Abstract
Background and Purpose
Epidemiologic studies have documented that plasma D-dimer, a fibrin degradation product, is a risk marker for coronary heart disease, but there is limited prospective evidence for stroke. Given that thrombosis is a key mechanism for many strokes, we studied whether D-dimer is a risk marker for ischemic stroke incidence in the Atherosclerosis Risk in Communities (ARIC) Study.
Methods
We measured D-dimer in 11,415 ARIC participants free of stroke and CHD in 1992–1995. We followed them for stroke, stroke subtype, and coronary heart disease events through 2012.
Results
Over a median of 18 years of follow-up, 719 participants had incident strokes (628 ischemic and 91 hemorrhagic). D-dimer was associated positively with risk of total, ischemic, and cardioembolic strokes, with risk elevated primarily for the highest quintile of D-dimer. After adjustment for other cardiovascular risk factors, the hazard ratio for the highest versus lowest quintile of D-dimer was 1.30 (95% CI 1.02–1.67) for total stroke, 1.33 (95% CI 1.02–1.73) for ischemic stroke, and 1.79 (95% CI 1.08–2.95) for cardioembolic stroke. There was no association with hemorrhagic, lacunar, or nonlacunar stroke categories. D-dimer was positively but weakly associated with CHD incidence.
Conclusions
A higher basal plasma D-dimer concentration in the general population is a risk marker for ischemic stroke, especially cardioembolic stroke.
Keywords: D-dimer, stroke, prospective study, coronary disease
Plasma D-dimer, a fibrin degradation product, is a valuable clinical test for detecting acute venous thrombosis. Because it is a broad thrombosis marker, the basal D-dimer concentration also serves as an epidemiological indicator of future cardiovascular disease risk, including coronary heart disease (CHD) and venous thromboembolism.1,2 A recent meta-analysis of 18 prospective studies reported a 23% greater risk of coronary heart disease per one standard deviation higher baseline concentration of D-dimer.1
Thrombosis is a key mechanism for many acute ischemic strokes. Yet, few epidemiological studies, and none to our knowledge in the U.S., have reported whether or not basal D-dimer concentrations are a marker of future ischemic stroke risk.3–6 Standard deviation higher D-dimer concentrations were associated with a 24% higher total stroke incidence rate in British men3 and a 21% higher ischemic stroke incidence rate in Italian adults.4 Two other prospective studies in the United Kingdom also found that D-dimer is associated positively with stroke incidence.5,6 Cross-sectional investigations of the Atherosclerosis Risk in Communities (ARIC) Study found higher D-dimer to be associated with greater prevalences of subclinical brain infarction7 and carotid atherosclerosis.8
To provide additional prospective evidence, we studied here the association of plasma D-dimer and incident ischemic stroke in the ARIC Study. We also updated the association of D-dimer with coronary heart disease in ARIC, previously reported in a small nested case-control study.9
Methods
Study Population
ARIC investigators have reported ARIC’s study design, methods, and stroke incidence rates in detail elsewhere.10,11 In brief, 15,792 men and women aged 45 to 64 years enrolled in the ARIC cohort in 1987–1989, and had subsequent examinations in 1990–92, 1993–95, 1996–98, and 2011–13, with ongoing regular telephone contact. The institutional review committees at each study center approved the methods and staff obtained informed participant consent.
Plasma D-dimer Measurements
We measured D-dimer concentrations on fasting citrate plasma that had been collected at ARIC visit 3 (in 1993–95) and stored unthawed at −70°C until analysis in 2014, using an immuno-turbidimetric assay (Liatest D-DI; Diagnostica Stago, Parsippany, NJ). The analytical coefficient of variation for this assay is 4 – 16%. Blind analysis of 73 pairs of ARIC samples split at the time of blood draw and stored until 2014 yielded an intra-class reliability coefficient of 0.92. The manufacturer’s normal reference range is 0.22 – 4.0 μg/mL, with most normal people’s values being <0.4 μg/mL.
Measurement of Risk Factors and Prevalent Medical Conditions
We analyzed risk factors measured by published methods9,10,12 at ARIC visit 3, the visit in which D-dimer was measured. ARIC technicians measured seated blood pressure three times with a random-zero sphygmomanometer, and we averaged the last two for analysis. We defined diabetes as a fasting serum glucose ≥126 mg/dL, nonfasting glucose ≥ 200 mg/dL, a physician diagnosis of diabetes, or use of antidiabetic medication in the past 2 weeks. ARIC assessed prevalent medical conditions at visit 3 using published definitions for heart failure,12 atrial fibrillation,13 left ventricular hypertrophy by electrocardiogram,14 and peripheral artery disease.
We defined history of stroke, for exclusion, as a baseline self-reported physician-diagnosed stroke or an incident stroke by ARIC criteria between baseline and visit 3. We similarly defined history of coronary heart disease at visit 3 as a myocardial infarction or a coronary revascularization.
For a supplemental analysis, we used ARIC visit 4 measurements of plasma N-terminal pro-B-type natriuretic peptide (NT-proBNP), high sensitivity troponin T (TnT), and C-reactive protein (CRP), because we had previously shown that these were risk markers for ischemic stroke.13 ARIC did not measure these biomarkers at visit 3.
Ascertainment of Stroke
We included stroke events occurring between ARIC visit 3 in 1993–95 and December 31, 2012. We did not ascertain transient ischemic attacks. We first identified participants’ hospitalizations and deaths via annual follow-up calls and by review of discharge lists from local hospitals. If the discharge diagnoses included a cerebrovascular disease code (International Classification of Diseases, Ninth Revision, code 430 to 438), if a cerebrovascular condition or procedure was mentioned in the discharge summary or on a neuroimaging report, abstractors recorded signs and symptoms and photocopied neuroimaging and other diagnostic reports.11 We classified hospitalized strokes from the abstracted diagnostic information as definite or probable via a computer algorithm and an expert reviewer,11 according to criteria adapted from the National Survey of Stroke.15 If certain diagnostic information was not obtained or not recorded, we considered the diagnostic criterion as not having been met. We further classified strokes into hemorrhagic stroke or ischemic stroke on the basis of neuroimaging studies or autopsies. We subclassified ischemic strokes as cardioembolic when there was either (1) medical record evidence of a potential cardiac source of embolus (e.g., valvular heart disease, atrial fibrillation or flutter, MI, cardiac or arterial operation or procedure, cardiac myxoma, bacterial endocarditis) or, rarely, (2) autopsy evidence of an infarcted area in the brain and a source of possible cerebral emboli in a vessel or the presence of an embolus in the brain. We also subclassified definite ischemic strokes as either nonlacunar or lacunar, on the basis of the recorded neuroimaging results, with lacunar requiring both of the following conditions: (1) typical location of the infarct (basal ganglia, brain stem, thalamus, internal capsule, or cerebral white matter) and (2) infarct size of ≤2 cm or unstated size. Lesions stated in imaging reports as being lacunar still needed to meet ARIC criteria, to be classified as lacunar.
Ascertainment of Coronary Heart Disease
Using published criteria, we defined CHD incidence as (1) a definite or probable MI,16 (2) a definite CHD death,16 or (3) a coronary revascularization.
Statistical Analysis
Of the 12,887 ARIC participants who attended visit 3, we excluded those without D-dimer measurement (n = 340), those with coronary heart disease or stroke prior to D-dimer assessment (n = 1,060), those who were either not white or not African American in Jackson or Forsyth County (n = 72). This left a maximum of 11,415 participants for analyses of incident stroke and subtypes. We computed time at risk from the date of D-dimer blood draw to the date for the earliest of the following: incident stroke, death, last contact, or end of follow-up. We treated the occurrence of a hemorrhagic stroke before an ischemic stroke as a censoring event and vice versa.
Using version 9.3 of SAS (SAS Institute, Cary, NC), we described participants’ characteristics by D-dimer quintile. We plotted Kaplan-Meier curves and used Poisson regression to compute incidence rates for quintiles of D-dimer. We used Cox proportional hazards models to estimate hazard ratios (HR) and 95% confidence intervals of incident ischemic stroke, with a test for trend in stroke using the quintile median D-dimer values to represent each quintile. We also computed hazard ratios in relation to continuous D-dimer and produced restricted cubic spline graphs with knots at the 5th, 35th, 65th, and 95th percentiles. We verified that the proportional hazards assumption held (p>0.05) for all outcomes by testing a log time by D-dimer interaction term. Because there was no evidence of multiplicative D-dimer by race or D-dimer by sex interactions (p>0.05), we pooled these subgroups. Model 1 for D-dimer and stroke adjusted for age, sex, race and ARIC field center; Model 2 additionally for systolic blood pressure, antihypertensive medication use (yes or no), diabetes status (yes or no), total and HDL cholesterol, body mass index, smoking status (never, former, current), alcohol intake, sports score, and education level (< high school, high school, > high school). Model 3 additionally adjusted for prevalent atrial fibrillation, heart failure, left ventricular hypertrophy by electrocardiogram, and peripheral artery disease (all yes or no). Model 4 additionally adjusted for NT-proBNP, TnT, and CRP from ARIC visit 4. For Model 4, we excluded events occurring between visits 3 and 4.
As a supplemental analysis, we repeated this strategy for incident coronary heart disease.
Results
Among the 11,415 participants free of stroke and CHD at ARIC visit 3, plasma D-dimer was higher in women than men, in African Americans than whites, and was associated positively with most stroke risk factors, other than lipids, and other prevalent cardiovascular conditions (Table 1). Over a median of 18 years of follow-up (max = 20 years), 719 participants had incident definite plus probable stroke events (628 ischemic and 91 hemorrhagic).
Table 1.
Characteristics of Participants According to Quintiles of Plasma D-dimer Concentration, the ARIC Study, 1993–1995.
| Characteristics | D-dimer quintiles (μg/ml)
|
||||
|---|---|---|---|---|---|
| Q1 (≤ 0.14) |
Q2 (0.15–0.22) |
Q3 (0.23–0.32) |
Q4 (0.33–0.49) |
Q5 (> 0.49) |
|
| N | 2308 | 2480 | 2283 | 2069 | 2275 |
| Age (years) | 58.4 (5.4) | 59.2 (5.5) | 59.8 (5.6) | 60.4 (5.8) | 61.3 (5.7) |
| Women (%) | 50.4 | 53.2 | 60.4 | 62.1 | 65.3 |
| Race, African American (%) | 10.1 | 14.8 | 24.9 | 32.7 | 35.3 |
| Education <high school (%) | 12.0 | 16.4 | 20.3 | 24.0 | 25.7 |
| Current Smoking (%) | 15.8 | 15.0 | 18.7 | 19.1 | 19.3 |
| Alcohol intake (g/d) | 6.7 (15.9) | 5.9 (14.2) | 6.1 (17.0) | 5.1 (15.3) | 5.2 (16.8) |
| Diabetes (%) | 11.0 | 13.0 | 13.8 | 17.6 | 16.0 |
| Systolic blood pressure (mm Hg) | 121 (17) | 123 (18) | 125 (19) | 127 (20) | 127 (20) |
| Hypertension meds (%) | 26.7 | 30.3 | 34.8 | 37.8 | 44.2 |
| Body mass index (kg/m2) | 27.1 (4.7) | 27.9 (5.0) | 28.8 (5.4) | 29.7 (6.2) | 29.2 (6.3) |
| Sports index (range 1–5) | 2.6 (0.8) | 2.6 (0.8) | 2.5 (0.8) | 2.5 (0.8) | 2.4 (0.8) |
| HDL-cholesterol (mg/dl) | 53.2 (17.9) | 52.1 (18.0) | 52.4 (17.6) | 53.3 (19.1) | 54.0 (18.7) |
| Total-cholesterol (mg/dl) | 211 (38) | 207 (37) | 207 (36) | 206 (38) | 207 (38) |
| Left ventricular hypertrophy (%) | 1.4 | 1.4 | 2.3 | 3.1 | 3.6 |
| History of atrial fibrillation (%) | 1.1 | 0.9 | 1.1 | 1.3 | 1.4 |
| History of peripheral artery disease (%) | 1.3 | 1.9 | 2.2 | 3.7 | 4.8 |
| History of heart failure (%) | 2.6 | 3.2 | 3.8 | 4.2 | 5.8 |
| C-reactive protein, (mg/L)* | 3.4 (4.9) | 3.7 (5.2) | 4.3 (5.9) | 5.3 (7.5) | 5.8 (9.1) |
| Troponin T (ng/L)* | 6 (4) | 7 (7) | 7 (9) | 8 (14) | 8 (14) |
| NT-proBNP, (pg/mL)* | 97 (207) | 109 (281) | 116 (238) | 170 (957) | 450 (8675) |
Values are mean (standard deviation) for continuous variables and percentages for categorical variables. ARIC, Atherosclerosis Risk in Communities; HDL, high density lipoprotein; NT-proBNP, N-terminal pro-brain natriuretic peptide.
From ARIC visit 4 (1996–1998)
As shown in Table 2 and Figure 1, D-dimer was associated positively with incidence rates of total stroke, total ischemic stroke, and its cardioembolic stroke subtype. In contrast, D-dimer was not associated significantly with hemorrhagic stroke or the lacunar and nonlacunar subtypes of ischemic stroke after adjustment for demographic factors (Table 2). Even though the tests for continuous D-dimer associations were statistically significant and cubic spline analyses (Supplemental Figures) confirmed that the associations were approximately linear, the incidence rates were elevated mainly for the highest versus lowest quintiles of D-dimer for total stroke (Model 1 HR = 1.43), ischemic stroke (HR = 1.47), and especially cardioembolic stroke (HR = 2.06). D-dimer was associated significantly with both definite and probable total stroke (Model 1 HRs = 1.33 and 1.86, respectively, for highest versus lowest quintile) (Supplemental Table).
Table 2.
Incidence Rates (95% CI) and Hazard Ratios (95% CI) of Stroke Subtypes in Relation to D-dimer, the ARIC Study, 1993–2012.
| Stroke subtype | D-dimer Quintiles, μg/ml
|
Continuous D-dimer
|
|||||
|---|---|---|---|---|---|---|---|
| Q1 (≤ 0.14) |
Q2 (0.15–0.22) |
Q3 (0.23–0.32) |
Q4 (0.33–0.49) |
Q5 (> 0.49) |
PTREND | Per 1-SD Loge increment* | |
| Total stroke | |||||||
| Events, n | 114 | 123 | 151 | 127 | 204 | ||
| Incidence rate† | 3.0 (2.5–3.6) | 3.0 (2.5–3.6) | 4.1 (3.5–4.8) | 3.9 (3.3–4.7) | 6.1 (5.3–6.9) | ||
| Model 1 HR | 1 (Referent) | 0.92 (0.71–1.18) | 1.13 (0.88–1.45) | 0.99 (0.76–1.28) | 1.43 (1.12–1.82) | 0.001 | 1.11 (1.03–1.20) |
| Model 2 HR | 1 (Referent) | 0.89 (0.69–1.16) | 1.04 (0.81–1.33) | 0.86 (0.66–1.12) | 1.30 (1.02–1.67) | 0.001 | 1.09 (1.002–1.18) |
| Model 3 HR | 1 (Referent) | 0.90 (0.70–1.17) | 1.04 (0.81–1.34) | 0.85 (0.65–1.11) | 1.30 (1.02–1.67) | 0.002 | 1.08 (1.00–1.17) |
| Model 4 HR | 1 (Referent) | 1.00 (0.74–1.35) | 1.16 (0.87–1.56) | 1.00 (0.73–1.36) | 1.36 (1.01–1.82) | 0.016 | 1.08 (0.98–1.18) |
| Hemorrhagic stroke | |||||||
| Events, n | 19 | 13 | 21 | 10 | 28 | ||
| Incidence rate† | 0.5 (0.3–0.8) | 0.3 (0.2–0.5) | 0.6 (0.4–0.9) | 0.3 (0.2–0.6) | 0.8 (0.6–1.2) | ||
| Model 1 HR | 1 (Referent) | 0.59 (0.29–1.19) | 0.95 (0.50–1.79) | 0.48 (0.22–1.06) | 1.22 (0.66–2.27) | 0.13 | 1.06 (0.86–1.32) |
| Model 2 HR | 1 (Referent) | 0.58 (0.28–1.17) | 0.82 (0.43–1.58) | 0.45 (0.21–1.00) | 1.20 (0.64–2.23) | 0.11 | 1.06 (0.85–1.33) |
| Ischemic stroke | |||||||
| Events, n | 95 | 110 | 130 | 117 | 176 | ||
| Incidence rate† | 2.5 (2.0–3.0) | 2.7 (2.2–3.3) | 3.5 (3.0–4.2) | 3.6 (3.0–4.3) | 5.2 (4.5–6.0) | ||
| Model 1 HR | 1 (Referent) | 0.98 (0.75–1.30) | 1.17 (0.89–1.53) | 1.09 (0.82–1.44) | 1.47 (1.13–1.91) | 0.001 | 1.12 (1.03–1.21) |
| Model 2 HR | 1 (Referent) | 0.96 (0.73–1.27) | 1.09 (0.83–1.43) | 0.94 (0.70–1.25) | 1.33 (1.02–1.73) | 0.006 | 1.09 (1.00–1.19) |
| Model 3 HR | 1 (Referent) | 0.97 (0.74–1.28) | 1.09 (0.83–1.44) | 0.94 (0.70–1.24) | 1.33 (1.02–1.74) | 0.006 | 1.09 (1.00–1.19) |
| Model 4 HR | 1 (Referent) | 1.12 (0.81–1.55) | 1.25 (0.90–1.72) | 1.09 (0.73–1.52) | 1.43 (1.04–1.98) | 0.028 | 1.10 (0.99–1.21) |
| Cardioembolic | |||||||
| Events, n | 24 | 32 | 32 | 27 | 60 | ||
| Incidence rate† | 0.6 (0.4–0.9) | 0.8 (0.6–1.1) | 0.9 (0.6–1.2) | 0.8 (0.6–1.2) | 1.8 (1.4–2.3) | ||
| Model 1 HR | 1 (Referent) | 1.12 (0.66–1.90) | 1.13 (0.66–1.94) | 1.02 (0.58–1.79) | 2.06 (1.25–3.38) | 0.001 | 1.23 (1.06–1.43) |
| Model 2 HR | 1 (Referent) | 1.06 (0.62–1.80) | 1.01 (0.59–1.74) | 0.85 (0.48–1.50) | 1.79 (1.08–2.95) | 0.002 | 1.20 (1.02–1.41) |
| Model 3 HR | 1 (Referent) | 1.10 (0.64–1.87) | 1.02 (0.59–1.76) | 0.86 (0.49–1.53) | 1.85 (1.11–3.06) | 0.001 | 1.21 (1.02–1.42) |
| Model 4 HR | 1 (Referent) | 1.63 (0.87–3.05) | 1.46 (0.76–2.80) | 1.50 (0.77–2.92) | 2.58 (1.40–4.78) | 0.001 | 1.25 (1.05–1.49) |
| Lacunar | |||||||
| Events, n | 21 | 22 | 32 | 36 | 31 | ||
| Incidence rate† | 0.5 (0.4–0.8) | 0.5 (0.4–0.8) | 0.9 (0.6–1.2) | 1.1 (0.8–1.5) | 0.9 (0.6–1.3) | ||
| Model 1 HR | 1 (Referent) | 0.90 (0.50–1.64) | 1.26 (0.72–2.21) | 1.44 (0.82–2.51) | 1.10 (0.62–1.98) | 0.77 | 1.04 (0.87–1.24) |
| Model 2 HR | 1 (Referent) | 0.91 (0.50–1.66) | 1.27 (0.72–2.24) | 1.31 (0.74–2.31) | 1.11 (0.62–2.00) | 0.77 | 1.04 (0.87–1.25) |
| Nonlacunar | |||||||
| Events, n | 50 | 56 | 66 | 54 | 85 | ||
| Incidence rate† | 1.3 (1.0–1.7) | 1.4 (1.1–1.8) | 1.8 (1.4–2.3) | 1.7 (1.3–2.2) | 2.5 (2.0–3.1) | ||
| Model 1 HR | 1 (Referent) | 0.95 (0.65–1.40) | 1.14 (0.79–1.66) | 0.96 (0.65–1.42) | 1.35 (0.94–1.96) | 0.04 | 1.09 (0.97–1.22) |
| Model 2 HR | 1 (Referent) | 0.93 (0.63–1.37) | 1.05 (0.72–1.53) | 0.82 (0.55–1.24) | 1.19 (0.82–1.73) | 0.18 | 1.05 (0.93–1.19) |
Model 1: Hazard ratio (HR) with 95% confidence interval from Cox proportional hazards model adjusted for age, sex and race–field center.
Model 2: Model 1 additionally adjusted for education level, smoking status, alcohol intake, body mass index, systolic blood pressure, antihypertensive medication use, diabetes status, total and HDL cholesterol.
Model 3: Model 2 additionally adjusted for prevalent left ventricular hypertrophy, atrial fibrillation, peripheral artery disease and heart failure.
Model 4: Model 3 additionally adjusted for C-reactive protein, troponin T and N-terminal pro-B-type natriuretic peptide from ARIC visit 4 (1996–1998).
1 Loge standard deviation (SD) = 0.97 loge μg/ml.
Unadjusted incidence rate per 1,000 person-years with 95% confidence interval.
ARIC, Atherosclerosis Risk in Communities; CI, confidence interval; HR, hazard ratio; SD, standard deviation.
Figure 1.
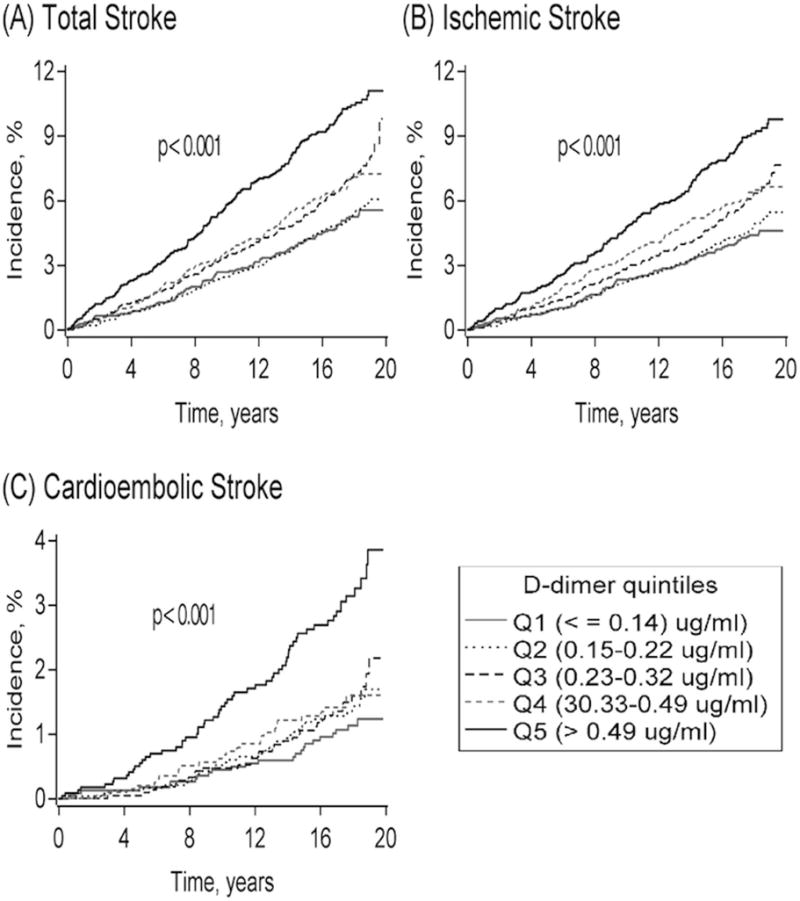
Kaplan-Meier cumulative incidence estimates of (A) total stroke, (B) ischemic stroke, and (C) cardioembolic stroke according to D-dimer quintiles in 11,415 ARIC participants, 1993–2012. The Log-rank p value of <0.001 represents differences among all groups (unadjusted).
Adjustment for other stroke risk factors somewhat attenuated the associations of D-dimer with stroke (Table 2). Yet, model 2 hazard ratios for the highest versus the lowest quintile of D-dimer remained statistically significant for total stroke (HR = 1.30), total ischemic stroke (HR = 1.33), and cardioembolic stroke (HR = 1.79). Adjustment of these three associations for prevalent cardiovascular conditions (Model 3) had little impact. Additional adjustment for NT-proBNP, TnT, and CRP (Model 4) slightly strengthened these associations.
To assess whether D-dimer may add to total stroke prediction, we computed the area under the receiver operating characteristic curve (AUC) for Model 2, with and without log D-dimer. The AUC was 0.797 without D-dimer and rose to only 0.798 with D-dimer.
For comparison with the associations for stroke, Table 3 shows that D-dimer was positively but more weakly associated with CHD incidence.
Table 3.
Incidence Rates (95% CI) and Hazard Ratios (95% CI) of D-dimer in Relation to Incident Coronary Heart Disease, the ARIC Study, 1993–2012.
| D-dimer quintiles, μg/ml
|
Continuous D-dimer
|
||||||
|---|---|---|---|---|---|---|---|
| Q1 (≤ 0.14) |
Q2 (0.15–0.22) |
Q3 (0.23–0.32) |
Q4 (0.33–0.49) |
Q5 (> 0.49) |
PTREND | Per 1−SD Loge increment* | |
| CHD | |||||||
| Events, n | 342 | 367 | 343 | 352 | 363 | ||
| Incidence rate† | 9.3 (8.4–10.4) | 9.5 (8.5–10.5) | 9.8 (8.8–10.9) | 11.4 (10.3–12.7) | 11.2 (10.1–12.4) | ||
| Model 1 HR | 1 (Referent) | 0.98 (0.85–1.14) | 1.07 (0.92–1.25) | 1.25 (1.07–1.46) | 1.24 (1.07–1.45) | 0.001 | 1.07 (1.02–1.12) |
| Model 2 HR | 1 (Referent) | 0.96 (0.83–1.12) | 1.03 (0.88–1.20) | 1.16 (0.99–1.35) | 1.14 (0.98–1.33) | 0.025 | 1.05 (1.002–1.11) |
Model 1: Hazard ratio (HR) with 95% confidence interval from Cox proportional hazards model adjusted for age, sex and race–field center.
Model 2: Model 1 additionally adjusted for education level, smoking status, alcohol intake, body mass index, systolic blood pressure, antihypertensive medication use, diabetes status, total and HDL cholesterol.
1 Loge standard deviation (SD) = 0.97 loge μg/ml.
Unadjusted incidence rate per 1,000 person-years with 95% confidence interval.
ARIC, Atherosclerosis Risk in Communities; CI, confidence interval; HR, hazard ratio; SD, standard deviation.
Discussion
This large prospective epidemiological investigation found that a higher plasma level of D-dimer in the general population, particularly being in the highest D-dimer quintile, was a moderately strong, independent risk factor for ischemic stroke, especially the cardioembolic stroke subtype. In contrast, D-dimer was not a risk factor for hemorrhagic stroke or the lacunar and noncardioembolic subtypes of ischemic stroke. We also corroborated a weak positive association of D-dimer with CHD, which has been well-documented.1 Despite the association of D-dimer with stroke incidence, suggesting a possible etiologic role for elevated D-dimer, D-dimer did not improve the prediction of future stroke, when added to other stroke risk factors.
D-dimer is a fibrinogen degradation product that reflects thrombus formation and breakdown. Prior studies have also documented that D-dimer levels vary in the general population and tend to correlate positively with other cardiovascular risk factors.17 Thus, some individuals seem to have an increased prothrombotic tendency, which may be in part due to elevated risk factors, but which may independently increase their risk of future atherothrombotic and venous thromboembolic events. All four previous prospective epidemiological studies of D-dimer and stroke also found a positive association for total or ischemic stroke.3–6 One reported a positive association with hemorrhagic stroke,4 in contrast with our findings. No previous study examined ischemic stroke subtypes, to evaluate particular risk for cardioembolic stroke. In addition, no previous study included a large group of non-white participants, and none adjusted for so many potential confounders as we did. It is striking that a small elevation in D-dimer measured in middle-aged ARIC participants was associated with increased ischemic stroke incidence over 20 years of follow-up, in addition to greater prevalences of subclinical brain infarction7 and carotid atherosclerosis.8
We anticipated and confirmed that D-dimer is associated most strongly with cardioembolic stroke, as it is the subtype most closely linked to fibrin thrombi. Adjustment for prevalent medical conditions associated with D-dimer, which might predispose to thrombi (i.e., atrial fibrillation, left ventricular hypertrophy by ECG, heart failure, and peripheral artery disease), did not affect the D-dimer association with cardioembolic stroke. However, certainly some participants would have developed these during the 20 years of follow-up, and the inability to control for this confounding might have exaggerated the hazard ratios for D-dimer. Adjustment for NT-pro BNP and TnT, which we previously showed were strong risk markers for cardioembolic stroke,13 had no impact on the D-dimer associations. A limitation to this adjustment was that these biomarkers were not available at the time of D-dimer assessment, but only three years later.
There are some other drawbacks to this study. First, we had only one measure of plasma D-dimer. Insofar as there is random biological or laboratory variability in D-dimer concentrations, the observed associations of D-dimer with stroke incidence would tend to underestimate the true associations. With a single measure, we also could not examine if change in D-dimer or D-dimer concentration just before stroke occurrence is etiologically important. Second, although our validation of stroke events was thorough, it was based, as in most epidemiological studies, on medical reviews and not standardized neurological examinations of all potential stroke patients. We likely misclassified some cardioembolic strokes, because even when the stroke records indicated a potential cardiac source of embolus, we (and the attending physicians) could not always verify that embolism truly occurred. We also could not readily separate our nonlacunar ischemic stroke group into large vessel disease versus other ischemic or cryptogenic ischemic strokes. Third, our prior publication demonstrating a positive association of D-dimer with subclinical lacunar infarcts7 is seemingly in conflict with the present results showing no association with clinical lacunar strokes. This may result from limitations in the classification process for clinical lacunar stroke or possibly from differences related to lacune subtypes, as the subtypes might have different probabilities of clinical presentation as well as contrasting risk factor associations.18
In conclusion, a higher basal plasma D-dimer concentration in the general population is a risk marker for ischemic stroke, especially cardioembolic stroke. Whether the association is causal is uncertain, and it seems unlikely that D-dimer could be clinically useful to identify patients at risk of stroke. On the other hand, recent evidence indicates that “ideal cardiovascular health” is associated with both lower D-dimer19 and lower stroke risk,20 suggesting that cardiovascular health may reduce risk for stroke by antithrombotic mechanisms.
Supplementary Material
Acknowledgments
The authors thank the staff and participants of the ARIC Study for their important contributions, and Elaine Cornell for supervising D-dimer measurements.
Sources of Funding
The National Heart, Lung, and Blood Institute (NHLBI) supported the D-dimer measurements via U01 HL096902, and ARIC via contracts HHSN268201100005C to HHSN268201100012C.
Footnotes
Journal Subject Codes: [10062] Epidemiology; [10173] Cerebrovascular Disease/Stroke; [10071] Risk factors; [10184] Acute coronary syndromes.
Disclosures
None.
References
- 1.Willeit P, Thompson A, Aspelund T, Rumley A, Eiriksdottir G, Lowe G, et al. Hemostatic factors and risk of coronary heart disease in general populations: New prospective study and updated meta-analyses. PLoS One. 2013;8:e55175. doi: 10.1371/journal.pone.0055175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cushman M, Folsom AR, Wang L, Aleksic N, Rosamond WD, Tracy RP, et al. Fibrin fragment D-dimer and the risk of future venous thrombosis. Blood. 2003;101:1243–1248. doi: 10.1182/blood-2002-05-1416. [DOI] [PubMed] [Google Scholar]
- 3.Wannamethee SG, Whincup PH, Lennon L, Rumley A, Lowe GD. Fibrin D-dimer, tissue-type plasminogen activator, von Willebrand factor, and risk of incident stroke in older men. Stroke. 2013;43:1206–1211. doi: 10.1161/STROKEAHA.111.636373. [DOI] [PubMed] [Google Scholar]
- 4.Di Castelnuovo A, Agnoli C, de Curtis A, Giurdanella MC, Sieri S, Mattiello A, et al. Elevated levels of D-dimers increase the risk of ischaemic and haemorrhagic stroke. Findings from the EPICORE Study. Thromb Haemost. 2014;112:941–946. doi: 10.1160/TH14-04-0297. [DOI] [PubMed] [Google Scholar]
- 5.Smith FB, Lee AJ, Fowkes FG, Price JF, Rumley A, Lowe GD. Hemostatic factors as predictors of ischemic heart disease and stroke in the Edinburgh Artery Study. Arterioscler Thromb Vasc Biol. 1997;17:3321–3325. doi: 10.1161/01.atv.17.11.3321. [DOI] [PubMed] [Google Scholar]
- 6.Smith A, Patterson C, Yarnell J, Rumley A, Ben-Schlomo Y, Lowe G. Which hemostatic markers add to the predictive value of conventional risk factors for coronary heart disease and ischemic stroke? The Caerphilly Study. Circulation. 2005;112:3080–3087. doi: 10.1161/CIRCULATIONAHA.105.557132. [DOI] [PubMed] [Google Scholar]
- 7.Gottesman RF, Cummiskey C, Chambless L, Wu KK, Aleksic N, Folsom AR, et al. Hemostatic factors and subclinical brain infarction in a community-based sample: the ARIC Study. Cerebrovasc Dis. 2009;28:589–594. doi: 10.1159/000247603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salomaa V, Stinson V, Kark JD, Folsom AR, Davis CE, Wu KK. Association of fibrinolytic parameters with early atherosclerosis. The ARIC Study. Atherosclerosis Risk in Communities Study. Circulation. 1995;91:284–290. doi: 10.1161/01.cir.91.2.284. [DOI] [PubMed] [Google Scholar]
- 9.Folsom AR, Aleksic N, Park E, Salomaa V, Juneja H, Wu KK. Prospective study of fibrinolytic factors and incident coronary heart disease: The Atherosclerosis Risk in Communities (ARIC) Study. Arterioscler Thromb Vasc Biol. 2001;21:611–617. doi: 10.1161/01.atv.21.4.611. [DOI] [PubMed] [Google Scholar]
- 10.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 11.Rosamond WD, Folsom AR, Chambless LE, Wang C-H, McGovern PG, Howard G, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) Cohort. Stroke. 1999;30:736–743. doi: 10.1161/01.str.30.4.736. [DOI] [PubMed] [Google Scholar]
- 12.Folsom AR, Yamagishi K, Hozawa A, Chambless LE. Absolute and attributable risks of heart failure incidence in relation to optimal risk factors. Circ Heart Fail. 2009;2:11–17. doi: 10.1161/CIRCHEARTFAILURE.108.794933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Folsom AR, Nambi V, Bell EJ, Oluleye OW, Gothesman RF, Lutsey PL, et al. Troponin T, N-terminal pro-B-type natriuretic peptide, and incidence of stroke: The Atherosclerosis Risk in Communities Study. Stroke. 2013;44:961–967. doi: 10.1161/STROKEAHA.111.000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crow RS, Prineas RJ, Rautaharju P, Hannah P, Liebson PR. Relation between electrocardiography and echocardiography for left ventricular mass in mild systemic hypertension (results from Treatment of Mild Hypertension Study) Am J Cardiol. 1995;75:1233–1238. [PubMed] [Google Scholar]
- 15.The National Survey of Stroke. National Institute of Neurological and Communicative Disorders and Stroke. Stroke. 1981;12(2 Pt 2 Suppl 1):I1–I91. [PubMed] [Google Scholar]
- 16.White AD, Folsom AR, Chambless LE, Sharrett AR, Yang K, Conwill D, et al. the ARIC Investigators Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: Methods and initial two years’ experience. J Clin Epidemiol. 1996;49:223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 17.Folsom AR, Delaney JC, Lutsey PL, Zakai NA, Jenny N, Polak J, et al. Associations of factor VIIIc, D-dimer, and plasmin-antiplasmin with incident cardiovascular disease and all-cause mortality. Am J Hematol. 2009;84:349–353. doi: 10.1002/ajh.21429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bezerra DC, Sharrett AR, Matsushita K, Gottesman RF, Shibata D, Mosley TH, Jr, et al. Risk factors for lacune subtypes in the Atherosclerosis Risk in Communities (ARIC) Study. Neurology. 2012;78:102–108. doi: 10.1212/WNL.0b013e31823efc42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xanthakis V, Enserro DM, Murabito JM, Polak JF, Wollert KC, Januzzi JL, et al. Ideal cardiovascular health: associations with biomarkers and subclinical disease and impact on incidence of cardiovascular disease in the Framingham Offspring Study. Circulation. 2014;130:1676–1683. doi: 10.1161/CIRCULATIONAHA.114.009273. [DOI] [PubMed] [Google Scholar]
- 20.Kulshreshtha A, Vaccarino V, Judd SE, Howard VJ, McClellan WM, Muntner P, et al. Life’s Simple 7 and risk of incident stroke: the reasons for geographic and racial difference in stroke study. Stroke. 2013;44:1909–1914. doi: 10.1161/STROKEAHA.111.000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


