Abstract
The World Health Organization has recommended the scale-up of voluntary medical male circumcision (VMMC) for HIV prevention in sub-Saharan Africa; however, men are often uninterested in undergoing VMMC. The Spear & Shield project enrolled 668 men and female partners from ten Zambian community health centers into parallel interventions promoting VMMC for HIV prevention or time-matched control conditions. A mediation model was utilized to examine the relationships between changes in women's acceptance of VMMC and men's readiness to undergo the procedure. Results demonstrated that, at 12 months post-intervention, a 5.9 % increase in the likelihood of undergoing VMMC among men in the experimental condition could be attributed to increased women's acceptance. From a public health perspective, involving women in VMMC promotion interventions such as the Spear & Shield project could significantly impact the demand for VMMC in Zambia.
Keywords: Voluntary medical male circumcision, Zambia, Women, Couples
Introduction
Several randomized controlled trials demonstrated that voluntary medical male circumcision (VMMC) provides at least 60 % protection against HIV acquisition in men [1–4]. In response, the World Health Organization recommended that VMMC be scaled-up as an essential component of HIV prevention efforts in areas of high HIV prevalence, such as sub-Saharan Africa [5]. It is estimated that widespread VMMC in Africa would avert 3.5 million new HIV infections by 2025, amounting to a cost savings of approximately $16.5 billion [6]. National efforts to promote VMMC as a HIV prevention strategy are underway in many high HIV prevalence countries; however, most African ethnic groups are culturally non-circumcising or practice "traditional" male circumcision, which may include only partial removal of the foreskin, non-sterile methods, and improper wound healing procedures. Consequently, many adult men express no interest in undergoing VMMC or allowing their sons to undergo VMMC [7–11]. Interventions to increase the availability and acceptability of VMMC for HIV prevention are therefore needed to optimize its potential benefits [12].
In addition to the benefits for men, VMMC protects female partners from STIs, cervical cancer, and indirectly provides protection against HIV infection in women through reduced male HIV prevalence [13–16]. Women report greater sexual satisfaction with circumcised partners, knowledge that VMMC protects against HIV, and strongly believe that VMMC improves health and hygiene [17, 18]. Additionally, women report that men's circumcision status is a salient factor when choosing sexual partners [18], and qualitative research has shown that the opinion of female partners can influence men's decision to undergo VMMC [19]. Studies have shown that women may also have reservations or misconceptions about VMMC, such as fear of increased promiscuity among circumcised men or the misconception that VMMC provides complete protection against HIV, and thus have called for women to be included in VMMC education programs [20, 21]. Despite this, the degree to which women are engaged in VMMC scale-up is unclear, and there is little data on the impact of women's involvement in VMMC promotion interventions [12]. Additionally, no studies have quantified the impact of women's support for male circumcision on men's readiness for VMMC.
The Republic of Zambia, identified by WHO as a priority country for VMMC scale-up, had completed only 32.6 % of their 2015 target of 1.9 million VMMCs as of July 2014 [22]. The Spear & Shield Zambia project was a bio-behavioral intervention designed to simultaneously increase the availability of VMMC in Zambian community health clinics (CHCs) and the uptake of VMMC among men who initially expressed little interest in undergoing the procedure. In addition, participating men were encouraged to invite their female partners to take part in a similar, parallel program. The study compared men receiving the intervention to those who received a time-matched attention control condition; it was hypothesized that men participating in the intervention would be more likely to undergo VMMC by the end of the study period as compared to those in the control condition. Additionally, it was hypothesized that the intervention would enhance women's positive attitudes and acceptability regarding VMMC, which would in turn positively influence their male partners to move along the behavioral continuum towards undergoing VMMC.
Methods
Prior to study initiation, ethical approval was obtained by the Research Ethics Committee of the University of Zambia School of Medicine and the Institutional Review Board at the University of Miami Miller School of Medicine. All study procedures were completed in accordance with ethical standards for human subjects research. The Spear & Shield trial protocol is registered on Clinicaltrials.gov, number NCT01688167.
Participants, Procedures, and Study Conditions
Thirteen CHCs were stratified by size and HIV voluntary counseling and testing (VCT) volume and randomized to the intervention condition (5 clinics), a time-matched control condition (5 clinics), or an “observation-only” condition (3 clinics) where monthly secular trend data on VMMC was collected and no participants were recruited. Eligible clinics in the Lusaka, Zambia area were identified in consultation with the Zambian Ministry of Health and were selected based on: (1) ≥50 VCT participants per month, (2) no CHC personnel currently performing circumcisions on a regular basis, (3) ≥3 providers available at each site for circumcision training, and (4) ≥2 VCT counselors (or equivalent) available at each site for sexual risk reduction training. Providers at each clinic (doctors, nurses, or other qualified clinic staff members) were trained to perform VMMC and supplies for VMMC were continually provided to all clinics to ensure sufficient access to VMMC services across clinics. Intervention and control clinics each recruited a total of 8–9 cohorts of 8–10 male participants.
As this was a cluster randomized trial, it was initially determined that the study would utilize a total of 12 clinics allocated in a 1:1 ratio (experimental, control) with each clinic recruiting 8 cohorts of 10 men for a total of 960 men. The sample size was determined by a power analysis which showed that the study would have at least 80 % power to detect a significant difference between conditions with VMMC rates of 5–10 % in the control arm, rates of 30–40 % in the Experimental arm, and intracluster correlation coefficients (ICC) up to 0.27 (depending on the two rates) with a two tailed test at the 0.05 level. However, due to logistic and staffing limitations, the total number of clinics was reduced to 10. The study power was re-assessed and it was determined that adequate power would be maintained with 10 clinics and 800 male participants. The sample size of female partners was not determined a priori, instead, all men were encouraged to invite their female partner to participate in a women's program comparable to their partner. Randomization of CHCs was conducted using a computer generated sequence of random numbers and was undertaken by Zambian investigators; trial statisticians did not participate in randomization.
Participants were recruited from VCT programs in CHCs in Lusaka, Zambia. All eligible participants, both experimental and control, were at least 18 years of age, HIV-negative, uncircumcised, and without plans to undergo VMMC in the future. Men were ineligible if they had plans to undergo VMMC, were HIV-positive, or had been diagnosed with a health condition that might preclude them from undergoing VMMC (e.g., penile cancer). Recruitment and enrollment were conducted in the same manner in both experimental and control clinics. For the purpose of this study, men were not restricted to enrolling with a sexual partner; female partners could also include friends or trusted advisors.
The Spear & Shield intervention was a weekly, four-session, manualized program delivered to men in a group format (~8–10 participants per group). The intervention was delivered by trained VCT counselors or nurses, and addressed issues related to HIV sexual risk reduction strategies. The role of VMMC in HIV risk reduction was highlighted in each of the sessions, including the benefits and drawbacks associated with VMMC, the VMMC procedure itself including the surgery and healing process, and the maintenance of sexual barrier/condom use following VMMC. A VMMC provider and a “spokesperson” who had previously undergone VMMC spoke to the group about their experiences and answered questions. Women's group sessions (~6–10 females per group) were similar in content to male sessions, focusing on sexual risk reduction strategies and the health benefits of VMMC for their partners and themselves. However, neither VMMC providers nor VMMC spokespersons visited the women's groups. Instead, experimental condition groups included additional emphasis on women's issues related to HIV prevention, such as prevention of intimate partner violence. All control sites offered time-matched health education video sessions to men's and women's groups. Participants were reimbursed K10 Zambian Kwacha (~US$2) for travel expenses related to attendance at intervention or control sessions and compensated K20 (~US$4) for completing assessments.
Measures
Data was collected using an audio computer-assisted self-interview (ACASI) system. Men completed assessments at baseline (T1), post-intervention (T2; approximately 2 months post-baseline), 6 months post-intervention (T3), and 12 months post-intervention (T4). Women completed assessments at baseline and post-intervention (T1 and T2).
Women's VMMC Attitudes and Acceptance
VMMC attitudes and acceptance were assessed using a measure adapted from the Uganda National Serosurvey [23, 24]. Items related to women's acceptance included the perceived cultural acceptability of VMMC among women, how strongly women preferred a circumcised or uncircumcised sexual partner, the degree to which women would advise a sexual partner to undergo VMMC, and whether or not women would like their current partner to undergo VMMC. Items were scored such that higher scores indicated higher preferences and more positive attitudes towards VMMC. In this study, the above items were used in a measurement model as indicators of continuous latent variables representing women's acceptance of VMMC.
Readiness to Undergo VMMC and Uptake of VMMC
Men's readiness to undergo VMMC was assessed using the stages of change model (SOC; [24, 25]). Men indicated whether they had never thought about undergoing VMMC or had thought about it but were not considering undergoing it within the next 6 months (precontemplation), were considering undergoing VMMC sometime within the next 6 months but had no specific plans to do so (contemplation), were considering undergoing VMMC sometime within the next 30 days and had taken a behavioral step towards the procedure (preparation), or had undergone VMMC (action) [26]. As this study recruited only uncircumcised men, no participants were in the action stage at baseline. Through the course of the study, participants who reported that they had undergone VMMC were verified as having actually undergone the procedure by study staff. Verification was completed through clinic record reviews (50 %), voluntary physical examinations (26 %), or VMMC provider interviews (24 %).
Statistical Analyses
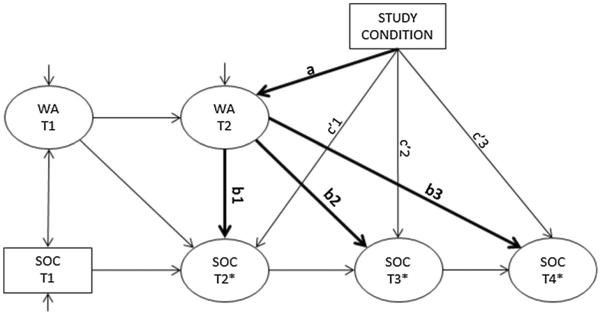
Although Spear & Shield enrolled a total of 800 men, as this manuscript focuses on the effect of female partners on men's decision to undergo VMMC, the analytic sample was limited to the N = 668 men who enrolled with a female partner (total N = 1336). In order to examine the longitudinal relationships between women's acceptance of VMMC and men's readiness, a cross-lagged mediation model was constructed. Latent variables representing women's VMMC acceptance at baseline (T1) and post-intervention (T2) were measured. Paths were estimated for the autocorrelations in women's acceptance and men's readiness, and between women's T1 acceptance and men's T2 readiness. The correlation between baseline acceptance and readiness was also estimated. Paths of interest for mediation included the effect of intervention condition on women's acceptance at T2 (the a path) and the effects of women's acceptance at T2 on men's readiness at T2, T3 (6 months post-intervention), and T4 (12 months post-intervention) (the b paths; b1, b2, and b3). The direct effects of condition on men's readiness at T2, T3, and T4 were also estimated (the c′ paths; c′1, c′2, and c′3; see Fig. 1).
Fig. 1.
A path model showing hypothesized mediation relationships between condition, women's VMMC acceptance, and men's readiness to undergo VMMC. Note: VMMC voluntary medical male circumcision, WA women's acceptance, SOC stages of change, T1 baseline, T2 post-intervention (approximately 2 months post-baseline), T3 6-month follow-up, T4 12-month follow-up
Estimation of the model required some restrictions on the treatment of the stages of change variable. Because the SOC measure is categorical with more than two levels, it would be a theoretically appropriate outcome variable in either an ordinal or multinomial logistic regression model (generally depending on the outcome of a test of proportional odds across levels of predictor variables). However, SOC at T2, T3, and T4 served as both outcomes and predictors in this path model. When the SOC is treated as a nominal variable with k categories and serves as a predictor, k − 1 dummy variables are needed to estimate k − 1 relationships with an outcome. However, when the SOC is a nominal outcome, it is treated as a single variable with k − 1 unordered thresholds. In this path model, there was not a theoretically or statistically appropriate method for reconciling this difference. On the other hand, an ordinal variable can be treated as an indicator of a continuous latent variable having certain “threshold” values that result in higher categories of the observed ordinal variable. The continuous latent variable can serve as both a predictor (requiring no dummy variables) and an outcome in a path model. Thus, stages of change at T2, T3, and T4 were analyzed as continuous latent variables (indicated as SOC T2*, T3*, and T4* in the path model), and at T1, SOC was treated as a continuous covariate. This method of estimation also ensures that the indirect path can be correctly computed as the product of the a and b paths when the observed mediator(s) and outcome(s) are not measured on the same scale [27]. Figure 1 presents a path model for this analysis; the bold lines indicate mediation paths of interest.
The model was estimated using mean- and variance-adjusted weighted least squares with empirical standard errors in order to control for the clustering of men within cohorts within clinics (n = 80 clusters). The final model was determined by comparing an unrestricted model to a model in which autoregressive paths were constrained to be equal over time using a χ2 difference test [28]. The test was significant, and the unrestricted model was retained. Coefficients on women's attitudes are reported as ordinary regression coefficients, and those on the stages of change are reported as probit regression coefficients. In addition, standardized coefficients were calculated according to the measurement scale of the predictor variable. If the predictor variable was continuous, the coefficient was calculated as β = b*SD(x)/SD(y) and represents the amount of change in the outcome, in standard deviations, per one unit change in the standard deviation of the predictor variable, holding all other relationships constant. If the predictor variable was categorical (i.e., study condition), the standardized coefficient was calculated as β = b/SD(y) and represents the difference in the outcome, in standard deviations, between the experimental and control conditions, holding all other relationships constant.
Indirect effects were estimated as the product of a and b paths. 95 % confidence intervals for the indirect effects were obtained by Monte Carlo simulation, using estimated path coefficients and standard errors obtained from the model [it is statistically more appropriate to obtain confidence intervals for indirect effects empirically (e.g., using Monte Carlo or bootstrap methods; [29]). Monte Carlo simulation was conducted using an R macro developed by Selig & Preacher [30]. All other analyses were completed using MPLUS version 6.11 at a two-tailed level of significance of p < .05.
Results
Demographics, Attendance, and Withdrawal
Male participants (n = 668 enrolled with a female partner, n = 340 in the experimental condition, n = 328 in the control condition; 40 cohorts from each condition) ranged from 18 to 57 years old (mean 27, SD = 9) with 11 ± 3 years of education. About half were unemployed (51 %, n = 341) and earned less than 500 ZMK (~$100) per year (53 %, n = 354). Thirty nine percent (n = 261) were married and 40 % (n = 269) had children. Most (65 %, n = 436) indicated that their partner had been tested for HIV, and 9 % of those (n = 38) reported that their partner was HIV seropositive. Female partners (n = 668) were 26 ± 8 years old with 10 ± 3 years of education. There was 82 % agreement on marital/relationship status between partners, which is expected given that in this study, enrollment was not restricted to primary sexual partners and the demographic questionnaire did not specifically refer to the enrolled partner. A higher percentage of female partners had children (49 %, n = 329). Table 1 presents further details on participant demographics.
Table 1.
Demographic information on N = 1336 men (n = 668) and women (n = 668) enrolled in Spear & Shield
| Characteristic | Men M(SD)/n (%) | Women |
|---|---|---|
| Age | 27 (9) | 26 (8) |
| Years of education | 11 (3) | 10 (3) |
| Employment status | ||
| Employed full or part time | 327 (49 %) | 185 (28 %) |
| Unemployed or volunteering | 341 (51 %) | 483 (72 %) |
| Annual income | ||
| ≥500 ZMK (~$100) | 314 (47 %) | 278 (42 %) |
| <500 ZMK | 354 (53 %) | 390 (58 %) |
| Marital status | ||
| Married | 261 (39 %) | 285 (43 %) |
| Not married | 407 (61 %) | 383 (57 %) |
| Children | ||
| At least one child | 269 (40 %) | 329 (49 %) |
| No children | 399 (60 %) | 339 (51 %) |
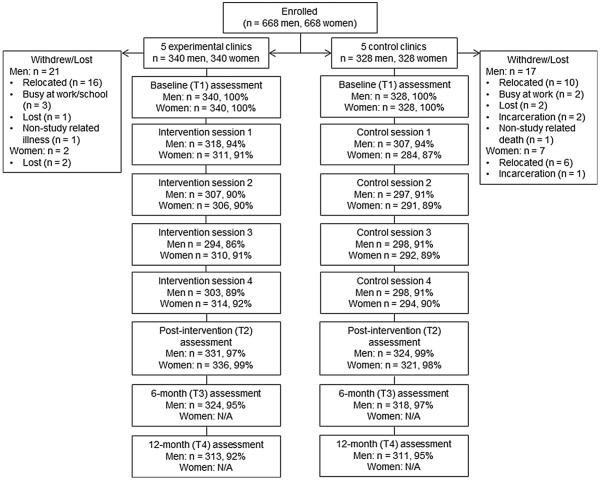
Attendance at the intervention and control sessions was high, averaging around 90 %, and there were very few missed assessments. In addition, of the N = 1336 participants (n = 668 men and 668 women), only 38 discontinued study participation. The most common reason for discontinuation was relocation (n = 26 participants relocated, 68 % of those withdrawn or lost). Figure 2 details attendance at the intervention or control sessions, assessment completion rates, and withdrawal/drop-out rates for each gender and study condition.
Fig. 2.
Attendance at the intervention or control sessions, assessment completion rates, and withdrawal/drop-out rates for N = 1336 men and women enrolled in the Spear & Shield project
Men's Uptake of VMMC Over Time
At baseline (T1), the majority of men were in the precontemplation stage of readiness to undergo VMMC (n = 376, 56 %); fewer were in the contemplation (n = 208, 32 %) and preparation stages (n = 84, 12 %). There was no difference in baseline readiness between treatment conditions [X2 (2 df) = 4.72, p = .095]. By post-intervention, 6.7 % of experimental condition men reached the action stage (i.e., underwent VMMC) as compared to 2.5 % of controls. At 6 months, the proportions were 24.7 % of the experimental condition vs. 11.7 % of controls. Ultimately, at 12 months follow-up, the proportions in the action stage were 41.4 % of men in the experimental condition and 20.9 % of controls.
Women's Acceptance of VMMC
Women's acceptance of VMMC was measured using four variables: perceived cultural acceptability, preference for a circumcised partner, the degree to which women would advise a partner to undergo VMMC, and whether or not they would like their current partner to undergo VMMC. Changes in these variables were measured from baseline to post-intervention, and the results are presented in Table 2. Generally, increases in acceptance were noted in both conditions, with greater increases in the experimental condition.
Table 2.
Means/frequencies of women's acceptance variables at baseline and post-intervention
| Variable | Baseline M(SD)/n(%) | Post-intervention | Mean/percent difference (Post minus Baseline) |
|---|---|---|---|
| Perceived cultural acceptability of VMMC | |||
| 1 = very unacceptable, 5 = very acceptable | |||
| Experimental | 4.14 (1.23) | 4.25 (1.14) | 0.11 |
| Control | 4.07 (1.22) | 4.00 (1.28) | −0.07 |
| Degree to which women would advise a partner to undergo | |||
| VMMC 1 = definitely NOT, 5 = definitely YES | |||
| Experimental | 4.21 (1.24) | 4.51 (0.97) | 0.30 |
| Control | 4.19 (1.24) | 4.30 (1.11) | 0.11 |
| Preference for a circumcised partner | |||
| 1 = definitely NOT prefer a circumcised partner, 5 = definitely prefer a circumcised partner | |||
| Experimental | 3.44 (1.28) | 3.92 (1.08) | 0.48 |
| Control | 3.22 (1.44) | 3.41 (1.29) | 0.19 |
| Would like their current partner to undergo VMMC | |||
| Experimental | |||
| Yes | 274 (81 %) | 317 (94 %) | 13 % |
| No | 66 (19 %) | 19 (6 %) | |
| Control | |||
| Yes | 227 (69 %) | 246 (77 %) | 8 % |
| No | 101 (31 %) | 75 (23 %) | |
The Impact of Women's Acceptance on Men's Readiness
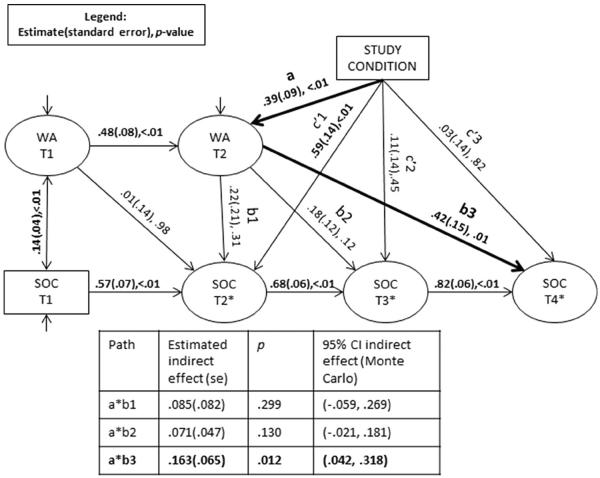
A path model was fit in order to examine the relationships between condition (experimental vs. control), women's VMMC acceptance, and men's readiness to undergo VMMC. Model fit statistics indicated that the model fit the data well [Root mean square error of approximation (RMSEA) = .045, 95 % CI RMSEA = (.035, .055); comparative fit index (CFI) = .936]. Examination of the model coefficients demonstrated that, as expected, the autoregressive paths in women's acceptance and men's readiness to undergo VMMC were all significant. Additionally, women's acceptance was significantly related to men's willingness to undergo VMMC at baseline (r = .139, p < .001), although the path between baseline acceptance and T2 readiness was not significant (see Fig. 3).
Fig. 3.
Unstandardized relationships between condition, women's VMMC acceptance, and men's readiness to undergo VMMC. Note Statistically significant coefficients are noted in bold; VMMC voluntary medical male circumcision, WA women's acceptance, SOC stages of change, T1 baseline, T2 post-intervention (approximately 2 months post-baseline), T3 6-month follow-up, T4 12-month follow-up
The first mediation pathway of interest, the a path, was significant, such that women in the experimental condition reported higher acceptance at T2 (b = .389, p < .001, standardized β = .680). The first b path, between acceptance and stages of change at T2, was not significant (b = .219, p = .306, β = .110). The product of these paths, a*b1, was 0.085 with a Monte Carlo 95 % confidence interval including zero [CI (−.059 to .269)]. Thus, women's acceptance did not mediate the relationship between condition and readiness at post-intervention (T2). At T2, men were primarily influenced by the intervention; the direct path between condition and readiness at T2, c′1, was significant (b = .593, p < .001; β = .516). The second b path, between acceptance at T2 and stages of change at T3, was also non-significant (b = .181, p = .115, β = .080). The product a*b2 was 0.071 [Monte Carlo 95 % CI (−.021 to .181)]; therefore, the relationship between the intervention and men's readiness to undergo VMMC at 6-months follow-up (T3) was not mediated by women's acceptance.
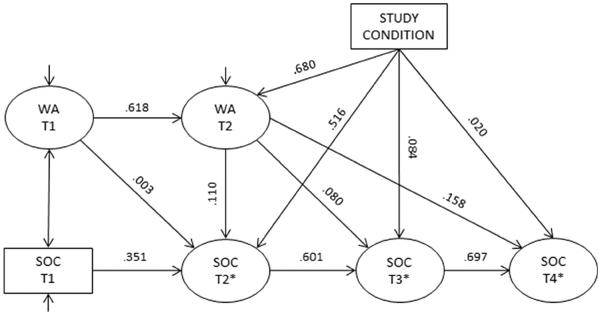
However, the third and final b path, between women's acceptance at T2 and men's readiness at 12 months (T4), was significant (b = .419, p = .006, β = .158). The product a*b3 was .163, with a Monte Carlo confidence interval that did not include zero [95 % CI (.042–.318)]. Thus, women's acceptance mediated the relationship between the intervention and men's readiness to undergo VMMC at 12 months follow-up. Figure 3 presents the path model with estimated unstandardized coefficients and indirect effects along with their standard errors and p values, and Fig. 4 presents the model with standardized coefficients.
Fig. 4.
Standardized path coefficients between condition, women's VMMC acceptance, and men's readiness to undergo VMMC. Note If the predictor variable is continuous, the coefficient was calculated as β = b*SD(x)/SD(y) and represents the amount of change in the outcome, in standard deviations, per one unit change in the standard deviation of the predictor variable, holding all other relationships constant. If the predictor variable is categorical (i.e., study condition), the standardized coefficient was calculated as β = b/SD(y) and represents the difference in the outcome, in standard deviations, between the experimental and control conditions, holding all other relationships constant
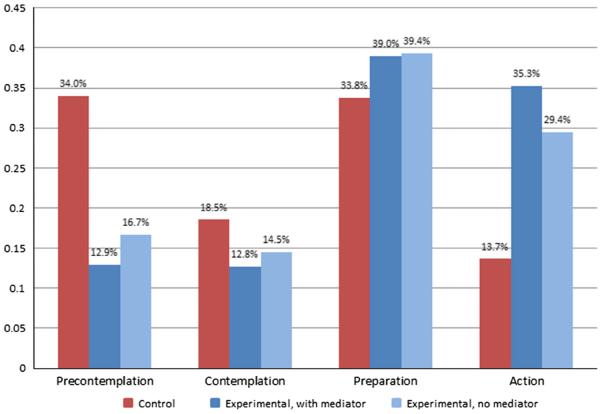
The indirect effect a*b3 can be interpreted in the context of the predicted thresholds for the latent continuous variable indicating stage of change and the regression paths between condition, women's acceptance and SOC to demonstrate the “amount” of mediation that occurred. Figure 5 shows the effects of women's acceptance on readiness among experimental condition participants at T3. The experimental condition is shown (1) including the mediation pathways (the “total effect” of condition on men's readiness) and (2) excluding the mediation pathways through women's acceptance (the “direct effect,” which does not include the mediator). As can be seen in the figure, the inclusion of the pathways through women's acceptance leads to a modest decrease in the expected percentage of men in the precontemplation and contemplation stages and an increase in the percentage of men in the preparation and action stages. At the action stage, the difference in percentage was 5.9 %, indicating that an additional 5.9 % of experimental condition men were predicted to undergo VMMC due to the positive changes in their female partners' acceptance from baseline to post-intervention. Applying the difference in the expected percentage of men reaching the action stage to the 340 experimental condition participants, it can be estimated that, in this study, approximately 20 VMMCs could be attributable to the increase in women's acceptance (an increase of 5.9 % reaching the action stage among 340 men).
Fig. 5.
Predicted stage of readiness for VMMC at T4, including estimates for the experimental condition (1) including mediation pathways through women's acceptance (indirect effect p = .013) and (2) excluding mediation pathways through women's acceptance, as well as the control condition
Discussion
This study examined whether some or all of the effect of an intervention to increase men's readiness to undergo VMMC could be attributable to enhanced female partner attitudes and VMMC acceptance. It was discovered that, immediately following the intervention, women's acceptance did not influence men's readiness; at that time, men were influenced primarily by the intervention itself. However, at 12 months post-intervention, improvements in women's attitudes and acceptance significantly influenced experimental condition men's readiness to undergo VMMC. At that time, an approximate 6 % increase in the likelihood of undergoing VMMC among experimental condition participants could be attributed to increased acceptance of VMMC among their female partners.
As VMMC continues to be scaled-up, it is estimated that 7 VMMCs will be needed to avert one case of HIV in Zambia [31], with fewer needed in areas of high HIV prevalence. Lusaka District, the location of this study and capital of Zambia, has the highest rates of HIV in the country (20.8 %; [32]). In Lusaka, progress has been made towards the goal of 80 % VMMC coverage by 2015 [22]. However, substantial gaps in coverage remain; the number of VMMCs completed in Lusaka was short of its 2012 target of ~32,000 by approximately one-third [33]. Implementing intensive VMMC promotion interventions such as the Spear & Shield project and involving women could help to close this gap. Scale-up of Spear & Shield was designed to be feasible; for example, the intervention was nested within the existing post-VCT process and utilized a trained member of the pre-existing community health center staff to facilitate the sessions. If Spear & Shield were scaled-up to reach 10,000 men and women in Lusaka, it could be anticipated that approximately 3530 VMMCs would be performed on men participating in the program, with 600 attributable to women's involvement. These VMMCs could result in ~504 cases of HIV averted, 85 of which would be due to women's influence. Finally, the VMMCs could also indirectly result in a substantial reduction in HIV, STI and HPV incidence in women.
In this study, the direct impact of the intervention on men's readiness for VMMC was highest immediately following the sessions. It is likely that men who were most amenable to undergoing VMMC were more immediately influenced, and the information and motivation presented during the intervention encouraged them to quickly move along the continuum towards VMMC. Conversely, the intervention may have had a “priming” effect on men who were less willing to consider VMMC immediately following the intervention, such that they might have become more sensitized than the control participants to the influence of their female partners as well as other environmental influences (e.g., media campaigns, community mobilization, changing social norms) on their readiness for VMMC. Additionally, despite the fact that “regression to the mean” is often noted in behavioral research, the Spear & Shield intervention continued to positively influence men indirectly through increasing women's acceptance 12 months after completion of the program. As has been found in other HIV prevention studies involving partners, targeting both members of a couple may lead to longer-lasting intervention effects than focusing on individuals [34–36]. This may be particularly salient in research on VMMC, in which the “behavior change” targeted by the intervention is not reversible.
This analysis was limited primarily by the inability to estimate different effects for predictor variables on each individual stage of change (i.e., treat SOC as a nominal variable). The method of analysis utilized assumes that the intervention and women's acceptance affected the likelihood of movement through each stage of change equally (for example, had the same impact on movement from precontemplation to contemplation as movement from contemplation to preparation), which may not have been the case. In addition, this analysis was unable to account for the nature of the relationship between men and their enrolled female partners. Enrollment of female partners was not limited to primary sexual partners, although sexual partners may have the most influence on men's decisions about whether to undergo VMMC. Future studies should examine whether “moderated mediation” occurs, i.e., whether partners of different types have more (or less) influence on men's decisions to undergo VMMC.
In summary, this study found that women's acceptance of VMMC significantly impacted men's decisions to undergo VMMC, supporting previous studies which underscore the importance of including partners in VMMC promotion efforts [17, 19]. Although women may have limited ability to negotiate safe sex in some African cultures [37], their preferences may nonetheless have a substantial influence on HIV prevention (e.g., [38, 39]). Thus, in order to increase the demand for and impact of VMMC, it is important to engage both men and women in biobehavioral interventions promoting VMMC as a potent HIV prevention strategy.
Acknowledgments
This study was supported by the National Institutes of Health, Grant number R01MH095539.
References
- 1.Bailey RC, Moses S, Parker CB, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007;369(9562):643–56. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 2.Gray RH, Kigozi G, Serwadda D, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369(9562):657–66. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 3.Gray R, Kigozi G, Kong X, et al. The effectiveness of male circumcision for HIV prevention and effects on risk behaviors in a posttrial follow-up study. AIDS. 2012;26(5):609–15. doi: 10.1097/QAD.0b013e3283504a3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta SD, Moses S, Agot K, et al. The long-term efficacy of medical male circumcision against HIV acquisition. AIDS. 2013;27(18):2899–907. doi: 10.1097/01.aids.0000432444.30308.2d. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization [Accessed 10 Dec 2014];New data on male circumcision and HIV prevention: policy and programme implications. 2007 http://libdoc.who.int/publications/2007/9789241595988_eng.pdf?ua=1.
- 6.Njeuhmeli E, Forsythe S, Reed J, et al. Voluntary medical male circumcision: modeling the impact and cost of expanding male circumcision for HIV prevention in eastern and southern Africa. PLoS Med. 2011;8(11):e1001132. doi: 10.1371/journal.pmed.1001132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hatzold K, Mavhu W, Jasi P, et al. Barriers and motivators to voluntary medical male circumcision uptake among different age groups of men in Zimbabwe: results from a mixed methods study. PLoS ONE. 2014;9(5):e85051. doi: 10.1371/journal.pone.0085051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mark D, Middelkoop K, Black S, et al. Low acceptability of medical male circumcision as an HIV/AIDS prevention intervention within a South African community that practises traditional circumcision. S Afr Med J. 2012;102(6):571–3. doi: 10.7196/samj.5477. [DOI] [PubMed] [Google Scholar]
- 9.Macintyre K, Andrinopoulos K, Moses N, et al. Attitudes, perceptions and potential uptake of male circumcision among older men in Turkana County, Kenya using qualitative methods. PLoS ONE. 2014;9(5):e83998. doi: 10.1371/journal.pone.0083998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simbayi LC, Shisana O, Rehle T, et al. [Accessed 10 Dec 2014];South African National HIV prevalence, incidence and behaviour survey, 2012. 2014 http://www.hsrc.ac.za/uploads/pageContent/4565/SABSSMIVLEOfinal.pdf.
- 11.Republic of Zambia Ministry of Health [Accessed 10 Dec 2014];Zambia sexual behavior survey, 2009. 2010 http://www.cpc.unc.edu/measure/publications/tr-10-73.
- 12.World Health Organization [Accessed 10 Dec 2014];Progress in scaling up voluntary medical male circumcision for HIV prevention in East and Southern Africa. 2013 http://www.malecircumcision.org/country_updates/documents/Progress%20in%20scaling%20up%20VMMC_Dec2013.pdf.
- 13.Weiss HA, Hankins CA, Dickson K. Male circumcision and risk of HIV infection in women: a systematic review and meta-analysis. Lancet Infect Dis. 2009;9(11):669–77. doi: 10.1016/S1473-3099(09)70235-X. [DOI] [PubMed] [Google Scholar]
- 14.Larke N. Male circumcision, HIV and sexually transmitted infections: a review. Br J Nurs. 2010;19(10):629–34. doi: 10.12968/bjon.2010.19.10.48201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hallett TB, Alsallaq RA, Baeten JM, et al. Will circumcision provide even more protection from HIV to women and men? New estimates of the population impact of circumcision interventions. Sex Transm Infect. 2011;87(2):88–93. doi: 10.1136/sti.2010.043372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis MA, Gray RH, Grabowski MK, et al. Male circumcision decreases high-risk human papillomavirus viral load in female partners: a randomized trial in Rakai, Uganda. Int J Cancer. 2013;133(5):1247–52. doi: 10.1002/ijc.28100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shacham E, Godlonton S, Thornton RL. Perceptions of male circumcision among married couples in Rural Malawi. J Int Assoc Provid AIDS Care. 2014;13(5):443–9. doi: 10.1177/2325957413508319. [DOI] [PubMed] [Google Scholar]
- 18.Riess TH, Achieng MM, Bailey RC. Women's beliefs about male circumcision, HIV prevention, and sexual behaviors in Kisumu, Kenya. PLoS ONE. 2014;9(5):e97748. doi: 10.1371/journal.pone.0097748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lanham M, L'Engle KL, Loolpapit M, Oguma IO. Women's roles in voluntary medical male circumcision in Nyanza Province, Kenya. PLoS ONE. 2012;7(9):e44825. doi: 10.1371/journal.pone.0044825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Layer EH, Beckham SW, Mgeni L, Shembilu C, Momburi RB, Kennedy CE. “After my husband's circumcision, I know that I am safe from diseases”: women's attitudes and risk perceptions towards male circumcision in Iringa, Tanzania. PLoS ONE. 2013;8(8):e74391. doi: 10.1371/journal.pone.0074391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mantell JE, Smit JA, Saffitz JL, et al. Medical male circumcision and HIV risk: perceptions of women in a higher learning institution in KwaZulu-Natal, South Africa. Sex Health. 2013;10(2):112–8. doi: 10.1071/SH12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization [Accessed 10 Dec 2014];WHO progress brief: voluntary medical male circumcision for HIV prevention in priority countries of East and Southern Africa. 2014 http://www.who.int/hiv/topics/malecircumcision/male-circumcision-info-2014/en/
- 23.Mugwanya KK, Baeten JM, Nakku-Joloba E, et al. Knowledge and attitudes about male circumcision for HIV-1 prevention among heterosexual HIV-1 serodiscordant partnerships in Kampala,Uganda. AIDS Behav. 2010;14(5):1190–7. doi: 10.1007/s10461-010-9696-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones D, Cook R, Arheart K, et al. Acceptability, knowledge, beliefs, and partners as determinants of Zambian men's readiness to undergo medical male circumcision. AIDS Behav. 2014;18(2):278–84. doi: 10.1007/s10461-013-0530-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prochaska JO, Redding CA, Evers K. The transtheoretical model and stages of change. In: Glanz K, Rimer BK, Viswanath KV, editors. Health behavior and health education: theory, research, and practice. 4th ed Jossey-Bass; San Francisco: 2008. [Google Scholar]
- 26.Prochaska JO, Velicer WF, Rossi JS, et al. Stages of change and decisional balance for 12 problem behaviors. Health Psychol. 1994;13(1):39–46. doi: 10.1037//0278-6133.13.1.39. [DOI] [PubMed] [Google Scholar]
- 27.MacKinnon DP, Lockwood CM, Brown CH, Wang W, Hoffman JM. The intermediate endpoint effect in logistic and probit regression. Clin Trials. 2007;4(5):499–513. doi: 10.1177/1740774507083434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muthen LK, Muthen BO. MPLUS user's guide. 6th ed Muthen & Muthen; Los Angeles: 2010. [Google Scholar]
- 29.Preacher KJ, Selig JP. Advantages of Monte Carlo confidence intervals for indirect effects. Commun Methods Meas. 2012;6:77–98. [Google Scholar]
- 30.Selig JP, Preacher KJ. [Accessed 10 Dec 2014];Monte Carlo method for assessing mediation: an interactive tool for creating confidence intervals for indirect effects. http://quantpsy.org/2008.
- 31.USAID [Accessed 27 Feb 2015];The potential cost and impact of expanding male circumcision in Zambia. 2009 http://www.malecircumcision.org/programs/documents/Zambia11309.pdf.
- 32.Lusaka Times [Accessed 10 Dec 2014];Lusaka ranks highest in HIV prevalence rate. 2013 http://www.lusakatimes.com/2013/06/26/lusaka-ranks-highest-inhiv-prevalence-rate/
- 33.Republic of Zambia Ministry of Health [Accessed 10 Dec 2014];Country operational plan for the scale-up of voluntary medical male circumcision in Zambia, 2012–2015. 2012 http://www.malecircumcision.org/country_updates/documents/Zambia_VMMC_operational_plan. pdf.
- 34.Jones DJ, Chitalu N, Ndubani P, et al. Sexual risk reduction among Zambian couples. Sahara J. 2009;6(2):69–75. doi: 10.1080/17290376.2009.9724932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.El-Bassel N, Gilbert L, Wu E, et al. Couple-based HIV prevention for low-income drug users from New York City: a randomized controlled trial to reduce dual risks. J Acquir Immune Defic Syndr. 2011;58(2):198–206. doi: 10.1097/QAI.0b013e318229eab1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karney BR, Hops H, Redding CA, Reis HT, Rothman AJ, Simpson JA. A framework for incorporating dyads in models of HIV-prevention. AIDS Behav. 2010;14(Suppl 2):189–203. doi: 10.1007/s10461-010-9802-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wechsberg WM, Myers B, Reed E, Carney T, Emanuel AN, Browne FA. Substance use, gender inequity, violence and sexual risk among couples in Cape Town. Cult Health Sex. 2013;15(10):1221–36. doi: 10.1080/13691058.2013.815366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones D, Kashy D, Chitalu N, et al. Risk reduction among HIV-seroconcordant and -discordant couples: the Zambia NOW2 intervention. AIDS Patient Care STDS. 2014;28(8):433–41. doi: 10.1089/apc.2014.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bailey RC, Muga R, Poulussen R, Abicht H. The acceptability of male circumcision to reduce HIV infections in Nyanza Province, Kenya. AIDS Care. 2002;14(1):27–40. doi: 10.1080/09540120220097919. [DOI] [PubMed] [Google Scholar]