Abstract
Increases in expression of α4βδ GABAA receptors (GABARs), triggered by fluctuations in the neurosteroid THP (3α-OH-5α[β]-pregnan-20-one), are associated with changes in mood and cognition. We tested whether α4βδ trafficking and surface expression would be altered by in vitro exposure to flumazenil, a benzodiazepine ligand which reduces α4βδ expression in vivo. We first determined that flumazenil (100 nM – 100 μM, IC50=~1 μM) acted as a negative modulator, reducing GABA (10 μM)-gated current in the presence of 100 nM THP (to increase receptor efficacy), assessed with whole cell patch clamp recordings of recombinant α4β2δ expressed in HEK-293 cells. Surface expression of recombinant α4β2δ receptors was detected using a 3XFLAG reporter at the C-terminus of α4 (α4F) using confocal immunocytochemical techniques following 48 h exposure of cells to GABA (10 μM) + THP (100 nM). Flumazenil (10 μM) decreased surface expression of α4F by ~60%, while increasing its intracellular accumulation, after 48 h. Reduced surface expression of α4β2δ after flumazenil treatment was confirmed by decreases in the current responses to 100 nM of the GABA agonist gaboxadol. Flumazenil-induced decreases in surface expression of α4β2δ were prevented by the dynamin blocker, dynasore, and by leupeptin, which blocks lysosomal enzymes, suggesting that flumazenil is acting to increase endocytosis and lysosomal degradation of the receptor. Flumazenil increased the rate of receptor removal from the cell surface by 2-fold, assessed using botulinum toxin B to block insertion of new receptors. These findings may suggest new therapeutic strategies for regulation of α4β2δ expression using flumazenil.
Keywords: flumazenil, GABA-A receptor, alpha-4, delta, receptor trafficking, pregnanolone
1. Introduction
The α4βδ GABAA receptor (GABAR) is a pentameric membrane protein which gates a Cl− conductance and is one of many possible subtypes which mediate inhibition in the brain (Olsen and Sieghart, 2009). This receptor expresses extrasynaptically (Wei et al., 2003) where it underlies a tonic inhibitory current (Smith et al., 2009). α4βδ GABARs normally have low expression in the CNS (Pirker et al., 2000; Wisden et al., 1992), but are capable of a high degree of plasticity. In vivo studies have shown that naturally occurring fluctuations in neuroactive steroids such as THP (allopregnanolone or 3α-OH-5α[β]-pregnan-20-one), a metabolite of the ovarian steroid progesterone (Compagnone and Mellon, 2000), can increase surface expression of this receptor at puberty (Shen et al., 2007), across the estrous cycle (Lovick et al., 2005; Maguire et al., 2005) and post-partum (Maguire and Mody, 2009; Sanna et al., 2009), in areas such as CA1 hippocampus, dentate gyrus and the midbrain central grey, as can direct administration of exogenous steroid to female rodents (Smith et al., 2006). Increased surface expression of α4βδ GABARs increases tonic inhibition (Shen et al., 2010), which has been shown to generate greater inhibitory current than phasic inhibition (Bai et al., 2000). This receptor is also a sensitive target for low dose alcohol (Sundstrom-Poromaa et al., 2002; Wallner et al., 2003) in cells which have high intracellular levels of protein kinase C-δ (Messing et al., 2007). Increased expression of α4β2δ GABARs produced by hormone fluctuations in vivo can in many cases be correlated with alterations in anxiety, seizure susceptibility as well as learning deficits, suggesting that these receptors may play an important role in pathophysiological conditions (Smith et al., 2007).
The biophysical and pharmacological properties of α4β2δ and α1β2/3δ GABARs are unique in that these receptors have a high sensitivity to GABA (EC50=0.5 μM) (Brown et al., 2002; Sundstrom-Poromaa et al., 2002; Zheleznova et al., 2008), which is, however, a partial agonist at these receptors. Thus, modulators such as THP and the related THDOC ((3α,5β)-3,21-dihydroxypregnan-20-one) increase receptor efficacy when acutely applied (Bianchi and Macdonald, 2003; Zheleznova et al., 2008), due to increases in the mean open time of the channel by the addition of a third longer open state (Bianchi and Macdonald, 2003). Our previous work suggests that prolonged exposure to drugs which increase receptor efficacy are also associated with increases in cell surface expression of α4β2δ (Kuver et al., 2012). Hence, a 48 h exposure of HEK-293 cells to THP in combination with GABA results in higher surface expression of α4β2δ GABAR than GABA alone, as do agonists (Bianchi and Macdonald, 2003; Brown et al., 2002) with increased efficacy at α4β2δ GABAR compared to GABA, gaboxadol (THIP or 4,5,6,7-tetrahydroisoxazolo(5,4-c)pyridin-3-ol) and β-alanine (Kuver et al., 2012).
α4β2δ GABARs are insensitive to modulation by benzodiazepine (BZ) agonists (Knoflach et al., 1996; Wafford et al., 1996). BZ agonists bind between the α and γ subunits (Sigel, 2002); thus binding of these agonists would be prevented in receptors such as α4β2δ which lack a γ subunit. In addition, an arginine at position 99 in the α4 (rather than histidine as found in α1–3, 5) also precludes binding of BZ agonists (Knoflach et al., 1996; Wieland et al., 1992). However, recent studies suggest that there is a modified BZ binding site on α4β3δ GABAR which can accommodate binding of other BZ ligands, including the BZ antagonist flumazenil (RO15-1788) and the BZ partial inverse agonist RO15-4513 (Hanchar et al., 2006). Binding of H3-RO15-4513 has been established in crude membrane fractions of recombinant α4β2δ GABARs expressed in HEK-293T cells, where it produces high affinity saturable binding (Hanchar et al., 2006). Flumazenil is effective as a competitive inhibitor of this binding, suggesting that in contrast to BZ agonists, flumazenil is able to bind to α4β3δ GABAR. Flumazenil is well known as a BZ antagonist at GABARs of the form α[1–3,5]βγ where it has no direct effect on its own, but when applied acutely blocks the effects of other BZ ligands on GABA-gated current and reduces sedation produced by BZ overdose (Olsen and Sieghart, 2009). Conversely, this drug has atypical effects at receptors containing the α4 subunit, such that a 10 μM concentration acutely potentiates current gated by GABA at recombinant α4β1/3γ2 GABARs (Wafford et al., 1996) recorded in the absence of a benzodiazepine agonist.
Recent in vitro studies have suggested that in addition to its acute effects on GABA-gated current, prolonged exposure to flumazenil can also regulate surface expression of GABARs containing α4 or the homologous α6 subunit (Biggio et al., 2007; Zheng et al., 1996), but there are conflicting reports on the direction of the effect of flumazenil on δ subunit expression. Flumazenil has been shown to decrease expression of the α4 subunit (Biggio et al., 2007), which was increased after withdrawal from 100 mM ethanol, when it coexpresses with γ2 (Biggio et al., 2007; Cagetti et al., 2003), without altering δ expression. However, another study showed that in vitro application of 10 μM flumazenil for 4–6 h to cultured cerebellar granule cells increases expression of the δ subunit in association with decreased expression of the homologous α6 subunit (Zheng et al., 1996). In contrast, a recent study from our lab showed that 48 h in vivo treatment with flumazenil reduces hippocampal expression of both α4 and δ subunits, which are increased by chronic treatment of rats with methamphetamine (Shen et al., 2013). Considering these diverse reports of flumazenil’s effects on α4 and δ, the present study sought to directly examine the effect of flumazenil in an isolated system, transfected HEK-293 cells, in order to determine whether flumazenil reduces α4βδ surface expression in vitro as a direct effect by altering receptor trafficking as a result of membrane insertion or endocytosis of the receptor. Although the in vivo approach has physiological relevance, it does not permit determination of the mechanism of flumazenil’s effect on α4βδ expression.
Although not yet tested rigorously, preliminary findings have appeared in abstract form suggesting that 100 nM flumazenil can act as a negative modulator at α4β3δ where it can reduce current gated by an EC20 of GABA (Dunn et al., 2003 abstract). The purpose of the present study was to confirm the effect of flumazenil at α4β2δ GABARs under conditions where their surface expression was increased, in the presence of THP. It was also our goal to test the effect of flumazenil on cell surface expression of α4β2δ GABARs using immunocytochemical techniques with a 3XFLAG-tagged α4 in HEK-293 cells following treating with GABA plus THP at concentrations we have shown produce maximal expression of the receptor (Kuver et al., 2012). This is a model of α4β2δ surface expression that produces consistent results in studies of receptor regulation (Kuver et al., 2012). Our findings suggest that flumazenil is a negative modulator at α4β2δ GABARs, reducing current generated by GABA plus THP. Sustained application of the drug can decrease cell surface expression of the α4β2δ GABARs which are increased by 48 h treatment of the cells with GABA plus THP.
2. Materials and Methods
2.1. Cell culture
This study used human embryonic kidney (HEK) 293 cells (ATCC, Manassas, VA), maintained in Dulbecco’s Modified Eagle’s Medium (DMEM/F-12, Invitrogen, Carlsbad, CA) which was supplemented with 10% fetal bovine serum (FBS, Sigma, St. Louis, MO), penicillin (100 IU/ml) and streptomycin (100μg/ml) (Invitrogen, Carlsbad, CA). Cells were grown on MatTek glass bottom dishes (MatTek Corp, Ashland, MA) at 37°C in a humidified incubation chamber (5% CO2, 95% O2).
2.2. cDNA
A mouse α4-3XFLAG (α4F) reporter was used for all studies. This construct uses three FLAG sequences (DYKDDDDK) at the C terminus of the GABAR α4 subunit for immunocytochemical detection with a high signal:noise ratio. It is expressed in a CMV-14 expression vector (Sigma, St. Louis, MO) and yields functional expression of the full receptor when transfected with β2 and δ cDNA. This construct has been described in a previous study (Kuver et al., 2012), where the GABA and gaboxadol concentration-responses of α4Fβ2δ and α4β2δ were shown to be indistinguishable, suggesting that the FLAG tag does not alter functional characteristics of the receptor.
cDNA for GABAR subunits mouse α4 (N.L. Harrison, Columbia U., New York), rat β2 (J. Bracamontes, Washington U, St. Louis) and human δ (K. Wafford, Merck, Sharp and Dohme, UK) were used for all studies, expressed in pcDNA3.1. (Mouse, rat and human cDNA sequences for β2 are nearly identical.)
2.3. Transfection
Cells were transfected with α4F, β2 and δ cDNA (1:1:1; α4(F):β2:δ) using a Nucleofector (Amaxa/Lonza, Walkersville, MD) with reagents and protocols optimized for HEK-293 cells (5 μg of cDNA was used per 100 μl reagent). In some cases, cells were also co-transfected with 2 μg eGFP cDNA (Amaxa/Lonza) for visualization of transfected cells under fluorescence microscopy where the transfection efficiency was consistently 70–80%. The final surface density of plated HEK-293 cells was 10,000 cells/plate.
2.4. Drug administration
Transfected HEK-293 cells were treated with GABA (10 μM) plus THP (pregnanolone or 3α-OH-5β-pregnan-20-one, 100 nM) or vehicle (0.01% dimethylsulfoxide) for 48 h. In some cases, they were also treated with flumazenil (10 μM) for varying lengths of time (0.5, 6, 24 or 48 h) culminating at the end of the 48 h GABA plus THP exposure period (a 2 d experiment). In initial studies, the 48 h GABA plus THP exposure period preceded flumazenil administration (a 4 d experiment). However, the 2 d experiment presented in this paper was chosen over the 4 d experiment because both produced similar results. In other cases they were treated with both flumazenil (10 μM) and/or botulinum toxin B (5 nM) across a time-course (1, 2, 4, 6 or 24 h). In all cases, cells were harvested at the end of the treatment so that all groups would be processed in parallel (Inset, Fig. 2). Other drugs used included leupeptin, an inhibitor of lysosomal degradation which has been shown to enter cells (Mirabilla et al., 2011; Lee et al., 2010) and dynasore, a cell-permeable inhibitory of dynamin, which prevents endocytosis (Macia et al., 2006). GABA, leupeptin, dynasore and flumazenil were from Sigma Chemical Co. (St. Louis, MO), THP was from Steraloids, Inc. (Newport, RI) and botulinum toxin B was from List Biological Laboratories, Inc. (Campbell, CA).
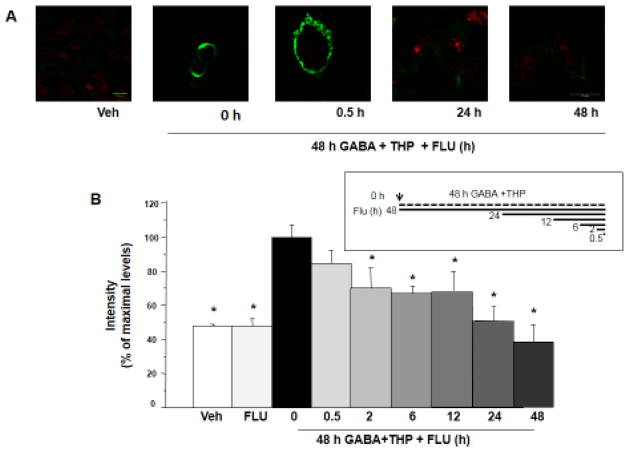
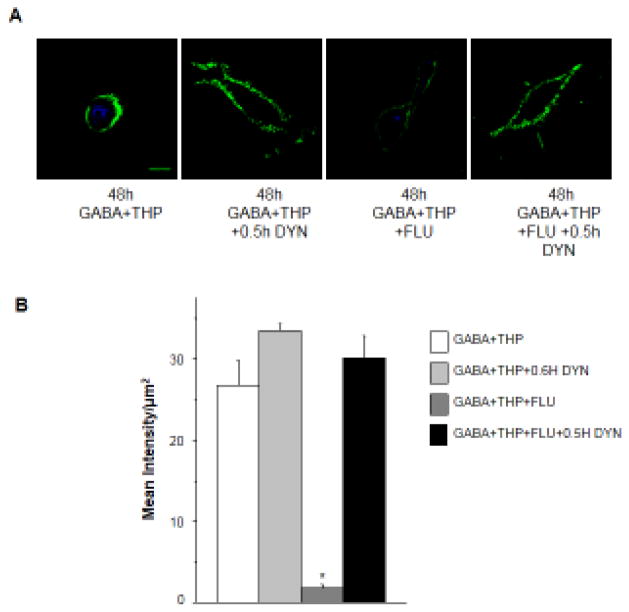
Figure 2. Flumazenil reduces surface expression of α4Fβ2δ after 48 h GABA plus THP exposure.
A, B, Cell surface expression of α4Fβ2δ expressed in HEK-293 cells. Cells were treated with vehicle (0.01% DMSO) or GABA(10 μM)+THP(100 nM) and harvested after 48 h. Inset, Flumazenil (FLU, F, 10 μM) was added at various times after GABA+THP treatment, so that all cells were harvested at the same time. FLAG expression was assessed with immunofluorescence (488 λ, F(ab′)2 fragment IgG). A, Representative images. Surface fluorescence intensity was greatest after 48 h GABA+THP treatment and reduced by FLU exposure. Cell stain, TO-PRO-3; scale bar, 10 μm. B, Averaged data. (n=9; *F(8,63)=13.23, P<0.0001, ANOVA; P<0.05 vs. 48 h GABA + THP, pos-hoc Tukey’s test). Scale, 10 μm.
2.5. Immunocytochemistry
2.5.1. Surface expression of α4F
Initially, live cells were probed for surface expression of α4F under non-permeable conditions which required that all steps be carried out on ice (Eshaq et al., 2010). Cells were first incubated with a mouse (Ms) monoclonal anti-FLAG M2 primary antibody (1:50–1:100) (Sigma, St. Louis, MO) followed by a goat (Gt) anti-Ms IgG F(ab′)2 fragment conjugated to Alexa Fluor 488 (1:500, Molecular Probes, Grand Island, NY). Cells were then fixed with 4% paraformaldehyde. In some cases, the cells were then stained with either DAPI (1:1000), a nuclear stain used as a cell marker, or permeabilized with 0.1% Triton X-100 for 5 minutes, and incubated with TO-PRO 3 (1:1000) a cell and nuclear marker (Molecular Probes) for 30 minutes. In the applicable figures, DAPI staining is in blue and TO-PRO 3 in red.
2.5.2. Intracellular expression of α4F
In order to detect intracellular immunostaining, cells were probed for α4F under permeabilized conditions at room temperature. Cells were initially fixed with 4% paraformaldehyde/4% sucrose and permeabilized with 0.1% Triton X-100. Cells were then blocked with 10% BSA and incubated with a Ms monoclonal anti-FLAG M2 primary antibody (1:100) (Sigma, St. Louis, MO) followed by a Gt anti-Ms IgG Alexa-488 secondary antibody (1:200) to detect α4F, and a Rb anti-calnexin antibody (1:500) (AbCam, Cambridge, MA) followed by Gt anti-Rb IgG Alexa-546 secondary antibody (1:100) to detect the endoplasmic reticulum (ER). All secondary antibody-Alexa fluor conjugates were from Molecular Probes (Grand Island, NY).
2.5.3. Immunofluorescence analysis
Images were visualized and captured on the Zeiss 710 or 510 inverted confocal microscope at 63X oil or 40X (Microscopy Core of NYU Langone Medical Center, NY, NY). The Image J program (NIH) (Kuver et al., 2012) was used for analysis of the immunofluorescence intensity of representative cells using the ROI (Region of Interest) manager in the Zen 2008 Light Edition program. After the threshold intensity was established, background fluorescence was subtracted from the image, and the intensity of fluorescent pixels around the circumference of the cell was calculated as the integrated density/total area (mean intensity/μm2). Images were captured of three cells per plate/group and the experiment repeated 3 times for each determination. Analysis of intracellular labeling was assessed using the same plane of focus that revealed ER staining.
2.6. Assessment of the rate of receptor removal from the cell surface
We assessed whether flumazenil increased the rate of removal of surface expressing α4Fβ2δ GABARs after GABA plus THP treatment of transfected HEK-293 cells. To this end, cells were treated with botulinum toxin B (5 nM) for varying lengths of time (1, 3, 5 and 24 or 48 h) to block insertion of newly formed receptors during 48 h exposure to GABA (10 μM) plus THP (100 nM), with or without flumazenil (10 μM). Botulinum toxin B cleaves vesicle associated membrane protein (VAMP/synaptobrevin) and prevents vesicle trafficking to the cell surface (Montecucco and Schiavo, 1995; Rummel 2013), as we have previously shown (Kuver et al., 2012). Cells were harvested at the end of the treatment so that all groups would be processed in parallel. Surface immunofluorescence was plotted as a function of time of exposure to botulinum toxin B. Analysis of the rate of receptor removal was accomplished with the least squares fit to the exponential decay function, y = A1^exp(−x/τ) + y0, where A1 is the initial amplitude minus the steady-state level, y0 is the steady-state level and τ is the decay time constant (Origin 8.5.1, Microcal, Piscataway, NJ).
2.7. Electrophysiology
2.7.1. Whole cell patch clamp
Whole cell currents were recorded from transfected HEK-293 cells in response to GABA (10 μM) plus THP (30 nM) or gaboxadol (100 nM) using voltage clamp techniques at a holding potential of −50 mV on a Nikon Diaphot inverted microscope. The bath perfusion solution contained (in mM): NaCl 120, CsCl 5, CaCl2 2, MgCl2 1, Hepes 10 and glucose 25, pH 7.4, 320 mOsm. Patch pipets (filament-capillary tubes, Sutter Instruments, Novato, CA) were fabricated from borosilicate glass using a Flaming-Brown puller to yield open tip resistances of 3 – 5 MΩ. The pipet solution contained (in mM): N-methyl-D-glucamine chloride 120, Cs4BAPTA 5 (Calbiochem, San Diego, CA), Mg-ATP 5, and an ATP regeneration system (20 mM Tris phosphocreatine and creatine kinase). Currents were recorded at room temperature (21–22°C) using an Axopatch 1D amplifier (Axon Instruments, Union city, CA) filtered at 2 kHz (four-pole Bessel filter) and detected at 10 kHz (pClamp 8.2).
2.7.2. Pharmacology
Following drug treatments, cells were first incubated in drug-free solution for 1 h prior to voltage clamp recording, which was carried out in the presence of 1 μM ZnCl2 to block any binary receptors (Meera et al., 2011). Flumazenil effects were examined on current generated by GABA (10 μM) plus THP (30 nM) to increase receptor efficacy as a model system with which to best determine potential inhibitory effects of flumazenil. This combined drug application was used to increase receptor efficacy because GABA alone generated insufficient current to reliably interpret the effect of flumazenil. The addition of THP generated robust current, and permitted consistent findings with flumazenil across a concentration range. Drugs were applied using a solenoid-controlled micropipette array 50 μm from the cell (Smith et al., 1998) which allowed for rapid onset of drug application (20 ms). Responses to drugs were recorded for 5–10 s. Flumazenil (100 nM – 100 μM) was pre-applied in the bath perfusion (30 s) and concomitantly with GABA plus THP when testing its effects on the current.
2.7.3. Data analysis
Analysis of peak current was accomplished with pClamp 10.1 (Axon Instruments, Union City, CA) and Origin (Microcal, Piscataway, NJ) software packages. In all cases, 4–5 current traces were averaged for each group. Flumazenil effects on the GABA-gated current were expressed as a ratio relative to current generated by 10 μM GABA plus 30 nM THP. A concentration-response curve was generated for flumazenil effects on GABA plus THP-generated current using the least square fit to a logistic (sigmoidal) function of the form y = A2 + (A1 − A2)(1 + (x/x0)^p, where A1,2 are the initial and final values, respectively, x0 is the IC50 and p is the Hill coefficient.
2.8. Statistics
Data are depicted as the mean ± SEM (Origin 7, Microcal, Piscataway, NJ). The analysis of variance (ANOVA) was used to evaluate significant differences between >2 groups, followed by a post-hoc Tukey’s test. The Student’s t-test was used to compare differences between 2 groups. In both cases, significance was established with a P<0.05.
3. Results
3.1. Flumazenil reduces GABA-gated current
In contrast to its role as a BZ antagonist at α1β2γ2 GABAR, flumazenil has been shown to act as a positive GABA modulator at α4βγ2 GABAR (Biggio et al., 2007; Wafford et al., 1996). Although the effect of flumazenil on δ-containing receptors has yet to be established conclusively, preliminary reports in abstract form have suggested that 100 nM of this BZ antagonist act as a negative GABA modulator at α4β2δ (Dunn et al., 2003 abstract). In order to confirm these findings, we examined the effect of flumazenil on current gated by α4β2δ in response to GABA plus THP. This combined treatment was used because there was insufficient current generated by GABA alone, which is a partial agonist at α4βδ GABARs (Brown et al., 2002), to see consistent effects of flumazenil. THP increases receptor efficacy (Bianchi and Macdonald, 2003) and generated sufficient current to observe consistent results with flumazenil.
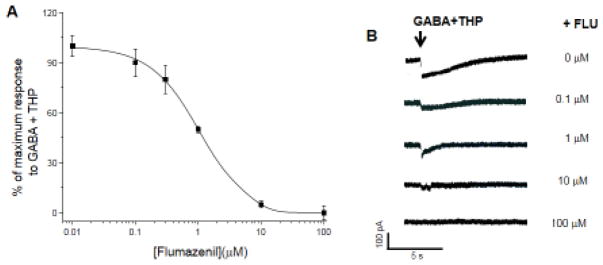
Whole-cell patch clamp recordings of HEK-293 cells transfected with α4β2δ show that flumazenil produced a concentration-dependent decrease in GABA-gated current (100 nM – 100 μM), with an IC50 =~1 μM (Fig. 1A, B). These findings indicate that low concentrations of flumazenil act as a negative modulator at α4β2δ, evaluated in the presence of THP. This newly discovered effect of flumazenil to decrease the amplitude of GABA-gated current at the extrasynaptic α4β2δ GABAR raises the possibility that it could influence the surface expression of this receptor.
Figure 1. Flumazenil reduces current gated by α4β2δ GABAA receptors.
A, Effect of FLU on GABA (10 μM) current gated by α4β2δ GABARs in the presence of 30 nM THP, included to increase receptor efficacy. Arrow, start of ligand application. FLU produced a concentration-dependent (0.01 – 100 μM) decrease in GABA current (IC50=1 μM). B, Representative traces. n=4–5 cells/group.
3.2. Flumazenil down-regulates surface expression of α4(3XFLAG)β2δ surface expression
Our previous findings established the effectiveness of the positive GABA modulator, THP, in increasing cell surface expression of α4β2δ GABARs (Kuver et al., 2012). Therefore, we tested whether flumazenil, as a negative modulator of α4β2δ GABARs (Fig. 2), would decrease surface expression of recombinant α4(3XFLAG)β2δ expressed in HEK-293 cells using our previously established model of maximal α4β2δ surface expression using GABA plus THP (Kuver et al., 2012).
To this end, we performed a time-based protocol (0.5, 2, 6, 12, 24, 48 h) of flumazenil (10 μM) treatment during a 48 h incubation of the HEK-293 cells with GABA (10 μM) plus THP (100 nM) to determine possible effects on surface expression of α4Fβ2δ. We have previously shown that 48 h exposure of HEK-293 cells with GABA plus THP at these concentrations produces peak levels of α4Fβ2δ surface expression (Kuver et al., 2012); surface expression levels are also increased by 1 μM GABA plus THP. Thus, we used this protocol as an optimized model to increase cell surface expression of α4Fβ2δ. All timed flumazenil treatments terminated at the conclusion of the 48 h incubation period (Fig. 2 timeline) so that cell processing for all groups proceeded in parallel.
Our results show that 10 μM flumazenil significantly (P<0.05) decreased cell surface expression produced by GABA plus THP exposure (Fig. 2), an effect first seen after a 2 h flumazenil exposure (initiated after 46 h exposure of cells to GABA plus THP). Maximal decreases in FLAG surface immunofluorescence were noted at 24 h (49.9%) and 48 h (62%) of flumazenil exposure with levels of surface expression not significantly different from cells treated with vehicle or flumazenil alone (Fig. 2A, B). Conversely, 30 min of flumazenil treatment had an insignificant effect on surface expression of the receptor compared to GABA plus THP alone, which increased surface expression 3-fold compared to vehicle (P<0.05). This timecourse of flumazenil effects is similar to our earlier studies, where flumazenil was administered following 48 h GABA plus THP exposure (data not shown). In that case, significant decreases were observed after 2 h flumazenil exposure, with maximal effects at 24–48 h flumazenil exposure.
Exposure of cells to flumazenil alone had no effect on surface expression compared to vehicle (Fig. 2B). Thus, our findings suggest that a negative modulator reduces expression of the α4(3XFLAG)β2δ receptor on the cell surface when expression levels of this receptor are increased by GABA plus THP.
3.3. Gaboxadol-gated current of α4β2δ GABARs decreases after 24–48 h of flumazenil exposure
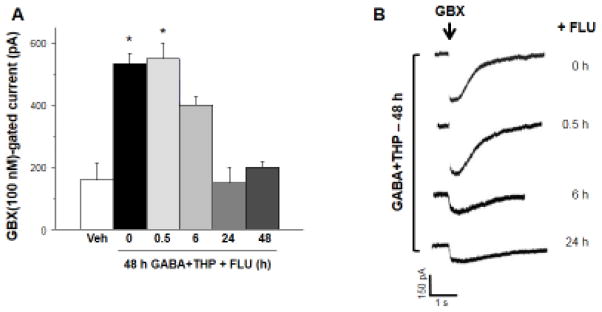
To determine if the decrease in observed cell surface expression of α4β2δ receptors produced by flumazenil reflected a decrease in functional receptors, we recorded responses of recombinant α4β2δ GABARs to 100 nM gaboxadol, a GABA agonist, which, at this concentration is selective for δ-containing GABAR (Brown et al., 2002; Meera et al., 2011). To this end, whole cell voltage clamp recordings were carried out after transfected HEK-293 cells were exposed to 48 h GABA plus THP with various time-based flumazenil treatments (0.5, 2, 6, 24 and 48 h) similar to the protocol used in Figure 2. Gaboxadol-induced currents decreased with increasing exposure to flumazenil beginning with 2 h of exposure (30% decrease, P<0.05), with maximal decreases occurring with 24–48 h of exposure (70% decrease, P<0.05, Fig. 3). The observed decreases in peak response to gaboxadol support our immunocytochemical results indicating that surface expression of α4Fβ2δ decreases following flumazenil administration in the presence of GABA plus THP.
Figure 3. Flumazenil exposure reduces responses of recombinant α4Fβ2δ GABARs to gaboxadol.
Whole cell patch clamp recordings of peak current responses (in pA) to acutely applied gaboxadol (GBX, 100 nM) in cells treated for 48 h with GABA plus THP or vehicle as well as FLU for the indicated times. A, Summary data and B, representative current traces show that long-term FLU treatment (6, 24, 48 h) reduces GBX-gated current. Arrow, start of ~500 ms ligand application. (n=5 cells/group, *F(6,28)=36.7, P<0.0001, ANOVA; P<0.05 vs. 48 h GABA+THP with 48 h FLU, post-hoc Tukey’s test).
3.4. Flumazenil increases the intracellular localization of α4Fβ2δ after GABA plus THP treatment
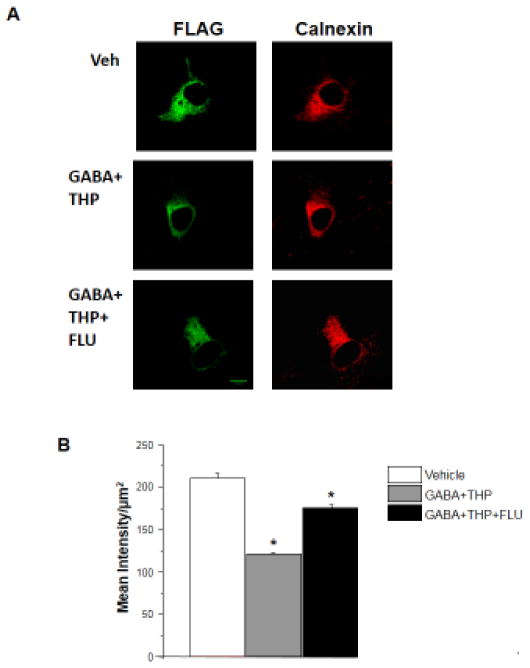
We measured intracellular α4 levels to identify the mechanism for down-regulation of its surface expression by flumazenil. After 48 h of treatment with vehicle or GABA plus THP, with or without flumazenil (48 h), the α4β2δ-transfected HEK cells were probed with an anti-FLAG antibody under permeabilized conditions. Intensity of labeling was determined within the intracellular compartment by using the same plane of focus which revealed ER staining in the merged image. Intracellular expression of the recombinant α4(3XFLAG)β2δ was highest in the control (vehicle) group (P<0.05, Fig. 4). 48 h treatment with GABA plus THP reduced this intracellular labeling by 38.1% (P<0.05) compared to vehicle-treated control. However, concomitant 48 h treatment with flumazenil partially reversed the effect of GABA plus THP treatment, increasing intracellular expression by ~50% compared to GABA plus THP (P<0.05), suggesting that it may have an effect on receptor trafficking or receptor re-cycling as detailed in the discussion.
Figure 4. Retention of α4Fβ2δ in the intracellular compartment after flumazenil administration to GABA plus THP-treated cells.
A, Representative images (40 X) showing (left to right) immunofluorescence for intracellular α4F (488 λ) and endoplasmic reticulum (ER, 546 λ) after 48 h treatment of transfected HEK-293 cells with GABA + THP with or without flumazenil (FLU, 10 μM). (Control, 0.01% DMSO) 48 h FLU (lower panel) increased intracellular α4F. B, Averaged data of the mean intracellular α4F intensity. (n=9, *F(2,6)=162.5, P<0.0001, ANOVA; P<0.05 vs. other groups, post-hoc Tukey’s test). Scale, 10 μm.
3.5. Flumazenil-induced reduction in surface levels of α4Fβ2δ after 48 h GABA plus THP are reversed by inhibiting dynamin
Internalization via the clathrin-coated pit has been considered the primary mechanism for reducing GABAAR surface expression at synaptic (Kittler et al., 2000) and extrasynaptic sites (Joshi and Kapur, 2009), but this mechanism has not yet been investigated in α4β2δ receptor down-regulation. To test if this same mechanism is responsible for down-regulation of extrasynaptic α4β2δ membrane receptors by flumazenil we used dynasore (80 μM) to block internalization through dynamin inhibition in HEK-293 cells. Dynasore acts immediately to block the dynamin protein (Macia et al., 2006), so an exposure time of 30 min has been used in internalization studies of membrane receptors (Oh et al., 2012). Cells were treated with dynasore during the final 30 min of a 48 h exposure of transfected HEK-293 cells to GABA plus THP with or without flumazenil, because GABA plus THP can increase α4β2δ surface expression within 20 min (Kuver et al., 2012). In fact, 30 min of dynasore treatment prevented the decrease in cell surface expression of α4Fβ2δ produced by flumazenil on GABA plus THP-treated cells (Fig. 5). Dynasore treatment resulted in more than a 10-fold increase in surface FLAG labeling compared to flumazenil-treated GABA plus THP cells (P<0.05). Surface labeling at this time-point was not significantly different than that observed for the GABA plus THP group not treated with flumazenil.
Figure 5. Dynasore effects on α4Fβ2δ surface expression after flumazenil administration to HEK-293 cells treated with GABA plus THP for 48 h.
Surface labeling of α4Fβ2δ expressed in HEK-293 cells after blockade of endocytosis with dynasore (DYN, 80 μM). Cells were treated for 48 h with vehicle (0.01% DMSO) or GABA (10 μM) + THP (100 nM) with or without flumazenil (FLU, 10 μM). DYN was added for 0.5 h at the end of the 48 h incubation. A, Representative (40 X) confocal images. Upper panel (left to right), Cells treated with GABA + THP alone, GABA + THP plus DYN, GABA+THP plus FLU; GABA+THP plus DYN and FLU. DYN prevented the decrease in surface FLAG immunofluorescence produced by FLU after 48 h GABA + THP treatment. Representative images were enhanced for visualization purposes (brightness (40%) and contrast (60%)). Cell stain, DAPI (546 λ), scale bar, 10 μm. B, Averaged data. (n=9, *F(3,32)=45.6, P<0.0001, ANOVA; P<0.05 vs. other groups, post-hoc Tukey’s test)
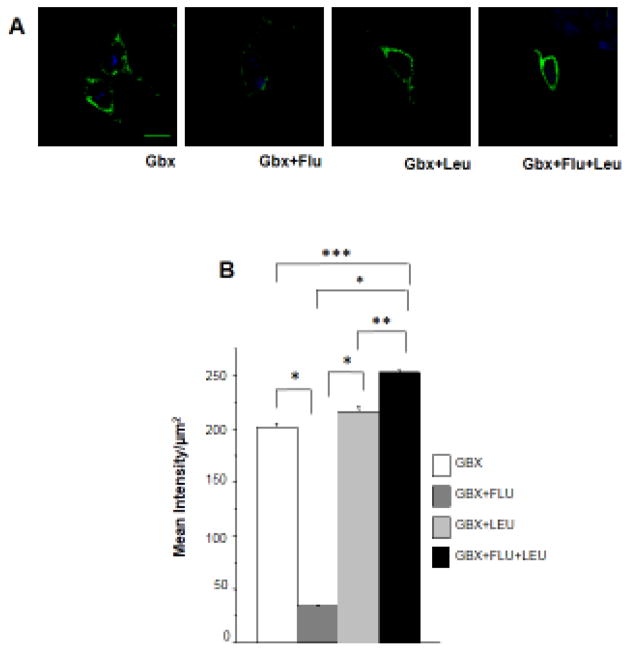
3.6. α4Fβ2δ surface levels increase after inhibition of lysosomal degradation following 48 h treatment of HEK-293 cells with flumazenil and gaboxadol
Other studies have implicated the endosomal pathway in the down-regulation of synaptic surface receptors via adaptin protein 2 (AP2) and β2 subunit interaction (Kittler et al., 2000). Therefore, we measured α4Fβ2δ cell surface expression after disabling lysosomal enzymes to determine if the observed effects on down-regulation of the α4/δ-containing receptor were linked to endocytic sorting. This was accomplished with leupeptin (1μM), an inhibitor of proteases found mainly in lysosomes responsible for protein degradation (Arancibia-Carcamo et al., 2009; Eriksen et al., 2010). In this study, we used gaboxadol, the high efficacy agonist of α4β2δ, to increase levels of its surface expression (Kuver et al., 2012) in order to compare flumazenil-induced effects on surface α4Fβ2δ expression with those from the studies using GABA plus THP.
Similar to its effect on GABA plus THP-treated cells, 48 h flumazenil exposure reduced surface expression of α4Fβ2δ by ~90% after gaboxadol treatment. However, leupeptin administration completely prevented this flumazenil-induced down-regulation of receptor surface expression following gaboxadol treatment (P<0.05, Fig. 6A, B). These data suggest that flumazenil increases degradation through endocytic sorting of α4β2δ.
Figure 6. Leupeptin effects on α4Fβ2δ surface expression after flumazenil administration to gaboxadol - treated HEK-293 cells.
Surface labeling of α4Fβ2δ expressed in HEK-293 cells. Cells were treated with gaboxadol (GBX, 10 μM) for 48 h in addition to either vehicle, flumazenil (FLU, 10 μM), leupeptin (LEU, 1 μM) or FLU plus LEU. A, Representative (40 X) confocal images. Surface FLAG immunofluorescence (488 λ) was reduced by FLU, but this effect was prevented by LEU. Cell stain, DAPI (350 λ), scale bar, 10 μM. B, Averaged data. (n=9, F(3,32)=614.4, P<0.0001, ANOVA; *P<0.05 vs. FLU, **P<0.05 vs. GBX+LEU, ***P<0.05 vs. GBX, post-hoc Tukey’s test.) Scale, 10 μm.
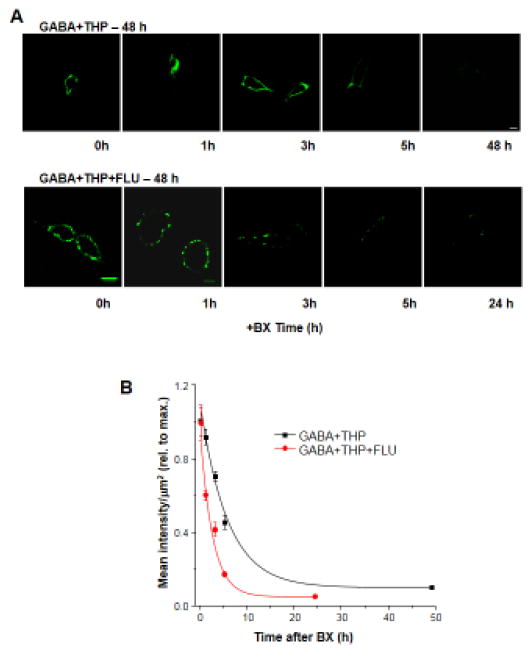
3.7. Flumazenil accelerates the rate of receptor removal from the cell surface
Although our data suggest that endocytosis plays a role in flumazenil-induced decreases in surface expression of α4β2δ GABARs, it does not directly address whether flumazenil alters the rate of removal of the receptor from the cell surface. Therefore, we examined this possibility in transfected HEK-293 cells treated with GABA plus THP, with or without flumazenil, for 48 h. To this end, we blocked receptor insertion for varying lengths of time (1, 3, 5 and 24 or 48 h) with application of botulinum toxin B (5 nM), known to cleave vesicle associated membrane protein-2 (VAMP2/synaptobrevin) and prevent vesicle trafficking to the cell surface (Montecucco and Schiavo, 1995; Rummel 2013). Endogenous VAMP2 is found in HEK293 cells (Singh et al., 2004) where it regulates membrane insertion of proteins, as we have shown in a previous study (Kuver et al., 2012). We then assessed surface labeling of α4F using the immunofluorescence techniques described above. Results were plotted as a function of surface labeling versus time of exposure to botulinum toxin B, and the curve fitted with a single exponential decay function.
The time constant for the decrease in surface labeling of α4F after blockade of receptor insertion was decreased by more than 50% by flumazenil (τ, 2.6 ± 0.23 h, flumazenil with GABA plus THP, versus τ, 5.71 ± 0.89 h, GABA plus THP; t(8) = 3.38, P <0.01, Fig. 7) in cells treated with GABA plus THP. These results suggest that the rate of receptor removal is significantly accelerated by 10 μM flumazenil. In contrast, GABA plus THP treatment does not alter the rate of receptor removal which is similar to vehicle (τ, 5.7 ± 0.64 h) as we have previously shown (Kuver et al., 2012.) This time-course is consistent with findings from recent studies (Joshi and Kapur, 2009) demonstrating that the surface half-life of δ-containing GABAR is on the order of hours, in contrast to γ-containing GABAR, where it is on the order of minutes.
Figure 7. Flumazenil accelerates the rate of α4Fβ2δ receptor removal from the cell surface.
Surface labeling of α4Fβ2δ expressed in HEK-293 cells was assessed after 48 h exposure to GABA (10 μM) + THP (100 nM) with or without flumazenil (FLU, 10 μM). Botulinum toxin B (BX, 5 nM) was added to block receptor insertion at various times prior to staining (0, 1, 3, 5 and 24 or 48 h). A, Representative confocal images (40 X) of surface α4F staining (488 λ, green) after treatment with BX for the indicated time periods, administered without (upper panel) or following 48 h treatment with FLU (lower panel). Scale, 10μm. Representative images were enhanced for visualization purposes (brightness (40%) and contrast (60%)). B, Averaged data. The rate of disappearance of surface α4F labeling is plotted as a function of time (n=5 cells per time point). The plot was best fit to a single exponential decay function, which was accelerated by FLU (τ, 2.6 ± 0.23 h) compared to GABA+THP alone (τ, 5.71 ± 0.89 h; t(8)=2.76, P=0.0247).
4. Discussion
Our findings suggest that when applied acutely, flumazenil can decrease current gated by α4β2δ GABARs, recorded in the presence of THP. Further, we show here that chronic 2 – 24 h exposure to flumazenil decreases cell surface expression of this receptor in transfected HEK-293 cells which had been increased by previous exposure to GABA plus THP. These studies used both immunocytochemical and electrophysiological/pharmacological techniques, suggesting a loss of functional receptors. Flumazenil-induced decreases in receptor surface expression were mediated via an acceleration in the rate of receptor removal from the cell surface through endocytotic and lysosomal degradation pathways. These findings may suggest novel therapeutic approaches for pathologies associated with hormonal states where α4β2δ GABARs are increased by fluctuations in endogenous THP (Maguire et al., 2007; Sabaliauskas et al., 2014).
4.1. BZ ligands: differences in binding to α4β2δ GABARs
The binding of BZ inverse agonists and antagonists, such as flumazenil, to α4β2δ GABARs is distinct from BZ agonists, which do not bind to these receptors due to an arginine to histidine substitution at residue 99 (Kleingoor et al., 1993) and the absence of a γ subunit which forms part of the binding pocket for BZ agonists (Sigel, 2002). However, reports suggest that flumazenil shares a binding site with ethanol (ETOH) (Hanchar et al. 2006). ETOH itself can be a positive modulator of α4βδ GABARs at low concentrations (3 – 30 mM) (Sundstrom-Poromaa et al., 2002; Wallner et al., 2003), an effect dependent upon protein kinase C-δ, although conflicting reports (Baur et al., 2009) have appeared. These low concentrations of ETOH act through a binding site which is also shared by the BZ partial inverse agonist RO15-4513; ETOH can prevent binding of [H3]-RO15-4513 to crude membranes of HEK-293T cells transfected with α4β3δ (Ki=~8 mM) (Hanchar et al. 2006). Binding of [H3]-RO15-4513 is also prevented by flumazenil (Ki=8.3 nM), suggesting that the BZ partial inverse agonist and antagonist share a similar binding site. However, binding of [H3]-RO15-4513 is not displaced by classic BZ agonists, including diazepam, midazolam and flunitrazepam; thus, these agonists do not share this binding site, as expected for a receptor containing an α4 subunit and lacking a γ subunit. Surprisingly, only RO15-4513, but not flumazenil, prevents the modulatory actions of ETOH at α4β3δ GABARs (Wallner et al., 2006). Although RO-15-4513 and flumazenil are structurally similar, an azido group at the C7 position of the BZ ring on RO15-4513 which is not present on flumazenil may be the moiety which overlaps with the ETOH-binding site. Earlier studies showed that mutations of histidine 101 in α1 of recombinant α1β2γ2 to residues (K or E) which prevent binding of a BZ agonist result in agonist-like effects of flumazenil (Dunn et al., 1999). These results are consistent with recent findings suggesting that flumazenil binds to different regions of the binding pocket occupied by BZ agonists (Kucken et al., 2003; Sigel and Luscher, 2011).
4.2. Flumazenil as a negative modulator and regulator of α4β2δ surface expression
In this study we establish the role of flumazenil as a negative modulator of α4β2δ GABARs, which was demonstrated robustly when GABA-gated current was recorded in the presence of THP. THP increases the efficacy of α4β2δ GABARs, at which GABA is only a partial agonist (Bianchi and Macdonald, 2003).
We have previously shown that high efficacy agonists and positive modulators of α4β2δ GABARs can increase cell surface expression of the receptor when applied for 24–48 h (Kuver et al., 2012). This is consistent with results from earlier studies showing that when GABA is a full agonist at GABARs such as α1β2γ2, GABA alone can increase surface expression of the receptor (Eshaq et al., 2010). Although the initial trigger for this effect is not established, it is noteworthy that flumazenil as a negative modulator of the receptor can exert the opposite effect, decreasing cell surface expression of α4β2δ GABARs, which are increased by either GABA plus THP or by the high efficacy agonist gaboxadol. Although the mechanism of its effect is not known, because HEK-293 cells were used in the present study, we can rule out potential effects of flumazenil on other GABARs which contain BZ binding sites. The concentration of flumazenil producing optimal effects in reducing surface expression was 10 μM, higher than the 1 μM concentration used for complete blockade of BZ-gated current in transfected cells (Kucken et al., 2000).
The present results are also consistent with a previous study (Zhou and Smith, 2009) suggesting that regulation of native α4-containing GABAR by GABAR modulators is dependent upon the effect of the drug, where positive modulators such as pentobarbital and THP increase receptor expression, while negative modulators or antagonists decrease receptor expression. The physiological mechanism for the observed flumazenil effects in the present study is not known and could be a result of potential allosteric effects of the drug on the receptor.
Flumazenil did not, however, decrease surface levels of α4 expression under control conditions. This finding is similar to those reported in an earlier study (Zhou and Smith, 2009) which demonstrated that α4 expression, assessed by Western blot, is correlated with changes in GABA-gated current, rather than simply through ligand-receptor interactions. In this study, α4 expression was increased by drugs which increase GABA-gated current, while α4 expression was decreased by drugs which decrease GABA-gated current. Reducing the driving force with bumetanide reduced the effect of the GABA-modulators to alter α4 expression. Under unstimulated conditions, flumazenil has no effect on the current and did not alter α4 surface expression in either the previous study or the current findings. Alternatively, the binding of GABA plus THP may allosterically change the receptor to permit flumazenil to reduce receptor surface expression.
4.3. Flumazenil effects on α4 surface expression
Flumazenil has previously been shown to decrease expression of native α4-containing GABARs without changing δ expression (Biggio et al., 2007) and the homologous α6 (Zheng et al., 1996 in cultured cerebellar granule cells, although in the latter study, it also increased α1 and δ expression, assessed using Western blot and immunocytochemical techniques. Our findings, however, suggest that flumazenil decreases in α4 surface expression are accompanied by reductions in δ surface expression, based on the electrophysiology results. The data from the present study are consistent with findings from the α4−/− mouse demonstrating that δ surface expression is significantly reduced in CA1 hippocampus and thalamus in the absence of α4 surface expression (Sabaliauskas et al., 2012; Peng et al., 2014), suggesting that the effects of flumazenil on δ surface expression may be both subunit and site-specific.
Our recent study also suggests that in vivo treatment of adult, male rats with flumazenil for 48 h reduces elevated hippocampal levels of α4β2δ expression, assessed by Western blot, produced by chronic methamphetamine (Shen et al., 2013). This decrease in α4β2δ expression prevented stress-triggered anxiety, which is one outcome of methamphetamine withdrawal (Shen et al., 2013). Interestingly, in one study, flumazenil was shown to be effective in reducing relapse in methamphetamine-dependent individuals (Urschel et al., 2009), suggesting a possible clinical application for the drug.
Early studies reported an effect of flumazenil in reversing the effects of chronic BZs on BZ binding and tolerance (Roca et al., 1990; Gonsalves and Gallager, 1988). Although the mechanism is not known, the insensitivity to BZs reported in these studies may reflect an increase in the BZ-insensitive α4 subunit. Thus the fact that flumazenil could reverse this insensitivity is consistent with the idea that flumazenil can decrease α4 surface expression.
4.4. Time-course of flumazenil’s effect on α4β2δ surface expression
Flumazenil effectively decreased receptor surface expression as early as 2–6 h after the onset of drug exposure, by accelerating the rate of receptor removal and decreasing the surface half-life from 5 to 1.3 h. In contrast, the mechanism for GABA plus THP’s effect to increase surface receptor expression was by increasing the rate of membrane insertion (Kuver et al., 2012). Thus, flumazenil is not acting to merely block the effect of GABA plus THP, which is consistent with the concept that the binding sites for GABA, steroids and flumazenil are distinct (Olsen and Sieghart, 2009).
This is further substantiated by the fact that flumazenil’s effect to decrease surface expression of α4β2δ was observed following elevated expression of the receptor (2–24 h flumazenil exposure occurring after 24–46 h GABA plus THP exposure) as well as during concomitant 48 h application of GABA plus THP with flumazenil. Our earlier studies, in fact, demonstrated that flumazenil rapidly reduces surface expression of α4β2δ GABARs following 48 h exposure to GABA plus THP (data not shown) across a similar time-course, with return to control levels by 2 h, again considerably faster than the 5 h surface half-life of untreated cells.
4.5. Flumazenil effects on α4β2δ internalization
In the present study, disabling dynamin and thereby inhibiting clathrin-dependent endocytosis with dynasore blocked the effect of flumazenil to reduce surface expression of α4Fβ2δ. This finding substantiates the conclusion that internalization is the putative mechanism underlying down-regulation by flumazenil. The fact that 30 min exposure to dynasore reversed flumazenil-induced reductions in cell surface expression of α4β2δ GABARs suggests that, unlike membrane insertion, regulation of endocytic mechanisms are rapid. This time-course is also sufficient to permit THP to increase membrane insertion of α4β2δ, which is significant by 30 min (Kuver et al., 2012). These findings suggest that flumazenil is causing the activation of proteins in the initial steps of the endocytic pathway and that this may be the rate-limiting step in its mechanism to down-regulate surface expression of α4β2δ receptors. Recent studies (Gonzalez et al., 2012) have shown that endocytosis of δ-containing GABARs induced by ethanol is produced by binding of the GABAR δ subunit to clathrin adaptor protein 2 (AP2). Similar processes mediate endocytosis of γ2-containing GABARs, which also require serine phosphorylation, the first step in the endocytic pathway (Kanematsu et al., 2007).
4.6. α4βδ GABARs and neuropsychological disorders
α4β2δ GABARs may play an important role in neuropsychological disorders associated with hormone fluctuations (Smith et al., 2007). These receptors are a sensitive target for neurosteroids such as THP (Belelli et al., 2002; Brown et al., 2002; Wohlfarth et al., 2002), a metabolite of the ovarian hormone progesterone, which is also released in response to sustained stress (Droogleever Fortuyn et al., 2004; Mukai et al., 2008; Purdy et al., 1991). Increases in α4βδ GABAR expression at puberty, across the ovarian cycle (Lovick et al., 2005; Lovick, 2007; Maguire et al., 2005; Maguire and Mody, 2007) and pregnancy (Maguire et al., 2009; Maguire and Mody, 2009; Sanna et al., 2009) are associated with anxiety, panic responses and seizure susceptibility, suggesting a role for this receptor in pathologies associated with hormonal fluctuations.
4.7. α4βδ GABARs and Premenstrual Dysphoric Disorder
Expression of α4βδ GABARs is also increased in CA1 hippocampus following withdrawal from the neuroactive steroid THP in a rodent model of premenstrual dysphoric disorder (PMDD) (Smith et al., 2006). This syndrome typically occurs in the luteal phase of the menstrual cycle (Cunningham et al., 2009), when fluctuations in circulating levels of THP and its parent compound progesterone occur. Similar to the rodent model, women with PMDD have abnormal, dysphoric responses to ovarian steroids such as THP or progesterone (Schmidt et al., 1998), respectively. Interestingly, flumazenil can trigger panic responses in PMDD (Le Melledo et al., 2000), despite a relative insensitivity to benzodiazepine agonists (Sundstrom et al., 1997), consistent with its role as a negative modulator of α4βδ, as reported in both human studies and in the rodent model of PMDD (Smith et al., 2006).
4.8. Conclusions
In conclusion, our findings suggest that flumazenil can reduce cell surface expression of α4β2δ GABARs that are up-regulated by steroid treatment. Flumazenil may thus have important therapeutic value in neuropsychological (Damgaard et al., 2011; Feng et al., 2010) and addictive disorders (Shen et al., 2013) which may involve this receptor.
Highlights.
Flumazenil is a negative modulator of α4βδ GABARs in the presence of THP
Flumazenil reduces THP-induced α4βδ GABAR surface expression
Flumazenil increases α4βδ receptor endocytosis
Flumazenil effects on α4βδ internalization require dynamin
Acknowledgments
The authors thank QH Gong for helpful technical assistance and J Celentano and C Czajkowski for a critical reading of the manuscript. This work was supported by NIH grants MH100561, DA09618 and AA12958 and a contract from Hythiam, Inc. to SSS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Arancibia-Carcamo IL, Yuen EY, Muir J, Lumb MJ, Michels G, Saliba RS, Smart TG, Yan Z, Kittler JT, Moss SJ. Ubiquitin-dependent lysosomal targeting of GABA(A) receptors regulates neuronal inhibition. Proc Natl Acad Sci US A. 2009;106:17552–17557. doi: 10.1073/pnas.0905502106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai D, Zhu G, Pennefather P, Jackson MF, Macdonald JF, Orser BA. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by y-aminobutyric acid(A) receptors in hippocampal neurons. Molec Pharmac. 2000;59:814–824. doi: 10.1124/mol.59.4.814. [DOI] [PubMed] [Google Scholar]
- Baur R, Kaur KH, Sigel E. Structure of α6β3δ GABA(A) receptors and their lack of ethanol sensitivity. J Neurochem. 2009;111:1172–1181. doi: 10.1111/j.1471-4159.2009.06387.x. [DOI] [PubMed] [Google Scholar]
- Belelli D, Casula A, Ling A, Lambert JJ. The influence of subunit composition on the interaction of neurosteroids with GABA(A) receptors. Neuropharm. 2002;43:651–661. doi: 10.1016/s0028-3908(02)00172-7. [DOI] [PubMed] [Google Scholar]
- Bianchi MT, Macdonald RL. Neurosteroids shift partial agonist activation of GABA(A) receptor channels from low- to high-efficacy gating patterns. J Neurosci. 2003;23:10934–10943. doi: 10.1523/JNEUROSCI.23-34-10934.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggio F, Gorini G, Caria S, Murru L, Sanna E, Follesa P. Flumazenil selectively prevents the increase in α4-subunit gene expression and an associated change in GABA(A) receptor function induced by ethanol withdrawal. J Neuro Chem. 2007;102:657–666. doi: 10.1111/j.1471-4159.2007.04512.x. [DOI] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human α4β3δ GABA(A) receptors. Br J Pharmacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagetti E, Liang J, Spigelman I, Olsen RW. Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABA-A receptors. Mol Pharmacol. 2003;63:53–64. doi: 10.1124/mol.63.1.53. [DOI] [PubMed] [Google Scholar]
- Compagnone NA, Mellon SH. Neurosteroids: Biosynthesis and Function of These Novel Neuromodulators. Frontiers in Neuroendocrinology. 2000;21:1–56. doi: 10.1006/frne.1999.0188. [DOI] [PubMed] [Google Scholar]
- Cunningham J, Yonkers KA, O’Brien S, Eriksson E. Update on research and treatment of premenstrual dysphoric disorder. Harv Rev Psychiatry. 2009;17:120–137. doi: 10.1080/10673220902891836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damgaard T, Plath N, Neill JC, Hansen SL. Extrasynaptic GABA-A receptor activation reverses recognition memory deficits in an animal model of schizophrenia. Psychopharmacology (Berl) 2011;214:403–413. doi: 10.1007/s00213-010-2039-9. [DOI] [PubMed] [Google Scholar]
- Droogleever Fortuyn HA, van Broekhoven F, Span PN, Backstrom T, Zitman FG, Verkes RJ. Effects of PhD examination stress on allopregnanolone and cortisol plasma levels and peripheral benzodiazepine receptors density. Psychoneuroendocrinol. 2004;29:1341–1344. doi: 10.1016/j.psyneuen.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Dunn SM, Davies M, Muntoni AL, Lambert JJ. Mutagenesis of the rat α1 subunit of the gamma-aminobutyric acid(A) receptor reveals the importance of residue 101 in determining the allosteric effects of benzodiazepine site ligands. Mol Pharmacol. 1999;56:768–774. [PubMed] [Google Scholar]
- Eriksen J, Bjorn-Yoshimoto WE, Jorgensen TN, Newman AH, Gether U. Postendocytic sorting of constitutively internalized dopamine transporter in cell lines and dopaminergic neurons. J Biol Chem. 2010;285:27289–27301. doi: 10.1074/jbc.M110.131003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshaq RS, Stahl LD, Stone R, 2nd, Smith SS, Robinson LC, Leidenheimer NJ. GABA acts as a ligand chaperone in the early secretory pathway to promote cell surface expression of GABA-A receptors. Brain Res. 2010;1346:1–13. doi: 10.1016/j.brainres.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, et al. Association of the GABRD gene and childhood-onset mood disorders. Genes Brain Behav. 2010;9:668–672. doi: 10.1111/j.1601-183X.2010.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonsalves SF, Gallager DW. Persistent reversal of tolerance to anticonvulsant effects and GABAergic subsensitivity by a single exposure to benzodiazepine antagonist during chronic benzodiazepine administration. J Pharmacol Exp Ther. 1988;244:79–83. [PubMed] [Google Scholar]
- Gonzalez C, Moss SJ, Olsen RW. Ethanol promotes clathrin adaptor-mediated endocytosis via the intracellular domain of δ-containing GABAA receptors. J Neurosci. 2012;32:17874–17881. doi: 10.1523/JNEUROSCI.2535-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanchar HJ, Chutsrinopkun P, Meera P, Supavilai P, Sieghart W, Wallner M, Olsen RW. Ethanol potently and competitively inhibits binding of the alcohol antagonist Ro15-4513 to α4/6β3δ GABA-A receptors. Proc Natl Acad Sci. 2006;103:8546–8551. doi: 10.1073/pnas.0509903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, Kapur J. Slow intracellular accumulation of GABA(A) receptor δ subunit is modulated by brain-derived neurotrophic factor. Neuroscience. 2009;164:507–519. doi: 10.1016/j.neuroscience.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanematsu T, Fujii M, Mizokami A, Kittler JT, Nabekura J, Moss SJ, Hirata M. Phospholipase C-related inactive protein is implicated in the constitutive internalization of GABA-A receptors mediated by clathrin and AP2 adaptor complex. J Neurochem. 2007;101:898–905. doi: 10.1111/j.1471-4159.2006.04399.x. [DOI] [PubMed] [Google Scholar]
- Kittler JT, Wang J, Connolly CN, Vicini S, Smart TG, Moss SJ. Analysis of GABA-A receptor assembly in mammalian cell lines and hippocampal neurons using gamma 2 subunit green fluorescent protein chimeras. Mol Cell Neurosci. 2000;16:440–452. doi: 10.1006/mcne.2000.0882. [DOI] [PubMed] [Google Scholar]
- Kleingoor C, Wieland HA, Korpi ER, Seeburg PH, Kettenmann H. Current potentiation by diazepam but not GABA sensitivity is determined by a single histidine residue. Neuroreport. 1993;4:187–190. doi: 10.1097/00001756-199302000-00018. [DOI] [PubMed] [Google Scholar]
- Knoflach F, Benke D, Wang Y, Scheurer L, Lüddens H, Hamilton BJ, Carter DB, Mohler H, Benson JA. Pharmacological modulation of the diazepam-insensitive recombinant gamma-aminobutyric acidA receptors α4β2γ2 and α6β2γ2. Mol Pharmacol. 1996;50:1253–1261. [PubMed] [Google Scholar]
- Kucken AM, Teissére JA, Seffinga-Clark J, Wagner DA, Czajkowski C. Structural requirements for imidazobenzodiazepine binding to GABA-A receptors. Mol Pharmacol. 2003;63:289–296. doi: 10.1124/mol.63.2.289. [DOI] [PubMed] [Google Scholar]
- Kuver A, Shen H, Smith SS. Regulation of the surface expression of α4β2δ GABA(A) receptors by high efficacy states. Brain Res. 2012;1463:1–20. doi: 10.1016/j.brainres.2012.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Melledo JM, Van Driel M, Coupland NJ, Lott P, Jhangri GS. Response to flumazenil in women with premenstrual dysphoric disorder. Am J Psychiatry. 2000;157:821–823. doi: 10.1176/appi.ajp.157.5.821. [DOI] [PubMed] [Google Scholar]
- Lee HHC, Jurd R, Moss SJ. Tyrosine phosphorylation regulates the membrane trafficking of the potassium chloride co-transporter KCC2. Mol Cell Neurosci. 2010;45:173–179. doi: 10.1016/j.mcn.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovick TA. GABA in the female brain - Oestrous cycle-related changes in GABAergic function in the periaqueductal grey matter. Pharacol Biochem Behav. 2007 doi: 10.1016/j.pbb.2007.12.014. epub. [DOI] [PubMed] [Google Scholar]
- Lovick TA, Griffiths JL, Dunn SM, Martin IL. Changes in GABA(A) receptor subunit expression in the midbrain during the oestrous cycle in Wistar rats. Neuroscience. 2005;131:397–405. doi: 10.1016/j.neuroscience.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell. 2006;10:839–50. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Maguire JL, Ferando I, Simonsen C, Mody I. Excitability changes related to GABA-A receptor plasticity during pregnancy. J Neurosci. 2009;29:9592–9601. doi: 10.1523/JNEUROSCI.2162-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire JL, Mody I. Neurosteroid synthesis-mediated regulation of GABA(A) receptors: relevance to the ovarian cycle and stress. J Neurosci. 2007;27:2155–2162. doi: 10.1523/JNEUROSCI.4945-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire JL, Mody I. GABA(A)R plasticity during pregnancy: relevance to postpartum depression. Neuron. 2009;59:207–213. doi: 10.1016/j.neuron.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Meera P, Wallner M, Otis T. Molecular basis for the high THIP/gaboxadol sensitivity of extrasynaptic GABA-A receptors. J Neurophysiology. 2011;106:2057–2011. doi: 10.1152/jn.00450.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing RO, Choi DS, Wei W, Kharazia VN, Deitchman JK, Lesscher HMB, Mody I. Protein kinase C-delta regulates GABA-mediated tonic inhibition and motor response to ethanol. Alc Clin Exp Res. 2007;31(Suppl):296A. [Google Scholar]
- Mirabilla AC, et al. Specific cell-permeable inhibitor of proteasome trypsin-like sites selectively sensitizes myeloma cells to bortezomib and carfilzomib. Chem Biol. 2011;18:608–18. doi: 10.1016/j.chembiol.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montecucco C, Schiavo G. Structure and function of tetanus and botulinum neurotoxins. Q Rev Biophys. 1995;28:423–472. doi: 10.1017/s0033583500003292. [DOI] [PubMed] [Google Scholar]
- Morlock EV, Czajkowski C. Different residues in the GABA-A receptor benzodiazepine binding pocket mediate benzodiazepine efficacy and binding. Mol Pharmacol. 2011;80:14–22. doi: 10.1124/mol.110.069542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai H, Higashi T, Nagura Y, Shimada K. Studies on neurosteroids XXV. Influence of 5α-reductase inhibitor, finasteride, on rat brain neurosteroid levels and metabolism. Biol Pharm Bull. 2008;31:1646–1650. doi: 10.1248/bpb.31.1646. [DOI] [PubMed] [Google Scholar]
- Oh P, Horner T, Witkiewicz H, Schnitzer JE. Endothelin induces rapid, dynamin-mediated budding of endothelial caveolae rich in ET-B. J Biol Chem. 2012;287:17353–17362. doi: 10.1074/jbc.M111.338897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. GABA-A receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–148. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z, Zhang N, Chandra D, Homanics GE, Olsen RW, Houser CR. Altered localization of the δ subunit of the GABAA receptor in the thalamus of α4 subunit knockout mice. Neurochem Res. 2014;39:1104–1117. doi: 10.1007/s11064-013-1202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore PH, Jr, Paul SM. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat. PNAS. 1991;88:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca DJ, Schiller GD, Friedman L, Rozenberg I, Gibbs TT, Farb DH. gamma-Aminobutyric acid-A receptor regulation in culture: Altered allosteric interactions following prololonged exposure to benzodiazepines, barbiturates, and methylxanthines. Mol Pharmacol. 1990;37:710–719. [PubMed] [Google Scholar]
- Rummel A. Double receptor anchorage of botulinum neurotoxins accounts for their exquisite neurospecificity. Curr Top Microbiol Immunol. 2013;364:61–90. doi: 10.1007/978-3-642-33570-9_4. [DOI] [PubMed] [Google Scholar]
- Sabaliauskas N, Shen H, Homanics GE, Smith SS, Aoki C. Knock-out of the γ-aminobutyric acid receptor subunit α4 reduces functional δ-containing extrasynaptic receptors in hippocampal pyramidal cells at the onset of puberty. Brain Res. 2012;1450:11–23. doi: 10.1016/j.brainres.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabaliauskas N, Shen H, Molla J, Gong QH, Kuver A, Aoki C, Smith SS. Neurosteroid effects at α4βδ GABAA receptors alter spatial learning and synaptic plasticity in CA1 hippocampus across the estrous cycle of the mouse. Brain Res. 2014 doi: 10.1016/j.brainres.2014.12.026. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna E, Mostallino MC, Murru L, Carta M, Talani G, Zucca S, Mura ML, Maciocco E, Biggio G. Changes in expression and function of extrasynaptic GABA-A receptors in the rat hippocampus during pregnancy and after delivery. J Neurosci. 2009;29:1755–1765. doi: 10.1523/JNEUROSCI.3684-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna E, Mostallino MC, Murru L, Carta M, Talani G, Zucca S, Mura ML, Maciocco E, Biggio G. Changes in expression and function of extrasynaptic GABA-A receptors in the rat hippocampus during pregnancy and after delivery. J Neurosci. 2009;29:1755–1765. doi: 10.1523/JNEUROSCI.3684-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt P, Nieman L, Danaceau M, Adams L, Rubinow D. Differential behavioral effects of gonadal steroids in women with premenstrual syndrome. New England J Med. 1998;338:209–216. doi: 10.1056/NEJM199801223380401. [DOI] [PubMed] [Google Scholar]
- Schulze H, Kolter T, Sandhoff K. Principles of lysosomal membrane degradation: Cellular topology and biochemistry of lysosomal lipid degradation. Biochim Biophys Acta. 2009;1793:674–683. doi: 10.1016/j.bbamcr.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Shen H, Gong QH, Aoki C, Yuan M, Ruderman Y, Dattilo M, Williams K, Smith SS. Reversal of neurosteroid effects at α4βδ GABAA receptors triggers anxiety at puberty. Nat Neurosci. 2007;10:469–477. doi: 10.1038/nn1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Mohammad A, Ramroop J, Smith SS. A stress steroid triggers anxiety via increased expression of α4βδ GABAA receptors in methamphetamine dependence. Neurosci. 2013;254:452–475. doi: 10.1016/j.neuroscience.2013.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Sabaliauskas N, Sherpa A, Fenton AA, Stelzer A, Aoki C, Smith SS. A critical role for α4βδ GABAA receptors in shaping learning deficits at puberty in mice. Science. 2010;327:1515–1518. doi: 10.1126/science.1184245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel E. Mapping of the benzodiazepine recognition site on GABA(A) receptors. Curr Top Med Chem. 2002;2:833–839. doi: 10.2174/1568026023393444. [DOI] [PubMed] [Google Scholar]
- Sigel E, Lüscher BP. A closer look at the high affinity benzodiazepine binding site on GABA-A receptors. Curr Top Med Chem. 2011;11:241–6. doi: 10.2174/156802611794863562. [DOI] [PubMed] [Google Scholar]
- Singh BB, et al. VAMP2-Dependent exocytosis regulates plasma membrane insertion of TRPC3 channels and contributes to agonist-stimulated Ca2+ influx. Mol Cell. 2004;15:635–646. doi: 10.1016/j.molcel.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Smith SS, Aoki C, Shen H. Puberty, steroids and GABA(A) receptor plasticity. Psychoneuroendocrinology. 2009;34(Suppl 1):S91–S103. doi: 10.1016/j.psyneuen.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Hsu FC, Markowitz RS, ffrench-Mullen JMH, Li X. GABAA receptor α4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature. 1998;392:926–929. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- Smith SS, Ruderman Y, Frye CA, Homanics GE, Yuan M. Steroid withdrawal in the mouse results in anxiogenic effects of 3α,5α-THP: A possible model of premenstrual dysphoric disorder. Psychopharmacology. 2006;186:323–333. doi: 10.1007/s00213-005-0168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Shen H, Gong QH, Zhou X. Neurosteroid regulation of GABA(A) receptors: Focus on the α4 and δ subunits. Pharm and Therapeutics. 2007;116:58–76. doi: 10.1016/j.pharmthera.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom I, Nyberg S, Backstrom T. Patients with premenstrual syndrome have reduced snsitivity to midazolam compared to control subjects. Neuropsychopharm. 1997;17:370–381. doi: 10.1016/S0893-133X(97)00086-9. [DOI] [PubMed] [Google Scholar]
- Sundstrom-Poromaa I, Smith DH, Gong Q, Sabado TN, Li X, Light A, Wiedmann M, Williams K, Smith S. Hormonally regulated α4β2δ GABA-A receptors are a target for alcohol. Nat Neurosci. 2002;5:721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urschel HC, Hanselka LL, Baron M. A controlled trial of flumazenil, gabapentin and hydroxyzine for initial treatment for methamphetamine dependence. J Psychopharm. 2009 doi: 10.1177/0269881109349837. epub. [DOI] [PubMed] [Google Scholar]
- Wafford KA, Thompson SA, Sikela J, Wilcox AS, Whiting PJ. Functional characterization of human GABA receptors containing the α4 subunit. Mol Pharmacol. 1996;50:670–678. [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Low dose ethanol actions on α4β3δ GABA-A receptors are reversed by the behavioral alcohol antaqgonist Ro15-4513. Proc Natl Acad Sci. 2006;103:8540–8545. doi: 10.1073/pnas.0600194103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances α4β3δ and α6β3δ gamma-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc Natl Acad Sci. 2003;100:15218–15223. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of δ subunit-containing GABA(A) receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland HA, Luddens H, Seeburg PH. A single histidine in GABA-A receptors is essential for benzodiazepine agonist binding. J Biol Chem. 1992;267:1426–1429. [PubMed] [Google Scholar]
- Wisden W, Laurie DJ, Monyer H, Seeburg P. The distribution of 13 GABA-A receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABA(A) receptors containing the δ subunit. J Neurosci. 2002;22:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheleznova N, Sedelnikova A, Weiss DS. α1β2δ, a silent GABA-A receptor: recruitment by tracazolate and neurosteroids. Brit J Pharmacol. 2008;153:1062–1071. doi: 10.1038/sj.bjp.0707665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng TM, Caruncho HJ, Zhu WJ, Vicini S, Ikonomovic S, Grayson DR, Costa E. Chronic flumazenil alters GABA(A) receptor subunit mRNA expression, translation product assembly and channel function in neuronal cultures. J Pharmacol Exp Ther. 1996;277:525–533. [PubMed] [Google Scholar]
- Zhou X, Smith SS. Expression levels of the α4 subunit of the GABA(A) receptor in differentiated neuroblastoma cells are correlated with GABA-gated current. Neuropharm. 2009;56:1041–1053. doi: 10.1016/j.neuropharm.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]