Abstract
Background
Studies have shown a modest relationship between depression and mortality in cancer patients. Our study addressed methodological weaknesses in the literature by restricting the sample to patients with one cancer type, adjusting for factors known to affect outcome, and following patients for a sufficient period of time.
Methods
We prospectively followed patients newly diagnosed with squamous cell oropharyngeal cancer from the start of radiation therapy until death or until date of last clinical visit. All patients were optimally treated with radiation and sometimes chemotherapy. After adjusting for tumor stage, treatment, comorbidities, smoking, excessive alcohol use, and demographic factors, we assessed the effects of baseline self-reported depression on overall survival and recurrence.
Results
One hundred thirty participants were followed for a median of 5 years. The average age was 56 years, and 83% were male. Eighteen participants died during the study and 15 experienced disease recurrence. Self-reported depression was associated with decreased overall survival duration (hazard ratio [HR] = 3.6; 95% confidence interval [CI] = 1.2-10.8), and disease recurrence (HR = 3.8; 95% CI = 1.2-12.2) in multivariate analysis. In addition, smoking was associated with disease recurrence.
Conclusion
Patients with oropharyngeal cancer may benefit from depression screening and evidence-based treatments, if appropriate. Future studies are needed to determine whether depression is an independent prognostic factor of outcome and to elucidate biobehavioral mechanisms involved in patients with oropharyngeal cancer.
Precis
This prospective study evaluated the impact of self-reported depression on outcomes of patients diagnosed with oropharyngeal cancer and treated with radiation (with or without chemotherapy). After following one hundred thirty participants for a median of 5 years, we found that patients' self-reported depression was associated with decreased overall survival duration and disease recurrence.
Keywords: Oropharynx, Depression, Survival, Recurrence
Background
Emerging evidence points to depression as an independent factor for survival.(1, 2) Two recent meta-analyses of prospective trials evaluating outcomes of patients with breast, lung, hepatobiliary cancers and leukemia reported that poorer outcomes occurred in patients who were also diagnosed with depression.(3, 4) Possible mechanisms underlying poorer outcomes in depressed cancer patients may include chronic activation of the hypothalamic-pituitary axis, which may weaken immune function by triggering a heightened inflammatory response, or noradrenergic-driven tumor angiogenesis.(5),(6-8) Another possible mechanism may be that depression is linked with poorer health behaviors such as decreased physical activity, tobacco and alcohol use.(9-12) However, the literature examining depression's effect on survival outcome in patients with cancer is marked with several methodological weaknesses. First, several studies did not adjust for known prognostic factors, such as tumor stage, histology, treatment, demographic factors, smoking, and comorbid disease at baseline.(3, 4) Second, some studies used convenience samples that included patients with various types of cancer and therefore prognostic profiles, which would confound any potential effects of depression on outcomes. Third, some studies followed patients for 1-3 years, which may not have been long enough to capture the natural progression of disease outcomes.4 A follow-up period of at least five years is preferable, as large cohort studies of oropharyngeal cancer patients have shown that rates of disease control stabilize by the fifth year.(13) Finally, few studies have investigated the effects of depression on tumor progression.4
We chose to study depression in oropharyngeal cancer patients because existing data show that they are more likely to experience high levels of distress compared to patients with other types of cancer.(14) Oropharyngeal cancer is diagnosed in nearly 10,000 Americans each year, and its age-adjusted incidence is rising by 5% annually owing to the increasing incidence of human papilloma virus (HPV)-related oropharyngeal squamous cell carcinomas.(15, 16) Oropharyngeal cancer affects twice as many men as women, and the average age at onset is in the mid-fifties. Despite the preponderance (85%) of cases diagnosed at stages III-IV, oropharyngeal cancer is highly curable, with overall 5-year survival rates ranging from 61% to 78%.(13, 17) Factors affecting survival include tumor stage at initial diagnosis, radiation dose and planning, use of chemotherapy, age, comorbid disease at diagnosis, smoking status, and HPV test results. Recurrence after initial treatment occurs in approximately 25% of patients within the first 2 years, but the recurrence rate varies depending on factors similar to those affecting survival.(18)
The goal of our study was to assess the effect of depression on overall survival, and disease recurrence in patients with oropharyngeal cancer. We sought to overcome some of the weaknesses of other studies evaluating depression in cancer patients by performing a prospective study in a homogenous population, adjusting for baseline tumor characteristics, cardiovascular comorbidity, smoking, excessive alcohol use, demographic factors, and cancer treatment, and following the patients for a sufficient time to capture nearly all outcome events.(13)
Methods
Patients
Following Institutional Review Board approval from the Office of Protocol Research at M. D. Anderson Cancer Center,, we recruited patients at The University of Texas M.D. Anderson Cancer Center (MDACC) who were had been diagnosed with nonrecurrent oropharyngeal cancer between March 2005 and June 2007. Patients were eligible if they a) had a primary diagnosis of squamous cell oropharyngeal cancer; b) had not begun radiation therapy; c) could read, write, and speak in English; and d) completed depression screening questionnaires at baseline. Patients were not eligible if they a) were diagnosed with recurrent oropharyngeal cancer, b) had distant metastasis at the time of diagnosis, or c) were currently being treated for other types of cancer. After providing informed consent, participants were enrolled onto the study 7-10 days prior to radiation treatment and assessed within 1-2 weeks for depressive symptoms. Depending on whether the patient had received neoadjuvant chemotherapy prior to radiation (which at MDACC lasted 3 cycles or 9 weeks), the start of radiation would begin as soon as 2-4 weeks post-referral to MDACC or as late as 3-4 months post-referral.
Design
Depression, smoking, excessive alcohol use, disease characteristics, and chemotherapy regimen were prospectively assessed at baseline entry into the study. As part of each participant's routine workup at the MDACC Head and Neck Center, information about comorbid diseases was prospectively assessed including cardiovascular comorbidities and factors affecting risks including blood pressure, cardiovascular family disease history, body mass index, diabetes mellitus, hyperlipidemia, history of myocardial infarction or stroke, and coronary artery disease. Participants were followed until time of death or last clinic visit..
Disease characteristics and demographics
Because most of the participants (92%) had late-stage (StageIII-IVb) disease, TNM stage was categorized into 3 levels: a) any T4 tumor, representing the highest risk for mortality; b) any N3/N2c tumor except those that were also T4 (meaning that bilateral or contralateral lymph nodes in the neck are affected (N2c) or that at least one lymph node is larger than 6 cm across (N3)), representing intermediate risk; or c) T < T4 and N < N3/N2c (referent category). Chemotherapy status was categorized into 3 levels: a) neoadjuvant induction and concurrent (highest risk), b) concurrent (intermediate risk), or c) induction chemotherapy only (no concurrent therapy) or no chemotherapy at all (referent category). Age was recorded at original diagnosis of oropharyngeal cancer and treated as a continuous variable in years.
HPV status
HPV tumor type was available for a subsample of 22 participants. HPV tumor typing was not routinely performed at MDACC until 2008, which was after the study period of our patients' initial cancer diagnosis (March 2005-June 2007).
Radiation therapy and chemotherapy
Disease management was individualized for each patient on the basis of the extent of disease. Overall strategies were decided between the patient and the treating physician, although all cases were reviewed in a multidisciplinary clinic prior to implementing final decisions. In general, the approach at MDACC during the years of this trial was to recommend neoadjuvant chemotherapy for patients with advanced regional disease, particularly in cases of multiple lymph node involvement or involvement of lymph nodes located in the lower neck. Various neoadjuvant regimens were used, but all were platin- and taxane-based. The use of concurrent chemotherapy during radiation was based on the extent of the primary tumor and was recommended for patients with tumors staged T3 or T4, and for patients with bulky disease but staged T2. Concurrent chemotherapy regimens varied but were most frequently single-agent cisplatin, carboplatin, or cetuximab.(13, 17) Radiation treatment planning was based on disease volume and location. Planned doses to gross disease (with margins) ranged from 66 to 72 Gy, and subclinical sites of disease considered at risk were prophylactically treated to 50 to 63 Gy.
Depressive symptomatology were measured using the Physicians Health Questionnaire (PHQ-9), which assesses the 9 Diagnostic and Statistical Manual IV (DSM-IV) criteria for major depressive episode.(19) PHQ-9 scores range from 0 to 27, and a cutoff score of 10 has a high positive predictive value for diagnosing major depression.(20-23) Depression was also measured with the Centers for Epidemiological Studies-Depression Scale (CES-D), which is a is a well-validated, widely-used 20-item self-report measure with possible scores ranging from 0 to 60. A cutpoint of 16 and above is considered to indicate clinically significant levels of depression. It has high internal consistency (alpha = .84 to 90) and moderate reliability (kappa =.51 to .70) Depressive symptomatology was treated as both a dichotomous variable (depressed if the PHQ-9 score was >9 or not depressed if the PHQ-9 score was ≤9) or as a continuous variable. Crohbach's alpha for our sample was 0.88.
Timing of depression assessment relative to diagnosis and treatment
All patients completed their depression assessment as they were beginning their radiation treatment. For patients who were dispositioned to neoadjuvant chemotherapy, radiation treatment started after the patient had completed 3 cycles of chemotherapy, with each chemotherapy administration occurring once every 3 weeks. Therefore patients who had neoadjuvant treatment completed their depression assessment 3-4 months after cancer diagnosis. For patients who were not dispositioned to neoadjuvant treatment, depression assessment was completed shortly after cancer diagnosis, between 2-4 weeks after being diagnosed. Crohbach's alpha for our sample was 0.88.
Baseline control risk factors
Smoking status was assessed using items from the Centers for Disease Control and Prevention Behavioral Risk Factor Surveillance Survey (BRFSS). Smoking status was categorized as a) current; b) recent, defined as having quit within the past 12 months; c) former, defined as having quit more than 12 months ago; or d) never, defined as having smoked fewer than 100 cigarettes within their lifetime. Excessive alcohol use was assessed using the Alcohol Use Disorders Identification Test (AUDIT); an AUDIT score ≥8 was considered an indicator of hazardous and harmful alcohol use. Number of comorbidities was treated as a continuous variable. Information about baseline comorbidities was retrospectively extracted from the online medical record: Diabetes mellitus was present if the online medical record system indicated a previously established clinical diagnosis, if the patient was taking diabetic medication, or if the patient's glucose level was recorded as ≥140 mg/dl on at least 2 occasions within a month of the cancer diagnosis. Hyperlipidemia was present if the patient's low-density lipoprotein level was recorded as ≥100 mg/dl on at least 1 occasion within a month of the cancer diagnosis or if the patient was taking medication for elevated cholesterol levels. Hypertension was present if the patient was undergoing antihypertensive therapy or if the patient's blood pressure was recorded as ≥140 mm/Hg systolic and ≥90 mm/Hg diastolic on at least 3 occasions within the first month after the cancer diagnosis. Tachycardia was present if the patient's baseline heartbeat was ≥100 beats per minute or if an abnormal echocardiogram had established tachycardia. Coronary artery disease was present if the patient exhibited coronary artery disease in baseline radiology reports, angiography documents, or positive stress tests within a month of the cancer diagnosis. Stroke and myocardial infarction were documented as any transcribed dictation from any service that confirmed their occurrence.
Overall survival duration was considered the time between the date of consent and either the date of the participant's death or date of the patient's last clinic visit.. Date of consent refers to the date on which newly diagnosed participants completed the study's informed consent documents. All patients consented to participate in the study before the beginning of radiation. The date of the participant's death was abstracted from the online Social Security death index and confirmed with the online medical record. All patients who were still alive at the end of follow-up were seen and assessed in clinic.
Time to recurrence was considered the time between the date of consent and the date of recurrence or the date of the patient's last clinic visit. Recurrence was defined as reappearance of oropharyngeal cancer in local, regional or distant metastatic sites, as confirmed by biopsy. Date of recurrence refers to the date of the biopsy. Participants who had a new primary were not counted as recurrent and were excluded from the time-to-recurrence analysis.
Statistical Analysis
For the initial univariate analyses, depression, demographic factors, tumor characteristics, treatment, smoking status, excessive alcohol use, and comorbidity variables were each analyzed to determine whether they were related to overall survival and recurrence. Log-rank tests were used for categorical variables and a Cox proportional hazards model was implemented for continuous variables. Kaplan-Meier survival curves were then plotted to visualize time to death and recurrence. Median overall survival durations, log-rank tests, and univariate proportional hazards models were also calculated.
A multivariate proportional hazards model was created to investigate the effects of potential risk factors on time to death and recurrence. The model was created containing all variables of interest. Then, backwards selection was run to select only the variables that were statistically significant at a level of p < 0.10 for inclusion in the final model. In the final model, all statistical tests for significance were conducted using a 2-sided α < 5%. Survival and recurrence proportional hazards were tested using time-dependent covariates in the model and with Schoenfeld and scaled Schoenfeld residuals. The overall goodness-of-fit model was evaluated using Cox-Snell residuals.
Results
One hundred thirty patients who met the eligibility criteria were enrolled in the study. Patients were followed for a median of 4.9 years with a minimum follow-up time of 0.1 years and a maximum of 6.0 years. All subjects were followed until death or last clinic visit. If the patient was alive at end of study completion they were censored as their date of last clinic visit. Patients who died but did not have a recurrence were censored at their time of death. A competing risk model was conducted to determine whether results changed when treating death as a competing event. It did not, so only time-to-recurrence results are presented. Three patients died without having recurred. Average age at entry into the study was 56 years, 92% were white, and 83% were male (Table 1). Nineteen patients (15%) scored higher than the PHQ-9 depression screening scale cutoff. More than 90% of patients were staged with III - IV disease, but only 32% had either T4 or N3/2c disease. One hundred twenty-seven patients (98%) received intensity-modulated radiation therapy, and 3 were treated with 3-D conformal therapy. The median dose was 70 Gy (66-72 Gy). Forty-seven patients (36%) received neoadjuvant chemotherapy and 51 (39%) received concurrent chemotherapy.
Table 1. Demographics (n = 130).
| Depression status | |||||||
|---|---|---|---|---|---|---|---|
| No (n = 111) | Yes (N = 19) | Total | |||||
| N | % | N | % | N | % | p-value | |
| Age, years | 0.14 | ||||||
| Mean (SD) | 56.8 (9.4) | 53.3 (10.2) | 56.3 (9.6) | ||||
| Min - Max | 30.7 - 78.5 | 28.4 - 69.1 | 28.4 - 79.5 | ||||
| Sex | 0.32 | ||||||
| Male | 94 | 84.7 | 14 | 73.7 | 108 | 83.1 | |
| Female | 17 | 15.3 | 5 | 26.3 | 22 | 16.9 | |
| Recurrence or new primary cancer during follow-up | 0.067 | ||||||
| None | 91 | 82.0 | 12 | 63.2 | 103 | 79.2 | |
| Recurred or progressed | 10 | 9.0 | 5 | 26.3 | 15 | 11.5 | |
| New Primary | 10 | 9.0 | 2 | 10.5 | 12 | 9.2 | |
| Death during follow-up | 0.14 | ||||||
| No | 98 | 88.3 | 14 | 73.7 | 112 | 86.2 | |
| Yes | 13 | 11.7 | 5 | 26.3 | 18 | 13.8 | |
| AJCC tumor stage | 0.36 | ||||||
| I-II | 10 | 9.1 | 0 | 0.0 | 10 | 7.8 | |
| III-IV | 100 | 90.9 | 19 | 100.0 | 119 | 92.2 | |
| Tumor size/nodal involvement | 0.93 | ||||||
| T < T4 and N < N3/N2c | 74 | 66.7 | 14 | 73.7 | 88 | 67.7 | |
| Any N3/N2c except T4 | 18 | 16.2 | 2 | 10.5 | 20 | 15.4 | |
| Any T4 | 19 | 17.1 | 3 | 15.8 | 22 | 16.9 | |
| Neoadjuvant chemotherapy | 0.80 | ||||||
| Did not Receive | 70 | 63.1 | 13 | 68.4 | 83 | 63.8 | |
| Received | 41 | 36.9 | 6 | 31.6 | 47 | 36.2 | |
| Concurrent chemotherapy | 0.80 | ||||||
| Did not Receive | 68 | 61.3 | 11 | 57.9 | 79 | 60.8 | |
| Received | 43 | 38.7 | 8 | 42.1 | 51 | 39.2 | |
| Prior history of cancer at baseline | >0.99 | ||||||
| No | 105 | 94.6 | 18 | 94.7 | 123 | 94.6 | |
| Yes | 6 | 5.4 | 1 | 5.3 | 7 | 5.4 | |
| Smoking history at baseline | 0.39 | ||||||
| Never | 45 | 40.5 | 6 | 31.6 | 51 | 39.2 | |
| Former | 43 | 38.7 | 8 | 42.1 | 51 | 39.2 | |
| Recent | 8 | 7.2 | 0 | 0.0 | 8 | 6.2 | |
| Current | 15 | 13.5 | 5 | 26.3 | 20 | 15.4 | |
| History of stroke at baseline | 0.47 | ||||||
| No | 108 | 97.3 | 18 | 94.7 | 126 | 96.9 | |
| Yes | 3 | 2.7 | 1 | 5.3 | 4 | 3.1 | |
| Excessive alcohol use | 0.47 | ||||||
| No | 108 | 97.3 | 18 | 94.7 | 126 | 96.9 | |
| Yes | 3 | 2.7 | 1 | 5.3 | 4 | 3.1 | |
| Comorbid hypertension at baseline | 0.31 | ||||||
| No | 67 | 61.5 | 9 | 47.4 | 76 | 59.4 | |
| Yes | 42 | 38.5 | 10 | 52.6 | 52 | 40.6 | |
| Comorbid diabetes at baseline | >0.99 | ||||||
| No | 95 | 88.8 | 17 | 89.5 | 112 | 88.9 | |
| Yes | 12 | 11.2 | 2 | 10.5 | 14 | 11.1 | |
| Comorbid hyperlipidemia at baseline | 0.27 | ||||||
| No | 74 | 69.2 | 15 | 83.3 | 89 | 71.2 | |
| Yes | 33 | 30.8 | 3 | 16.7 | 36 | 28.8 | |
| Prior history of myocardial infarction at baseline | 0.59 | ||||||
| No | 103 | 94.5 | 19 | 100.0 | 122 | 95.3 | |
| Yes | 6 | 5.5 | 0 | 0.0 | 6 | 4.7 | |
| Comorbid coronary artery disease at baseline | 0.30 | ||||||
| No | 90 | 83.3 | 18 | 94.7 | 108 | 85.0 | |
| Yes | 18 | 16.7 | 1 | 5.3 | 19 | 15.0 | |
| Comorbid tachycardia at baseline | >0.99 | ||||||
| No | 103 | 95.4 | 18 | 94.7 | 121 | 95.3 | |
| Yes | 5 | 4.6 | 1 | 5.3 | 6 | 4.7 | |
| Number of comorbidities at baseline | 0.82 | ||||||
| 0 | 39 | 36.4 | 8 | 44.4 | 47 | 37.6 | |
| 1-3 | 63 | 58.9 | 10 | 55.6 | 73 | 58.4 | |
| >=4 | 5 | 4.7 | 0 | 0.0 | 5 | 4.0 | |
HR, hazard ratio; UB, upper bound; LB, lower bound. Boldface indicates statistical significance.
Survival
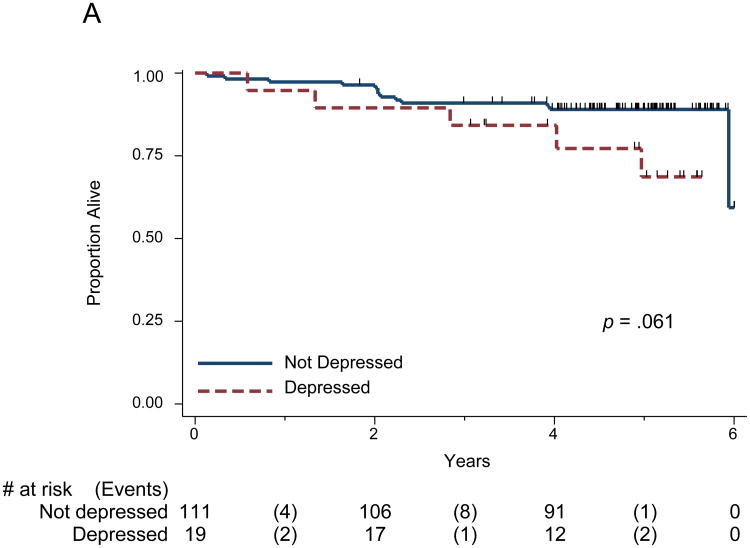
The median follow-up time for all patients was 5 years; 112 patients (86%) were alive at the end of the study period and were censored for all survival time analyses. The remaining 18 patients (14%), died either from their cancer, or treatment (or complications thereof). In addition, one patient died from Alzheimer's disease one year after radiation had ended. In the univariate analysis of PHQ-9 depression and overall survival, depression's association with survival was borderline (log rank p = 0.061, Figure 1A) and significant in the full multivariate analysis (p= 0.022, Table 2). None of the other factors (chemotherapy, tumor stage, age, sex, smoking, excessive alcohol use, and number of comorbidities) were significant in the full multivariate analysis. In the reduced multivariate model, PHQ-9 depression, dichotomized using the cutoff of 10, was significantly associated with overall survival (hazard ratio [HR] = 3.6; 95% confidence interval [CI] = 1.2-10.8, p = 0.022). The number of comorbidities at baseline approached significance in the reduced multivariate model (Table 2). When PHQ-9 depression was entered into the multivariate models as a continuous variable, depression was again significantly associated with overall survival: for every unit increase of the PHQ-9, participants' risk for reduced survival was increased by a factor of 1.1, or 10% (Table 3). Depression measured with the CES-D, either dichotomized using the conventional cutoff of 16 or as a continuous variable, resulted in nonsignificant risks for mortality (HR=2.5; 95% CI = 0.9-7.6, p=.10) see Tables S1 and S2, Supplemental Digital Content 1).
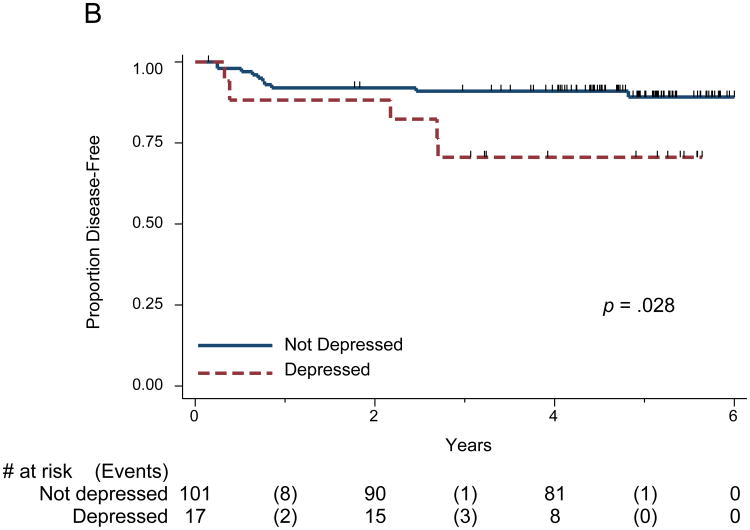
Figure 1.
(A) Kaplan Meier Survival Curve for Overall Survival and PHQ-9 Depression, categorical; (B) Survival Curve for Time to Recurrence and PHQ-9 Depression, categorical.
The survival graphs indicate the number of participants under observation at years 0, 2, 4, and 6, as well as the number of participants who experienced events during those intervals. The tick marks represent those patients censored at last clinic visit.
Table 2. Cox Proportional Hazards Model for Overall Survival Duration and PHQ-9 Depression (Categorical).
| Full model | Reduced model | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Effect | HR | 95% LB | 95% UB | p value | HR | 95% LB | 95% UB | p value |
| Age | 1.0 | 0.94 | 1.07 | 0.9 | ||||
| Female | 0.8 | 0.14 | 4.14 | 0.8 | ||||
| Chemotherapy (referent: none/neoadjuvant) | ||||||||
| Concurrent only | 1.5 | 0.34 | 6.29 | 0.6 | ||||
| Neoadjuvant and concurrent | 1.4 | 0.27 | 6.99 | 0.7 | ||||
| Excessive alcohol use | 3.5 | 0.36 | 34.67 | 0.3 | ||||
| Smoking status (referent: never) | ||||||||
| Former | 1.4 | 0.36 | 5.56 | 0.6 | ||||
| Recent | 4.3 | 0.37 | 49.75 | 0.2 | ||||
| Current | 3.1 | 0.73 | 13.18 | 0.1 | ||||
| Tumor size (referent: not T4/N3/N2c) | ||||||||
| Any N3/N2c | 2.4 | 0.52 | 10.65 | 0.3 | ||||
| Any T4 | 1.4 | 0.31 | 6.30 | 0.7 | ||||
| Total comorbidities | 1.3 | 0.78 | 2.10 | 0.3 | 1.4 | 0.98 | 2.02 | 0.062 |
| PHQ-9 Depression (Categorical) | 3.5 | 1.01 | 12.30 | 0.049 | 3.6 | 1.21 | 10.82 | 0.022 |
HR, hazard ratio; UB, upper bound; LB, lower bound. Boldface indicates statistical significance.
Table 3. Cox Proportional Hazards Model for Overall Survival Duration and PHQ-9 Depression (Continuous).
| Effect | Full model | Reduced model | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| HR | 95% LB | 95% UB | p value | HR | 95% LB | 95% UB | p value | |
| Age | 1.0 | 0.9 | 1.1 | 0.9 | ||||
| Female | 1.1 | 0.2 | 5.9 | 0.9 | ||||
| Chemotherapy (referent: none/neoadjuvant) | ||||||||
| Concurrent only | 1.2 | 0.3 | 5.9 | 0.8 | ||||
| Neoadjuvant and concurrent | 1.1 | 0.2 | 5.9 | 0.9 | ||||
| Excessive alcohol use | 3.9 | 0.4 | 37.5 | 0.2 | ||||
| Smoking status (referent: never) | ||||||||
| Former | 1.9 | 0.5 | 8.3 | 0.4 | ||||
| Recent | 5.3 | 0.5 | 63.2 | 0.2 | ||||
| Current | 3.9 | 0.8 | 18.5 | 0.1 | ||||
| Tumor size (referent: not T4/N3/N2c) | ||||||||
| Any N3/N2c | 2.6 | 0.6 | 12.3 | 0.2 | ||||
| Any T4 | 1.8 | 0.4 | 9.5 | 0.5 | ||||
| Total comorbidities | 1.3 | 0.8 | 2.1 | 0.3 | 1.4 | 1.0 | 2.0 | 0.070 |
| PHQ-9 Depression (continuous) | 1.1 | 1.0 | 1.2 | 0.040 | 1.1 | 1.0 | 1.2 | 0.010 |
HR, hazard ratio; UB, upper bound; LB, lower bound. Boldface indicates statistical significance.
Recurrence
Fifteen patients (11.5%) had disease recurrence during follow-up. Two of the 15 patients did not die. Depression (log rank p = 0.028 was associated with disease recurrence in the univariate analysis (Figure 1B) as well as in multivariate analysis (p = 0.025 Table 4). Smoking status was significantly associated with time to recurrence in the multivariate full model (p = 0.026 Table 4). In the reduced multivariate model, depression (HR = 3.8; 95% CI = 1.2-12.2) and smoking status (HR = 0.3; 95% CI = 0.08-0.9) were significant. When the reduced multivariate model was conducted using depression as a continuous variable instead of a dichotomous variable, depression remained significantly associated with increased risk for recurrence: for every unit increase of the PHQ-9, the risk for recurrence was increased by a factor of 1.1, or 10%. Smoking status also remained significant (Table 5). Proportional hazards were not violated in our overall survival or time to recurrence models. Depression measured with the CES-D, either dichotomized using the conventional cutoff of 16 or as a continuous variable, was associated with significant risk for recurrence (HR=3.7; 95% CI =1.2-11.9, p=.0025; see Tables S3 and S4, Supplemental Digital Content 1).
Table 4. Cox Proportional Hazards Model for Time to Recurrence and PHQ-9 Depression (Categorical).
| Effect | Full model | Reduced model | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| HR | 95% LB | 95% UB | p value | HR | 95% LB | 95% UB | p value | |
| Age | 1.0 | 0.97 | 1.1 | 0.2 | ||||
| Female | 0.4 | 0.05 | 3.7 | 0.5 | ||||
| Chemotherapy (referent: none/neoadjuvant) | ||||||||
| Concurrent only | 1.3 | 0.27 | 5.9 | 0.8 | ||||
| Neoadjuvant and concurrent | 0.4 | 0.04 | 3.9 | 0.4 | ||||
| Excessive alcohol use | 2.8 | 0.26 | 30.5 | 0.4 | ||||
| Total comorbidities | 0.9 | 0.44 | 1.6 | 0.6 | ||||
| Tumor size (referent: not T4/N3/N2c) | ||||||||
| Any N3/N2c | 2.9 | 0.67 | 12.5 | 0.2 | ||||
| Any T4 | 1.5 | 0.24 | 8.93 | 0.7 | ||||
| Never/former/recent smoker | ||||||||
| (referent: current smoker) | 0.3 | 0.06 | 1.03 | 0.55 | 0.27 | 0.08 | 0.86 | 0.026 |
| PHQ-9 Depression (categorical) | 5.0 | 1.39 | 17.7 | 0.014 | 3.8 | 1.2 | 12.2 | 0.025 |
HR, hazard ratio; UB, upper bound; LB, lower bound. Boldface indicates statistical significance.
Table 5. Cox Proportional Hazards Model for Time to Recurrence and PHQ-9 Depression (Continuous).
| Effect | Full model | Reduced model | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| HR | 95% LB | 95% UB | p value | HR | 95% LB | 95% UB | p value | |
| Age | 1.0 | 1.0 | 1.1 | 0.3 | ||||
| Female | 0.5 | 0.1 | 4.0 | 0.5 | ||||
| Chemotherapy (referent: none/neoadjuvant) | ||||||||
| Concurrent only | 0.9 | 0.2 | 4.9 | 0.9 | ||||
| Neoadjuvant and concurrent | 0.3 | 0.0 | 2.8 | 0.3 | ||||
| Excessive alcohol use | 3.4 | 0.3 | 33.4 | 0.3 | ||||
| Total comorbidities | 0.8 | 0.4 | 1.6 | 0.6 | ||||
| Tumor size (referent: not T4/N3/N2c) | ||||||||
| Any N3/N2c | 2.9 | 0.7 | 12.9 | 0.2 | ||||
| Any T4 | 1.7 | 0.3 | 11.2 | 0.6 | ||||
| Never/former/recent smoker | ||||||||
| (referent: current smoker) | 0.2 | 0.1 | 0.9 | 0.032 | 0.3 | 0.1 | 0.9 | 0.036 |
| PHQ-9 Depression (continuous) | 1.1 | 1.0 | 1.2 | 0.017 | 1.1 | 1.0 | 1.2 | 0.038 |
HR, hazard ratio; UB, upper bound; LB, lower bound. Boldface indicates statistical significance.
HPV status
HPV tumor type was available for 22 participants: 15 had HPV-related tumor test results and 7 had HPV-unrelated results. We did not detect any differences between the 2 groups (HPV-related vs HPV-unrelated) in terms of baseline tumor stage, T category, demographic factors, smoking, or depression.
Timing of depression assessment relative to diagnosis and treatment
The time between documented diagnosis of oropharynx cancer and depression assessment ranged from 6 days to 473 days (M=53.7 days, SD=60.7, Median= 37.5 days). The time between oropharynx cancer diagnosis and time of radiation treatment ranged from 14 days to 483 days (M =65.2 days, SD=60.4; Median= 49 days). One participant was an outlier, waiting more than one year from the time of diagnosis to begin radiation treatment in order to pursue alternative cancer therapies, consequently he was enrolled onto study 473 days post-diagnosis and started treatment 10 days later.
The distribution of the number of days from time of cancer diagnosis to time of depression assessment was assessed to determine whether timing of depression assessment affected study outcomes. There was no difference in prevalence of depression between those who were assessed within 4 weeks of diagnosis versus those who were assessed more than 4 weeks post-diagnosis (p=.613). Nor was there a difference between those who were assessed within 6 weeks of diagnosis versus those were assessed more than 6 weeks post-diagnosis (p=.319). Finally, we examined time to death & time to relapse/progression using only those subjects who were assessed more than 6-weeks post-diagnosis. Excessive alcohol use was excluded from these models because only two subjects who were assessed more than 6-weeks post-diagnosis excessively used alcohol and neither of them died. Otherwise, the model assessments were the same. Depression continued to negatively affect time to death & time to relapse/progression.
Discussion
The key finding of this study is that depression was independently associated with overall survival and disease recurrence in non-recurrent, late-stage oropharyngeal cancer patients after adjusting for tumor stage, chemotherapy, number of baseline comorbid diseases, age, sex, baseline smoking status, and baseline excessive alcohol use. It should be stressed that our results are preliminary, based on a small number of events and should therefore be interpreted with caution.
In our study, tumor burden and treatment variables were not significantly related to survival. This may have been due to treatment intensification for patients with relatively larger tumor size and/or greater nodal involvement. In addition, many of our patients were suspected to have had HPV-associated disease, due to the high preponderance of non-smokers (39% were never-smokers and 39% former) and of non-drinkers (43% reported zero drinks in the past week). This is significant because HPV-associated disease has been shown to have high control rates.(18) Finally, analysis of the distribution of timing of depression assessment in relation to cancer diagnosis did not reveal significant differences in the prevalence of depression whether depression was measured within 4 weeks of diagnosis or after 4 weeks of diagnosis (similar findings were found when comparing the distribution of depressed patients within and after 6 weeks of diagnosis). Therefore we feel the potential impact of misclassification is minimized because the prevalence of depression is similar regardless of the timing of depression assessment in relation to cancer diagnosis.
While we did not measure inflammatory cytokine levels, it is unlikely that high levels of circulating inflammatory cytokines drove depression's association with poorer outcome in our study of oropharyngeal cancer patients. Past research with breast, ovarian and pancreatic cancer have shown significant relationships between higher levels of cytokines, depression and poorer outcomes, however these cancers are relatively bulky and frequently uncontrolled compared to oropharyngeal cancer. (1, 2) Oropharyngeal tumors are very small and highly chemosensitive and therefore unlikely to emit levels of cytokines into the periphery. Accordingly, we did not find that depression was related to disease stage (p=.36), tumor size or nodal involvement (p=.93; Table 1).
Other potential mechanisms to explore in future studies may be flattened diurnal cortisol expression or lower total cortisol concentration which have been linked to decreased survival in breast, lung, renal cell and ovarian cancer patients, and has also been strongly linked to chronic major depression in non-cancer patient populations.(24-27) Sustained, excessive activation of the hypothalamic-pituitary-adrenal (HPA) axis is linked with major depression, as evidenced by hypersecretion of corticotropin-releasing factor (CRF) and elevated glucocorticoid levels. (28) Interestingly, CRF-driven markers of prolonged sympathetic activity, such as elevated resting heart rate, have been shown to be prognostic for survival in metastatic breast cancer (29) and in ovarian cancer (HR=1.02 for every HR unit increase (CI=1.01-1.04).(30)
Compared to other studies which examined whether depression was significantly related to survival in cancer, our results showed higher adjusted HRs for depression related to outcome (HRs ranged from 3.6 to 3.8) than recent studies showing independent effects of chronic stress and depression on mortality after adjustment for tumor characteristics and treatment in breast, lung, and hepatobiliary cancers and leukemia (HRs ranging from 1.07-1.33).(3, 4) Our results also showed higher adjusted HRs for depression than those found in three other prospective studies examining distress in heterogeneous samples of head and neck cancer. Patients with oral cavity, pharyngeal or laryngeal cancer who also had high scores on the Life Orientation Test pessimism subscale had an increased odds ratio of 1.12 for mortality after adjusting for age, disease stage, and cohabitation.(31) de Graeff et al. followed 208 patients for 45 months and found that distress was not related to survival nor recurrence after adjusting for sociodemographic factors, smoking, disease stage, disease site, type of cancer treatment, and functional status. However, this may have been due to the fact that they used a convenience sample of patients with different types of head and neck cancer (oral cavity, laryngeal, hypopharyngeal or oropharyngeal cancer).(32) This is significant because tumors of each site have a unique natural history and prognostic profile. For example, patients with hypopharyngeal cancer have worse prognoses than patients with oropharyngeal cancer.(33) In addition, while oropharyngeal cancer has been historically classified as a head and neck cancer, it is somewhat distinct from the other types of head and neck cancer in that the recent epidemic of HPV infection has pointed to distinct epidemiologic profile wherein patients with oropharyngeal cancer are younger, have higher SES, and tend to be non-smokers.(15, 18) That depression was related to both mortality and recurrence in our study was not surprising, as the separate analyses included nearly the same patients (all but two of the patients who recurred also died). De Boer et al. followed 133 patients with laryngeal, oral cavity, oropharynx or hypopharynx cancer for six years and found no effect for distress after adjusting for sociodemographic variables, smoking, alcohol use, and for disease and tumor treatment variables. However, this sample included a sizable proportion (n=57) of participants who already had recurrent disease at the beginning of the study.(34) Possible reasons for the higher HRs in our study are the restriction of our sample to newly-diagnosed oropharyngeal cancer, adjustment for multiple factors known to affect survival, sufficient length of follow-up and the use of a rigorous screening tool, the PHQ-9, to measure depression.
Tumor and disease variables were not significantly related to survival. This may have been due to treatment intensification for patients with larger tumor size and/or nodal involvement, or that many of our patients were suspected to have had HPV-associated disease, due to the high preponderance of non-smokers (39% were never-smokers and 39% former) and of non-drinkers (43% reported zero drinks in the past week). HPV-associated disease has been shown to have high control rates.(18)
Limitations
This study was performed upon a relatively small study population; therefore the results of this study are not necessarily generalizable to a larger population. There were few deaths and recurrences observed in this study (n = 18 and n = 15, respectively). Additionally, only 19 subjects met the PHQ-9 cutoff for depression, and of these only 5 died. It is possible that the small cell size inflated our test-statistic, biasing results towards the alternative hypothesis of association. The low number of events did result in wide confidence intervals, which limited the degree of certainty in determining the strength of depression's impact on recurrence and survival. While the sample size for our study was small, the depression's association with survival and recurrence was strong enough that an effect was still detected.
In addition, because we did not measure depression continually throughout treatment and follow-up, we are limited in our ability to draw inferences as to the mechanism of baseline depression on subsequent outcomes of survival and recurrence 5 years later. We were not able to test for an interaction effect between depression and smoking on overall survival, since there were too few participants who identified as current smokers and scored above the PH-9 cutoff. Therefore, future studies should be powered to measure possible interactions between smoking and depression. Finally, survivor bias was possible in that depression level was assessed after the cancer diagnosis. Even so, the bias was minimal because the mean difference between consent date and diagnosis date was 1.8 months.
Conclusion
Our study is unique in that our sample was narrowly restricted to a precise type of head and neck cancer which was treated in a uniform manner, adjusted for smoking status with a psychometrically-validated measure, and was followed for a sufficient period of time to detect outcome events. Provided that future studies confirm our findings, the results of our study indicate that future screening and appropriate follow-up for depression in newly-diagnosed oropharyngeal cancer patients may be beneficial. For example, self-administered depression screening tools administered at the beginning of treatment could identify patients who are in need of referral for further assessment of depressive disorders by psychiatric or social work services. Future research is needed to confirm these results in a separate sample, especially to determine whether treatment for depression ameliorates the apparently harmful results of depression that were found in our sample.
Supplementary Material
Acknowledgments
We would like to thank E. Neely Atkinson, Ph.D. for his consultation on this study.
Funding sources: This study was supported by the following grants: NCI R03 CA108358, NCI P30 CA016672, NCI K07 CA 093512, NCI R25 CA056452 and NIDCR R01 DE019141.
Abbreviations
- HPV
Human Papilloma Virus
- MDACC
M.D. Anderson Cancer Center
- TNM
Tumor size, Nodal involvement, Metastasis Staging
- Gy
Gray (1 Joule/kg)
- PHQ-9
Physicians Health Questionnaire
- DSM-IV
Diagnostic and Statistical Manual IV
- CES-D
Centers for Epidemiological Studies-Depression Scale
- BRFSS
Behavioral Risk Factor Surveillance Survey
- AUDIT
Alcohol Use Disorders Identification Test
- HR
Hazard ratio
- CI
Confidence interval
- SES
Socioeconomic Status
Footnotes
Financial Disclosure: None of the authors have any financial disclosures nor conflicts of interest to report.
References
- 1.Armaiz-Pena G, Lutgendorf S, Cole S, Sood A. Neuroendocrine modulation of cancer progression. Brain, Behavior and Immunity. 2009;23:10–15. doi: 10.1016/j.bbi.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lutgendorf S, Sood AK, Antoni M. Host factors and cancer progression: Biobehavioral signaling pathways and interventions. Journal of Clinical Oncology. 2010;28:4094–4099. doi: 10.1200/JCO.2009.26.9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chida Y, Hamer M, Wardle J, Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nature Reviews Clinical Oncology. 2008;5:466–475. doi: 10.1038/ncponc1134. [DOI] [PubMed] [Google Scholar]
- 4.Satin J, Linden W, Phillips M. Depression as a predictor of disease progression and mortality in cancer patients: a meta-analysis. Cancer. 2009;115:5349–5361. doi: 10.1002/cncr.24561. [DOI] [PubMed] [Google Scholar]
- 5.Antoni M, Lutgendorf S, Cole S, Dhabhar F, Sephton S. The influence of bio-behavioral factors on tumour biology: pathways and mechanisms. Nature Reviews Cancer. 2006;6:240–248. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McEwen BS. Protective and damaging effects of stress mediators. NEJM. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 7.McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. European Journal of Pharmacology. 2008;174:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McEwen BS, Lasley E. Allostatic load: When protection gives way to damage. Advances. 2003;19:28–33. [PubMed] [Google Scholar]
- 9.Anda R, Williamson D, Escobedo L, Mast E, Giovino G, Remington P. Depression and the dynamics of smoking: a national perspective. JAMA. 1990;264:1541–1545. [PubMed] [Google Scholar]
- 10.Farmer M, Locke B, Moscicki E, Dannenberg A, Larson D, Radloff LS. Physical activity and depressive symptoms: the NHANES I epidemiologic follow-up study. American Journal of Epidemiology. 1988;128:1340–1351. doi: 10.1093/oxfordjournals.aje.a115087. [DOI] [PubMed] [Google Scholar]
- 11.Steptoe A, Wardle J, Fuller R, Holte A, Justo J, Sanderman R, Wichstrom L. Leisure-time physical exercise: prevalence, attitudinal correlates, and behavioral correlates among young europeans from 21 countries. Preventive Medicine. 1997;26:845–854. doi: 10.1006/pmed.1997.0224. [DOI] [PubMed] [Google Scholar]
- 12.Grant BF, Harford TC. Comorbidity between DSM-IV alcohol use disorders and major depression: results of a national survey. Drug and Alcohol Dependence. 1995;39:197–206. doi: 10.1016/0376-8716(95)01160-4. [DOI] [PubMed] [Google Scholar]
- 13.Garden A, Kies M, Morrison W, Weber R, Frank S, Glisson B, Gunn B, Beadle B, Ang K, Rosenthal D, Sturgis E. Outcomes and patterns of care of patients with locally advanced oropharyngeal carcinoma treated in the early 21st century. Radiation Oncology. 2013;8:21–31. doi: 10.1186/1748-717X-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zabora J, Brintzenhofeszoc K, Curbow B, Hooker C, Piantadosi S. The prevalence of psychological distress by cancer site. Psycho-Oncology. 2001;10:19–28. doi: 10.1002/1099-1611(200101/02)10:1<19::aid-pon501>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 15.Chaturvedi A, Engels E, Anderson W, ML G. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. Journal of Clinical Oncology. 2008;26:612–619. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 16.Sturgis E, Ang KK. The epidemic of hpv-associated oropharyngeal cancer is here: is it time to change our treatment paradigms? Journal of the National Comprehensive Cancer Network. 2011;9:665–673. doi: 10.6004/jnccn.2011.0055. [DOI] [PubMed] [Google Scholar]
- 17.Howlader N, Noone A, Krapcho M, Neyman N, Aminou R, Alterkruse S, Kosary C, Ruhl J, Tatlovich Z, Cho H, Mariotto A, Eisner M, Lewis D, Chen H, Feuer E, Cronin Ke. SEER Cancer Statistics Review, 1975-2009. N. C. Institute (ed); Bethesda, MD: 2011. [Google Scholar]
- 18.Ang KK, Harris J, Wheeler R, Weber RS, Rosenthal D, Nguyen-Tan P, Westra W, chung C, Jordan R, Lu C, Kim H, Axelrod R, Silverman C, Redmond K, Gillison M. Human papillomavirus and survival of patients with oropharyngeal cancer. New England Journal of Medicine. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spitzer RL, Kroenke K, Williams JB group tPHQPs. Validation and utility of a self-report version of PRIME-MD: The PHQ Primary Care Study. Journal of the American Medical Association. 1999;282:1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 20.Rost K, Duan N, Rubenstein L, Ford D, Sherbourne C, Meredith L, Wells K. The Quality Improvement for Depression collaboration: General analytic strategies for a coordinated study of quality improvement in depression care. General Hospital Psychiatry. 2001;23:239–253. doi: 10.1016/s0163-8343(01)00157-8. [DOI] [PubMed] [Google Scholar]
- 21.Rost K, Nutting P, Smith J, Werner J, Duan N. Improving depression outcomes in community primary care practice. Journal of General Internal Medicine. 2001;16:143–149. doi: 10.1111/j.1525-1497.2001.00537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fechner-Bates S, Coyne J, Schwenk T. The relationship of self-reported distress to depressive disorders and other psychopathology. Journal of Consulting and Clinical Psychology. 1994;62:550–559. doi: 10.1037//0022-006x.62.3.550. [DOI] [PubMed] [Google Scholar]
- 23.Gilbody S, House A, Sheldon T. Routinely administered questionnaires for depression and anxiety: a systematic review. BMJ. 2001;322:406–409. doi: 10.1136/bmj.322.7283.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Irwin M, Olmstead R, CGanz P, Haque R. Sleep disturbance, inflammation and depression risk in cancer survivors. Brain, Behavior and Immunity. 2013;30:S58–S67. doi: 10.1016/j.bbi.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sephton S, Lush E, Dedert E, Floyd A, Rebholz W, Dhabhar F, Spiegel D, Salmon P. Diurnal cortisol rhythm as a predictor of lung cancer survival. Brain, Behavior and Immunity. 2013;30:S163–170. doi: 10.1016/j.bbi.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 26.Schrepf A, Thaker P, Goodheart M, Bender D, Slavich G, Dahmoush L, Penedo F, DeGeest K, Mendez L, Lubaroff D, Cole S, Sood A, Lutgendorf S. Diurnal cortisol and survival in epithelial ovarian cancer. Psychoneuroendocrinology. 2015;53:256–267. doi: 10.1016/j.psyneuen.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu S, Yang H, Thayer J, Andersen B. Assocition of the physiological stress response with depressive symptoms in patients with breast cancer. Psychosomatic Medicine. 2014;76:252–256. doi: 10.1097/PSY.0000000000000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 29.Giese-Davis J, Wilhelm FH, Tamagawa R, Palesh O, Neri E, Taylor CB, Kraemer HC, Spiegel D. Higher Vagal Activity as Related to Survival in Patients With Advanced Breast Cancer: An Analysis of Autonomic Dysregulation. Psychosomatic Medicine. 2015;77:346–355. doi: 10.1097/PSY.0000000000000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shinn EH, Lenihan DJ, Urbauer DL, Basen-Engquist KM, Valentine A, Palmero L, Woods ML, Patel P, Nick AM, Shahzad MM, Stone RL, Golden A, Atkinson E, Lutgendorf SK, Sood AK. Impact of cardiovascular comorbidity on ovarian cancer mortality. Cancer Epidemiol Biomarkers Prev. 2013;22:2102–2109. doi: 10.1158/1055-9965.EPI-13-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allison P, Guichard C, Fung K, Gilain L. Dispositional optimism predicts survival status 1 year after diagnosis in head and neck cancer patients. Journal of Clinical Oncology. 2003;21:543–548. doi: 10.1200/JCO.2003.10.092. [DOI] [PubMed] [Google Scholar]
- 32.de Graeff A, de Leeuw J, Ros W, Hordijk G, Blijham G, Winnubst J. Sociodemographic factors and quality of life as prognostic indicators in head and neck cancer. European Journal of Cancer. 2001;37:332–339. doi: 10.1016/s0959-8049(00)00385-3. [DOI] [PubMed] [Google Scholar]
- 33.Society AC. Facts & Figures. 2013;2012 [Google Scholar]
- 34.De Boer M, Van den Borne B, Pruyn J, Ryckman R, Volovics L, Knegt P, Meeuwis CA, Mesters I, Verwoerd C. Psychosocial and physical correlates of survival and recurrence in patients with head and neck carcinoma. Cancer. 1998;83:2567–2579. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.