Abstract
Objective
To assess the correlation of clinician identified myofascial taut bands with their presence and characteristics on Magnetic Resonance Elastography (MRE) imaging.
Design
Cross-sectional study.
Setting
An MRI research laboratory.
Participants
A convenience sample of 65 adults (45 women, 20 men) identified by skilled musculoskeletal physicians as having upper trapezius myofascial pain associated taut bands.
Interventions
Subjects had their taut bands outlined and were positioned within a 1.5T MRI machine. Shear waves were induced with a pneumatic transducer located over the belly of the involved muscle. Wave propagation was visualized with MRE images across a vibration-cycle. Imaging data was assessed independently by two skilled MRE interpreters.
Main Outcome Measures
The primary outcome measure was the determination of the intra- and inter-rater reliabilities of MRE taut band identification and their correlation with clinician identification of band presence. Secondary outcomes consisted of the elucidation of the physical characteristics of taut bands and their surrounding muscle tissue.
Results
MRE intra- and inter-rater reliability was excellent with Kappa's and 95% Confidence intervals (CI) of 0.86, [0.68, 1.00]; and 0.93, [0.79, 1.00], respectively. Stiffness in MRE identified taut bands was elevated at a mean of 11.5 KPa (±2.4 KPa) and fell to a mean of 5.8 KPa (± 0.9 KPa) in surrounding muscle tissue (p<0.001); muscular tone in trapezius muscles without a taut band was relatively uniform at a mean of 6.6 KPa (±2.1 KPa). Agreement between the physicians and the MRE raters, however, was relatively poor (63.1%, 95% CI [50.2%, 74.7%]).
Conclusions
Our findings suggest that while clinicians may overestimate, and current MRE techniques may underestimate, the presence of taut bands, that these bands exist, can be assessed quantitatively, and do represent localized areas of increased muscle stiffness.
Keywords: Magnetic Resonance Elastography, Myofascial Pain, Myofascial Taut Band
The advancement of clinical medicine is dependent on the ability to accurately diagnose disease and impartially assess the effects of treatment. The last few decades have resulted in remarkable progress. Nonetheless, the identification of a condition and its treatment often remains more dependent on an examiner's skills and judgment than we would like. This dependence is especially marked in musculoskeletal medicine, where a lack of quantitative measures limits research efforts and ensures that the diagnosis, treatment, and study of a variety of soft tissue conditions remains more subjective than we would wish.
Fibromyalgia and myofascial pain are emblematic of this situation in that while they affect millions of people,1 their diagnosis remains based on clinical criteria, their treatment controversial, and their findings dependent on a clinician's interviewing and examination skills. For example, the diagnosis of fibromyalgia is in large part based on the identification of tender points in a criteria-based distribution.2, 3 Conversely, a diagnosis of myofascial pain is centered on the findings of bands of increased muscle tone (“taut bands”) in association with small areas of tenderness capable of generating reproducible patterns of referred pain.4
There have been numerous attempts to reliably quantify the presence and nature of these entities. Among these efforts have been electromyographic investigations, 5,6, 7 histochemical analyses,8, 9 thermography, and pressure algometry. 10-13 A variety of findings have been reported but none, with the possible exception of algometry, have approached the level of a criterion standard. However, even algometry has its limits in that it is user dependent and, at best, provides a semi-quantitative assessment of tenderness and tissue tone.
This picture is beginning to improve with new work suggesting improvements in our ability to characterize the salient aspects of these conditions. Three approaches seem particularly promising. The first is exemplified by the work of Shah and colleagues14, 15 and involves a microanalytic and biochemical analysis of the nature of taut bands and trigger points. The remaining two approaches examine muscle characteristics on a more macroscopic level. The first of these, Magnetic Resonance Elastography (MRE),16, 17 is an MRI imaging technique that assesses changes in shear waves as they pass through muscle.18-21 The second, ultrasound elastography, uses ultrasound to image the elastic properties of soft tissue in a manner somewhat similar to MRE. This technique, while promising, is still exploratory and remains in the earlier stages of development. 22, 23
Each of these approaches offer exciting insights into the etiologies and treatment of muscular disease. However, the benefits of the first are limited by its invasive nature and restriction to the micro milieu of a small area. Ultrasound elastography, on the other hand, while less constrained in the amount of area that can be examined, remains in a more exploratory status. Given this, we believed that the best approach for the time being was to investigate the correlation of observations of skilled clinicians with their corresponding quantitative findings on MRE.
Fibromyalgia and myofascial pain are each painful musculoskeletal conditions that lend themselves to MRE quantification. While the nuances of their similarities and differences24-27 are intriguing, the pertinent factor for this investigation is that myofascial taut bands are far larger in size than the more localized areas associated with the trigger points of myofascial pain24, 26, 28 or the tender points of fibromyalgia. We, therefore, decided to perform a preliminary study to investigate the correspondence of clinically identified myofascial taut bands with their presence on MRE images. Our secondary goal was to further our understanding of their nature and characteristics.
Methods
Subject Recruitment
Subjects with myofascial pain were recruited with the assistance of informal word of mouth advertising and institutional notices. Eligibility was determined by screening examinations performed by either of two physicians with extensive experience in musculoskeletal medicine. Inclusion criteria4, 29, 30 included an age between 21 and 70 years; pain in one or both upper trapezius muscles of at least 3 months’ duration; examination findings of a taut band within either trapezius; identification of a tender point within the band; and the subject reporting reproduction of their “usual pain” upon palpation. Once these criteria had been met, the examining physician marked the course of an identified taut band on the skin of the subject with a felt tip marker.
The study protocol was reviewed and approved by our Institutional Review Board. All participants provided written consent before entry into the study.
MRE Image Data Collection
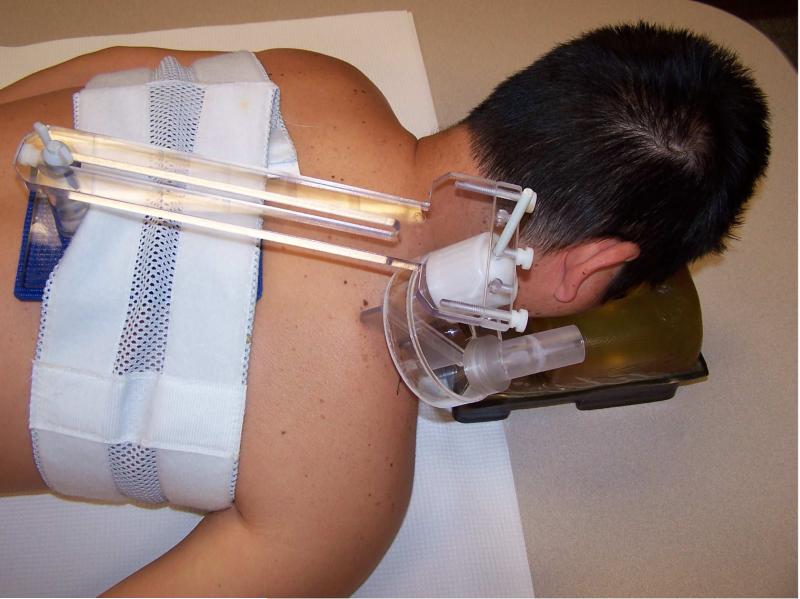
MRE imaging was performed within an hour of a subject's examination in a manner that has been described previously.31, 32 In brief, subjects lay prone with their torsos within the coil of a 1.5T MRI unit a. A pneumatically driven applicator oriented perpendicularly to the long axis of the taut band and 2 cm lateral to its palpated trigger point was used to introduce 150 Hz vibrations into the studied muscle (Figure 1). The MRE scan plane was adjusted to pass through the muscle's principal plane of the muscle (Figure 2). MRE images were collected using a 17.8 cm (7-inch) diameter surface coilb with a gradient-echo MRE pulse sequence gated to the transducer's vibration. Wave propagation was visualized with 8 equally offset images across a vibration-cycle.
Figure 1.
Apparatus setup for the MRE examination.
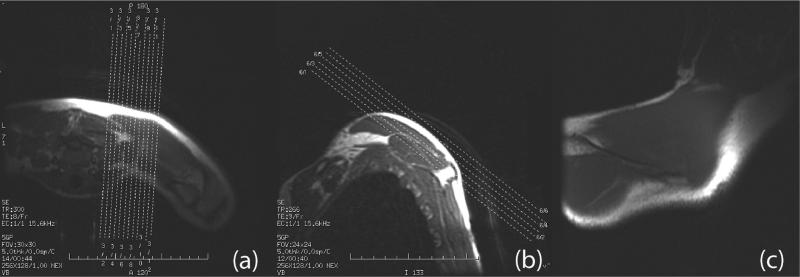
Figure 2.
Scout planes that lead to the final MRE scan plane. (a) Oblique scout planes made on the axial images; (b) further scout planes made on an oblique axis. (c) The final scout image.
MRE Image Analysis
Image analysis of the raw MRE wave data was accomplished using a matched filter algorithm in a manner that has been previously described.16, 33 Muscle stiffness was calculated at the voxel (i.e., a three-dimensional pixel) level using an adaptive smoothed matched filter and its second derivative to solve the Helmholtz wave equationc.
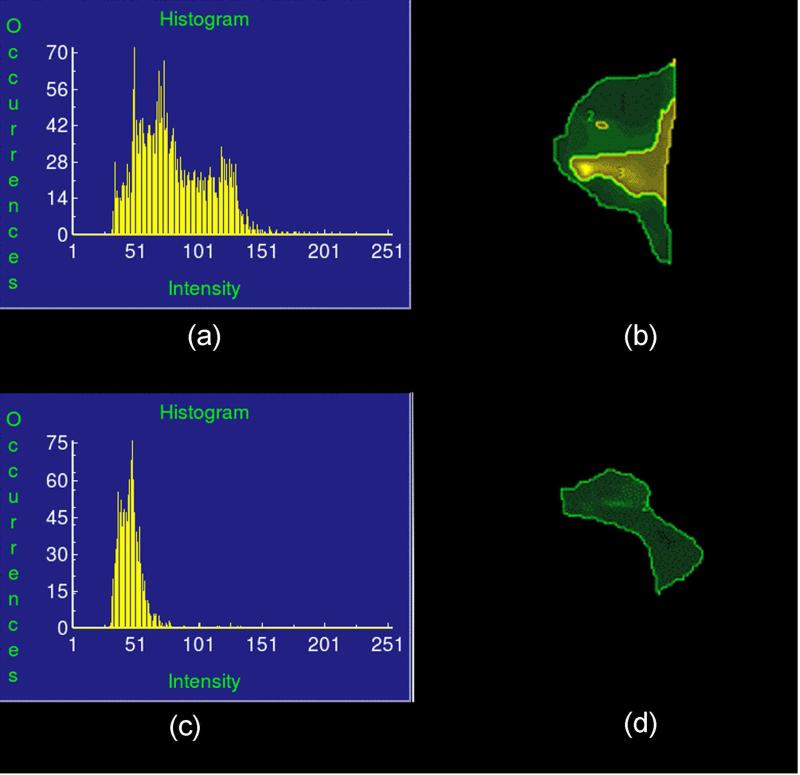
Taut bands in the resulting “elastograms” were then identified in the following manner: a broad region of interest (ROI) was first selected and the elastogram cropped to encompass the region in which shear wave propagation was observed in the raw MRE wave image data. A stiffness histogram of the region, based on the elastogram, was then analyzed using an automated thresholding process,d as described in the next paragraph. Two maximum-likelihood thresholds were then identified and utilized to divide the stiffness range into three statistically significant classes: Class 1 was the taut band itself, Class 2 the surrounding muscle tissue, and Class 3 non-muscular background. In addition, a third maximum-likelihood threshold was identified to exclude occasionally observed signal artifacts and outlier spots from the analysis. The corresponding ROI for each stiffness class was then identified and the taut band region determined. The ratio (ζ) of the mean stiffness in the taut band region divided by the mean stiffness of the surrounding muscle tissue was calculated and termed the “stiffness ratio” (Figure 3).
Figure 3.
The automated thresholding process (ANALYZE®, Mayo Clinic, Rochester, MN) to determine the three maximum-likelihood thresholds that divide the stiffness range into four statistically significant classes.
(a) Histogram of a typical case with taut band region and surrounding normal tissue region divided by the thresholding algorithm.
(b) The division of stiffness classes in the region of interest for (a).
(c) Histogram of a typical case which no threshed can be identified in the region of interest (ROI) that divides the stiffness range into any statistically significant classes.
(d) The division of stiffness classes in the region of interest for (c). Based on the thresholding process, the whole ROI is one single class (excluding the image background), thus a homogenous region.
The above mentioned automated thresholding process produces a multidimensional vector that encodes the complex histogram shape. The approach is based on a previously published algorithm that identifies the thresholds that maximize interclass variance in a grayscale image.34
In the event that only two statistically different stiffness classes (i.e., one class being the image background and the other being the muscle) could be identified by the automated thresholding process, the entire ROI was considered as a single homogenous class with a stiffness ratio ζ of 1.
Each elastogram was then categorized using two different methods to assess the presence and stiffness of a taut band. The first method was a two-value system that categorized the elastograms into group I, with no taut band identified (i.e., homogenous with stiffness ratio = 1) and group II, when a taut band was identified. The second grading method was a three-value system that aimed at further characterizing the “severity” of taut bands, with group I having no taut band identified, group II having a taut band with a stiffness ratio below the median of the bands as a group, and group III having a taut band with a stiffness ratio above the median.
Intra-rater Reliability
Subjects were scanned by a single operator (Operator A) and after an initial scan removed from the MRI machine before being repositioned and scanned a second time 10 minutes later. MRE image data collected from the two scans were then analyzed at the end of the study by the same operator.
Inter-rater Reliability
Subjects were first scanned by one operator and removed from the scanner. Ten minutes later they were placed back in the scanner and scanned by a second operator following the protocol described above. Image data was analyzed at the completion of the study by the operator who had collected the images.
The line marking the taut band placed by the physician was used only to guide placement of the vibration applicator prior to the initiation of a scan. The operators did not examine the subjects, and, to avoid possible bias, image analysis was not begun until data collection had been completed for all subjects. The categorized stiffness ratio (both the 2-value categorization and 3-value categorization methods) was used as the final measurements for repeatability analysis.
Statistical Analysis
Inter-rater and intra-rater reliability were estimated by calculating Kappa coefficients, which are reported with their corresponding 95% confidence intervals. Separate analyses were calculated using both the two-value and three-value stiffness categorization methods outlined above. For the two-level scheme, simple Kappa statistics were used, while the three-level classification system was analyzed by calculating weighted Kappa statistics using Cicchetti-Allison weights.35 All analyses were conducted using SAS version 9.2e. The absolute stiffness of the taut band area was compared to that of the surrounding muscle tissue using a paired t-test.
Results
A total of 70 subjects was imaged, but in 5 the wave quality was too poor to be accurately analyzed. Consequently, these 5 subjects were excluded from analysis, yielding an analysis cohort of 65 subjects.
MRE Intra-rater Reliability
Typical MRE wave images and elastograms for an intra-rater reliability subject are shown in Figure 4. Among the 30 intra-rater reliability subjects, 16 (53.3%) were consistently identified as having a taut band in their elastogram while 12 (40.0%) subjects were consistently found to have homogenous stiffness (i.e., no taut band region, or stiffness ratio = 1). Two subjects yielded inconsistent observations, with one scan showing a taut band while the other did not. The Kappa coefficient (0.86, 95% CI [0.68, 1.00]) of the two-value categorized stiffness ratio method is considered ”almost perfect”36, suggesting excellent intra-rater reliability. (Table 1)
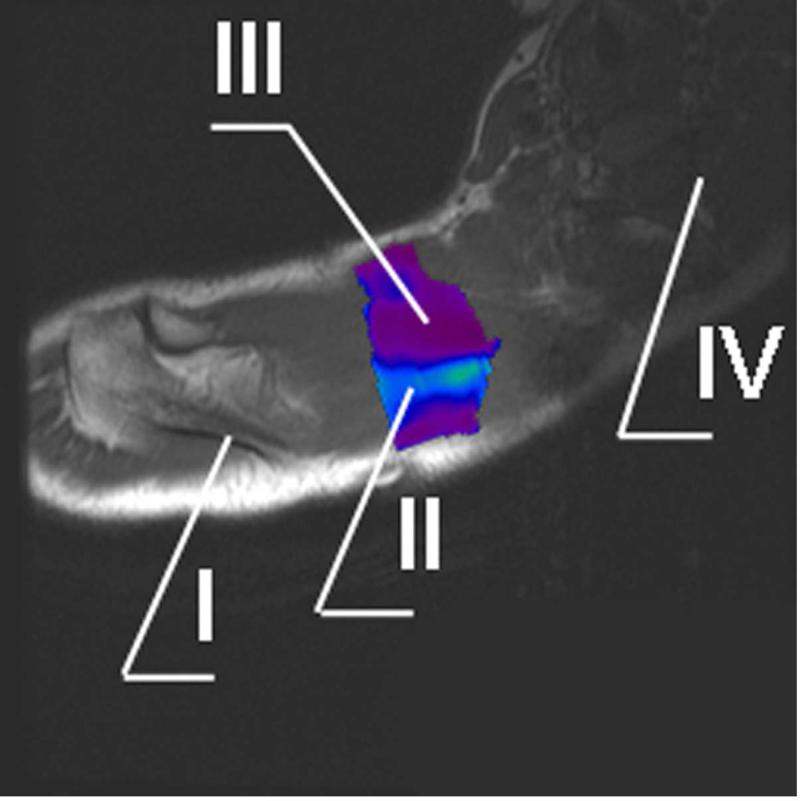
Figure 4.
Typical MRE elastogram of region of interest (ROI) superimposed onto the regular MR image.
I. Spine of scapula
II. Taut band
III. Surrounding muscle tissues
IV. Cervical spine
Table 1.
Intra-rater Agreement
| Second Scan | ||||
|---|---|---|---|---|
| Taut Band identified | ||||
| No | Yes | Total | ||
| Initial Scan Taut Band Identified | No | 12 | 1 | 13 |
| Yes | 1 | 16 | 17 | |
| Total | 13 | 17 | 30 | |
MRE Inter-rater Reliability
Of the 35 subjects evaluated for inter-rater reliability, 25 (71.4%) consistently showed taut band regions, while 9 (25.7%) were consistently found to have homogenous stiffness in the ROI. Only 1 case involved a disagreement between the two operators. The Kappa coefficient of the two-value categorized stiffness ratio method was extremely high (0.93, 95% CI [0.79, 1.00]), connoting “almost perfect” agreement.36 (Table 2)
Table 2.
Inter-rater Agreement
| Operator B Taut Band Identified | ||||
|---|---|---|---|---|
| No | Yes | Total | ||
| Operator A Taut Band Identified | No | 9 | 0 | 9 |
| Yes | 1 | 25 | 26 | |
| Total | 10 | 25 | 35 | |
Taut Band Characteristics
The mean stiffness of the taut bands observed in the subjects in whom a taut band was consistently identified (16 intra-rater and 25 inter-rater subjects) was 11.5 KPa (±2.4 KPa). Stiffness in surrounding muscle fell within a few mm to a mean of 5.8 KPa (±0.9 KPa). Stiffness in trapezii without a taut band was relatively uniform at 6.6 KPa (±2.1 KPa).
In an effort to further characterize the severity of a taut band, the three-value categorization method described above was further studied, with categories II and III defined respectively as being either below or above the median stiffness ratio among all positive subjects. The weighted Kappa coefficients based on the three-value categorized stiffness ratio measurement method were in the “substantial” range for intra-rater reliability (0.75, 95% CI [0.58, 0.93]) as well as for inter-rater reliability (0.68, 95% CI [0.49, 0.87]).
Correlation between Clinician and MRE Findings
All 65 subjects of the current study were identified by skilled musculoskeletal physicians as having taut bands. Of these, 41 (63.1%, 95% CI [50.2%, 74.7%]) had their band presence confirmed by MRE. Only 3 subjects had inconsistent findings among the two successive scans.
Discussion
To our knowledge, this is the first study to assess the intra-rater and inter-rater reliability of an imaging modality with the potential to visualize and quantify myofascial taut bands. The Kappa statistics associated with these findings (0.86 and 0.93, respectively) are considered “almost perfect”.36 As such, they testify to the reliability and reproducibility of this imaging approach.
Our observations confirm a number of other clinical impressions. First, although not dwelt on above, the taut bands observed were oriented in the direction of the muscle fibers and therefore support the clinical expectation that these bands are associated with bundles of muscle fibers rather than with a fiber-independent area of muscle. Second, as clinically expected, taut bands are indeed stiffer than the surrounding muscle in which they are found. Specifically, taut band stiffness was found to have a mean value of 11.5KPa while stiffness in uninvolved muscle only a few mm away dropped to 5.8KPa. The stiffness of trapezii which did not harbor a taut band was 6.6KPa. These differences compare well with that of previous, albeit less extensive, work.31, 32
It is worthwhile to realize that baseline muscle stiffness varies from subject to subject, depending on age, gender, physical activities, etc. Therefore, an absolute stiffness value is neither the best, nor most sensitive, indicator of a taut band's presence. Instead, the stiffness ratio, which is defined as the ratio of stiffness between the identified taut band and the surrounding muscle tissue in the same individual, provides a normalized and, to us, a more reliable indicator of a taut band's nature.
The Kappa statistic is a typical method for evaluating categorized measurements. It is generally accepted that a Kappa coefficient of 0.61-0.80 represents substantial agreement, and values above 0.81 represent “almost perfect” agreement.36 Kappa coefficients of manual palpation investigations of taut band presence vary widely with some reporting acceptable values of agreement between investigators (0.66-1)37, 38 and others finding poor (0.21-0.38)39-41 or inconsistent results.42 In contrast, both the three (no taut band, low stiffness and high stiffness band) and the two-value (band, no band) categorization schemes of our study resulted in high Kappa values and an improved level of reliability relative to that of manual palpation.
One of the more interesting findings in this study was that while the physicians each had more than 20 years’ experience in musculoskeletal medicine, about one third of subjects they identified as having a taut band were not found to have one on imaging. This finding was unexpected. A number of explanations for this discrepancy exist. One is that the physicians’ assessments of a band's presence may have been biased (as one of them suggested) at times by the presence of a myofascial pain pattern. Alternatively, it may be that there are taut bands identifiable by the clinicians that lie below the level of our ability to detect them on MRE. This latter potential is an important issue, and its examination requires attention to the physics of a wave traveling though matter.
More specifically, we are limited by the physics of wave propagation as the choice of the vibration frequency (and hence resolution) used in depends on the nature of the tissue being examined and how rapidly a shear wave attenuates as it passes through it. For example, much attention has been devoted in the medical context to tumors and tissues, such as the fibrotic liver and cartilage. These tissues can be quite stiff, and frequencies on the order of 300 to 600 or even 1000 Hz have been used.43 However, waves attenuate rapidly in softer tissues, such as muscle, at these higher frequencies. This forces a trade-off between the goal of maximal sensitivity and the ability to penetrate tissue. The result is the use of a vibration frequency of about the 150 Hz used in this study. Given this, it turns out that taut bands with widths less than about 10 mm may have their stiffness underestimated. Where this underestimation would blend into an inability to detect their presence is unclear. The deficit, however, would result in clinicians having a higher probability of identifying narrower bands than MRE.
This inference requires future investigation, but as the delay between examination and imaging was no more than an hour, the possibility of a spontaneous loss of a band in someone with longstanding myofascial pain seems unlikely.
Study Limitations
This study has a number of strengths. Among these were the large size of its sample (n=65), its blinding, and the complementary expertise of its physicians in musculoskeletal medicine and its MRE evaluators in MRE image acquisition and analysis.
The study, of course, also has its limitations. A major concern is the lack of a control group. However, this choice was not made lightly. More specifically, we chose the design for this initial investigation for three reasons. First, the driving question for the study's clinicians was whether “If I felt a band, was it there on imaging?” Second, we were comfortable from our previous, but limited, imaging work that if a muscle was not identified clinically as having a taut band, it did not have one on imaging. Third, while it isn't what we would like, cost is a factor in research as well as practice the current study included more than 140 MRE scans, and our budget and machine time constraints simply did not allow for the addition of a large group of control subjects.
It should also be noted that we would have preferred to have blinded the MRE operators prior to their performing the scans. However, we believe that the consequences of not doing so (created by the limited number of trained personnel available to us) were minimal given that we didn't use the markings over the taut bands other than to ensure correct positioning in the scanner. The fact that the operators had seen the skin markings was immaterial given that MRE image interpretations were done blindly and not until after all imaging had been completed.
We are comfortable with the findings but in hindsight realize that a small change in our protocol would have thrown much more light on the discrepancy between physician identification of a taut band's occurrence and its presence on MRE imaging. This change (i.e., rather than the physician merely identifying and marking a taut band during their examination, but also having them qualitatively assess its nature on a three point scale of increasing stiffness (e.g., mild, moderate, and severe)) would have permitted a far deeper study of the correlation of physical findings and their MRE images. We hope to pursue this issue in the future.
Conclusions
Our findings suggest that while clinicians may overestimate, and current MRE methods may underestimate, the presence of taut bands, that these bands exist, can be reliably assessed quantitatively and, do represent localized areas of increased muscle stiffness.
Highlights.
Magnetic Resonance Elastography (MRE) is an MRI technique that can image changes in tissue stiffness
Myofascial taut bands can be imaged and quantified by Magnetic Resonance
Taut bands stiffness is about twice that (11.5 vs 5.8 KPa) of adjacent muscle tissue
Stiffness of muscle adjacent to a taut band is similar to that of uninvolved muscles
Acknowledgement
We thank Ms. Kristin Fruth from the Department of Biomedical Statistics and Informatics, Mayo Clinic, for her assistance with statistical analysis. In addition, we would like to acknowledge and thank Mr. Thomas Hulshizer from the Center for Advanced Imaging Research, Mayo Clinic, for his assistance in MR image data collection.
This study was funded by NIH NCCAM 1-R21-AT-004908-01A2 and NIH EB001981. The Mayo Clinic and Richard E. Ehman hold intellectual property and receive royalties for patents related to the imaging technique used in this study. This study was not previously published, submitted, or presented at a conference.
List of Abbreviations
- MRE
Magnetic Resonance Elastography
- MRI
Magnetic Resonance Imaging
- ROI
Region of Interest
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: There is no financial benefit to any of the other authors, nor is there a conflict of interest.
Signa, 3135 Easton Turnpike, General Electric, Fairfield, CT 06828 USA
Midwest RF, 1050 Walnut Ridge Drive, Hartland, WI 53029 USA
MRELAB®, Mayo Clinic, 200 First Street SW, Rochester, MN 55905 USA
ANALYZE®, Mayo Clinic, 200 First Street SW, Rochester, MN 55905 USA
SAS Institute Inc, 100 SAS Campus Dr, Cary, NC 27513
References
- 1.Gerwin RD. Classification, epidemiology, and natural history of myofascial pain syndrome. Current Pain & Headache Reports. 2001;5(5):412–20. doi: 10.1007/s11916-001-0052-8. [DOI] [PubMed] [Google Scholar]
- 2.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Hauser W, Katz RS, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol. 2011;38(6):1113–22. doi: 10.3899/jrheum.100594. [DOI] [PubMed] [Google Scholar]
- 3.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 2010;62(5):600–10. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]
- 4.Travell J, Simons LS, Simons DG. Travell and Simon's Myofascial Pain and Dysfunction. Vol 1 Upper Half of Body. 2nd ed. Williams & Wilkins; Baltimore: 1999. [Google Scholar]
- 5.Chou LW, Hsieh YL, Kao MJ, Hong CZ. Remote influences of acupuncture on the pain intensity and the amplitude changes of endplate noise in the myofascial trigger point of the upper trapezius muscle. Arch Phys Med Rehabil. 2009;90(6):905–12. doi: 10.1016/j.apmr.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 6.Ge HY, Monterde S, Graven-Nielsen T, Arendt-Nielsen L. Latent myofascial trigger points are associated with an increased intramuscular electromyographic activity during synergistic muscle activation. J Pain. 2014;15(2):181–7. doi: 10.1016/j.jpain.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Quintner JL, Bove GM, Cohen ML. A critical evaluation of the trigger point phenomenon. Rheumatology (Oxford) 2015;54(3):392–9. doi: 10.1093/rheumatology/keu471. [DOI] [PubMed] [Google Scholar]
- 8.Awad E. Interstitial myofibrositis: hypothesis of the mechanism. Arch Phys Med Rehabil. 1973;54(10):449–53. [PubMed] [Google Scholar]
- 9.Brendstrup P, Jespersen K, Asboe H. Morphological and chemical connective tissue changes in fibrositic muscles. Ann Rheum Dis. 1957;16(4):438–40. doi: 10.1136/ard.16.4.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byrt CS, Grof CP, Furbank RT. C4 plants as biofuel feedstocks: optimising biomass production and feedstock quality from a lignocellulosic perspective. J Integr Plant Biol. 2011;53(2):120–35. doi: 10.1111/j.1744-7909.2010.01023.x. [DOI] [PubMed] [Google Scholar]
- 11.Fischer AA. Pressure threshold meter: its use for quantification of tender spots. Arch Phys Med Rehabil. 1986;67(11):836–8. [PubMed] [Google Scholar]
- 12.Fischer AA. Reliability of the pressure algometer as a measure of myofascial trigger point sensitivity. Pain. 1987;28(3):411–4. doi: 10.1016/0304-3959(87)90074-1. [DOI] [PubMed] [Google Scholar]
- 13.Fischer AA. Documentation of myofascial trigger points. Arch Phys Med Rehabil. 1988;69(4):286–91. [PubMed] [Google Scholar]
- 14.Shah JP, Danoff JV, Desai MJ, Parikh S, Nakamura LY, Phillips TM, et al. Biochemicals associated with pain and inflammation are elevated in sites near to and remote from active myofascial trigger points. Arch Phys Med Rehabil. 2008;89(1):16–23. doi: 10.1016/j.apmr.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 15.Shah JP, Phillips TM, Danoff JV, Gerber LH. An in vivo microanalytical technique for measuring the local biochemical milieu of human skeletal muscle. J Appl Physiol (1985) 2005;99(5):1977–84. doi: 10.1152/japplphysiol.00419.2005. [DOI] [PubMed] [Google Scholar]
- 16.Manduca A, Oliphant TE, Dresner MA, Mahowald JL, Kruse SA, Amromin E, et al. Magnetic resonance elastography: Non-invasive mapping of tissue elasticity. Medical Image Analysis. 2001;5:237–54. doi: 10.1016/s1361-8415(00)00039-6. [DOI] [PubMed] [Google Scholar]
- 17.Muthupillai R, Rossman PJ, Lomas DJ, Greenleaf JF, Riederer SJ, Ehman RL. Magnetic resonance imaging of transverse acoustic strain waves. Magn Reson Med. 1995;36:266–74. doi: 10.1002/mrm.1910360214. [DOI] [PubMed] [Google Scholar]
- 18.Bensamoun SF, Ringleb SI, Littrell L, Chen Q, Brennan M, Ehman RL, et al. Determination of thigh muscle stiffness using magnetic resonance elastography. Journal of Magnetic Resonance Imaging. 2006;23(2):242–7. doi: 10.1002/jmri.20487. [DOI] [PubMed] [Google Scholar]
- 19.Debernard L, Robert L, Charleux F, Bensamoun SF. Characterization of muscle architecture in children and adults using magnetic resonance elastography and ultrasound techniques. Journal of Biomechanics. 2011;44(3):397–401. doi: 10.1016/j.jbiomech.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 20.Sack I, Bernarding J, Braun J. Analysis of wave patterns in MR elastography of skeletal muscle using coupled harmonic oscillator simulations. Magnetic Resonance Imaging. 2002;20(1):95–104. doi: 10.1016/s0730-725x(02)00474-5. [DOI] [PubMed] [Google Scholar]
- 21.Uffmann K, Maderwald S, Ajaj W, Galban CG, Mateiescu S, Quick HH, et al. In vivo elasticity measurements of extremity skeletal muscle with MR elastography. NMR in Biomedicine. 2004;17(4):181–90. doi: 10.1002/nbm.887. [DOI] [PubMed] [Google Scholar]
- 22.Ballyns JJ, Shah JP, Hammond J, Gebreab T, Gerber LH, Sikdar S. Objective sonographic measures for characterizing myofascial trigger points associated with cervical pain. Journal of Ultrasound in Medicine. 2011;30(10):1331–40. doi: 10.7863/jum.2011.30.10.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sikdar S, Shah JP, Gebreab T, Yen RH, Gilliams E, Danoff J, et al. Novel applications of ultrasound technology to visualize and characterize myofascial trigger points and surrounding soft tissue. Arch Phys Med Rehabil. 2009;90(11):1829–38. doi: 10.1016/j.apmr.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bennett RM, Goldenberg DL. Fibromyalgia, myofascial pain, tender points and trigger points: splitting or lumping? Arthritis Res Ther. 2011;13(3):117. doi: 10.1186/ar3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chou LW, Kao MJ, Lin JG. Probable mechanisms of needling therapies for myofascial pain control. Evid Based Complement Alternat Med. 2012;2012:705327. doi: 10.1155/2012/705327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ge HY, Fernandez-de-Las-Penas C, Yue SW. Myofascial trigger points: spontaneous electrical activity and its consequences for pain induction and propagation. Chin Med. 2011;6:13. doi: 10.1186/1749-8546-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ge HY, Wang Y, Fernandez-de-las-Penas C, Graven-Nielsen T, Danneskiold-Samsoe B, Arendt-Nielsen L. Reproduction of overall spontaneous pain pattern by manual stimulation of active myofascial trigger points in fibromyalgia patients. Arthritis Res Ther. 2011;13(2):R48. doi: 10.1186/ar3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ge HY, Arendt-Nielsen L. Latent myofascial trigger points. Curr Pain Headache Rep. 2011;15(5):386–92. doi: 10.1007/s11916-011-0210-6. [DOI] [PubMed] [Google Scholar]
- 29.Fischer AA, Muller K, Scholtissek C. Specific inhibition of the synthesis of influenza virus late proteins and stimulation of early, M2, and NS2 protein synthesis by 3-deazaadenosine. Virology. 1990;177(2):523–31. doi: 10.1016/0042-6822(90)90517-u. [DOI] [PubMed] [Google Scholar]
- 30.Simons DG, Travell J. Myofascial origins of low back pain. 1. Principles of diagnosis and treatment. Postgraduate Medicine. 1983;73(2):66, 8–70. doi: 10.1080/00325481.1983.11697756. [DOI] [PubMed] [Google Scholar]
- 31.Chen Q, Basford JR, An KN. Ability of Magnetic Resonance Elastography to Assess Taut Bands. Clinical Biomechanics. 2008;23(5):7. doi: 10.1016/j.clinbiomech.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Q, Bensamoun SF, Basford JR, Thompson J, An KN. Identification and Quantification of Myofascial Taut Bands with Magnetic Resonance Elastography. Arch Phys Med Rehabil. 2007;88:1658–61. doi: 10.1016/j.apmr.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 33.Manduca A, Muthupillai R, Rossman PJ, Greenleaf JF, Ehman RL. Image processing for magnetic resonance elastography. SPIE Medical Imaging. 1996;2710:616–23. [Google Scholar]
- 34.Reddi SS, Rudin SF, Keshaven HR. An optimal multiple threshold scheme for imaging segmentation. IEEE Transactions on Systems, Man and Cybernetics. 1984;SMC-14:661–5. [Google Scholar]
- 35.Byrt T, Bishop J, Carlin JB. Bias, prevalence and kappa. J Clin Epidemiol. 1993;46(5):423–9. doi: 10.1016/0895-4356(93)90018-v. [DOI] [PubMed] [Google Scholar]
- 36.Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med. 2005;37(5):360–3. [PubMed] [Google Scholar]
- 37.Al-Shenqiti AM, Oldham JA. Test-retest reliability of myofascial trigger point detection in patients with rotator cuff tendonitis. Clin Rehabil. 2005;19(5):482–7. doi: 10.1191/0269215505cr791oa. [DOI] [PubMed] [Google Scholar]
- 38.Njoo KH, Van der Does E. The occurrence and inter-rater reliability of myofascial trigger points in the quadratus lumborum and gluteus medius: a prospective study in non-specific low back pain patients and controls in general practice. Pain. 1994;58(3):317–23. doi: 10.1016/0304-3959(94)90125-2. [DOI] [PubMed] [Google Scholar]
- 39.Hsieh CY, Hong CZ, Adams AH, Platt KJ, Danielson CD, Hoehler FK, et al. Interexaminer reliability of the palpation of trigger points in the trunk and lower limb muscles. Arch Phys Med Rehabil. 2000;81(3):258–64. doi: 10.1016/s0003-9993(00)90068-6. [DOI] [PubMed] [Google Scholar]
- 40.Lew PC, Lewis J, Story I. Inter-therapist reliability in locating latent myofascial trigger points using palpation. Manual Therapy. 1997;2(2):87–90. doi: 10.1054/math.1997.0289. [DOI] [PubMed] [Google Scholar]
- 41.Riddle DL, Rothstein JM. Intertester reliability of McKenzie's classifications of the syndrome types present in patients with low back pain. Spine (Phila Pa 1976) 1993;18(10):1333–44. doi: 10.1097/00007632-199308000-00013. [DOI] [PubMed] [Google Scholar]
- 42.Bron C, Franssen J, Wensing M, Oostendorp RA. Interrater reliability of palpation of myofascial trigger points in three shoulder muscles. Journal of Manual & Manipulative Therapy. 2007;15(4):203–15. doi: 10.1179/106698107790819477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lopez O, Amrami KK, Manduca A, Ehman RL. Characterization of the dynamic shear properties of hyaline cartilage using high-frequency dynamic MR elastography. Magn Reson Med. 2008;59(2):356–64. doi: 10.1002/mrm.21474. [DOI] [PubMed] [Google Scholar]