Abstract
The type 1 parathyroid hormone receptor (PTH1R) is a key regulator of calcium homeostasis and bone turnover. Here, we employed SILAC-based quantitative mass spectrometry combined with bioinformatic pathways analysis to examine global changes in protein phosphorylation following short-term stimulation of endogenously expressed PTH1R in osteoblastic cells in vitro. Following 5 min exposure to the conventional agonist, PTH(1-34), we detected significant changes in the phosphorylation of 224 distinct proteins. Kinase substrate motif enrichment demonstrated that consensus motifs for PKA and CAMK2 were the most heavily upregulated within the phosphoproteome, while consensus motifs for mitogen-activated protein kinases were strongly downregulated. Signaling pathways analysis identified ERK1/2 and AKT as important nodal kinases in the downstream network and revealed strong regulation of small GTPases involved in cytoskeletal rearrangement, cell motility, and focal adhesion complex signaling. Our data illustrate the utility of quantitative mass spectrometry in measuring dynamic changes in protein phosphorylation following GPCR activation.
Keywords: G protein-coupled receptor, mass spectrometry, parathyroid hormone receptor, phosphoproteomics, osteoblast
1. Introduction
As our appreciation of the “pluridimensionality” of G protein-coupled receptor (GPCR) efficacy [1] has grown, it has become increasingly important to consider ligand action in the context of signaling networks. At physiological levels of expression, individual GPCRs may engage multiple downstream signaling pathways simultaneously, using both heterotrimeric G proteins and non-G protein effectors, e.g. arrestins, as conduits [2,3]. As these initial signals propagate, the potential for crosstalk expands exponentially, rapidly exceeding the capacity of simple pathway-focused approaches, e.g. protein immunoblots, to capture the complexity. Adding to the problem is the knowledge that ligand structure can “bias” the efficiency with which a GPCR activates its downstream effectors relative to the native ligand [4,5]. “Biasing” GPCR signaling by varying ligand structure holds the promise of novel therapeutics, but at the same time introduces the specter of unexpected “on-target” effects arising from activating GPCRs in “unnatural” ways [6]. Yet another concern is the impact of system factors, e.g. cell background-specific variations in receptor density and expression of downstream effectors, which can introduce wide variations in the observed response [5]. Given these challenges, an “ideal” approach to understanding GPCR signal transduction would involve studying GPCRs in a native context using methods capable of both capturing high-dimensionality data and deconvoluting it to generate information about the net effect on biological pathways and processes.
Quantitative phosphoproteomic analysis provides an avenue for elucidating transient phosphorylation-mediated signaling events following receptor-ligand interactions [7,8]. Dynamic post-translational modifications impact signal transduction by affecting protein interactions, subcellular localization, enzyme activity, and protein half-life. Recent in-depth phosphoproteomic studies indicate that seventy-five percent of proteins are phosphorylated, the majority of which (85%) are multiply phosphorylated [9], and phosphorylation is a key post-translational mediator of cellular signaling necessary for growth, proliferation, motility, differentiation, survival, and the response to hormones and therapeutics. While subject to its own limitations, mass spectrometric approaches provide the capacity to capture a global “snapshot” of stimulus-induced changes in the phosphoproteome that reflect the first few minutes of receptor activation. Combining this information with bioinformatic approaches that translate regulated phosphorylation sites into kinase substrate consensus motifs, link kinases to potential substrates, and identify signaling pathways and regulated biological processes, can produce a holistic picture of signaling at the network level, and generate testable hypotheses about the links between proximal signaling events and downstream phenotypic responses. Indeed, proteomic studies investigating the phosphorylation regulated by stimulation of GPCRs are revealing unanticipated signaling intermediates, finding non-canonical signaling events, and enabling the prediction of activated kinases [10–15].
Here, we apply quantitative stable isotopic labeling by amino acids in cell culture (SILAC)-based mass spectrometry to probe the protein phosphorylation networks regulated by short term exposure to hPTH(1-34), a conventional agonist of the type 1 parathyroid hormone (PTH1R). PTH1R signaling is complex, resulting from activation of diverse heterotrimeric G protein species, i.e. Gs, Gi, and Gq/11, as well as non-canonical arrestin-mediated signaling events [16–18], so to reduce artifacts related to receptor overexpression our experiments were performed in cultured differentiating osteoblastic cells expressing native levels of receptor. In addition to detecting the expected contributions of Gs-cAMP and Gq/11-calcium signaling, our results demonstrate a robust phosphorylation network regulating small GTPases and cytoskeletal rearrangement, and provide insights into PTH1R-mediated signaling in a physiological context.
2. Materials and Methods
2.1. General Considerations
Continued advances in methodology, instrumentation, and computational proteomics are enabling the identification and functional assessment of differentially regulated post-translational modifications [19]. For in vitro studies, SILAC is considered the gold standard [7,20]. Utilizing SILAC, changes in protein expression and phosphorylation can be measured, providing a comprehensive view of the dynamic phosphorylation events occurring following ligand-receptor stimulation. As compared to isobaric tagging and label free approaches, SILAC-labeled proteins are combined in the first step of the sample preparation workflow thereby reducing the effect of technical errors introduced when preparing multiple samples in parallel. This yields a higher sensitivity to measure smaller changes in the extent of phosphorylation.
On the other hand, SILAC requires metabolic incorporation of amino acids into proteins prior to cell stimulation, so that individual peptide tandem mass spectra can be assigned to their sample of origin, e.g. stimulated versus non-stimulated, when the mixed samples are analyzed. Thus the approach is not amenable to analysis of proteins prepared directly from tissue. Further, comparison of the phosphoproteomes of multiple murine tissues has revealed the occurrence of tissue-specific sites of protein phosphorylation necessitating analysis in the cell type of interest [21]. To circumvent these limitations, we chose to metabolically label MC3T3-E1 pre-osteoblast cells, an immortalized cell line derived from newborn mouse calvaria, to establish a system to investigate the receptor-proximal effects of PTH1R activation in a near native cell background.
2.2. Cell Culture and Stable Isotope Labeling with Amino Acids
MC3T3-E1 subclone 4 pre-osteoblast cells (CRL-2593, ATCC) were used as a model of osteoblast differentiation and function. When grown in osteogenic media containing ascorbic acid and β-glycerophosphate, the cells differentiate, express markers reflecting different stages of osteoblast differentiation, and secrete a mineralized hydroxyapatite matrix [22–25]. The presence of the PTH1R in these cells has been confirmed and the cAMP response to receptor stimulation is readily detectable after 4 days in culture [26–28]. This response is maintained after 10 days of culture in osteogenic media.
Reagents for cell culture and SILAC labeling of MC3T3-E1 cells
-
Stable isotope labeled amino acids:
Light Arg: Arg0 L-Arginine-HCl (Fisher Scientific: PI-89989)
Light Lys: Lys0 L-Lysine-2HCl (Fisher Scientific: PI-89987)
Medium Arg: Arg6 (13C6 L-Arginine-HCL) (Fisher Scientific: PI-88210)
Medium Lys: Lys4 (4,4,5,5-D4 L-Lysine-2HCl) (Cambridge Isotopes: DLM-2640-0.5)
Heavy Arg: Arg10 (13C6 15N4 L-Arginine-HCL) (Fisher Scientific: PI-89990)
Heavy Lys: Lys8 (13C6 15N2 L-Lysine-2HCl) (Cambridge Isotopes: CNLM-291-0.1)
SILAC Media: For “light”, “medium”, or “heavy” SILAC media add labeled amino acids to custom MEMα media lacking arginine and lysine (Gibco®). For a final concentration of 0.5 mM arginine add 0.015 ml of 10 mg/ml arginine per ml media. For a final concentration of 0.4 mM lysine add 0.0073 ml of 10 mg/ml lysine per ml of media.
SILAC Growth Media: For growth media add 10% dialyzed fetal calf serum (FCS) (Fisher Scientific) and 1% penicillin/streptomycin to SILAC media. The FCS is dialyzed to eliminate a source of unlabeled lysine or arginine.
SILAC Osteogenic Media: For osteogenic differentiation media (MEMα, 10% FCS, 50 μg/ml ascorbic acid and 10 mM β-glycerophosphate) add 1.53 mg beta glycerol phosphate per ml of media and 1 ul of 1000x (5 mg of L-ascorbic acid per ml of media) to SILAC growth media.
Protocol for cell culture and SILAC labeling of MC3T3-E1 cells
To enable full incorporation of isotopically labeled arginine and lysine into the cellular protein pool, grow pre-osteoblastic MC3T3-E1 cells subconfluently at 37°C and 5% CO2 for at least 5 doublings in SILAC growth media light ([12C614N2] Lys, [12C614N4] Arg), medium ([2H4] Lys, [13C6] Arg), or heavy ([13C615N2] Lys, [13C615N4] Arg) amino acids.
Efficient metabolic labeling of cellular proteins is confirmed by LC-MS/MS [29]. Once the labels are fully incorporated (≥95%), cells can be seeded or aliquots stored in liquid nitrogen for subsequent experiments.
Seed the SILAC-labeled cells at 1 × 105 cells per cm2 and grow to confluence in growth media. To obtain sufficient starting material for TiO2 enrichment of phosphopeptides from osteoblasts, we use five to ten 100 mm culture plates with 9 ml of media per dish for each condition of a triple SILAC experiment. This yields 5–7 mg of protein per treatment condition.
For experiments in differentiating osteoblasts, after reaching confluence cells are incubated in osteogenic media with media changes every other day. As the cells differentiate, mineralized matrix accrues in the dish. At day 10 of differentiation (day 13 in culture) the cells are visibly differentiated and bone nodules have begun to form in the dish.
NOTE: Sources of potential quantification error include:
Incomplete incorporation of isotopically labeled amino acids. After five doublings, the efficiency of metabolic labeling must be checked by analyzing an aliquot of cell lysate protein by LC-MS/MS.
High levels of endogenous arginase activity in some cell types result in arginine-to-proline conversion contributing to isotopically labeled proline residues. This may be overcome by increasing the concentration of proline in the media as described by Park et al [30].
To correct for potential technical errors when combining equal amounts the SILAC-labeled protein from each treatment condition, the median ratio of all peptides observed in one LC-MS/MS analysis is subtracted from the ratio of each peptide.
Errors can also occur from the breakdown of proteins in the fetal calf serum and incorporation of unlabeled “light” amino acids into the proteins. This is controlled for by using a “label swap” i.e. switching the untreated condition to a different isotopic label in a biological replicate. This also corrects for potential false positives arising from reagent-specific changes such as isotope effects on cellular metabolism [30]. These false positives will exhibit ratios that change in opposite directions and are filtered out by statistical analyses based on the reproducibility of the measurements.
2.3. Receptor Stimulation and Protein Preparation
2.3.1 Acute Stimulation of MC3T3-E1 cells
Eighteen hours prior to stimulation, cells are serum-starved by reducing the concentration of dialyzed FCS to 1% v/v in osteogenic SILAC media. Stimulate light, medium, or heavy labeled SILAC cells for the desired time with 0.1 μM human PTH N-terminal residues 1-34 [hPTH(1-34)]. Dissolve hPTH(1-34) (Tocris) to 0.1 mM in MEMα medium and dispense in cell plate at a ratio of 1:1000.
Immediately lyse cells with freshly made urea buffer [9.0 M urea, 20 mM HEPES (pH 8.0), 1 mM sodium orthovanadate, 2.5 mM sodium pyrophosphate, 1 mM β-glycerol-phosphate in HPLC grade water] and incubate lysate on a rotator for 4 hrs at 4°C.
Remove cellular debris by centrifugation at 17,000 × g for 10 minutes at 4°C.
Determine protein concentration by using a Bradford assay, and combine equal amounts of protein (~5 mg) from each treatment group at 1:1:1 ratio.
NOTE: Additional considerations:
The method of protein extraction will affect the efficiency of protein recovery from different cellular compartments, e.g. subcellular organelles, integral membrane proteins, etc. Thus, consideration should be given to the best method based on the tissue, cell type, organelle, and protein size of interest [31,32].
We chose urea buffer in order to quickly denature the protein following ligand stimulation. An advantage of using urea is that it increases the ability to detect peptides from integral membrane proteins. The approach however does not reveal ligand-induced changes in subcellular localization of proteins. Rather, the dynamic changes in phosphorylation are averaged across the cell. Subcellular fractionation coupled to SILAC-labeling [7] and estimates of changes in the stoichiometry of phosphorylation [9] will reveal more in depth coverage of the dynamic nature of the phosphoproteome.”
For conditions of receptor stimulation exceeding one hour an aliquot of the SILAC labeled protein can be removed for analysis of differential protein expression. This can be done using SDS-PAGE separation, whereby the lane is cut into 12–24 fractions followed by in-gel tryptic digestion, and the resulting peptides are analyzed by LC-MS/MS. During processing of the data, the changes in phosphorylation can be normalized to correct for changes in protein expression.
2.3.2 Reduction, Alkylation and Tryptic Digestion in Solution
Dilute the protein sample to 2 M urea with water (~50 ml).
Reduce cysteines in 1 mM of dithiothreitol for 30 minutes at room temperature.
Alkylate thiols in 5.5 mM of iodoacetamide for 15 minutes at room temperature in the dark.
Check pH and adjust if needed to 7.8–8.7 for effective trypsin digestion.
Digest protein with trypsin (Sigma) at a 1:100 (w/w) enzyme to protein ratio overnight at 37°C.
Quench the digestion reaction by adding 4.0 ml of 10% v/v trifluoroacetic acid (TFA) in water.
Desalt digested peptides (15–20 mg) using a 6cc C18 Sep-Pak cartridge (Waters) and dry under vacuum. Store at −80°C until further use.
2.4. Peptide Fractionation Approaches
Prior to enrichment of post-translationally modified peptides, fractionation of the complex tryptic peptide mixture provides a greater separation space enabling detection of less abundant analytes by LC-MS/MS. Several alternatives, including strong cation exchange chromatography (SCX), off-gel electrophoresis, and high pH reversed phase HPLC are commonly utilized methods for peptide fractionation.
2.4.1. Strong Cation Exchange Chromatography
SCX is routinely used as an orthogonal approach to fractionate peptides into less complex mixtures prior to phosphopeptide enrichment and C18RP-LC-MS/MS [31]. The low to high salt gradient of the SCX chromatography allows for fractionation of peptides by net charge state. Negatively charged phosphopeptides are minimally retained and elute early within the gradient [33] The separation of multiply phosphorylated peptides from mono-phosphorylated peptide can reduce the bias of enrichment of multi-phosphorylated peptides by titanium dioxide.
Materials and Buffers for SCX
Column: 6.4 mm × 30 mm (15 μm) Resource S methylsulfonate column (GE Healthcare).
Buffer A: 5 mM KH2PO4 (pH 2.7), 30% acetonitrile. Typically 600 ml is required per separation.
Buffer B: 5 mM KH2PO4 (pH 2.7), 350 mM KCl, 30% acetonitrile. Typically 400 ml is required per separation.
NOTE: Before adding acetonitrile to the aqueous buffer containing potassium salts, vacuum filter buffers to remove particulates and adjust the pH with phosphoric acid if necessary.
Protocol for SCX
Solubilize desalted, dried peptides (15–20 mg) in 1–2 mL of SCX Buffer A.
Separate peptides using a gradient of 0% B for 10 min; 0–30% B in 30 min; 30–100% B in 5 min; 100% B for 10 min at a flow rate of 1 ml/min.
Monitor the chromatographic separation at 214 nm and collect fractions every minute starting with the unretained flow-through peak.
Combine adjacent fractions into desired number of fractions.
2.4.2. Off-Gel Electrophoresis (OGE)
Using off-gel electrophoresis, peptides separated by isoelectric focusing can be conveniently removed in the liquid phase without necessitating extraction from an immobilized pH gradient gel.
Materials and Buffers for OGE
Immobilized pH gradient (IPG) strips: 1 mm Immobilon Drystrip pH 3–10, 24 cm (GE Healthcare).
Rehydration buffer: 1X Off Gel solution (Agilient Technologies).
3100 Off-Gel Fractionator (Agilent Technologies).
Protocol for OGE
The maximum recommended capacity of the IPG strips is 5 mg per strip. For a large-scale fractionation of 15 mg of peptides, five IPG strips can be loaded with 3 mg per strip to prevent overloading.
Set up the Off-Gel fractionator for 24 wells per strip following the manufacturer’s instructions.
Pipette 20 μL rehydration buffer into each well and equilibrate for 15 min.
Place wetted electrode pads at both ends of the strip.
Resuspend trypsin digested peptides (~15 mg) in 80% v/v OffGel stock solution in water (18 ml). Pipette 150 μL of peptide solution into each well.
Cover the wells with seals.
Use mineral oil at both ends of the strip to avoid evaporation of the electrode wetting solution.
Perform isoelectric focusing with a maximum current of 50 μA, 8000 V, 200 mW until 50 kVh is reached.
Remove fractionated peptides from the wells by pipetting (120–150 μl per fraction per strip).
Acidify and desalt peptides using a C18 SepPak cartridge (Waters) prior to drying under vacuum.
NOTE: In a direct comparison of separation methods using the same starting material fractionated into 12 fractions we identified 2,076 phosphopeptides following OGE and 5,277 phosphopeptides after SCX.
2.4.3. High-pH reversed phase chromatography
The separation selectivity of high pH reversed phase liquid chromatography (HpH RPLC) is based on the redistribution of charges within peptide chains at high-pH (~10) which alters peptide hydrophobicity and shifts the retention times as compared to low-pH separations [34]. HpH RPLC provides improved resolution yielding up to 80% more peptide identifications than prefractionation by SCX [35, 36]. Additional advantages of this method include the employment of salt-free buffers, eliminating the need for an additional desalting step. In contrast to SCX and OGE, HpH RPLC does not concentrate bulk phosphopeptides but results in a more even distribution of the phosphopeptides across the gradient. While the resolution of HpH RP chromatography is superior to SCX, the orthogonality with subsequent low pH-RP is achieved by combining the fractions using a concatenation approach [37]. The protocol below is based on [38, 39].
Materials and Buffers for HpH RPLC
Buffer A: 10 mM ammonium hydroxide in HPLC grade water.
Buffer B: 10% 10 mM ammonium hydroxide in HPLC grade water, 90% acetonitrile.
Column: Zorbax 300 Extend-C18 column (9.4 × 250 mm, 5 μm, 300 Å) (Agilent Technologies).
Agilent 1100 HPLC pump equipped with a 2 mL injection loop.
Protocol for HpH RPLC
Desalt digested peptides (15–20 mg) using a 6cc SepPak C18 cartridge (Waters).
Resuspend tryptic peptides (15 mg) in 1–2 ml buffer A.
Separate peptides with a gradient of 1%–25% B in 50 minutes; 25–60% B in 4 minutes; and 60–70% B in 2 min with a flow rate of 3 ml/min.
Monitor the elution profile at 214 nm and collect fractions every 30 seconds between 0 and 70 minutes.
-
Keep fractions on ice and combine using a concatenation approach according the matrix below:
Combined Fraction # Individual Fraction # 1 1,2,25,26,49,50,73,74,97,98,121,122 2 3,4,27,28,51,52,75,76,99,100,123,124 3 5,6,29,30,53,54,77,78,101,102,125,126 4 7,8,31,32,55,56,79,80,103,104,127,128 5 9,10,33,34,57,58,81,82,105,106,129,130 6 11,12,35,36,59,60,83,84,107,108,131,132 7 13,14,37,38,61,62,85,86,109,110,133,134 8 15,16,39,40,63,64,87,88,111,112,135,136 9 17,18,41,42,65,66,89,90,113,114,137,138 10 19,20,43,44,67,68,91,92,115,116,139,140 11 21,22,45,46,69,70,93,94,117,118 12 23,24,47,48,71,72,95,96,119,120 Dry combined fractions under vacuum, and store at −20 °C until enrichment.
2.5. Phosphopeptide Enrichment
Due to the relatively low concentration of post-translationally modified peptides in proteolytically digested cell or tissue lysates, an enrichment step is necessary for robust identification and quantitation. Enrichment strategies utilizing immobilized metal affinity chromatography (IMAC), titanium dioxide (TiO2), or a combination thereof (Ti4+-IMAC), are capable of yielding up to 99% enrichment of phosphopeptides from complex mixtures [15, 40]. Coupled with increased mass accuracy and enhanced sensitivity of modern mass spectrometers, these enrichment techniques allow the application of quantitative proteomic methods to the characterization of dynamic phosphorylation [41]. We found phosphopeptide enrichment as described by Larsen, et al TiO2 simple and effective. Due to the low abundance and stoichiometry of phosphoTyr as compared to phosphoSer/Thr, a more selective enrichment approach is necessary for global quantitative analysis of phosphotyrosine. As described by White et al, immobilized anti-phosphotyrosine antibodies can be used to robustly enrich these peptides prior to LC-MS/MS analysis [42].
Reagents and Materials for TiO2 phosphopeptide enrichment
Buffer A: 80% acetonitrile, 0.5% trifluoroacetic acid
Buffer B: buffer A and lactic acid, 3:1 (v/v). 21.6 ml of buffer A and 7.2 ml lactic acid.
Elution solution: 40% acetonitrile, 5% NH4OH in water (~1–2ml)
Low-Bind 1.5 mL microcentrifuge tubes
C4 ZipTips
5 mg TiO2 beads per fraction (Titansphere, 10 μm, GL Sciences)
Protocol for TiO2 phosphopeptide enrichment
Phosphopeptides from each fraction are enriched using a slurry of 5 mg TiO2 beads, fresh buffers and elution solution [43].
Reconstitute peptides in 200 μl of HPLC grade water and add 1.3 ml of buffer B.
Wash 5 mg of TiO2 beads (per tube) in 200 μl buffer A and equilibrate in 200 μl buffer B. Centrifuge and remove the buffers after each step.
Incubate peptides with beads for 45 minutes at room temperature with mixing.
Centrifuge and remove supernatant. Wash beads sequentially with 200 μl of buffers B and A. Remove buffer after each step.
Resuspend TiO2 beads in 50 μl of buffer A and transfer to the top of a C4 Ziptip, which serves as a frit.
Remove buffer by centrifugation.
To elute peptides, place C4 Ziptip in a clean, low-bind 1.5 ml centrifuge tube, and add 50 μl of elution buffer to top of tip.
Centrifuge at 1,000 × g for 5 minutes twice. It may be necessary to use a second tube for the second spin as the backpressure of the liquid from the first elution may prevent the second elution.
Immediately neutralize the eluted peptide solution with 10 μl of neat formic acid.
Dry samples under vacuum, then desalt with a C18 ZipTip.
To increase the efficiency of phosphopeptide enrichment, a second enrichment can be performed [44]. The enrichment efficiency is dependent on the cell type and prefractionation method utilized. A single enrichment of SCX fractions by TiO2 yields between 50–75% phosphopeptides, whereas double serial enrichment can yield up to 99% phosphopeptides.
2.6. Mass Spectrometric Analysis (LC-MS/MS)
Peptides are loaded onto a trap column, eluted, and separated on a 75 μm × 15 cm fused- silica column packed in-house with C18 reversed-phase resin (YMC-ODS-AQ; 5-μm particles; 200-Å pore; Waters) using an acetonitrile gradient of 5–50% in 180 min containing 0.2% formic acid on a Dionex Ultimate 3000 nano LC system at flow rate of 200 nl/min. The sample is introduced via nano-electrospray ionization (nESI) with an uncoated silica emitter (360 μm OD, 50 μm ID, with a 5 μm tip, New Objective) to a hybrid dual-pressure linear ion trap-orbitrap mass spectrometer (Orbitrap Elite, Thermo Scientific).
Mass spectra are acquired in data dependent mode using a TOP10 method collecting one FTMS survey MS scan within a mass range of m/z 400–1800 followed by acquisition of tandem mass spectra (MS/MS) of the ten most intense ions in the survey scan. Depending on the speed of the instrument, a lower number of MS/MS scans in between survey scans will yield more points along the chromatographically resolved peak which is necessary for accurate quantitation at the MS1 level.
The automatic gain control target value in the Orbitrap was 106 for the survey MS scan at a resolution of 60,000 at m/z 400.
Peptides can be fragmented in the ion trap by collision-induced dissociation (CID) with an automatic gain control target value of 1000 ions and a minimum threshold of 500 counts.
A decision tree approach can be utilized to fragment large, highly charged peptides by electron transfer dissociation (ETD) rather than CID. For ETD reactions using fluoranthene we engage the reaction time per charge state setting [45]. To facilitate fragmentation of phosphopeptides, supplemental activation can be turned on which yields primarily c, z, and y ions, and possible neutral loss of phosphoric acid (98 Da) from the precursor and product ions.
2.7. Data Processing and Analysis
Many peptides have multiple potential sites of phosphorylation, introducing the need to confidently assign the site of modification prior to downstream analysis. Large-scale identification of site-specific changes in phosphorylation and subsequent prediction of activated kinases have been enabled by algorithms that assess and score the assignment of the position of the modification during automated database searches [46–50]. Manual inspection of the annotated tandem mass spectra is necessary to confirm the appropriate threshold parameters have been set and to verify the site-assignment prior to proceeding with site-directed mutagenesis or modification-specific antibody generation. Peptides with confidently assigned sites of phosphorylation can be further examined for enrichment of kinase substrate motifs [51–53]. Freely availability software platforms also provide the ability to readily access gene/protein annotation, known protein interactions, and site-specific functional annotation stored in multiple shared data repositories [54,55]. As the data quality and modification-and site-specific informatic tools and databases advance, the potential to assimilate and functionally interrogate post-translationally regulated signaling networks will improve. Together these complementary approaches and advances are pushing the field forward and provide robust workflows for phosphoproteomics that can be adapted to other post-translational modifications as well.
2.7.1. Peptide Identification, Site-Assignment, and Quantitation
Multiple software platforms are available for the identification and quantification of SILAC labeled phosphopeptides. We chose to use MaxQuant since the output contains the protein residue number that is modified, sequence windows of 15 residues on either side of the site of modification, and the ability to easily interrogate the identified sites against databases of known and functionally annotated post-translational modifications. Additionally, hundreds of LC-MS/MS analyses can be search simultaneously with the output assimilated into multiple text files to simplify downstream data processing. Specifically regarding SILAC-based experiments, after the initial database search MaxQuant provides the ability to look for and quantify a SILAC peptide partner that was not selected for sequencing by tandem mass spectrometry.
The MaxQuant/Perseus platforms are freely available (version1.5.0.22 Max Planck Institute of Biochemistry, Dept. of Proteomics and Signal Transduction, Munich, Germany) [50,56,57]. Workshops and on-line tutorials are kindly made available for users.
All of the raw files from multiple biological replicate experiments including label swap controls can be searched simultaneously. Andromeda, the database search algorithm embedded in MaxQuant, can be configured to search specific database of interest or for mass shifts of interest, neutral loss ions, and diagnostic fragment ions. Prior to searching, the tandem mass spectra are processed such that 12 ions per 100 m/z are retained. To correct for drift in the calibration during data acquisition, a recalibration option can be selected in which the first database search is performed at 20 ppm precursor mass tolerance. Following recalibration of the m/z ratios, the search is repeated with a more stringent mass tolerance of 4.5 ppm for precursor ions measured in the Orbitrap. The mass tolerance is 0.5 Da for fragment ions generated by CID and ETD and measured in the ion trap. Cysteine carbamidomethylation is selected as a fixed modification if peptides were reduced and alkylated with iodoacetamide. Methionine oxidation and phosphorylation on serine, threonine, and tyrosine are included as variable modifications. Trypsin cleaves on the C-terminal side of lysine and arginine and we specify that trypsin also cleaves between lysine and proline. Only fully tryptic peptides are reported with a maximum of two missed cleavages.
The tandem mass spectra are searched against a concatenated forward and reversed database supplemented with protein sequences of common contaminants, such as porcine trypsin and human keratin.
To establish the false discovery rate for peptide identification and phosphorylation site assignment, a “Revert” decoy database mode is selected [9] and the results are filtered by a 1% false discovery rate (FDR) at the peptide spectral match (PSM) and site levels.
Minimum Andromeda and delta scores of 40 and 6, respectively, are the default parameters required for identification of modified peptides. These values are adjusted if necessary after manual inspection of the spectral matches. To search for missing SILAC partners after the initial database search the “Requantify” option is employed. If biological or technical replicates are performed under the same chromatographic conditions, the “match between runs” feature can be enabled to increase the number of quantified pairs that are identified. This feature matches identifications in adjacent fractions and among biological replicate experiments with measured ratios of SILAC pairs that may have not been sequenced.
In MaxQuant, the relative quantitation is achieved by comparison of the integrated isotopic envelope (summed centroids) from each member of the SILAC pair.
MaxQuant 1.5 generates a comprehensive set of tab-delimited text files. The “Evidence” file includes the modified tryptic peptides observed, the m/z of the precursor ion selected for sequencing, the recalibrated m/z of the precursor, charge state, and number of missed cleavages. The PhosphoSTY file features the “sequence window” for each site of phosphorylation identified with the site of phosphorylation in the center and 15 amino acids on each side of the phosphosite, facilitating kinase substrate motif analysis. The PhosphoSTY file also contains the residue number of the site of modification within the leading protein sequence. The “Tables” pdf file contains a description of each text file generated by MaxQuant and the data that are reported in each text file.
To process the data, the text files can be uploaded into the Perseus platform, which contains visualization tools for quality control, statistical tests, easily accessible databases of site-specific modifications, and functional and structural annotation associated with specific sites of modification [58]. The normalized SILAC ratios (_1,_2,_3) can be added from the PhosphoSTY file to the expression column, potential contaminants to the categorical column, and all additional information into the numerical or text columns. Normalization of the ratios is achieved by subtracting the median of all ratios per column of ratios [56]. Up to date databases of known sites of post-translational modifications, kinase-substrate interactions, regulatory sites, and disease associated sites can be downloaded into the configuration folder of Perseus from Phosphosite Plus (PSP) Resource [50] for annotation.
Reproducibly regulated phosphosites can be determined using the moderated t-statistic function within the LIMMA package of Bioconductor R [59]. Ratios from the biological replicate experiments are evaluated using a moderated t-statistic with a Benjamini-Hochberg correction for multiple hypothesis testing [60]. After statistical analysis, text files can be reimported into Perseus for further processing and analyses for GO enrichment, and other downstream algorithms to interrogate phosphosite consensus motifs, predicted kinase activation, and pathway/network analyses.
3. Results
3.1. Defining the PTH Regulated Phosphoproteome in Osteoblastic Cells Expressing Endogenous Levels of PTH1R
The original estimates of the relative abundance of S/T/Y phosphorylation based on autoradiographic measurements made in 1980 (Ser 90%, Thr 10%, and Tyr 0.05%) [61] have been recapitulated in global proteomic analyses of cells at basal conditions (Ser 84.5%, Thr 15.1%, and Tyr 0.04%) [9]. Quantitatively, the stoichiometry of S/T/Y phosphorylation was found to be low in unstimulated cells. However, during highly coordinated cellular events such as mitosis, occupancy of the majority of S/T phosphosites rises from <20% to >60% while Y phosphorylation remains low [9]. Tyrosine phosphorylation occurs much less frequently and is maintained at lower stoichiometric levels rising from 10% to 50% following receptor tyrosine kinase stimulation [9]. The specificity of ligand-initiated events is achieved, in part, through the temporal regulation of phosphorylation at a subset of protein S/T/Y residues.
3.1.1. Detection and Identification of Osteoblast Phosphoproteins
The experimental workflow employed to identify phosphoproteins and phosphorylation sites regulated by acute stimulation with the conventional PTH1R agonist, hPTH(1-34), is summarized in Figure 1A. SILAC-labeled MC3T3-E1 cells differentiated in osteogenic media for 10 days were incubated in 1% FCS containing media for 18 hours prior to stimulation with hPTH[1-34] for varying times. Proteolytically digested peptides were separated into twelve fractions by SCX chromatography and phosphopeptides were enriched from each fraction using TiO2. The resulting peptides were analyzed by LC-MS/MS with an Orbitrap Elite mass spectrometer.
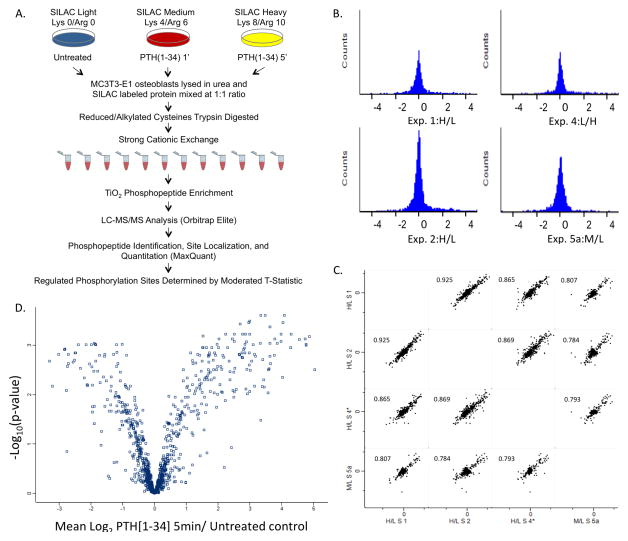
Figure 1.
Capturing the PTH1R regulated phosphoproteome. A) Experimental workflow for measuring differential changes in PTH(1-34)-induced phosphorylation events following acute stimulation of the receptor. SILAC labeled cells were differentiated for 10 days in light, medium, or heavy SILAC osteogenic media, serum starved in 1% FCS overnight and stimulated with hPTH[1-34] for varying times. Cells were lysed and the protein was mixed 1:1. The mixture was trypsin digested and the resulting peptides fractionated by strong-cation exchange (SCX). Phosphopeptides were enriched from each fraction with TiO2 and analyzed by LC-MS/MS (Orbitrap Elite, Thermo Scientific). Peptide identification and relative changes in the extent of phosphorylation were determined using MaxQuant. Statistical analysis to determine significantly regulated sites was performed using a moderated t-test. B) The distribution of normalized, log2 transformed ratios of phosphopeptides enriched from hPTH(1-34) MC3T3-E1 cells treated for 5 minutes compared to control cells. The histograms of SILAC encoded peptide ratios measured in four biological replicate experiments including a label swap control exhibited a normal distribution. C) Multi-scatter plots representing pairwise comparisons of the normalized log2 ratios measured in each experiment demonstrate high Pearson correlation coefficients (>0.75) indicating reproducibility of measurements between biological replicate experiments. D) Volcano plot showing the reproducibility of measured ratios for each phosphopeptide that was observed at least twice.
Tandem mass spectra were acquired on the ten most abundant ions in each survey mass spectral scan following CID or a CID-ETD decision tree approach. For peptide identification, tandem mass spectra from 9 SILAC experiments including technical replicates for 2 experiments (132 LC-MS/MS analyses) were searched simultaneously against a murine protein database (Uniprot; December 2013) with MaxQuant v1.5.1.0 on a 24 core, 48 GB RAM high performance server. A false discovery rate (FDR) threshold of 0.01 was used for peptide spectral matches, protein identifications, and site identifications. For modified peptides an Andromeda score greater than 40 and a minimum delta score of 6 was required. The text files generated in MaxQuant were processed using Perseus v1.5.0.8. Matches to the reversed database and common potential contaminants were removed. From the modification-specific peptides table, 6,621 phosphopeptides were identified (Supplemental Table 1). These phosphopeptides corresponded to 2,060 protein groups, 85 of which were derived from multiple gene products, 1561 corresponded to a unique protein group, and 414 were mapped to a unique protein. A single titanium dioxide enrichment step yielded 44% phosphopeptides. In subsequent studies we have been able to increase the enrichment to 95% using a double enrichment strategy. All of the protein groups identified (3,148) are shown in Supplemental Table 2A. We observed 146 protein kinases (GO:0004672) (Supplemental Table 2B) and 123 sequence-specific DNA binding transcription factors (GO:0003700) including Runx1 and 41% sequence coverage of the osteogenic transcription factor Runx2 (Supplemental Table 2C).
As very few phosphoproteomic studies have been carried out in osteoblasts, the phosphoSTY file was processed using the Perseus software to identify how many novel sites of phosphorylation were observed. A total of 7,237 sites of phosphorylation were identified, 4,635 were “Class I” sites with a localization probability ≥0.75 and a minimum score difference of 5 (see Supplemental Table 3 A–B). An example annotated tandem mass spectrum with the predicted fragment ions is provided demonstrating peptide identification and assignment of the site of phosphorylation (Supplemental Figure 1). Known phosphorylation sites, kinase-substrate relations, known regulatory and disease-related sites from databases including PhosphositePlus, Uniprot, and the Human Protein Reference database accessible via Perseus were used for protein, site, and modification-specific annotation. Five hundred and forty of the Class I sites identified have not been reported in Uniprot or PhosphositePlus (Supplemental Table 3C). Novel sites of phosphorylation were observed on many proteins that regulate osteoblast differentiation and function including transcriptional regulators such as Sp7 (osterix), Twist and Prrx-1,2.
3.1.2. Identification of Phosphopeptides Regulated after 5 Minute hPTH(1-34) Stimulation
The relative changes in the extent of phosphorylation were measured in MaxQuant and are based on the ratio of chromatographically resolved peak areas of the isotopic clusters for the SILAC phosphopeptide pairs. To demonstrate the information that can be obtained by this quantitative approach we are reporting the phosphorylation events that were regulated after 5 minutes of stimulation with PTH(1-34) in five biological replicate experiments including a label swap control. To determine the regulated sites of phosphorylation, the normalized ratios of singly, doubly, and multiply phosphorylated peptides associated with a particular site were transposed from a single row in the phosphoSTY table into columns yielding 3 potential ratio measurements per site. The ratios from the label swap experiment were inverted and all of the ratios were then log2 transformed. Data quality was assessed using the Perseus platform. To ensure the data in each biological replicate experiment were normally distributed and properly normalized, the log2 transformed peptide ratios were viewed as histograms (Figure 1B). To assess the reproducibility of measured ratios for each peptide between biological replicate experiments, the correlation between experiments was examined pairwise. Four out of five experiments comparing the relative changes in phosphorylation after 5 minutes of stimulation yielded a Pearson correlation coefficient greater than 0.75 and were utilized for subsequent analyses (Figure 1C). In the four replicates, a total of 4,720 SILAC-labeled, phosphopeptide pairs were quantified. Of these, 924, 522, and 260 phosphopeptide pairs were measured in 2, 3, or all 4 biological replicates, respectively. Since most of the phosphopeptides were only quantified in one experiment, we also extracted the monophosphorylated peptides observed once that exhibited >2 fold change (Supplemental Table 4). This list of 589 potentially regulated phosphorylation sites included decreased phosphorylation of MAPK1 Y185, which has been shown in previous studies by immunoblotting [62] and increased phosphorylation at PTH1R S491, a known PTH-regulated site [63–65].
A more rigorous method was applied to determine the sites of modification that were significantly regulated. The moderated t test was performed on the log2 transformed ratios using the LIMMA package of Bioconductor R and the p values were corrected for multiple hypothesis testing with the Benjamini Hochberg (BH) method [59,60,66]. Using BH corrected p value threshold of 0.1 and n≥2 observations, 376 phosphosites corresponding to 224 protein groups were regulated. Of the regulated peptides, 361 were localized Class I sites and 16 were novel (see Supplemental Table 5). 162 sites were decreasing in phosphorylation and 214 were increasing in phosphorylation. To view the reproducibility of each phosphopeptide ratio measured within these four experiments, the −log10 p value for each peptide was plotted against the log2 transformed ratios (Figure 1D).
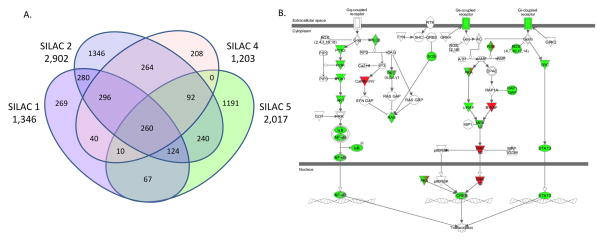
Because tissue-specific sites of phosphorylation are as-yet incompletely catalogued [21], there is no ‘gold standard’ database against which to objectively assess how comprehensively an experimental dataset covers the entire phosphoproteome. Moreover, the conditions used for phosphopeptide isolation/enrichment and analysis can lead to systematic underrepresentation of certain species, e.g. integral membrane proteins and phosphotyrosine containing peptides. The limited dynamic range and stochastic nature in sampling by the mass spectrometer also limits the depth of coverage. The Venn diagram in Figure 2A illustrates number/overlap of phosphorylation sites observed between the four biological replicates utilized for subsequent analyses. Rapid advances in experimental design, instrumentation, software and improvement at each step of the workflow, continue to reveal the breadth and complexity of the phosphoproteome. In our hands, subsequent studies employing HpH RPLC fractionation provided additional quantitative measurements and increased the number of observations between experiments. While these advances yield higher confidence in peptide identification and quantification, from a systems perspective, the bioinformatic analysis of signaling networks only requires that the input dataset be large/accurate enough to generate reliable predictions of pathway regulation. To crudely assess pathway coverage, we overlaid our list of observed/regulated phosphoproteins onto the Ingenuity® Systems canonical GCPR signaling pathway network. As shown in Figure 2B, our dataset provided very good coverage of signaling pathways classically associated with short term GPCR activation.
Figure 2.
Reproducibility and coverage of the MC3T3-E1 osteoblast phosphoproteome. A) Four-way Venn diagram depicting the number of SILAC phosphopeptide pairs identified in each of the four biological replicate experiments. Of 4,720 unique phosphopeptide pairs, 260 were observed in all four experiments, 522 in three of four experiments, 924 in at least two experiments, and 3,014 in a single experiment. Regulated phosphopeptides, defined as those with BH corrected p value <0.1 and n≥2 observations, were used in subsequent analyses. B) Observed (green) and regulated (red) phosphoproteins were overlaid on the IPA canonical GPCR signaling pathway network. Symbols with both colors, e.g. PKA and PDE, represent multi-subunit proteins in which at least one subunit contained a regulated site of phosphorylation.
3.2. Exploring the PTH Regulated Kinome
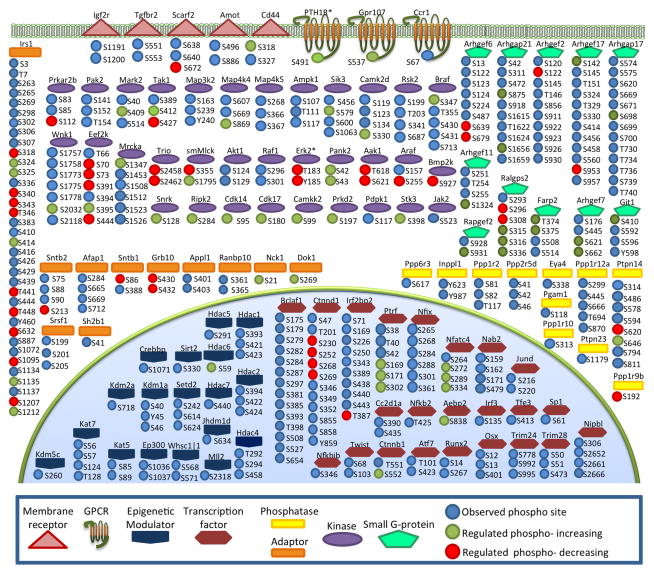
Protein kinases are one of the largest families of genes in eukaryotes, with genetic studies estimating that these enzymes account for 2% of the total genome. In humans, 518 genes code for kinases [67] of which 510 orthologues exist in mice [68]. Based on the prevalence of the most frequently phosphorylated residues serine, threonine and tyrosine, an estimated 700,000 possible phosphorylation sites are available in the cellular environment [69,70]. Figure 3 depicts a selected subset of phosphoproteins organized by molecular function and cellular localization to provide a sense of the distribution of basal and regulated phosphorylation events observed across the cellular landscape in our dataset.
Figure 3.
Selected phosphoproteins observed in hPTH(1-34) stimulated osteoblasts. The full list of identified phosphoproteins (see Supplemental Table 3) was sorted by molecular function. Selected proteins classified as kinases, phosphatases, transcription factors, epigenetic modulators, GPCRs, membrane receptors, regulators of small GTPases, and proteins with the GObp annotation “bone formation”, are shown grouped according to GO cellular compartment. All proteins shown were observed at least twice, with the exception of ERK1/2 and PTH1R, which were only observed in single biological experiments.
As many protein kinases are themselves regulated by phosphorylation, quantitative phosphoproteomic datasets can provide empiric information about the phosphorylation state of functionally annotated regulatory sites on specific kinases, thus allowing inferences about their activation/inhibition. The quality of such direct observational data is dependent on multiple factors, including the extent of coverage in the input dataset, i.e. whether relevant phosphorylation sites are “seen” in the data; and the arbitrary threshold(s) assigned to define significant change, i.e. p value and number of observations. A complementary approach, kinase substrate motif enrichment analysis using Motif-X [51], which searches all observed phosphorylation sites to identify the most abundant consensus phosphorylation motifs, can implicate kinases that may not have been directly observed and begin to link specific kinases to their downstream substrates. Due to the exponential growth of protein and post-translational modification databases, we are now able to add contextual information to substrate predictions. NetworKIN is a kinase prediction tool that incorporates consensus motifs with a probabilistic protein interaction network (STRING) for a context-specific analysis of kinase/substrate interactions [55,71,72]. Based on the idea that signaling complexes are modular in nature, the linear motif information is coupled to a corresponding signaling module for increased prediction accuracy. This allows for integration of more of the nuanced aspects of kinase substrate specificity.
3.2.1. Observed and Regulated Kinases in PTH(1-34) Stimulated MC3T3-E1 Osteoblasts
To identify kinases regulated by acute hPTH(1-34) stimulation, a list of phosphorylated protein kinases was extracted using GO annotations. Figure 4A depicts the kinases observed in 10-day cultures of differentiating MC3T3-E1 osteoblasts in a circular phylogenetic tree, aligned based on the homology of the kinase domains [73,74]. The identified kinases span all of the major kinase families. The number, location, and direction of significantly regulated sites of phosphorylation are displayed in the concentric bar graph (p value <0.1; n≥2 observations; with the exception of ERK2, >2 fold decrease, n=1). Based on the observed changes in site-specific phosphorylation of kinases, the predicted activity of BRAF, ERK2, and EEF2K would be attenuated, whereas the predicted activity of ARAF and PAK2 would be enhanced following 5 minute PTH1R stimulation. Increased phosphorylation of BRAF at S347 (homologous to human S365) is associated with inhibited kinase activity [75–77]. Consistent with reduced activity of BRAF, which lies upstream of ERK2, we observed decreased phosphorylation of the ERK2 peptide containing residues T183 and Y185. Increased phosphorylation of ARAF at S255 (homologous to human S257) has been shown to enhance its kinase activity [78]. Increased phosphorylation of p21 protein (Cdc42/Rac)-activated kinase 2 (PAK2) was observed at residues S141 and T154. Phosphorylation at these sites increases PAK2 kinase activity [79]. The only other functionally annotated, regulated phosphorylation site observed was on calcium/calmodulin-dependent eukaryotic elongation factor 2 kinase (EEF2K) at S444. Phosphorylation of the homologous site in human EEF2K decreases the half-life of the kinase [80].
Figure 4.
Substrate motif analysis of PTH(1-34) regulated protein kinases. A) All protein kinases identified in differentiated MC3T3-E1 cells are displayed according to phylogenetic similarity of the kinase domain. Domain sequences were retrieved from KinBase, aligned in Clustal Ω, and displayed using iTOL. Phosphorylation sites regulated following 5 min stimulation with hPTH(1-34) are indicated by the concentric bar graph. Directionality and number of sites regulated are denoted by the size and color of the bar, with green indicating increased phosphorylation and red indicating decreased phosphorylation. B) The sequence windows of regulated mono-phosphorylated peptides were analyzed using the Motif-X algorithm. The dominant motifs for sites with increased phosphorylation included sites of basophilic kinases (RRxS, RKxS, RxS, RxxS) such as PKA. The dominant motifs for sites decreasing in phosphorylation were proline-directed sites (PxSP) and basic sites (RxxS). C) Western blots of total cell protein isolated from MC3T3-E1 cells treated for 5 min with 1 μM hPTH(1-34) or vehicle control (CNTL) probed with phosphorylation substrate motif antisera recognizing the PKA consensus motif RxxpS/T (upper panel) or the MAPK/CDK consensus motif PxpSP (lower panel).
3.2.2. Linking Kinases to Substrates by Substrate Motif Analysis
The activation state of upstream kinases should be reflected in the phosphorylation of downstream targets bearing their consensus substrate motifs. To confirm predicted regulation of kinase activity based on the observed changes in their phosphorylation state and identify likely downstream targets, regulated monophosphorylated peptides with high confidence site-assignments (localization probability >0.75) were evaluated for kinase substrate motif enrichment using Motif-X [49]. Motif-X enables matching of input peptide sequences to non-redundant output consensus motifs and provides an output file indicating which peptide substrates matched to a particular sequence motif. 298 peptides were monophosphorylated with 118 sites decreasing and 180 sites increasing in phosphorylation. The pre-aligned sequence windows obtained from the phospho-S/T/Y file generated by MaxQuant were decreased to a width of 15 amino acids and submitted to Motif-X to test for enrichment against a background of the mouse proteome. A minimum of 20 occurrences and p value threshold of 0.000001 were required. An insufficient number of regulated phosphoTyr and phosphoThr residues limited the analysis to peptides containing regulated phosphoSer. As shown in Figure 4B, Motif-X analysis revealed increased phosphorylation of basophilic kinases which would include PKA/CAMK2, and decreased phosphorylation by proline directed kinases such as ERK/MAPK/CDK. To confirm enrichment of these regulated linear phosphorylated consensus motifs using an alternative strategy, protein isolated from day 10 of osteoblast differentiation exposed to vehicle or hPTH(1-34) for 5 minutes were immunoblotted using antisera (Cell Signaling Technologies) recognizing the phosphorylated consensus motifs for PKA (RxxpS/T) or MAPK/CDK (PxpSP). As shown in Figure 4C, robust increases in PKA substrate motifs and decreases in MAPK/CDK consensus motifs occurred with 5 min stimulation. Since activation of Gs-cAMP signaling is an early consequence of PTH1R activation [18] and phosphorylation of 3 isoforms of the PKA regulatory subunit, Prkar1a, Prkar2a, and Prkar2b were observed in the phosphoprotein dataset (Supplemental Table 4), the finding of increased phosphorylation among PKA consensus phosphorylation sites was predictable. Similarly, the observed increase in BRAF S327 phosphorylation (Figure 4A), corresponding to a predicted decrease in ERK activity, was substantiated by the global decline in phosphorylation of MAPK substrate consensus sites.
3.2.3. Prediction of Activated Kinases
To link kinases to their predicted substrates, we utilized KinomeExplorer 3.0 with the NetworKIN and NetPhorest algorithms. For this application we expanded the list of PTH(1-34)-regulated, phosphosites by including those sites observed once with >2 fold change in order to increase the number of sites for prediction of kinase activation and kinase substrate interactions. To facilitate uploading data into NetworKIN, which encompasses a database of 200 human kinases and their substrate interactions, sequence windows of twelve amino acids were converted from mouse to human using PhosphositePlus. Approximately 65% of the murine sequences had identical human homologs. The sequences were submitted to Motif-X as described above.
The sets of sequence windows assigned to each non-redundant motif were individually analyzed by NetworKIN and matched to kinases in the embedded database. The output includes a calculated NetworKIN score, which is a combination of the STRING network proximity score and the Netphorest probability score [55]. When the score for an “id” kinase approaches zero so does the chances of it being the perpetrator of the phosphorylation. The NetworKIN output was filtered by a minimum score of 0.22, a max score difference of 4, and a maximum of ten kinase predictions per site. Because of the redundancy and overlap of substrates with functionally-related kinases, it was not considered appropriate to exclusively analyze the top scoring kinase predicted for each site. For example, phosphorylation of a member of the RRxS motif bearing set, Anaphase Promoting Complex Subunit 1 (ANAPC1) at serine 355 was predicted to be extremely likely by both Cdk1 and Cdk2, with assigned scores of 8.5 and 9.6 respectively. The predicted kinases were then filtered to retain only the kinases observed in our mass spectrometric analyses of MC3T3-E1 osteoblasts. Due to the reported ambiguities associated with phosphosites within the protein ANHAK these sites were removed [50]. To calculate the frequency of each predicted kinase for the motif heatmap, the total number of occurrences of each kinase in the predictions for each substrate motif were tallied then divided by the number of individual substrate motifs (i.e. unique phosphorylation sites) for a percent occurrence frequency. Occurrence frequencies of less than 20% were discarded. The heat map generated with NetworKIN output [72] illustrates the relationship between observed consensus site motifs in individual protein substrates and the kinases that phosphorylate them (Figure 5A). While this analysis is limited by the redundancy in consensus phosphorylation site motifs amongst different kinases, the data output clearly shows a high degree of regulation at sites predicted to be substrates of PKA, PKC, PAK, CDK, and MAPK isoforms.
Figure 5.
NetworKIN analysis of phosphorylated substrate peptides changing with treatment predicts kinases involved with GPCR signaling and cytoskeletal regulation. A) Kinase activation analysis performed on regulated (BH adjusted p-value of <0.1, moderated t-statistic) phosphopeptides separated into increasing and decreasing sets and matched to kinases in NetworKIN. The resultant rank-ordered predictions for each kinase were filtered to include only kinases observed in MC3T3-E1 cells and then evaluated for changes in activation status by a one-sided Kolmogorov-Smirnov test with a 0.05 FDR filter. B) Linear phosphorylation motif analysis of the MC3T3-E1 phosphoproteome. To gain more site-specific information, significantly overrepresented phosphorylation motifs were determined by Motif-X and matched to kinases with NetworKIN. NetworKIN output was filtered by score minimum of 0.22, maximum score difference of 4, and maximum of ten predictions per site. For the purposes of this analysis, lysine and arginine were considered degenerate. Predictions were ranked by frequency of presence in total number of sites bearing the indicated motif analyzed by NetworKIN. Due to NetworKIN’s human protein-limited analysis abilities, the peptides were converted from mouse to human site numbers in PhosphositePlus. This conversion process provided varying levels of efficiency (ranging from 50–100%) per motif set and introduced moderate bias in the analysis. For example, ‘SP down’ motif sites were converted at 50% efficiency (n= 67/134), yet RxxS down motif sites were converted at 100% efficiency (42/42). This biases the output as both motifs were also present in the increasing sets.
To probe the data using a more general substrate-driven approach, the entire set of regulated phosphopeptides was divided into increasing or decreasing phosphorylation status and each full set analyzed by Networkin. The resultant scores for each set were compared to unregulated phosphopeptide scores using the Kolmogorov-Smirnov test. The Kolmogorov-Smirnoff Test (KS-test) is a non-parametric and distribution free statistical test for determining if samples arose from the same population (i.e. treated and non-treated) and is often used with data that contain rank-based scores such as NetworKIN produces (http://www.mayo.edu/mayo-edu-docs/center-for-translational-science-activities-documents/berd-5-6.pdf). Using the NetworKIN analysis of the unregulated sites of phosphorylation as background, the scores for each predicted kinase in the increasing or decreasing regulated set were compared to the reference set using the one-sided KS-test [14,15,38]. A 0.05 p-value threshold was chosen for determining changes in kinase activity. As shown in Figure 5B, the results corroborate our previous findings such as changes in activity of PKA and PAK isoforms, while indicating additional kinases likely involved such as RSK2, and MRCKa/b, which are annotated for regulation of ERK signaling and cytoskeletal rearrangement, respectively.
3.3. PTH regulated signaling networks
An advantage of “unbiased” high dimensionality datasets is that they harbor encrypted information about the higher order pathways and biological processes that are affected by experimental perturbation of a complex system, e.g. living cells. The challenge is to produce a rational and biologically relevant condensation of complex data into outputs that predict the functional activities of the genes/proteins modulated between the control and test datasets. Geneset enrichment analysis (GSEA) is a statistical approach that compares an experimentally determined list of regulated factors, e.g. phosphoproteins or mRNA transcripts, to a curated database of genes/proteins involved in specific signaling pathways/biological processes, to determine the probability of pathway regulation based on the overrepresentation of observed factors in the database pathway/process cluster. There are multiple freely available pathway databases and facile calculation programs to facilitate these analyses [81–83]. Because co-regulated geneset databases are themselves based on empiric data and not fully, or even correctly, annotated, such analyses should be considered exploratory in nature. The interpretation of acutely regulated phosphosites adds another layer of complexity as phosphorylation may alter protein function in multiple ways including altering activity, half-life, localization, and interactions. In addition, many proteins are targeted by multiple kinases and have sites that are increasing or decreasing in phosphorylation at specific residues. Regulated phosphoproteins can be considered nodes in signaling networks that are engaged by hormone/drug stimulation. Thus, it is preferable to employ multiple tools and interrogation strategies to explore the experimental dataset [81], and to undertake independent validation experiments before conclusions are drawn.
3.3.1. Analysis of Signaling Pathways
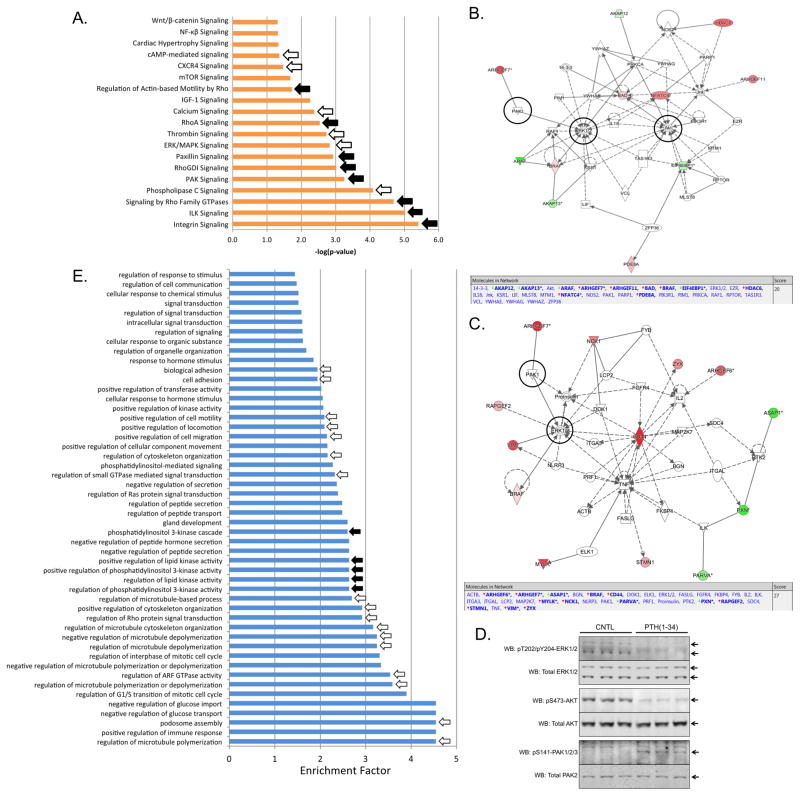
Figure 6A depicts the output of a parametric GSEA performed using the list of regulated class I phosphorylation sites (Supplemental Table 5) detected in PTH(1-34)-stimulated MC3T3-E1 osteoblasts using the Ingenuity® Systems Pathways Analysis (IPA) tool. As expected, many of the top-scoring pathways; e.g. cAMP-mediated signaling, calcium signaling, phospholipase C signaling (white arrows), correspond to the known heterotrimeric G protein coupling profile of PTH1R. Interestingly, seven of the top ten scoring pathways relate to regulation of small GTPases involved in cytoskeletal rearrangement, cell motility, and signaling via focal adhesion complexes; e.g. integrin signaling, integrin-linked kinase (ILK) signaling, paxillin/p21 protein (Cdc42/Rac)-activated kinase (PAK) signaling, and regulation of Rho GTPases (black arrows). IPA analysis also suggested robust crosstalk between PTH1R and insulin-like growth factor type 1 signaling pathways; e.g. IGF-1 signaling, mTor signaling, which may reflect the large number of regulated phosphorylation sites seen on insulin receptor substrate 1 (IRS-1; Figure 2).
Figure 6.
Informatic analysis of PTH(1-34) regulated signaling pathways and biological processes. A) Ingenuity® Pathways Analysis (www.ingenuity.com/) performed using the list regulated phosphorylation sites detected in PTH(1-34)-stimulated MC3T3-E1 osteoblasts. Significantly regulated pathways were defined as; ≥2 genes per group, p<0.05 enrichment compared to a standard murine background database. Pathways related to canonical GPCR signaling (white arrows) and small GTPase regulation (black arrows) are indicated. B) The top-scoring IPA network diagram generated by combining the list of observed regulated phosphorylation events associated with classical GPCR signaling pathways. The network components, along with the identity and direction of regulation of observed factors (red arrows, increasing; green arrows, decreasing) are shown at the top of each panel. C) The top-scoring IPA network diagram generated by combining the list of observed regulated phosphorylation events associated with small GTPase signaling pathways. The network components, along with the identity and direction of regulation of observed factors are shown at the top of each panel. In panels B and C, black circles denote the position of AKT, ERK, and PAK within the predicted networks. D) Total cell protein isolated from differentiated MC3T3-E1 treated for 5 min with 0.1 μM PTH(1-34) or vehicle control (CNTL) was probed using antisera specific for activated (pT202/pY204) or total ERK1/2; active (pS473) or total AKT; and active (pS141) or total PAK2. Representative immunoblots of triplicate samples are shown. E) Significantly populated GObp terms determined in Perseus. The Top 50 enriched GObp terms are shown. GObp terms related to regulation of cytoskeletal dynamics (white arrows) and regulation of PI3K/AKT and lipid kinase signaling (black arrows) are indicated.
Figures 6B and 6C depict the top-scoring IPA network diagrams generated by combining the list of observed regulated phosphorylation events associated with classical GPCR signaling pathways (Figure 6A; white arrows) or small GTPase signaling pathways (Figure 6A; black arrows), respectively. The components of the IPA predicted network, along with the identity and direction of regulation of observed factors, are shown below each network. Since observational proteomic datasets are inevitably incomplete, either due to incomplete coverage of the regulated phosphoproteome or the simple fact that not all pathway components are regulated by phosphorylation, network diagrams such as these often predict the involvement of unobserved pathway intermediates and provide testable hypotheses for experimental validation. In this analysis, ERK1/2 and AKT were predicted to act as nodal kinases in the downstream PTH1R signaling network, while PAK1/2 was predicted to be involved in cross regulation of Rac1 and ERK1/2 signaling via the PAK-interacting Cdc42/Rac guanine nucleotide exchange factor ARHGEF7. ARHGEF7, also called β-Pix/COOL-1, is regulated via PKA-dependent phosphorylation [84,85]. Notably, β-PIX forms a dimeric complex with GIT1, an ADP ribosylation fator (ARF)-GTPase activating protein (GAP) that transiently localizes PAKs to remodeling focal adhesions through binding to paxillin [86], and increased GIT1 S410 phosphorylation was experimentally observed (Figure 3). Although regulation of ERK1/2-pT183/pY185 and PAK2-pS141 were observed in one or two biological replicates, and regulated changes in AKT phosphorylation were not directly observed by mass spectrometry, targeted immunoblotting based on the IPA network output confirmed strong negative regulation of ERK1/2 and AKT phosphorylation and increased PAK2 phosphorylation (Figure 6D).
3.3.2. From Pathways to Biological Processes
Using Perseus, we next examined the GO biological process terms associated with the regulated phosphorylation sites (Supplemental Table 5) using a Fisher exact test, BH FDR of 0.02, and unregulated phosphosites as background. As with the IPA pathways analysis output, which heavily populated pathways related to regulation of small GTPases, the most strongly populated GObp terms related to cytoskeletal rearrangement and cell motility; e.g. microtubule polymerization/depolymerization, cell motility, Rho signal transduction, and ARF GTPase activity (Figure 6E; white arrows). Notably, hPTH(1-34) stimulates migration of primary pre-osteoblasts in vitro [87], and regulation of Rho, Rac1 and Cdc42 signaling in osteoblasts is known to affect osteoblast mechanotransduction, survival, and motility [88-90]. Other commonly represented GObp terms related to regulation of phosphatidylinositol 3′-kinase activity (PI3K) (black arrows), reflecting the observed regulation of Rac1-PAK signaling and regulation of AKT demonstrated in the IPA analysis. Thus, our data suggest that downstream of the initial cAMP-PKA regulated signaling events, the short-term responses of differentiating osteoblastic cells to PTH1R activation revolve around regulation of Rac/Rho/Cdc42 control of cytoskeletal dynamics involved in cell-matrix signaling, and cell survival pathways regulated by PI3K-AKT signaling.
4. Discussion
The consequences of GPCR activation are encoded by receptor coupling to a relatively small number of proximal effectors, e.g. heterotrimeric G proteins, arrestins, and other non-G protein effectors. Although the initial number of signaling conduits is limited, tremendous complexity in both the short and long term biological response arises from the interplay of signal strength/duration and the cellular context in which the receptor is expressed. Capturing this level of complexity in a native cell context requires the ability to generate high-dimensionality data that reflect, as much as possible, all of the downstream changes resulting from receptor activation. Here, we employed SILAC-based quantitative mass spectrometry combined with bioinformatic pathways analysis to examine global changes in protein phosphorylation following short-term stimulation of endogenously expressed PTH1R with the conventional agonist PTH(1-34). Our results reveal both comforting familiarities and some surprising insights. Given its known activation of Gs-cAMP and Gq/11-calcium signaling pathways, it is not surprising that global phosphorylation of consensus PKA/CAMK sites rose dramatically in the first 5 min of stimulation. Likewise, cAMP signaling is known to affect ERK1/2 activity, producing either activation or inhibition depending on cellular context [91]. In 13-day old differentiating MC3T3-E1 cells, PTH(1-34) stimulation increased phosphorylation of an inhibitory site on BRAF, leading to decreased ERK1/2 activity, and a global fall in the phosphorylation of consensus MAPK sites. Somewhat less expected were the central roles of ERK1/2 and AKT detected in the IPA pathways analysis, and the heavy bias toward regulation of small GTPase signaling. Early in the response, ERK1/2 and AKT signaling were depressed, while signaling via ARHGEF6 (α-PIX) and ARHGEF7(β-PIX)-PAK2 was activated, suggesting that regulation of Rac1/Cdc42 signaling, dynamic cytoskeletal rearrangement, and crosstalk with focal adhesion/paxillin-based signaling are important contributors to PTH1R signaling in osteoblasts.
Although metabolic labeling with SILAC is considered the gold standard for quantitative proteomic assessment of post-translational modifications [7, 20], the technique is not without limitations. Caveats of the approach include: 1) redundant sequencing of SILAC-labeled peptide pairs which reduces the depth of the analysis; 2) limitations in multiplexing; and, 3) incompatibility with protein prepared directly from tissue. Recent studies have demonstrated the ability of alternative technologies, such as label free quantification, to probe deeper into the phosphoproteome [9] and to assay dynamic phosphorylation profiles following GPCR-stimulation in vitro [38] and in vivo [19]. Advances in instrumentation are also permitting the evaluation of multiplexed approaches such as 18-plex NeuSILAC [92] and 10-plex TMT isobaric labeling with phosphorylation-directed data acquisition [93]. Beyond technological limitations, phosphoproteomic analysis of signal transduction on the time scale of a few minutes may not capture the information needed to link activation of signaling pathways with longer-term transcriptional regulatory events that determine the phenotypic response of a tissue to ligand stimulation. To fully appreciate the complexity, it would be necessary to relate high-dimensionality mass spectrometric datasets to longer term in vitro or in vivo transcriptomic and conventional phenotypic data [87,94].
Using informatic approaches to analyze complex datasets likewise calls for an appreciation of the limitations and exercise of judgement. High-dimensionality, e.g. proteomic and transcriptomic, datasets are prone to both false discovery and missing data, depending on the filters applied to determine significance. If very stringent criteria are applied, then the resulting data will be highly reliable, i.e. contain a low percentage of false positives, but may yield datasets too small for reliable pathways analysis due to a high false negative rate if the entire genome is used as the background for enrichment. While the output of pathways analysis is dependent on the quality of the input data, GSEA approaches are able to tolerate a relatively high degree of “noise”, as they rely on calculating the statistical probability that the curated pathway geneset was not populated by random chance [81,83]. Datasets that are too small, or datasets that are too large, i.e. contain a high percentage of false positives, will both decrease the sensitivity of the analysis. As a result, it is often most rewarding to perform pathways analysis using factor lists extracted at different levels of significance, to determine by trial-and-error which parameters yield the most informative results from a given dataset. Because of their exploratory nature, it is likewise preferable to employ multiple tools to explore the experimental dataset [83], and to undertake independent validation experiments before drawing conclusions. Despite these limitations, our results illustrate how technological advances in mass spectrometry-based proteomics have enabled more in-depth study of endogenous GPCR signaling in a native cell background. Given its ability to capture high-dimensionality data without pre-conceived hypotheses about function, these approaches should be of particular value in settings where the signaling outcomes are less predictable, e.g. characterization of non-canonical GPCR signaling pathways activated by arrestin pathway-selective “biased” agonists.
Supplementary Material
Representative annotated tandem mass spectrum.
All modification-specific peptides identified by LC-MS/MS in MC3T3-E1 osteoblasts.
All proteins observed (2A); All protein kinases observed (2B); All DNA binding transcription factors observed (2C), in MC3T3-E1 osteoblasts.
All phosphoSTY sites observed (3A–B); Novel phosphoSTY sites observed (3C), in MC3T3-E1 osteoblasts.
Monophosphorylated STY sites observed in a single biological replicate with ≥2-fold change
Significantly regulated monophosphorylated STY sites observed in ≥2 biological replicates.
Highlights.
Dynamic phosphorylation events in osteoblasts stimulated with hPTH(1-34) were measured.
Acute stimulation of the PTH1R engaged canonical GPCR signaling pathways.
Novel signaling intermediates stimulated by PTH[1-34] were revealed.
Quantitative LC-MS/MS methods were established to assess PTH1R signaling networks.
Acknowledgments
The authors thank Susana Comte-Walters, Michael Schilling and Benjamin Neely for assistance. Mass spectrometry was performed in the MUSC Mass Spectrometry Facility, a University Research Resource Facility funded through the Office of the Provost and the SC COBRE in Oxidants, Redox Balance and Signaling (P20 GM103542). This work was supported by grants R01 DE020925 (LEB), S10 D010731(LEB), R01 DK055524 (LML) and R01 GM095497 (LML).
Abbreviations
- CID
collision induced dissociation
- ETD
electron transfer dissociation
- FTMS
Fourier transform mass spectrometry
- GO
Gene Ontology
- GPCR
G protein-coupled receptor
- GSEA
Geneset Enrichment Analysis
- HpH RPLC
high pH reversed phase liquid chromatography
- IPA
Ingenuity® Systems Pathways Analysis
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- MS
mass spectrometry
- OGE
off gel electrophoresis
- PTH
parathyroid hormone
- PTH1R
type 1 parathyroid hormone receptor
- RANKL
Receptor activator of nuclear factor kappa-B ligand
- SCX
strong cation exchange chromatography
- SILAC
stable isotopic labeling by amino acids in cell culture
- S/T/Y
serine, threonine, tyrosine
- TiO2
titanium dioxide
- TMT
tandem mass tags
- Runx
Runt-related transcription factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Galandrin S, Bouvier M. Distinct signaling profiles of beta1 and beta2 adrenergic receptor ligands toward adenylyl cyclase and mitogen-activated protein kinase reveals the pluridimensionality of efficacy. Mol Pharmacol. 2006;70:1575–1584. doi: 10.1124/mol.106.026716. [DOI] [PubMed] [Google Scholar]
- 2.Luttrell LM, Gesty-Palmer D. Beyond desensitization: Physiological relevance of arrestin-dependent signaling. Pharmacol Rev. 2010;62:305–330. doi: 10.1124/pr.109.002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kenakin T, Miller LE. Seven transmembrane receptors as shapeshifting proteins: the impact of allosteric modulation and functional selectivity on new drug discovery. Pharmacol Rev. 2010;62:265–304. doi: 10.1124/pr.108.000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christopoulos A, Kenakin T. G protein-coupled receptor allosterism and complexing. Pharmacol Rev. 2002;54:323–374. doi: 10.1124/pr.54.2.323. [DOI] [PubMed] [Google Scholar]
- 5.Kenakin TP. Functional selectivity through protean and biased agonism: who steers the ship? Mol Pharmacol. 2007;72:1393–1401. doi: 10.1124/mol.107.040352. [DOI] [PubMed] [Google Scholar]
- 6.Luttrell LM. Minireview: More than just a hammer: ligand “bias” and pharmaceutical discovery. Mol Endocrinol. 2014;28:281–294. doi: 10.1210/me.2013-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, Mann M. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell. 2006;127:635–648. doi: 10.1016/j.cell.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 8.Roux PP, Thibault P. The coming of age of phosphoproteomics--from large data sets to inference of protein functions. Mol Cell Proteomics. 2013;12:3453–3464. doi: 10.1074/mcp.R113.032862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma K, D’Souza RC, Tyanova S, Schaab C, Wisniewski JR, Cox J, Mann M. Ultradeep human phosphoproteome reveals a distinct regulatory nature of Tyr and Ser/Thr-based signaling. Cell Rep. 2014;8:1583–1594. doi: 10.1016/j.celrep.2014.07.036. [DOI] [PubMed] [Google Scholar]
- 10.Xiao K, Sun J, Kim J, Rajagopal S, Zhai B, Villen J, Haas W, Kovacs JJ, Shukla AK, Hara MR, Hernandez M, Lachmann A, Zhao S, Lin Y, Cheng Y, Mizuno K, Ma’ayan A, Gygi SP, Lefkowitz RJ. Global phosphorylation analysis of beta-arrestin-mediated signaling downstream of a seven transmembrane receptor (7TMR) Proc Natl Acad, Sci U S A. 2010;107:15299–15304. doi: 10.1073/pnas.1008461107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christensen GL, Kelstrup CD, Lyngso C, Sarwar U, Bogebo R, Sheikh SP, Gammeltoft S, Olsen JV, Hansen JL. Mol Cell Proteomics. 2010;9:1540–1553. doi: 10.1074/mcp.M900550-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kendall RT, Strungs EG, Rachidi SM, Lee MH, El-Shewy HM, Luttrell DK, Janech MG, Luttrell LM. The beta-arrestin pathway-selective type 1A angiotensin receptor (AT1A) agonist [Sar1,Ile4,Ile8]-angiotensin II regulates a robust G protein-independent signaling network. J Biol Chem. 2011;286:19880–19891. doi: 10.1074/jbc.M111.233080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Douglass J, Gunaratne R, Bradford D, Saeed F, Hoffert JD, Steinbach PJ, Knepper MA, Pisitkun T. Identifying protein kinase target preferences using mass spectrometry. Am J Physiol Cell Physiol. 2012;303:C715–727. doi: 10.1152/ajpcell.00166.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bogebo R, Horn H, Olsen JV, Gammeltoft S, Jensen LJ, Hansen JL, Christensen GL. Predicting kinase activity in angiotensin receptor phosphoproteomes based on sequence-motifs and interactions. PloS One. 2014;9:e94672. doi: 10.1371/journal.pone.0094672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lundby A, Andersen MN, Steffensen AB, Horn H, Kelstrup CD, Francavilla C, Jensen LJ, Schmitt N, Thomsen MB, Olsen JV. In vivo phosphoproteomics analysis reveals the cardiac targets of β-adrenergic receptor signaling. Sci Signal. 2013;6:rs11. doi: 10.1126/scisignal.2003506. [DOI] [PubMed] [Google Scholar]
- 16.Gesty-Palmer D, Chen M, Reiter E, Ahn S, Nelson CD, Wang S, Eckhardt AE, Cowan CL, Spurney RF, Luttrell LM, Lefkowitz RJ. Distinct conformations of the parathyroid hormone receptor mediate G protein and beta-arrestin dependent activation of ERK1/2. J Biol Chem. 2006;281:10856–10864. doi: 10.1074/jbc.M513380200. [DOI] [PubMed] [Google Scholar]
- 17.Gesty-Palmer D, Flannery P, Yuan L, Spurney R, Lefkowitz RJ, Luttrell LM. A beta-arrestin biased agonist of the parathyroid hormone receptor (PTH1R) promotes bone formation independent of G protein activation. Science – Transl Med. 2009;1:1ra1. doi: 10.1126/scitranslmed.3000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Appleton KM, Lee MH, Alele C, Alele C, Luttrell DK, Peterson YK, Morinelli TA, Luttrell LM. Biasing the parathyroid hormone receptor: relating in vitro ligand efficacy to in vivo biological activity. Methods Enzymol. 2013;522:229–262. doi: 10.1016/B978-0-12-407865-9.00013-3. [DOI] [PubMed] [Google Scholar]
- 19.Olsen JV, Mann M. Status of large-scale analysis of post-translational modifications by mass spectrometry. Mol Cell Proteomics. 2013;12:3444–3452. doi: 10.1074/mcp.O113.034181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ong SE, Blagoev B, Kratchmarova I, Kristensen DB, Steen H, Pandey A, Mann M. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol Cell Proteomics Molecular & cellular proteomics. 2002;1:376–386. doi: 10.1074/mcp.m200025-mcp200. [DOI] [PubMed] [Google Scholar]
- 21.Huttlin EL, Jedrychowski MP, Elias JE, Goswami T, Rad R, Beausoleil SA, Villen J, Haas W, Sowa ME, Gygi SP. A tissue-specific atlas of mouse protein phosphorylation and expression. Cell. 2010;143:1174–1189. doi: 10.1016/j.cell.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang D, Christensen K, Chawla K, Xiao G, Krebsbach PH, Franceschi RT. Isolation and characterization of MC3T3-E1 preosteoblast subclones with distinct in vitro and in vivo differentiation/mineralization potential. J Bone Miner Res. 1999;14:893–903. doi: 10.1359/jbmr.1999.14.6.893. [DOI] [PubMed] [Google Scholar]
- 23.Franceschi RT, Iyer BS, Cui Y. Effects of ascorbic acid on collagen matrix formation and osteoblast differentiation in murine MC3T3-E1 cells. J Bone Miner Res. 1994;9:843–854. doi: 10.1002/jbmr.5650090610. [DOI] [PubMed] [Google Scholar]
- 24.Franceschi RT. The role of ascorbic acid in mesenchymal differentiation. Nutr Rev. 1992;50:65–70. doi: 10.1111/j.1753-4887.1992.tb01271.x. [DOI] [PubMed] [Google Scholar]
- 25.Franceschi RT, Iyer BS. Relationship between collagen synthesis and expression of the osteoblast phenotype in MC3T3-E1 cells. J Bone Miner Res. 1992;7:235–246. doi: 10.1002/jbmr.5650070216. [DOI] [PubMed] [Google Scholar]
- 26.Schiller PC, D’Ippolito G, Roos BA, Howard GA. Anabolic or catabolic responses of MC3T3-E1 osteoblastic cells to parathyroid hormone depend on time and duration of treatment. J Bone Miner Res. 1999;14:1504–1512. doi: 10.1359/jbmr.1999.14.9.1504. [DOI] [PubMed] [Google Scholar]
- 27.Spurney RF. Regulated expression of G protein-coupled receptor kinases (GRK’s) and beta-arrestins in osteoblasts. Calcif Tissue Int. 2003;73:153–160. doi: 10.1007/s00223-002-1018-5. [DOI] [PubMed] [Google Scholar]
- 28.Sudo H, Kodama HA, Amagai Y, Yamamoto S, Kasai S. In vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. J Biol Chem. 1983;96:191–198. doi: 10.1083/jcb.96.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lanucara F, Eyers CE. Mass spectrometric-based quantitative proteomics using SILAC. Meth Enzymol. 2011;500:133–150. doi: 10.1016/B978-0-12-385118-5.00008-6. [DOI] [PubMed] [Google Scholar]
- 30.Park SS, Wu WW, Zhou Y, Shen RF, Martin B, Maudsley S. Effective correction of experimental errors in quantitative proteomics using stable isotope labeling by amino acids in cell culture (SILAC) J Proteomics. 2012;75:3720–3732. doi: 10.1016/j.jprot.2012.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cañas B, Piñeiro C, Calvo E, López-Ferrer D, Gallardo JM. Trends in sample preparation for classical and second generation proteomics. J Chromatogr A. 2007;1153:235–258. doi: 10.1016/j.chroma.2007.01.045. [DOI] [PubMed] [Google Scholar]
- 32.Hu L, Ye M, Zou H. Recent advances in mass spectrometry-based peptidome analysis. Expert Rev Proteomics. 2009;6:433–447. doi: 10.1586/epr.09.55. [DOI] [PubMed] [Google Scholar]
- 33.Villén J, Gygi SP. The SCX/IMAC enrichment approach for global phosphorylation analysis by mass spectrometry. Nat Protoc 2008. 2008;3:1630–1638. doi: 10.1038/nprot.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gilar M, Jaworski A, Olivova P, Gebler JC. Peptide retention prediction applied to proteomic data analysis. Rapid Commun Mass Spectrom. 2007;21:2813–2821. doi: 10.1002/rcm.3150. [DOI] [PubMed] [Google Scholar]
- 35.Gilar M, Olivova P, Daly AE, Gebler JC. Orthogonality of separation in two-dimensional liquid chromatography. Anal Chem. 2005;77:6426–6434. doi: 10.1021/ac050923i. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Yang F, Gritsenko MA, Wang Y, Clauss T, Liu T, Shen Y, Monroe ME, Lopez-Ferrer D, Reno T, Moore RJ, Klemke RL, Camp DG, 2nd, Smith RD. Reversed-phase chromatography with multiple fraction concatenation strategy for proteome profiling of human MCF10A cells. Proteomics. 2011;11:2019–2026. doi: 10.1002/pmic.201000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang F, Shen Y, Camp DG, 2nd, Smith RD. High-pH reversed-phase chromatography with fraction concatenation for 2D proteomic analysis. Expert Rev Proteomics. 2012;9:129–134. doi: 10.1586/epr.12.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Udeshi ND, Mertins P, Svinkina T, Carr SA. Large-scale identification of ubiquitination sites by mass spectrometry. Nat Protoc. 2013;8:1950–1960. doi: 10.1038/nprot.2013.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Batth TS, Francavilla C, Olsen JV. Off-line high-pH reversed-phase fractionation for in-depth phosphoproteomics. J Proteome Res. 2014;13:6176–6186. doi: 10.1021/pr500893m. [DOI] [PubMed] [Google Scholar]
- 40.de Graaf EL, Giansanti P, Altelaar AF, Heck AJ. Single-step enrichment by Ti4+-IMAC and label-free quantitation enables in-depth monitoring of phosphorylation dynamics with high reproducibility and temporal resolution. Mol Cell Proteomics. 2014;13:2426–2434. doi: 10.1074/mcp.O113.036608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Erickson BK, Jedrychowski MP, McAlister GC, Everley RA, Kunz R, Gygi SP. Evaluating multiplexed quantitative phosphopeptide analysis on a hybrid quadrupole mass filter/linear ion trap/orbitrap mass spectrometer. Anal Chem. 2015;87:1241–1249. doi: 10.1021/ac503934f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang YI, Wolf-Yadlin A, Ross PL, Pappin DJ, Rush J, Lauffenburger DA, White FM. Time-resolved mass spectrometry of tyrosine phosphorylation sites in the epidermal growth factor receptor signaling network reveals dynamic modules. Mol Cell Proteomics. 2005;4(9):240–50. doi: 10.1074/mcp.M500089-MCP200. [DOI] [PubMed] [Google Scholar]
- 43.Larsen MR, Thingholm TE, Jensen ON, Roepstorff P, Jørgensen TJ. Highly selective enrichment of phosphorylated peptides from peptide mixtures using titanium dioxide microcolumns. Mol Cell Proteomics. 2005;4:873–86. doi: 10.1074/mcp.T500007-MCP200. [DOI] [PubMed] [Google Scholar]
- 44.Mertins P, Qiao JW, Patel J, Udeshi ND, Clauser KR, Mani DR, Burgess MW, Gillette MA, Jaffe JD, Carr SA. Integrated proteomic analysis of post-translational modifications by serial enrichment. Nat Methods. 2013;10:634–637. doi: 10.1038/nmeth.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Compton PD, Strukl JV, Bai DL, Shabanowitz J, Hunt DF. Optimization of electron transfer dissociation via informed selection of reagents and operating parameters. Anal Chem. 2012;84(3):1781–5. doi: 10.1021/ac202807h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beausoleil SA, Villen J, Gerber SA, Rush J, Gygi SP. A probability-based approach for high-throughput protein phosphorylation analysis and site localization. Nat Biotechnol. 2006;24:1285–1292. doi: 10.1038/nbt1240. [DOI] [PubMed] [Google Scholar]
- 47.Taus T, Kocher T, Pichler P, Paschke C, Schmidt A, Henrich C, Mechtler K. Universal and confident phosphorylation site localization using phosphoRS. J Proteome Res. 2011;10:5354–5362. doi: 10.1021/pr200611n. [DOI] [PubMed] [Google Scholar]
- 48.Bern M, Kil YJ, Becker C. Byonic: advanced peptide and protein identification software. Curr Protoc Bioinformatics Chapter. 2012;13(Unit 13):20. doi: 10.1002/0471250953.bi1320s40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baker PR, Trinidad JC, Chalkley RJ. Modification site localization scoring integrated into a search engine. Mol Cell Proteomics. 2011;10:M111.008078. doi: 10.1074/mcp.M111.008078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cox J, Neuhauser N, Michalski A, Scheltema RA, Olsen JV, Mann M. Andromeda: a peptide search engine integrated into the MaxQuant environment. J Proteome Res. 2011;10:1794–1805. doi: 10.1021/pr101065j. [DOI] [PubMed] [Google Scholar]
- 51.Schwartz D, Gygi SP. An iterative statistical approach to the identification of protein phosphorylation motifs from large-scale data sets. Nat Biotechnol. 2005;23:1391–1398. doi: 10.1038/nbt1146. [DOI] [PubMed] [Google Scholar]
- 52.Hornbeck PV, Zhang B, Murray B, Kornhauser JM, Latham V, Skrzypek E. PhosphoSitePlus, 2014: mutations, PTMs and recalibrations. Nucleic Acids Res. 2015;43:D512–520. doi: 10.1093/nar/gku1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maddelein D, Colaert N, Buchanan I, Hulstaert N, Gevaert K, Martens L. The iceLogo web server and SOAP service for determining protein consensus sequences. Nucleic Acids Res. 2015;(Pii):gkv385. doi: 10.1093/nar/gkv385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cox J, Mann M. 1D and 2D annotation enrichment: a statistical method integrating quantitative proteomics with complementary high-throughput data. BMC Bioinformatics. 2012;13(Suppl 16):S12. doi: 10.1186/1471-2105-13-S16-S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Horn H, Schoof EM, Kim J, Robin X, Miller ML, Diella F, Palma A, Cesareni G, Jensen LJ, Lindin R. KinomeXplorer: an integrated platform for kinome biology studies. Nat Methods. 2014;11:603–604. doi: 10.1038/nmeth.2968. [DOI] [PubMed] [Google Scholar]
- 56.Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol. 2008;26:1367–1372. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- 57.Cox J, Matic I, Hilger M, Nagaraj N, Selbach M, Olsen JV, Mann M. A practical guide to the MaxQuant computational platform for SILAC-based quantitative proteomics. Nat Protocol. 2009;4:698–705. doi: 10.1038/nprot.2009.36. [DOI] [PubMed] [Google Scholar]
- 58.Geiger T, Velic A, Macek B, Lundberg E, Kampf C, Nagaraj N, Uhlen M, Cox J, Mann M. Initial quantitative proteomic map of 28 mouse tissues using the SILAC mouse. Mol Cell Proteomics. 2013;12:1709–1722. doi: 10.1074/mcp.M112.024919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society, Series B (Methodological) 1995;57:289–300. [Google Scholar]
- 61.Hunter T, Sefton BM. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980;77:1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Datta NS, Kolailat R, Fite A, Pettway G, Abbou-Samra AB. Distinct roles for mitogen-activated protein kinase phosphatase-1 (MKP-1) and ERK-MAPK in PTH1R signaling during osteoblast proliferation and differentiation. Cell Signal. 2010;3:457–66. doi: 10.1016/j.cellsig.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miedlich SU, Abou-Samra AB. Eliminating phosphorylation sites of the parathyroid hormone receptor type 1 differentially affects stimulation of phospholipase C and receptor internalization. Am J Physiol Endocrinol Metab. 2008;295:E665–671. doi: 10.1152/ajpendo.00036.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tawfeek HA, Qian F, Abou-Samra AB. Phosphorylation of the receptor for PTH and PTHrP is required for internalization and regulates receptor signaling. Mol Endocrinol. 2002;16:1–13. doi: 10.1210/mend.16.1.0760. [DOI] [PubMed] [Google Scholar]
- 65.Blind E, Bambino T, Huang Z, Bliziotes M, Nissenson RA. Phosphorylation of the cytoplasmic tail of the PTH/PTHrP receptor. J Bone Miner Res. 1996;11:578–86. doi: 10.1002/jbmr.5650110505. [DOI] [PubMed] [Google Scholar]
- 66.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–34. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 67.Udeshi ND, Mani DR, Eisenhaure T, Mertins P, Jaffe JD, Clauser KR, Hacohen N, Carr SA. Methods for quantification of in vivo changes in protein ubiquitination following proteasome and deubiquitinase inhibition. Mol Cell Proteomics. 2012;11:148–159. doi: 10.1074/mcp.M111.016857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Caenepeel S, Charydczak G, Sudarsanam S, Hunter T, Manning G. The mouse kinome: discovery and comparative genomics of all mouse protein kinases. Proc Natl Acad Sci U S A. 2004;101:11707–12. doi: 10.1073/pnas.0306880101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Echols N, Harrison P, Balasubramanian S, Luscombe NM, Bertone P, Zhang Z, Gerstein M. Comprehensive analysis of amino acid and nucleotide composition in eukaryotic genomes, comparing genes and pseudogenes. Nucleic Acids Res. 2002;30:2515–23. doi: 10.1093/nar/30.11.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ubersax JA, Ferrell JE., Jr Mechanisms of specificity in protein phosphorylation. Nat Rev Mol Cell Biol. 2007;8:530–41. doi: 10.1038/nrm2203. [DOI] [PubMed] [Google Scholar]
- 71.Linding R, Jensen LJ, Ostheimer GJ, van Vugt MS, Jørgensen C, Miron IM, Diella F, Colwill K, Taylor L, Elder K, Metalnikov P, Nguyen V, Pasculescu A, Jin J, Park JG, Samson LD, Woodgett JR, Russell RB, Bork P, Yaffe MB, Pawson T. Systematic discovery of in vivo phosphorylation networks. Cell. 2007;129:1415–26. doi: 10.1016/j.cell.2007.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Linding R, Jensen LJ, Pasculescu A, Olhovsky M, Colwill K, Bork P, Yaffe MB, Pawson T. NetworKIN: a resource for exploring cellular phosphorylation networks. Nucleic Acids Res. 2008;36:D695–9. doi: 10.1093/nar/gkm902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Letunic I, Bork P. Interactive Tree Of Life v2: online annotation and display of phylogenetic trees made easy. Nucleic Acids Res. 2011;39:W475–478. doi: 10.1093/nar/gkr201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rigbolt KT, Prokhorova TA, Akimov V, Henningsen J, Johansen PT, Kratchmarova I, Kassem M, Mann M, Olsen JV, Blagoev B. System-wide temporal characterization of the proteome and phosphoproteome of human embryonic stem cell differentiation. Sci Signal. 2011;4:rs3. doi: 10.1126/scisignal.2001570. [DOI] [PubMed] [Google Scholar]
- 75.Cheung M, Sharma A, Madhunapantula SV, Robertson GP. Akt3 and mutant V600E B-Raf cooperate to promote early melanoma development. Cancer Res. 2008;68:3429–3439. doi: 10.1158/0008-5472.CAN-07-5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang BH, Guan KL. Regulation of the Raf kinase by phosphorylation. Exp Lung Res. 2001;27:269–295. doi: 10.1080/019021401300054046. [DOI] [PubMed] [Google Scholar]
- 77.Guan KL, Figueroa C, Brtva TR, Zhu T, Taylor J, Barber TD, Vojtek AB. Negative regulation of the serine/threonine kinase B-Raf by Akt. J Biol Chem. 2000;275:27354–27359. doi: 10.1074/jbc.M004371200. [DOI] [PubMed] [Google Scholar]
- 78.Baljuls A, Schmitz W, Mueller T, Zahedi RP, Sickmann A, Hekman M, Rapp UR. Positive regulation of A-RAF by phosphorylation of isoform-specific hinge segment and identification of novel phosphorylation sites. J Biol Chem. 2008;283:27239–27254. doi: 10.1074/jbc.M801782200. [DOI] [PubMed] [Google Scholar]
- 79.Li T, Zhang J, Zhu F, Wen W, Zykova T, Li X, Liu K, Peng C, Ma W, Shi G, Dong Z, Bode AM, Dong Z. P21-activated protein kinase (PAK2)-mediated c-Jun phosphorylation at 5 threonine sites promotes cell transformation. Carcinogenesis. 2011;32:659–666. doi: 10.1093/carcin/bgq271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kruiswijk F, Yuniati L, Magliozzi R, Low TY, Lim R, Bolder R, Mohammed S, Proud CG, Heck AJ, Pagano M, Guardavaccaro D. Coupled activation and degradation of eEF2K regulates protein synthesis in response to genotoxic stress. Sci Signal. 2012;5:ra40. doi: 10.1126/scisignal.2002718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim SY, Volsky DJ. PAGE: Parametric analysis of geneset enrichment. BMC Bioinformatics. 2005;6:144–156. doi: 10.1186/1471-2105-6-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maudsley S, Chadwick W, Wang L, Zhou Y, Martin B, Park SS. Bioinformatic approaches to metabolic pathways analysis. Methods Mol Biol. 2011;756:99–130. doi: 10.1007/978-1-61779-160-4_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maudsley S, Siddiqui S, Martin B. Systems analysis of arrestin pathway functions. Prog Mol Biol Transl Sci. 2013;118:431–467. doi: 10.1016/B978-0-12-394440-5.00017-6. [DOI] [PubMed] [Google Scholar]
- 84.ten Klooster JP, Jaffer ZM, Chernoff J, Hordijk PL. Targeting and activation of Rac1 are mediated by the exchange factor beta-Pix. J Cell Biol. 2006;172:759–769. doi: 10.1083/jcb.200509096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chahdi A, Sorkin A. Protein kinase A-dependent phosphorylation modulates beta1Pix guanine nucleotide exchange factor activity through 14-3-3beta binding. Mol Cell Biol. 2008;28:1679–1687. doi: 10.1128/MCB.00898-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Premont RT, Perry SJ, Schmalzigaug R, Roseman JT, Xing Y, Claing A. The GIT/PIX complex: an oligomeric assembly of GIT family ARF GTPase-activating proteins and PIX family Rac1/Cdc42 guanine nucleotide exchange factors. Cell Signal. 2004;16:1001–1011. doi: 10.1016/j.cellsig.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 87.Gesty-Palmer D, Yuan L, Martin B, Wood WH, 3rd, Lee MH, Janech MG, Tsoi LC, Zheng WJ, Luttrell LM, Maudsley S. β-arrestin-selective G protein-coupled receptor agonists engender unique biological efficacy in vivo. Mol Endocrinol. 2013;27:296–314. doi: 10.1210/me.2012-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yoshida T, Clark MF, Stern PH. The small GTPase RhoA is crucial for MC3T3-E1 osteoblastic cell survival. J Cell Biochem. 2009;106:896–902. doi: 10.1002/jcb.22059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hamamura K, Swarnkar G, Tanjung N, Cho E, Li J, Na S, Yokota H. RhoA-mediated signaling in mechanotransduction of osteoblasts. Connect Tissue Res. 2012;53:398–406. doi: 10.3109/03008207.2012.671398. [DOI] [PubMed] [Google Scholar]
- 90.Wan Q, Cho E, Yokota H, Na S. Rac1 and Cdc42 GTPases regulate shear stress-driven β-catenin signaling in osteoblasts. Biochem Biophys Res Commun. 2013;433:502–507. doi: 10.1016/j.bbrc.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lefkowitz RJ, Pierce KL, Luttrell LM. Dancing with different partners: protein kinase a phosphorylation of seven membrane-spanning receptors regulates their G protein-coupling specificity. Mol Pharmacol. 2002;62:971–974. doi: 10.1124/mol.62.5.971. [DOI] [PubMed] [Google Scholar]
- 92.Merrill AE, Hebert AS, MacGilvray ME, Rose CM, Bailey DJ, Bradley JC, Wood WW, El Masri M, Westphall MS, Gasch AP, Coon JJ. NeuCode labels for relative protein quantification. Mol Cell Proteomics. 2014;13:2503–2512. doi: 10.1074/mcp.M114.040287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Paulo JA, McAllister FE, Everley RA, Beausoleil SA, Banks AS, Gygi SP. Effects of MEK inhibitors GSK1120212 and PD0325901 in vivo using 10-plex quantitative proteomics and phosphoproteomics. Proteomics. 2015;15:462–473. doi: 10.1002/pmic.201400154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Maudsley S, Martin B, Gesty-Palmer D, Cheung H, Johnson C, Patel S, Becker KG, Wood WH, 3rd, Zhang Y, Lehrmann E, Luttrell LM. Delineation of a conserved arrestin-biased signaling repertoire in vivo. Mol Pharmacol. 2015;87:706–717. doi: 10.1124/mol.114.095224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative annotated tandem mass spectrum.
All modification-specific peptides identified by LC-MS/MS in MC3T3-E1 osteoblasts.
All proteins observed (2A); All protein kinases observed (2B); All DNA binding transcription factors observed (2C), in MC3T3-E1 osteoblasts.
All phosphoSTY sites observed (3A–B); Novel phosphoSTY sites observed (3C), in MC3T3-E1 osteoblasts.
Monophosphorylated STY sites observed in a single biological replicate with ≥2-fold change
Significantly regulated monophosphorylated STY sites observed in ≥2 biological replicates.