Abstract
Background
Childhood pneumonia declined following introduction of pneumococcal conjugate vaccine (PCV7) in the United States. Recent reports suggest an increase in childhood pneumonia complicated by empyema. We assessed whether early declines in pneumonia hospitalization rates were sustained and trends in such hospitalizations complicated by empyema in United States children aged <5 years.
Methods
Nationwide Inpatient Sample and Census data were used to calculate annual all-cause and pneumococcal pneumonia hospitalization rates for pre-PCV7(1996–1999) and post-PCV7 years(2001–2007) and to analyze trends in total and pathogen-specific pneumonia-associated empyema.
Results
Among children aged <2 years, all-cause pneumonia hospitalizations decreased 33% (95%CI: 28–37) from 1267 per 100000 in pre-PCV7 years to 852 in post-PCV7 years. Pneumococcal pneumonia hospitalization rates declined 61% (95%CI: 55–67) post-PCV7 compared with pre-PCV7 years. Pneumonia hospitalizations complicated by empyema increased 2.01-fold from 3.5 per 100000 in 1996–1998 to 7.0 in 2005–2007. Rates of pneumococcal and streptococcal empyema remained stable, whereas rates of staphylococcal and other or unspecified empyema increased 4.08 and 1.89-fold. Among children aged 2–4 years, all-cause pneumonia rates remained stable, whereas pneumococcal pneumonia decreased by 26% (95%CI: 16–34). Pneumonia complicated by empyema increased 2.81-fold from 3.7 per 100000 in 1996–1998 to 10.3 in 2005–2007. In this age group, there were 2.17, 2.80, 3.76 and 3.09-fold increases in rates of pneumococcal, streptococcal, staphylococcal and other or unspecified empyema, respectively.
Conclusion
Declines in childhood pneumonia hospitalizations following PCV7 introduction were sustained. Although empyema complicated only a small fraction of pneumonia hospitalizations, the observed increases were due to several pathogens and warrant monitoring.
INTRODUCTION
In the United States (US), routine infant immunization with a seven-valent pneumococcal conjugate vaccine (PCV7) began in 2000. After PCV7 introduction, invasive pneumococcal disease (IPD) declined substantially in all age groups. In randomized clinical trials, pneumococcal conjugate vaccines also provided protection against pneumonia.[1–4] Pneumonia is a much more frequent cause of hospitalization than IPD, and the leading killer of children worldwide.[5–7] Following PCV7 introduction in the US, rates of pneumonia hospitalizations and ambulatory visits among children aged <2 years declined through 2004.[8–10] However, IPD caused by serotypes not covered by PCV7 has increased during recent years[11] and the sustainability of the initial declines in pneumonia is unknown.
Empyema is a relatively rare complication of pneumonia. In the US, regional and multistate studies[12–14] reported increases in childhood pneumonia complicated by empyema due primarily to pneumococcal serotypes not included in PCV7. In those studies, the increases began in the 1990s[13] and continued after PCV7 introduction.[14] Nevertheless, national trends in empyema incidence are unknown.
We assessed whether the previously observed decreases in the incidence of childhood pneumonia hospitalizations continued seven years after PCV7 introduction. We also evaluated national trends in pneumonia hospitalizations complicated by empyema, including trends in empyema associated with specific pathogens.
METHODS
Nationwide Inpatient Sample
The Nationwide Inpatient Sample is the largest source of inpatient data publicly available in the US. These databases contain information on inpatient stays from States that participate in the Healthcare Cost and Utilization Project (HCUP). Information recorded includes clinical and resource-utilization data for 5–8 million hospitalizations per year from a sample of approximately 1000 hospitals. These hospitals constitute an estimated 20% sample of community hospitals, including nonfederal short-term, general, and specialty hospitals. Participating hospitals are sampled by stratified probability sampling, with sampling probabilities proportional to the number of community hospitals in each stratum.[15]
The Nationwide Inpatient Sample collects data on all hospitalizations regardless of payment source, and sampling variables are provided to calculate national estimates. Up to 15 ICD9-CM coded discharge diagnoses and procedures are recorded, with first-listed diagnoses (principal) regarded as the primary reason for hospitalization.[16] Since these data are publicly available and have no personal identifiers, this study was considered exempt from review by the institutional review boards of Vanderbilt University and the CDC.
Outcomes
Pneumonia hospitalizations were identified using algorithms based on coded discharge diagnoses. All-cause pneumonia hospitalizations were defined by a principal diagnosis of pneumonia, or a principal diagnosis of septicemia, meningitis or empyema and a pneumonia code in another diagnosis field. Pneumococcal pneumonia hospitalizations met the all-cause pneumonia definition but also had either a specific pneumococcal pneumonia code or an unspecified pneumonia code plus another code, indicating pneumococcal infection.[8] Diagnosis and procedure codes identified empyema and thoracentesis-related procedures associated with pneumonia hospitalizations. Diagnosis codes for pneumonia or bacteremia/septicemia indicating specific etiologies determined empyema classification into mutually exclusive groups: pneumococcal, streptococcal, staphylococcal and other or unspecified.
Overall rates of acute respiratory tract illness (ARTI) and non-pneumonia ARTI hospitalizations were examined to assess whether changes occurred in coding practices from pneumonia to non-pneumonia ARTI(See appendix for ICD9-CM codes).
Statistical analyses
Pneumonia hospitalization rates
Annual hospitalization rates for all-cause and pneumococcal pneumonia were computed using weighted frequencies as numerators and annual, mid-year census population estimates as denominators to estimate person-time. Annual rates were calculated per 100,000 population and age-stratified by <2 and 2–4 year age groups. To estimate the effect attributable to the PCV7 immunization program, we compared the average annual weighted hospitalization rates for post-PCV7 years(2001–2007) with pre-PCV7 years(1996–1999). Year 2000 was considered a transition year and excluded from these analyses.[17]
Rates and rate ratios (RR) were estimated by fitting outcome-specific Poisson regression models that included parameters for age group, study period, and their interaction, while accounting for the data sampling design. Population estimates for each age group and calendar year represented the offset term for the models. Comparisons of rates before and after PCV7 introduction were obtained through linear combinations of model coefficients.[18, 19]
Empyema hospitalization rates
Annual rates of all-cause pneumonia complicated by empyema and procedures associated with these complicated pneumonias were analyzed separately. Due to an increasing trend in empyema incidence from 1996 through 1999 among children aged <2 years(test for trend p=0.001), all pre-PCV7 years were not combined. Among children aged 2–4 years, a non-significant increasing trend pre-PCV7 was also observed(p=0.157). The slopes for trends in empyema rates pre- and post-PCV7 declined among children aged <2 years(p=0.029) but were not significantly different among children 2–4 years(p=0.695). Therefore, these secular trends were assessed and described over the whole 12 year study period. Per HCUP recommendations, estimates based on <10 un-weighted observations were considered unreliable and were not reported. To obtain reliable pathogen-specific empyema rate estimates, we combined annual data in three-year periods. To perform parallel analyses of total and pathogen-specific empyema and to quantify secular trends, we compared rates in combined years 2005–2007 with those in 1996–1998. All reported p values were two-tailed and accounted for the sampling design. A p value of <0.05 or the exclusion of 1 from the RR 95% confidence intervals indicated statistical significance. Statistical analyses used the survey applications of SAS 9.1.3 and Stata 10.0.
RESULTS
Characteristics of Pneumonia Hospitalizations
During the 12-year study period, there were an estimated 1.5 million all-cause pneumonia hospitalizations among US children aged <5 years. In 2007, a total of 59,980 children aged <2 and 42,356 children aged 2–4 years were hospitalized for all-cause pneumonia. There were more boys (56.0%) than girls; 35.3.8% were white, 17.5.% Hispanic, 14.2% African American, 5.9% other race, and 27.1% were missing race information.
Among children aged <2 years, the overall proportion of all-cause pneumonia hospitalizations complicated by empyema and with a thoracentesis related procedure performed was 0.51% (95% CI: 0.45–0.58) and 0.67% (95% CI: 0.59–0.76), respectively. Similarly, among children aged 2–4 years empyema and thoracentesis procedures were recorded in 1.59% (95% CI: 1.41–1.80) and 1.86% (95% CI: 1.66–2.08) of all-cause pneumonia hospitalizations, respectively. The overall proportion of in-hospital deaths during all-cause pneumonia hospitalizations was 0.20% (95% CI: 0.17–0.23) and 0.17% (95% CI: 0.14–0.20) for children aged <2 and 2–4 years, respectively.
Among children aged <2 years hospitalized due to all-cause pneumonia, the mean number of discharge diagnoses recorded increased from 2.71 (95% CI: 2.62–2.81) in 1996 to 3.29 (95% CI: 3.11–3.48) in 2007(p<0.001), and the mean number of procedures recorded was 0.33 (95% CI: 0.24–0.42) in 1996 and 0.31 (95% CI: 0.25–0.37) in 2007(p=0.656). Among children aged 2–4 years, the mean number of discharge diagnoses recorded increased from 2.96 (95% CI: 2.91–3.02) in 1996 to 3.41 (95% CI: 3.21–3.60) in 2007(p<0.001), and the mean number of procedures was 0.29 (95% CI: 0.25–0.32) in 1996 and 0.30 (95% CI: 0.24–0.36) in 2007(p=0.881).
Approximately 1.65% (95% CI: 1.53–1.79) and 2.61% (95% CI: 2.43–2.79) of all-cause pneumonia hospitalizations met the definition of pneumococcal pneumonia among children aged <2 years and 2–4 years, respectively. In 2007, a total of 834 children aged <2 and 1062 children aged 2–4 years had hospitalizations coded as pneumococcal pneumonia. During the study years, empyema was recorded in 7.92% (95% CI: 6.75–9.27) and 16.87% (95% CI: 14.95–18.99) of pneumococcal pneumonia hospitalizations in children aged <2 and 2–4 years, respectively. For both age groups, approximately 83% of all-cause pneumonia hospitalizations complicated by empyema had a thoracentesis procedure performed throughout the study years.
Pneumonia hospitalization trends
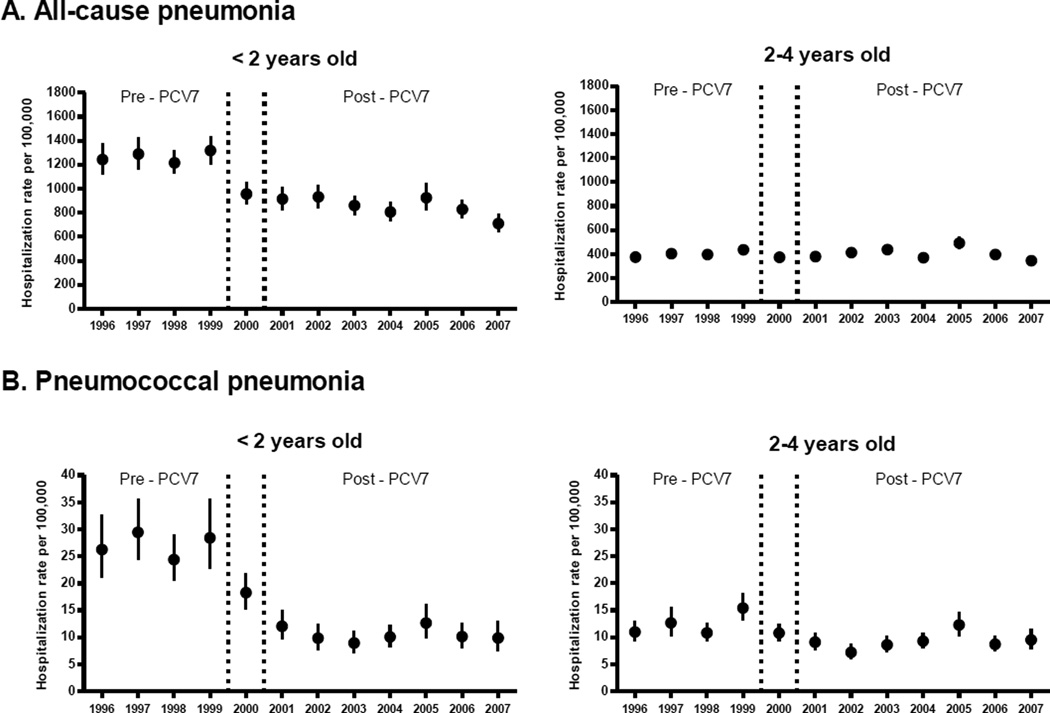
Among children aged <2 years, annual rates of all-cause pneumonia hospitalizations declined by 33%, from 1267 per 100000 children in pre-PCV7 years to 852 during post-PCV7 years. Among children aged 2–4 years, the rates of all-cause pneumonia did not change (Table 1). After the initial decline, pneumonia hospitalizations remained relatively stable after PCV7 introduction with the exception of a transient increase in 2005(Figure 1).
Table 1.
Rates of ARTI, all-cause pneumonia and pneumococcal pneumonia hospitalizations, United States children aged <5 years, 1996–2007
| Rate per 100000. Pre- PCV7 (1996– 1999) |
Rate per 100000. Post-PCV7 (2001–2007) |
Rate difference (95% CI) |
Rate ratio (95% CI) | |
|---|---|---|---|---|
| All ARTI | ||||
| <2 years | 3984 | 3272 | −712 (−935 – −474) | 0.82 (0.77 – 0.88) |
| 2 – 4 years | 969 | 1011 | 43 (−1 – 88) | 1.04 (1.00 – 1.09) |
| Non-pneumonia ARTI | ||||
| <2 years | 2718 | 2419 | −298 (−479 – −104) | 0.89 (0.82 – 0.96) |
| 2 – 4 years | 567 | 607 | 40 (11 – 71) | 1.07 (1.02 – 1.12) |
| Pneumonia (all-cause) | ||||
| <2 years | 1267 | 852 | −414 (−471 – −353) | 0.67 (0.63 – 0.72) |
| 2 – 4 years | 402 | 404 | 2 (−21 – 27) | 1.01 (0.95 – 1.07) |
| Pneumococcal pneumonia | ||||
| <2 years | 27 | 11 | −17 (−18 – −15) | 0.39 (0.33 – 0.45) |
| 2 – 4 years | 12 | 9 | −3 (−4 – −2) | 0.74 (0.66 – 0.84) |
ARTI, acute respiratory tract illness. Numbers in brackets are 95% confidence intervals. All-cause pneumonia includes pneumococcal pneumonia.
Figure 1.
Annual hospitalization rates for all-cause and pneumococcal pneumonia among children aged <5 years, United States, 1996–2007
Footnote: Error bars indicate 95% confidence intervals. Vertical lines indicate the year of PCV7 introduction
Among children aged <2 years, annual rates of pneumococcal pneumonia hospitalizations declined by 61%, from 27 per 100000 children during pre-PCV7 years to 11 during post-PCV7 years. Similarly, rates of pneumococcal pneumonia declined by 26% among children aged 2–4 years(Table 1). Rates of pneumococcal pneumonia hospitalizations remained relatively stable during post-PCV7 years in both age groups(Figure 1).
ARTI hospitalization trends
Among children aged <2 years, overall annual rates of ARTI(including all-cause pneumonia) decreased by 18%, from 3984 per 100000 during pre-PCV7 years to 3272 during post-PCV7 years. In this age group, rates of non-pneumonia ARTI declined by 11% from 2718 per 100000 during pre-PCV7 years to 2419 during post-PCV7 years. Among children aged 2–4 years, overall rates of ARTI remained stable. However, rates of non-pneumonia ARTI increased by 7% from pre to post-PCV7 years(Table 1).
Empyema trends
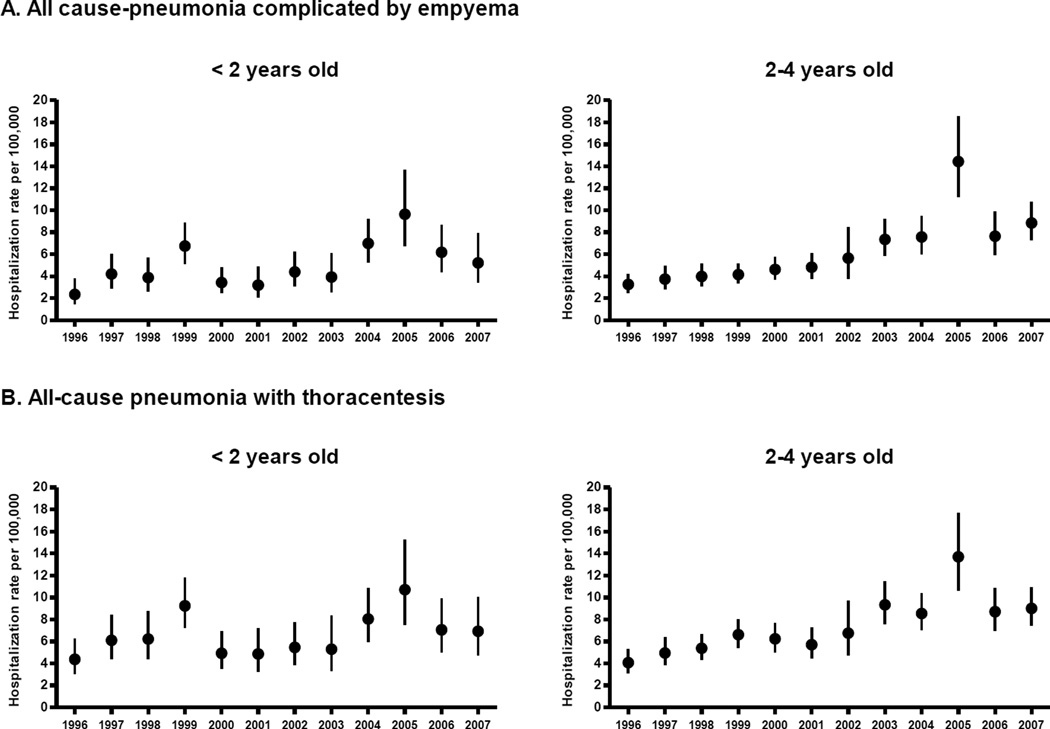
Among children aged <2 years, annual rates of pneumonia complicated by empyema increased 2.01-fold from 3.5 per 100000 in 1996–1998 to 7.0 per 100000 in 2005–2007(441 cases in 2007). Similarly, empyema hospitalization rates increased 2.81–fold among children aged 2–4 years, from 3.7 per 100000 to 10.3 per 100000(1088 cases in 2007). Among children aged <2 years, annual rates of thoracentesis performed during pneumonia hospitalizations increased 1.48-fold. Among children aged 2–4 years, rates of thoracentesis increased 2.18–fold (Table 2 and Figure 2).
Table 2.
Rates of overall and pathogen-specific pneumonia hospitalizations complicated by empyema, United States children aged <5 years, 1996–2007
| Rate per 100000 (1996–1998) |
Rate per 100000 (2005–2007) |
Rate difference (95% CI) |
Rate ratio (95% CI) | |
|---|---|---|---|---|
| Empyema | ||||
| <2 years | 3.5 | 7.0 | 3.5 (1.5 – 6.3) | 2.01 (1.44 – 2.8) |
| 2 – 4 years | 3.7 | 10.3 | 6.6 (4.5 – 9.3) | 2.81 (2.24 – 3.54) |
| Thoracentesis | ||||
| <2 years | 5.6 | 8.2 | 2.6 (0.6 – 5.5) | 1.48 (1.10 – 1.98) |
| 2 – 4 years | 4.8 | 10.5 | 5.7 (3.6 – 8.2) | 2.18 (1.76 – 2.7) |
| Pneumococcal empyema | ||||
| <2 years | 1.1 | 1.3 | 0.1 (−0.4 – 1.0) | 1.13 (0.68 – 1.88) |
| 2 – 4 years | 1.1 | 2.5 | 1.3 (0.6 – 2.3) | 2.17 (1.55 – 3.03) |
| Streptococcal empyema | ||||
| <2 years | 0.4 | 0.7 | 0.3 (0 – 0.8) | 1.74 (0.98 – 3.07) |
| 2 – 4 years | 0.4 | 1.0 | 0.7 (0.3 – 1.3) | 2.8 (1.72 – 4.57) |
| Staphylococcal empyema | ||||
| <2 years | 0.6 | 2.5 | 1.9 (0.9 – 3.5) | 4.08 (2.51 – 6.63) |
| 2 – 4 years | 0.2 | 0.8 | 0.6 (0.2 – 1.4) | 3.76 (1.98 – 7.15) |
| Other or unspecified empyema | ||||
| <2 years | 1.3 | 2.5 | 1.2 (0.3 – 2.5) | 1.89 (1.23 – 2.9) |
| 2 – 4 years | 1.9 | 6.0 | 4.0 (2.6 – 6.0) | 3.09 (2.33 – 4.1) |
Numbers in brackets are 95% confidence intervals.
Figure 2.
Annual hospitalization rates for all-cause pneumonia complicated by empyema and thoracentesis-related procedures among children aged <5 years, United States, 1996–2007
Footnote: Error bars indicate 95% confidence intervals.
Pathogen-specific empyema trends
Among children aged <2 years, annual rates of pneumococcal empyema per 100000 children were 1.1 in 1996–1998 and 1.3 in 2005–2007 (RR: 1.13, 95% CI: 0.68–1.88). Among children aged 2–4 years, pneumococcal empyema increased from 1.1 per 100000 in 1996–1998 to 2.5 per 100000 in 2005–2007, a 2.17-fold increase.
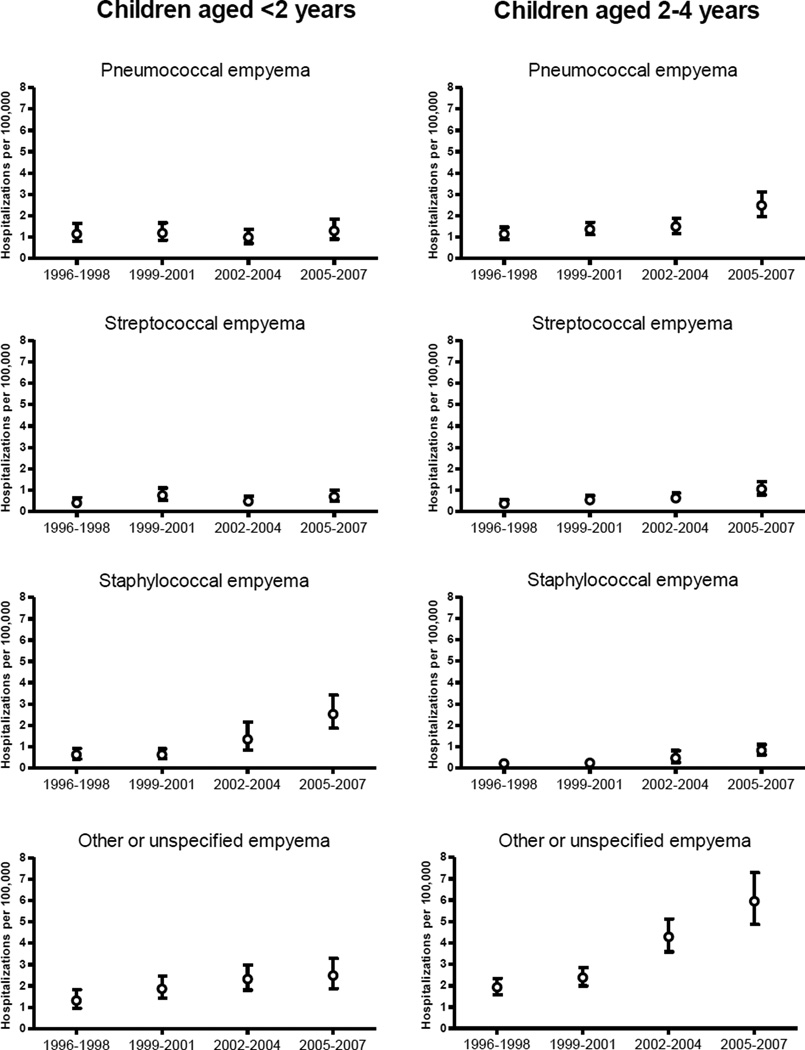
Among children aged <2 years, rates of streptococcal empyema were 0.4 per 100000 in 1996–1998 and 0.7 per 100000 in 2005–2007 (RR: 1.74, 95% CI: 0.98–3.07). Staphylococcal empyema increased 4.08-fold from 0.6 per 100000 in 1996–1998 to 2.5 per 100000 in 2005–2007. Empyema rates due to other or unspecified pathogens increased 1.89-fold during the study years. Among children aged 2–4 years, streptococcal empyema rates increased 2.80-fold, whereas staphylococcal empyema rates increased 3.76-fold. Empyema due to other or unspecified pathogens increased 3.09-fold(Table 2 and Figure 3).
Figure 3.
Rates of pneumonia hospitalizations complicated by empyema by associated pathogens, United States children aged <5 years (1996–2007)
Footnote: Error bars indicate 95% confidence intervals.
During the study period, there were some years with strong clustering of empyema cases in relatively few hospitals. For instance, in 2005, 20% of hospitals reporting empyema in children <2 years accounted for half of the reported cases. For the same year, 10% of hospitals reporting empyema in children aged 2–4 years accounted for half of the reported cases. This clustering of empyema resulted in wide confidence intervals around some estimates(Figure 2).
DISCUSSION
The declines observed in all-cause and pneumococcal pneumonia hospitalizations following introduction of PCV7 in the US were sustained through 2007. Despite the major impact of PCV7 on pneumonia, rates of pneumonia complicated by empyema increased gradually throughout the study period. Our findings indicate that these increases began before PCV7 introduction and that several pathogens contributed to these changes. Among young children aged <2 years, empyema caused by Staphylococcus aureus accounted for most of the increase, whereas among children aged 2–4 years, most of the increase in empyema was recorded as due to unspecified pathogens.
By the end of 2007, the estimated coverage with three and four doses of PCV7 among US children aged 19–35 months was 90% and 75.3%, respectively.[20] Although IPD caused by vaccine serotypes has been virtually eliminated in young children, IPD caused by non-vaccine serotypes increased. However, IPD incidence due to these non-vaccine serotypes remains small.[11] After the initial declines, the overall incidence of IPD has remained relatively constant and well below historical values through the end of 2007.[21] Consistent with these changes, declines in pneumonia hospitalizations were sustained and pneumonia hospitalization rates remained relatively stable after the initial declines.[8]
Our findings indicate that the observed declines in pneumonia were not the result of changes in coding practices from pneumonia to other non-pneumonia diagnoses.[22] Changes in admission practices do not appear to account for the reduction in pneumonia hospitalizations since no compensatory increases in rates of pneumonia ambulatory visits have been observed after PCV7 introduction.[23] On the contrary, ambulatory visits for pneumonia have also declined, further supporting the beneficial effects of the immunization program.[9]
We also considered whether the increase in empyema may have been due at least in part to changes in coding practices. We observed an overall trend of increasing number of diagnoses recorded for all hospitalizations (data not shown). Both the overall increase in the number of recorded diagnoses and thoracentesis procedures suggests that some of the observed increase in empyema may be associated with enhanced coding and improved diagnosis. However, the magnitude of the increase was consistent with other local and regional reports of an increase in this complication. An increasing number of empyemas was reported in Utah before PCV7 introduction, from 1993 through 1999[13] and similar increases were documented in Texas.[24] Moreover, a multistate surveillance initiative documented an increase in complicated pneumococcal pneumonia (mainly empyema), from 1996 through 1999.[12] Similar increases have also been observed in other countries.[25–29]
Nationally, empyema was a relatively rare occurrence, complicating less than 1% of all-cause pneumonia hospitalizations among children aged <5 years. In 2007, an estimated 1500 US children aged <5 years were hospitalized for pneumonia complicated by empyema. Nevertheless, our analysis of twelve years of national data, allowed the assessment of trends in this rare outcome. The increasing incidence of pneumonia complicated by empyema during the study years is intriguing. Previous reports suggest that pneumococcal serotype 1 (not covered by PCV7) was a leading cause of this complication, even before PCV7 introduction.[12, 13, 24] Although no serotype information was available in our study database, a recent surveillance report suggested that serotype 1 accounted for <1% of invasive pneumococcal diseases among US children aged <5 years in 2007.[30] Peaks of empyema hospitalizations were observed during some years (e.g. 1999, 2005), possibly associated with epidemic patterns of disease caused by certain serotypes. Although overall rates of empyema increased over time, pneumococcal empyema rates remained stable in children aged <2 years but increased among children aged 2–4 years. Rates of staphylococcal empyema increased substantially in children aged <5 years. These findings are consistent with results of a study performed in Texas (1993–2002) that documented a gradual increase in staphylococcal empyema, especially among young children.[24]
Empyema due to other or unspecified pathogens also increased among children aged <5 years. Defining the etiology of empyema is difficult[12, 13] and whether these increases in empyema due to other or unspecified pathogens indicates true emerging trends, reduced or delayed laboratory testing or increased use of antibiotics prior to hospitalization[31], is unclear. Interestingly, several studies that used molecular diagnostic techniques found that a sizable proportion of culture-negative empyema in children was caused by pneumococci, mainly serotype 1.[26, 32–35] Even though those studies suggest that pneumococcus remains the leading cause of empyema in children, our data also suggest that the etiology of empyema is changing and monitoring empyema trends is warranted.
The observed reductions in pneumonia hospitalization rates preceded the formal recommendations for influenza immunization of young children that started in 2004.[36] The national estimated coverage of influenza vaccine among children aged 6–23 months (fully vaccinated) was 17.8%, 20.6% and 21.3% for the 2004–05, 2005–06 and 2006–07 influenza seasons, respectively.[37] We considered that this low influenza vaccine coverage was an unlikely explanation for the substantial pneumonia declines observed. Of interest, the national incidence of empyema associated with Staphylococcus aureus infections was highest during 2005–2007 coinciding temporally with increases in mortality due to co-infection of influenza and Staphylococcus aureus among young children.[38] Continuous surveillance will be necessary to explore the effects of increasing coverage with influenza vaccine on pneumonia and its complications.
Several caveats in the interpretation of our results must be considered. First, in this ecologic study we assessed the population effects of the PCV7 immunization program regardless of the individual’s immunization status, thus reflecting direct and indirect vaccine effects as well as effects unrelated to vaccination. Second, since our main analyses focused on children aged <2 years, our estimates included vaccine effects on young infants who were not yet eligible for immunization but likely benefited from reduced exposure to the pneumococcus.[39] Third, our databases do not contain information on pneumococcal serotypes or patterns of antibiotic resistance. Since serotyping is not part of routine diagnostic work-up and this information is not recorded in medical charts, our estimates represent changes in disease caused by both vaccine and non-vaccine serotypes. Similarly, the available information did not allow the distinction between empyema due to methicillin-resistant and non methicillin-resistant Staphylococcus aureus. Finally, the accuracy of our approach to identify pneumonia and empyema cannot be determined with the information available and changes in coding and recording practices could affect our estimates (i.e. artificially increasing the number of empyema during recent years). Nevertheless, approximately 83% of empyema hospitalizations had thoracentesis procedures recorded, suggesting high specificity of the discharge diagnosis codes. Nationwide Inpatient Sample data are de-identified before public release so chart reviews cannot be performed to confirm diagnoses. The identification of pneumonia using any listed discharge diagnosis in administrative databases can be suboptimal in identifying pneumonia as the main reason for hospitalization.[10] That approach would include nosocomial pneumonia that developed during a hospital stay. In this study, we attempted to exclude nosocomial pneumonia or empyema by focusing our outcome definitions on the principal discharge diagnosis (first listed). Discharge diagnoses are recorded at the end of the hospitalization episode and principal discharge diagnoses are considered to represent the main reason for hospitalization in the Nationwide Inpatient Sample data.[16]
For the analyses of pneumonia hospitalizations, our study averaged the annual incidence rates observed before and after PCV7 introduction. This approach ignored secular trends and provided conservative estimates compared with those obtained modeling monthly disease rates.[8] However, hospitalization monthly data were not consistently available for all study years and empyema was a rare outcome, necessitating pooling data in three-year time periods.
Our findings indicate that the initial declines observed in rates of all-cause and pneumococcal pneumonia hospitalizations were sustained. Routine immunization with PCV7 has substantially reduced the burden of childhood pneumonia hospitalizations in the US. The increasing trend in empyema preceded PCV7 introduction suggesting that a direct association with the PCV7 immunization program is unlikely. Although empyema was associated with only a small fraction of pneumonias, the observed increase is of concern and warrants monitoring.
ACKNOWLEDGMENTS
We gratefully acknowledge CDC investigator: Drew Baughman, M.S, for critical, non-compensated review of the manuscript
Potential conflicts of interest. Dr. Grijalva received lecture fees from Wyeth. Dr. Griffin received grant support from MedImmune and Pfizer. Drs. Grijalva and Griffin receive research support from Wyeth.
Financial support. This study was funded by the Centers for Disease Control and Prevention through a Cooperative Agreement with the Association for Prevention Teaching and Research (TS-1454). Dr. Grijalva is supported by a CDC career development award (K01 CI000163).
APPENDIX. ICD9-CM codes
Pneumonia: 481†, 480*, 482*, 483*, 484*, 485*, 486*, 487·0
Meningitis: 3201, 00321, 0360, 0361, 047, 0470, 0471, 0491, 0530, 05472, 0721, 09181, 0942, 09882, 10081, 11283, 1142, 11501, 11511, 11591, 1300, 3200, 3202, 3203, 3207, 32081, 32082, 32089, 321*, 0478, 0479, 320, 3208, 3209, 322, 3220, 3229
Septicemia or bacteremia: 0382, 0031, 0202, 0223, 0312, 0362, 0380, 0381*, 0383, 0384*, 0545, 038, 0388, 0389, 78552, 7907, 99591, 99592
Pneumococcal infection not otherwise specified: 041·2
Empyema: 510*
Thoracentesis‡: 34.04, 34.09, 34.91, 34.51, 34.21
ARTI: 381*, 382*, 383*, 460*, 461*, 462*, 463*, 464*, 465*, 466*, 480*, 481*, 482*, 483*, 484*, 485*, 486*, 487*, 490*, 493*
*All ICD9-CM codes starting with this number. †Pneumococcal pneumonia. ‡Thoracentesis-related procedure codes.
Footnotes
No other potential conflict of interest relevant to this article was reported.
Disclaimer. The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Black S, Shinefield H, Fireman B, Lewis E, Ray P, Hansen JR. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatric Infectious Disease Journal. 2000;19:187–195. doi: 10.1097/00006454-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Klugman KP, Madhi SA, Huebner RE, Kohberger R, Mbelle N, Pierce N. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med. 2003;349(14):1341–1348. doi: 10.1056/NEJMoa035060. [DOI] [PubMed] [Google Scholar]
- 3.Cutts FT, Zaman SM, Enwere G, et al. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomised, double-blind, placebo-controlled trial. Lancet. 2005;365(9465):1139–1146. doi: 10.1016/S0140-6736(05)71876-6. [DOI] [PubMed] [Google Scholar]
- 4.Lucero MG, Nohynek H, Williams G, et al. Efficacy of an 11-valent pneumococcal conjugate vaccine against radiologically confirmed pneumonia among children less than 2 years of age in the Philippines: a randomized, double-blind, placebo-controlled trial. Pediatr Infect Dis J. 2009 Jun;28(6):455–462. doi: 10.1097/INF.0b013e31819637af. [DOI] [PubMed] [Google Scholar]
- 5.Mulholland K. Childhood pneumonia mortality--a permanent global emergency. Lancet. 2007 Jul 21;370(9583):285–289. doi: 10.1016/S0140-6736(07)61130-1. [DOI] [PubMed] [Google Scholar]
- 6.McIntosh K. Community-acquired pneumonia in children. N Engl J Med. 2002;346(6):429–437. doi: 10.1056/NEJMra011994. [DOI] [PubMed] [Google Scholar]
- 7.Wardlaw T, Salama P, Johansson EW, Mason E. Pneumonia: the leading killer of children. Lancet. 2006 Sep 23;368(9541):1048–1050. doi: 10.1016/S0140-6736(06)69334-3. [DOI] [PubMed] [Google Scholar]
- 8.Grijalva CG, Nuorti JP, Arbogast PG, Martin SW, Edwards KM, Griffin MR. Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the USA: a time-series analysis. Lancet. 2007 Apr 7;369(9568):1179–1186. doi: 10.1016/S0140-6736(07)60564-9. [DOI] [PubMed] [Google Scholar]
- 9.Zhou F, Kyaw MH, Shefer A, Winston CA, Nuorti JP. Health care utilization for pneumonia in young children after routine pneumococcal conjugate vaccine use in the United States. Arch Pediatr Adolesc Med. 2007 Dec;161(12):1162–1168. doi: 10.1001/archpedi.161.12.1162. [DOI] [PubMed] [Google Scholar]
- 10.Nelson JC, Jackson M, Yu O, et al. Impact of the introduction of pneumococcal conjugate vaccine on rates of community acquired pneumonia in children and adults. Vaccine. 2008 Jul 25; doi: 10.1016/j.vaccine.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 11.Hicks LA, Harrison LH, Flannery B, et al. Incidence of Pneumococcal Disease Due to Non-Pneumococcal Conjugate Vaccine (PCV7) Serotypes in the United States during the Era of Widespread PCV7 Vaccination, 1998–2004. J Infect Dis. 2007 Nov 1;196(9):1346–1354. doi: 10.1086/521626. [DOI] [PubMed] [Google Scholar]
- 12.Tan TQ, Mason EO, Jr, Wald ER, et al. Clinical characteristics of children with complicated pneumonia caused by Streptococcus pneumoniae. Pediatrics. 2002 Jul;110(1 Pt 1):1–6. doi: 10.1542/peds.110.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Byington CL, Spencer LY, Johnson TA, et al. An epidemiological investigation of a sustained high rate of pediatric parapneumonic empyema: risk factors and microbiological associations. Clin Infect Dis. 2002 Feb 15;34(4):434–440. doi: 10.1086/338460. [DOI] [PubMed] [Google Scholar]
- 14.Byington CL, Korgenski K, Daly J, Ampofo K, Pavia A, Mason EO. Impact of the pneumococcal conjugate vaccine on pneumococcal parapneumonic empyema. Pediatr Infect Dis J. 2006 Mar;25(3):250–254. doi: 10.1097/01.inf.0000202137.37642.ab. [DOI] [PubMed] [Google Scholar]
- 15.Agency for Healthcare Research and Quality. The Healthcare cost and utilization project. Overview of the Nationwide Inpatient sample [Internet site] [Accessed August 10th. 2008]; Available from http://www.hcup-us.ahrq.gov/nisoverview.jsp.
- 16.Agency for Healthcare Research and Quality. The Healthcare cost and utilization project. Hospital Inpatient Statistics, 1996 [Internet site] [Accessed August 10th. 2008]; Available from http://www.hcup-us.ahrq.gov/reports/natstats/his96/clinclas.htm.
- 17.Nuorti JP, Martin SW, Smith PJ, Moran JS, Schwartz B. Uptake of pneumococcal conjugate vaccine among children in the 1998–2002 United States birth cohorts. Am J Prev Med. 2008 Jan;34(1):46–53. doi: 10.1016/j.amepre.2007.09.028. [DOI] [PubMed] [Google Scholar]
- 18.Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004;292(11):1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 19.Tsai CJ, Griffin MR, Nuorti JP, Grijalva CG. Changing epidemiology of pneumococcal meningitis after the introduction of pneumococcal conjugate vaccine in the United States. Clin Infect Dis. 2008 Jun 1;46(11):1664–1672. doi: 10.1086/587897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. The National Center for Health Statistics. The National Immunization Survey [Internet site] [Accessed October 1st 2009];2009 Available from http://www.cdc.gov/nis/.
- 21.Centers for Disease Control and Prevention. Active Bacterial Core Surveillance (ABCs). Surveillance reports [internet site] [Accessed October 1st. 2009]; Available from http://www.cdc.gov/ncidod/dbmd/abcs/survreports.htm.
- 22.Nuorti JP, Grijalva CG, Walter ND, Griffin MR. Monitoring national trends in pneumonia hospitalizations in children <5 years, United States, 1997–2005. International Symposium on Pneumococci and Pneumococcal Diseases; Reykjavik; Iceland. 2008. [Google Scholar]
- 23.Grijalva CG, Poehling KA, Nuorti JP, et al. National impact of universal childhood immunization with pneumococcal conjugate vaccine on outpatient medical care visits in the United States. Pediatrics. 2006 Sep;118(3):865–873. doi: 10.1542/peds.2006-0492. [DOI] [PubMed] [Google Scholar]
- 24.Schultz KD, Fan LL, Pinsky J, et al. The changing face of pleural empyemas in children: epidemiology and management. Pediatrics. 2004 Jun;113(6):1735–1740. doi: 10.1542/peds.113.6.1735. [DOI] [PubMed] [Google Scholar]
- 25.Roxburgh CS, Youngson GG, Townend JA, Turner SW. Trends in pneumonia and empyema in Scottish children in the past 25 years. Arch Dis Child. 2008 Apr;93(4):316–318. doi: 10.1136/adc.2007.126540. [DOI] [PubMed] [Google Scholar]
- 26.Obando I, Munoz-Almagro C, Arroyo LA, et al. Pediatric parapneumonic empyema, Spain. Emerg Infect Dis. 2008 Sep;14(9):1390–1397. doi: 10.3201/eid1409.071094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eastham KM, Freeman R, Kearns AM, et al. Clinical features, aetiology and outcome of empyema in children in the north east of England. Thorax. 2004 Jun;59(6):522–525. doi: 10.1136/thx.2003.016105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsieh YC, Hsueh PR, Lu CY, Lee PI, Lee CY, Huang LM. Clinical manifestations and molecular epidemiology of necrotizing pneumonia and empyema caused by Streptococcus pneumoniae in children in Taiwan. Clin Infect Dis. 2004 Mar 15;38(6):830–835. doi: 10.1086/381974. [DOI] [PubMed] [Google Scholar]
- 29.Goldbart AD, Leibovitz E, Porat N, et al. Complicated community acquired pneumonia in children prior to the introduction of the pneumococcal conjugated vaccine. Scand J Infect Dis. 2009;41(3):182–187. doi: 10.1080/00365540802688378. [DOI] [PubMed] [Google Scholar]
- 30.Nuorti JP, Rosen J, Thomas A, et al. Invasive pneumococcal disease caused by serotypes in the new 13-valent pneumococcal conjugate vaccine - implications for immunization strategies. Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); San Francisco, CA. 2009. [Google Scholar]
- 31.Hendrickson DJ, Blumberg DA, Joad JP, Jhawar S, McDonald RJ. Five-fold increase in pediatric parapneumonic empyema since introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2008 Nov;27(11):1030–1032. doi: 10.1097/INF.0b013e31817e5188. [DOI] [PubMed] [Google Scholar]
- 32.Eltringham G, Kearns A, Freeman R, et al. Culture-negative childhood empyema is usually due to penicillin-sensitive Streptococcus pneumoniae capsular serotype 1. J Clin Microbiol. 2003 Jan;41(1):521–522. doi: 10.1128/JCM.41.1.521-522.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tarrago D, Fenoll A, Sanchez-Tatay D, et al. Identification of pneumococcal serotypes from culture-negative clinical specimens by novel real-time PCR. Clin Microbiol Infect. 2008 Sep;14(9):828–834. doi: 10.1111/j.1469-0691.2008.02028.x. [DOI] [PubMed] [Google Scholar]
- 34.Ploton C, Freydiere AM, Benito Y, et al. Streptococcus pneumoniae thoracic empyema in children: rapid diagnosis by using the Binax NOW immunochromatographic membrane test in pleural fluids. Pathol Biol (Paris) 2006 Oct-Nov;54(8–9):498–501. doi: 10.1016/j.patbio.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 35.Le Monnier A, Carbonnelle E, Zahar JR, et al. Microbiological diagnosis of empyema in children: comparative evaluations by culture, polymerase chain reaction, and pneumococcal antigen detection in pleural fluids. Clin Infect Dis. 2006 Apr 15;42(8):1135–1140. doi: 10.1086/502680. [DOI] [PubMed] [Google Scholar]
- 36.Harper SA, Fukuda K, Uyeki TM, Cox NJ, Bridges CB. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2004;53(RR-6):1–40. [PubMed] [Google Scholar]
- 37.Influenza vaccination coverage among children aged 6–23 months--United States, 2006–07 influenza season. MMWR Morb Mortal Wkly Rep. 2008 Sep 26;57(38):1039–1043. [PubMed] [Google Scholar]
- 38.Finelli L, Fiore A, Dhara R, et al. Influenza-associated pediatric mortality in the United States: increase of Staphylococcus aureus coinfection. Pediatrics. 2008 Oct;122(4):805–811. doi: 10.1542/peds.2008-1336. [DOI] [PubMed] [Google Scholar]
- 39.Poehling KA, Talbot TR, Griffin MR, et al. Invasive pneumococcal disease among infants before and after introduction of pneumococcal conjugate vaccine. Jama. 2006 Apr 12;295(14):1668–1674. doi: 10.1001/jama.295.14.1668. [DOI] [PubMed] [Google Scholar]