Abstract
Background and Purpose
Emerging work has linked menopausal vasomotor symptoms (VMS) to subclinical cardiovascular disease (CVD) among women. However, VMS are dynamic over time. No studies have considered how temporal patterns of VMS may relate to subclinical CVD. We tested how temporal patterns of VMS assessed over 13 years were related to carotid intima media thickness (IMT) among midlife women.
Methods
The Study of Women’s Health Across the Nation is a longitudinal cohort study of midlife women. 811 Caucasian, African American, Hispanic, and Chinese participants with a well-characterized final menstrual period completed measures of VMS, a blood draw, and physical measures approximately annually for 13 years. Women underwent a carotid artery ultrasound at study visit 12.
Results
Four trajectories of VMS were identified by trajectory analysis (consistently high, early onset, late onset, persistently low VMS) and tested in relation to carotid indices in linear regression models. Results indicated that women with early onset VMS had both greater mean IMT [beta, b(standard error, SE)=.03(.01), p=0.03] and greater maximal IMT [b(SE)=.04(.01), p=.008] than women with consistently low VMS, adjusting for demographics and CVD risk factors.
Conclusions
This is first study to test trajectories of VMS in relation to subclinical CVD. Women with VMS early in the menopause transition had higher mean IMT and maximal IMT than those with consistently low VMS across the transition. Associations were not accounted for by demographic factors nor by CVD risk factors. Results can signal to women in need of early CVD risk reduction.
Keywords: atherosclerosis, epidemiology, menopause, sex, women
Introduction
CVD is the leading cause of death among women, with its incidence increasing postmenopausally.1 An understanding of how menopause-related factors may be related to CVD risk among women has long been of interest. Vasomotor symptoms (VMS) are the classic menopausal symptom, experienced by over 70% of women.2 While VMS are known to be associated with poorer quality of life,3 VMS have been linked to physical health outcomes, including cardiovascular disease (CVD) risk. Multiple studies show relations between VMS and subclinical CVD4–7 and CVD risk factors.8–10 However, the literature is not entirely consistent,11, 12 and further understanding of VMS-CVD risk relations is warranted.
While most women will experience VMS during the menopause transition, the patterns of VMS vary dramatically.13, 14 Some women experience VMS early when they are still menstruating; others only postmenopausally; and still others have VMS for decades.14 These variations may reflect different etiologies of VMS with varying physiologic sequelae. Preliminary work indicates that the timing of VMS may be important to CVD risk.5, 12, 15 However, these studies were modest in size, had few assessments, and/or asked women to recall their VMS occurring years earlier. They were not adequately designed to address variations in trajectories of VMS over the transition. In order to do so, a large cohort study with prospective assessments of VMS is needed.
The Study of Women’s Health Across the Nation (SWAN) is a large longitudinal cohort study of women transitioning through the menopause. Women were recruited in the pre- or early perimenopause and have been followed for over a decade. VMS have been assessed approximately annually, making SWAN an ideal cohort to prospectively characterize trajectories of VMS over the menopause transition. At visit 12, participants underwent a carotid ultrasound to assess carotid artery IMT, a well-validated subclinical CVD index predictive of later clinical CVD.16 We tested whether different trajectories of VMS over the menopause transition were related to later IMT and considered whether associations were accounted for by standard CVD risk factors.
Methods
SWAN is a prospective cohort study of women conducted at seven sites: Boston; Chicago; the Detroit area; Los Angeles; Newark, New Jersey; Pittsburgh, Pennsylvania; and Oakland, California.17 Each site recruited Caucasian women and one additional racial/ethnic group. The six sites participating in carotid measurements recruited Caucasian women plus African American (Pittsburgh, Chicago, Michigan, Boston), Chinese (Oakland) or Hispanic (Newark) women. Women were recruited from lists of names or household addresses, and select sites supplemented primary sampling frames to obtain adequate numbers of racial/ethnic minority women. Baseline eligibility criteria included being aged 42–52 years, having a uterus and ≥one ovary, not being pregnant or lactating, not using oral contraceptives/hormone therapy (HT), and having ≥one menstrual cycle in the prior 3 months. 51% (N=3302) of eligible women enrolled. Annual clinic assessments began in 1996–1997. Ultrasound data were collected at visit 12. SWAN protocols were approved by the institutional review boards at each site, and each participant provided written informed consent. This study investigated associations between VMS trajectories from baseline through the 12th annual SWAN visit and carotid outcomes at visit 12.
Of the 1512 women who had valid carotid data, 637 women were excluded from analyses due to a lack of a discernable final menstrual period (FMP; due to surgery or hormone use) or <3 visits with VMS data (required to construct trajectories). An additional 64 women were excluded due to a history of stroke or myocardial infarction. 811 women were included in analyses. Women excluded differed from women included in that they were less often Chinese and more often African American or Caucasian (p<0.001) and, consistent with the CVD exclusion, had a poorer risk factor profile (higher BMI, higher SBP, lower HDL, higher trigycerides, higher HOMA, more often diabetic, more often taking cardiovascular medications, p’s<0.05).
Vasomotor Symptoms
VMS were assessed via questionnaire at each of 12 annual visits. Women responded to two questions which asked separately how often they experienced: 1) hot flashes and 2) night sweats in the past two weeks (not at all, 1–5 days, 6–8 days, 9–13 days, every day). For each visit, women were categorized as having VMS if they reported any hot flashes or night sweats at that visit. Patterns of experiencing VMS (trajectories) across visits were identified (see data analyses).
Ultrasound measures
At each site, centrally trained and certified sonographers obtained carotid ultrasound images using a Terason t3000 Ultrasound System (Teratech Corp, Burlington, MA) equipped with a variable frequency 5–12 Mhz linear array transducer. Two digitized images were obtained of each of the left and right distal common carotid artery. From each of these 4 images, using the AMS semi-automated edge detection software,18 near and far wall common carotid artery IMT measures were obtained by electronically tracing the lumen-intima interface and the media-adventitia interface across a 1-cm segment proximal to the carotid bulb; one measurement was generated for each pixel over the area, for a total of approximately 140 measures for each segment. The average and maximal values for these measures were recorded, with the mean of the average and maximal readings of all 4 images used in analyses. Common carotid artery inter-adventitial diameter (AD) was measured directly as the distance from the adventitial-medial interface on the near wall to the medial-adventitial interface on the far wall at end-diastole across the same common carotid artery segments used for IMT measurement. Images were read centrally at the SWAN Ultrasound Reading Center (University of Pittsburgh Ultrasound Research Laboratory). Technicians at study sites were trained by the University of Pittsburgh Ultrasound Research Laboratory and monitored during the study for reliability. Reproducibility was excellent [intraclass correlation coefficients (ICC) ≥ 0.77 (between sonographers), ICC>0.90 (between readers)].19
Covariates
At baseline, race/ethnicity was reported and education assessed (high school, some college/vocational, ≥college). Other covariates were taken from visit 12 (concurrent with the carotid ultrasound). Age, smoking (current vs. past/never), anxiety, and medication use were derived from questionnaires/interviews. Use of cardiovascular medications (blood pressure lowering, lipid-lowering, blood thinning) was classified. Height and weight were measured and BMI calculated (kg/m2). Blood pressure was averaged from two seated measurements, and the measure with the strongest association with the outcome included as a covariate (systolic). Women were considered diabetic if they reported diabetes or had fasting glucose levels ≥126 mg/dL, or reported any use of insulin/anti-diabetic agents at ≥70% of the visits and/or for ≥3 consecutive visits.
Phlebotomy was performed following overnight fast within 90 days of the annual visit. Blood was separated, frozen (−80°C), and sent to the University of Michigan Pathology Laboratory, CLIA-certified and accredited by the College of American Pathologists. Measurements were performed on a Siemens ADVIA 2400 automated chemistry analyzer utilizing Siemens ADVIA chemistry system reagents. Glucose was measured using a two-step enzymatic reaction and serum insulin measured using radioimmunoassay. HOMA-IR was calculated [(insulin*glucose)/22.5]. Lipid fractions were determined from EDTA-treated plasma.
Data Analyses
Group based growth trajectory modeling20 was used (Proc Traj in SAS) to identify trajectories of VMS over time. Preliminary analyses in the full SWAN cohort identified four distinct trajectories.14 For the present analyses, VMS trajectories were re-identified among participants who had a carotid ultrasound, a discernible FMP, and ≥3 visits with VMS data. Visits in which women reported HT use were dropped. Trajectories were adjusted for study site and age. The time scale was anchored to the FMP, with a maximum time before and FMP of 8.74 and 10.41 years, respectively. Trajectories were based on model fit statistics and scientific plausibility; four VMS trajectories were identified that each woman occupied based on her highest posterior (predicted) probability.
The four VMS trajectories were next linked to carotid outcomes. Associations between VMS trajectories and outcomes were estimated in linear regression adjusted for age, race/ethnicity, education, and site, and covariates associated with outcomes at p<0.05. IMT, HOMA-IR, and triglyceride values were natural log transformed. Interactions between VMS trajectories and race/ethnicity and BMI were examined as cross product terms. In sensitivity analyses, 24 women reporting using medications that could impact VMS [selective estrogen receptor modulators (SERMs), aromatase inhibitors, selective serotonin reuptake inhibitors or serotonin norepinepherine reuptake inhibitors (SSRI/SNRIs), gabapentin] were excluded. Residual analysis and diagnostic plots were used to verify model assumptions. Analyses were performed with SAS v9.2 (SAS, Cary, NC).
Results
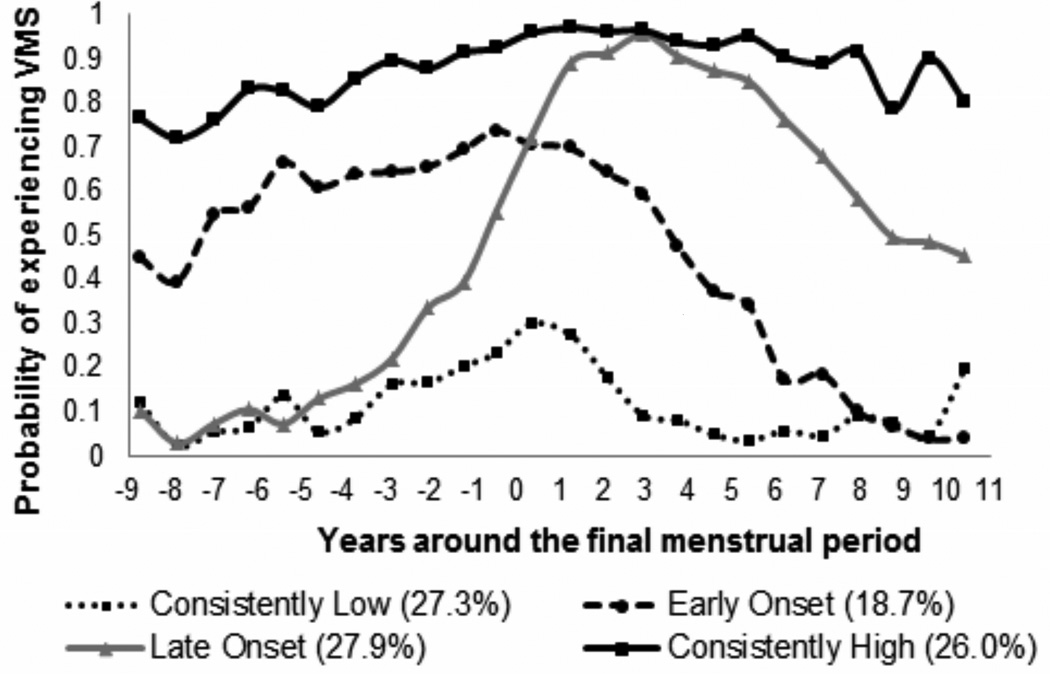
At visit 12, the participants were on average 59 years old, overweight, nonsmoking, and normotensive (Table 1). Four trajectories of VMS were identified: 1) consistently low probability of having VMS, 2) consistently high probability of having VMS, 3) VMS early in the transition that decreased shortly after the FMP, and 4) VMS that developed largely after the FMP (Figure 1), similar to the full SWAN cohort.14 African American women and women with lower education were most likely to have consistently high VMS; and Non-Hispanic Caucasian, Chinese, and more highly educated women were more likely to have consistently low VMS (Table 1). Women with consistently high VMS and early onset VMS also had a more adverse CVD risk factor profile.
Table 1.
Characteristics of women by vasomotor symptom trajectory group
| Consistently Low | Early Onset | Late Onset | Consistently High | Overall P value |
|
|---|---|---|---|---|---|
| Age, years, Mean±SD | 59.8±2.6 | 59.7±2.6 | 59.2±2.6 | 59.5±2.8 | .06 |
| Race, n (%) | <.0001 | ||||
| Black | 30(13.2) | 43(32.1) | 67(29.8) | 98(43.8) | |
| Caucasian | 125(54.8) | 63(47.0) | 116(51.6) | 86(38.4) | |
| Chinese | 60(26.3) | 22(16.4) | 33(14.7) | 22(9.8) | |
| Hispanic | 13(5.7) | 6(4.5) | 9(4.0) | 18(8.0) | |
| Education, n (%) | <.0001 | ||||
| High school | 43(19.3) | 29(21.8) | 45(20.1) | 69(30.9) | |
| Some college/vocational | 51(22.9) | 39(29.3) | 67(29.9) | 86(38.6) | |
| ≥College | 129(57.9) | 65(48.9) | 112(50.0) | 68(30.5) | |
| BMI, kg/m2, Mean±SD | 28.3±7.3 | 30.5±7.4* | 28.0±6.2 | 31.3±7.7* | <.0001 |
| SBP, mmHg, Mean±SD | 117.9±15.5 | 124.5±18.5* | 119.0±15.7 | 125.2±19.0* | <.0001 |
| DBP, mmHg, Mean±SD | 73.0 ±9.8 | 75.6 ±11.1* | 72.8 ±10.1 | 74.5 ±9.7* | .03 |
| HDL, mg/dL, Mean±SD | 64.6±17.2 | 60.3±15.0* | 64.5±16.7 | 60.0±14.2* | .002 |
| LDL, mg/dL, Mean±SD | 123.9±34.6 | 126.4±29.5 | 128.7±36.6 | 123.3±35.6 | .40 |
| Triglycerides, mg/dL, Median (Q1,Q3) | 91.5(71.0, 125.5) | 101.0(76.0, 145.0)* | 87.0(69.0, 126.0) | 103.0(76.0, 140.0)* | .03 |
| HOMA index, Median (Q1,Q3) | 1.7(1.1, 3.4) | 2.6(1.5, 4.0)* | 1.7(1.1, 3.0) | 2.6(1.4, 4.2)* | <.0001 |
| Anxiety, Median (Q1,Q3) | 1.0(.0, 3.0) | 2.0(.0, 4.0) | 1.0(.0, 3.0) | 3.0(1.0, 6.0)* | <.0001 |
| Smoker, n (%) | 11 (4.9) | 9 (6.8) | 15 (6.7) | 30(13.6) | .004 |
| Diabetes, n(%) | 22(9.7) | 15(11.2) | 7(3.1) | 39(17.4) | <.0001 |
| Cardiovascular medication use, n (%)† | 103(45.4) | 75(56.4) | 103(46.4) | 144(64.6) | <.0001 |
Significant (p<0.05) difference compared to consistently low VMS;
Ever use during the study; Cardiovascular medications: antihypertensive, lipid lowering, or anticoagulants
BMI = body mass index; DBP = diastolic blood pressure; HDL = high density lipoprotein, HOMA = homeostatic model assessment, LDL = low density lipoprotein; Q = quartile; SBP = systolic blood pressure; SD = standard deviation
Figure 1.
Trajectories of VMS over the menopause transition (N=811)
Note: Adjusted for study site and age
We next considered trajectories of VMS in relation to IMT. Women with consistently high VMS or early onset VMS had higher IMT than women with consistently low VMS (Table 2). Early onset VMS remained associated with higher mean and maximal IMT when adjusting for demographic and CVD risk factors (Table 3).
Table 2.
Unadjusted IMT by vasomotor symptom group
| Consistently Low | Early Onset | Late Onset | Consistently High | Overall P value | |
|---|---|---|---|---|---|
| IMT, M (SD), mm | .77(.11) | .82(.12)* | .77(.11) | .80(.12)* | .0001 |
| Maximal IMT, M (SD), mm | .90(.13) | .96(.15)* | .90(.13) | .94(.14)* | <.0001 |
p<0.05 relative to consistently low vasomotor symptoms
IMT = intima media thickness; M = mean
Table 3.
Multivariable associations between vasomotor symptom (VMS) trajectories and IMT
| Mean IMT | Maximum IMT | |||
|---|---|---|---|---|
| β(SE) | P | β(SE) | P | |
| Model 1 | ||||
| VMS trajectory | ||||
| Consistently low | ------ | ----- | ||
| Early onset | .04(.01) | .004 | .05(.01) | .0006 |
| Late onset | −.01(.01) | .50 | −.01(.01) | .50 |
| Consistently high | .01(.01) | .30 | .02(.01) | .20 |
| Model 2 | ||||
| VMS trajectory | ||||
| Consistently low | ------ | ----- | ||
| Early onset | .03(.01) | .03 | .04(.01) | .008 |
| Late onset | −.002(.01) | .90 | −.001(.01) | .90 |
| Consistently high | −.001(.01) | .90 | .002(.01) | .90 |
Model 1 covariates: site, age, ethnicity, education
Model 2 covariates: Adjusted for site, age, ethnicity, education, body mass index, systolic blood pressure, high density lipoproteins; low density lipoproteins, triglycerides, homeostatic model assessment, smoking status, diabetes, anxiety, use of cardiovascular medications
IMT = intima media thickness; VMS=vasomotor symptoms
We next tested for interactions between VMS trajectory group and race/ethnicity or BMI in relation to IMT. None of these interactions were significant (p’s>.05). We also considered AD given its association with vascular remodeling21 and sensitivity to reproductive hormones.22 Whereas women with early onset VMS had higher AD [B(SE)=.14(.07), p=.04, vs. consistently low VMS] in minimally-adjusted models, relations did not persist when additionally adjusting for CVD risk factors [B(SE)=.06(.07), p=.40]. Finally, we conducted analyses excluding women taking medications that might impact VMS (SERMS, aromatase inhibitors, SSRI/ SNRIs, gabapentin). Findings were unchanged (data not shown).
Discussion
This is the first study to examine trajectories of VMS over the course of the menopause transition in relation to subclinical CVD. SWAN is uniquely able to address this question, given the repeated prospective assessment of VMS over a decade, the well-characterized cohort, and the measurement of IMT. Although women with persistent VMS over the menopause transition had the worst CVD risk factor profile, it was the women with early onset VMS (VMS occurring up to a decade prior to the FMP and declining several years after the FMP) who had the highest IMT. Associations were not accounted for by demographics or by CVD risk factors.
A notable aspect of VMS is that they are dynamic, changing dramatically as women progress through the menopause. Emerging work suggests that VMS may be related to higher subclinical CVD cross-sectionally.4, 5, 7, 23 However, given the dynamic nature of VMS, a single assessment is inadequate to characterize a woman’s true burden of VMS. The few studies that have considered VMS over time in relation to CVD risk generally show more persistent VMS associated with subclinical CVD.5, 6 However, these studies had few assessments,5 limited sample sizes,5, 6 lack of ethnic diversity,6 or failed to capture the early transition.6 The Women’s Health Initiative reports have shown complex relations between VMS and CVD risk over time,12, 24 yet analyses were limited by exclusion of women with high burden of VMS and reliance upon women recalling their VMS up to a decade earlier, the accuracy of which is likely low. Thus, the relation of VMS over time to subclinical CVD has not been rigorously tested.
As most women get VMS, refining the understanding of what types of VMS are most relevant to cardiovascular health is warranted. Like other research on reproductive factors and midlife women’s cardiovascular health,25 timing matters. These data indicate that early-occurring VMS (starting up to a decade before the FMP) appear to have specific implications for a woman’s cardiovascular health. The magnitude of the effects observed here is clinically significant, comparable to >4 years of aging in the present cohort. Prior work has shown that some women start experiencing VMS early in the transition (often when they are still cycling), particularly African American or obese women.26 However, the present results controlled for race/ethnicity and BMI. Other work has indicated that VMS are associated with a more adverse adipokine profile,27 reduced cardiac vagal control,28 more adverse inflammatory or hemostatic profile,29 and poorer endothelial function.4, 7 A closer examination of mechanisms linking early onset VMS to CVD risk is warranted.
This study had several limitations. VMS were self-reported and recalled over the prior two weeks, reports which may contain more error than diaries or physiologic VMS indices. To characterize VMS trajectories relative to the FMP, women without a discernable FMP due to HT use, hysterectomy, or oophorectomy were excluded. Results may not generalize to these women. Other conditions relevant to development of atherosclerosis (e.g., chronic obstructive pulmonary disease, autoimmune disorders), were not rigorously assessed. Aspects of vessel morphology linked to CVD risk (i.e., dolichocarotids30, 31) were not systematically assessed and should be considered in future work. Further, IMT was assessed once at visit 12; thus trajectories of IMT could not be characterized. IMT was assessed only at the CCA and not at other sites. This approach is consistent with guidelines, as IMT at the CCA is most reliably measured and predictive of events,16 yet atherosclerosis at other sites would not have been captured here.
SWAN has multiple strengths, including it being a large cohort of women who have been assessed prospectively and repeatedly over the course of the menopause transition. VMS are measured approximately annually up to 13 times, allowing the unique opportunity to characterize VMS trajectories. The FMP, menopausal stage, and HT are rigorously assessed, allowing anchoring of VMS trajectories relative to the FMP and reducing confounding effects of HT. SWAN included a group of ethnically diverse women. Finally, carotid ultrasounds were included in this large cohort, and multiple CVD risk factors assessed repeatedly and prospectively.
This study was the first to examine trajectories of VMS over the menopause transition in relation to subclinical CVD, showing that women with VMS beginning a decade prior to the FMP had the highest IMT. Associations were not accounted for by CVD risk factors. Findings underscore that work investigating relations between VMS and CVD risk should consider the timing of VMS. Findings on VMS and CVD may ultimately be used to further understand the pathophysiology of CVD in women as well as to assist in CVD risk prediction among midlife women.
Acknowledgments
Funding Sources
SWAN has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495).
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Appendix
Clinical Centers: University of Michigan, Ann Arbor–Siobán Harlow, PI 2011–present, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA–Joel Finkelstein, PI 1999–present; Robert Neer, PI 1994–1999; Rush University, Rush University Medical Center, Chicago, IL–Howard Kravitz, PI 2009–present; Lynda Powell, PI 1994–2009; University of California, Davis/Kaiser–Ellen Gold, PI; UCLA–Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY–Carol Derby, PI 2011–present, Rachel Wildman, PI 2010–2011; Nanette Santoro, PI 2004–2010; University of Medicine and Dentistry–NJ Medical School, Newark–Gerson Weiss, PI 1994–2004; and the University of Pittsburgh, Pittsburgh, PA–Karen Matthews, PI.
NIH Program Office: NIA, Bethesda, MD–Winifred Rossi 2012 - present; Sherry Sherman 1994 –2012; Marcia Ory 1994–2001; NINR, Bethesda, MD–Program Officers.
Central Laboratory: University of Michigan, Ann Arbor–Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA–Maria Mori Brooks, PI 2012 - present; Kim Sutton-Tyrrell, PI 2001–2012; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995–2001.
| Steering Committee: | Susan Johnson, Current Chair |
| Chris Gallagher, Former Chair |
Footnotes
Disclosures: Thurston: None; El Khoudary: None; Tepper: None; Jackson: Consulting: McKesson, American College of Cardiology; Authorships/editorial: American Journal of Medicine, Up-To-Date, Spry Publishing; Joffe: Grant support: Cephalon/Teva, Merck Advisory board/consulting: Merck, Noven, Tanaka Mitsubishi; Chen: None; Matthews: None
References
- 1.Mosca L, Barrett-Connor E, Wenger NK. Sex/gender differences in cardiovascular disease prevention: What a difference a decade makes. Circulation. 2011;124:2145–2154. doi: 10.1161/CIRCULATIONAHA.110.968792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gold E, Colvin A, Avis N, Bromberger J, Greendale G, Powell L, et al. Longitudinal analysis of vasomotor symptoms and race/ethnicity across the menopausal transition: Study of women’s health across the nation (SWAN) Am J Public Health. 2006;96:1226–1235. doi: 10.2105/AJPH.2005.066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avis NE, Colvin A, Bromberger JT, Hess R, Matthews KA, Ory M, et al. Change in health-related quality of life over the menopausal transition in a multiethnic cohort of middle-aged women: Study of women's health across the nation. Menopause. 2009;16:860–869. doi: 10.1097/gme.0b013e3181a3cdaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thurston RC, Sutton-Tyrrell K, Everson-Rose SA, Hess R, Matthews KA. Hot flashes and subclinical cardiovascular disease: Findings from the study of women's health across the nation heart study. Circulation. 2008;118:1234–1240. doi: 10.1161/CIRCULATIONAHA.108.776823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thurston RC, Sutton-Tyrrell K, Everson-Rose SA, Hess R, Powell LH, Matthews KA. Hot flashes and carotid intima media thickness among midlife women. Menopause. 2011;18:352–358. doi: 10.1097/gme.0b013e3181fa27fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thurston RC, Kuller LH, Edmundowicz D, Matthews KA. History of hot flashes and aortic calcification among postmenopausal women. Menopause. 2010;17:256–261. doi: 10.1097/gme.0b013e3181c1ad3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bechlioulis A, Kalantaridou SN, Naka KK, Chatzikyriakidou A, Calis KA, Makrigiannakis A, et al. Endothelial function, but not carotid intima-media thickness, is affected early in menopause and is associated with severity of hot flushes. J Clin Endocrinol Metab. 2010;95:1199–1206. doi: 10.1210/jc.2009-2262. [DOI] [PubMed] [Google Scholar]
- 8.Gast GC, Grobbee DE, Pop VJ, Keyzer JJ, Wijnands-van Gent CJ, Samsioe GN, et al. Menopausal complaints are associated with cardiovascular risk factors. Hypertension. 2008;51:1492–1498. doi: 10.1161/HYPERTENSIONAHA.107.106526. [DOI] [PubMed] [Google Scholar]
- 9.Thurston R, El Khoudary S, Sutton-Tyrrell K, Crandall C, Gold E, Sternfeld B, et al. Vasomotor symptoms and lipid profiles in women transitioning through menopause. Obstet Gynecol. 2012;119:753–761. doi: 10.1097/AOG.0b013e31824a09ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thurston RC, El Khoudary SR, Sutton-Tyrrell K, Crandall CJ, Sternfeld B, Joffe H, et al. Vasomotor symptoms and insulin resistance in the study of women's health across the nation. J Clin Endocrinol Metab. 2012;97:3487–3494. doi: 10.1210/jc.2012-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tuomikoski P, Ebert P, Groop PH, Haapalahti P, Hautamaki H, Ronnback M, et al. Evidence for a role of hot flushes in vascular function in recently postmenopausal women. Obstet Gynecol. 2009;113:902–908. doi: 10.1097/AOG.0b013e31819cac04. [DOI] [PubMed] [Google Scholar]
- 12.Szmuilowicz ED, Manson JE, Rossouw JE, Howard BV, Margolis KL, Greep NC, et al. Vasomotor symptoms and cardiovascular events in postmenopausal women. Menopause. 2011;18:603–610. doi: 10.1097/gme.0b013e3182014849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freeman EW, Sammel MD, Lin H, Liu Z, Gracia CR. Duration of menopausal hot flushes and associated risk factors. Obstet Gynecol. 2011;117:1095–1104. doi: 10.1097/AOG.0b013e318214f0de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tepper P, Randolph J, Jones B, Crawford S, Gold E, El Khoudary S, et al. Trajectory patterns of vasomotor symptoms over the menopausal transition in the study of women’s health across the nation (abstract) Menopause. 2013;20:1356. [Google Scholar]
- 15.Thurston R, Kuller L, Edmundowicz D, Matthews K. Hot flashes and aortic calcification among postmenopausal women. Menopause. 2010;17:256–261. doi: 10.1097/gme.0b013e3181c1ad3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: A consensus statement from the american society of echocardiography carotid intima-media thickness task force. J Am Soc Echocardiography. 2008;21:93–111. doi: 10.1016/j.echo.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 17.Sowers M, Crawford S, Sternfeld B, Morganstein D, Gold EB, Greendale GA, et al. Swan: A multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, Lobo AR, editors. Menopause: Biology and pathology. New York: Academic Press; 2000. pp. 175–188. [Google Scholar]
- 18.Wendelhag I, Gustavsson T, Suurkula M, Berglund G, Wikstrand J. Ultrasound measurement of wall thickness in the carotid artery: Fundamental principles and description of a computerized analysing system. Clinical Physiology. 1991;11:565–577. doi: 10.1111/j.1475-097x.1991.tb00676.x. [DOI] [PubMed] [Google Scholar]
- 19.Sutton-Tyrrell K, Wolfson SK, Thompson T, Kelsey SF. Measurement variability in duplex scan assessment of carotid atherosclerosis. Stroke. 1992;23:215–220. doi: 10.1161/01.str.23.2.215. [DOI] [PubMed] [Google Scholar]
- 20.Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6:109–138. doi: 10.1146/annurev.clinpsy.121208.131413. [DOI] [PubMed] [Google Scholar]
- 21.Kiechl S, Willeit J. The natural course of atherosclerosis. Part ii: Vascular remodeling. Bruneck study group. Arteriosc Thromb Vasc Biol. 1999;19:1491–1498. doi: 10.1161/01.atv.19.6.1491. [DOI] [PubMed] [Google Scholar]
- 22.El Khoudary SR, Wildman RP, Matthews K, Thurston RC, Bromberger JT, Sutton-Tyrrell K. Endogenous sex hormones impact the progression of subclinical atherosclerosis in women during the menopausal transition. Atherosclerosis. 2012;225:180–186. doi: 10.1016/j.atherosclerosis.2012.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lambrinoudaki I, Augoulea A, Armeni E, Rizos D, Alexandrou A, Creatsa M, et al. Menopausal symptoms are associated with subclinical atherosclerosis in healthy recently postmenopausal women. Climacteric. 2012;15:350–357. doi: 10.3109/13697137.2011.618564. [DOI] [PubMed] [Google Scholar]
- 24.Allison MA, Manson JE, Aragaki A, Langer RD, Rossouw J, Curb D, et al. Vasomotor symptoms and coronary artery calcium in postmenopausal women. Menopause. 2010;17:1136–1145. doi: 10.1097/gme.0b013e3181e664dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lenfant F, Tremollieres F, Gourdy P, Arnal JF. Timing of the vascular actions of estrogens in experimental and human studies: Why protective early, and not when delayed? Maturitas. 2011;68:165–173. doi: 10.1016/j.maturitas.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 26.Freeman EW, Sammel MD, Grisso JA, Battistini M, Garcia-Espagna B, Hollander L. Hot flashes in the late reproductive years: Risk factors for african american and caucasian women. J Womens Health Gend Based Med. 2001;10:67–76. doi: 10.1089/152460901750067133. [DOI] [PubMed] [Google Scholar]
- 27.Thurston RC, Chang Y, Mancuso P, Matthews KA. Adipokines, adiposity, and vasomotor symptoms during the menopause transition: Findings from the study of women's health across the nation. Fertil Steril. 2013;100:793–800. doi: 10.1016/j.fertnstert.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thurston R, Christie I, Matthews K. Hot flashes and cardiac vagal control during women’s daily lives. Menopause. 2012;19:406–412. doi: 10.1097/gme.0b013e3182337166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thurston RC, El Khoudary SR, Sutton-Tyrrell K, Crandall CJ, Gold E, Sternfeld B, et al. Are vasomotor symptoms associated with alterations in hemostatic and inflammatory markers? Findings from the study of women's health across the nation. Menopause. 2011;18:1044–1051. doi: 10.1097/gme.0b013e31821f5d39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matteo Ciccone M, K Sharma R, Scicchitano P, Cortese F, Salerno C, Berchialla P, et al. Dolichocarotids: Echo-color doppler evaluation and clinical role. J Atheroscler Thromb. 2014;21:56–63. doi: 10.5551/jat.18085. [DOI] [PubMed] [Google Scholar]
- 31.Ciccone MM, Scicchitano P, Palumbo V, Cortese F, Valecce R, Dentamaro I, et al. Dolichocarotids and dilated cardiomyopathy: Is there a relationship? Int J Cardiol. 2012;158:123–125. doi: 10.1016/j.ijcard.2012.04.052. [DOI] [PubMed] [Google Scholar]