Abstract
Objective
Depression and inflammation may independently promote breast cancer (BCa) disease progression and poorer clinical outcomes. Depression has been associated with increased levels of inflammatory markers in medically healthy individuals and cancer patients. However, inconsistencies in study time frames complicate interpretation of results within specific cancer types. This study examined relationships between depressive symptoms and inflammation in women with early stage BCa before beginning adjuvant treatment.
Method
Women with stage 0–III BCa were recruited approximately 4–8 weeks post-surgery. Depressive symptoms were assessed using the Hamilton Rating Scale for Depression and blood samples were collected to quantify circulating levels of IL-1β, IL-6, and TNF-α by ELISA. ANCOVAs were used to test for group differences (elevated vs. low depressive symptoms) in levels of cytokines. Multiple regression analyses were used to examine relationships between continuous severity of depressive symptoms and levels of cytokines adjusting for relevant biobehavioral covariates.
Results
Thirty-six of 89 (40%) patients showed elevated levels of depressive symptoms, and in adjusted models had marginally higher levels of IL-1β (M=14.49, 95% CI [6.11, 32.65] vs. M=4.68, 95% CI [1.96, 9.86]) and significantly higher levels of TNF-α (M=17.07, 95% CI [8.27, 34.32] vs. M=6.94, 95% CI [3.58, 12.80]) than women with low depressive symptoms. Across the spectrum of depressive symptoms, greater magnitude of depressive symptoms was related to greater levels of IL-1β (β=0.06, p=0.006, R2=0.25) and TNF-α (β=0.06, p=0.003, R2=0.27).
Conclusions
Post-surgery and pre-adjuvant treatment for early stage BCa, depressive symptoms covary with elevated levels of multiple pro-inflammatory cytokines. Findings have implications for psychosocial and biological interventions concurrently focusing on depression and inflammation.
Keywords: breast cancer, depression, pro-inflammatory cytokines
INTRODUCTION
Breast cancer (BCa) patients often report elevated levels of depression (1), which are linked to decreased quality of life (1), poorer perceived health status (2), and increased mortality (3). Examinations of biological mechanisms by which depression might affect physical health outcomes in BCa have focused on inflammation as one of several processes (4), as depression has been related to increased levels of circulating inflammatory indicators (5–7). Studies suggest stress and depression may prime the inflammatory system to be more reactive to subsequent stress (for a review see 8). This is particularly salient in the context of cancer, as cancer is a major life stressor that causes a natural inflammatory response (9) involving release of pro-inflammatory cytokines (10) that may promote processes related to disease progression through interaction with circulating tumor cells that remain post-surgery (11).
Individuals with comorbid cancer and depression exhibit greater levels of inflammation than individuals with cancer alone (6), and it is possible that depressed cancer patients may be at greater risk of disease progression. Thus, clarifying relationships between magnitude of depressive symptoms and indicators of inflammation in cancer populations warrants attention. A body of work has established relationships between depression and increased inflammatory indicators in medically healthy subjects (5), individuals with various cancers (6), and BCa patients (7). However, methodological issues limit the impact of this work.
One limitation is that various measurement methodologies make direct comparison of results between studies difficult. There are discrepancies in the point in treatment at which data are collected, how depression is measured, and which inflammatory markers are targeted. It remains unclear whether parallel elevations in depressive symptoms and inflammation are due to BCa itself or secondary to treatment (7, 12). One study of BCa patients with Major Depressive Disorder (MDD) who had not begun adjuvant treatment found an association between depression and inflammation (7), yet another study of women who had received adjuvant treatment did not (12). Thus, examining depression and inflammation after the onset of adjuvant therapy may obscure real associations.
The present study captures women with stage 0-III BCa post-surgery but before beginning active systemic or local adjuvant treatment, as the post-surgical, pre-adjuvant period may be a time when treatment-related factors (chemotherapy, radiation and immunotherapy) can be excluded as potential confounders of the depression-inflammation association (13). We expanded upon previous research in multiple ways. We explored relationships between depressive symptoms and inflammatory markers in a non-clinically depressed sample rather than restricting analyses to clinically depressed vs. non-depressed patients. Thus, any results may be applicable to the majority of women diagnosed with BCa without MDD. Inflammation was measured with three circulating pro-inflammatory cytokines (Interleukin-1 beta [IL-1β], Tumor Necrosis Factor-alpha [TNF-α], and IL-6) rather than a single inflammatory marker in order to provide converging evidence for a depression-inflammation association. These cytokines were targeted because they are commonly studied in both depression (8) and cancer (14) literature. Clinical depression has been associated with elevated levels of IL-6 in women with BCa (7), and the present study extends beyond this single inflammatory marker to include the pro-inflammatory cytokines IL-1β and TNF-α, which have been implicated as particularly relevant to the process of angiogenesis and pro-metastatic signaling (10) and together form a more comprehensive panel of cytokines. In addition, we have previously reported that women post-surgery for BCa with greater levels of negative affect (in proportion to positive affect) exhibit greater leukocyte gene expression for IL1B, TNF, and IL6 in circulating peripheral blood mononuclear cells (PBMCs; 15). Examining circulating levels of IL-1β, TNF-α, and IL-6 serves to replicate and extend the literature relating depression and inflammation in women with BCa across the range of depressive symptoms. We considered the relationships between depressive symptoms and pro-inflammatory cytokines in the context of multiple covariates shown to be relevant to inflammatory processes (12, 16). In addition, because monocyte-derived pro-inflammatory signaling has been proposed to account for the negative mood-inflammation association in BCa patients (15), we examined relationships between depressive symptoms and circulating levels of five lymphocyte subpopulations to rule out influence of lymphocyte counts on circulating cytokine levels: total T cells (CD3+ CD19−), T helper (CD3+ CD4+), T cytotoxic (CD3+ CD8+), NK (CD56+ CD3−), and B cells (CD19+). We explored potential demographic, and clinicopathological/medical moderators of the relationships between magnitude of depressive symptoms and levels of pro-inflammatory cytokines. Finally, the present sample is ethnically diverse with approximately one third of the sample self-identifying as an ethnic minority, allowing for greater generalizability of results to a wider population of BCa patients.
We examined associations between depressive symptoms and pro-inflammatory cytokines in two ways, first using a categorical classification of depression based on accepted clinical cut-offs on the Hamilton Depression Rating Scale (HRSD, 17) and second using a continuous HRSD score. We hypothesized that women with elevated depressive symptoms (above the clinical cutoff) would display greater levels of IL-1β, TNF-α, and IL-6 than women with lower depressive symptoms (below the clinical cutoff). We also hypothesized that greater magnitude of depressive symptoms would be associated with greater concentrations of these cytokines.
Method
Participants
Participants were part of a larger trial of a stress management intervention approved by the Human Subjects Research Office of the University of Miami Institutional Review Board described elsewhere (18). Women with stage 0-III BCa were recruited 4–8 weeks post-surgery (lumpectomy or mastectomy) from a public hospital, a university-based cancer center, and surgical oncology practices in South Florida between December 1998 and February 2005. Inclusion criteria were: surgical treatment of non-metastatic BCa (i.e. not stage IV), ages 21–75, and fluent in English. Women were required to have not received neo-adjuvant or begun post-surgical adjuvant treatment, to never have been previously diagnosed with cancer (except minor skin cancer such as squamous or basal cell carcinomas), to have no major comorbid medical conditions, to have never been diagnosed with psychosis, panic disorder, or a major depressive episode, and to not endorse suicidality. The present study included a subgroup of women who provided blood samples at time of study enrollment.
There were no differences between women in the present study (N=89) and women from the parent study (N=151) who did not provide blood samples on demographic characteristics (age, race/ethnicity), education level, clinicopathological/medical variables (stage of disease, type of surgical procedure, ER status, PR status, HER-2/neu status, triple negative status), time since surgery, lifestyle factors (smoking status, BMI), prescription medication (anti-depressants, anti-anxiety medications, sleep medications, pain-reducing medications), or symptom intensity (fatigue severity); all p values >.10. The present (M=0.87, SD=2.46) and parent (M=1.98, SD=3.68) study samples were significantly different (p=0.012) on number of positive lymph nodes. The samples did not differ on either operationalization of depression (categorical, p=0.063; or continuous, p=0.549). Thus, with the exception of number of positive lymph nodes, we conclude that this sample is representative of the parent study sample.
Procedures
Following full explanation of study procedures, signed informed consent was obtained. Information was collected regarding socioeconomic and medical status via self-report measures and verified via medical chart review. Depressive symptoms were assessed via structured interview, participants completed a self-report measure of fatigue, and blood samples were obtained within one week in most cases. Assessments were conducted post-surgery for BCa but before participants began active adjuvant treatment to avoid effects of adjuvant therapies on mood and pro-inflammatory cytokines.
Psychosocial measures
Demographic and medical characteristics
At time of enrollment, self-reported demographic information was collected regarding age and socioeconomic status (race/ethnicity, years of education). Measures of medical status were: stage of disease, surgical procedure, number of positive lymph nodes, estrogen receptor (ER) status, progesterone receptor (PR) status, HER-2/neu status, triple negative status, time from surgery to assessment, smoking status (smoker or non-smoker), body mass index (BMI), and use of prescription medications (for depression, anxiety, sleep, pain).
Depressive symptoms
The 17-item interview-based HRSD (17) was used to assess presence and severity of depressive symptoms over the past week. High inter-rater reliability, internal consistency, and discriminant validity have been shown for this scale previously (19). Reliability in the present study was adequate (α=0.79). A clinical psychologist with extensive training in use of the HRSD trained study assessors based on the structured interview guide (20). Depressive symptoms were treated in two ways: First, HRSD scores were used to categorize women as either elevated (HRSD score > 7) or low depressive symptoms (HRSD score ≤ 7) based on previous validation of this cutoff (21). Second, magnitude of HRSD was considered on a continuum ranging from 0–23. The HRSD has previously been used in samples of women with BCa (22, 7).
Fatigue
The 14-item self-report Fatigue Symptom Inventory (23) was used to assess aspects of fatigue (frequency, severity, interference with daily functioning) over the past week. It was designed to assess fatigue in cancer populations, and has demonstrated acceptable psychometric properties (24). We focused on fatigue severity based on previous studies showing associations with inflammation among BCa patients (12). Fatigue severity was represented as the average of 4 items targeting levels of fatigue, each scored on a 9-point scale. Higher scores indicate greater fatigue severity.
Sample collection
Blood samples (30–40 ml) were taken at time of study enrollment. Participants were asked to abstain from alcohol use, recreational drug use, and caffeinated beverages on the day of blood draw. Women who self-reported infections within the past week or active use of antibiotic or allergy medications were rescheduled for a blood draw at least one week after remission of the illness or completion of medication use. Blood was drawn by venipuncture in Vacutainer tubes (BD, USA) without anticoagulant (for serum isolation) between 4:00–6:30pm to maximize convenience for participants. Samples were kept overnight at room temperature and processed the following morning. Serum samples were separated according to standard procedures and stored at −20 °C for subsequent batch testing.
Pro-inflammatory cytokines
Analyses focused on serum concentrations of the pro-inflammatory cytokines IL-1β, TNF-α, and IL-6, measured using ultrasensitive ELISA kits from Life Technologies (USA). The lowest level of detection for cytokines was 0.06 pg/ml, 0.09 pg/ml, and 0.10 pg/ml for IL-1β, TNF-α, and IL-6 respectively. Assays were performed according to manufacturer’s instructions and yielded the following intra- and inter-assay coefficients of variability (%), respectively: IL-1β (6.4, 7.2), TNF-α (6.7, 8.2), and IL-6 (8.3, 10.0).
Isolation of PBMCs
Blood samples were separated on Ficoll density gradient (Lymphocyte Separation Medium, ICN Biochemicals, USA). PBMCs were collected from the gradient interface, washed twice with Phosphate Buffered Saline (Gibco-BRL, USA), resuspended in RPMI-1640 (Gibco-BRL, USA), and supplemented with the following: 10% fetal bovine serum, 100 U/ml penicillin (Gibco-BRL, USA), 100 µg/ml streptomycin (Gibco-BRL, USA), 1 mM sodium pyruvate (Gibco-BRL, USA), 1 mM non-essential amino acids (Gibco-BRL, USA) and 5×10−2 mM 2-mercaptoethanol, referred to as complete tissue culture media (cRPMI). Cell counts were performed by 0.4% Trypan blue dye exclusion. Assays were performed with fresh samples within 20h after blood was drawn.
Lymphocyte phenotypes
To examine whether results were affected by proportion of different cell types, PBMCs were stained and analyzed by flow cytometry. Fluorochrome-conjugated antibodies (anti-CD3, -CD4, -CD8, -CD56 and -CD19) and corresponding isotype controls (all from Beckman-Coulter, USA) were used to enumerate total T cells (CD3+ CD19−), T helper (CD3+ CD4+), T cytotoxic (CD3+ CD8+), NK (CD56+ CD3−), and B cells (CD19+). Briefly, 5×105 PBMCs were labeled with antibody according to manufacturer's instructions in 50µl of FACS buffer/tube (HBSS with 0.02% sodium azide and 0.1% BSA) and incubated in the dark on ice for 30 min; cells were washed with FACS buffer, then fixed with 0.25% paraformaldehyde and stored at 4°C in the dark until analyzed (1–2 days). Analyses were performed in a Becton Dickinson FACScan (San Jose, CA, USA) equipped with FACScan research software.
Statistical analyses
Analyses were conducted to determine whether women included in this study (N=89) differed on study variables compared to women from the parent study who did not provide blood samples (N=151). Psychological and immunologic variables were analyzed for outliers and normality. Immunologic variables were logarithmically transformed using log(x+1) to achieve normal distributions and maintain positive values. Transformed variables were used in all subsequent analyses, and descriptive values were back transformed to report results in pg/mL. Analyses of Covariance (ANCOVAs) were used to test for differences between elevated and low depressive symptoms groups on each of the three cytokine variables. Multiple regression analyses were used to assess associations between magnitude of depressive symptoms and cytokine values, for relationships between depressive symptoms and levels of five lymphocyte subsets measured, and for moderated relationships between depressive symptoms and cytokine values. Analyses were conducted using SPSS version 20 (SPSS, Chicago, IL, USA).
Selection of Covariates
Study analyses adjusted for demographic (age, ethnicity), medical (stage of disease, time from surgery to study assessment, use of pain medication, smoking status), and symptom intensity (fatigue severity) variables known to influence systemic inflammation (12, 16). Ethnicity was dummy coded with Reference Group – White/Non-Hispanic, Group 1 – Hispanic, Group 2 –Other. Stage of disease was categorized as non-invasive (stage 0) or invasive (stage I, II, or III). With this model, ratio of observations to predictors was approximately 10:1, adhering to proposed guidelines of 10–15 observations per predictor (25).
BMI has been suggested as an important covariate when assessing circulating inflammatory markers (16). However, BMI data was only available for 70% (62 women) of the sample. To maximize sample size, analyses were first conducted and reported adjusting for main study covariates. Then analyses were conducted with inclusion of BMI as an additional covariate, reducing the proportion of observations/predictors to approximately 6:1. This ratio is not ideal, and increases risk of over-fitting the model. Results of these analyses must be interpreted with caution (25).
It has been suggested that use of anti-depressant medications may be related to levels of pro-inflammatory cytokines (16, 26). However, excluding participants currently taking anti-depressant medications decreased our sample by 9 women. Nonetheless, regression analyses were conducted in the subsample of participants not currently taking anti-depressant medications adjusting for the main study covariates, and results are reported. Proportion of observations/predictors in these final analyses was approximately 9:1, so results must be interpreted with caution (25).
Results
On average, women in the present study were 50.40 years old (SD=7.69), had completed 15.45 years of education (SD=2.54), and approximately two thirds of the sample identified as White/Non-Hispanic (63 women, 70.8%). Seventeen women (19.1%) were diagnosed with stage 0 (carcinoma in-situ), 36 (40.4%) with stage I, 33 (37.1%) with stage II, and 3 (3.4%) with stage III BCa. Other patient characteristics are shown in Table 1.
Table 1.
Patient Characteristics
| Variable | Full Study Sample (N=89) |
Elevated Depressive Symptoms (N=36) |
Low Depressive Symptoms (N=53) |
Elevated vs. Low Depressive Symptoms Group Differences |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SEM | Mean | SEM | t | df | p | |
| Age (Years) | 50.40 | 7.69 | 49.25 | 1.35 | 51.19 | 1.01 | 1.17 | 87 | 0.25 |
| Education (Years) | 15.45 | 2.54 | 15.06 | 0.50 | 15.72 | 0.30 | 1.21 | 87 | 0.23 |
| Time from Surgery to Assessment (Days) | 37.63 | 22.67 | 40.40 | 4.26 | 35.79 | 2.87 | −0.93 | 86 | 0.35 |
| BMI | 26.32 | 5.24 | 28.55 | 1.29 | 25.03 | 0.66 | −2.69 | 61 | 0.009 |
| Number of positive lymph nodes | 0.87 | 2.46 | 0.83 | 0.30 | 0.89 | 0.31 | 0.10 | 87 | 0.92 |
| Fatigue Intensity | 4.37 | 1.70 | 4.89 | 0.27 | 3.99 | 0.23 | −2.50 | 84 | 0.014 |
| HRSD Continuous | 7.25 | 5.55 | 12.83 | 0.71 | 3.45 | 0.26 | |||
| N | % | N | % | N | % | χ2 | df | p | |
| Race/Ethnicity | 12.67 | 2 | 0.002 | ||||||
| White non-Hispanic | 63 | 70.8 | 18 | 50.0 | 45 | 84.9 | |||
| Hispanic | 17 | 19.1 | 12 | 33.3 | 5 | 9.4 | |||
| Other | 9 | 10.1 | 6 | 16.7 | 3 | 5.7 | |||
| Stage | 1.98 | 3 | 0.58 | ||||||
| 0 | 17 | 19.1 | 9 | 25.0 | 8 | 12.7 | |||
| I | 36 | 40.4 | 12 | 33.3 | 24 | 38.1 | |||
| II | 33 | 37.1 | 14 | 38.9 | 19 | 30.2 | |||
| III | 3 | 3.4 | 1 | 2.8 | 2 | 3.2 | |||
| Surgical Procedure | 0.26 | 1 | 0.61 | ||||||
| Lumpectomy | 49 | 55.1 | 21 | 58.3 | 28 | 52.8 | |||
| Mastectomy | 40 | 44.9 | 15 | 41.7 | 25 | 47.2 | |||
| ER Status | 2.04 | 2 | 0.36 | ||||||
| Positive | 57 | 64.0 | 20 | 55.6 | 37 | 69.8 | |||
| Negative | 11 | 12.4 | 6 | 16.7 | 5 | 9.4 | |||
| Unreported | 21 | 23.6 | 10 | 27.8 | 11 | 20.8 | |||
| PR Status | 1 | 2 | 0.61 | ||||||
| Positive | 40 | 44.9 | 14 | 38.9 | 26 | 49.1 | |||
| Negative | 19 | 21.3 | 8 | 22.2 | 11 | 20.8 | |||
| Unreported | 30 | 33.7 | 14 | 38.9 | 16 | 30.1 | |||
| HER-2/neu Status | 4.65 | 2 | 0.098 | ||||||
| Positive | 9 | 10.1 | 5 | 13.9 | 4 | 7.5 | |||
| Negative | 34 | 38.2 | 9 | 25.0 | 25 | 47.2 | |||
| Unreported | 46 | 51.7 | 22 | 61.1 | 24 | 45.3 | |||
| Triple Negative Status | 1.5 | 2 | 0.47 | ||||||
| Triple Negative | 4 | 4.5 | 1 | 2.8 | 3 | 5.7 | |||
| Not Triple Negative | 61 | 68.5 | 23 | 63.9 | 38 | 71.7 | |||
| Unreported | 24 | 27.0 | 12 | 33.3 | 12 | 22.6 | |||
| Smoking Status | 0 | 1 | 0.98 | ||||||
| Smoker | 5 | 5.6 | 2 | 5.6 | 3 | 5.7 | |||
| Non-Smoker | 84 | 94.4 | 34 | 94.4 | 50 | 94.3 | |||
| Anti-depressant medication use | 0.95 | 1 | 0.33 | ||||||
| Yes | 9 | 10.1 | 5 | 13.9 | 4 | 7.5 | |||
| No | 80 | 89.9 | 31 | 86.1 | 49 | 92.5 | |||
| Anxiety medication use | 1.36 | 1 | 0.24 | ||||||
| Yes | 17 | 80.9 | 9 | 25.0 | 8 | 15.1 | |||
| No | 72 | 80.9 | 27 | 75.0 | 45 | 84.9 | |||
| Sleep medication use | 6.62 | 1 | 0.010 | ||||||
| Yes | 14 | 15.7 | 10 | 27.8 | 4 | 7.5 | |||
| No | 75 | 84.3 | 26 | 72.2 | 49 | 92.5 | |||
| Pain medication use | 2.27 | 1 | 0.13 | ||||||
| Yes | 20 | 22.5 | 11 | 30.6 | 9 | 17.0 | |||
| No | 69 | 77.5 | 25 | 69.4 | 44 | 83.0 | |||
Note. Data represent descriptive statistics and differences between patients with elevated vs. low depressive symptoms.
As elevated vs. low depressive symptoms groups are defined by scores on the HRSD, tests of group differences on this measure are not meaningful and have been omitted.
Categorically, 36 women fell in the elevated depressive symptoms group (40.4%, HRSD M=12.83, SD=4.25) and 53 women (59.6%) fell in the low depressive symptoms group (HRSD M=3.45, SD=1.93). Mean HRSD score in the low depressive symptoms group was similar to a meta-analytic mean HRSD score among healthy controls (27).
The elevated and low depressive symptoms groups showed no differences on most demographic variables (with the exception of ethnicity) and clinicopathological/medical variables (with the exception of BMI and use of sleep medication). Groups differed on reported fatigue severity (see Table 1).
Categorical Depression Groupings and Serum Cytokines
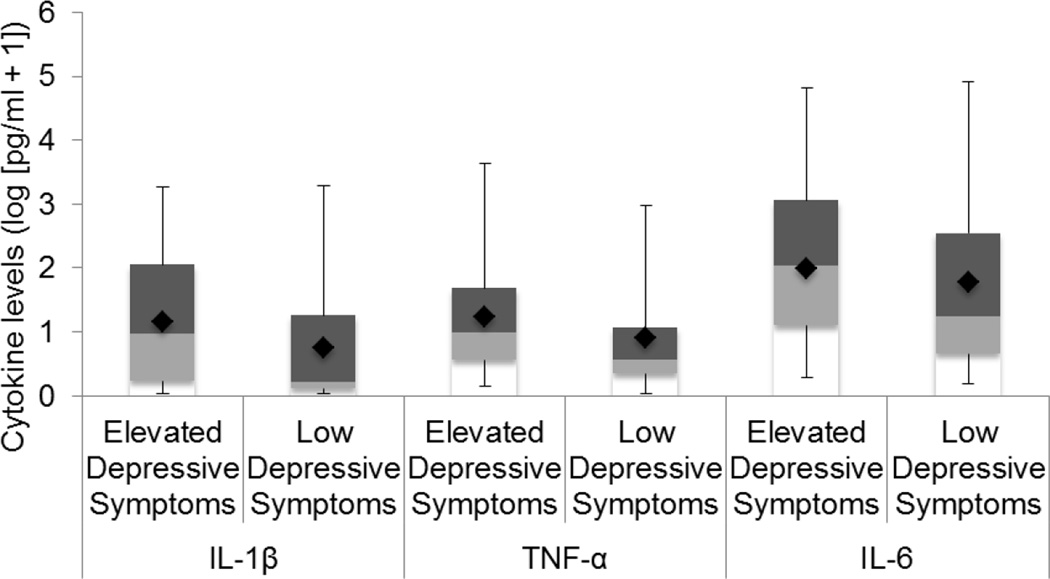
Analyses were conducted with depression dichotomized (see Table 2). Adjusting for age, ethnicity, stage of disease, time from surgery to study assessment, use of pain medications, fatigue severity, and smoking status, the elevated depressive symptoms group had marginally higher levels of serum IL-1β (unadjusted M=13.79, SD=9.47 pg/mL; adjusted M=14.49, 95% CI [6.11, 32.65]) than the low depressive symptoms group (unadjusted M=4.13, SD=6.41 pg/mL; adjusted M=4.68, 95% CI [1.96, 9.86]), F(9, 71)=1.95, p=0.059, η2=0.20. The elevated depressive symptoms group had higher levels of TNF-α (unadjusted M=15.98, SD=5.76 pg/mL; adjusted M=17.07, 95% CI [8.27, 34.32]) than the low depressive symptoms group (unadjusted M=6.59, SD=5.17 pg/mL; adjusted M=6.94, 95% CI [3.58, 12.80]), F(9, 72)=2.13, p=0.038, η2=0.21. The elevated depressive symptoms group (unadjusted M=127.82, SD=15.22; adjusted M=88.74, 95% CI [33.28, 233.96]) had higher levels of IL-6 than the low depressive symptoms group (unadjusted M=43.67, SD=16.38 pg/mL; adjusted M=61.52, 95% CI [27.44, 136.40]), F(9, 75)=2.01, p=0.050, η2=0.19 (see Figure 1). With BMI included, group differences in IL-1β became significant (p=0.050), and differences in TNF-α (p=0.13) and IL-6 (p=0.17) became non-significant.
Table 2.
The Associations Between Depressive Symptoms and Inflammatory Markers
| Immune Marker |
Elevated Depressive Symptoms |
Low Depressive Symptoms |
Analyses | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Lower Limit |
Upper Limit |
Mean | Lower Limit |
Upper Limit |
df | F | p | η2 | N | |
| IL-1β | 14.49 | 6.11 | 32.65 | 4.68 | 1.96 | 9.86 | 9, 71 | 1.95 | 0.059 | 0.20 | 81 |
| TNF-α | 17.07 | 8.27 | 34.32 | 6.94 | 3.58 | 12.80 | 9, 72 | 2.13 | 0.038 | 0.21 | 82 |
| IL-6 | 88.74 | 33.28 | 233.96 | 61.52 | 27.44 | 136.40 | 9, 75 | 2.01 | 0.050 | 0.19 | 85 |
Note. Results of 2-way ANCOVAs. Cytokine data are reported in pg/mL. Means and 95% confidence intervals are reported adjusted for age, ethnicity, stage of disease, time from surgery to study assessment, pain medication use, fatigue intensity, and smoking status.
Figure 1. Group Differences on Levels of Pro-Inflammatory Cytokines.
Box and whisker plots with cytokine levels reflecting unadjusted data values are represented as log(pg/ml+1). Each plot represents raw data values in quartiles including minimum values (limit of lower whisker), median values (cutoff between lower and upper boxes), and maximum values (limit of upper whisker). Diamonds represent means of cytokines adjusted for study covariates.
Continuous Levels of Depressive Symptoms and Serum Cytokines
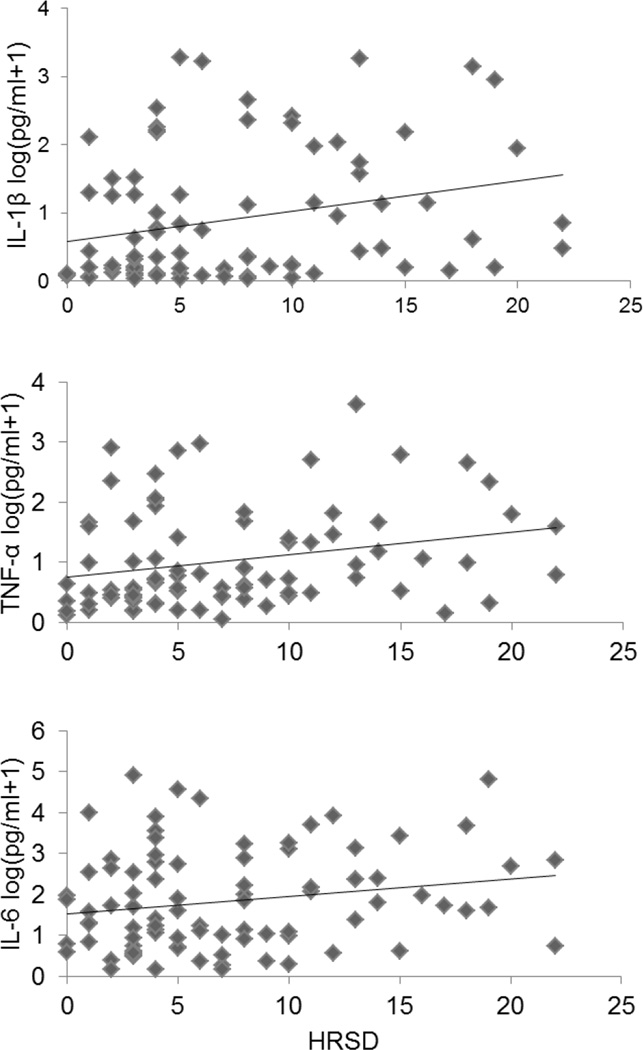
Analyses were conducted with magnitude of depressive symptoms on a continuum (see Table 3). Adjusting for age, ethnicity, stage of disease, time from surgery to study assessment, use of pain medications, fatigue severity, and smoking status, levels of pro-inflammatory cytokines were regressed on HRSD scores. Depressive symptoms were positively associated with IL-1β (β=0.06, t[80]=2.85, SE=0.02, p=0.006), and the model accounted for 25% of variance in IL-1β (R2=0.25, F[9, 71]=2.56, p=0.013). Depressive symptoms were positively associated with TNF-α (β=0.06, t[81]=3.12, SE=0.02, p=0.003), and the model accounted for 27% of variance in TNF-α (R2=0.27 F[9, 72]=3.02, p=0.004). Depressive symptoms were not significantly associated with IL-6 (β=0.03, t[84]=0.97, SE=0.03, p=0.34, R2=0.20, F[9, 75]=2.09, p=0.041, see Figure 2). With BMI included, associations between depressive symptoms and IL-1β (p=0.006) and TNF-α (p=0.007) remained significant, and associations between depressive symptoms and IL-6 remained non-significant (p=0.19). Excluding women taking anti-depressant medications, associations between depressive symptoms and IL-1β (p=0.006) and TNF-α (p=0.0006) remained significant, and associations between depressive symptoms and IL-6 remained non-significant (p=0.27).
Table 3.
Multivariate Analyses of the Association Between Continuous Depressive Symptom Scores with Pro-Inflammatory Cytokine levels
| β | t | SE | p | Model Statistics | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| df | F | p | R2 | N | ||||||
| IL-1β | ||||||||||
| Age | 0.04 | 3.21 | 0.01 | 0.002 | ||||||
| Ethnicity dummy code 1 | 0.42 | 1.50 | 0.28 | 0.14 | ||||||
| Ethnicity dummy code 2 | −0.05 | −0.14 | 0.35 | 0.89 | ||||||
| Stage (non-invasive vs. invasive) | 0.07 | 0.26 | 0.27 | 0.80 | ||||||
| Time since surgery | 0.00 | −0.83 | 0.01 | 0.41 | ||||||
| Pain medication use | −0.20 | −0.77 | 0.26 | 0.44 | ||||||
| Fatigue Intensity | −0.01 | −0.21 | 0.07 | 0.84 | ||||||
| Smoking status | −0.71 | −1.68 | 0.42 | 0.10 | ||||||
| HRSD | 0.06 | 2.85 | 0.02 | 0.006 | 9, 71 | 2.56 | 0.013 | 0.25 | 81 | |
| TNF-α | ||||||||||
| Age | 0.04 | 3.21 | 0.01 | 0.002 | ||||||
| Ethnicity dummy code 1 | 0.13 | 0.56 | 0.23 | 0.58 | ||||||
| Ethnicity dummy code 2 | 0.29 | 0.95 | 0.31 | 0.35 | ||||||
| Stage (non-invasive vs. invasive) | 0.42 | 1.88 | 0.22 | 0.064 | ||||||
| Time since surgery | 0.00 | −0.18 | 0.00 | 0.859 | ||||||
| Pain medication use | −0.19 | −0.86 | 0.22 | 0.40 | ||||||
| Fatigue Intensity | −0.01 | −0.09 | 0.06 | 0.93 | ||||||
| Smoking status | −0.78 | −2.20 | 0.36 | 0.031 | ||||||
| HRSD | 0.06 | 3.12 | 0.02 | 0.003 | 9, 72 | 3.02 | 0.004 | 0.27 | 82 | |
| IL-6 | ||||||||||
| Age | 0.03 | 1.93 | 0.02 | 0.058 | ||||||
| Ethnicity dummy code 1 | 0.45 | 1.28 | 0.35 | 0.21 | ||||||
| Ethnicity dummy code 2 | 1.30 | 2.91 | 0.45 | 0.005 | ||||||
| Stage (non-invasive vs. invasive) | 0.60 | 1.83 | 0.33 | 0.071 | ||||||
| Time since surgery | 0.01 | 1.17 | 0.01 | 0.244 | ||||||
| Pain medication use | 0.23 | 0.70 | 0.33 | 0.49 | ||||||
| Fatigue Intensity | −0.01 | −0.09 | 0.08 | 0.93 | ||||||
| Smoking status | −0.42 | −0.78 | 0.54 | 0.44 | ||||||
| HRSD | 0.03 | 0.97 | 0.03 | 0.34 | 9, 75 | 2.09 | 0.041 | 0.20 | 85 | |
Note. Analyses reported are adjusted for age, ethnicity, stage, time from surgery to study assessment, pain medication use, fatigue intensity, and smoking status.
Figure 2. Associations Between HRSD Scores and Inflammatory Markers.
Cytokine levels are represented as log(pg/ml+1). Figures reflect unadjusted data values.
Supplementary Analyses
Supplemental analyses examined associations between magnitude of depressive symptoms and circulating lymphocyte subsets adjusting for main study covariates. There were no significant associations between depressive symptoms and circulating levels of total T cells (CD3+, p=0.46; CD19−, p=0.093), T helper (CD3+, p=0.46; CD4+, p=0.77), T cytotoxic (CD3+, p=0.46; CD8+, p=0.71), NK (CD56+, p=0.83; CD3−, p=0.46), or B cells (CD19+, p=0.093). In addition, analyses examined whether associations between magnitude of depressive symptoms and serum pro-inflammatory cytokines differed as a function of demographic and clinicopathological/medical factors. There were no significant interactions between depressive symptoms and participant age (p=0.31, p=0.61, p=0.88), ethnicity (p=0.75, p=0.67, p=0.90), education (p=0.075, p=0.060, p=0.51), stage of disease (p=0.97, p=0.81, p=0.37), ER status (p=0.096, p=0.079, p=0.75), PR status (p=0.84, p=0.90, p=0.65), surgery type (p=0.12, p=0.31, p=0.15), fatigue severity (p=0.20, p=0.73, p=0.40), or BMI (p=0.19, p=0.46, p=0.18) on levels of IL-1β, TNF-α, or IL-6 respectively.
Discussion
This study found associations between interview-based depressive symptoms and serum levels of pro-inflammatory cytokines in women recently diagnosed with non-metastatic BCa who had undergone surgery but had not begun adjuvant therapy. Analyses were conducted adjusting for age, ethnicity, stage of disease, time from surgery to study assessment, use of pain medications, fatigue severity, and smoking status, which have been shown to affect systemic inflammatory markers (12, 16). Women with elevated levels of depressive symptoms had higher serum levels of TNF-α and IL-6, and marginally higher levels of IL-1β than women with low depressive symptoms. Increasing levels of depressive symptoms were associated with greater serum levels of IL-1β, TNF-α, but were not associated with IL-6.
Previous studies relating depression to inflammation in women with BCa have yielded mixed results (7, 12, 28). Discrepancies between studies could be explained by methodological differences in terms of study assessment time frames and measures of inflammation. To date, only one prior study has examined depression status and pro-inflammatory cytokines among women with non-metastatic BCa before beginning active adjuvant treatment (7).
In support of this work, we found that women with elevated depressive symptoms had higher levels of TNF-α and IL-6, and marginally higher levels of IL-1β than women with low depressive symptoms after adjusting for covariates, with medium effect sizes (29). This adds support to the literature showing a relationship between poor psychological adaptation (e.g., depression and depressed mood) and biobehavioral processes such as increased inflammation (10, 7, 28) via sympathetic nervous system signaling (30). These biological alterations, in turn, may interact with the tumor microenvironment to impact tumor growth (31), invasion (32), and metastatic signaling (33, 34) in cancer patients (for a review see 35).
Our findings that magnitude of depressive symptoms was significantly related to greater serum levels of IL-1β and TNF-α after adjusting for covariates indicate that as severity of depressive symptoms increase, so do levels of circulating pro-inflammatory cytokines. Paired with the observation that the range of depressive symptoms in this sample represents a lower range of depressive symptoms than would be observed in a clinically-defined depressive sample, these findings suggest that levels of depressive symptoms may be relevant for inflammatory processes within this lower range. Showing that findings held after excluding the small number of cases currently using anti-depressants reinforces this notion. Further, these findings suggest that a dichotomous conceptualization of depression within the non-clinical range as well as the actual magnitude of depressive symptoms may relate to indicators of inflammation in this population.
We extended our findings by including BMI as an additional covariate given the relationship observed between depressive symptoms, BMI, and systemic inflammation (16). When BMI was included, we found that women with elevated depressive symptoms had significantly higher levels of serum IL-1β than women with low depressive symptoms. As magnitude of depressive symptoms increased, so did levels of IL-1β and TNF-α. Categorically and continuously, depressive symptoms were not related to levels of IL-6. However, inclusion of BMI reduced our sample size and brought the observation-to-predictor ratio to approximately 6:1. This is considered an over-fitted model (25) and results should be interpreted with caution. While speculative, it is possible that the loss of the association between depressive symptoms and two of the cytokine measures upon addition of BMI suggests that BMI may at least partially mediate the association between depressive symptoms and inflammation in this sample. This is further supported by the observation that women in our sample who met the criterion for elevated depressive symptoms also revealed greater BMI levels. While these results add to the overall picture of the depressive symptoms-inflammation association, they warrant replication in a larger sample.
Supplementary analyses seeking insight into cellular sources of cytokine effects revealed no associations between magnitude of depressive symptoms and circulating lymphocyte phenotypes, suggesting that associations between depressive symptoms and cytokines are not likely due to covarying lymphocytes. It is possible that observed associations are due to differences in lymphocyte production of cytokines or activities of other cell types (e.g., monocytes or circulating tumor cells). However neither monocytes nor circulating tumor cells were enumerated in the present study. We previously reported that post-surgical BCa patients reporting greater negative affect in proportion to positive affect revealed greater leukocyte gene expression for IL1B, TNF, and IL6 in circulating PBMCs (15). Review of gene libraries (e.g., Gene Ontology) and Transcript Origin Analysis—a bioinformatics analysis (36, 37)—suggested that this pattern of gene expression reflects up-regulation of nuclear factor-kappa B (NFκB)-mediated pathways in myeloid cell lines (monocytes and plasmocytoid dendritic cells) (15). It is plausible that HRSD scores may relate to greater serum cytokine levels of IL-1β, TNF-α, and IL-6 in the present study via monocyte activation. These findings are not limited to BCa patients, as depression has been linked to up-regulation of leukocyte pro-inflammatory genes (IL1A, TNF and IL6) in patients with renal cell carcinoma (RCC), along with greater numbers of tumor-associated macrophages, and greater mortality over a 5-year period post diagnosis (38).
A final set of supplemental analyses sought insight into potential moderators of relationships between depressive symptoms and cytokines, and revealed no significant interactions between depressive symptoms and demographic (age, ethnicity, education), and clinicopathological/medical factors (stage of disease, ER status, PR status, surgery, fatigue severity, BMI). This suggests that the main study findings may be generalizable to individuals across many demographic and medically-defined subgroups of BCa patients.
Notably, the elevated and low depressive symptoms groups showed no significant differences on age, education, surgery type, clinicopathological variables, and other medications that could explain differences in inflammatory status (39, 9, 40). Methodologically, limiting our sample to women with no signs of current infections allowed us to rule out the likelihood that depressive symptoms and inflammatory levels were driven by acute infectious processes (41).
Strengths and Limitations
This study was cross-sectional: direction of causality between study variables cannot be determined. However, previous research has longitudinally demonstrated that depressive symptoms predict later inflammation in BCa patients (28). An additional limitation was lack of data available pertaining to more specific medication use (e.g., beta blockers, aspirin, cox inhibitors), post-surgical residual disease and complications, surgical margins, and additional clinicopathological variables to further distinguish cancer subtypes (e.g., luminal a, luminal b, histology, grade). However, complete data was obtained regarding stage of disease and number of positive lymph nodes. Nonetheless, additional studies of depression and inflammation among women with BCa would benefit from including these additional variables, as they may impact findings. BMI data was only available for 70% of the sample, limiting power to detect associations between depressive symptoms and cytokines while being mindful of the observation–to-predictor ratio in light of additional covariates (25). The present study focused on a sample of women predominantly highly educated and non-Hispanic White who were potentially motivated to participate in health-related research, limiting generalizability of findings. However, one third of the sample identified as ethnic minority, mostly Hispanic women, distinguishing the present study from previous work (e.g. 7). The fact that a greater proportion of depressed women were of Hispanic ethnicity is interesting and demands further attention, since Hispanic women may have greater difficulties adjusting to BCa treatment (42). Additional strengths of the study were inclusion of theoretically supported covariates (age, ethnicity, stage of disease, use of pain medications, fatigue severity, and smoking status), exclusion of women with active infections, and exploration of potential moderators of associations between depressive symptoms and cytokines. Finally, the study captured women with BCa at a distinct point in cancer treatment, namely after surgery but before beginning active systemic or local adjuvant treatment. Thus, treatment related factors may be excluded as potential confounders of the depression-inflammation association.
Future Research
Future research would benefit from examining longitudinal relationships between depression and cytokines, allowing for time-lagged analyses to clarify direction of causality between depressive symptoms and cytokine levels that could not be determined in the present study. Such analyses could investigate whether relationships between depression and cytokines change over treatment and survivorship. To date, one study has demonstrated a relationship between depression and time-lagged inflammation (i.e. white blood cell count, neutrophil count, ratio of helper T to suppressor T cells) in the context of a psychosocial intervention targeting depressive symptoms for women with BCa (28). In addition, we previously demonstrated that a psychosocial intervention may down-regulate leukocyte expression of pro-inflammatory genes (15). Additional longitudinal research could similarly focus on how changes in depression precede, follow, or parallel changes in pro-inflammatory cytokines in the context of psychosocial interventions known to reduce depressive symptoms in BCa patients undergoing primary treatment (43).
Recent work demonstrates that pharmacological interventions targeting depressive symptoms may impact levels of inflammation (44). Extending this research to samples of women with BCa would be beneficial. An alternative conceptualization of biological treatments of depression and inflammation would be to target inflammation and subsequently depression through anti-inflammatory medications. One study reported a decrease of depressive symptoms in response to a TNF antagonist in medically healthy individuals with treatment-resistant MDD (45). There is much potential for expanding this research by examining anti-inflammatory treatments for inflammation and depression in the general population and BCa patients (for a review see 46).
The present findings have implications for treatment of BCa patients with comorbid depressive symptoms. By identifying specific biological mechanisms affected by depression, it is possible to target these mechanisms in the treatment of BCa in an effort to reduce probability of tumor growth, invasion, and metastasis. Reducing depression through psychological or pharmacologic interventions early in treatment (for review see 13) seems a promising approach for modulating these biological mechanisms.
Conclusions
The present study suggests that in the period after surgery for non-metastatic BCa, women with greater depressive symptomology had higher serum levels of pro-inflammatory cytokines than non-depressed women. Research investigating longitudinal associations between depression and inflammation is warranted in BCa patients and other populations given the suspected effects of depression and inflammation on health outcomes in cancer patients (3, 35). It is important to explore effects of psychosocial and biological interventions on concurrently reducing depression and inflammation early in treatment of BCa and how this affects long-term health outcomes.
Acknowledgments
Michael H. Antoni the principal investigator of this study, as well as Bonnie B. Blomberg, Alain Diaz, Suzanne Lechner, Stefan Glück, and Charles S. Carver received funding for this study through the National Cancer Institute of the National Institutes of Health, 5R01 CA64710.
Acronyms used
- BCa
breast cancer
- MDD
Major Depressive Disorder
- TNF
tumor necrosis factor
- IL
interleukin
- HRSD
Hamilton Rating Scale for Depression
- ER
estrogen receptor
- PR
progesterone receptor
- BMI
body mass index
- ANCOVA
analysis of covariance
- NFκB
Nuclear factor kappa B
Footnotes
Trial Registration: NCT01422551.
Conflicts of interest and sources of funding
References
- 1.Bower J. Behavioral symptoms in patients with breast cancer and survivors. J Clin Oncol. 2008;26:768–777. doi: 10.1200/JCO.2007.14.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badger T, Braden C, Mishel M, Longman A. Depression burden, psychological adjustment, and quality of life in women with breast cancer: Patterns over time. Res Nurs Health. 2004;27:19–28. doi: 10.1002/nur.20002. [DOI] [PubMed] [Google Scholar]
- 3.Giese-Davis J, Collie K, Rancourt K, Neri E, Kraemer H, Spiegel D. Decrease in depressive symptoms is associated with longer survival in patients with metastatic breast cancer: A secondary analysis. J Clin Oncol. 2011;29:413–420. doi: 10.1200/JCO.2010.28.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cole S. Chronic inflammation and breast cancer recurrence. J Clin Oncol. 2009;27:3418–3419. doi: 10.1200/JCO.2009.21.9782. 27: 3418–3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howren M, Lamkin D, Suls J. Associations of depression with c-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosom Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 6.Musselman D, Miller A, Porter M, Manatunga A, Gao F, Penna S, Pearce B, Landry J, Glover S, McDaniel J, Nemeroff C. Higher than normal plasma interleukin-6 concentrations in cancer patients with depression: Preliminary findings. Am J Psychiatry. 2001;158:1252–1257. doi: 10.1176/appi.ajp.158.8.1252. [DOI] [PubMed] [Google Scholar]
- 7.Soygur H, Palaoglu O, Akarsu E, Cankurtaran E, Ozalp E, Turhan L, Ayhan I. Interleukin-6 levels and HPA axis activation in breast cancer patients with major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1242–1247. doi: 10.1016/j.pnpbp.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Slavich G, Irwin M. From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychol Bull. 2014;140:774–815. doi: 10.1037/a0035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 10.Armaiz-Pena G, Cole S, Lutgendorf S, Sood A. Neuroendocrine influences on cancer progression. Brain Behav Immun. 2013;30:S19–S25. doi: 10.1016/j.bbi.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paterlini-Brechot P, Benali NL. Circulating tumor cells (CTC) detection: Clinical impact and future directions. Cancer Lett. 2007;253:180–204. doi: 10.1016/j.canlet.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 12.Bower J, Ganz P, Tao M, Wenhau H, Belin T, Sepah S, Cole S, Aziz N. Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clin Cancer Res. 2009;15:5534–5540. doi: 10.1158/1078-0432.CCR-08-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antoni M. Psychosocial intervention effects on adaptation, disease course, and biobehavioral processes in cancer. Brain Behav Immun. 2013;30:S88–S98. doi: 10.1016/j.bbi.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aggarwal B, Shishodia S, Sandur S, Pandey M, Sethi G. Inflammation and cancer: How hot is the link? Biochem Pharmacol. 2006;72:1605–1621. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 15.Antoni M, Lutgendorf S, Blomberg B, Carver C, Lechner S, Diaz A, Stagl J, Arevalo J, Cole S. Cognitive-behavioral stress management reverses anxiety-related leukocyte transcriptional dynamics. Biol Psychiatry. 2012;71:366–372. doi: 10.1016/j.biopsych.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Connor M, Bower J, Jin Cho H, Creswell J, Simitrov S, Hamby M, Hoyt M, Martin J, Robles T, Sloan E, Thomas K, Irwin M. To assess, to control, to exclude: Effects of biobehavioral factors on circulating inflammatory markers. Brain Behav Immun. 2009;23:887–897. doi: 10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antoni M, Lechner S, Kazi A, Wimberly S, Sifre T, Urcuyo K, Phillips K, Gluck S, Carver C. How stress management improves quality of life after treatment for breast cancer. J Consult Clin Psychol. 2006;74:1143–1152. doi: 10.1037/0022-006X.74.6.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller I, Bishop S, Norman W, Maddever H. The modified Hamilton rating scale for depression: reliability and validity. Psychiatry Res. 1985;14:131–142. doi: 10.1016/0165-1781(85)90057-5. [DOI] [PubMed] [Google Scholar]
- 20.Williams J. Structured interview guide for the Hamilton depression rating scale (SIGH-D) SIGHD-IDSC. 1989;45:1–16. doi: 10.1001/archpsyc.1988.01800320058007. [DOI] [PubMed] [Google Scholar]
- 21.Zimmerman M, Posternak M, Chelminski I. Implications of using different cut-offs on symptom severity scales to define remission from depression. Int Clin Psychopharm. 2004;19:215–220. doi: 10.1097/01.yic.0000130232.57629.46. [DOI] [PubMed] [Google Scholar]
- 22.Poleshuck E, Katz J, Andrus C, Hogan L, Jung B, Kulick D, Dworkin R. Risk factors for chronic pain following breast cancer surgery: A prospective study. J Pain. 2006;7:626–634. doi: 10.1016/j.jpain.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hann D, Jacobsen P, Azzarello L, Martin S, Curran S, Fields K, Greenberg H, Lyman G. Measurement of fatigue in cancer patients: development and validation of the Fatigue Symptom Inventory. Qual Life Res. 1998;7:301–310. doi: 10.1023/a:1024929829627. [DOI] [PubMed] [Google Scholar]
- 24.Donovan K, Jacobsen P, Andrykowski M, Winters E, Balducci L, Malik U, Kenady D, McGrath P. Course of fatigue in women receiving chemotherapy and/or radiotherapy for early stage breast cancer. J Pain Symptom Manage. 2004;28:373–380. doi: 10.1016/j.jpainsymman.2004.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Babyak M. What you see may not be what you get: A brief, nontechnical introduction to overfitting in regression-type models. Psychosom Med. 2004;66:411–421. doi: 10.1097/01.psy.0000127692.23278.a9. [DOI] [PubMed] [Google Scholar]
- 26.Hamer M, Batty GD, Marmot MG, Singh-Manoux A, Kivimaki M. Anti-depressant medication use and C-reactive protein: Results from two population-based studies. Brain Behav Immun. 2011;25:168–173. doi: 10.1016/j.bbi.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zimmerman M, Chelminski I, Posternak M. A review of studies of the Hamilton depression rating scale in healthy controls. J Nerv Ment Dis. 2004;192:595–601. doi: 10.1097/01.nmd.0000138226.22761.39. [DOI] [PubMed] [Google Scholar]
- 28.Thornton L, Andersen B, Schuler T, Carson W., III A psychological intervention reduces inflammatory markers by alleviating depressive symptoms: Secondary analysis of a randomized controlled trial. Psychosom Med. 2009;71:715–724. doi: 10.1097/PSY.0b013e3181b0545c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen J. Statistical power analysis for the behavioral sciences. New York, NY: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 30.Kiecolt-Glaser J, McGuire L, Robles T, Glaser R. Psychoneuroimmunology: Psychological influences on immune function and health. J Consult Clin Psychol. 2002;70:537–547. doi: 10.1037//0022-006x.70.3.537. [DOI] [PubMed] [Google Scholar]
- 31.Lutgendorf S, Johnson E, Cooper B, Anderson B, Sorosky J, Buller R, Sood A. Vascular endothelial growth factor and social support in patients with ovarian carcinoma. Cancer. 2002;95:808–815. doi: 10.1002/cncr.10739. [DOI] [PubMed] [Google Scholar]
- 32.Sood A, Bhatty R, Kamat A, Landen C, Ham L, Thaker P, Li Y, Gershenson D, Cole S. Stress hormone-mediated invasion of ovarian cancer cells. Clin Cancer Res. 2006;12:369–375. doi: 10.1158/1078-0432.CCR-05-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sloan E, Priceman S, Cox B, Yu S, Pimental M, Tangkanangnukul V, Arevalo J, Morizono K, Karanikolas B, Wu L, Sood A, Cole S. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010;70:7042–7052. doi: 10.1158/0008-5472.CAN-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang E, Kim S, Donovan E, Chen M, Gross A, Webster Marketon J, Barsky S, Glaser R. Norepinephrine upregulates VEGF, IL-8, and IL-6 expression in human melanoma tumor cell lines: Implications for stress-related enhancement of tumor progression. Brain Behav Immun. 2009;23:267–275. doi: 10.1016/j.bbi.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lutgendorf S, Sood A, Antoni M. Host factors and cancer progression: Biobehavioral signaling pathways and interventions. J Clin Oncol. 2010;28:4094–4099. doi: 10.1200/JCO.2009.26.9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cole S, Hawkley L, Arevalo J, Sung C, Rose R, Cacioppo J. Social regulation of gene expression in human leukocytes. Genome Biol. 2007;8:R189.1–R189.13. doi: 10.1186/gb-2007-8-9-r189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cole S. Elevating the perspective on human stress genomics. Psychoneuroendocrinology. 2010;35:955–962. doi: 10.1016/j.psyneuen.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen L, Cole S, Sood A, Prinsloo S, Kirschbaum C, Arevalo J, Jennings N, Scott S, Vence L, Wei Q, Kentor D, Radvanyi L, Tannir N, Jonasch E, Tamboli P, Pisters L. Depressive symptoms and cortisol rhythmicity predict survival in patients with renal cell carcinoma: Role of inflammatory signaling. PLOS ONE. 2012;7:e42324. doi: 10.1371/journal.pone.0042324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hannestad J, DellaGioia N, Bloch M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: A meta-analysis. Neuropsychopharmacology. 2011;36:2452–2459. doi: 10.1038/npp.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Owen N, Poulton T, Hay F, Mohamed-Ali V, Steptoe A. Socioeconomic status, C-reactive protein, immune factors, and responses to acute mental illness. Brain Behav Immun. 2003;17:286–295. doi: 10.1016/s0889-1591(03)00058-8. [DOI] [PubMed] [Google Scholar]
- 41.Dantzer R. Cytokine-induced sickness behavior: Mechanisms and implications. Annals NY Academy Sciences. 2001;933:222–234. doi: 10.1111/j.1749-6632.2001.tb05827.x. [DOI] [PubMed] [Google Scholar]
- 42.Yanez B, Thompson E, Stanton A. Quality of life among Latina breast cancer patients: A systematic review of the literature. J Cancer Surviv. 2011;5:191–207. doi: 10.1007/s11764-011-0171-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Antoni M, Lehman J, Kilbourn K, Boyers A, Culver J, Alferi S, Yount S, McGregor B, Arena P, Harris S, Price A, Carver C. Cognitive-behavioral stress management intervention decreases the prevalence of depression and enhances benefit finding among women under treatment for early-stage breast cancer. Health Psychol. 2001;20:20–32. doi: 10.1037//0278-6133.20.1.20. [DOI] [PubMed] [Google Scholar]
- 44.Tuglu C, Kara S, Caliyurt O, Vardar E, Abay E. Increased serum tumor necrosis factor-alpha levels and treatment response in major depressive disorder. Psychopharmacology. 2003;170:429–433. doi: 10.1007/s00213-003-1566-z. [DOI] [PubMed] [Google Scholar]
- 45.Raison C, Rutherford R, Woolwine B, Shup C, Schettler P, Drake D, Haroon E, Miller A. A randomized controlled trial of the tumor necrosis factor antagonist Infliximab for treatment-resistant depression: The role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70:31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller A, Maletic V, Raison C. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]