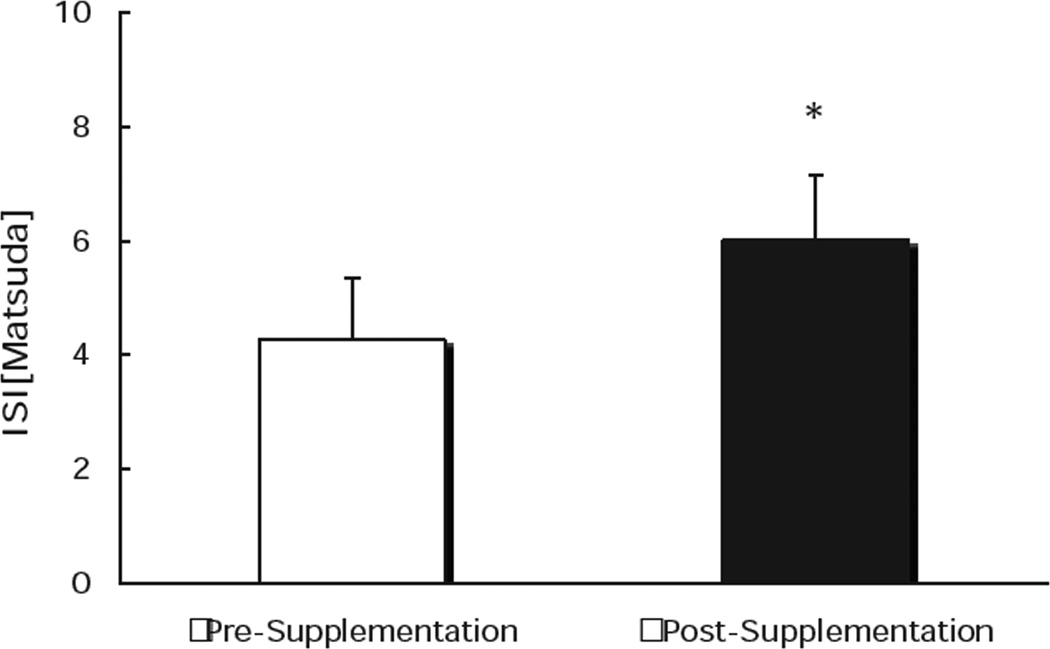
Figure 2.
EAA/Whey Protein/Phytosterol supplement-based changes in insulin sensitivity derived from Matsuda index. * Indicates significant increase from pre- to post-supplementation.
Single Nucleotide Polymorphisms of Select Genes
The changes in triglyceride concentrations did not appear to be related to the starting level in either the entire group (r2=0.21; P=0.21) or the six responsive individuals (r2=0.1774; P=0.4056) who had reduced TG favorably to the nutritional supplementation. However, of the three subjects who did not have a reduction in triglyceride level in response to the nutritional supplementation (Subjects 4,7 and 9 in Table 2), two of them (7 and 9) had a single T allele of the SNP rs1260326, which has been reported to be associated with elevated triglycerides [23–25]. The other common SNPs of the three genes that were also tested (ZNF259, LPL and APOB) did not appear to show any differences between the responders and non-responders.
Glucose Metabolism
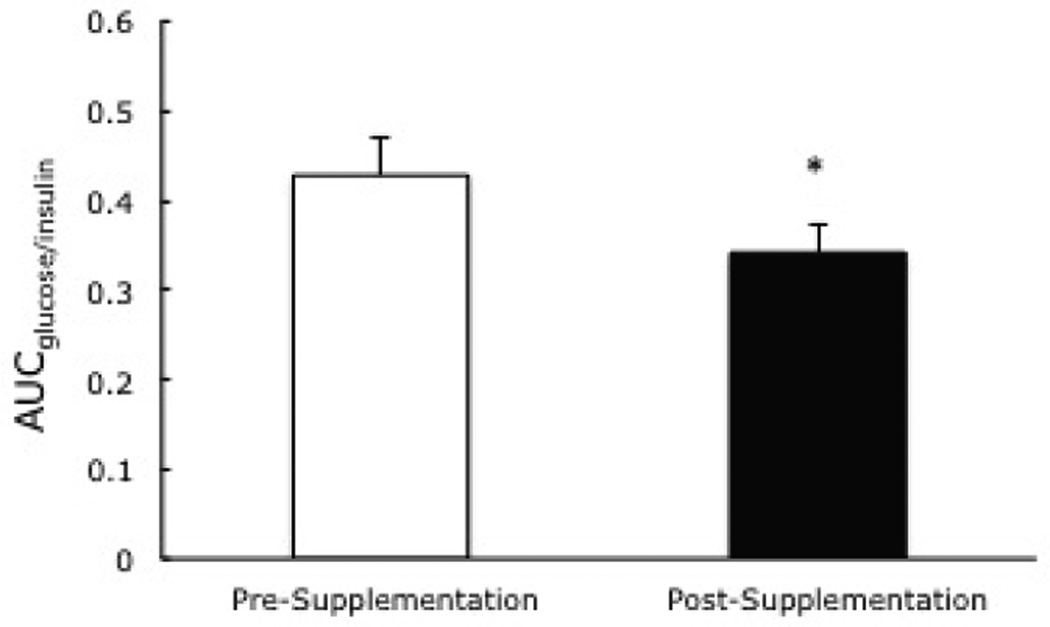
There was a trend (P=0.09) towards a reduction in the fasting plasma glucose from 106±5 mg/dl to 99±3, but it was not significant. However, the insulin sensitivity index (ISI) was increased significantly from 4.2±0.8 to 5.9±0.9, pre- to post-supplementation (P=0.008) (Figure 2). The total AUCins/glucose (an OGTT derived index of insulin secretion) was also improved, from 0.43±0.4 to 0.34±0.3 at pre- to post-supplementation (Figure 3). In addition to a reduced insulin response during the OGTT, there was also a significant decline in the basal plasma insulin from 9.7±0.9 to 7.1±1.1 uU/ml from pre- to post-supplementation, after treatment with the nutritional supplementation for four weeks.
Figure 3.
EAA/Whey Protein/Phytosterol supplement-based change in the area under the curveglucose/insulin. * Indicates significant reduction from pre- to post-supplementation.
DISCUSSION
The results of the present study demonstrate that supplementation with high Leucine, essential amino acids and phytosterols promoted favorable alterations in plasma lipids and insulin sensitivity in individuals with mild hypercholesterolemia. Unlike other approaches that might utilize exercise, weight loss and/or pharmaceuticals, supplementation with high Leucine/EAAs and phytosterols three times daily for only four weeks prompted adaptations that are associated with a substantial reduction in the risk factors for metabolic syndrome and cardiovascular disease. In previous studies utilizing amino acid supplementation in the elderly, the combination of lower plasma TGs and lower liver TGs is consistent with the hypothesis that the essential amino acids stimulated the peripheral clearance of TGs [18]. Therefore, we suggest a potential combined or synergistic influence of EAAs and phytosterols on beneficial alterations in blood lipids and glucose metabolism.
Plasma triglycerides were not decreased in three of the nine individuals. Although the favorable plasma lipid responses were not associated with a single nucleotide polymorphism in the gene of ZNF259, GCKR, LPL, or APOB, it is of interest that in two of the three TG non-responders, there was a single T allele of the SNP rs1260326 of the GCKR gene. In older individuals, there has been found an association between this GCKR SNP, triglycerides and glucose, suggesting the presence of gene-environment interactions [23–25]. In addition the magnitude of the significant reduction in the six TG responders was at least as large or greater than that which would have been expected to be achieved by chronic fenofibrate therapy (95±13 mg/dl, P=0.008) [19]. The simplicity of this short-term supplement warrants further investigation in a larger double blind, randomized, placebo-controlled trial that would explore the potential genetic and molecular mechanisms responsible for these promising results.
While fibrates and niacin are commonly prescribed for the treatment of hyperlipidemia, it is likely that the efficacy of pharmacologic therapy may be determined by the genotype of the individual being treated. It is noteworthy that 3 out of the 9 subjects (33%) demonstrated a complete lack of responsiveness in their triglyceride levels to Leucine/EAAs and phytosterols. In order to more clearly examine the role of gene polymorphisms in the modulation of triglycerides through nutritional therapy, we evaluated certain single nucleotide polymorphisms (SNPs) that have been identified through genome-wide association studies(GWAs) that might be associated with dyslipidemia. We included the common SNPs for the ZNF259, GCKR, LPL, and APOB genes in our study. Although we did not find an association between the SNPs studied and the response to supplementation in our study, the finding of a single T allele in the rs1260226 SNP of the GCKR gene in two of the three subjects who did not show a triglyceride reduction following supplementation suggests that it may be of interest to evaluate a larger cohort in the future. Clearly, it is likely to be a complex interaction of multiple genes and may also include other dietary and nutritional factors as well as epigenetic and gene-environment interactions [22–25, 28–32].
There are also other factors that may have been responsible for the different responses with respect to the triglyceride levels. A number of polymorphisms of the APOA5 gene have been identified that are strongly linked to persistent elevations in triglycerides [29]. However, the APOA5 and the ZNF259 genes are located within 1.3 Kb of each other on chromosome 11q23, so it is likely that crosstalk between APOA5 and ZNF259 would modulate the TG levels [33]. Differences in the liver × receptors (LXRs) also interact with variations in dietary intake, and may have variable influences on plasma triglycerides as well [34]. This supports the notion that the non-responsive nature of these individuals could have also been associated with differences in their dietary intake.
The provision of leucine in the present study represented an important constituent of the formulation, due to its ability to act on multiple metabolic and signaling pathways that are central to the development of the metabolic syndrome [35]. A slight increase in dietary leucine has been shown to prevent hepatic steatosis and reduce adipose tissue inflammation in mice provided with a high fat diet [35]. Since chronic inflammation has been linked to excessive lipolysis [36], leucine may have a beneficial influence on lowering lipids through its ability to counteract excess inflammation in adipose tissue. This notion fits well with the observation that inflammation coexists with a decrease in expression of lipogenic enzymes in adipose tissue [37], suggesting a disruption of adipogenesis that might have a negative influence on the reesterification of free fatty acids back to adipose tissue. Future studies are needed to link leucine’s action on inflammation in adipose tissue to changes in lipid kinetics that could potentially foster adequate regulatory control of triglycerides and cholesterol.
In addition to the potential benefits of supplementation with leucine and other EAAs, the addition of phytosterols may have also contributed to the lowering of cholesterol in this study. As part of the formulation, we included ~2 grams/day of phytosterols that have been previously shown in a meta-analysis of 41 clinical trials to effectively lower LDL-cholesterol by at least 10% [38]. It is relatively well accepted that consumption of phytosterols helps to lower intestinal cholesterol absorption and in turn promotes a reduction in plasma LDL-cholesterol [39]. While it has been suggested that the effectiveness of phytosterols is augmented by their concomitant ingestion with meals, additional studies have confirmed that their effectiveness is not necessarily dependent upon meal ingestion [40]. The supplement in the current study was taken between meals.
The subjects in the present study demonstrated significant improvements in insulin sensitivity, as derived from the Matsuda ISI index. This occurred in conjunction with an overall lowering of insulin secretion (ie., total AUCins/gluc), as assessed during the OGTT. The results are in agreement with previous studies that have utilized the supplementation of essential amino acid mixtures, and found simultaneous reductions in blood glucose in the fasted state and during the OGTT in the presence of an overall lowering of plasma insulin [41]. While the recovery of insulin action might have been facilitated by improvements in lean mass in the previous study, we did not find a significant increase in lean tissue, presumably due to short-term (ie., 4 weeks) supplementation. Nonetheless, we have previously shown that Leucine/EAAs stimulates muscle protein synthesis [42], and that when ingested, the effect is extended over 16 weeks while it also increases lean body mass [43]. The stimulation of muscle protein synthesis may promote a more anabolic environment that increases glucose utilization and may serve to lower circulating blood glucose. In addition, supplementation with leucine alone in the presence of a high fat diet has been shown to normalize the otherwise abnormal expression of glucokinase in the liver and inflammatory cytokines such as TNF-α in adipose tissue in mice fed a high fat diet. This was shown to occur in the presence of increased protein synthesis mediated by activation of the mTOR/p70s6K pathway [35]. Therefore, increased leucine availability alone contributes to a normalization of deleterious factors in the liver and adipose tissue in combination with an increasingly anabolic environment in skeletal muscle that consumes glucose in the context of muscle preservation.
CONCLUSION
Four weeks of supplementation with High Leucine/ EAAs and phytosterols thrice daily promoted significant reductions in plasma triglycerides in six of the nine enrolled volunteers who all had elevated TGs at the outset of the study. In those three without a reduction in plasma triglycerides, two of three non-responders had a single T allele in the GCKR rs1260326 SNP. There was a significant reduction in total cholesterol and LDL-cholesterol in all nine of the individuals enrolled into the study following the nutritional supplementation. In addition to the promising results related to blood lipids, there was also a significant improvement in insulin sensitivity that occurred in conjunction with a reduction in fasting insulin and insulin secretion. While future larger studies with a randomized, placebo-controlled design are needed, the positive results of the current study suggest that dietary supplements of Leucine/ EAAs and phytosterols can favorably impact risk factors for metabolic syndrome and cardiovascular disease, even without weight loss or any change in physical activity.
ACKNOWLEDGEMENT
Supported by NIH SBIR GRANT 0-0496870 and NIH P30 grant AG28718. All authors read and approved the final manuscript. We extend our sincere appreciation to our volunteers for their effort towards the completion of this demanding study.
Disclosure Statement
The authors^ were compensated as consultants through the NIH SBIR grant.
ABBREVIATIONS
- EAA
Essential Amino Acids
- OGTT
Oral Glucose Tolerance Test
- LDL
Low Density Lipoprotein
- TG
Triglyceride
- ISI
Insulin Sensitivity Index
- SNPs
Single-Nucleotide Polymorphisms
- FFA
Free Fatty Acids
- PPAR-α
Peroxisome proliferator-activated receptor alpha
- VLDL
Very Low-Density Lipoproteins
- ZNF259
Zinc Finger Protein 259
- GCKR
Glucokinase (hexokinase 4) Regulator
- LPL
Lipoprotein Lipase
- APOB
Apo lipoprotein
REFERENCES
- 1.Watts GF, Karpe F. Triglycerides and atherogenic dyslipidaemia: extending treatment beyond statins in the high-risk cardiovascular patient. Heart. 2011;97:350–356. doi: 10.1136/hrt.2010.204990. [DOI] [PubMed] [Google Scholar]
- 2.Taskinen MR. Diabetic dyslipidaemia: from basic research to clinical practice. Diabetologia. 2003;46:733–749. doi: 10.1007/s00125-003-1111-y. [DOI] [PubMed] [Google Scholar]
- 3.Chan DC, Watts GF. Pharmacological regulation of dyslipoproteinaemia in insulin resistant states. Curr Vasc Pharmacol. 2008;6:67–77. doi: 10.2174/157016108783331277. [DOI] [PubMed] [Google Scholar]
- 4.Kannel WB. Risk stratification of dyslipidemia: insights from the Framingham Study. Curr Med Chem Cardiovasc Hematol Agents. 2005;3:187–193. doi: 10.2174/1568016054368250. [DOI] [PubMed] [Google Scholar]
- 5.Gibbons LW1, Gonzalez V, Gordon N, Grundy S. The prevalence of side effects with regular and sustained-release nicotinic acid. Am J Med. 1995;99:378–385. doi: 10.1016/s0002-9343(99)80185-5. [DOI] [PubMed] [Google Scholar]
- 6.Vega GL, Grundy SM. Lipoprotein responses to treatment with lovastatin, gemfibrozil, and nicotinic acid in normolipidemic patients with hypoalphalipoproteinemia. Arch Intern Med. 1994;154:73–82. [PubMed] [Google Scholar]
- 7.Knopp RH, Brown WV, Dujovne CA, Farquhar JW, Feldman EB, Goldberg AC, et al. Effects of fenofibrate on plasma lipoproteins in hypercholesterolemia and combined hyperlipidemia. Am J Med. 1987;83:50–59. doi: 10.1016/0002-9343(87)90871-0. [DOI] [PubMed] [Google Scholar]
- 8.Forcheron F, Cachefo A, Thevenon S, Pinteur C, Beylot M. Mechanisms of the triglyceride- and cholesterol-lowering effect of fenofibrate in hyperlipidemic type 2 diabetic patients. Diabetes. 2002;51:3486–3491. doi: 10.2337/diabetes.51.12.3486. [DOI] [PubMed] [Google Scholar]
- 9.Wolfe BM, Piché LA. Replacement of carbohydrate by protein in a conventional-fat diet reduces cholesterol and triglyceride concentrations in healthy normolipidemic subjects. Clin Invest Med. 1999;22:140–148. [PubMed] [Google Scholar]
- 10.Nuttall FQ, Gannon MC, Saeed A, Jordan K, Hoover H. The metabolic response of subjects with type 2 diabetes to a high-protein, weight-maintenance diet. J Clin Endocrinol Metab. 2003;88:3577–3583. doi: 10.1210/jc.2003-030419. [DOI] [PubMed] [Google Scholar]
- 11.Wolfe BM. Potential role of raising dietary protein intake for reducing risk of atherosclerosis. Can J Cardiol. 1995;11(Suppl G):127G–131G. [PubMed] [Google Scholar]
- 12.Wolfe BM, Giovannetti PM. Short-term effects of substituting protein for carbohydrate in the diets of moderately hypercholesterolemic human subjects. Metabolism. 1991;40:338–343. doi: 10.1016/0026-0495(91)90142-j. [DOI] [PubMed] [Google Scholar]
- 13.Furtado JD, Campos H, Appel LJ, Miller ER, Laranjo N, Carey VJ. Effect of protein, unsaturated fat, and carbohydrate intakes on plasma apoliprotein B and VLDL and LDL containing apolipoprotein C-III: results from the OmniHeart Trial. Am J Clin Nutr. 2008;87:1623–1630. doi: 10.1093/ajcn/87.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alaupovic P. Significance of apolipoproteins for structure, function, and classification of plasma lipoproteins. Methods Enzymol. 1996;263:32–60. doi: 10.1016/s0076-6879(96)63004-3. [DOI] [PubMed] [Google Scholar]
- 15.Kashyap ML, Barnhart RL, Srivastava LS, Perisutti G, Vink P, Allen C, et al. Effects of dietary carbohydrate and fat on plasma lipoproteins and apolipoproteins C-II and C-III in healthy men. J Lipid Res. 1982;23:877–886. [PubMed] [Google Scholar]
- 16.McAuley KA, Hopkins CM, Smith KJ, McLay RT, Williams SM, Taylor RW. Comparison of high-fat and high-protein diets with a high-carbohydrate diet in insulin resistant, obese women. Diabetologia. 2005;48:8–16. doi: 10.1007/s00125-004-1603-4. [DOI] [PubMed] [Google Scholar]
- 17.Appel LJ, Sacks FM, Carey VJ, Obarzanek E, Swain JF, Miller ER, et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the Omni Heart randomized trial. JAMA. 2005;294:2455–2464. doi: 10.1001/jama.294.19.2455. [DOI] [PubMed] [Google Scholar]
- 18.Børsheim E, Bui QU, Tissier S, Cree MG, Rønsen O, Morio B, et al. Amino acid supplementation decreases plasma and liver triacylglycerols in elderly. Nutrition. 2009;25:281–288. doi: 10.1016/j.nut.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cree MG, Newcomer BR, Read LK, Sheffield-Moore M, Paddon-Jones D, Chinkes DL. Plasma triglycerides are not related to tissue lipids and insulin sensitivity in elderly following PPAR-alpha agonist treatment. Mech Ageing Dev. 2007;128:558–565. doi: 10.1016/j.mad.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kassis AN, Vanstone CA, AbuMweis SS, Jones PJ. Efficacy of plant sterols is not influenced by dietary cholesterol intake in hypercholesterolemic individuals. Metabolism. 2008;57:339–346. doi: 10.1016/j.metabol.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Poli A, Marangoni F, Paoletti R, Mannarino E, Lupattelli G, Notarbartolo A, et al. Non-pharmacological control of plasma cholesterol levels. Nutr Metab Cardiovasc Dis. 2008;18:S1–S16. doi: 10.1016/j.numecd.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Waterworth DM, Ricketts SL, Song K, Chen L, Zhao JH, Ripatti S, et al. Genetic variants influencing circulating lipid levels and risk of coronary artery disease. Arterioscler Thromb Vasc Biol. 2010;30:2264–2276. doi: 10.1161/ATVBAHA.109.201020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alfred T, Ben-Shlomo Y, Cooper R, Hardy R, Deary IJ, Elliott J, et al. Associations between a polymorphism in the pleiotropic GCKR and Age-related phenotypes: the HALCyon programme. PLoS One. 2013;8:e70045. doi: 10.1371/journal.pone.0070045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petersen AK, Stark K, Musameh MD, Nelson CP, Römisch-Margl W, Kremer W, et al. Genetic associations with lipoprotein subfractions provide information on their biological nature. Hum Mol Genet. 2012;21:1433–1443. doi: 10.1093/hmg/ddr580. [DOI] [PubMed] [Google Scholar]
- 25.Johansen CT, Hegele RA. Allelic and phenotypic spectrum of plasma triglycerides. Biochemica et Biophysica Acta. 2012;1821:833–842. doi: 10.1016/j.bbalip.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 27.Ahrén B, Pacini G. Importance of quantifying insulin secretion in relation to insulin sensitivity to accurately assess beta cell function in clinical studies. Eur J Endocrinol. 2004;150:97–104. doi: 10.1530/eje.0.1500097. [DOI] [PubMed] [Google Scholar]
- 28.Ress C, Moschen AR, Sausgruber N, Tschoner A, Graziadei I, Weiss H, et al. The role of apolipoprotein A5 in non-alcoholic fatty liver disease. Gut. 2011;60:985–991. doi: 10.1136/gut.2010.222224. [DOI] [PubMed] [Google Scholar]
- 29.Nilsson SK, Heeren J, Olivecrona G, Merkel M. Apolipoprotein A-V; a potent triglyceride reducer. Atherosclerosis. 2011;219:15–21. doi: 10.1016/j.atherosclerosis.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 30.Kulminski AM, Culminskaya I, Arbeev KG, Ukraintseva SV, Stallard E, Arbeeva L, et al. The role of lipid-related genes, aging-related processes, and environment in healthspan. Aging Cell. 2013;12:237–246. doi: 10.1111/acel.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim DS, Burt AA, Ranchalis JE, Jarvik ER, Rosenthal EA, Hatsukami TS, et al. Novel gene-by-environment interactions: APOB and NPC1L1 variants affect the relationship between dietary and total plasma cholesterol. J Lipid Res. 2013;54:1512–1520. doi: 10.1194/jlr.P035238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petta S, Miele L, Bugianesi E, Cammà C, Rosso C, Boccia S, et al. Glucokinase Regulatory Protein Gene Polymorphism Affects Liver Fibrosis in Non-Alcoholic Fatty Liver Disease. PLoS ONE. 2014;9:e87523. doi: 10.1371/journal.pone.0087523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cha S, Yu H, Park AY, Song KH. Effects of apolipoprotein A5 haplotypes on the ratio of triglyceride to high-density lipoprotein cholesterol and the risk for metabolic syndrome in Koreans. Lipids Health Dis. 2014;13:45. doi: 10.1186/1476-511X-13-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lehrke M, Lebherz C, Millington SC, Guan HP, Millar J, Rader DJ, et al. Diet-dependent cardiovascular lipid metabolism controlled by hepatic LXRalpha. Cell Metab. 2005;1:297–308. doi: 10.1016/j.cmet.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Macotela Y, Emanuelli B, Bång AM, Espinoza DO, Boucher J, Beebe K, et al. Dietary leucine--an environmental modifier of insulin resistance acting on multiple levels of metabolism. PLoS One. 2011;6:e21187. doi: 10.1371/journal.pone.0021187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poulain-Godefroy O, Lecoeur C, Pattou F, Frühbeck G, Froguel P. Inflammation is associated with a decrease of lipogenic factors in omental fat in women. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1–R7. doi: 10.1152/ajpregu.00926.2007. [DOI] [PubMed] [Google Scholar]
- 38.Katan MB, Grundy SM, Jones P, Law M, Miettinen T, Paoletti R, et al. Efficacy and safety of plant stanols and sterols in the management of blood cholesterol levels. Mayo Clin Proc. 2003;78:965–978. doi: 10.4065/78.8.965. [DOI] [PubMed] [Google Scholar]
- 39.Miettinen TA, Tilvis RS, Kesäniemi YA. Serum plant sterols and cholesterol precursors reflect cholesterol absorption and synthesis in volunteers of a randomly selected male population. Am J Epidemiol. 1990;131:20–31. doi: 10.1093/oxfordjournals.aje.a115479. [DOI] [PubMed] [Google Scholar]
- 40.Plat J, Mensink RP. Plant stanol and sterol esters in the control of blood cholesterol levels: mechanism and safety aspects. Am J Cardiol. 2005;96:15D–22D. doi: 10.1016/j.amjcard.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 41.Solerte SB, Gazzaruso C, Bonacasa R, Rondanelli M, Zamboni M, Basso C, et al. Nutritional supplements with oral amino acid mixtures increases whole-body lean mass and insulin sensitivity in elderly subjects with sarcopenia. Am J Cardiol. 2008;101:69E–77E. doi: 10.1016/j.amjcard.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 42.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab. 2006;291:E381–E387. doi: 10.1152/ajpendo.00488.2005. [DOI] [PubMed] [Google Scholar]
- 43.Børsheim E, Bui QU, Tissier S, Kobayashi H, Ferrando AA, Wolfe RR. Effect of amino acid supplementation on muscle mass, strength and physical function in elderly. Clin Nutr. 2008;27:189–195. doi: 10.1016/j.clnu.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]