Abstract
Objectives
Bioactive glass (BAG) is known to possess antimicrobial and remineralizing properties; however, the use of BAG as a filler for resin based composite restorations to slow recurrent caries has not been studied. Accordingly, the objective of this study was to investigate the effect of 15 wt% BAG additions to a resin composite on bacterial biofilms penetrating into marginal gaps of simulated tooth fillings in vitro during cyclic mechanical loading.
Methods
Human molars were machined into approximately 3 mm thick disks of dentin and 1.5–2 mm deep composite restorations were placed. A narrow 15–20 micrometer wide dentin-composite gap was allowed to form along half of the margin by not applying dental adhesive to that region. Two different 72 wt% filled composites were used, one with 15 wt% BAG filler (15BAG) and the balance silanated strontium glass and one filled with OX-50 and silanated strontium glass without BAG (0BAG – control). Samples of both groups had Streptococcus mutans biofilms grown on the surface and were tested inside a bioreactor for two weeks while subjected to periods of cyclic mechanical loading. After post-test biofilm viability was confirmed, each specimen was fixed in glutaraldehyde, gram positive stained, mounted in resin and cross-sectioned to reveal the gap profile. Depth of biofilm penetration for 0BAG and 15BAG was quantified as the fraction of gap depth. The data were compared using a Student’s t-test.
Results
The average depth of bacterial penetration into the marginal gap for the 15BAG samples was significantly smaller (~61%) in comparison to 0BAG, where 100% penetration was observed for all samples with the biofilm penetrating underneath of the restoration in some cases.
Significance
BAG containing resin dental composites reduce biofilm penetration into marginal gaps of simulated tooth restorations. This suggests BAG containing composites may have the potential to slow the development and propagation of secondary tooth decay at restoration margins.
Keywords: Resin Composite, Marginal Gap, Biofilm, Streptococcus mutans, Bioactive Glass, Secondary Caries
1. Introduction
The U.S. National Institutes of Health estimates that >122 million composite tooth restorations are placed in the United States annually; however, annual failure rates up to 15% have been reported [1] and a review of the literature suggests the average lifetime of posterior dental composites is only six years [2]. Furthermore, a majority of restorations are replacements of failed restorations [3]. The most common reason for the replacements is secondary caries occurring at the margins [4–7]. Secondary caries is caused by bacterial microflora [8,9] and the formation of a biofilm (plaque) at and within the restoration-tooth margin [10] most likely facilitated by a gap forming between the two that allows bacterial colonization [11]. While cyclic loading is a known potential cause of failure for both tooth tissue and restorative materials [12–17], margin failure and gap propagation may also occur due to the cyclic loading experienced by restorations during mastication [18–21]. While resin composites have relatively good adhesive/sealing properties, polymerization shrinkage of the resin during curing imposes stresses on the interface that increase the chance of interfacial failure when combined with cyclic occlusal loading [11].
While it is known that after successful gap colonization, bacteria consume saccharides and produce lactic acid as the byproduct [22] that demineralizes tooth structure, there is currently a poor understanding of the factors influencing biofilm development and propagation into interfacial gaps. While bacterial biofilm formation is considered a necessary ingredient, a biofilm alone does not guarantee tooth decay [6,23]. Furthermore, a recent study suggests that cyclic loading plays an important role in aiding biofilm penetration deep into marginal gaps [24]. The decreased pH due to acid production then shifts the equilibrium dissolution reaction of hydroxylapatite towards demineralization with calcium and phosphate ions leaching out of the tissue, causing caries propagation [25–27]. Streptococcus mutans strains have been identified as the most abundant bacterial species under the restoration in cases of secondary caries [28,29]. Such findings suggest a strong need for restorative composites with antimicrobial and/or remineralization properties to slow secondary caries formation and extend the life of composite restorations.
Many materials have antimicrobial properties: copper, zinc, silver, various silica-based glasses etc. [30,31]. Bioactive glass (BAG) has been shown to have both an antimicrobial effect on oral bacteria and the ability to remineralize adjacent mineralized tissues [32–37]. The antimicrobial effect of BAG is attributed in part to the release of ions (e.g., calcium and phosphate) that have a toxic effect on the cells and cause neutralization of the local acidic environment [38], the latter leads to a local increase in pH that is not well tolerated by many oral bacteria [39]. However, the exact mechanism responsible for the antimicrobial effect of BAG is not completely elucidated.
Although the first BAG was developed more than 40 years ago [40], exploration into its potential for use in resin based dental restorative composites has only very recently begun [41–43]. It has been shown that composites containing up to 15% by weight non-silanated BAG filler can have mechanical properties comparable to, or superior to, commercial composites [42] and that the additional of BAG fillers does not compromise the degree of monomer conversion [43]. Furthermore, despite BAG ions leaching out of the composite the degradation of mechanical properties with ageing is no worse, or better than, many commercial composites [42]. Tauböck et al. showed that BAG particles embedded in a resin matrix can still induce bioactivity and increase the pH of a buffered saline solution [43]. Finally, another recent study has looked at doping the BAG with Ag to enahnce its antimicrobial effect [41].
While it appears clear that BAG-containing composites can meet the mechanical property requirements for dental restorations, there is a need to study the secondary caries resistance of tooth restorations made from BAG-containing composites. Accordingly, the goal of this research was to examine the effect of BAG additions on bacterial biofilm formation along marginal gaps of simulated tooth fillings in vitro. This study will use a previously developed in-vitro testing model that applies cyclic loading to a restored tooth while in a living oral bacteria environment [24]. It is hypothesized that BAG-containing composites will have a negative effect on biofilm formation in marginal gaps due to the antimicrobial effects of BAG.
2. Materials and methods
2.1. Bioactive glass composite preparation
BAG filler was produced by the sol-gel process, which was reported in detail previously in [44]. Breifly, pure alkoxides (tetraethyl orthosilicate (TEOS, Si(OC2H5)4), calcium methoxyethoxide (CMOE, C6H14O4Ca), and triethyl phosphate (TEP, (C2H5)3PO4)) were used for BAG synthesis. CMOE was synthesized from Ca metal and methoxyethanol, but all other reagents were obtained from Sigma Aldrich. Solutions of the alkoxides in methoxyethanol were prepared in an inert dry nitrogen atmosphere glovebox.
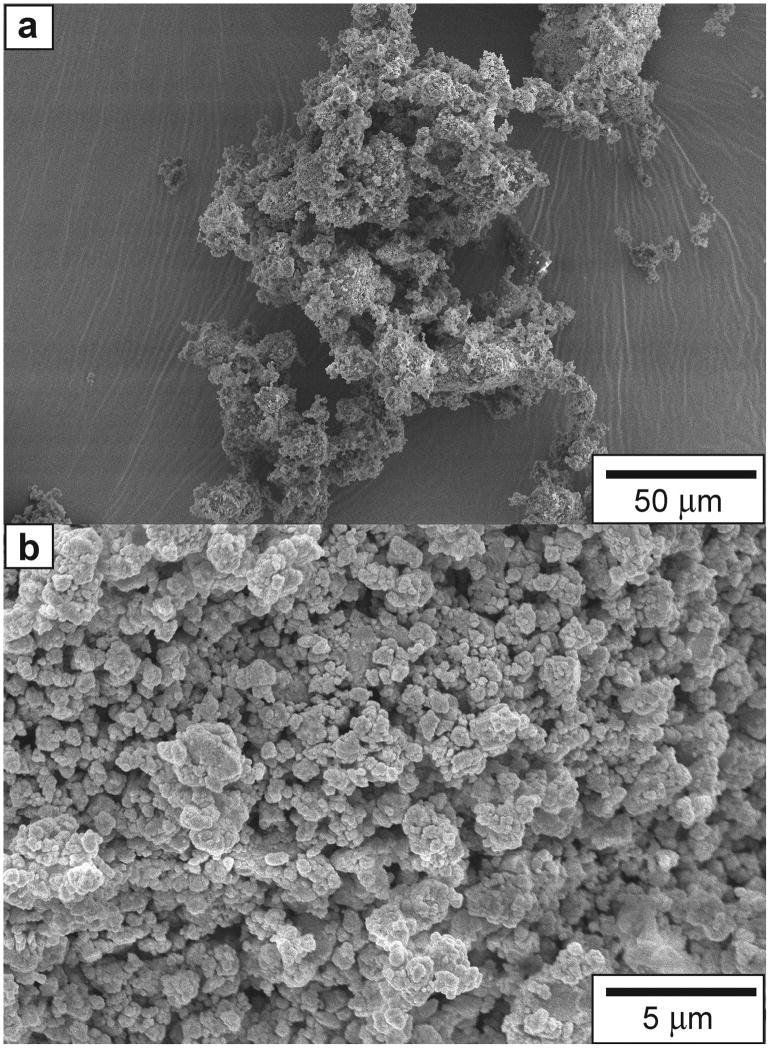
The solution was aged, air-dried and then dried at elevated temperatures to evaporate the solvent completely and stabilize the glass. The obtained product was first ball milled, and then processed in a Micronizer jet-mill (Sturtevant Inc., Hanover, MA) to a final fine particle size of 0.04 – 3 μm. The final composition of the BAG filler used was approximately 65% SiO2, 31% CaO and 4% P2O5 (mol%). SEM micrographs of agglomerated BAG filler particles can be seen in Fig. 1.
Fig. 1.
Scanning electron micrograph of the bioactive glass filler used in this study. a) An agglomerate of BAG particles. b) A higher magnification view of the BAG particles.
The composite was prepared by combining 57 wt% silanated strontium glass (Bisco Inc.) and 15 wt% BAG with resin matrix containing a 50:50 mixture of bisphenol A glycidyl methacrylate (BisGMA):triethylene glycoldimethacrylate (TEGDMA) monomers with 0.4 wt% of camphorquinone (CQ), 0.8 wt% of 4-dimethylaminobenzoic acid ethyl ether (EDMAB), and 0.05 wt% of 3, 5-di-tertbutyl-4-hydroxytoluene (BHT)). The 15% BAG addition was selected based on previous work that demonstrated no significant reduction in mechanical properties when adding up to 15% BAG [42]. The control composite (0BAG), was filled with 5 wt% aerosol silica (OX-50, Degussa) and 67 wt% silanated strontium glass. The 5 wt % silica addition was necessary to achieve adequate handling of the uncured composite. Both composites had a total filler content of 72 wt.% and full details on the composite preparation and mechanical properties may be found in [42].
2.2. Sample preparation
Recently extracted human molars were used to produce simulated tooth restoration samples. The complete procedure for sample preparation was described previously in detail in [24]. In brief, the cusps of each molar were sectioned off, and a second section was made across the tooth immediately above the pulp. This sectioning resulted in a disk-shaped slab of tooth ~2.5–3 mm thick and ~9 mm in diameter, composed mostly of dentin with some enamel on the circumference. A cavity was machined into the center of each sample 1.5–2 mm deep (to always leave a pulpal floor 1 mm thick) and 5 mm in diameter using a computer controlled milling system (CNC specimen former, U. of Iowa). After machining, the samples were soaked in 1% chloramine T for one week for sterilization. After initial sterilization, the bottom of the cavity and half of its circumference were treated with a dentin bonding agent (Clearfil SE Bond) following the manufacturers instructions. First the primer component was actively applied to the cut dentin surfaces with a disposable micro brush tip for 20 seconds. Then a mild stream of air was blown on the primer to evaporate volatile components until no observable liquid movement could be detected. Next, the bonding component was applied to all primed surfaces with a disposable micro brush tip, then brush thinned with a separate disposable brush tip to ensure a uniform thickness of the adhesive layer. The bonding agent was cured for 10 seconds using a Kerr Demetron Demi light curing unit with an approximate 1000 mW/cm2 irradiance. Finally, the composite was inserted in a single increment (max thickness of 2 mm) and cured for 20 seconds with the same light curing unit.
Due to polymerization shrinkage, a ~15–20 μm gap was formed around approximately half of the circumference where the dentin bonding agent was not originally applied. A 1 mm thick shim was used to protect the half of the circumference where the gaps were created (Fig. 2). The composite surface of the sample was polished with fine abrasive disks (Sof-flex, 3M ESPE) to reveal the cavosurface margin and the gap. The samples were sterilized in 70% ethanol for one hour and placed in sterile brain heart infusion (BHI) media for storage, thus allowing ethanol to diffuse out of the specimen and to check for successful sterilization. Six (N =6) 15BAG and six (N = 6) 0BAG samples were prepared.
Fig. 2.
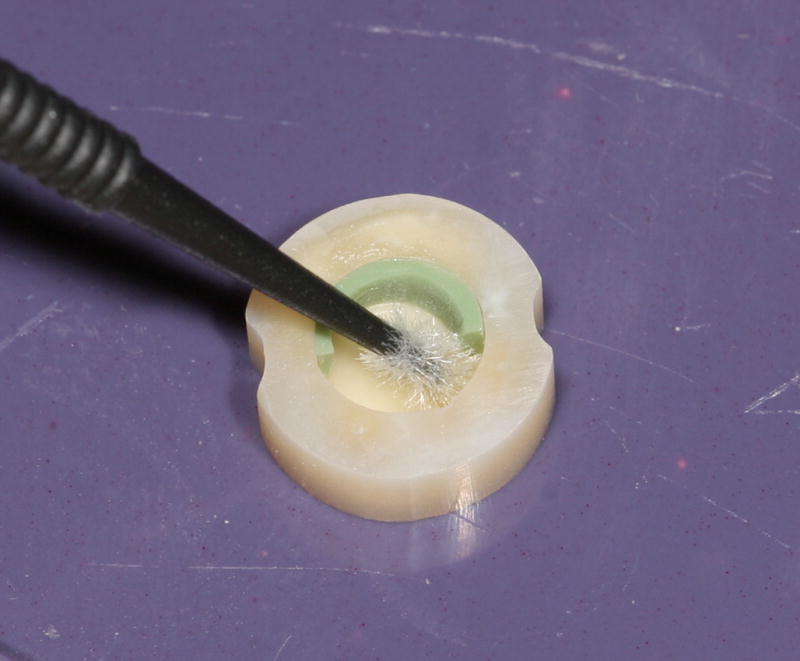
Sample during adhesive application with the 1 mm thick shim in place.
Biofilms were grown on each sample by incubating at 37°C in 5% CO2 and 95% relative humidity (BBD 6220 incubator, Thermo, Asheville, NC, USA) in separate petri dishes with approximately 15 ml of initially sterilized BHI media with 3% sucrose. One milliliter of Streptococcus mutans bacteria culture at an optical density of 0.8 was added to each dish. Incubation lasted approximately 4 days and the media was refreshed each day, until the biofilm could be observed with a naked eye. The samples were then placed in sterile bioreactors for further testing.
2.3. Test method
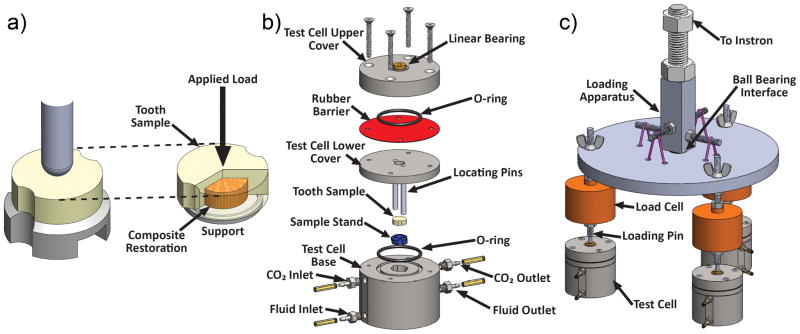
A novel bioreactor capable of in-situ cyclic loading was used for the demineralization studies (Fig. 3), and has been described previously in full detail [24]. Samples were placed in the bioreactor composite side down on top of a 7 mm diameter ring shaped stand (Fig. 3a). A combined biaxial bending and shear loading was applied by a semispherical ended loading rod (3 mm diameter) at the center of the sample which was aligned and fixed in the center of the bioreactor by a linear bearing in the top cover (Figs. 3a, 3b). When assembled, the entire bioreactor was completely sealed using rubber seals. Previous testing with these bioreactors demonstrated no external contamination over the entire two week experiment time [24]. Three specimens were tested simultaneously using three separate bioreactors (Fig. 3c). During the experiments, incubator conditions (see above) were reproduced inside each bioreactor. Fresh sterilized BHI media was pumped into the lower pipe fitting (fluid inlet in Fig. 3b) of the bioreactor at approximately 1 ml/min flow rate and pumped out of the test cell at the level just above the sample through the outlet pipe fitting to ensure the media covered the entire sample. Fluid motion was produced with peristaltic pumps (Model FB 70381, Thermo Fischer Scientific Inc., Waltham, MA, USA) at both the inlet and outlet. Each bioreactor was submerged to roughly half of its height in a 37°C water bath controlled by a digital immersion circulator (Cole-Palmer, Vernon Hills, IL, USA).
Fig. 3.
(a) – Sample schematic and relative position of sample, sample stand ring support and loading rod. (b) – Detailed assembly of a bioreactor test cell. (c) – Schematic of the test setup for loading three samples at a time.
Cyclic loading was applied using a computer controlled servo-hydraulic test system (Model 8872, Instron Corporation, Norwood, MA, USA). Fig. 3c shows how the load was equally distributed between three bioreactors using a ball bearing interface. The load for each specimen was measured using individual load cells (Model LCF300, FUTEK Advanced Sensor Technology Inc., Irvine, CA, USA), with the difference between each cell load always <5%. Specimen loading was done by alternating blocks of cyclic loading and resting periods. Cyclic loading blocks were 2 hours of cycling with the maximum load of ~113 N and a minimum load of ~11.3 N which corresponds to 25% and 2.5%, respectively, of the measured mean breaking force (450 N). During the resting periods, samples were kept at the minimum (11.3 N) load for 4 hours. The two block sequence was repeated 56 times to give a total of time of ~2 weeks for each experiment.
After each experiment, the samples were removed from the bioreactors and were subjected to a live/dead staining procedure (Life Technologies Live/Dead BacLight Bacterial Viability Kit) following the kit manufacture’s protocol and the biofilm was evaluated under confocal laser scanning microscopy (CSLM) to ensure the biofilm was still viable at the end of the experiments. Once sterility or biofilm viability were confirmed the samples were fixed in 10 ml 4%-gluteraldehyde and held at a temperature of 4°C overnight.
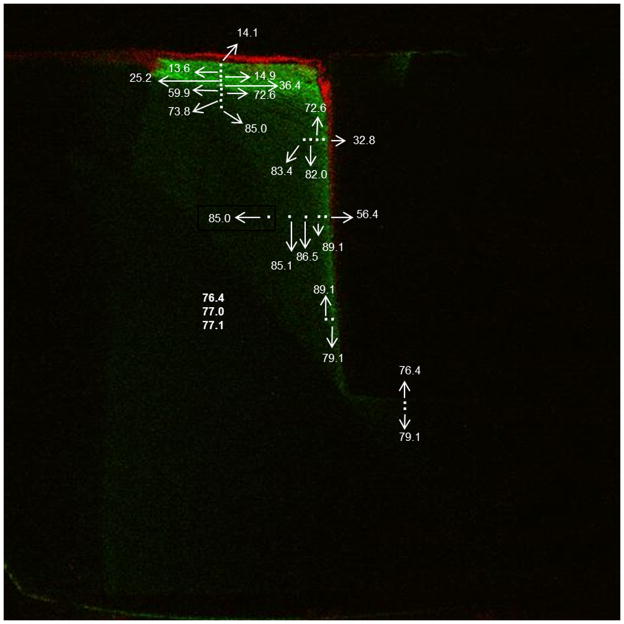
For gap analysis the biofilm was carefully removed from the surface with a swab, avoiding the gap area. The specimens were gram positive stained by applying crystal violet dye (Difco Laboratories, Detroit MI, USA), rinsing, and applying iodine to bind the dye to the bacteria. Samples were then mounted in LR white resin (London Resin Company Ltd, Reading, Berkshire England), sectioned in half on a slow speed diamond saw, and finally examined under the stereomicroscope for the presence of dentin demineralization and the extent of penetration of the stained bacterial biofilm. The sample half with the greatest apparent demineralization from the stereomicroscopic evaluation was further examined with CSLM (BioRad/Zeiss Radiance 2100 confocal laser scanning system) using 4x/0.2 objective. An ion Argon laser source with excitation at 488 nm and a 500–550 nm band pass filter was used to detect the autofluorescence while a GRE/Ne laser source with excitation band at 543 nm wavelength and a 570 nm long pass filter was used to detect the stained bacteria in the biofilm. Demineralization of the dentin was revealed by the autofluorescence as bright green [49–52] and the presence of the biofilm was revealed by the gram stain as red. Since dentin is naturally fluorescent, demineralization was correlated to the brightest green autofluorescence regions by Knoop micro-hardness mapping (Duramin 5 hardness tester, Struers Inc., Cleveland, OH, USA). A 100 gf load was used with a 20 s dwell time and the imprint sizes were measured using optical microscopy. Image J (U. S. National Institutes of Health, Bethesda, Maryland, USA) was used to assess gap sizes and bacterial penetration, the latter of which was quantified as a fraction of the total depth of the gap and a Student’s t-test (α ≤ 0.05) was used to compare the 0BAG and 15BAG cases.
3. Results
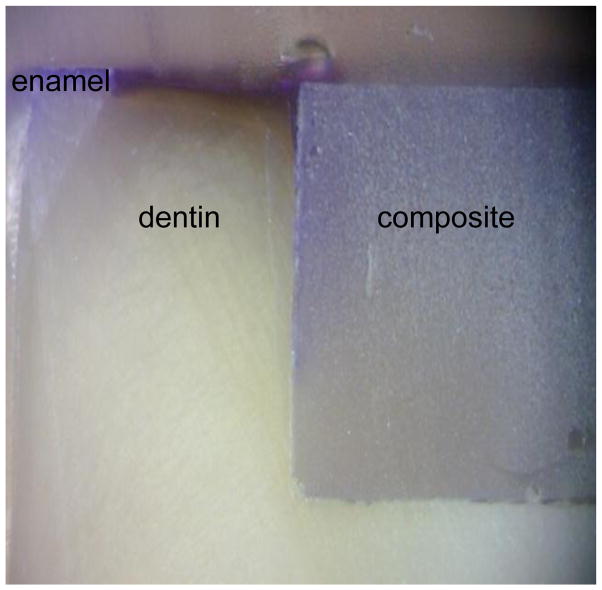
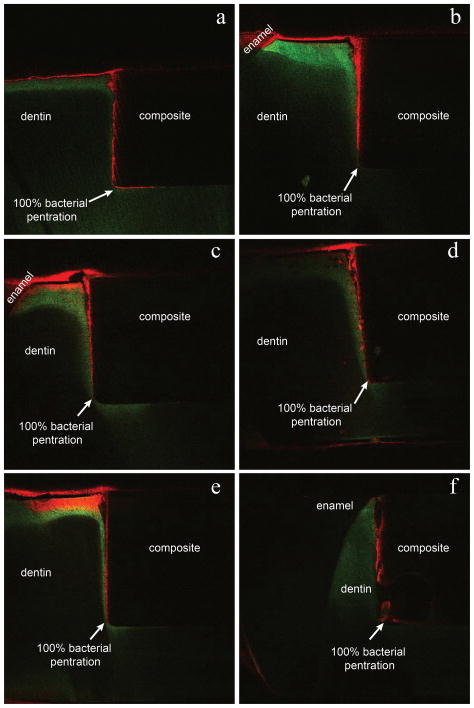
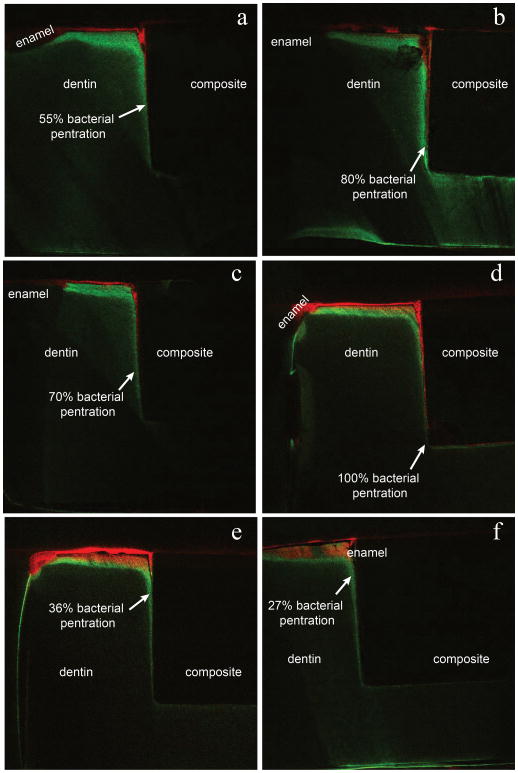
Post-test evaluation using the live/dead assay and fluorescent confocal laser scanning microscopy confirmed a live biofilm on the surface of each sample. An optical micrograph of an example cross section is shown in Fig. 4. Fluorescent microscopy images of the sample cross sections verify the presence of bacteria in the gap seen as red in the panel images in Figs. 5 and 6 for the 0BAG and 15BAG specimens, respectively. Knoop indentation results show that the brightest green fluorescent regions at the surface and along the gaps were soft, and thus demineralized (Fig. 7). However, the paler green color that runs along the gap past the red bacteria stain generally did not show a decline in hardness, suggesting minimal if any demineralization (Fig. 7).
Fig. 4.
Example sample cross section showing the orientation of the dentin, composite, and in some cases enamel in Figs. 3 & 4.
Fig. 5.
Fluorescence image of the 0BAG control sample cross sections. Bacterial penetration (red) is seen extending deep into the gap between the dentin and composite filling. Green areas represent protein fluorescence from areas of demineralized dentin.
Fig. 6.
Fluorescence image of the 15BAG sample cross sections. Bacterial penetration (red) is seen extending only partially into the gap between the dentin and composite filling. Green areas represent protein fluorescence from areas of demineralized dentin.
Fig. 7.
Knoop micro-hardness results are overlaid onto a confocal microscopy image to show the quantitative extent of demineralization. The white spots are the indent locations, while the white arrows point to the associated Knoop Hardness Number.
Comparing Figs. 5 & 6, it is seen that bacterial penetration is consistently much deeper for the 0BAG composite than for 15BAG while there was no statistically significant difference in gap size (Table 1). A summary of all six samples for each case is shown in Table 1 in terms of the percentage of bacteria penetration. Bacterial penetration for 0BAG composite samples was always to the full depth of the restoration (100% average), sometimes propagating along the floor of the cavity under the restoration. In contrast, the average depth of penetration for the 15 BAG samples was only 61% of the depth of the gap with a standard deviaton of 27%. The Student’s t-test verified this difference in bacterial penetration to be significantly different (p < 0.05).
Table 1.
Gap sizes and bacterial penetration in the gap for 0BAG and 15BAG composite samples.
| 0BAG | 15BAG | |||
|---|---|---|---|---|
| Sample | Gap size (μm) | Bacterial penetration (%) | Gap size (μm) | Bacterial penetration (%) |
| 1 | 15.3 | 100 | 18.1 | 27 |
| 2 | 18.8 | 100 | 17.6 | 36 |
| 3 | 17.6 | 100 | 15.1 | 55 |
| 4 | 16.5 | 100 | 16.5 | 70 |
| 5 | 20.1 | 100 | 16.3 | 80 |
| 6 | 48.2 | 100 | 15.3 | 100 |
| mean (stdv) | 22.8 (12.6) | 100 (0) | 16.5 (1.2) | 61 (27) |
4. Discussion
Two weeks was chosen for the total test time because demineralization studies without cyclic loading have shown that length to be necessary to achieve reliably measurable demineralization [45,46]. An average person chews at approximately 1.5 Hz [47], and the actual chewing time per day may be conservatively estimated as about 20 minutes, equating to 1800 chews/day or 657,000 chews/year. An intermittent cycling phase of two hours followed by four hours without cycling gives a total of 43,200 cycles per day, or approximately 605,000 cycles in two weeks. Thus, the total number of cycles is equivalent to nearly one year of normal human chewing. The four hour resting time was chosen to represent a normal time between meals and to provide ample opportunity for the biofilm to grow and potentially double [48] before being subjected to another loading block.
The post-test live/dead assay results indicated that the conditions needed for successful biofilm growth (nutrition supply, carbon dioxide level and temperature) were maintained throughout the entire length of the experiments. For the 0BAG control composite, the degree of bacterial penetration into the gaps was consistently very deep, reaching the bottom of the cavity. It is important to note that while the floor of the prepared cavity was treated with dentin bonding agent, an approximately 1mm thick shim was used to prevent application of the bonding system to half of the cavity wall, and so the initial 1mm of the floor was not bonded to the composite. However, in many cases bacterial penetration underneath the 0BAG restoration was observed (Fig. 5) suggesting the possibility that the synergistic effect of cyclic mechanical loading allowed gap propagation and further bacterial penetration in some samples. Indeed, the important role of cyclic loading in aiding bacterial penetration into interfacial gaps between the dentin and composite has been reported previously [24].
A significantly lower degree of bacterial penetration into the gap was found for samples that were restored with 15BAG resin composite. On average, for 15BAG composite the penetration was 61% of the gap depth with consistently no penetration underneath the filling. Moreover, there was no statistically significant difference in gap size that might account for this difference. While the mean gap size for the control samples appears somewhat higher, this is due to a single outlier and the mean values are indistinguishable if that outlier is excluded. Furthermore, a t-test both with or without the outlier included showed no significant difference in gap size between the groups. This suggests that the release of BAG ions into the gap can help control the local gap chemistry and create an antimicrobial environment that slows biofilm development and propagation.
Furthermore, this study verified the usefulness of this model to create detectable dentin demineralization, akin to recurrent caries, in gaps associated with dental composite restorations. The use of autofluorescence is generally thought of as a qualitative measure to detect demineralization [49–52]. Accordingly, it is seen qualitatively in Figs. 5 & 6 that the brightest green areas, suggestive of significant amounts of demineralization, occurred on the outer dentin surfaces and along the gaps for all samples. The challenge of interpreting the autofluorescence is that sound dentin is also naturally fluorescent, though in most cases this does not appear at all intense. According, pairing the confocal microscopy images with micro-hardness tests provided a better quantitative measure of the extent of demineralization (Fig. 7).
Demineralization occurred adjacent to both the control and the BAG-containing composites, and in general, was more limited as one proceeded deeper into the gap and more distant from the acidogenic bacterial biofilm. Furthermore, the micro-hardness evaluations reveled that demineralization extended roughly to the extent of bacterial penetration (Fig. 7). This is attributed to the creation of the low pH environment by the viable biofilm in the gap. While pale green fluorescence, when present, always extended beyond the bacteria (Fig. 6), hardness results consistently showed that these were regions of sound, or essentially unaffected, dentin. This is reasonable since the acid would tend to be more readily neutralized and its effect more limited the further away it was from the acidogenic bacterial biofilm.
As mentioned above, the exact mechanisms of the antimicrobial effect of BAG remain unclear, but it may be related to a local rise in pH in the gap [39], one or more of the ions directly affecting the bacteria, or a combination of both factors [38]. While more studies will be needed to understand the exact antimicrobial mechanisms associated with BAG, the results of the present study suggest that BAG containing composites have some potential to slow biofilm penetration into, and demineralization within, marginal gaps.
Finally, while the focus of this study was to develop anti-bacterial composites, it is important to note that attention to the adhesives used is equally important and that work is also underway. This is because when a well applied adhesive detaches from the cavity wall, it could potentially seal the beneficial BAG inside the composite, or at least greatly slow the ion leaching. Accordingly, this paper represents part of a systematic study to first evaluate the effect of using a BAG composite alone, while ongoing and future work includes examining the effect of using a BAG containing adhesive with conventional composites, and then finally the combination of both. The clinical relevance of using a BAG containing composite is to address the situation where the adhesive is not perfectly applied and an anti-bacterial agent is needed where gaps first form at the location(s) of missing adhesive. Ultimately, it is suspected though that the best solution will be an anti-bacterial adhesive used in conjunction with an anti-bacterial composite.
5. Conclusions
Based on an in vitro study of simulated tooth filling samples cyclically loaded in a custom bioreactor system, it was found that 15 wt% additions of bioactive glass (BAG) fillers to a resin-based dental composite demonstrated a significant antimicrobial effect in reducing the extent of bacterial biofilm penetration into pre-existing marginal gaps.
Highlights.
Novel bioreactor system was used for in vitro cyclic loading of tooth filling samples
We grew biofilms on simulated tooth restorations with bioactive and control composites
Bacteria penetrated less into marginal gaps for the bioactive glass samples samples
Bioactive glass fillers may have promise for slowing secondary caries formation
Acknowledgments
This work was supported by NIH/NIDCR GRANT DE021372. The authors thank Steven Naleway, Jonathon Cummins and Max Breedlove for their assistance developing the bioreactor system. The authors thank Drs. Fernanda Gwinner, Michael Danilchik, Satin Salehi, and Gamze Karacolak for their help with sample preparation, confocal microscopy, image analysis, and hardness mapping, respectively.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hickel R, Kaaden C, Paschos E, Buerkle V, Garcia-Godoy F, Manhart J. Longevity of occlusally-stressed restorations in posterior primary teeth. Am J Dent. 2005;18:198–211. [PubMed] [Google Scholar]
- 2.Downer M, Azli N, Bedi R, Moles D, Setchell D. Dental Restorations: How long do routine dental restorations last? A systematic review. British Dental Journal. 1999;187:432–439. doi: 10.1038/sj.bdj.4800298a1. [DOI] [PubMed] [Google Scholar]
- 3.Deligeorgi V, Mjör IA, Wilson NH. An overview of reasons for the placement and replacement of restorations. Prim Dent Care. 2001;8:5–11. doi: 10.1308/135576101771799335. [DOI] [PubMed] [Google Scholar]
- 4.Marks LAM, Weerheijm KL, van Amerongen WE, Groen HJ, Martens LC. Dyract versus Tytin class II restorations in primary molars: 36 months evaluation. Caries Research. 1999;33:387–392. doi: 10.1159/000016538. [DOI] [PubMed] [Google Scholar]
- 5.Mjör IA. Glass-ionomer cement restorations and secondary caries: a preliminary report. Quintessence Int. 1996;27:171–174. [PubMed] [Google Scholar]
- 6.Mjör IA, Toffenetti OF. Secondary caries: A literature review with case reports. Quintessence Int. 2000;31:165–179. [PubMed] [Google Scholar]
- 7.Wilson NH, Burke FJ, Mjör IA. Reasons for placement and replacement of restorations of direct restorative materials by a selected group of practitioners in the United Kingdom. Quintessence Int. 1997;28:245–248. [PubMed] [Google Scholar]
- 8.Aas JA, Griffen AL, Dardis SR, Lee AM, Olsen I, Dewhirst FE, Leys EJ, Paster BJ. Bacteria of dental caries in primary and permanent teeth in children and young adults. Journal of clinical microbiology. 2008;46:1407–1417. doi: 10.1128/JCM.01410-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selwitz RH, Ismail AI, Pitts NB. Dental caries. The Lancet. 2007;369:51–59. doi: 10.1016/S0140-6736(07)60031-2. [DOI] [PubMed] [Google Scholar]
- 10.Kidd E, Beighton D. Prediction of secondary caries around tooth-colored restorations: a clinical and microbiological study. Journal of dental research. 1996;75:1942–1946. doi: 10.1177/00220345960750120501. [DOI] [PubMed] [Google Scholar]
- 11.Choi K, Condon J, Ferracane J. The effects of adhesive thickness on polymerization contraction stress of composite. Journal of dental research. 2000;79:812–817. doi: 10.1177/00220345000790030501. [DOI] [PubMed] [Google Scholar]
- 12.Shah MB, Ferracane JL, Kruzic JJ. Mechanistic aspects of fatigue crack growth behavior in resin based dental restorative composites. Dent Mater. 2009;25:909–916. doi: 10.1016/j.dental.2009.01.097. [DOI] [PubMed] [Google Scholar]
- 13.Kruzic JJ, Ritchie RO. Fatigue of mineralized tissues: Cortical bone and dentin. Journal of the Mechanical Behavior of Biomedical Materials. 2008;1:3–17. doi: 10.1016/j.jmbbm.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Kruzic JJ, Nalla RK, Kinney JH, Ritchie RO. Mechanistic aspects of in vitro fatigue-crack growth in dentin. Biomaterials. 2005;26:1195–1204. doi: 10.1016/j.biomaterials.2004.04.051. [DOI] [PubMed] [Google Scholar]
- 15.Nalla RK, Imbeni V, Kinney JH, Staninec M, Marshall SJ, Ritchie RO. In vitro fatigue behavior of human dentin with implications for life prediction. J Biomed Mater Res A. 2003;66:10–20. doi: 10.1002/jbm.a.10553. [DOI] [PubMed] [Google Scholar]
- 16.Kruzic JJ, Ritchie RO. Kitagawa-Takahashi diagrams define the limiting conditions for cyclic fatigue failure in human dentin. J Biomed Mater Res A. 2006;79:747–751. doi: 10.1002/jbm.a.30939. [DOI] [PubMed] [Google Scholar]
- 17.Arola D, Bajaj D, Ivancik J, Majd H, Zhang D. Fatigue of biomaterials: Hard tissues. Int J Fatigue. 2010;32:1400–1412. doi: 10.1016/j.ijfatigue.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arisu H, Üçtasli M, Eligüzeloglu E, Özcan S, Ömürlü H. The effect of occlusal loading on the microleakage of class V restorations. Operative dentistry. 2008;33:135–141. doi: 10.2341/07-49. [DOI] [PubMed] [Google Scholar]
- 19.Campos P, Barceleiro MO, Sampaio-Filho H, Martins L. Evaluation of the cervical integrity during occlusal loading of class II restorations. Operative dentistry. 2008;33:59–64. doi: 10.2341/07-35. [DOI] [PubMed] [Google Scholar]
- 20.Pongprueksa P, Kuphasuk W, Senawongse P. Effect of elastic cavity wall and occlusal loading on microleakage and dentin bond strength. Operative dentistry. 2007;32:466–475. doi: 10.2341/06-132. [DOI] [PubMed] [Google Scholar]
- 21.Vandewalle KS, Ferracane JL, Hilton TJ, Erickson RL, Sakaguchi RL. Effect of energy density on properties and marginal integrity of posterior resin composite restorations. Dental Materials. 2004;20:96–106. doi: 10.1016/s0109-5641(03)00124-6. [DOI] [PubMed] [Google Scholar]
- 22.Loesche WJ. Microbiology of Dental Decay and Periodontal Disease. In: Baron S, editor. Medical Microbiology. 4. University of Texas Medical Branch at Galveston; Galveston, TX: 1996. [PubMed] [Google Scholar]
- 23.Fejerskov O. Changing paradigms in concepts on dental caries: consequences for oral health care. Caries Res. 2004;38:182–191. doi: 10.1159/000077753. [DOI] [PubMed] [Google Scholar]
- 24.Khvostenko D, Salehi S, Naleway SE, Hilton TJ, Ferracane JL, Mitchell JC, Kruzic JJ. Cyclic mechanical loading promotes bacterial penetration along composite restoration marginal gaps. Dent Mater. 2015;31:702–710. doi: 10.1016/j.dental.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stephan RM. Intra-oral hydrogen-ion concentrations associated with dental caries activity. Journal of dental research. 1944;23:257–266. [Google Scholar]
- 26.Keyes PH. Research in dental caries. J Am Dent Assoc. 1968;76:1357–1373. doi: 10.14219/jada.archive.1968.0186. [DOI] [PubMed] [Google Scholar]
- 27.Keyes PH. The infectious and transmissible nature of experimental dental caries. Findings and implications. Arch Oral Biol. 1960;1:304–320. doi: 10.1016/0003-9969(60)90091-1. [DOI] [PubMed] [Google Scholar]
- 28.Loesche W, Rowan J, Straffon L, Loos P. Association of Streptococcus mutans with human dental decay. Infection and immunity. 1975;11:1252–1260. doi: 10.1128/iai.11.6.1252-1260.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loesche WJ. Role of Streptococcus mutans in human dental decay. Microbiological reviews. 1986;50:353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Syafiuddin T, Hisamitsu H, Toko T, Igarashi T, Goto N, Fujishima A, Miyazaki T. In vitro inhibition of caries around a resin composite restoration containing antibacterial filler. Biomaterials. 1997;18:1051–1057. doi: 10.1016/s0142-9612(97)88072-6. [DOI] [PubMed] [Google Scholar]
- 31.Gilchrist T, Healy DM. Antimicrobial composition composed of controlled release glasses. 2000. [Google Scholar]
- 32.Waltimo T, Brunner T, Vollenweider M, Stark W, Zehnder M. Antimicrobial effect of nanometric bioactive glass 45S5. Journal of Dental Research. 2007;86:754–757. doi: 10.1177/154405910708600813. [DOI] [PubMed] [Google Scholar]
- 33.Vollenweider M, Brunner TJ, Knecht S, Grass RN, Zehnder M, Imfeld T, Stark WJ. Remineralization of human dentin using ultrafine bioactive glass particles. Acta Biomaterialia. 2007;3:936–943. doi: 10.1016/j.actbio.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Brown ML, Davis HB, Tufekci E, Crowe JJ, Covell DA, Mitchell JC. Ion release from a novel orthodontic resin bonding agent for the reduction and/or prevention of white spot lesions. An in vitro study. The Angle orthodontist. 2011;81:1014–1020. doi: 10.2319/120710-708.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manfred L, Covell DA, Crowe JJ, Tufekci E, Mitchell JC. A novel biomimetic orthodontic bonding agent helps prevent white spot lesions adjacent to brackets. The Angle orthodontist. 2013;83:97–103. doi: 10.2319/110811-689.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greenspan DC, West JK, Lee S, Meyers JL, Diamond M. Anti-inflammatory and antimicrobial uses for bioactive glass compositions. 2004. [Google Scholar]
- 37.Zehnder M, Soderling E, Salonen J, Waltimo T. Preliminary evaluation of bioactive glass S53P4 as an endodontic medication in vitro. J Endodont. 2004;30:220–224. doi: 10.1097/00004770-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 38.Gubler M, Brunner TJ, Zehnder M, Waltimo T, Sener B, Stark WJ. Do bioactive glasses convey a disinfecting mechanism beyond a mere increase in pH? Int Endod J. 2008;41:670–678. doi: 10.1111/j.1365-2591.2008.01413.x. [DOI] [PubMed] [Google Scholar]
- 39.Allan I, Newman H, Wilson M. Antibacterial activity of particulate bioglass against supra- and subgingival bacteria. Biomaterials. 2001;22:1683–1687. doi: 10.1016/s0142-9612(00)00330-6. [DOI] [PubMed] [Google Scholar]
- 40.Hench LL, Splinter RJ, Allen W, Greenlee T. Bonding mechanisms at the interface of ceramic prosthetic materials. J Biomed Mater Res. 1971;5:117–141. [Google Scholar]
- 41.Chatzistavrou X, Fenno JC, Faulk D, Badylak S, Kasuga T, Boccaccini AR, Papagerakis P. Fabrication and characterization of bioactive and antibacterial composites for dental applications. Acta Biomater. 2014;10:3723–3732. doi: 10.1016/j.actbio.2014.04.030. [DOI] [PubMed] [Google Scholar]
- 42.Khvostenko D, Mitchell JC, Hilton TJ, Ferracane JL, Kruzic JJ. Mechanical performance of novel bioactive glass containing dental restorative composites. Dent Mater. 2013:1139–1148. doi: 10.1016/j.dental.2013.08.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tauböck TT, Zehnder M, Schweizer T, Stark WJ, Attin T, Mohn D. Functionalizing a dentin bonding resin to become bioactive. Dent Mater. 2014;30:868–875. doi: 10.1016/j.dental.2014.05.029. [DOI] [PubMed] [Google Scholar]
- 44.Mitchell JC, Musanje L, Ferracane JL. Biomimetic dentin desensitizer based on nano-structured bioactive glass. Dental Materials. 2011;27:386–393. doi: 10.1016/j.dental.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 45.Buren JL, Staley RN, Wefel J, Qian F. Inhibition of enamel demineralization by an enamel sealant, Pro Seal: an in-vitro study. American Journal of Orthodontics and Dentofacial Orthopedics. 2008;133:S88–S94. doi: 10.1016/j.ajodo.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 46.Cho A, Suzuki S, Hatakeyama J, Haruyama N, Kulkarni AB. A method for rapid demineralization of teeth and bones. The open dentistry journal. 2010;4:223–229. doi: 10.2174/1874210601004010223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Braem M, Lambrechts P, Vanherle G. Clinical relevance of laboratory fatigue studies. J Dent. 1994;22:97–102. doi: 10.1016/0300-5712(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 48.Wong L, Sissons CH. Human dental plaque microcosm biofilms: effect of nutrient variation on calcium phosphate deposition and growth. Arch Oral Biol. 2007;52:280–289. doi: 10.1016/j.archoralbio.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 49.Banerjee A, Boyde A. Autofluorescence and mineral content of carious dentine: scanning optical and backscattered electron microscopic studies. Caries Res. 1998;32:219–226. doi: 10.1159/000016456. [DOI] [PubMed] [Google Scholar]
- 50.de Carvalho FG, de Fucio SB, Sinhoreti MA, Correr-Sobrinho L, Puppin-Rontani RM. Confocal laser scanning microscopic analysis of the depth of dentin caries-like lesions in primary and permanent teeth. Brazilian dental journal. 2008;19:139–144. doi: 10.1590/s0103-64402008000200010. [DOI] [PubMed] [Google Scholar]
- 51.van der Veen MH, ten Bosch JJ. The influence of mineral loss on the auto-fluorescent behaviour of in vitro demineralised dentine. Caries Res. 1996;30:93–99. doi: 10.1159/000262143. [DOI] [PubMed] [Google Scholar]
- 52.Banerjee A, Gilmour A, Kidd E, Watson T. Relationship between S. mutans and the autofluorescence of carious dentin. Am J Dent. 2004;17:233–236. [PubMed] [Google Scholar]