Abstract
Idiopathic pulmonary fibrosis (IPF) is an intractable disease for which the pathological findings are characterized by temporal and spatial heterogeneity. The pathogenesis is composed of myriad factors, including repetitive injuries to epithelial cells, alterations in immunity, the formation of vascular leakage and coagulation, abnormal wound healing, fibrogenesis, and collagen accumulation. Therefore, the molecular target drugs that are used or attempted for treatment or clinical trials may not cover the myriad therapeutic targets of IPF. In addition, the complicated pathogenesis results in a lack of informative biomarkers to diagnose accurately the status of IPF. These facts point out the necessity of using a combination of drugs, that is, each single drug with molecular targets or a single drug with multiple therapeutic targets. In this review, we introduce a humoral factor, stanniocalcin-1 (STC1), which has myriad functions, including the maintenance of calcium homeostasis, the promotion of early wound healing, uncoupling respiration (aerobic glycolysis), reepithelialization in damaged tissues, the inhibition of vascular leakage, and the regulation of macrophage functions to keep epithelial and endothelial homeostasis, which may adequately cover the myriad therapeutic targets of IPF.
Keywords: idiopathic pulmonary fibrosis (IPF), stanniocalcin-1 (STC1), biomarker, wound healing, macrophage
Introduction
What is idiopathic pulmonary fibrosis?
Idiopathic pulmonary fibrosis (IPF) is a chronic lung disease in which the lung volume (vital capacity), diffusing capacity for carbon monoxide, 6-minute walking distance, and oxygen contents in the blood are reduced irreversibly through the progressive fibrosis.1,2 The pathological findings of IPF usually show the usual interstitial pneumonia, which is characterized by temporal and spatial heterogeneity with fibrotic foci, mature fibrosis, honeycombing, etc.3 The prognosis is poor, and the mean survival time is within three to five years. The quality of life (QOL) in patients with IPF is also very poor because of progressive dyspnea and intractable cough. New molecular target drugs, pirfenidone and nintedanib, reduce the annual decline of the vital capacity and may improve the prognosis,4–6 but the effects of these drugs are weak and insufficient to satisfy the needs of patients. Therefore, new therapies for improving the prognosis and/or QOL of IPF patients are needed.7,8
The lack of reliable biomarkers leads to the treatment failure using single drug targeting specific molecules of IPF
Although several biomarkers, including KL-6, SP-D, MUC5, MMP7, spirometry, breath gases, and fibrocytes, have been explored by clinicians, there are no useful markers that accurately predict the diagnosis and prognosis.9 The reason is that, since the pathogenesis of IPF is very complicated, the combination of only a few biomarkers will not be adequate for the evaluation. Therefore, a combination of drugs or a single drug with broad actions will be needed to cover the myriad therapeutic targets of IPF.
What is stanniocalcin-1 (STC1)?
STC1 is a mitochondrial- related hormone first derived from the corpuscles of Stannius located on the ventral surface of the kidney of bony fish.10,11 In fish, STC1 inhibits excessive calcium influx from sea water through the gills, intestine, etc., to inside the body and protects cells and organs from high calcium levels that could otherwise cause cell death. In mammals, the STC1 gene is widely expressed in tissues including the brain, thyroid, spleen, thymus, parathyroid, muscle, pancreas, intestine, heart, lung, liver, adrenal grand, kidney, prostate, placenta, testis, and ovary. The wide expression of STC1 suggests that its function is exhibited in an autocrine and paracrine fashion to maintain calcium homeostasis and to inhibit apoptosis. Furthermore, its localization to the spleen and thymus suggests a role in the immune/inflammatory responses.12–14
Mesenchymal stem cells secrete huge amounts of STC1 into microenvironment to maintain homeostasis of organ and tissue under harmful conditions
Mesenchymal stem cells (MSCs) have been shown to ameliorate lung injuries and fibrotic changes in animal models of bleomycin-, naphthalene-, and lipopolysaccharide-induced lung injuries.10,15–18 MSCs are known to home to the sites of injuries, inhibit the inflammation, and promote the tissue repair through various functions, including differentiation into specific cells and secretion of trophic factors promoting wound healing and Immunomodulation. Recently, paracrine effects of MSCs have been reported to be among the most important factors for wound healing (Fig. 1).10,15,19–23
Figure 1.
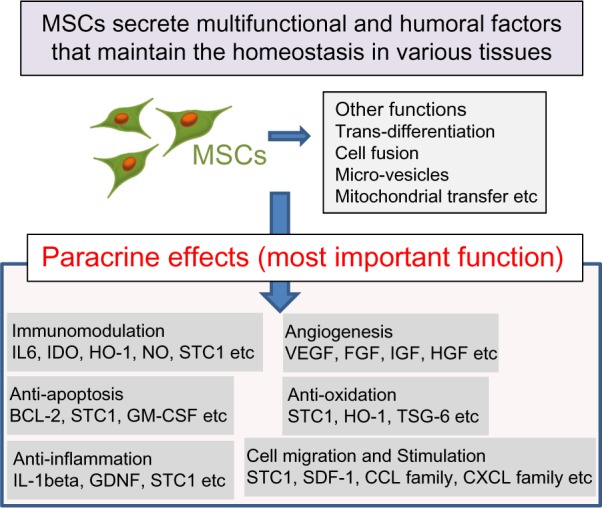
MSCs secrete multifunctional and humoral factors that maintain the homeostasis in various tissues. MSCs maintain the homeostasis of organs through multiple functions, including transdifferentiation to specific cells, cell fusion with sick cells, mitochondrial transfer to sick cells, and secretion of microvesicles and trophic factors. Many researchers have suggested that a paracrine effect is the most important function of MSCs to maintain the homeostasis of organs and tissues.
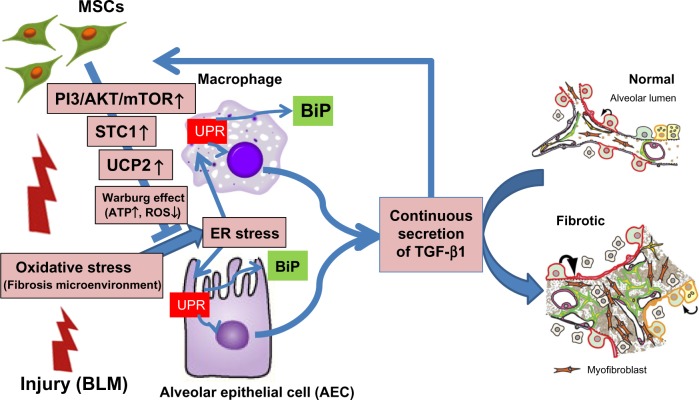
We found that MSCs secrete huge amounts of STC1 in comparison with alveolar epithelial cells (AECs), alveolar macrophages (AMs), and fibroblasts under multiple insults, including hypoxia, acidosis (eg, lactic acid and hydrochloric acid), and oxidative stress (eg, hydrogen peroxide).24,25 We found that the profibrotic growth factors, TGF-β1, PDGFB, and FGF2, stimulate MSCs to secrete much STC1 in comparison to AECs and fibroblasts. We also found that STC1 derived from MSCs ameliorated lung injuries and fibrogenesis in a bleomycin-induced pulmonary fibrosis animal model (Fig. 2).10 STC1 is considered an important initiator of wound healing through regulating calcium and Adenosine Tri-Phosphate (ATP) efflux into cells.26,27 These insights suggest that STC1 is a kind of shock protein released from MSCs to maintain homeostasis and initiate the first step of wound healing.23
Figure 2.
MSCs reduce a central profibrotic factor, TGF-β1, using STC1 in bleomycin-induced pulmonary fibrosis. The model of our study quoted in Ref. 10 is shown in this figure. Oxidative stress induces ER stress and unfolded protein response (UPR) in AECs and AMs. Continuous UPR causes continuous secretion of TGF-β1 from AECs and AMs. On the other hand, TGF-β1 stimulates MSCs to secrete STC1 through PI3/AKT/mTOR pathway. STC1 increases UCP2 and UCP2 induces a reduction of ER stress through the reduction of ROS in AECs and AMs. Finally, STC1 derived from MSCs reduces the secretion of TGF-β1 from AECs and AMs.
Abbreviation: BLM, Bleomycin.
The purpose of this review was to summarize and provide the expected effects and side effects of STC1 treatments for IPF.
STC1 has Myriad Functions: the Possibility of Covering Complicated Therapeutic Targets of IPF
Oxidative stress and profibrotic growth hormones induce MSCs to secrete STC1
Injuries and stress, such as viral infections, gastroesophageal reflux, smoking, inhaled particles, genetic vulnerability of epithelial cells, hypoxia, acidosis, and other environmental exposures, cause the accumulation of intracellular reactive oxygen species (ROS), which activate various pathways including those involved in inflammation, wound healing, metabolism, cell proliferation, differentiation, and death in AECs and AMs.7,28 Intracellular ROS induce endoplasmic reticulum (ER) stress that is characterized by the accumulation of unfolded proteins in the ER. An excessive unfolded protein response (UPR) induces cell death, but moderate and continuous UPR results in the prolonged activation of profibrotic inflammatory pathways containing NF-κB, P38, and JNK and the inadequate secretion of profibrotic growth factors, such as TGF-β1, PDGF-BB, and FGF2, from AECs and AMs.29–33 Interestingly, ROS and profibrotic growth factors derived from AECs and AMs induce MSCs to secrete huge STC1 in tissues.10 We found that the ability of MSCs to ameliorate lung injuries and fibrosis is dependent on STC1 secretion.10
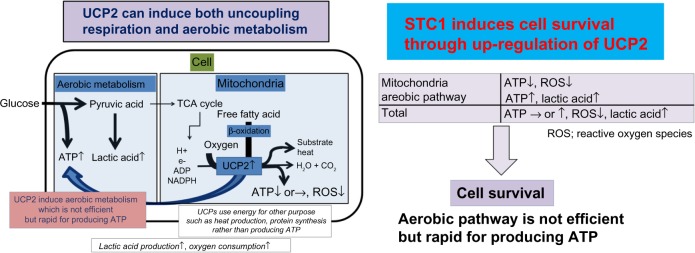
STC1 induces uncoupling respiration in AECs and AMs
STC1 induces the upregulation of uncoupling protein 2 (UCP2) in AECs and AMs. UCP2 induces uncoupling respiration even under aerobic conditions.10,24,25 Uncoupling respiration reduces mitochondrial membrane potential, calcium efflux into mitochondria, and the production of ROS derived from mitochondria. Uncoupling respiration also stimulates the anaerobic pathway producing ATP and the lipid utilization of mitochondria and maintains total ATP production in cells. Therefore, uncoupling respiration results in a reduction of oxidative stress and the survival of cells under harmful microenvironments (Fig. 3). These dynamic changes in metabolism status may be the main functions of STC1. We also confirmed that STC1 derived from MSCs reduces intracellular ROS, ER stress, and TGF-β1 production in AECs and AMs on the dependency of UCP2.10 When MSCs are injected intravenously, almost all the MSCs are trapped in the lung and the MSCs release STC1 in the microenvironment of pulmonary fibrosis in an autocrine or paracrine manner. The administration of STC1 via the trachea (inhalation) also reduces the injuries and fibrosis in bleomycin-induced pulmonary fibrosis.10 This result suggests that the inhalation of STC1 can inhibit AECs and AMs directly. The efficiency of STC1 in reducing intracellular ROS measured by flow cytometry was 1500 times higher than that with the same quantities of N-AcetylCysteine (NAC).10
Figure 3.
The schema of UCP2 and STC1 functions in the intracellular ATP and ROS production. STC1 induces the upregulation of UCP2 in AECs and AMs, which induces uncoupling respiration even under aerobic conditions. Uncoupling respiration reduces the mitochondrial membrane potential and the production of ROS derived from mitochondria in cells. Uncoupling respiration also stimulates the anaerobic pathway production of ATP and the lipid utilization of mitochondria and maintains the total ATP production. Therefore, uncoupling respiration results in a reduction of oxidative stress and the survival of cells under harmful microenvironments.
STC1 promotes wound healing in epithelial cells
Wound healing is essential for maintaining homeostasis and for the survival of all creatures. The healing process is always the result of sequential steps, each taking the damaged area one step closer to restoring the cellular architecture and function. The turbulence of these processes may cause fibrogenesis and carcinogenesis. Generally, the processes of wound healing, in the order of occurrence, are clot formation, inflammation (macrophages and neutrophils), cell migration and remodeling (reepithelialization), and resolution.27
Until recently, little was known about the immediate signals that initiate the process and the reepithelialization signal that terminates wound healing. Recent studies revealed that ATP and Ca2+ from damaged cells are needed for the initiation of wound healing. The influx of ATP and Ca2+ into epithelial cells via P2X and P2Y receptors regulates the ATP-induced calcium wave that stimulates the PI3-AKT and ERK-MAPK signaling pathways to promote wound healing. STC1 sensitizes the incorporation of ATP and Ca2+ influx into epithelial cells via the P2X and P2Y receptors.11,26,27,34
Extracellular ATP also stimulates inflammatory cells, including macrophages, neutrophils, and lymphocytes, and these cells remove damaged cells.35 Interestingly, at this point, STC1 is upregulated in epithelial cells and initiates reepithelialization with the surge of Ca2+ influx.26,27,34 STC1 inhibits Ca2+ signaling in the migration of macrophages and endothelial cells to damaged tissues.12,36,37 STC1 may regulate the beginning and end of wound healing using different mechanisms depending on the type, whether epithelial or mesenchyme. STC1 may be a very important factor for the recovery of abnormal wound healing, which is a major problem in the pathogenesis of IPF.27
STC1 inhibits the activation of AMs
Alveolar injuries and stress in alveolar spaces activate the innate immune system, especially, AMs. A conceptual classification divides activated macrophages into two major groups, M1 and M2. M1 macrophages recognize damage-associated molecular patterns and pathogen-associated molecular patterns.23,38 The activation of NF-κB, P38, and JNK pathways in M1 macrophages induces the transcription of proinflammatory genes, NLRP3 inflammasome, and causes M1 macrophages to release the proinflammatory cytokines such as IL-1β and TNFα.23,39 These cytokines promote fibrosis through the upregulation of ROS production. Alternatively activated M2a-like macrophages secrete large amounts of profibrotic growth factors, insulin-like growth factor (IGF) binding protein 5, CCL18, arginase, etc., which promote fibroblast differentiation and collagen synthesis.7 On the other hand, TH-2 cytokines such as IL-4 and IL-13 induce the activation of Mreg/M2c-like macrophages through KL4, PPAR, and STAT6 pathways located downstream of the IL-4 receptor. These macrophages can promote the resolution of fibrosis through multiple mechanisms, including the production of suppressive cytokines such as IL−10.39 STC1 also inhibits NLRP3 inflammasome through decreasing mitochondrial ROS.40 Furthermore, STC1 inhibits the production of TGF-β1 through the downregulation of ER stress and ROS, which is dependent on UCP2.10 These results suggest that STC1 can inhibit the activation of M1 and M2a macrophages in alveolar spaces.
STC1 may promote the early inhibition of vascular leakage and reepithelialization in damaged alveolar spaces
When the alveolar structures are injured by various insults, the desquamation of AECs from the basement membrane and transmigration of circulating monocytes into alveolar spaces, vascular leakage, and coagulation occur in the alveolar spaces. Early inhibition of the vascular leakage and reepithelialization may prevent fibroblast accumulation and collagen genesis in the damaged alveolar spaces. STC1 blocks the TNF-α-induced monolayer permeability of coronary artery endothelial cells through maintaining occluden-1 and claudin-1, which comprise the tight junction.41 Furthermore, the increase of vascular permeability after ischemia/reperfusion injury by clamping renal pedicles is inhibited in STC1 transgenic mice.36 STC1 promotes reepithelialization in wound healing through inducing migration by keratinocyte lamellipodia formation and inhibits the metzincin metalloproteinase, pregnancy-associated plasma protein-A, which modulates IGF signaling.34,42 These studies suggest that STC1 can inhibit vascular leakage and fibroblast accumulation and promote reseal by the epithelial cell migration in the earlier stages of the injuries.
Is STC1 carcinogenetic?
Numerous papers have mentioned higher expression of STC1 in many cancers, although the mechanisms have not been clarified.11 Normal tissues also have higher STC1 expression under ischemic or hypoxia conditions. Therefore, the higher expression of STC1 is not specific to cancer.11,24,25 The higher expression of STC1 in ischemic tissues and cancer can be explained as an adaptation for avoiding apoptosis through reducing ROS production from mitochondria and ER stress. Perhaps STC1 shifts the metabolic status of cells into uncoupling respiration for adaptation of the cells to the harmful condition.24
Several papers have described STC1 has an anticarcinogenesis function in cancers under the control of NF-κB, P53, etc.43–45 In addition, it has been reported that STC1 stimulates anticarcinogenesis factors, such as AMPK, sirtuin3, and UCP2, in renal ischemia/reperfusion injury.46 MSCs cultured in a 3D sphere expressed higher levels of STC1 and showed reduced proliferation and differentiation. They also expressed high levels of three anticancer proteins: IL-24, TNFα-related apoptosis-inducing ligand, and CD82.15,47 STC1 transgenic mice do not show any carcinogenic character in their natural history.48 Intermittent inhalation of STC1 might be safe in terms of carcinogenesis, but further data are needed to confirm its safety as a treatment for IPF.
Summary
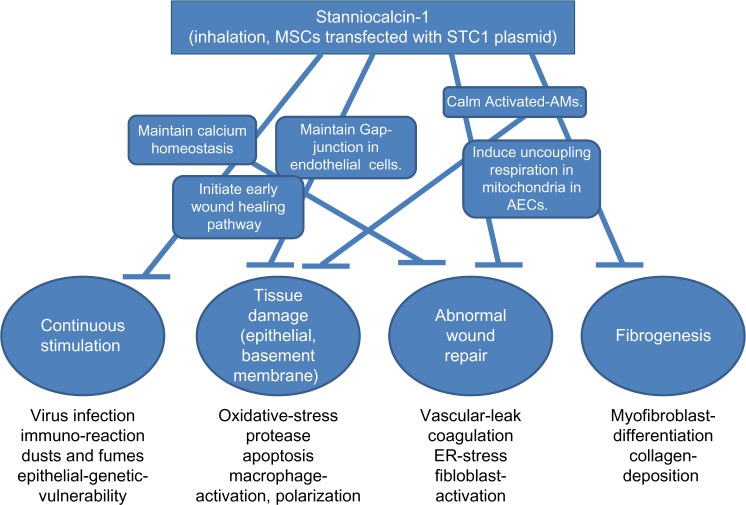
STC1 has myriad functions including the maintenance of calcium homeostasis, the promotion of early wound healing and uncoupling respiration (aerobic glycolysis), the inhibition of vascular leakage, the reepithelialization in damaged tissues, and the regulation of macrophage functions (Fig. 4). In addition, small quantities of STC1 are sufficient to reduce BLM-induced pulmonary fibrosis. Since STC1 is a local hormone found in all animals, it will likely not have any significant side effects that may enable its use in combination with other molecular target drugs for the treatment of IPF. The inhalation method can deliver STC1 to AMs directly. Inhalation of STC1 may have the great possibility of promoting normal wound healing in the lungs of IPF.
Figure 4.
Myriad functions of STC1 cover the various therapeutic targets of IPF. STC1 has myriad functions including the maintenance of calcium homeostasis, the promotion of early wound healing, uncoupling respiration (aerobic glycolysis), the inhibition of vascular leakage, reepithelialization in damaged tissues, and the regulation of macrophage functions. These numerous functions of STC1 cover various therapeutic targets of IPF.
Acknowledgments
We thank Dr. Hiroshi Furukawa, Tsukuba University, Japan, and Dr. Shu Hisata, Columbia University, USA, for giving us this opportunity.
Footnotes
ACADEMIC EDITOR: Hussein D. Foda, Editor in Chief
PEER REVIEW: Two peer reviewers contributed to the peer review report. Reviewers’ reports totaled 268 words, excluding any confidential comments to the academic editor.
FUNDING: This work was supported by grants from the Japan Society for the Promotion of Science (JSPS-15K09205). The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Wrote the first draft of the manuscript: SO. Contributed to the writing of the manuscript: MO, MK, TH, YT, SH. Agree with manuscript results and conclusions: TI, HO, HK. Jointly developed the structure and arguments for the paper: MI. Made critical revisions and approved final version: SO. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188:733–48. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barkauskas CE, Noble PW. Cellular mechanisms of tissue fibrosis. 7. New insights into the cellular mechanisms of pulmonary fibrosis. Am J Physiol Cell Physiol. 2014;306:C987–96. doi: 10.1152/ajpcell.00321.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–82. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 5.Karimi-Shah BA, Chowdhury BA. Forced vital capacity in idiopathic pulmonary fibrosis – FDA review of pirfenidone and nintedanib. N Engl J Med. 2015;372:1189–91. doi: 10.1056/NEJMp1500526. [DOI] [PubMed] [Google Scholar]
- 6.King TE, Jr, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2083–92. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 7.Ahluwalia N, Shea BS, Tager AM. New therapeutic targets in idiopathic pulmonary fibrosis. Aiming to rein in runaway wound-healing responses. Am J Respir Crit Care Med. 2014;190:867–78. doi: 10.1164/rccm.201403-0509PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woodcock HV, Maher TM. The treatment of idiopathic pulmonary fibrosis. F1000prime Rep. 2014;6:16. doi: 10.12703/P6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ley B, Brown KK, Collard HR. Molecular biomarkers in idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2014;307:L681–91. doi: 10.1152/ajplung.00014.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ono M, Ohkouchi S, Kanehira M, et al. Mesenchymal stem cells correct inappropriate epithelial-mesenchyme relation in pulmonary fibrosis using stanniocalcin-1. Mol Ther. 2015;23:549–60. doi: 10.1038/mt.2014.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeung BH, Law AY, Wong CK. Evolution and roles of stanniocalcin. Mol Cell Endocrinol. 2012;349:272–80. doi: 10.1016/j.mce.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Kanellis J, Bick R, Garcia G, et al. Stanniocalcin-1, an inhibitor of macrophage chemotaxis and chemokinesis. Am J Physiol Renal Physiol. 2004;286:F356–62. doi: 10.1152/ajprenal.00138.2003. [DOI] [PubMed] [Google Scholar]
- 13.Sheikh-Hamad D. Mammalian stanniocalcin-1 activates mitochondrial antioxidant pathways: new paradigms for regulation of macrophages and endothelium. Am J Physiol Renal Physiol. 2010;298:F248–54. doi: 10.1152/ajprenal.00260.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Huang L, Abdelrahim M, et al. Stanniocalcin-1 suppresses superoxide generation in macrophages through induction of mitochondrial UCP2. J Leukoc Biol. 2009;86:981–8. doi: 10.1189/jlb.0708454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartosh TJ, Ylostalo JH, Mohammadipoor A, et al. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc Natl Acad Sci U S A. 2010;107:13724–9. doi: 10.1073/pnas.1008117107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brody AR, Salazar KD, Lankford SM. Mesenchymal stem cells modulate lung injury. Proc Am Thorac Soc. 2010;7:130–3. doi: 10.1513/pats.200908-091RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ortiz LA, Gambelli F, McBride C, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A. 2003;100:8407–11. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serikov VB, Popov B, Mikhailov VM, Gupta N, Matthay MA. Evidence of temporary airway epithelial repopulation and rare clonal formation by BM-derived cells following naphthalene injury in mice. Anat Rec (Hoboken) 2007;290:1033–45. doi: 10.1002/ar.20574. [DOI] [PubMed] [Google Scholar]
- 19.Ionescu L, Byrne RN, van Haaften T, et al. Stem cell conditioned medium improves acute lung injury in mice: in vivo evidence for stem cell paracrine action. Am J Physiol Lung Cell Mol Physiol. 2012;303:L967–77. doi: 10.1152/ajplung.00144.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ortiz LA, Dutreil M, Fattman C, et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci U S A. 2007;104:11002–7. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Danchuk S, Ylostalo JH, Hossain F, et al. Human multipotent stromal cells attenuate lipopolysaccharide-induced acute lung injury in mice via secretion of tumor necrosis factor-alpha-induced protein 6. Stem Cell Res Ther. 2011;2:27. doi: 10.1186/scrt68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foskett AM, Bazhanov N, Ti X, Tiblow A, Bartosh TJ, Prockop DJ. Phase-directed therapy: TSG-6 targeted to early inflammation improves bleomycin-injured lungs. Am J Physiol Lung Cell Mol Physiol. 2014;306:L120–31. doi: 10.1152/ajplung.00240.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol Ther. 2012;20:14–20. doi: 10.1038/mt.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Block GJ, Ohkouchi S, Fung F, et al. Multipotent stromal cells are activated to reduce apoptosis in part by upregulation and secretion of stanniocalcin-1. Stem Cells. 2009;27:670–81. doi: 10.1002/stem.20080742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohkouchi S, Block GJ, Katsha AM, et al. Mesenchymal stromal cells protect cancer cells from ROS-induced apoptosis and enhance the Warburg effect by secreting STC1. Mol Ther. 2012;20:417–23. doi: 10.1038/mt.2011.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Block GJ, DiMattia GD, Prockop DJ. Stanniocalcin-1 regulates extracellular ATP-induced calcium waves in human epithelial cancer cells by stimulating ATP release from bystander cells. PLoS One. 2010;5:e10237. doi: 10.1371/journal.pone.0010237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cordeiro JV, Jacinto A. The role of transcription-independent damage signals in the initiation of epithelial wound healing. Nat Rev Mol Cell Biol. 2013;14:249–62. doi: 10.1038/nrm3541. [DOI] [PubMed] [Google Scholar]
- 28.Ueha S, Shand FH, Matsushima K. Cellular and molecular mechanisms of chronic inflammation-associated organ fibrosis. Front Immunol. 2012;3:71. doi: 10.3389/fimmu.2012.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawson WE, Cheng DS, Degryse AL, et al. Endoplasmic reticulum stress enhances fibrotic remodeling in the lungs. Proc Natl Acad Sci U S A. 2011;108:10562–7. doi: 10.1073/pnas.1107559108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanjore H, Blackwell TS, Lawson WE. Emerging evidence for endoplasmic reticulum stress in the pathogenesis of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2012;302:L721–9. doi: 10.1152/ajplung.00410.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baek HA, Kim do S, Park HS, et al. Involvement of endoplasmic reticulum stress in myofibroblastic differentiation of lung fibroblasts. Am J Respir Cell Mol Biol. 2012;46:731–9. doi: 10.1165/rcmb.2011-0121OC. [DOI] [PubMed] [Google Scholar]
- 32.Korfei M, Ruppert C, Mahavadi P, et al. Epithelial endoplasmic reticulum stress and apoptosis in sporadic idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;178:838–46. doi: 10.1164/rccm.200802-313OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lawson WE, Crossno PF, Polosukhin VV, et al. Endoplasmic reticulum stress in alveolar epithelial cells is prominent in IPF: association with altered surfactant protein processing and herpesvirus infection. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1119–26. doi: 10.1152/ajplung.00382.2007. [DOI] [PubMed] [Google Scholar]
- 34.Yeung BH, Wong CK. Stanniocalcin-1 regulates re-epithelialization in human keratinocytes. PLoS One. 2011;6:e27094. doi: 10.1371/journal.pone.0027094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawamura H, Kawamura T, Kanda Y, Kobayashi T, Abo T. Extracellular ATP-stimulated macrophages produce macrophage inflammatory protein-2 which is important for neutrophil migration. Immunology. 2012;136:448–58. doi: 10.1111/j.1365-2567.2012.03601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang L, Belousova T, Chen M, DiMattia G, Liu D, Sheikh-Hamad D. Overexpression of stanniocalcin-1 inhibits reactive oxygen species and renal ischemia/reperfusion injury in mice. Kidney Int. 2012;82:867–77. doi: 10.1038/ki.2012.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang L, Garcia G, Lou Y, et al. Anti-inflammatory and renal protective actions of stanniocalcin-1 in a model of anti-glomerular basement membrane glomerulonephritis. Am J Pathol. 2009;174:1368–78. doi: 10.2353/ajpath.2009.080476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duffield JS, Lupher M, Thannickal VJ, Wynn TA. Host responses in tissue repair and fibrosis. Annu Rev Pathol. 2013;8:241–76. doi: 10.1146/annurev-pathol-020712-163930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oh JY, Ko JH, Lee HJ, et al. Mesenchymal stem/stromal cells inhibit the NLRP3 inflammasome by decreasing mitochondrial reactive oxygen species. Stem Cells. 2014;32:1553–63. doi: 10.1002/stem.1608. [DOI] [PubMed] [Google Scholar]
- 41.Chen C, Jamaluddin MS, Yan S, Sheikh-Hamad D, Yao Q. Human stanniocalcin-1 blocks TNF-alpha-induced monolayer permeability in human coronary artery endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:906–12. doi: 10.1161/ATVBAHA.108.163667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kloverpris S, Mikkelsen JH, Pedersen JH, et al. Stanniocalcin-1 potently inhibits the proteolytic activity of the metalloproteinase pregnancy-associated plasma protein-A. J Biol Chem. 2015;290(36):21915–24. doi: 10.1074/jbc.M115.650143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo F, Li Y, Wang J, Li Y, Li Y, Li G. Stanniocalcin1 (STC1) inhibits cell proliferation and invasion of cervical cancer cells. PLoS One. 2013;8:e53989. doi: 10.1371/journal.pone.0053989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lai KP, Law AY, Yeung HY, Lee LS, Wagner GF, Wong CK. Induction of stanniocalcin-1 expression in apoptotic human nasopharyngeal cancer cells by p53. Biochem Biophys Res Commun. 2007;356:968–75. doi: 10.1016/j.bbrc.2007.03.074. [DOI] [PubMed] [Google Scholar]
- 45.Ching LY, Yeung BH, Wong CK. Synergistic effect of p53 on TSA-induced stanniocalcin 1 expression in human nasopharyngeal carcinoma cells, CNE2. J Mol Endocrinol. 2012;48:241–50. doi: 10.1530/JME-11-0159. [DOI] [PubMed] [Google Scholar]
- 46.Pan JS, Huang L, Belousova T, et al. Stanniocalcin-1 inhibits renal ischemia/reperfusion injury via an AMP-activated protein kinase-dependent pathway. J Am Soc Nephrol. 2015;26:364–78. doi: 10.1681/ASN.2013070703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Higuera GA, Fernandes H, Spitters TW, et al. Spatiotemporal proliferation of human stromal cells adjusts to nutrient availability and leads to stanniocalcin-1 expression in vitro and in vivo. Biomaterials. 2015;61:190–202. doi: 10.1016/j.biomaterials.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 48.Johnston J, Ramos-Valdes Y, Stanton LA, Ladhani S, Beier F, Dimattia GE. Human stanniocalcin-1 or -2 expressed in mice reduces bone size and severely inhibits cranial intramembranous bone growth. Transgenic Res. 2010;19:1017–39. doi: 10.1007/s11248-010-9376-7. [DOI] [PubMed] [Google Scholar]