Abstract
Objectives
Our goal is to review design strategies for the fabrication of calcium phosphate ceramic scaffolds (CPS), in light of their transient role in bone tissue engineering and associated requirements for effective bone regeneration.
Methods
We examine the various design options available to meet mechanical and biological requirements of CPS and later focus on the importance of proper characterization of CPS in terms of architecture, mechanical properties and time-sensitive properties such as biodegradability. Finally, relationships between in vitro vs. in vivo testing are addressed, with an attempt to highlight reliable performance predictors.
Results
A combinatory design strategy should be used with CPS taking into consideration 3D architecture, adequate surface chemistry and topography, all of which are needed to promote bone formation. CPS represent the media of choice for delivery of osteogenic factors and anti-infectives. Non-osteoblast mediated mineral deposition can confound in vitro osteogenesis testing of CPS and therefore the expression of a variety of proteins or genes including collagen type I, bone sialoprotein and osteocalcin should be confirmed in addition to increased mineral content.
Conclusions
CPS are a superior scaffold material for bone regeneration because they actively promote osteogenesis. Biodegradability of CPS via calcium and phosphate release represents a unique asset. Structural control of CPS at the macro, micro and nanoscale and their combination with cells and polymeric materials is likely to lead to significant developments in bone tissue engineering.
Keywords: Calcium phosphate ceramic, scaffold, hydroxyapatite, bioactive glass, bone tissue engineering
Introduction
Autologous bone grafts remain the gold standard in bone replacement procedures with the highest success rates for bone regeneration1. It is well established, however, that harvest of bone tissue is associated with several clinical drawbacks, including limited availability of healthy bone, secondary surgery cost and burden, harvest site morbidity and long-term pain issues2. There is therefore a critical need for synthetic bone graft materials capable of promoting successful bone regeneration. Indeed the past two decades have been associated with sustained interdisciplinary efforts to design and develop synthetic scaffolds encompassing a wide range of materials from ceramics3 to polymers, including composite scaffolds, cell-bearing, protein-loaded or growth factor-carrying scaffolds mixing both inorganic and organic phases4–7.
Amongst available scaffold materials, calcium phosphate-based ceramics represent a unique avenue based on tunable similarities in both crystalline structure and chemistry between calcium phosphate ceramics and bone apatite, the mineral phase of bone tissue that is similar, albeit distinct, from hydroxyapatite (HA) due to its carbonate content and reduced or absent hydroxyl groups.8 A literature search associating the terms “calcium” and “phosphate” and “scaffolds” returned a total of more than 7,000 articles. This interest appears to have gathered momentum in the past 15 years, although HA, and more generally calcium phosphate-based ceramics have long been the focus of extensive research9–13. Calcium phosphate ceramics have been shown to enhance bone formation depending on crystallinity, crystalline phase and Ca/P ratio, which results in calcium and phosphate ion release needed for bone mineralization14, 15. This characteristic uniquely differentiates them from other metal oxide ceramics used in orthopedics, such as alumina or zirconia that are considered chemically inert. The importance of a scaffold-type architecture stems from the fact that interconnected porosity is a condition for osteoconductivity and promotes angiogenesis. Furthermore, there is ample literature showing that calcium phosphate bioceramic scaffolds promote both osteogenesis and osseointegration, which are directly related to surface charge, chemistry and topography. However, it should be noted that the target application for calcium phosphate scaffolds (CPS) is transient bone replacement. Therefore, the degree of mimicry with regard to bone does not extend beyond chemistry, surface topography and architecture. Bone becomes stiffer and stronger as it matures while CPS should biodegrade and become weaker, with the end point of being completely replaced by newly formed bone.
CPS are manufactured using a palette of techniques from polymer foam replication to ceramic foaming, inclusion of porogens, 3D printing and gel casting. This variety of manufacturing techniques illustrates the difficulty of producing ceramic scaffolds with controlled pore size, porosity and mechanical integrity. Regardless of manufacturing technique, the last step is a thermal treatment or sintering step. This high temperature step has traditionally triggered design issues due to the competition between the high temperatures required for sintering and crystalline phase thermal decomposition. Additionally, for bioactive glass-ceramics, competition between sintering and crystallization processes renders sintering to full density difficult to achieve.
Our goal is to review design strategies for the fabrication of CPS, in light of their transient role in bone tissue engineering and associated requirements for effective bone regeneration. We later focus on the importance of proper characterization of CPS in terms of architecture, mechanical properties and time-sensitive properties such as biodegradability. Finally, relationships between in vitro vs. in vivo testing are addressed, with an attempt to highlight reliable performance predictors.
CPS Design - Requirements for CPS as bone graft substitutes
As mentioned earlier, an ideal scaffold material for synthetic bone grafts should be osteoinductive, osteoconductive, promote osseointegration, be able to deliver osteogenic agents, anti-infectives and stem cells, and degrade at the same rate as new bone forms16. Calcium phosphate ceramic scaffolds are therefore excellent candidates, offering a large palette of design options as detailed below.
Osteoinduction and biodegradation
Osteoinduction can be defined as the chemical stimulation of human mesenchymal stem cells into bone-forming osteoblasts, thereby inducing osteogenesis17. Osteoinduction is best demonstrated by the ability of a material to form bone in an ectopic site18. Calcium phosphate ceramics have been shown to be osteoinductive19. It is postulated that osteoinductivity of CPS stems from the combination of micro and macroporosity capable of entrapping and concentrating growth factors that are directly involved in mesenchymal stem cell differentiation into an osteoblastic lineage20. The surface and bulk chemistry of the crystalline phases involved is also likely to play a role. The most frequently used phases for CPS are hydroxyapatite (HA), beta tri-calcium phosphate (β-TCP) and combinations of HA and β-TCP. It is well established that the dissolution rate of calcium phosphate phases is directly related to their Ca/P ratio, TCP with a ratio of 1.5, is more soluble than HA, with a ratio of 1.67. The solubility of the final product can be tuned by using biphasic calcium phosphate (BCP), a combination of HA and α or β-TCP. The solubility of calcium phosphate phases is also affected by ionic substitutions. The HA crystalline structure includes several highly exchangeable sites, with both anionic and cationic substitutions possible. The most studied dopants for hydroxyapatite are Mg2+, Si4+ and Sr2+. For a detailed list of ionic substitutions and the mechanism of action of metallic trace elements, the reader is referred to the excellent review by Bose et al.21. Briefly, Mg2+ promotes angiogenesis22, 23, Sr2+ enhances osteogenesis24–27 and Si4+ induces angiogenesis28 and has been shown to play a key role in mineralization processes29. Silicon substitution for phosphorous is also possible with tricalcium phosphate and leads to the stabilization of the high temperature alpha polymorph of TCP, which is more soluble at physiological pH, due to a less compact crystallographic structure30, 31. Due to the smaller radius of the silicon ion, silicon for phosphorous substitution in the HA unit cell leads to a distortion of PO4 tetrahedra. These defects in the crystalline structure are responsible for an increase in solubility. Gibson et al. also showed that silicon doping prevented grain growth in HA ceramics and led to an increase in the temperature needed to achieve adequate sintering32. Charge imbalance created by the replacement of PO43− with SiO44− leads to a more electronegative surface, which has been linked to increased surface adhesion29. Indeed silicon-doped calcium phosphates have generated tremendous interest due to the clinical confirmation of their important role in the formation of bone and cartilage systems. The degree of Si doping is limited to a maximum of about 2 wt.% for HA, in order to prevent the formation of other calcium silicate phases32. This small amount has limited effect on the lattice parameters of Si-HA, which are very similar to those of stoichiometric HA (JCPDS 09-432).
Strontium or magnesium-substituted hydroxyapatites also exhibit greater solubility than undoped HA, with a larger unit cell for Sr-HA and a smaller unit cell for Mg-HA33, due to respective ionic radii. The optimal amount of magnesium that can be incorporated in the HA structure is about 7–8%34, while larger amounts of strontium can be incorporated, up to a full substitution of strontium for calcium35–38. Combinations of dopants have the potential to synergistically enhance the osteoinductive properties of calcium phosphate ceramics. The highly exchangeable nature of both HA and TCP crystalline structures therefore represents a powerful design tool in bone tissue engineering39. Lattice parameters of various doped hydroxyapatites are listed in Table 1.
Table 1.
Space group and lattice parameters of pure and doped hydroxyapatites.
Osteoconduction given by scaffold architecture
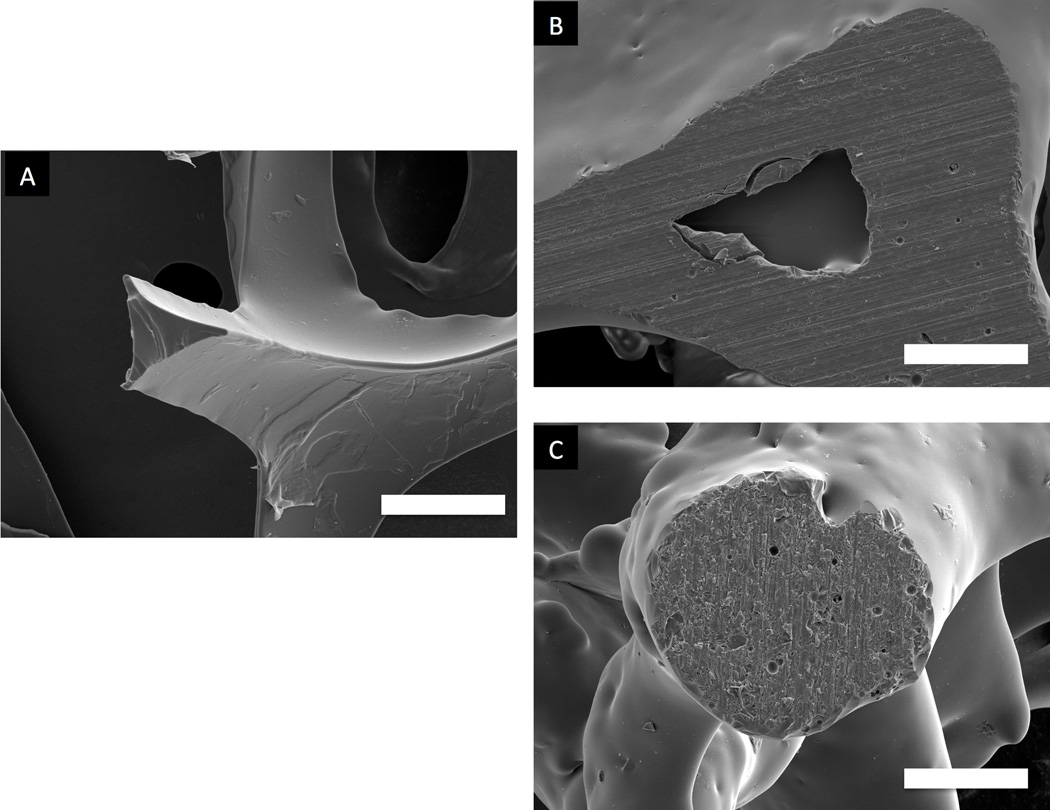
Osteoconductivity is another highly desirable property for a synthetic bone graft substitute and means that new “bone can grow onto a surface”17 or volume. To this effect, an ideal scaffold should incorporate macropores of 150 to 500 micrometers in diameter and exhibit 60–80% interconnected porosity. This description corresponds to that of open cell or cellular ceramics40. The difference between cellular and porous ceramics lies in the interconnectivity of the porous network. In addition to porous bone graft substitutes, cellular ceramics have numerous technological applications such as filters for molten metals, refractory linings, thermal barriers and heat exchangers40. Consequently, a wide palette of processing techniques is available for the production of open cell ceramics. One of the most widely used of these techniques is the replication technique41, in which a polymer foam is coated with a well dispersed ceramic slurry. The coated foam is then slowly dried and burned out, leading to an open cell ceramic construct. The choice of polymer is critical as it dictates reproducibility through its modulus of elasticity, it must also burnout with minimal residue. Key processing steps are the slurry optimization, the drying process after slurry impregnation and the burnout/sintering heat treatment. The burnout of the polymer foam occurs in the 300–400°C temperature range, slow heating rates are therefore recommended in order to prevent the collapse of the green ceramic scaffolds. HA42–44 as well as bioglasses45 and combinations of HA and bioglass46 scaffolds have been successfully processed using this technique. The polymer foam replication technique is frequently used due to its simplicity; however, major drawbacks are inherent fabrication defects with sharp apices at the center of the hollow struts due to polymer burnout. Figure 1 shows an uncoated polymer strut (1A) and a typical defect at the center of a sintered scaffold strut from polymer burnout (1B). This issue can be addressed either by performing a second coating with a lighter ceramic slurry, the resulting characteristic strut is shown in Figure 1C, showing a fully dense strut cross section.
Figure 1.
SEM of a polymeric strut cross section (A); the resulting defect in a sintered scaffold (B) and successful defect elimination after a second coating followed by sintering (C). Bar=100 µm.
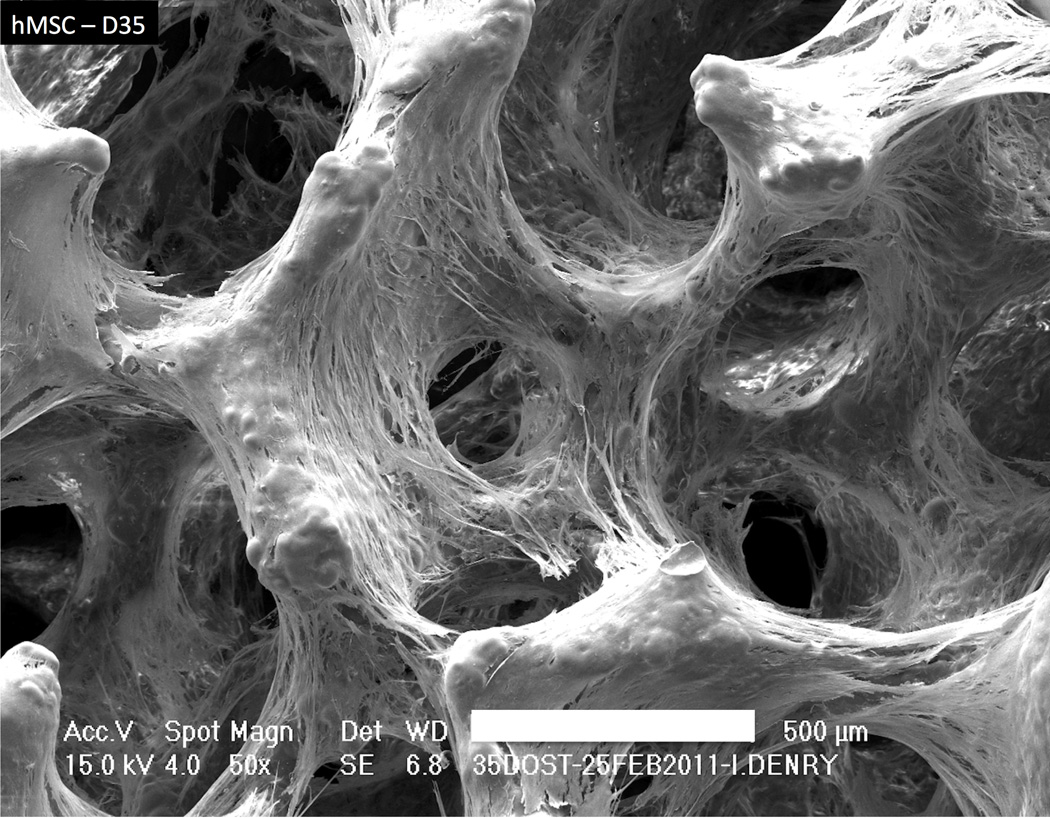
Other green fabrication techniques available for producing open cell ceramics include addition of porogens, foaming and gel casting and rapid prototyping, with a large number of variants40, 47–54. HA inks have been successfully developed for production of 3D scaffolds by direct ink writing55. A common final step for all ceramic scaffold production techniques is the firing or sintering step, typically associated with shrinkage, potential crystallization and phase transformation. In this regard, bioactive glasses offer tremendous versatility as their chemical composition can be tailored to widen the sintering window, allowing full sintering to occur prior to the onset of crystallization56, 57. Figure 2 shows how mesenchymal stem cells successfully colonized a bioglass scaffold after 5 weeks of culture.
Figure 2.
Scanning electron micrograph (SEM) showing human mesenchymal stem cell (hMSC) colonisation of a fluorapatite glass-ceramic scaffold at 35 days. Bar=500 µm.
Osseointegration
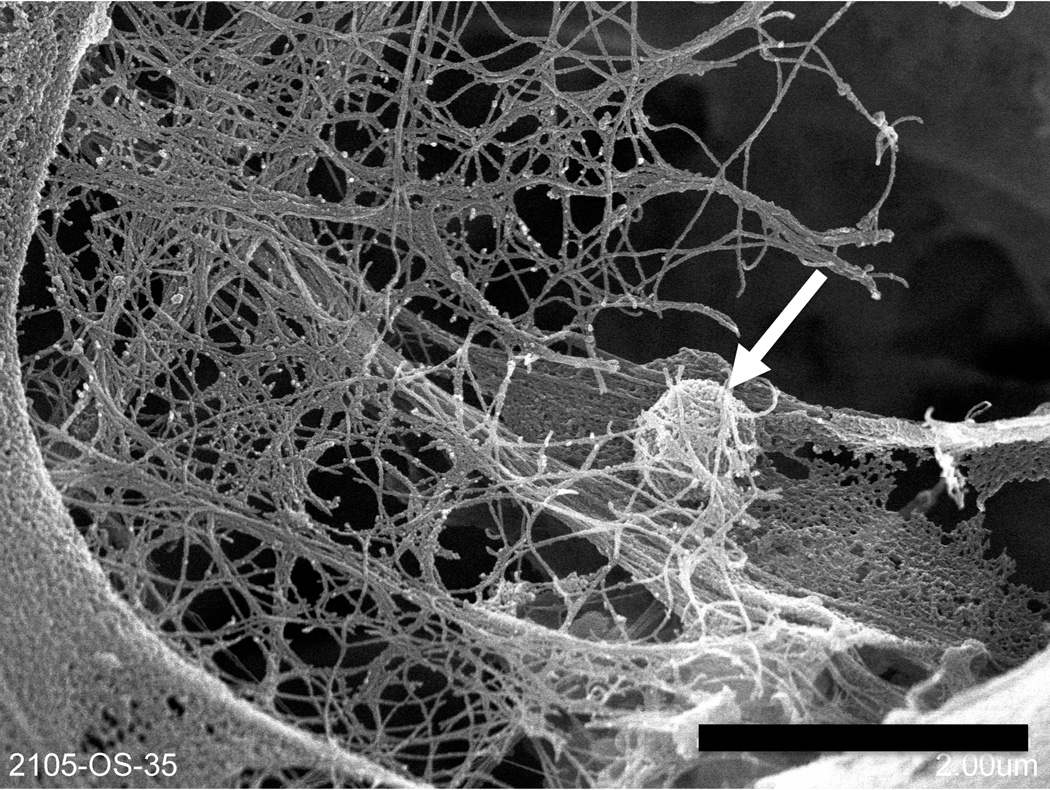
Although CPS are designed to biodegrade over time, they should promote osseointegration, which is defined as the formation of a chemical bond between bone and the surface of an implanted material without formation of fibrous tissue. Osseointegration is promoted by surface charge58, 59, wettability, nanotopography60, 61, microporosity62 and hemocompatibility63, 64. Seyfert et al.64 proposed an excellent discussion on in vitro testing of hemocompatibility according to ISO standard 10993-465 and clearly demonstrated the clinical relevance of hemocompatibility testing for implantable devices, while others have shown that every single surface parameter plays a key role in implant/blood interactions63. If surface charge and wettability are rather complex to tailor with ceramic materials, nanotopography and microporosity can be easily controlled through thermal treatment by altering heating rate, temperature or duration. Figure 3 illustrates interaction between a human mesenchymal stem cell (hMSC) and a fluorapatite glass-ceramic at 4 days, facilitated by a microstructure characterized by submicrometer spherical crystals. Figure 4 illustrates hMSC differentiation into an osteoblastic lineage after 35 days. A fibrillar network is present, together with a spherical nodule consisting of amorphous calcium phosphate.
Figure 3.
SEM of a hMSC on a fluorapatite glass-ceramic scaffold at 4 days with filopodia extending towards submicrometer fluorapatite spherical crystals. Bar=2 µm.
Figure 4.
SEM of hMSC on a fluorapatite glass-ceramic scaffold after 35 days, a fibrillar network is present, together with a spherical nodule consisting of amorphous calcium phosphate (arrow). Bar=2 µm.
Delivery of osteogenic factors and anti-infectives by CPS
Strategies to enhance successful bone regeneration by CPS include the addition of growth factors, cytokines, stem cells, and anti-infectives. Given the propensity of CPS to adsorb and concentrate osteoinductive and angiogenic molecules naturally present in the body and thereby enhance bone healing66, many of these same factors have been pre-loaded on CPS scaffolds prior to implantation as a means to enhance bone formation. The list of osteogenic factors that have been delivered by CPS for orthopaedic and dental applications is growing and includes bone morphogenetic proteins (e.g. BMP-2, BMP-7)67–69, human growth hormone (hGF)70, platelet derived growth factor (PDGF-BB)71, 72, transforming growth factor beta-3 (TGF-β3)73, fibroblast growth factor-2 (FGF-2)74, platelet rich plasma (PRP)75 and vascular endothelial growth factor (VEGF)69, 76. While many CPS-growth factor combinations have been tested in animals, we are only aware of one commercially available CPS-growth factor product used clinically: GEM21S® (Osteohealth) which delivers PDGF-BB in a controlled manner through the use of a β-TCP delivery vehicle for dental applications.
It is important to use a controlled release delivery vehicle for growth factors because they are proteins that are rapidly denatured if administered systemically. Even more importantly, a delivery system is needed in order to release the growth factor at a low dose locally in a contained manner within the wound site. Growth factors have a broad range of activity on many different cell types throughout the body and uncontrolled non-local administration can lead to tissue malformation associated with painful and life threatening side effects. Complication rates between 10% and 50% have been reported in spine fusion when using BMP-2 delivered from a weakly confining collagen sponge that resulted in ectopic bone formation in and around the spinal canal, post-operative radiculitis, vertebral osteolysis and allergic/hyperinflammatory response, new malignancies77. Growth factor activity is normally tightly regulated in the body through the production of inhibitors, such as growth factor receptor antagonists, e.g. noggin for BMP-2. The artificial delivery of high doses of growth factors instigates the counter productive expression of antagonists, providing further rationale for efficient low dose delivery from biomaterial scaffolds such as CPS.
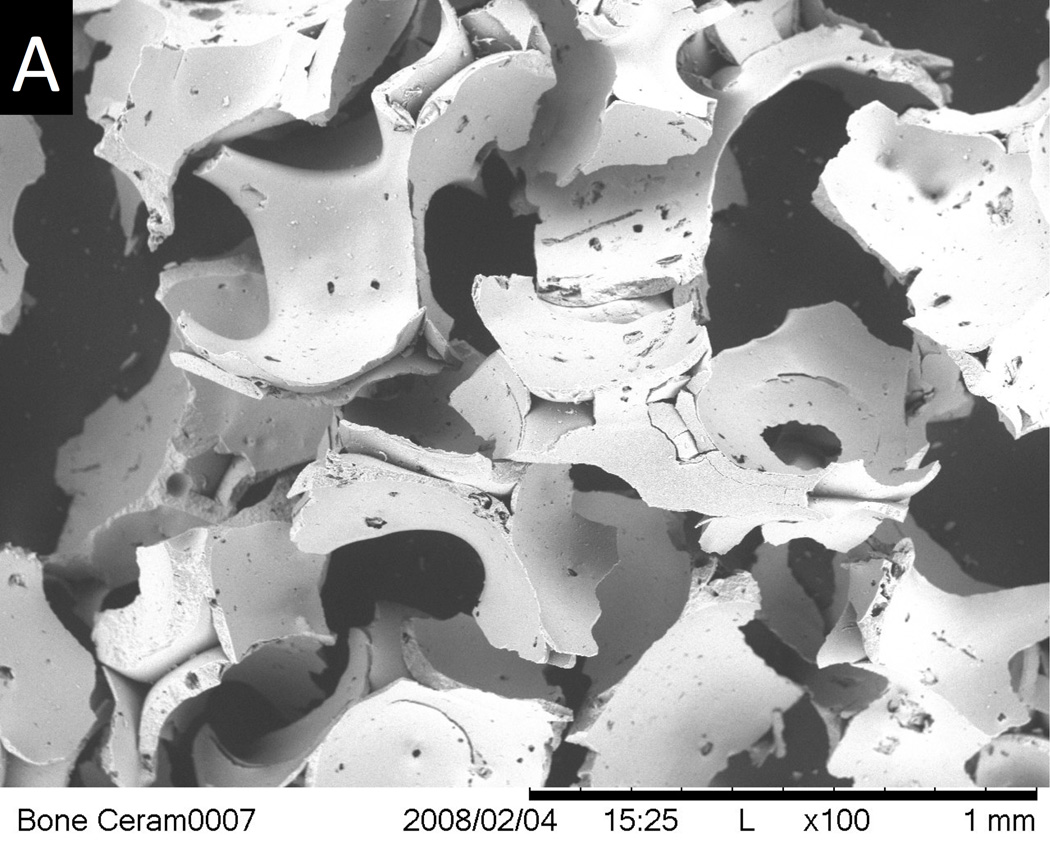
The final thermal treatment or sintering step during CPS manufacture requires that the addition of osteogenic molecules occur after the structural CPS component is manufactured to avoid destroying their biological activity by the heat. Application of a growth factor as a surface coating is thus the only technique available for combining growth factors with CPS. Simple adsorption of the active biomolecule to the CPS surface was the initial approach used, but that technique is associated with an undesirable burst release67, 68, 76. The release kinetics critically affects the biological effects of growth factors and a slow and consistent release is beneficial. To fine tune the release of BMP-2, the surface area and porosity of a commercial sintered biphasic calcium phosphate ceramic was enhanced by applying a biomimetic nanocrystalline apatite coating to increase BMP-2 binding and to slow the release67. Another approach to reduce burst release and to enhance growth factor biological effects is to co-precipitate the growth factor within a CaP coating on the CPS by dissolving the growth factor in a simulated body fluid solution and allowing for direct co-precipitation on the CPS. This was demonstrated for BMP-2 and VEGF delivery from CPS by Liu et al. 68 and Wernike et al., respectively76. Choy et al.78 increased the degradation of β-TCP ceramics through the incorporation of receptor activator of nuclear factor κ-B ligand (RANKL) using the co-precipitation technique. Superficially adsorbed RANKL did not induce the formation of osteoclasts on β-TCP ceramics in their studies. Overcoat of an additional polymer layer above the adsorbed growth factor, or encapsulation of the growth factor within a polymer that is then applied as a coating to the CPS are other strategies to optimize growth factor delivery from CPS. Polak et al.79 incorporated BMP-2 within gelatin microspheres and then infiltrated them into micropores on the surface of a porous HA CPS. Figure 5 demonstrates successful BMP-2 delivery and osseointegration from a biphasic 60% HA/40% β-TCP particulate bone graft substitute combined with a polyethylene glycol hydrogel (HA/TCP/PEG) (Institut Straumann, Basel, Switzerland). The scanning electron micrograph of Figure 5 A shows the micro and macro-porosity of this product that encouraged new bone tissue growth. The histology image of Figure 5B shows new bone tissue growth into the HA/TCP/PEG around a dental implant that was retrieved after 6 weeks from a rabbit mandible as described in Wen et al. 201580. The use of a small amount of polymer as an outer layer, particularly a polymer that does not destroy the biological activity of the growth factors, can enhance the ability of CPS to serve the dual purpose of an osteogenic drug delivery vehicle and scaffold.
Figure 5.
(A) SEM of an HA/TCP bone ceramic scaffold showing macro and microporosity. Bar = 1 mm. (B) Histological section showing bone osteoconduction into the HA/TCP bone ceramic when applied around a dental implant that was placed horizontally in rabbit mandibular bone. Bar = 2 mm.
Antibiotic delivery from CPS continues to be investigated since infection remains a significant problem associated with orthopaedic and dental surgeries. Silver has antibacterial properties and because of its non-organic nature can be doped into CPS by mixing Ag2O with TCP powder and then sintering81. Alternatively AgNO3 can be dissolved in a calcification solution and then coprecipitated to form a silver containing hydroxyapatite coating on titanium implants82. The biomimetic precipitation method has also been used for incorporation and release of cephalothin, carbenicillin, amoxicillin, cefamandol, tobramycin, gentamicin and vancomycin in carbonated HA coatings on titanium alloy plates83. The chemical nature and concentration of the antibiotic being incorporated had a significant influence on the carbonated HA coating thickness and thus their release, in addition to their chemical nature. Antibiotics containing carboxylate groups like cephalothin were slower released than others. Kim et al.84 mixed an antibiotic drug, tetracycline hydrochloride, within a polycaprolactone (PCL)-HA powder combination in a solvent and applied it through dip coating onto a porous HA scaffold. The burst release of antibiotic was reduced and the poor mechanical properties of the highly porous HA scaffold (87%) with pore size of 150–200 µm were enhanced by the PCL-HA coating which blunted or covered the flaws in the CPS. Increased percentage of HA in the coating mixture enhanced release, but decreased the mechanical strength. In a different study, polycaprolactone and polyethylene glycol were mixed and then combined with morselized granules of a corraline calcium carbonate/calcium phosphate CPS and tobramycin sulfate antibiotic and compacted in a mold to form a highly porous biodegradable CPS85. This fabrication technique led to a moldable, carvable product with favorable mechanical properties and drug release kinetics providing bactericidal activity to 10 weeks in vitro when polycaprolactone exceeded 98% w/w of the total polymer fraction. Drug release in both cases was considerably affected by coating resorption: more degradation leading to more release. A slow degrading polymer coating like polycaprolactone was fine-tuned by the addition of more resorbable CaP powder. One major downside to these coating techniques, as pointed out in the recent and thorough review of the various types of CaP coating techniques with and without growth factors, is that while bioactive hybrid composite CaP-based coatings is an exciting area of research, the adhesion between coating and substrate and high costs related to industrial upscaling are the most influential factors restricting wide spread application of these techniques on a commercial scale86.
Cell Delivery applications of CPS
Bone regeneration induced by the delivery of mesenchymal stem cells (MSCs) and other progenitor cells currently suffers from a lack of reproducibility for many reasons including donor variability, heterogeneity within the cell source, and loss of multipotency with expansion87. Interestingly, animal and human studies have shown that a CPS implantation vehicle can increase the probability of successful bone regeneration outcomes from stem cell-based therapies88, 89. In fact, the very first study demonstrating the osteogenic capability of marrow derived MSCs in an ectopic subcutaneous implantation assay used biphasic CPS made of 60% HA and 40% β-TCP88. It was concluded that the ceramic graft technique was a sensitive assay for identifying the osteogenic potential of marrow derived stem cells as compared to diffusion chambers in which the cells were not implanted on a CPS. Martin et al.89 assessed osteogenicity of bone marrow stromal cells treated with FGF-2 prior to seeding on 100% HA scaffolds with 70–80% porosity and the majority of pores greater than 150 µm in an ectopic bone formation assay. They only found consistent new bone formation when cells were implanted on the HA scaffold, bone was never observed if the cells were implanted on a type I collagen sponge. They postulated that the positive results occurred because the mineralized surface of the ceramic served as a primer for the initiation of bone matrix deposition.
As to which CPS is the best to guide the osteogenic differentiation of MSCs, it seems to depend on whether MSCs are seeded on the scaffolds or if the scaffolds are being implanted without cells and endogenous MSCs self-seed the scaffold. For example, Matsushima et al.90 compared the subcutaneous bone formation in rats from MSCs implanted on HA or β-TCP ceramics and found more bone in the HA ceramic than that in the β-TCP ceramic in 6 of 7 cases. In a comparison study of the scaffolds alone without cells in dogs, rabbits and rats, ectopic bone was most pronounced in the biphasic calcium phosphate ceramics (60 wt% HA and 40 wt% TCP)19. Ectopic bone formation without cells reproducibly occurred in dogs, while in rabbits and rats the new tissue formation was mainly limited to osteoid. The mechanism behind the biomaterial-driven osteogenic differentiation of seeded progenitor stem cells was studied by Barradas et al.91. They completed in vitro studies with MSCs seeded on both types of materials and concluded that increased attachment and cell spreading on β-TCP as compared to HA, and induced expression of G-protein coupled receptor 5A associated with protein kinase A signaling pathway, is the earliest evidence of an osteogenic signal from β-TCP. It should be noted that attempts to precondition stem cells in vitro by culturing them in the presence of Ca(2+) and P(i) supplements resulted in partial or complete abrogation of in vivo ectopic bone formation on CPS indicating it is not the ions alone that are the predominant mechanism92. Wang et al.93 found that a biphasic CPS (30% hydroxyapatite HA and 70% tricalcium phosphate (β-TCP)) promoted the highest expression of BMP-2 after intramuscular implantation in mice. Tang et al.94 also found that BMP Smad signaling is active in MSCs affected by osteoinductive CPS. A likely reason for the difference between results from cell seeding studies on CPS vs implantation of naked CPS is due to poor cell viability of cells seeded directly on dry CPS95. To enhance survival of MSCs seeded directly on dry CPS, CPS surfaces have been coated with fibronectin and arginine-glycine-aspartic acid (RGD)96. The live cell density of scaffolds made with an 0.1% RGD coating was 4x that of the CPS control. Therefore uncoated HA scaffolds may be better for direct cell seeding than β-TCP since they are less resorbable. During implantation of CPS without cells the initial contact with blood primes the surface and prepares it for viable in situ cell seeding.
Characterization of scaffold architecture
The unique architecture of open cell ceramics has such a significant impact on their mechanical properties that it becomes critical to fully characterize all structural parameters if any modeling of structure vs. properties relationships is to be attempted97. Some important parameters include relative density, cell (pore) size and distribution, cell window opening, strut thickness and distribution, strut shape and infrastructure and degree of anisotropy98, 99. The relative density is defined by the ratio of the density of the cellular solid to that of the strut material. True cellular solids have a relative density of less than 0.3.
As indicated earlier, pore size is a critical parameter for CPS. For polymeric foam products, the pore size is often determined by measuring the number of pores per inch on digital micrographs of a sectioned foam, using the well established linear intercept method. However, this technique is not applicable to CPS for the following reasons: 1) there is no distinction made between pore and window, 2) the pore size will depend on the location of the section, 3) there is likely to be a significant scattering of the data.
Other techniques for determining pore size involve digital image analysis routines. Either optical or scanning electron micrographs can be analyzed and both pore diameter and pore size distribution are derived automatically. However, as for the linear intercept technique, great care should be taken to account for the fact that these images are a 2D projection of a 3D pore structure. Assuming that the pores are spherical, a correction factor of 1.62 can be derived100.
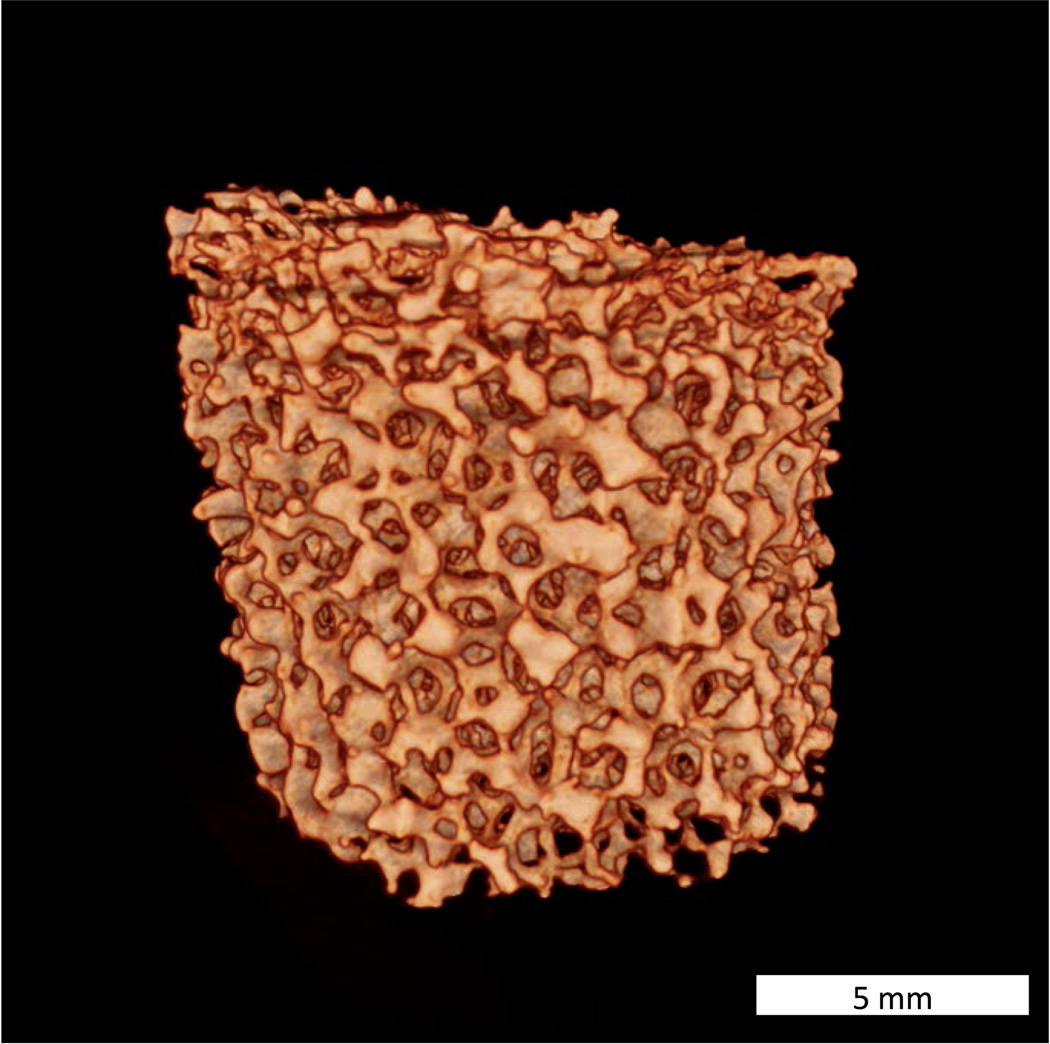
Micro computed tomography (micro-CT) permits a more precise representation of pore size, shape and distribution101, by providing a complete slice by slice 3D scaffold representation (Figure 6). The morphometric structure model index (SMI), originally developed to analyze human bone102 can be used to characterize the 3D architecture of ceramic scaffolds. Mean pore size, surface to volume ratio and strut thickness are obtained via a distance transformation method103. In summary, due to the complexity of CPS architecture, micro-CT appears to be the technique of choice for characterization, algorithms developed for human bone morphometric analysis already exist and can be conveniently adapted for CPS analysis.
Figure 6.
Micro-CT reconstructed image of a fluorapatite glass-ceramic scaffold. Bar=5 mm.
Mechanical properties
Perhaps the most commonly tested mechanical property for ceramic scaffolds is the compressive strength. Typically, specimens are kept unconstrained and loaded in compression. The compressive strength is calculated from the stress-strain curve. Although this appears to be a straightforward test, the lack of standardization across laboratories renders comparisons very difficult. Referring to ASTM standard F2883-11104, applicable to implantable ceramic scaffolds, the compressive strength should be tested according to either ASTM standard C1424 for advanced ceramics105, or ASTM standard D1621 for rigid cellular plastics106. One issue is that neither of these standards is helpful for compression testing of cellular ceramics. Since stepwise failure is unavoidable due to the cellular structure, different values of failure loads can be selected. As mentioned earlier, this lack of consensus on compressive strength measurement makes comparisons between materials and laboratories very difficult. Nevertheless, the three most important characteristics that should be kept in mind for ceramic scaffolds are the intrinsic strength and density of the bulk ceramic and the relative density of the scaffolds. From these, it is possible to predict a performing envelope for the final scaffold.
Solubility
Solubility is another important property of calcium phosphate scaffolds that is required by design. It is best measured according to ISO standard 10993-14. Results may vary considerably depending on the form and particle size. Inclusion of a control ceramic of known solubility may help validate the data obtained for both types of media.
In vivo performance vs in vitro: performance predictors
It would be of extreme clinical value to be able to reliably predict human in vivo bone regeneration performance of a new bone graft material or other bone therapeutic. In vivo animal experimentation involving implantation of the potential therapeutic material in a bone defect is currently the best pre-clinical screening method. Detailed procedures for how to conduct reliable in vivo evaluation in bone defects can be found in several recent American Society of Testing and Materials (ASTM) International standards107–109. CPS have played an important role in the in vivo screening of osteogenic MSC preparations88, 89 as described above. The cost of in vivo animal studies and the loss of animal lives continue to motivate the development of in vitro screening assays. There are a number of different types of in vitro osteogenesis assays currently used that attempt to predict in vivo performance listed here in historical order (1) in vitro apatite forming ability measured by a simulated body fluid test110, (2) in vitro osteogenic differentiation assays involving seeding of human or rodent osteoprogenitor cells such as MSCs, calvarial bone progenitors, or cells lines derived from an osteosarcoma and evaluating their differentiation via bone protein expression and mineral content111, (3) high throughput assays involving flow cytometry screening of cells after exposure to osteogenic agents112. No method is fully predictive due to a variety of bone formation mechanisms that may be operational depending on the conditions of in vivo implantation, such as endochondral ossification in which cartilage formation precedes bone formation. There are also pros and cons to each test method. The apatite forming ability test was recently reviewed113 and it was concluded that the success of the method is dependent on the expected bioactivity mechanism. If nucleation of an apatite matrix is anticipated, it may be predictive. However, as reviewed here, it has been shown that chemical, biological, or topographical mechanisms may be important in determining the in vivo bioactivity of biomaterials and the SBF immersion tests cannot capture the effects of biological or topographical mechanisms.
Due the variability of the in vitro screening assays involving cell seeding leading to a many false positives associated with dystrophic or non-osteoblast mediated mineral, ASTM recently developed a guidance document for in vitro osteogenesis assays114 and a standard practice for in vitro calcification assays115. Measurements of proliferation and alkaline phosphatase activity only are not sufficient as shown by tests with MSCs116. The important point of the ASTM standards is that assessment of in vitro osteogenesis must include more than alkaline phosphatase expression and mineral content. A thorough analysis will use conditions that do not promote non-osteoblast mediated mineral and will include quantifying the expression of a variety of proteins or genes including collagen type I, bone sialoprotein and osteocalcin in addition to mineral content. To confirm that osteoblasts are co-located with the mineral and to eliminate the possibility that the biomaterial scaffold being tested has nucleated mineral on its own, use of progenitor cells from type I collagen reporter that fluoresce when they become osteoblasts in combination with fluorescent staining of calcium are invaluable117.
Conclusions
There are a large number of combinatory design strategies available for CPS that should be considered in order to generate a suitable 3D architecture, adequate surface chemistry and topography, all of which are needed to promote bone formation. CPS represent the media of choice for delivery of osteogenic factors and anti-infectives. CPS are a superior scaffold material for bone regeneration because they actively promote osteogenesis. Biodegradability with calcium and phosphate release represents a unique asset. Structural control of CPS at the macro, micro and nanoscale and their combination with cells and polymeric materials is likely to lead to significant developments in bone tissue engineering.
Acknowledgements
This work was supported in part by the National Institute Of Dental & Craniofacial Research of the National Institutes of Health (NIDCR NIH) under awards number R01DE019972 (PI: Denry) and R01DE021103 (PI: Kuhn). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Fernandez-Yague MA, Abbah SA, McNamara L, et al. Biomimetic approaches in bone tissue engineering: Integrating biological and physicomechanical strategies. Advanced Drug Delivery Reviews. 2015;84:1–29. doi: 10.1016/j.addr.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Finkemeier CG. Bone-grafting and bone-graft substitutes. Journal of Bone and Joint Surgery-American Volume. 2002;84A(3):454–464. doi: 10.2106/00004623-200203000-00020. [DOI] [PubMed] [Google Scholar]
- 3.Dorozhkin SV. Bioceramics of calcium orthophosphates. Biomaterials. 2010;31(7):1465–1485. doi: 10.1016/j.biomaterials.2009.11.050. [DOI] [PubMed] [Google Scholar]
- 4.Samavedi S, Whittington AR, Goldstein AS. Calcium phosphate ceramics in bone tissue engineering: A review of properties and their influence on cell behavior. Acta Biomaterialia. 2013;9(9):8037–8045. doi: 10.1016/j.actbio.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 5.Vallet-Regi M, Ruiz-Hernandez E. Bioceramics: From Bone Regeneration to Cancer Nanomedicine. Advanced Materials. 2011;23(44):5177–5218. doi: 10.1002/adma.201101586. [DOI] [PubMed] [Google Scholar]
- 6.Rezwan K, Chen QZ, Blaker JJ, Boccaccini AR. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials. 2006;27(18):3413–3431. doi: 10.1016/j.biomaterials.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 7.Porter JR, Ruckh TT, Popat KC. Bone Tissue Engineering: A Review in Bone Biomimetics and Drug Delivery Strategies. Biotechnology Progress. 2009;25(6):1539–1560. doi: 10.1002/btpr.246. [DOI] [PubMed] [Google Scholar]
- 8.Rey C, Combes C, Drouet C, Glimcher MJ. Bone mineral: update on chemical composition and structure. Osteoporosis International. 2009;20(6):1013–1021. doi: 10.1007/s00198-009-0860-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vallet-Regi M. Ceramics for medical applications. Journal of the Chemical Society-Dalton Transactions. 2001;(2):97–108. [Google Scholar]
- 10.LeGeros R, LeGeros J. Bioceramics: Calcium phosphate ceramics: past, present and future; Paper presented at: 15th International Symposium on Ceramics in Medicine.2002. Sydney. [Google Scholar]
- 11.LeGeros RZ. Biodegradation and bioresorption of calcium phosphate ceramics. Clin Mater. 1993;14(1):65–88. doi: 10.1016/0267-6605(93)90049-d. [DOI] [PubMed] [Google Scholar]
- 12.LeGeros RZ. Properties of osteoconductive biomaterials: calcium phosphates. Clin Orthop. 2002;395(2):81–98. doi: 10.1097/00003086-200202000-00009. [DOI] [PubMed] [Google Scholar]
- 13.LeGeros RZ, LeGeros JP. Dense hydroxyapatite. In: Hench LL, Wilson J, editors. An introduction to bioceramics. River Edge, NJ: World Scientific Publishing Co. Pte. Ltd; 1993. pp. 139–180. [Google Scholar]
- 14.Dorozhkin SV. Calcium Orthophosphate-Based Bioceramics. Materials. 2013;6(9):3840–3942. doi: 10.3390/ma6093840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan AF, Saleem M, Afzal A, et al. Bioactive behavior of silicon substituted calcium phosphate based bioceramics for bone regeneration. Materials Science & Engineering C-Materials for Biological Applications. 2014;35:245–252. doi: 10.1016/j.msec.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Calori GM, Mazza E, Colombo M, Ripamonti C. The use of bone-graft substitutes in large bone defects: Any specific needs? Injury-International Journal of the Care of the Injured. 2011;42:S56–S63. doi: 10.1016/j.injury.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Albrektsson T, Johansson C. Osteoinduction, osteoconduction and osseointegration. European Spine Journal. 2001;10:S96–S101. doi: 10.1007/s005860100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minardi S, Corradetti B, Taraballi F, et al. Evaluation of the osteoinductive potential of a bio-inspired scaffold mimicking the osteogenic niche for bone augmentation. Biomaterials. 2015;62:128–137. doi: 10.1016/j.biomaterials.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 19.Wang L, Zhang B, Bao C, et al. Ectopic osteoid and bone formation by three calcium-phosphate ceramics in rats, rabbits and dogs. PLoS One. 2014;9(9):e107044. doi: 10.1371/journal.pone.0107044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daculsi G, Fellah BH, Miramond T. The Essential Role of Calcium Phosphate Bioceramics in Bone Regeneration. In: BenNissan B, editor. Advances in Calcium Phosphate Biomaterials. Berlin: Springer-Verlag Berlin; 2014. pp. 71–96. [Google Scholar]
- 21.Bose S, Fielding G, Tarafder S, Bandyopadhyay A. Understanding of dopant-induced osteogenesis and angiogenesis in calcium phosphate ceramics. Trends in Biotechnology. 2013;31(10):594–605. doi: 10.1016/j.tibtech.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landi E, Logroscino G, Proietti L, et al. Biomimetic Mg-substituted hydroxyapatite: from synthesis to in vivo behaviour. Journal of Materials Science-Materials in Medicine. 2008;19(1):239–247. doi: 10.1007/s10856-006-0032-y. [DOI] [PubMed] [Google Scholar]
- 23.Maier JAM, Bernardini D, Rayssiguier Y, Mazur A. High concentrations of magnesium modulate vascular endothelial cell behaviour in vitro. Biochimica Et Biophysica Acta-Molecular Basis of Disease. 2004;1689(1):6–12. doi: 10.1016/j.bbadis.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Marie PJ, Ammann P, Boivin G, Rey C. Mechanisms of action and therapeutic potential of strontium in bone. Calcified Tissue International. 2001;69(3):121–129. doi: 10.1007/s002230010055. [DOI] [PubMed] [Google Scholar]
- 25.Marquis P, Roux C, Diaz-Curiel M, et al. Long-term beneficial effects of strontium ranelate on the quality of life in patients with vertebral osteoporosis (Soti study) Calcified Tissue International. 2007;80:S137–S38. [Google Scholar]
- 26.Ortolani S, Vai S. Strontium ranelate: An increased bone quality leading to vertebral antifracture efficacy at all stages. Bone. 2006;38(2):19–22. doi: 10.1016/j.bone.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 27.Pors Nielsen S. The biological role of strontium. Bone. 2004;35(3):583–588. doi: 10.1016/j.bone.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 28.Li H, Chang J. Bioactive silicate materials stimulate angiogenesis in fibroblast and endothelial cell co-culture system through paracrine effect. Acta Biomaterialia. 2013;9(6):6981–6991. doi: 10.1016/j.actbio.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 29.Pietak AM, Reid JW, Stott MJ, Sayer M. Silicon substitution in the calcium phosphate bioceramics. Biomaterials. 2007;28(28):4023–4032. doi: 10.1016/j.biomaterials.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Bose S, Tarafder S, Banerjee SS, Davies NM, Bandyopadhyay A. Understanding in vivo response and mechanical property variation in MgO, SrO and SiO2 doped beta-TCP. Bone. 2011;48(6):1282–1290. doi: 10.1016/j.bone.2011.03.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carrodeguas RG, De Aza S. alpha-Tricalcium phosphate: Synthesis, properties and biomedical applications. Acta Biomaterialia. 2011;7(10):3536–3546. doi: 10.1016/j.actbio.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 32.Gibson IR, Best SM, Bonfield W. Effect of silicon substitution on the sintering and microstructure of hydroxyapatite. Journal of the American Ceramic Society. 2002;85(11):2771–2777. [Google Scholar]
- 33.Farzadi A, Bakhshi F, Solati-Hashjin M, Asadi-Eydivand M, abu Osman NA. Magnesium incorporated hydroxyapatite: Synthesis and structural properties characterization. Ceramics International. 2014;40(4):6021–6029. [Google Scholar]
- 34.Landi E, Tampieri A, Celotti G, Sprio S. Densification behaviour and mechanisms of synthetic hydroxyapatites. Journal of the European Ceramic Society. 2000;20(14–15):2377–2387. [Google Scholar]
- 35.Bigi A, Boanini E, Capuccini C, Gazzano M. Strontium-substituted hydroxyapatite nanocrystals. Inorganica Chimica Acta. 2007;360(3):1009–1016. [Google Scholar]
- 36.Bigi A, Falini G, Gazzano M, Roveri N, Tedesco E. Structural refinements of strontium substituted hydroxylapatites. Materials Science Forum. 1998:814–819. [Google Scholar]
- 37.Landi E, Sprio S, Sandri M, Celotti G, Tampieri A. Development of Sr and CO3 co-substituted hydroxyapatites for biomedical applications. Acta Biomaterialia. 2008;4(3):656–663. doi: 10.1016/j.actbio.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 38.Landi E, Tampieri A, Celotti G, et al. Sr-substituted hydroxyapatites for osteoporotic bone replacement. Acta Biomaterialia. 2007;3(6):961–969. doi: 10.1016/j.actbio.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 39.Supova M. Substituted hydroxyapatites for biomedical applications: A review. Ceramics International. 2015;41(8):9203–9231. [Google Scholar]
- 40.Scheffler M, Colombo P. Cellular Ceramics: Structure, Manufacturing, Properties and Applications. Weinheim: Wiley-VCH; 2005. [Google Scholar]
- 41.Schwartzwalder K, Somers AV inventors; General Motors Corporation, assignee. Method of making porous ceramic articles. US patent. 1963 May 21; 3,090,094. [Google Scholar]
- 42.Chang BS, Lee CK, Hong KS, et al. Osteoconduction at porous hydroxyapatite with various pore configurations. Biomaterials. 2000;21(12):1291–1298. doi: 10.1016/s0142-9612(00)00030-2. [DOI] [PubMed] [Google Scholar]
- 43.Saiz E, Gremillard L, Menendez G, et al. Preparation of porous hydroxyapatite scaffolds. Materials Science & Engineering C-Biomimetic and Supramolecular Systems. 2007;27(3):546–550. [Google Scholar]
- 44.Tian JT, Tian JM. Preparation of porous hydroxyapatite. Journal of Materials Science. 2001;36(12):3061–3066. [Google Scholar]
- 45.Padilla S, Sanchez-Salcedo S, Vallet-Regi M. Bioactive glass as precursor of designed-architecture scaffolds for tissue engineering. Journal of Biomedical Materials Research Part A. 2007;81A(1):224–232. doi: 10.1002/jbm.a.30934. [DOI] [PubMed] [Google Scholar]
- 46.Santos JD, Knowles JC, Reis RL, Monteiro FJ, Hastings GW. Microstructural characterization of glass-reinforced hydroxyapatite composites Biomaterials. 1994;15(1):5–10. doi: 10.1016/0142-9612(94)90188-0. [DOI] [PubMed] [Google Scholar]
- 47.Colombo P, Hellmann JR. Ceramic foams from preceramic polymers. Materials Research Innovations. 2002;6(5–6):260–272. [Google Scholar]
- 48.Shepherd JH, Best SM. Calcium phosphate scaffolds for bone repair. JOM. 2011;63(4):83–92. [Google Scholar]
- 49.Lewis JA, Smay JE. Three-Dimensional Periodic Structures. Cellular Ceramics: Structure, Manufacturing, Properties and Applications. 2005:87–100. [Google Scholar]
- 50.Lewis JA, Smay JE, Stuecker J, Cesarano J., III Direct ink writing of three-dimensional ceramic structures. Journal of the American Ceramic Society. 2006;89(12):3599–609. [Google Scholar]
- 51.Simon JL, Michna S, Lewis JA, et al. In vivo bone response to 3D periodic hydroxyapatite scaffolds assembled by direct ink writing. Journal of Biomedical Materials Research Part A. 2007;83A(3):747–758. doi: 10.1002/jbm.a.31329. [DOI] [PubMed] [Google Scholar]
- 52.Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21(24):2529–2543. doi: 10.1016/s0142-9612(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 53.Descamps M, Duhoo T, Monchau F, et al. Manufacture of macroporous beta-tricalcium phosphate bioceramics. Journal of the European Ceramic Society. 2008;28(1):149–157. [Google Scholar]
- 54.Descamps M, Richart O, Hardouin P, Hornez JC, Leriche A. Synthesis of macroporous beta-tricalcium phosphate with controlled porous architectural. Ceramics International. 2008;34(5):1131–1137. [Google Scholar]
- 55.Michna S, Wu W, Lewis JA. Concentrated hydroxyapatite inks for direct-write assembly of 3-D periodic scaffolds. Biomaterials. 2005;26(28):5632–5639. doi: 10.1016/j.biomaterials.2005.02.040. [DOI] [PubMed] [Google Scholar]
- 56.Denry I, Holloway JA. Low temperature sintering of fluorapatite glass-ceramics. Dental Materials. 2014;30(2):112–121. doi: 10.1016/j.dental.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fu Q, Saiz E, Rahaman MN, Tomsia AP. Bioactive glass scaffolds for bone tissue engineering: state of the art and future perspectives. Materials Science & Engineering C-Materials for Biological Applications. 2011;31(7):1245–1256. doi: 10.1016/j.msec.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barrere F, Mahmood TA, de Groot K, van Blitterswijk CA. Advanced biomaterials for skeletal tissue regeneration: Instructive and smart functions. Materials Science & Engineering R-Reports. 2008;59(1–6):38–71. [Google Scholar]
- 59.Barrere F, van Blitterswijk CA, de Groot K. Bone regeneration: molecular and cellular interactions with calcium phosphate ceramics. International Journal of Nanomedicine. 2006;1(3):317–332. [PMC free article] [PubMed] [Google Scholar]
- 60.Davies JE. Bone bonding at natural and biomaterial surfaces. Biomaterials. 2007;28(34):5058–5067. doi: 10.1016/j.biomaterials.2007.07.049. [DOI] [PubMed] [Google Scholar]
- 61.Davies JE. Understanding peri-implant endosseous healing. J Dent Educ. 2005;67(8):932–949. [PubMed] [Google Scholar]
- 62.Woodard JR, Hilldore AJ, Lan SK, et al. The mechanical properties and osteoconductivity of hydroxyapatite bone scaffolds with multi-scale porosity. Biomaterials. 2007;28(1):45–54. doi: 10.1016/j.biomaterials.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 63.Diaz-Rodriguez P, Gonzalez P, Serra J, Landin M. Key parameters in blood-surface interactions of 3D bioinspired ceramic materials. Materials Science & Engineering C-Materials for Biological Applications. 2014;41:232–239. doi: 10.1016/j.msec.2014.04.058. [DOI] [PubMed] [Google Scholar]
- 64.Seyfert UT, Biehl V, Schenk J. In vitro hemocompatibility testing of biomaterials according to the ISO 10993-4. Biomolecular Engineering. 2002;19(2–6):91–96. doi: 10.1016/s1389-0344(02)00015-1. [DOI] [PubMed] [Google Scholar]
- 65.ISO. Standard 10993-4 Biological evaluation of medical devices - Part 4: Selection of tests for interactions with blood. Geneva, Switzerland: 2002. [Google Scholar]
- 66.LeGeros RZ. Properties of osteoconductive biomaterials: calcium phosphates. Clin Orthop Relat Res. 2002;(395):81–98. doi: 10.1097/00003086-200202000-00009. [DOI] [PubMed] [Google Scholar]
- 67.Autefage H, Briand-Mesange F, Cazalbou S, et al. Adsorption and release of BMP-2 on nanocrystalline apatite-coated and uncoated hydroxyapatite/beta-tricalcium phosphate porous ceramics. J Biomed Mater Res B Appl Biomater. 2009;91(2):706–715. doi: 10.1002/jbm.b.31447. [DOI] [PubMed] [Google Scholar]
- 68.Liu Y, de Groot K, Hunziker EB. BMP-2 liberated from biomimetic implant coatings induces and sustains direct ossification in an ectopic rat model. Bone. 2005;36(5):745–757. doi: 10.1016/j.bone.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 69.Roldan JC, Detsch R, Schaefer S, et al. Bone formation and degradation of a highly porous biphasic calcium phosphate ceramic in presence of BMP-7, VEGF and mesenchymal stem cells in an ectopic mouse model. J Craniomaxillofac Surg. 2010;38(6):423–430. doi: 10.1016/j.jcms.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 70.Guicheux J, Gauthier O, Aguado E, et al. Human growth hormone locally released in bone sites by calcium-phosphate biomaterial stimulates ceramic bone substitution without systemic effects: a rabbit study. J Bone Miner Res. 1998;13(4):739–748. doi: 10.1359/jbmr.1998.13.4.739. [DOI] [PubMed] [Google Scholar]
- 71.Nevins M, Giannobile WV, McGuire MK, et al. Platelet-derived growth factor stimulates bone fill and rate of attachment level gain: results of a large multicenter randomized controlled trial. J Periodontol. 2005;76(12):2205–2215. doi: 10.1902/jop.2005.76.12.2205. [DOI] [PubMed] [Google Scholar]
- 72.Irokawa D, Ota M, Yamamoto S, Shibukawa Y, Yamada S. Effect of beta tricalcium phosphate particle size on recombinant human platelet-derived growth factor-BB-induced regeneration of periodontal tissue in dog. Dent Mater J. 2010;29(6):721–730. doi: 10.4012/dmj.2010-033. [DOI] [PubMed] [Google Scholar]
- 73.Steffen T, Stoll T, Arvinte T, Schenk RK. Porous tricalcium phosphate and transforming growth factor used for anterior spine surgery. Eur Spine J. 2001;(10 Suppl 2):S132–S140. doi: 10.1007/s005860100325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jeong I, Yu HS, Kim MK, Jang JH, Kim HW. FGF2-adsorbed macroporous hydroxyapatite bone granules stimulate in vitro osteoblastic gene expression and differentiation. J Mater Sci Mater Med. 2010;21(4):1335–1342. doi: 10.1007/s10856-009-3971-2. [DOI] [PubMed] [Google Scholar]
- 75.Paderni S, Terzi S, Amendola L. Major bone defect treatment with an osteoconductive bone substitute. Chir Organi Mov. 2009;93(2):89–96. doi: 10.1007/s12306-009-0028-0. [DOI] [PubMed] [Google Scholar]
- 76.Wernike E, Montjovent MO, Liu Y, et al. VEGF incorporated into calcium phosphate ceramics promotes vascularisation and bone formation in vivo. Eur Cell Mater. 2010;19:30–40. doi: 10.22203/ecm.v019a04. [DOI] [PubMed] [Google Scholar]
- 77.Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11(6):471–491. doi: 10.1016/j.spinee.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 78.Choy J, Albers CE, Siebenrock KA, et al. Incorporation of RANKL promotes osteoclast formation and osteoclast activity on beta-TCP ceramics. Bone. 2014;69:80–88. doi: 10.1016/j.bone.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 79.Polak SJ, Levengood SK, Wheeler MB, et al. Analysis of the roles of microporosity and BMP-2 on multiple measures of bone regeneration and healing in calcium phosphate scaffolds. Acta Biomater. 2011;7(4):1760–1771. doi: 10.1016/j.actbio.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 80.Wen B, Kuhn L, Charles L, et al. Comparison of bone morphogenetic protein-2 delivery systems to induce supracrestal bone guided by titanium implants in the rabbit mandible. Clin Oral Implants Res. 2015 doi: 10.1111/clr.12645. [DOI] [PubMed] [Google Scholar]
- 81.Seeley Z, Bandyopadhyay A, Bose S. Influence of TiO2 and Ag2O addition on tricalcium phosphate ceramics. J Biomed Mater Res A. 2007;82(1):113–121. doi: 10.1002/jbm.a.31077. [DOI] [PubMed] [Google Scholar]
- 82.Chen Y, Zheng X, Xie Y, et al. Anti-bacterial and cytotoxic properties of plasma sprayed silver-containing HA coatings. J Mater Sci Mater Med. 2008;19(12):3603–3609. doi: 10.1007/s10856-008-3529-8. [DOI] [PubMed] [Google Scholar]
- 83.Stigter M, Bezemer J, de Groot K, Layrolle P. Incorporation of different antibiotics into carbonated hydroxyapatite coatings on titanium implants, release and antibiotic efficacy. J Control Release. 2004;99(1):127–137. doi: 10.1016/j.jconrel.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 84.Kim HW, Knowles JC, Kim HE. Hydroxyapatite/poly(epsilon-caprolactone) composite coatings on hydroxyapatite porous bone scaffold for drug delivery. Biomaterials. 2004;25(7–8):1279–1287. doi: 10.1016/j.biomaterials.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 85.Brooks BD, Sinclair KD, Davidoff SN, et al. Molded polymer-coated composite bone void filler improves tobramycin controlled release kinetics. J Biomed Mater Res B Appl Biomater. 2014;102(5):1074–1083. doi: 10.1002/jbm.b.33089. [DOI] [PubMed] [Google Scholar]
- 86.Surmenev RA, Surmeneva MA, Ivanova AA. Significance of calcium phosphate coatings for the enhancement of new bone osteogenesis--a review. Acta Biomater. 2014;10(2):557–579. doi: 10.1016/j.actbio.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 87.Siddappa R, Licht R, van Blitterswijk C, de Boer J. Donor variation and loss of multipotency during in vitro expansion of human mesenchymal stem cells for bone tissue engineering. J Orthop Res. 2007;25(8):1029–1041. doi: 10.1002/jor.20402. [DOI] [PubMed] [Google Scholar]
- 88.Haynesworth SE, Goshima J, Goldberg VM, Caplan AI. Characterization of cells with osteogenic potential from human marrow. Bone. 1992;13(1):81–88. doi: 10.1016/8756-3282(92)90364-3. [DOI] [PubMed] [Google Scholar]
- 89.Martin I, Muraglia A, Campanile G, Cancedda R, Quarto R. Fibroblast growth factor-2 supports ex vivo expansion and maintenance of osteogenic precursors from human bone marrow. Endocrinology. 1997;138(10):4456–4462. doi: 10.1210/endo.138.10.5425. [DOI] [PubMed] [Google Scholar]
- 90.Matsushima A, Kotobuki N, Tadokoro M, et al. In vivo osteogenic capability of human mesenchymal cells cultured on hydroxyapatite and on beta-tricalcium phosphate. Artif Organs. 2009;33(6):474–481. doi: 10.1111/j.1525-1594.2009.00749.x. [DOI] [PubMed] [Google Scholar]
- 91.Barradas AM, Monticone V, Hulsman M, et al. Molecular mechanisms of biomaterial-driven osteogenic differentiation in human mesenchymal stromal cells. Integr Biol (Camb) 2013;5(7):920–931. doi: 10.1039/c3ib40027a. [DOI] [PubMed] [Google Scholar]
- 92.Chai YC, Roberts SJ, Desmet E, et al. Mechanisms of ectopic bone formation by human osteoprogenitor cells on CaP biomaterial carriers. Biomaterials. 2012;33(11):3127–3142. doi: 10.1016/j.biomaterials.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 93.Wang J, Chen Y, Zhu X, et al. Effect of phase composition on protein adsorption and osteoinduction of porous calcium phosphate ceramics in mice. J Biomed Mater Res A. 2014;102(12):4234–4243. doi: 10.1002/jbm.a.35102. [DOI] [PubMed] [Google Scholar]
- 94.Tang Z, Wang Z, Qing F, et al. Bone morphogenetic protein Smads signaling in mesenchymal stem cells affected by osteoinductive calcium phosphate ceramics. J Biomed Mater Res A. 2015;103(3):1001–1010. doi: 10.1002/jbm.a.35242. [DOI] [PubMed] [Google Scholar]
- 95.Sun H, Yang HL. Calcium phosphate scaffolds combined with bone morphogenetic proteins or mesenchymal stem cells in bone tissue engineering. Chin Med J (Engl) 2015;128(8):1121–1127. doi: 10.4103/0366-6999.155121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen W, Zhou H, Weir MD, Bao C, Xu HK. Umbilical cord stem cells released from alginate-fibrin microbeads inside macroporous and biofunctionalized calcium phosphate cement fir bone regeneration. Acta Biomaterialia. 2012;8(6):2297–2306. doi: 10.1016/j.actbio.2012.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gibson LJ, Ashby MF. The mechanics of 3-dimensional cellular materials Proceedings of the Royal Society of London Series a-Mathematical Physical and Engineering Sciences. 1982;382(1782):43. [Google Scholar]
- 98.Mullens S, Luyten J, Zeschky J. Characterization of Structure and Morphology. Cellular Ceramics: Structure, Manufacturing, Properties and Applications. 2005:227–266. [Google Scholar]
- 99.Gibson L, Ashby M. Cellular Solids: Structure and Properties. 2nd ed. Cambridge, UK: Cambridge University Press; 1999. [Google Scholar]
- 100.ASTM. 3576 Standard test method for cell size of rigid cellular plastics. 1977:919–922. [Google Scholar]
- 101.Cancedda R, Cedola A, Giuliani A, et al. Bulk and interface investigations of scaffolds and tissue-engineered bones by X-ray microtomography and X-ray microdiffraction. Biomaterials. 2007;28(15):2505–2524. doi: 10.1016/j.biomaterials.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 102.Hildebrand T, Ruegsegger P. Bone. 3 SUPPL. Vol. 19. New York: 1996. Structure model index: A new method to describe remodeling of trabecular bone; p. 143S. [Google Scholar]
- 103.Hildebrand T, Ruegsegger P. A new method for the model-independent assessment of thickness in three-dimensional images. Journal of Microscopy-Oxford. 1997;185:67–75. [Google Scholar]
- 104.ASTM. Book of Standards Volume: 13.02. West Conshohocken, PA: ASTM International; 2011. F2883 - 11 Standard Guide for Characterization of Ceramic and Mineral Based Scaffolds used for Tissue-Engineered Medical Products (TEMPs) and as Device for Surgical Implant Applications. [Google Scholar]
- 105.ASTM. C1424-10 Standard Test Method for Monotonic Compressive Strength of Advanced Ceramics at Ambient Temperature. West Conshohocken, PA: ASTM International; 2015. [Google Scholar]
- 106.ASTM. D1621-10, Standard Test Method for Compressive Properties Of Rigid Cellular Plastics. West Conshohocken, PA: ASTM International; 2010. [Google Scholar]
- 107.ASTM. Book of Standards Volume: 13.02. West Conshohocken, PA: ASTM International; 2014. F2721-09 Standard Guide for Pre-clinical in vivo Evaluation in Critical Size Segmental Bone Defects - Developed by Subcommittee: F04.44. [Google Scholar]
- 108.ASTM. Book of Standards Volume: 13.02. West Conshohocken, PA: ASTM International; 2012. F2884 - 12 Standard Guide for Pre-clinical in vivo Evaluation of Spinal Fusion. [Google Scholar]
- 109.ASTM. Book of Standards Volume: 13.02. West Conshohocken, PA: ASTM International; 2013. F2529 - 13 Standard Guide for in vivo Evaluation of Osteoinductive Potential for Materials Containing Demineralized Bone (DBM) [Google Scholar]
- 110.Kokubo T. Bioactive glass ceramics: properties and applications. Biomaterials. 1991;12(2):155–163. doi: 10.1016/0142-9612(91)90194-f. [DOI] [PubMed] [Google Scholar]
- 111.Boskey AL, Roy R. Cell culture systems for studies of bone and tooth mineralization. Chem Rev. 2008;108(11):4716–4733. doi: 10.1021/cr0782473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Walmsley GG, Atashroo DA, Maan ZN, et al. High-Throughput Screening of Surface Marker Expression on Undifferentiated and Differentiated Human Adipose-Derived Stromal Cells. Tissue Eng Part A. 2015;21(15–16):2281–2291. doi: 10.1089/ten.tea.2015.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zadpoor AA. Relationship between in vitro apatite-forming ability measured using simulated body fluid and in vivo bioactivity of biomaterials. Mater Sci Eng C Mater Biol Appl. 2014;35:134–143. doi: 10.1016/j.msec.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 114.ASTM. Book of Standards Volume: 13.02. West Conshohocken, PA: ASTM International; 2014. F3106 - 14 Standard Guide for in vitro Osteoblast Differentiation Assays -Developed by Subcommittee: F04.43. [Google Scholar]
- 115.ASTM. Book of Standards Volume: 13.02. West Conshohocken, PA: ASTM International; 2013. F2997-13 Standard Practice for Quantification of Calcium Deposits in Osteogenic Culture of Progenitor Cells Using Fluorescent Image Analysis - Developed by Subcommittee: F04.43. [Google Scholar]
- 116.Cassiede P, Dennis JE, Ma F, Caplan AI. Osteochondrogenic potential of marrow mesenchymal progenitor cells exposed to TGF-beta 1 or PDGF-BB as assayed in vivo and in vitro. J Bone Miner Res. 1996;11(9):1264–1273. doi: 10.1002/jbmr.5650110911. [DOI] [PubMed] [Google Scholar]
- 117.Kuhn LT, Liu Y, Advincula M, et al. A nondestructive method for evaluating in vitro osteoblast differentiation on biomaterials using osteoblast-specific fluorescence. Tissue Eng Part C Methods. 2010;16(6):1357–1366. doi: 10.1089/ten.TEC.2009.0701. [DOI] [PubMed] [Google Scholar]
- 118.Markovic M, Fowler BO, Tung MS. Preparation and comprehensive characterization of a calcium hydroxyapatite reference material. Journal of Research of the National Institute of Standards and Technology. 2004;109(6):553–568. doi: 10.6028/jres.109.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gibson IR, Best SM, Bonfield W. Chemical characterization of silicon-substituted hydroxyapatite. Journal of Biomedical Materials Research. 1999;44(4):422–428. doi: 10.1002/(sici)1097-4636(19990315)44:4<422::aid-jbm8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 120.Ergun C, Webster TJ, Bizios R, Doremus RH. Hydroxylapatite with substituted magnesium, zinc, cadmium, and yttrium. I. Structure and microstructure. Journal of Biomedical Materials Research. 2002;59(2):305–311. doi: 10.1002/jbm.1246. [DOI] [PubMed] [Google Scholar]
- 121.Sudarsanan K, Young R. Structure of strontium hydroxide phosphate, Sr5(PO4)3OH. Acta Cryst. 1972;B28:3668–3670. [Google Scholar]