Abstract
Study Objectives
To evaluate the effect of Roux-en-Y gastric bypass surgery (RYGB) on the pharmacokinetics of midazolam (a CYP3A4 substrate) and digoxin (a P-glycoprotein substrate).
Design
Prospective, nonblinded, longitudinal, single-dose pharmacokinetic study in three phases: presurgery baseline and postoperative assessments at 3 and 12 months.
Patients
Twelve obese patients meeting current standards for bariatric surgery.
Measurements and Main Results
At each study visit, patients received a single dose of oral digoxin and midazolam at 8 a.m. Blood samples were collected at regular intervals for 24 hours after dosing. Continuous 12-lead electrocardiogram (EKG), heart rate, blood pressure, and respiratory rate were monitored, and pharmacokinetic parameters from the three visits were compared. The peak plasma concentration (Cmax) of midazolam increased by 66% and 71% at 3- and 12-month post-RYGB (p=0.017 and p=0.001, respectively), whereas the median time to peak concentration (Tmax) was reduced by 50%. The mean Cmax for 1′-hydroxymidazolam increased by 87% and 80% at 3 and 12 months (p=0.001 and p<0.001, respectively). However, neither the area under the concentration-time curve (AUC) for midazolam nor the metabolite-to-parent AUC ratio changed significantly over time. For digoxin, the median Tmax decreased from 40 minutes at baseline to 30 and 20 minutes at 3 and 12 months, respectively. The mean AUC for digoxin, heart rate, and EKG patterns were similar across the three study phases.
Conclusion
Contemporary proximal RYGB increases the rate of drug absorption without significantly changing the overall exposure to midazolam and digoxin. The Cmax of a CYP3A4 substrate with a high extraction ratio was substantially increased after RYGB.
Keywords: Roux-en-Y gastric bypass, bariatric surgery, pharmacokinetics, absorption, obesity, CYP3A4, P-glycoprotein
Bariatric surgery is an effective means of facilitating and maintaining significant weight loss and improving comorbidities in patients with class II and class III obesity.1–4 In the last decade, an estimated 1 million bariatric surgeries were performed in the United States, with an annual rate of 50–60 procedures per 100,000 adults.5, 6 In 2010 alone, ~101,600 procedures were performed in the United States and Canada.7 These statistics, together with the continued high prevalence of obesity in the United States, implies that most clinicians will be involved in the care of patients who have had bariatric procedures.8 Roux-en-Y gastric bypass (RYGB) surgery is the most frequently performed bariatric procedure worldwide.2, 7 The RYBG is primarily a restrictive procedure with a mild malabsorptive component that involves altering the anatomy and physiology of the upper gastrointestinal (GI) tract. In brief, the stomach is partitioned by surgical stapling into a small gastric pouch (~20–30 ml in size) in the upper fundus and the remnant stomach. The jejunum is divided in the proximal region ~50–75 cm from the ligament of Treitz to form the biliopancreatic duodenal limb that includes the remnant stomach, duodenum, and the proximal jejunum. The “Roux limb” connects the distal small intestine to the small gastric pouch, allowing for passage of food. The biliopancreatic duodenal limb is reconnected to the distal jejunum to allow the mixing of food with bile salts and pancreatic digestive enzymes downstream. Most of the stomach, the entire duodenum, and a fraction of the proximal jejunum are excluded from food while the remaining part of the GI tract, including the distal jejunum, the entire ileum and the colon, remains intact for digesting and absorbing nutrients and fluid. The reduced time and distance for the mixing of food and digestive enzymes may diminish nutrient absorption. Some element of malabsorption accompanied by decreased caloric intake is believed to promote weight loss.
The altered anatomy in the upper GI tract after RYGB suggests that the pharmacokinetic profile of drugs, especially during the absorption phase, may be changed. Currently, very few studies have addressed this issue, and their results are conflicting.9–12 Research conducted in the last decade has also provided insight into the physiology of the small intestine, which is now considered to be important in regulating the oral absorption of both drugs and nutrients. Cytochrome P450 (CYP) 3A4 and P-glycoprotein (P-gp) are among the two most significant proteins affecting oral drug absorption.13, 14 CYP3A4 is expressed along the entire small intestine, with slightly increased expression from the duodenum to the middle section of the jejunum and a gradually reduced expression in the distal jejunum and ileum.15 Based on the relative distribution of intestinal CYP3A4, the duodenum and proximal jejunum account for 20–40% of the total intestinal CYP3A4 activity. The RYGB procedure could result in a significant increase in the oral bioavailability of some CYP3A4 substrates. Similarly, P-gp expression differs along the length of the human small intestine. Using both Western blot and quantitative measurement of messenger RNA concentrations, the level of P-gp expression is lowest in the duodenum and highest in the distal ileum and the colon.16–18 Therefore, it is possible that the effect of RYGB on the absorption of P-gp substrates would be limited because RYGB does not affect the distal intestine. With increasing numbers of patients undergoing RYGB, understanding the potential effects of RYGB on drug absorption would enhance patient care and further our understanding of intestinal physiology. The aim of this pilot study was to determine if RYGB affects the pharmacokinetics of CYP3A4 and P-gp substrates. Specifically, we compared the pattern and magnitude of oral absorption of midazolam and digoxin, established probes of CYP3A4 and P-gp, respectively, in obese patients before and after undergoing RYGB.19, 20
Methods
Patients
All obese patients meeting the practice standard established by the Center for Bariatric Surgery at the University of Washington Medical Center for RYGB were eligible to participate in this prospective, nonblinded, longitudinal study. The standard includes specific body mass index (BMI) requirements with significant obesity-related comorbidities, such as diabetes or sleep apnea. Eligible patients experienced only short-term success with previous serious weight-loss attempts, were under the care of a primary physician, and were committed to making significant and sustained changes in eating habits and lifestyle including exercising at least 90 minutes per week. Patients with contraindications to midazolam or digoxin, with documented liver, kidney, or heart failure, or who were unwilling to return to the clinical research unit for postoperative follow-up visits were excluded. Patients were recruited by the research coordinators between October 2006 and December 2007. All surgeries were performed by one of three surgeons. Written informed consent was obtained from all patients prior to study initiation. The study protocol was reviewed and approved by the institutional review board of the University of Washington.
Study Procedures
The study comprised three phases: presurgical baseline and postoperative assessments at 3 and 12 months. The 3-month postoperative evaluation was chosen to allow for complete surgical wound healing and to coincide with the time most patients begin to consume solid food. The 12-month postoperative evaluation was chosen to provide information indicative of long-term metabolic and physiologic changes. Further, 12 months is the time point at which most recipients of RYGB achieve maximal weight loss.
Patients were admitted to the University of Washington General Clinical Research Center (UW-GCRC) for a 24-hour stay on each of the 3 study days. The baseline visit was scheduled approximately 1 week before the RYGB surgery. Patients were instructed to abstain from consuming citrus juice, apple juice, dietary supplements, or drugs known to affect intestinal CYP3A4 and P-gp activity for at least 5 days prior to study initiation. The patients were fasted overnight before each of the three scheduled visits to the clinical research unit. At 8 a.m., midazolam 2 mg oral solution (prepared by diluting injectable midazolam solution in water) and digoxin 0.5 mg (oral tablets) were administered orally with no more than 120 ml of water. Blood samples were collected at the following times after the administration of the study drugs: 0 (pre-dose), 10, 20, 30, 40, and 50 minutes, 1, 1.25, 1.5, 2, 3, 4, 6, 12, and 24 hours. Continuous 12-lead electrocardiogram (EKG) monitoring was performed for the first 12 hours. Vital signs including heart rate, blood pressure, and respiratory rate were measured every 30 minutes for the first 2 hours, then hourly for the next 2 hours, and again at the 6th hour. Patients were allowed to have a standardized meal after hour 3. Plasma was harvested and stored at −80°C until analysis. Study procedures were repeated for the 3- and 12-month visits. At the 12-month visit, all patients underwent an endoscopic gastroduodenoscopy. A fiberoptic endoscope was passed down the esophagus, through the gastric pouch and into the jejunum at the Roux limb through the gastrojejunostomy. A mucosal biopsy specimen was obtained and saved for future genomic analysis.
Assays
Midazolam, 1′-hydroxymidazolam, d4-midazolam, and d4-1′-hydroxymidazolam were purchased from Cerilliant Corporation (Round Rock, TX). Digoxin was purchased from Sigma-Aldrich Corporation (St. Louis, MO), and d3-digoxin was provided by Dr. Danny Shen (University of Washington). All other reagents and solvents were high-performance liquid chromatography (HPLC) grade or higher.
Plasma midazolam and 1′-hydroxymidazolam concentrations were measured using liquid chromatography (LC)/mass spectrometry. Samples were prepared with 0.5 ml of plasma, diluted with 0.5 ml of water, and spiked with 30 pmol each of d4-midazolam and d4-1′-hydroxymidazolam. One milliliter of saturated sodium borate (pH 10) and 5 ml of toluence:dichloromethane (7:3) was added to each sample. Samples were mixed on a horizontal shaker for 20 minutes and centrifuged at 1500 g for 20 minutes. The organic layer was transferred and evaporated at 47°C under nitrogen gas. Samples were reconstituted with 120 μl methanol, transferred to tubes containing 100 μl of water, vortexed and centrifuged for 10 minutes at 13,000 g, and transferred to LC vials. Detection of midazolam (m/z 325.9), 1′-hydroxymidazolam (m/z 341.9), and the stable labeled internal standards (m/z 330.9 and 346.9, respectively) was performed on a Waters 2690 separations module (Waters Corp, Milford, MA) coupled to a Waters Micromass platform LCZ mass spectrometer using electrospray positive ionization and gradient HPLC elution on an Agilent Technologies (Santa Clara, CA) Zorbax Eclipse XDB-C18 analytical column (2.1 × 50 mm, 5 μm) with a Phenomenex guard column (Phenomenex Inc., Torrance, CA). Analytes were eluted following a 10-μl injection using a linear gradient of water with 0.0037% formic acid (A) and methanol with 0.0037% formic acid (B). Initial conditions were 45% B for 0.5 minutes, increasing to 60% B at 2 minutes, held at 60% B until 2.5 minutes, increasing to 90% B at 3 minutes, held at 90% B until 4 minutes, and returning to 45% B at 5 minutes. The total run time was 8 minutes.
For digoxin analysis, the procedure was modified.21 Plasma (500 ll) was spiked with 3 ng of d3-digoxin; 500 μl of 25% ammonium chloride (pH 9.5), and 5 ml of methyl tert-butyl ether was added to each tube. Samples were mixed on a horizontal shaker for 30 minutes and centrifuged at 1500 g for 10 minutes. The organic layer was transferred and evaporated at 40°C. Samples were reconstituted with 80 μl of the initial mobile phase conditions. Each sample (10 μl) was injected onto an Agilent 1100 HPLC coupled to an Agilent MSD. Separation was achieved using an Agilent Zorbax RX-C8 (150 × 2.1 mm, 5 lm, 80A pore size) and a Phenomenex guard column held at 35°C at a flow rate of 0.25 ml/minute using an isocratic gradient of 10 mM ammonium acetate and 1 mM ammonium chloride (35%) and methanol (65%). The chloride adducts of digoxin (m/z 815.4) and d3-digoxin (m/z 818.4) were monitored using atmospheric pressure ionization-electrospray in negative mode. The total run time was 6 minutes.
Data Analysis
For each subject, noncompartmental analysis of plasma concentration versus time data was performed using Phoenix WinNonlin (v.6.3, Certera, St. Louis, MO). Peak plasma concentration (Cmax), and time to peak concentration (Tmax) were taken from the observed data. The terminal rate constant (k) was estimated from the terminal log-linear concentration versus time points for each patient. The terminal half-life (t1/2) was calculated from the equation t1/2 = ln2/k. The area under the plasma concentration-time curve (AUC) was calculated using the linear trapezoidal rule. Due to the limited duration of plasma collection compared with the half-life of digoxin (~40 hrs), extrapolation of the data to infinite time was not performed and the terminal t1/2, apparent volume of distribution, and oral clearance of digoxin were not calculated. The pharmacokinetic parameters of the drugs from the three study phases were compared.
Statistical Analysis
All results are expressed as mean ± SDs. Data from each of the two postoperative visits were compared with the presurgical baseline data with paired Student t test using Excel 2013 (Microsoft Corporation, Redmond, WA). Graphs were plotted using KaleidaGraph (Synergy Software, Inc., Reading, PA). A p value < 0.05 was considered significant.
Results
Patients and Weight Loss
Twelve patients were enrolled in the study, and nine completed all three phases. One patient completed the first two study visits only (i.e., baseline and 3-mo post-RYGB), one patient completed the baseline and 12-month visits only, and one patient completed the baseline visit without incidence but could not complete the subsequent postoperative visits because of sustained asymptomatic bradycardia (resting heart rate < 50 beats/min) before receiving any study drugs. The median age of the 12 patients was 41 years (range 37–55 yrs) (Table 1). The mean baseline weight was 150.8 ± 41.4 kg. The mean BMIs of patients completing each study phase were 51.9 kg/m2 (baseline), 42.2 kg/m2 (3 mo), and 35.0 kg/m2 (12 mo). The average length of the Roux limb was 128 ± 25 cm (range 90–150 cm). The average weight loss at 3 and 12 months was 26.7 ± 8.5 kg and 51.9 ± 28.0 kg, respectively, which translates to a total weight loss of 18.1% at 3 months and 36.6% at 12 months. No patient experienced changes in heart rate or EKG during any phase of the study.
Table 1.
Demographics of Study Population
| Baseline (n=12) | 3 mo (n=10) | 12 mo (n=10) | |
|---|---|---|---|
| Females, n (%) | 9 (75) | 8 (80) | 8 (80) |
| Race | |||
| White | 11 | 9 | 9 |
| Native American | 1 | 1 | 1 |
| Mean weight, kg, SD | 150.8 ± 41.4 | 121.7 ± 36.3a | 113.2 ± 29.2a |
| Body mass index, kg/m2, SD | 51.9 ± 12.1 | 42.4 ± 11.7 | 35.0 ± 16.6a |
| Weight loss from baseline | |||
| Absolute weight, kg, SD | – | 26.7 ± 8.5 | 51.9 ± 28.0 |
| Percentage weight, %, SD | – | 18.1 ± 3.4 | 36.6 ± 25.7 |
p<0.05 vs baseline.
Midazolam Pharmacokinetics
Following RYGB, the mean peak concentration of midazolam was higher and occurred earlier compared with baseline (Figure 1, Table 2). Using the data from the nine patients who completed all three phases, the midazolam Cmax was 66% and 71% higher than baseline values (p=0.017 and p=0.001) at 3 and 12 months, respectively. The median Tmax decreased by 50% at both postoperative intervals compared with baseline, from 40 to 20 minutes, suggesting a more rapid onset and rate of absorption after RYGB. The mean Tmax at baseline was 0.61 ± 0.22 hours, whereas the Tmax at the 3- and 12-month visits were 0.28 ± 0.08 hour (p=0.001) and 0.26 ± 0.08 hour (p=0.002), respectively. The mean midazolam apparent volume of distribution was significantly decreased at both the 3- and 12-month postoperative phases compared with presurgical baseline (553 ± 218 L [p=0.03] and 206 ± 260 L [p=0.04] vs 763 ± 254 L). The midazolam oral clearance and AUC were not significantly different among the three phases. There was a trend, although not significant, for a shorter terminal t1/2 of midazolam over the course of the study. The terminal t1/2 of midazolam at baseline, 3 months, and 12 months was estimated to be 8.0 ± 3.7 hours, 5.3 ± 3.7 hours, and 4.5 ± 4.3 hours, respectively.
Figure 1.
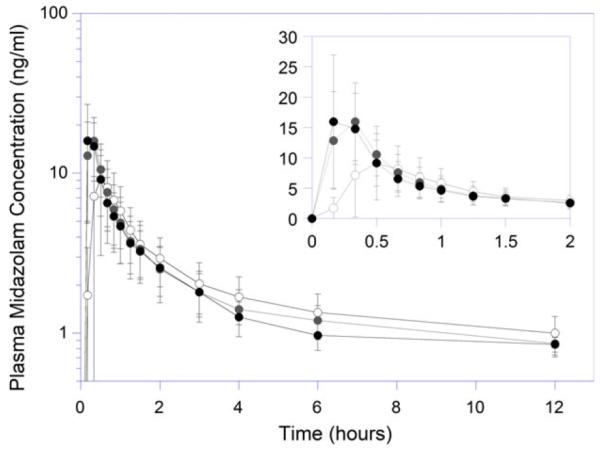
Plasma midazolam concentration versus time profiles in patients undergoing Roux-en-Y gastric bypass. Open, gray, and black circles represent presurgical baseline, 3- and 12-month postoperative study days, respectively.
Table 2.
Summary of Midazolam and 1′-hydroxymidazolam Pharmacokinetics Before and After Roux-en-Y Gastric Bypass (mean ± SD)
| Baseline (n=9) | 3 mo (n=9) | 12 mo (n=9) | |
|---|---|---|---|
| Midazolam | |||
| Cmax, ng/ml | 9.67 ± 6.72 | 16.09 ± 6.65a | 16.56 ± 7.55a |
| Tmax, hr | 0.61 ± 0.22 | 0.28 ± 0.08a | 0.26 ± 0.08a |
| Vd/F, L | 763 ± 254 | 553 ± 219a | 506 ± 260a |
| Cl/F, L/hr | 91.9 ± 74.4 | 100.6 ± 58.6 | 100.1 ± 40.2 |
| t1/2, hr | 8.0 ± 3.7 | 5.3 ± 3.7 | 4.5 ± 4.3 |
| AUC0–last, ng·hr/ml | 20.8 ± 10.7 | 20.3 ± 10.8 | 18.3 ± 7.9 |
| AUC0–∞, ng·hr/ml | 31.2 ± 15.7 | 26.8 ± 14.7 | 23.8 ± 11.2 |
| 1′-Hydroxymidazolam | |||
| Cmax, ng/ml | 3.19 ± 2.01 | 5.97 ± 2.11a | 5.75 ± 2.00a |
| Tmax, hr | 0.69 ± 0.29 | 0.35 ± 0.10a | 0.31 ± 0.05a |
| t1/2, hr | 3.3 ± 1.3 | 2.5 ± 1.2 | 2.1 ± 1.3 |
| AUC0–last, ng·hr/ml | 6.5 ± 3.9 | 8.1 ± 3.4 | 7.1 ± 1.9 |
| AUC0–∞, ng·hr/ml | 8.5 ± 4.2 | 9.8 ± 3.9 | 8.2 ± 2.0 |
| 1′OH MDZ:MDZ AUC ratio | 0.35 ± 0.27 | 0.47 ± 0.28 | 0.40 ± 0.14 |
AUC = area under the concentration-time curve; Cl/F = oral clearance; Cmax = peak plasma concentration; t1/2 = serum half-life; Tmax = time to peak plasma concentration; Vd/F = apparent volume of distribution.
p<0.05 compared with baseline.
To specifically assess the change in CYP3A4 activity, concentrations of 1′-hydroxymidazolam were measured, and the AUC ratio between 1′-hydroxymidazolam and midazolam were determined. The mean 1′-hydroxymidazolam peak concentrations were higher and occurred earlier following RYGB. Among the nine individuals who completed all three study visits, 1′-hydroxymidazolam Cmax values increased by 87% and 80%, at 3 and 12 months, respectively (Figure 2, Table 2). However, the AUC and terminal half-life of 1′-hydroxy-midazolam remained unchanged postoperatively. The metabolite-to-parent AUC ratios were not significantly different among the three phases.
Figure 2.
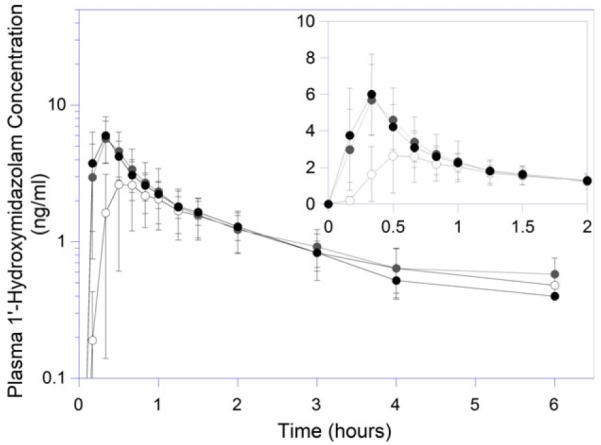
Plasma 1′-hydroxymidazolam concentration versus time profiles in patients undergoing Roux-en-Y gastric bypass. Open, gray, and black circles represent presurgical baseline, 3- and 12-month postoperative study days, respectively.
Digoxin Pharmacokinetics
Following RYGB, the mean peak digoxin concentration was unchanged; however, the median time to peak concentration decreased from 40 minutes at baseline to 30 and 20 minutes at 3 and 12 months, respectively (Table 3, Figure 3). There appeared to be a reduction in the lag time for digoxin absorption in the postoperative periods (Figure 3). The mean AUCs of digoxin, from 0 to 4 hours and 0 to 24 hours, and the ratio of AUCs were unchanged at the 3- and 12-month postoperative visits compared with baseline.
Table 3.
Summary of Digoxin Pharmacokinetics Before and After Roux-en-Y Gastric Bypass (mean ± SD)
| Baseline (n=9) | 3 mo (n=9) | 12 mo (n=9) | |
|---|---|---|---|
| Digoxin | |||
| Cmax, ng/ml | 3.08 ± 1.38 | 3.05 ± 0.85 | 3.41 ± 1.13 |
| Tmax, hr | 0.82 ± 0.29 | 0.50 ± 0.19a | 0.39 ± 0.15a |
| Vd/F, L | 889 ± 172 | 815 ± 279 | 811 ± 221 |
| Cl/F, L/hr | 19.9 ± 5.6 | 18.5 ± 7.2 | 17.1 ± 6.1 |
| AUC 0→Cmax, ng·hr/ml | 1.3 ± 0.6 | 1.0 ± 0.4 | 0.8 ± 0.4a |
| AUC0–4, ng·hr/ml | 5.6 ± 1.3 | 6.1 ± 1.6 | 6.3 ± 1.6 |
| AUC0–24, ng·hr/ml | 13.0 ± 2.5 | 14.6 ± 3.8 | 14.3 ± 2.9 |
| AUC0–∞ ng·hr/ml | 26.8 ± 6.7 | 30.3 ± 10.0 | 32.8 ± 11.7 |
| AUC0→Cmax/AUC0→24 (%) | 9.4 ± 3.0 | 6.5 ± 2.4a | 5.7 ± 2.4a |
AUC = area under the concentration-time curve; Cl/F = oral clearance; Cmax = peak plasma concentration; Tmax = time to peak plasma concentration; Vd/F = apparent volume of distribution.
p<0.05 compared with baseline.
Figure 3.
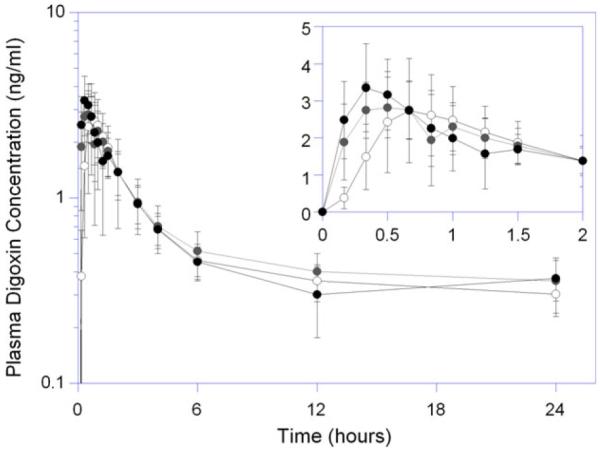
Plasma digoxin concentration versus time profiles in patients undergoing Roux-en-Y gastric bypass. Open, gray, and black circles represent presurgical baseline, 3- and 12-month postoperative study days, respectively.
Safety Monitoring
No changes in vital signs, mental alertness, or sedation were noted. Heart rate and EKG patterns at the postoperative visits were also similar compared with baseline.
Discussion
Interpretation of findings from many existing pharmacokinetic studies in patients undergoing bariatric surgery is hindered by design limitations including single patient case studies, insufficient sample collection, inconsistent surgical techniques, or variable surgical procedures in the study cohorts. Most studies compared the pharmacokinetics in post-RYGB patients with values from nonsurgical control subjects, instead of using a before and after surgery design. Therefore, the results could be greatly confounded by interindividual variability in the disposition of the drugs.
To our knowledge, our study is the first to use a prospective longitudinal design with an adequate number of blood samples to evaluate how RYGB affects the pattern and extent of oral drug absorption. Pharmacokinetic profiles of the probe drugs were compared in patients before and at 3 and 12 months after RYGB surgery performed using comparable techniques. This design minimizes the variance related to interindividual variations in drug disposition by allowing individuals to serve as their own control. The probes we used are specific for evaluating the impact of RYGB on intestinal and hepatic CYP3A-mediated metabolism and intestinal P-gp transport. Given the alteration of the upper GI anatomy after RYGB, changes in oral drug absorption would most likely involve altered transit time or CYP3A4 and P-gp activity. Our results suggest that for CYP3A4 or P-gp substrates, the onset of drug absorption occurs much earlier after proximal RYGB using the current surgical standards (i.e., gastric pouch ~20–30 ml; Roux limb up to 150 cm) as suggested by the decrease in Tmax. Moreover, no evidence suggested that oral bioavailability was significantly decreased after RYGB as speculated by others.22, 23
Although the overall apparent oral clearance of midazolam was unchanged at the 3- and 12-month visits after RYGB, peak concentrations were significantly higher at both postoperative time points. This finding differs from the results of another study that examined oral midazolam bioavailability after RYGB.11 Another study also reported that the Tmax of oral midazolam was decreased but did not find significant changes in Cmax or AUC. It compared the pharmacokinetics of midazolam in RYGB recipients with nonsurgical control subjects. Moreover, it collected fewer blood samples during the midazolam absorption phase and may have missed the actual Tmax and Cmax.11 Because midazolam was administered as an oral liquid in both studies, one would expect the time to reach Cmax to be within 10–15 minutes after drug administration in the postoperative patients.
Our finding of increased midazolam Cmax is supported by studies of atorvastatin, another substrate of CYP3A4 with low baseline oral bioavailability.24, 25 Therefore, for a drug that is a CYP3A4 substrate with a relatively low oral bioavailability, contemporary RYGB appears to increase the rate of absorption, and the patient may achieve a much higher serum concentration initially, including Cmax, that could increase the risk of dose-related side effects. This observation is consistent with our clinical experience where some patients receiving other CYP3A substrates, such as alprazolam, triazolam, or carbamazepine, experienced increased central nervous system–related side effects shortly after RYGB. An increase in the Cmax was not associated with an overall change in oral clearance of midazolam or digoxin. Conclusions about the absolute bioavailability of CYP3A or P-gp substrates cannot be made without intravenous data. However, it appears that the contemporary RYGB procedure alters the pattern of absorption without significantly changing the overall exposure to the drug, at least for drugs with metabolic and transport profiles similar to midazolam and digoxin.
Our observation of a trend toward decreased t1/2 of midazolam is of interest and likely related to the change in CYP3A4 activity associated with weight or dietary changes. The presurgical half-lives of midazolam in our obese patients were significantly longer than the data obtained from normal weight individuals but comparable with the published data in obese subjects.26, 27 Obesity or increased consumption of fructose are associated with mild to moderate nonalcoholic fatty liver disease, characterized by fatty infiltration of the liver.28, 29 In most cases, this condition reverses upon significant weight loss if the liver disease has not progressed to the cirrhotic stage. A study involving 21 obese patients showed a negative correlation between BMI with both hepatic CYP3A4 expression and the oral clearance of atorvastatin lactone, a CYP3A4 substrate.30 The association between nonalcoholic fatty liver disease from high intake of dietary fat and fructose with decreased CYP3A4 expression was demonstrated in an animal model.31 It is likely that hepatic CYP3A4 activity in the RYBG patients was slightly improved at the 3- and 12-month time points after substantial weight loss and significant dietary changes. As noted earlier, the RYGB procedure bypasses the duodenum and proximal jejunum, which may lead to a reduction of intestinal CYP3A4-mediated presystematic metabolism.15 This may also be the basis of the significant increase in the Cmax of midazolam we observed at the postoperative phases. Moreover, oral bioavailability in these patients may be altered due to decreases in intestinal CYP3A4-mediated presystemic metabolism due to RYGB and increases in hepatic CYP3A4-mediated first-pass clearance due to weight loss. These changes may be of clinical importance. However the magnitude and direction of change would depend on the individual patient and characteristics of the drug administered (e.g., intestinal vs hepatic first-pass metabolism). The relationship between obesity and weight loss on the regulation and function of CYP3A4 warrants further investigation.
The lack of change in the AUC of digoxin is consistent with the hypothesis that RYGB would have a negligible impact on a P-gp substrate because of the regional distribution of this transport protein.17 The decrease in the Tmax of digoxin is consistent with the case of oral midazolam, suggesting that oral to small intestinal transit time is shortened by the altered upper GI anatomy associated with proximal RYGB. However, as currently performed, RYGB apparently provides adequate small intestinal luminal surface for optimal absorption. Digoxin oral bioavailability is preserved in patients with small intestinal resection and bypass as long as adequate intestinal cell mass is preserved.32, 33 It appears that P-gp substrates with high intrinsic oral bioavailability would be minimally affected by the contemporary RYGB and continued weight loss.
The decrease in Tmax for digoxin after RYGB was not as large as seen with midazolam. One possible explanation may be the use of digoxin tablets but midazolam solution. Digoxin oral tablets need to undergo dissolution before being absorbed. The data suggest that the post-RYGB anatomy still provides a favorable environment for digoxin tablets to undergo complete dissolution. Given the short oral to small intestinal transit time and the relatively achlorhydric environment of the gastric pouch, it is possible that some drugs or formulations requiring long periods of time or acid for dissolution will not be adequately absorbed. Future research should be directed to investigate which dosage form or formulation characteristics are more significantly affected by RYGB.
Our study has several limitations. This is an exploratory pilot study with a small sample size. Some of the trends we observed in the results could have occurred by chance and should be confirmed in future investigations. Because most drugs are eliminated by multiple CYP enzymes and/or transport proteins, the change we observed in this study may not be applicable to all CYP3A4 and P-gp substrates. More importantly, other factors such as genetic polymorphism, gender, dietary intake, drug-drug interactions, drug-nutrient interactions, and chronic illnesses may also alter CYP and P-gp functions. Future investigations addressing these potential confounders should be conducted to provide a more population-specific observation. In addition, the study was aimed to evaluate the effect of RYGB on oral drug absorption. Weight loss and the drastic changes in diet may also alter systemic clearance by altering the hepatic expression and activities of CYP3A4 and P-gp. Administration of both probes via intravenous and oral routes is needed to determine the impact of RYGB on intestinal enzyme or transporters versus hepatic processes. Finally, the volume of distribution for midazolam has been shown to be significantly increased with obesity.26 Extreme weight loss as experienced by patients undergoing RYGB may result in a significant decrease in volume of distribution and related pharmacokinetic parameters. Intravenous administration of the probe drugs would enable better estimations of their pharmacokinetic parameters.
In summary, using oral midazolam solution and digoxin tablets as probes, we observed that contemporary proximal RYGB is associated with an earlier onset of absorption without any significant change to the overall exposure of the drugs following oral dosing. The peak plasma concentration (i.e., Cmax) of midazolam was substantially increased after RYGB. For both midazolam and digoxin, Tmax was significantly reduced after RYGB. Patients receiving high extraction ratio drugs should be closely monitored in the first few hours after dose administration for concentration-related side effects.
Acknowledgments
The authors would like to thank Sarah Hammond and Nickolas Dasher for their tremendous efforts in coordinating this study.
This research is supported by ACCP Research Institute Frontiers Research Award; NIH National Center for Research Resources grants UL1 RR025014, KL2 RR025015, TL1 RR025016, and M01-RR-00037 of the University of Washington General Clinical Research Center and the University of Washington School of Pharmacy DMTPR funding.
References
- 1.Chang SH, Stoll CR, Song J, Varela JE, Eagon CJ, Colditz GA. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003–2012. JAMA Surg. 2014;149:275–87. doi: 10.1001/jamasurg.2013.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Courcoulas AP, Christian NJ, Belle SH, et al. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA. 2013;310:2416–25. doi: 10.1001/jama.2013.280928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hallersund P, Sjöström L, Olbers T, et al. Gastric bypass surgery is followed by lowered blood pressure and increased diuresis—long term results from the Swedish Obese Subjects (SOS) study. PLoS ONE. 2012;7:e49696. doi: 10.1371/journal.pone.0049696. Erratum in: PLoS One. 2013;8. doi:10.1371/annotation/88dbb5b7-3f7e-4b26- a5b6-f020d33f0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlsson LM, Peltonen M, Ahlin S, et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med. 2012;367:695–704. doi: 10.1056/NEJMoa1112082. [DOI] [PubMed] [Google Scholar]
- 5.How many bariatric surgeries are taking place? Available from http://www.amednews.com/assets/pdf/2012gbisa0423b.pdf. Accessed October 08, 2014. [Google Scholar]
- 6.Nguyen NT, Masoomi H, Magno CP, Nguyen XM, Laugenour K, Lane J. Trends in use of bariatric surgery, 2003–2008. J Am Coll Surg. 2011;213:261–6. doi: 10.1016/j.jamcollsurg.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 7.Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2011. Obes Surg. 2013;23:427–36. doi: 10.1007/s11695-012-0864-0. [DOI] [PubMed] [Google Scholar]
- 8.Ogden CL, Carroll MD, Kit BK, Glegal KM. Prevalence of Obesity in the United States, 2009–2010. NCHS Data Brief. 2012 Jan;(82) Available from http://www.cdc.gov/nchs/ data/databriefs/db82.pdf. Accessed August 20, 2014. [PubMed]
- 9.Yska JP, van der Linde S, Tapper VV, et al. Influence of bariatric surgery on the use and pharmacokinetics of some major drug classes. Obes Surg. 2013;23:819–25. doi: 10.1007/s11695-013-0882-6. [DOI] [PubMed] [Google Scholar]
- 10.Smet J, Van Bocxlaer J, Boussery K. The influence of bypass procedures and other anatomical changes in the gastrointestinal tract on the oral bioavailability of drugs. J Clin Pharmacol. 2013;53:361–76. doi: 10.1002/jcph.65. [DOI] [PubMed] [Google Scholar]
- 11.Tandra S, Chalasani N, Jones DR, Mattar S, Hall SD, Vuppalanchi R. Pharmacokinetic and pharmacodynamic alterations in the Roux-en-Y gastric bypass recipients. Ann Surg. 2013;258:262–9. doi: 10.1097/SLA.0b013e31827a0e82. [DOI] [PubMed] [Google Scholar]
- 12.Padwal R, Brocks D, Sharma AM. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes Rev. 2010;11:41–50. doi: 10.1111/j.1467-789X.2009.00614.x. [DOI] [PubMed] [Google Scholar]
- 13.Darwich AS, Neuhoff S, Jamei M, Rostami-Hodjegan A. Interlay of metabolism and transport in determining oral drug absorption and gut wall metabolism: a simulation assessment using the “Advanced Dissolution, Absorption, Metabolism (ADAM)” model. Curr Drug Metab. 2010;11:716–29. doi: 10.2174/138920010794328913. [DOI] [PubMed] [Google Scholar]
- 14.Croft M, Keely B, Morris I, Tann L, Lappin G. Predicting drug candidate victims of drug-drug interactions, using microdosing. Clin Pharmacokinet. 2012;51:237–46. doi: 10.2165/11597070-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 15.Paine MF, Khalighi M, Fisher JM, et al. Characterization of interintestinal and intranintestinal variations in human CYP3A-dependent metabolism. J Pharmacol Exp Ther. 1997;283:1552–62. [PubMed] [Google Scholar]
- 16.Fricker G, Drewe J, Huwyler J, et al. Relevance of P-glycopro- tein for the enteral absorption of cyclosporin A: in vitro-in vivo correlation. Br J Pharmacol. 1996;118:1841–7. doi: 10.1111/j.1476-5381.1996.tb15612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mouly S, Paine MF. P-glycoprotein increases from proximal to distal regions of human small intestine. Pharm Res. 2003;20:1595–9. doi: 10.1023/a:1026183200740. [DOI] [PubMed] [Google Scholar]
- 18.Tang H, Pak Y, Mayersohn M. Protein expression pattern of P-glycoprotein along the gastrointestinal tract of the Yucatan micropig. J Biochem Mol Toxicol. 2004;18:18–22. doi: 10.1002/jbt.20001. [DOI] [PubMed] [Google Scholar]
- 19.Giacomini KM, Huang SM, Tweedie DJ, et al. Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9:215–36. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu YT, Hao HP, Liu CX, Wang GJ, Xie HG. Drugs as CYP3 A probes, inducers, and inhibitors. Drug Metab Rev. 2007;39:699–721. doi: 10.1080/03602530701690374. [DOI] [PubMed] [Google Scholar]
- 21.Kaiser P, Kramer U, Meissner D, Kress M, Wood WG, Reinauer H. Determination of the cardiac glycosides digoxin and digitoxin by liquid chromatography combined with isotope-dilution mass spectrometry (LC-IDMS)—a candidate reference measurement procedure. Clin Lab. 2003;49:329–43. [PubMed] [Google Scholar]
- 22.Smith A, Henriksen B, Cohen A. Pharmacokinetic considerations in Roux-en-Y gastric bypass patients. Am J Health Syst Pharm. 2011;68:2241–7. doi: 10.2146/ajhp100630. [DOI] [PubMed] [Google Scholar]
- 23.Rogers CC, Alloway RR, Alexander JW, Cardi M, Trofe J, Vinks AA. Pharmacokinetics of mycophenolic acid, tacrolimus and sirolimus after gastric bypass surgery in end-stage renal disease and transplant patients: a pilot study. Clin Transplant. 2008;22:281–91. doi: 10.1111/j.1399-0012.2007.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skottheim IB, Stormark K, Christensen H, et al. Significantly altered systemic exposure to atorvastatin acid following gastric bypass surgery in morbidly obese patients. Clin Pharmacol Ther. 2009;86:311–8. doi: 10.1038/clpt.2009.82. [DOI] [PubMed] [Google Scholar]
- 25.Jakobsen GS, Skottheim IB, Sandbu R, et al. Long-term effects of gastric bypass and duodenal switch on systemic exposure of atorvastatin. Surg Endosc. 2013;27:2094–101. doi: 10.1007/s00464-012-2716-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenblatt DJ, Abernethy DR, Locniskar A, Harmatz JS, Limjuco RA, Shader RI. Effect of age, gender, and obesity on midazolam kinetics. Anesthesiology. 1984;61:27–35. [PubMed] [Google Scholar]
- 27.Brill MJ, van Rongen A, Houwink AP, et al. Midazolam pharmacokinetics in morbidly obese patients following semi-simul-taneous oral and intravenous administration: a comparison with healthy volunteers. Clin Pharmacokinet. 2014;53:931–41. doi: 10.1007/s40262-014-0166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yilmaz Y, Younossi ZM. Obesity-associated nonalcoholic fatty liver disease. Clin Liver Dis. 2014;18:19–31. doi: 10.1016/j.cld.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 29.Schultz A, Neil D, Aguila MB, Mandarim-de-Lacerda CA. Hepatic adverse effects of fructose consumption independent of overweight/obesity. Int J Mol Sci. 2013;14:21873–86. doi: 10.3390/ijms141121873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ulvestad M, Skottheim IB, Jakobsen GS, et al. Impact of OATP1B1, MDR1, and CYP3A4 expression in liver and intestine on interpatient pharmacokinetic variability of atorvastatin in obese subjects. Clin Pharmacol Ther. 2013;93:275–82. doi: 10.1038/clpt.2012.261. [DOI] [PubMed] [Google Scholar]
- 31.Patoine D, Levac X, Pilote S, Drolet B, Simard C. Decreased CYP3A expression and activity in guinea pig models of diet induced metabolic syndrome: is fatty liver infiltration involved? Drug Metab Dispos. 2013;41:952–7. doi: 10.1124/dmd.112.050641. [DOI] [PubMed] [Google Scholar]
- 32.Marcus FI, Quinn EJ, Horton H, et al. The effect of jejunoileal bypass on the pharmacokinetics of digoxin in man. Circulation. 1977;55:537–41. doi: 10.1161/01.cir.55.3.537. [DOI] [PubMed] [Google Scholar]
- 33.Gerson CD, Lowe EH, Lindenbaum J. Bioavailability of digoxin tablets in patients with gastrointestinal dysfunction. Am J Med. 1980;69:43–9. doi: 10.1016/0002-9343(80)90498-2. [DOI] [PubMed] [Google Scholar]


