Abstract
Objectives
Information concerning physical growth among small-scale populations remains limited, yet such data are critical to local health efforts and to foster basic understandings of human life history and variation in childhood development. Using a large dataset and robust modeling methods, this study aims to describe growth from birth to adulthood among the indigenous Shuar of Amazonian Ecuador.
Methods
Mixed-longitudinal measures of height, weight, and BMI were collected from Shuar participants (n = 2,463; age 0–29 years). Centile growth curves and tables were created for each anthropometric variable of interest using GAMLSS. Pseudo-velocity and LMS curves were generated to further investigate Shuar patterns of growth and to facilitate comparison with U.S. CDC and multinational WHO growth references.
Results
The Shuar are small throughout life and exhibit complex patterns of growth that differ substantially from those of international references. Similar to other Amazonians, Shuar growth in weight compares more favorably to references than growth in height, resulting in BMI curves that approximate international medians. Several additional characteristics of Shuar development are noteworthy, including large observed variation in body size early in life, significant infant growth faltering, extended male growth into adulthood, and a markedly early female pubertal growth spurt in height. Phenotypic plasticity and genetic selection in response to local environmental factors may explain many of these patterns.
Conclusions
Providing a detailed reference of growth for the Shuar and other Amazonian populations, this study possesses direct clinical application and affords valuable insight into childhood health and the ecology of human growth.
Keywords: Shuar, growth and development, life history, human biological variation, Amazonia
INTRODUCTION
Understanding variation in human growth and development has long been a primary objective for the fields of human biology and public health (Tanner, 1981; WHO, 1995). Although operating under occasionally conflicting research paradigms (Schell and Magnus, 2007), both fields continue to emphasize the importance of obtaining descriptions of growth from diverse human populations to realize existing research goals (Eveleth and Tanner, 1990; Stinson, 2012). Despite this agreement, small-scale indigenous populations, comprising a significant portion of human genetic and cultural diversity (Henn et al., 2011; Karafet et al., 2002; Kent, 1996; Wang et al., 2007), remain greatly underrepresented in the literature on childhood growth. The little data available from these groups are notable, however, in that they suggest large variation in body size between populations and patterns of growth and development that frequently differ from those of Western children (Eveleth and Tanner, 1990; Ulijaszek, 1995; Walker et al., 2006). This observation underscores the diversity and complexity of physical development in challenging environments (Cameron, 2007) and highlights the central importance of obtaining new descriptions of growth among small-scale populations to better understand human biological variation, phenotypic plasticity, and health.
In addition to their utility to researchers, population-level descriptions of growth may also serve as important tools in the clinical evaluation of children. Descriptions of growth that incorporate centile distributions are particularly valuable to clinicians as references of expected growth (De Onis et al., 2004). While international references such as those of the World Health Organization (WHO) (De Onis, 2007; WHO, 2006) and the United States Center for Disease Control and Prevention (CDC) (Kuczmarski et al., 2002) are critical for comparison of growth between regions or countries, population-specific references more accurately describe the physical development of local children. Indeed, recently created growth references for a variety of groups demonstrate that international references may not be appropriate for the clinical assessment of growth in many populations, particularly those of non-Western descent (Gleiss et al., 2013; Guedes et al., 2010; Hakeem et al., 2004; Hasan et al., 2001; Marwaha et al., 2006; Mushtaq et al., 2012; Neyzi et al., 2006). This finding, along with a strong preference for population-specific growth charts by most clinicians and parents (Albertsson-Wikland et al., 2002; De Onis et al., 2004), illustrates the present demand for local descriptions of growth throughout much of the developing world.
This article attempts to address the needs of researchers and local South American clinicians by providing a detailed description of physical growth from birth to adulthood among the indigenous Shuar of southeastern Ecuador. Previous research has demonstrated that the Shuar, who inhabit a nutritionally and pathogenically challenging Amazonian environment, experience high rates of stunting, moderate rates of underweight, and low rates of wasting during childhood (Blackwell et al., 2009). However, the growth patterns underlying these observations have never been fully modeled or described and their clinical significance remains unclear. Taking advantage of a newly-compiled and large mixed-longitudinal dataset, this study develops centile growth references for Shuar height, weight, and BMI using popular GAMLSS (Generalized Additive Models for Location, Scale, and Shape) methods (Rigby and Stasinopoulos, 2005). LMS (Lambda-Mu-Sigma) curves (Cole and Green, 1992) and pseudo-velocity curves for each anthropometric measure are further generated from GAMLSS model parameters to facilitate direct comparison of Shuar growth with CDC and WHO references.
METHODS
Study population
The Shuar are a natural fertility indigenous population of ≈ 40,000–110,000 individuals (CODENPE, 2012; Rubenstein, 2001) inhabiting the neotropical lowland Amazonas region of southeastern Ecuador. Similar to other members of the Jivaroan language group, the Shuar have traditionally lived in small, scattered households and have practiced a mixed subsistence pattern of horticulture, foraging, hunting, and fishing (Harner, 1984; Karsten, 1935; Rubenstein, 2001; Stirling, 1938). Regular contact between the Shuar and outsiders began with Christian missionization during the 1940s, leading to the gradual formation of centralized villages and the creation of the Federación Interprovincial de Centros Shuar (FICSH) in 1964. Currently, the Shuar reside predominantly in 668 communities dispersed across ≈ 900,000 hectares of land (CODENPE, 2012). The last decade has seen acceleration in the development of modern infrastructure in Shuar territory, leading to varying degrees of integration into the local, regional, and national economy (Liebert et al., 2013; Lu, 2007). Most development has been limited to the Upano Valley where many Shuar communities are now connected by road to larger towns, have electricity, and sell goods at market or occasionally participate in wage labor. Other communities, particularly those east of the Cutucú mountain range (cross-Cutucú), are only beginning to experience integration into the market economy and continue to practice a largely traditional way of life.
Despite recent development in Shuar territory, most Shuar continue to consume a generally low-quality diet, with increasingly diminished returns from foraging, hunting, and fishing (Descola and Lloyd, 1996; Harner, 1984; Liebert et al., 2013). Overall, garden foods (including plantains, sweet manioc, bananas, papaya, and sweet potato) are estimated to provide ≈ 65% of the total number of calories consumed by the Shuar (Liebert et al., 2013). This bulky dietary base is commonly supplemented by fish, palm grubs, small game, domesticates, and, in some cases, purchased market items (Liebert et al., 2013; Lu, 2007). Once obtained, food is typically boiled, accompanied by salt, and distributed narrowly within the household. In addition to being eaten, manioc is fermented to make nihamanch (referred to more generally by the Quichua term, chicha), a mildly alcoholic drink consumed daily by most individuals (Colehour et al., 2014).
As is the case in much of the developing world, the burden of infectious disease remains high throughout Shuar territory. Many Shuar have difficulty obtaining clean water, lack basic sanitation devices such as latrines, and are often in close contact with animal disease vectors (Jokisch and McSweeney, 2006). One study recently found that 54% of investigated Shuar adults and 74% of investigated Shuar children were infected with at least one species of soil-transmitted helminth, with only slightly lower rates of infection in the more market-integrated Upano Valley than cross-Cutucú (Cepon-Robins et al., 2014). Rates of acute respiratory infections and vector-borne diseases (e.g., malaria, dengue, yellow fever, etc.) are similarly high among the Shuar (Jokisch and McSweeney, 2006) and are comparable to published rates from elsewhere in the Ecuadorian Amazon (Kuang-Yao Pan et al., 2010). Access to modern medical care is typically limited, with 96% of individuals living in communities without a resident doctor and travelling on average 2.9 (± 3.2) hours to the nearest health center for basic treatment (Jokisch and McSweeney, 2006; Jokisch and McSweeney, 2011). Although exact vaccination rates are unknown, available data suggest that they are very low. One large study, for example, recently found that only 28% of investigated Shuar and Achuar children under the age of 5 years had received at least one documented vaccination while, of these, only 11% had completed a full regime (Jokisch and McSweeney, 2006).
Data collection
Data were collected as part of the Shuar Health and Life History Project (SHLHP; http://www.bonesandbehavior.org/shuar), an interdisciplinary research effort initiated in Ecuador in 2005. Mixed-longitudinal data for height, weight, and BMI from 2,463 Shuar participants age 0–29 years, providing a total of 5,140 measurement occasions, were included in the present study (Table 1 and detail in online Supplemental File S1). All participants gave informed consent or assent, with both parental consent and child assent for subjects under age 15 years. Research approval was obtained from village leaders, FICSH, and the Institutional Review Boards of the University of Oregon and Harvard University.
Table 1.
Shuar sample sizes and BCPE smoothing parameters for GAIC best-fit GAMLSS models
| Nindividuals | nobservations | Age power transformation (λ) | df(μ) | df(σ) | df(ν) | df(τ) | ||
|---|---|---|---|---|---|---|---|---|
| Height | Males | 1,143 | 1,646 | 0.55 | 15a | 8 | 1 | 1 |
| Females | 1,197 | 1,927 | 0.55 | 11 | 7 | 1 | 1 | |
| Weight | Males | 1,189 | 2,216 | 0.70 | 19a | 5 | 4 | 1 |
| Females | 1,256 | 2,662 | 0.80 | 15 | 7 | 1 | 1 | |
| BMI | Males | 1,140 | 1,638 | 0.35 | 14 | 1 | 2 | 1 |
| Females | 1,172 | 1,896 | 0.35b | 12 | 4 | 1 | 1 |
Operations performed using a GAIC penalty of 3.5 (see Methods).
Male λ-value used in modeling (see Methods). Sample distribution by age is provided in online Supplemental File S1.
Data were drawn from the following three sources:
SHLHP survey data: Mixed-longitudinal data from 1,035 participants age 0–29 years (n = 3,085 measurement occasions) were collected directly by the authors between 2007 and 2013 as part of ongoing SHLHP survey research. Data were collected following conventional methods (Lohman et al., 1988) in diversely market-integrated communities across Shuar territory. Height was measured to the nearest 1.0 mm using a portable stadiometer (Seca Corporation 214, Hanover, MD). Infant length was measured to the nearest 1.0 mm using a portable infantometer (Seca Corporation 231, Hanover, MD). Weight was measured to the nearest 0.1 kg using an electronic scale (Tanita Corporation BC-534/BF-689, Tokyo, Japan). Dates of birth were available for most participants from official school records. Ages were also obtained and cross-checked using overlapping genealogies constructed from information given by parents and other community members.
Health diagnostic study: Cross-sectional data from 1,251 participants age 0–25 years were collected during a health diagnostic study conducted by FICSH and the hospital Pio XII in collaboration with the SHLHP in 2005. This study has been previously described (Blackwell et al., 2009) and consisted of teams of physicians and trained health professionals who visited schools in both the Upano Valley and cross-Cutucú regions of Morona Santiago province. Height and weight were measured for nearly 100% of the individuals in attendance on the day of the visit (as well as a few non-attendees) using standard methods. Ethnicity was assessed based on self-report, with only individuals identifying as Shuar being included in the present study. Birthdates were obtained from official school records and were cross-checked with SHLHP genealogy data when available.
Health center records: Mixed-longitudinal data from 177 participants age 0–28 years (n = 804 measurement occasions) were obtained from health center records drawn from a single Upano Valley Shuar community participating in SHLHP research in 2009. These records include conventional height (or length) and weight measurements obtained by a trained local clinician during routine and illness-related visits. In general, health center data are from very young individuals, with information from children < 3 years of age constituting 75% of the dataset. Birthdates recorded on health center records were cross-checked with available SHLHP genealogical information to ensure age estimation accuracy.
Centile curve modeling
Centile curves for height, weight, and BMI were constructed for each sex between the ages of 0–29 years using GAMLSS (Rigby and Stasinopoulos, 2005), an extension of the popular LMS method for modeling growth (Cole and Green, 1992). Following an evaluation of 30 existing methods for growth curve construction (Borghi et al., 2006), GAMLSS was recently found to outperform other modeling methods and was selected to generate the WHO childhood growth standards (De Onis, 2007). GAMLSS modeling involves two general procedures: (i) fitting a parametric distribution of an outcome variable at each continuous value of age in the data; and, (ii) smoothing the distribution on age for each parameter of the selected parametric distribution function using cubic splines or a number of other methods. The two GAMLSS procedures are estimated simultaneously across the complete dataset by iterative maximization of a penalized likelihood function using the R-package “gamlss” (http://www.gamlss.org/) (Stasinopoulos and Rigby, 2007) in R 3.0.3 (http://cran.us.r-project.org/). This study attempted, whenever possible, to replicate the GAMLSS curve fitting procedures of the WHO (Borghi et al., 2006; De Onis, 2007; WHO, 2006) by fitting growth curves using the 4-parameter Box–Cox power exponential (BCPE) distribution (Rigby and Stasinopoulos, 2004) with smoothing degrees of freedom determined in a stepwise fashion.
Shuar centile curves were fit using GAMLSS as follows, with final model parameters given in Table 1:
Models were first fit as BCPE(x=ageλ, df(μ)=10, df(σ)=5, ν=1, τ=2) with values of the age-transformation power λ ranging from 0.05 to 1.5. The λ parameter from the model with the smallest global deviance was selected. For one model (female BMI) the λ selected by this procedure (1.15) resulted in over-smoothing of young ages. For this model the λ parameter from the male BMI model (0.35) was used.
Using the selected λ model, outliers with predicted z-scores > 3 or < −3 were removed.
Generalized Akaike information criterion (GAIC) was used to determine the appropriate degrees of freedom for μ, σ, ν, and τ. While the WHO methods used standard Akaike information criterion (penalty term of 2) for μ and GAIC for other parameters, given the smaller sample size of this study, it was found that GAIC criterion was less likely to result in over-fitting. Degrees of freedom for μ and σ were selected by comparing all models with df(μ) ranging from 1 to 20 and df(σ) ranging from 1 to 10. For two models (male height and weight) the GAIC penalty of 3 resulted in models that over-fit idiosyncrasies in the data sampling. For these two models only, a GAIC penalty term of 3.5 was used to produce smoother fits. Models with ν = 1 or df(ν) ranging from 1 to 8 were then compared. Finally, models with τ = 2 or df(ν) ranging from 1 to 8 were compared to determine whether a τ parameter was needed. For all but two models the minimal GAIC was obtained with τ = 2. For the other two, allowing τ to vary (df = 1) reduced GAIC, but only slightly, and had no significant effect on centile curves. We therefore followed the WHO in fixing τ = 2 in all models, resulting in the reduction of distribution functions to the simpler 3-parameter Box-Cox Cole and Green (BCCG) distribution (Cole and Green, 1992), equivalent to the LMS method.
Goodness of fit for all final models was assessed using grid tests to compare observed and expected proportions of observations above and below specific centiles (Borghi et al., 2006; Healy et al., 1988).
It should be noted that the WHO constructed growth curves separately for children 0–5 years old (WHO, 2006) and 5–19 years old (De Onis, 2007). The advantage of fitting separate curves is that it allows for different power transformations and model parameters for different age ranges. However, this technique also introduces new assumptions when merging the two curves and may create “edge effects” at transition points. Shuar curves were fit two ways: (i) curves were fit separately for ages 0–5 years and 5–29 years; and, (ii) curves were fit singly for the entire age range of 0–29 years. The two methods produced essentially identical μ curves but did create several mismatches in σ curves (despite similar overall values). As a result, it was decided to differ from the WHO in the use of a single GAMLSS model for the entire age range in all analyses. From a clinical standpoint, this has essentially no effect on the centiles produced. The upper age range was included in all analyses to act as an “anchor” for modeling (Indrayan, 2014) and is uninformative. Only information for age 0–25 years is presented.
To assist other researchers in future analyses involving LMS output data, R code for calculating z-scores and centiles from LMS values is provided in online Supplemental File S2.
LMS and pseudo-velocity curve modeling
Final GAMLSS model parameters were used to produce LMS curves for each sex and anthropometric measure of interest. The L, M, and S parameters from growth models can be interpreted as showing asymmetry or skew (L), median (M), and variability (S) in the distribution of data. Additionally, pseudo-velocity curves were created for each sex and measure of interest using the first derivatives of the median (μ) curves obtained from GAMLSS models. The following four parameters were obtained for each measure of interest: (i) average growth rate from age 3 to 10 years; (ii) age at takeoff velocity (ATO); (iii) age at peak velocity (APV); and, (iv) age at return to takeoff velocity (ARTO). The first measure provides an estimate of “child-juvenile” growth velocity between the approximate ages of weaning and puberty. ATO, APV, and ARTO represent the ages of the beginning, peak, and end of the pubertal growth spurt, respectively. APV was calculated as the age of maximum growth velocity after the first 7 years of life. ATO was calculated as the age at which growth acceleration first attained a value > 0 units/year−1 immediately prior to the APV (Gasser et al., 1985). ARTO was calculated as the age of return to the growth velocity at ATO immediately following APV. Because individuals have growth spurts at different ages, using mixed-longitudinal data from multiple participants creates overlapping spurt periods that can obscure the true peak growth velocity of a population (Hauspie and Molinari, 2004). For this reason, the magnitude of adolescent growth spurts is not reported.
To facilitate comparison with international references, Shuar LMS and pseudo-velocity curves for each anthropometric measure of interest were plotted alongside CDC (Kuczmarski et al., 2002) and WHO (De Onis, 2007; WHO, 2006) reference data.
RESULTS
Centile curves
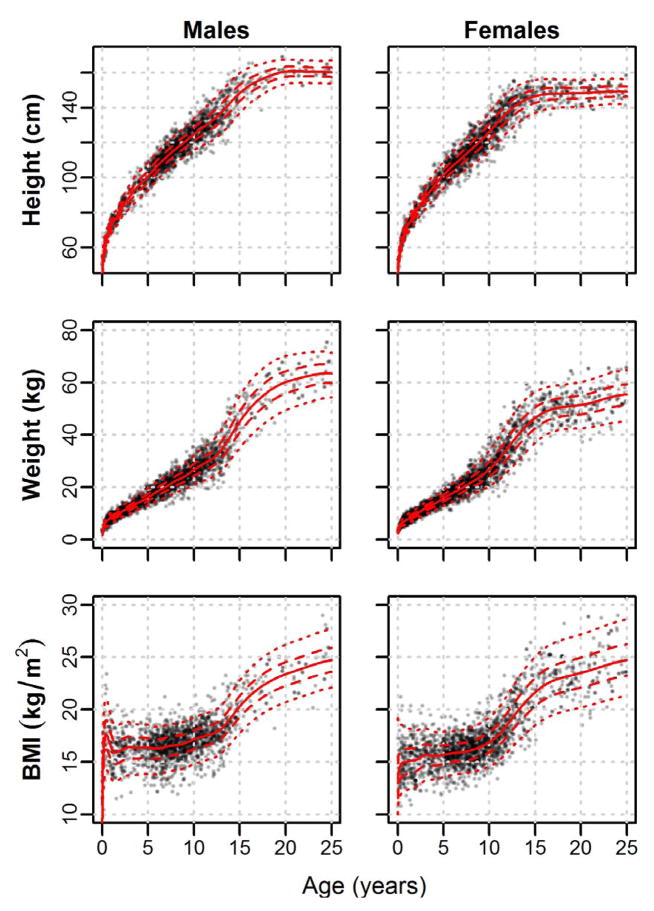
Centile curves for Shuar height, weight, and BMI from age 0 to 25 years are plotted by sex in Figure 1. Complete tables of centile values (2nd, 5th, 25th, 50th, 75th, 95th, and 98th) and accompanying LMS parameters may be found in online Supplemental Files S3–S5.
Fig. 1.
Sex-specific centile curves for Shuar height, weight, and BMI from age 0 to 25 years. Solid lines = 50th centile; Dashed lines = 25th and 75th centiles; Dotted lines = 5th and 95th centiles. Complete centile values and accompanying LMS parameters are provided in online Supplemental Files S3–S5.
LMS curves
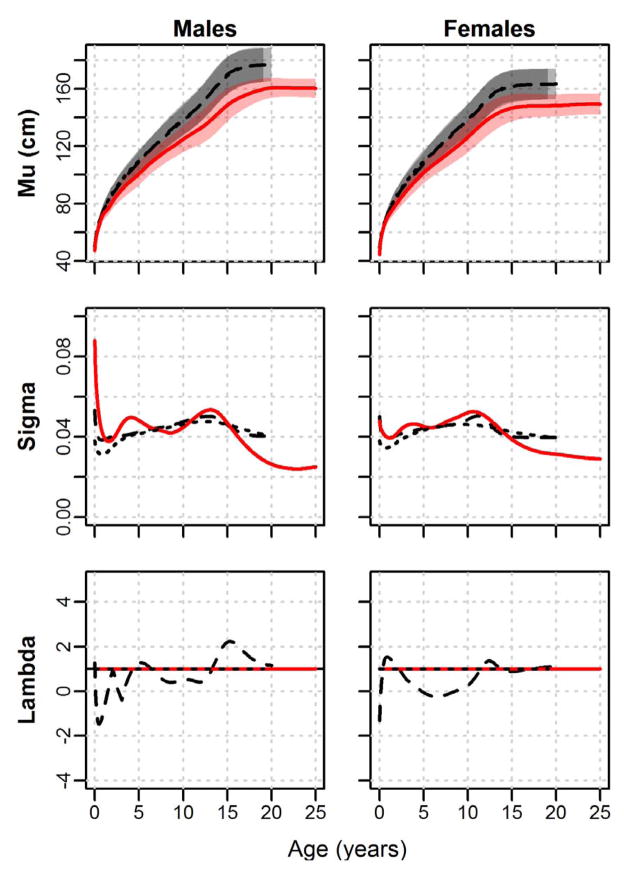
Height LMS curves for the Shuar, CDC, and WHO are presented in Figure 2. Height M-curves (displaying median values along with 5th to 95th centile ranges) illustrate that the Shuar are short relative to international references throughout development. M-curves for both sexes decline from near the WHO 40th centile in early infancy to the ≈ 3rd centile for males and the ≈ 5th centile for females by age 2 years. Shuar M-curves for both sexes remain generally stable at these reference levels during early childhood but begin to fall further below WHO and CDC M-curves beginning near the age of 6 years, a pattern that continues for much of the remainder of development and is associated with Shuar 95th centiles that are consistently below international reference medians. In adulthood, Shuar 95th centiles for height approximate the WHO 10th and 15th centiles for males and females, respectively. Shuar height S-curves (illustrating variability in the distribution of height at a given age) follow the general pattern of international references, with values falling from birth and peaking near puberty; however, Shuar height S-curves in both sexes are distinct from references in possessing a clear second peak in variation during early childhood as well as low levels of variation in adulthood. Similar to WHO references, Shuar height L-curves are flat along a value of λ = 1, indicating no modeling of skew in the distribution of height around age.
Fig. 2.
LMS curves for height by sex. Shuar (solid red line, red shade), CDC references (dashed black line, light grey shade), and WHO references (dotted black line, dark grey shade). Mu = median height (5th to 95th centiles shaded); Sigma = coefficient of variation; Lambda = Box-Cox transformation (skew).
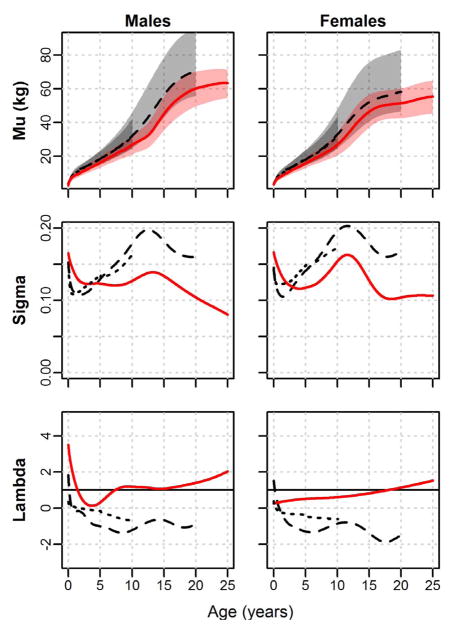
LMS curves for weight are presented in Figure 3. Similar to results for height, Shuar weight M-curves are well below those of the CDC and WHO throughout development, beginning near international reference 30th centiles in early infancy and subsequently falling to ≈ 5th centiles by age 2 years in both sexes. In sharp contrast to the pattern observed for height, however, Shuar weight M-curves begin rising progressively relative to international references in early childhood and approximate reference 20th centiles by 5 years of age. Male M-curves curves fall again near puberty relative to CDC references, to about the 5th centile, before rising once more to around the 20th centile in adulthood. Females, in contrast, gain relative to CDC references for weight continuously from early childhood, reaching the ≈ 35th centile in adulthood. Shuar weight 95th centile lines for both sexes are close to the CDC 95th centile lines in infancy, drop to around the 40th centile by age 2 years, and then rise to around the 65th centile in childhood. The female 95th centile curve remains around that of the CDC 65th centile into adulthood, while the male curve falls to around the CDC 50th centile. Shuar weight S-curves exhibit the same overall pattern as those of CDC and WHO references, illustrating high variation in infancy that drops into childhood and then rises during puberty. Despite overall similarity in the shape of S-curves, the Shuar demonstrate greater levels of variation than references during the first several years of life and relatively lower levels of variation at later ages. Shuar weight L-curves reflect modest distributional skew that is generally positive (i.e. λ < 1) across age but is significantly less exaggerated than the positive skew of references.
Fig. 3.
LMS curves for weight by sex. Shuar (solid red line, red shade), CDC references (dashed black line, light grey shade), and WHO references (dotted black line, dark grey shade). Mu = median weight (5th to 95th centiles shaded); Sigma = coefficient of variation; Lambda = Box-Cox transformation (skew).
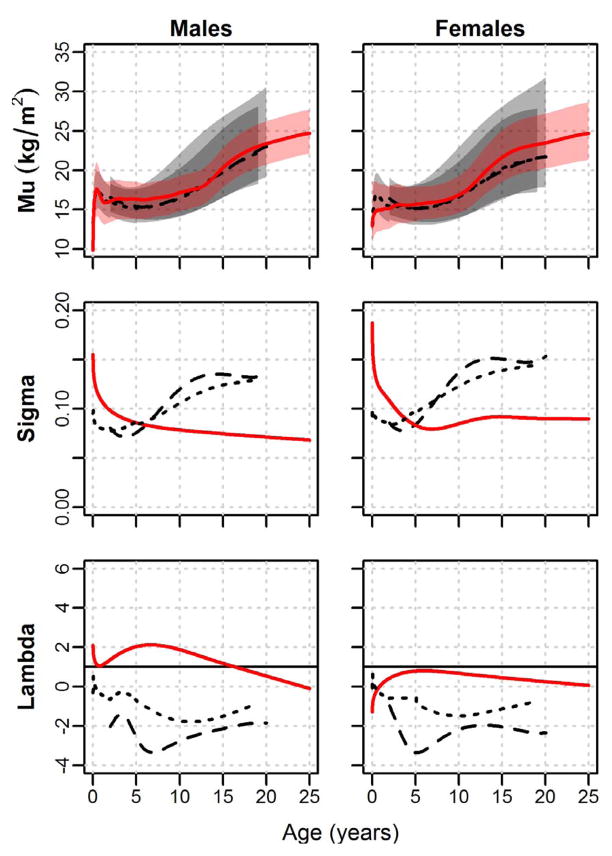
BMI LMS curves are presented in Figure 4. Shuar M-curves exhibit low median BMI values early in infancy that, similar to WHO curves, demonstrate early and rapid increases, particularly in males. Unlike CDC and WHO references, the Shuar do not appear to experience a significant BMI “dip” during early childhood, with median BMI values generally increasing following infancy. From early childhood, Shuar BMI M-curves lie near or slightly above those of international references. Shuar 5th and 95th centiles generally fall well within the 5th and 95th centiles of both WHO and CDC references. Consistent with the relatively narrow distribution observed in the range of the Shuar 5th and 95th centile lines, Shuar BMI S-curves are markedly different than those of CDC and WHO references, exhibiting relatively high values at birth that fall below international reference values near age 7 years and remain low into adulthood. Shuar BMI L-curves exhibit modestly greater λ-values than references (indicating more negative skew) and show a pattern of generally decreasing λ-values following early childhood that contrasts the increasing curves of the CDC and WHO.
Fig. 4.
LMS curves for BMI by sex. Shuar (solid red line, red shade), CDC references (dashed black line, light grey shade), and WHO references (dotted black line, dark grey shade). Mu = median BMI (5th to 95th centiles shaded); Sigma = coefficient of variation; Lambda = Box-Cox transformation (skew).
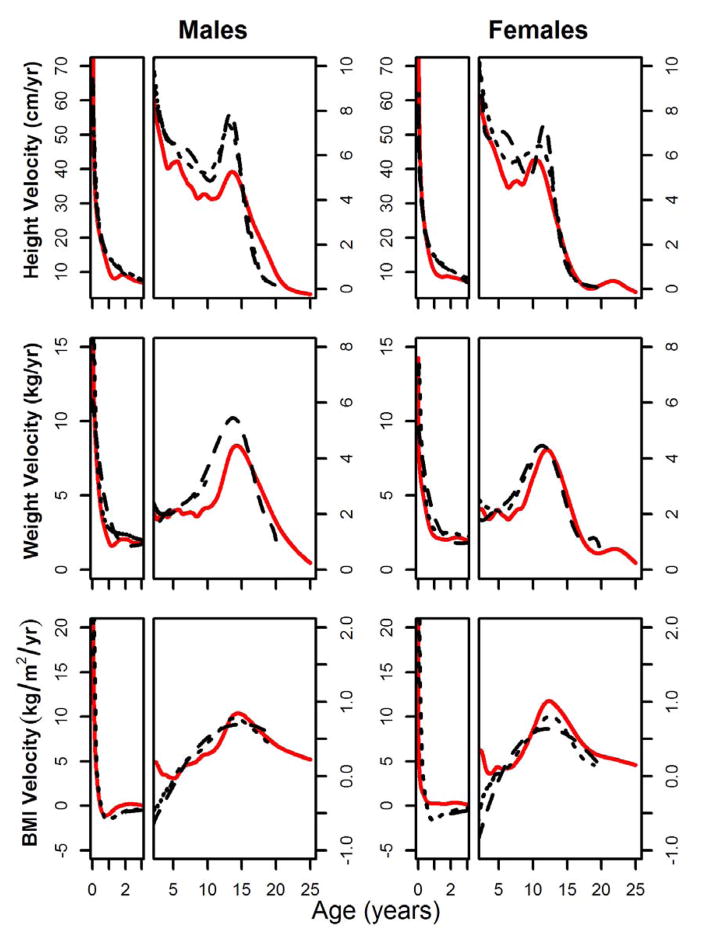
Pseudo-velocity curves
Pseudo-velocity curves for height, weight, and BMI are presented in Figure 5. Associated model parameters are provided in Table 2.
Fig. 5.
Pseudo-velocity curves for height, weight, and BMI plotted by sex. Shuar (solid red line), CDC references (dashed black line), and WHO references (doted black line). Curves represent the velocity of the median (μ) GAMLSS model parameter. Curves for age ≤ 3 years (left) are enhanced for detail.
Table 2.
Growth pseudo-velocity parameters for the Shuar and international references
| Child-juvenile velocity (units/year) | ATO (years) | APV (years) | ARTO (years) | |||
|---|---|---|---|---|---|---|
| Height (cm) | Males | Shuar | 5.1 | 11.0 | 13.6 | 15.5 |
| CDC | 6.2 | 10.4 | 13.3 | 15.0 | ||
| WHO | 6.0 | 9.7 | 13.1 | 14.8 | ||
| Females | Shuar | 5.4 | 8.4 | 10.2 | 12.1 | |
| CDC | 6.3 | 9.3 | 11.6 | 12.9 | ||
| WHO | 6.2 | 7.0 | 11.0 | 12.2 | ||
| Weight (kg) | Males | Shuar | 2.0 | 8.4 | 14.2 | 19.6 |
| CDC | 2.5 | 7.8 | 13.7 | 17.5 | ||
| WHO | 2.4 | - | - | - | ||
| Females | Shuar | 2.2 | 8.4 | 12.1 | 15.6 | |
| CDC | 2.7 | 7.2 | 11.4 | 14.4 | ||
| WHO | 2.6 | - | - | - | ||
| BMI (kg/m2) | Males | Shuar | 0.11 | - | 14.5 | - |
| CDC | 0.09 | - | 15.0 | - | ||
| WHO | 0.12 | - | 13.9 | - | ||
| Females | Shuar | 0.20 | - | 12.3 | - | |
| CDC | 0.16 | - | 12.1 | - | ||
| WHO | 0.17 | - | 12.5 | - |
Child-juvenile velocity = average growth velocity between the ages of 3 to 10 years; ATO = age at takeoff velocity; APV = age at peak velocity; ARTO = age at return to takeoff velocity.
Height pseudo-velocity curves illustrate several significant differences in growth between the Shuar and international references. Growing relatively quickly over the first several months of life, the Shuar track the height curves of the CDC and WHO to the age of ≈ 9 months. Shuar pseudo-velocity curves for males and females drop well below those of references at this time, where they remain throughout infancy. Between the ages of 3 to 10 years, Shuar curves are consistently below references, with average child-juvenile growth rates in both sexes that are ≈ 1 cm/year less than those of the CDC and WHO. Beginning near puberty, Shuar growth exhibits marked sex differences. The growth of Shuar males at this time can be characterized as moderately slow relative to references, with lower growth velocities, a clear but slightly delayed pubertal growth spurt, and growth that persists at marked velocity to the age of 20.9 years. A very different pattern is observed in the post-juvenile growth of Shuar females. Shuar females experience a growth spurt in height that is substantially earlier than that of CDC and WHO references (APVShuar = 10.2 years, APVCDC = 11.6 years, APVWHO = 11.0 years) and possess a height pseudo-velocity curve that is nearly identical to that of references beyond the age of 13 years, demonstrating no significant prolongation of growth.
Shuar weight pseudo-velocity curves generally follow those of international references early in life, particularly those of the WHO. Beginning at the age of ≈ 1 year, however, male and female Shuar curves clearly fall below those of references. Shuar growth rates in weight for both sexes remain, on average, below those of the CDC and WHO throughout juvenility. Later in life, the Shuar experience clear pubertal growth spurts in weight that are modestly delayed (males = + 0.5 years; females = + 0.7 years) relative to CDC references. As observed in height curves, Shuar males, but not females, exhibit clear extension of weight growth into adulthood.
BMI pseudo-velocity curves illustrate similar growth between the Shuar and WHO references early in infancy, with Shuar females experiencing moderately lower growth rates than references. Between the ages of ≈ 1 to 5 years, Shuar BMI curves lie above those of references. This pattern is reversed between the ages of ≈ 5 to 10 years, however, resulting in average child-juvenile growth rates for BMI that are similar between the Shuar, CDC, and WHO. Shuar pseudo-velocity curves for BMI more closely resemble those of the WHO than the CDC during puberty, exhibiting clear growth spurts during this time. In general, Shuar pubertal BMI growth velocities are greater than those of references, a pattern that is particularly apparent in females.
DISCUSSION
This study provides the first published use of GAMLSS methods to describe growth among a small-scale indigenous population. Following its application for generating the WHO childhood growth standards (De Onis, 2007), GAMLSS has recently been chosen to model physical development in a number of Western populations (Gleiss et al., 2013; Nagy et al., 2014; Saari et al., 2011). GAMLSS offers several modeling and interpretive advantages over competing methods but requires a relatively large dataset (Borghi et al., 2006). Mixed-longitudinal data from 2,463 individuals living throughout Shuar territory were included in the present study. This sample size far exceeds those of most previous studies of growth among small-scale groups, including those among other Amazonians (e.g., Black et al., 1977; Dangour, 2001; Hill and Hurtado, 1996; Hodge and Dufour, 1991; Stinson, 1989), and represents ≈ 2–6% of the current Shuar population (CODENPE, 2012; Rubenstein, 2001).
Shuar comparative growth
Consistent with previous investigations performed using limited cross-sectional data (Blackwell et al., 2009; Blackwell et al., 2010; Houck et al., 2013), the results of the present study document that the Shuar are small throughout life, with growth in height that compares less favorably to international references than growth in weight. This general pattern of growth, reflecting high rates of stunting but low rates of underweight and wasting, strongly characterizes indigenous Amazonians collectively (Dufour, 1992; Foster et al., 2005; Orr et al., 2001; Piperata et al., 2011; Santos and Coimbra Jr, 1991; Zonta et al., 2010). Indeed, Amazonian populations have long been recognized as among the shortest but not the lightest people in the world (Stinson, 1990). With an overall rate of stunting of ≈ 40% (Blackwell et al., 2009), the Shuar approximate the median of a wide range of stunting rates among other indigenous Amazonians (Foster et al., 2005) and fall near, for example, rates of 38% for the Pueblo Tacana and Esse-Ejjas of Bolivia (Benefice et al., 2006) and 43% for the Achuar of Ecuador (Orr et al., 2001). Shuar median adult heights of 160.8 cm and 149.3 cm for males and females, respectively, closely resemble averages calculated for 20 other lowland South American groups (males = 161.7 ± 5.5 cm; females = 149.8 ± 4.8 cm) (Godoy et al., 2006), further supporting the notion that general growth outcomes among the Shuar are typical of other small-scale Amazonian populations.
Unlike most previous studies of growth among small-scale societies, the use of GAMLSS modeling in the present study provides a series of parameters that make in-depth description of population-level growth patterns possible. Results suggest that, in general, Shuar growth is complex and differs substantially from that of international references. Shuar males and females grow rapidly over the first few months of life but experience subsequent declines in infant growth that track near the CDC and WHO 5th centiles for height and weight in early childhood. Growth diverges markedly from international references during mid-childhood, however, with Shuar height experiencing progressive decreases and Shuar weight progressive increases relative to references for most of the remaining growth period. Resulting Shuar child-juvenile median growth rates in both sexes are ≈ 1.0 cm/year less and ≈ 0.5 kg/year less than that of references for height and weight, respectively. During puberty, clear sex differences in Shuar growth patterns are apparent. Relative to international references, Shuar males exhibit modest delays in the timing of pubertal growth spurts in height and weight and extend growth in both of these measures into adulthood. Shuar females experience a similar slight delay in the pubertal growth spurt in weight, but, unlike males, demonstrate a growth spurt in height that is approximately one full year earlier than that of international references and do not appear to extend growth into adulthood. Interestingly, the S and L parameters describing Shuar growth vary significantly from WHO and CDC references, reflecting differences in sample distribution and skew. The Shuar demonstrate substantially lower variation than references in measures of height, weight, and BMI during adulthood but somewhat greater variation in these same measures early in life. Lower adult variation in body size among the Shuar is likely the result of reduced genetic and environmental diversity relative to the more ethnically heterogeneous samples of the WHO and CDC. Greater variation in Shuar body size during infancy and childhood may conversely reflect heightened sensitivity of Shuar growth to environmental influences during early life (see below). Findings further suggest that the Shuar possess significantly less positive skew than references in the distribution of body size throughout life, particularly in weight and BMI. This observation appears to be related to a lower incidence of overweight in Shuar children.
Few comparably detailed data are currently available to assess the degree to which nuanced characteristics of Shuar growth reflect those of other Amazonians and small-scale indigenous groups. Considering a wider range of human populations, it is well documented that rapid growth in height and weight over the first few months of life (Karlberg et al., 1997; Khandelwal et al., 2014) as well as subsequent faltering in these measures between the age of 3 to 24 months (Shrimpton et al., 2001; WHO, 1995) are common patterns of growth in many developing populations. This observation includes limited data from the Bolivian Amazon suggesting declines from international growth references during infancy among the indigenous Tsimane’, Pueblo Tacana, and Esse-Ejjas (Benefice et al., 2006; Foster et al., 2005). The progressive falling of Shuar height curves and rising of Shuar weight curves relative to CDC and WHO references beginning in mid-childhood illustrates that the Shuar do not consistently track the growth of reference children beyond infancy. A similar pattern of divergence from references, evidenced by lower height-for-age and greater weight-for-age reference z-scores in older versus younger children, has been documented among several other Amazonian groups (Benefice et al., 2006; Foster et al., 2005; Wilson et al., 2011). While more data are needed, this finding suggests that preferential growth in weight over that of height following early childhood may be a common developmental characteristic among indigenous Amazonian populations.
Shuar pubertal growth is also noteworthy from a comparative perspective. While there exists substantial global variation in the dynamics of human growth near puberty (Eveleth and Tanner, 1990; Ulijaszek, 2001), the typical pattern of growth at this time in the developing world is characterized by delayed, longer, and less dramatic growth spurts often accompanied by the extension of growth into adulthood (Cameron, 2007; Eveleth and Tanner, 1990). Shuar males largely follow this pattern of slow and delayed pubertal growth, conforming closely to limited available data from small-scale groups and other Amazonians (Bogin et al., 1990; Eveleth and Tanner, 1990; Sellen, 1999; Walker et al., 2006). Although Shuar females also experience an expected delay relative to international references in pubertal growth in weight, the finding of relatively early pubertal growth in height in Shuar females contrasts starkly with previous observations among other small-scale groups (Walker et al., 2006). Among Amazonians, the timing of the Shuar female growth spurt in height (APVheight = 10.2 years) is significantly earlier than reported for the Tsimane’ (APVheight = 12.0 years) and the Paraguayan Ache (APVheight = 13.0 years) (Walker et al., 2006). Notably, however, the growth spurt parameters for the Tsimane’ and Ache were estimated visually from curves constructed using a very modest number of individuals. As such, comparison of the timing of pubertal growth in height between the Shuar and these populations must be made cautiously. Abundant evidence documenting early age at menarche among many lowland South American populations (Kramer et al., 2009), as well as a close relationship between the timing of menarche and the timing of the human pubertal growth spurt in height (Elizondo, 1992; Ellison, 1981), suggests the likelihood that other Amazonian populations will be found to experience early female pubertal growth in height, similar to that of the Shuar, as sufficient data become available.
Environmental and selective explanations for Shuar growth patterns
The extent of genetic contribution to Amazonian growth characteristics remains unclear (Orr et al., 2001; Stinson, 1990); however, environmental factors are thought to explain much of the observed variation in growth within and between human populations globally (Eveleth and Tanner, 1990; Ulijaszek, 2006; WHO, 1995). In the developing world, including Amazonia, nutritional inadequacy and infectious disease are typically considered the primary causes of slow childhood growth and delayed maturation (Foster et al., 2005; Hodge and Dufour, 1991; Santos and Coimbra Jr, 1991; WHO, 1995). Developmental responses to these environmental factors likely explain some, but not all, aspects of Shuar growth. As noted above, the Shuar do indeed face substantial rates of infectious and parasitic disease. Although the precise relationship between infection and the growth of Shuar children is currently unknown, ample evidence suggests that soil-transmitted helminths and other pathogens that are common among the Shuar negatively affect growth in many populations (Crompton and Nesheim, 2002; Goodman et al., 2011; Moffatt et al., 2001; Rowland et al., 1988; Sackey et al., 2003). The pathways through which these infections impact childhood growth are numerous and complex (Stephensen, 1999). Among the Shuar, the large predicted energetic cost associated with frequent pathogen-driven activation of the immune system (Blackwell et al., 2011; Blackwell et al., 2010; Mcdade et al., 2012), in conjunction with low-nutrient-density diets (Dufour, 1992; Liebert et al., 2013), may play a key role in limiting resources available for growth, particularly early in life and near weaning at ≈ 15 months of age (Madimenos et al., 2012). This hypothesis requires further testing but receives initial support from evidence indicating that stature in Shuar subadults is inversely related to levels of various biomarkers of immune activation (Blackwell et al., 2010).
While developmental response to environmental stimuli such as nutrition and infection likely explains some aspects of Shuar growth, consideration of explanations involving genetic adaptation may also provide useful insight into the patterns observed in this study. Several possible adaptive explanations for the small body size of tropical forest populations have previously been suggested and may apply to the Shuar. These include selection for heat dissipation (Cavalli-Sforza, 1986; Hiernaux and Froment, 1976; Roberts, 1953), efficient mobility (Diamond, 1991; Stinson, 1990; Tobias, 1972), and low body maintenance requirements (Gurven and Walker, 2006; Stini, 1969). When adult mortality is high, short stature may also reflect the indirect outcome of selection for early reproductive maturation (Migliano et al., 2007; Walker et al., 2006). In some cases, mortality-driven selection for early reproduction may additionally lead to earlier and more rapid growth (Case, 1978; Charnov and Berrigan, 1993; Stearns, 1992). This latter scenario may explain the early female growth spurt in height observed among the Shuar. Although Shuar age-specific mortality curves are not available, data from interviews (SHLHP, unpublished data), other indigenous Amazonians (Gurven et al., 2007; Hill and Hurtado, 1996; Walker et al., 2006) and elsewhere in the Ecuadorian Amazon (Kuang-Yao Pan et al., 2010; WHO, 2011) suggest that Shuar adult mortality is likely quite high. Direct evidence documents that Shuar females do indeed initiate reproduction early in life, possessing a mean age at menarche (13.0 years) and a mean age at first birth (17.5 years) (Madimenos et al., 2012) that fall near the very low end of ranges observed among 22 other small-scale populations (Walker et al., 2006). Recognizing that skeletal maturation is the key physiological factor determining age at reproductive maturation in females (Elizondo, 1992; Ellison, 1981), extrinsic mortality should more strongly influence female growth in height than growth in weight. This, along with possible energy-partitioning benefits relating to the temporal staggering of growth spurts in different soma, may explain why Shuar females possess early pubertal growth spurts in height but not weight. The particularly strong influence of mortality factors on life history parameters in females (Stearns, 1992; Walker et al., 2006), as well as possible male target body size selection pressures relating to male-male competition, may further explain why Shuar females but not males experience early pubertal growth in height.
Finally, it must be noted that developmental plasticity and genetic adaptation are not independent explanations of Shuar growth. Developmental reaction norms are themselves the product of natural selection, and different populations may, for example, respond to energetic and disease stressors differently as a result of distinct histories of selection on reaction norms. The Shuar and other Amazonians appear to prioritize growth in weight over growth in height relative to populations from many parts of the world (Eveleth and Tanner, 1990; Foster et al., 2005; Victora, 1992). This apparent pattern of preferential allocation of energy into gains in weight rather than height may facilitate energy availability for female reproduction early in life. Alternatively, it may reflect selection for robust immune function in response to persistent pathogen exposure, for which central body fat may be particularly important (Samaras et al., 2010; Wells and Cortina-Borja, 2013). Future research is needed to investigate these hypotheses.
Clinical application of Shuar growth references
In addition to its utility for understanding human biological variation and life history, the description of Shuar growth presented in this study may also serve as an important tool for use in the clinical assessment of childhood development and health. Growth references provide authorities with information about the growth status of children and are critical for identifying groups and individuals who are at risk for disease or require urgent care (De Onis et al., 2004). Although some researchers advocate the use of a single growth reference for all children globally (De Onis et al., 2004), evidence is mounting to suggest that international references may not be appropriate for assessing growth in all populations, particularly among non-Western and indigenous groups (Gleiss et al., 2013; Guedes et al., 2010; Hakeem et al., 2004; Hasan et al., 2001; Marwaha et al., 2006; Mushtaq et al., 2012; Neyzi et al., 2006; Ulijaszek, 1994; Ulijaszek, 2001). In line with this finding, a number of factors suggest that the growth references presented in this study may be used to supplement international references in the clinical assessment of Shuar children. First, while CDC and WHO references appear to model Shuar infant growth with fair accuracy, the Shuar do not closely follow the growth of reference children at older ages. This observation is most apparent following early childhood, a time when variation in the distribution of Shuar height, weight, and BMI are low and genetic factors are thought to become increasingly important in producing differences in growth between populations (Haas and Campirano, 2006; Ulijaszek, 2001). Second, there is little evidence of skew in Shuar growth distributions, implying that Shuar medians are not the consequence of an unhealthy subset of individuals shifting the median downward but rather that the entire population distribution of the Shuar is lower than the distribution of international references and remains more-or-less normally distributed at all ages. Finally, many notable aspects of Shuar growth appear to be common among other indigenous Amazonian and South American populations living in diverse socio-ecological contexts, further supporting a genetic basis for at least some differences in growth between the Shuar and CDC/WHO references.
CONCLUSIONS
This study has used a large dataset and robust GAMLSS modeling to provide a detailed, population-level description of Shuar physical growth that may be useful to both researchers and clinicians. Such descriptions are rare among small-scale indigenous groups, and these data contribute significantly to the global database of variation in human growth and development. Results demonstrate that the Shuar are small throughout life and possess patterns of growth that differ markedly from international references, particularly following early childhood and near puberty. Phenotypic plasticity and genetic selection in response to local environmental and life history factors, among them a heavy burden of infectious disease and high predicted rates of extrinsic mortality, likely explain many noteworthy aspects of Shuar growth. Future research will test hypotheses linking ecological variables, growth, reproduction, and health in this population.
Supplementary Material
Acknowledgments
SUPPORTING GRANTS:
National Science Foundation (BNS9157-449; BCS-0824602; BCS-0925910); Leakey Foundation General Grant; Wenner-Gren Foundation; UCSB Center for Evolutionary Psychology (via NIH grant number: 5DP1O000516-04); Harvard University Frederick Sheldon Travelling Fellowship; James S. McDonnell Foundation; Fulbright Foundation; UO Anthropology Department and Office of Research Grants; Ryoichi Sasakawa Young Leaders Fellowship; UO Institute of Cognitive and Decision Sciences Research Grants; Ministerio de Salud Pública de Morona Santiago, Ecuador; UCSB Faculty Career Development Award.
The authors express their gratitude to the Shuar participants that made this research possible and rewarding. We thank Peter Ellison and two anonymous reviewers for their helpful comments on earlier versions of this manuscript. Additional appreciation is directed to funding sources and our many collaborators, including José Pozo, Washington Tiwia, FICSH, the Ecuadorian Ministerio de Salud Pública, and the hospital Pio XII.
Footnotes
The authors declare no conflict of interest with this work.
LITERATURE CITED
- Albertsson-Wikland K, Luo ZC, Niklasson A, Karlberg J. Swedish population-based longitudinal reference values from birth to 18 years of age for height, weight and head circumference. Acta paediatrica (Oslo, Norway : 1992) 2002;91(7):739–754. doi: 10.1080/08035250213216. [DOI] [PubMed] [Google Scholar]
- Benefice E, Lopez R, Monroy SL, Rodríguez S. Fatness and overweight in women and children from riverine Amerindian communities of the Beni River (Bolivian Amazon) American Journal of Human Biology. 2006;19(1):61–73. doi: 10.1002/ajhb.20580. [DOI] [PubMed] [Google Scholar]
- Black F, Hierholzer W, Black D, Lamm S, Lucas L. Nutritional status of Brazilian Kayapó Indians. Human Biology. 1977:139–153. [PubMed] [Google Scholar]
- Blackwell AD, Gurven MD, Sugiyama LS, Madimenos FC, Liebert MA, Martin MA, Kaplan HS, Snodgrass JJ. Evidence for a Peak Shift in a Humoral Response to Helminths: Age Profiles of IgE in the Shuar of Ecuador, the Tsimane of Bolivia, and the U.S. NHANES. PLoS neglected tropical diseases. 2011;5(6):e1218. doi: 10.1371/journal.pntd.0001218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell AD, Pryor G, Pozo J, Tiwia W, Sugiyama LS. Growth and market integration in Amazonia: A comparison of growth indicators between Shuar, Shiwiar, and nonindigenous school children. American Journal of Human Biology. 2009;21(2):161–171. doi: 10.1002/ajhb.20838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell AD, Snodgrass JJ, Madimenos FC, Sugiyama LS. Life history, immune function, and intestinal helminths: Trade-offs among immunoglobulin E, C-reactive protein, and growth in an Amazonian population. American Journal of Human Biology. 2010;22(6):836–848. doi: 10.1002/ajhb.21092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogin B, Wall M, Macvean RB. Longitudinal growth of high socioeconomic status Guatemalan children analyzed by the Preece-Baines function: An international comparison. American Journal of Human Biology. 1990;2(3):271–281. doi: 10.1002/ajhb.1310020309. [DOI] [PubMed] [Google Scholar]
- Borghi E, de Onis M, Garza C, Van den Broeck J, Frongillo EA, Grummer-Strawn L, Van Buuren S, Pan H, Molinari L, Martorell R, et al. Construction of the World Health Organization child growth standards: selection of methods for attained growth curves. Statistics in medicine. 2006;25(2):247–265. doi: 10.1002/sim.2227. [DOI] [PubMed] [Google Scholar]
- Cameron N. Growth patterns in adverse environments. American Journal of Human Biology. 2007;19(5):615–621. doi: 10.1002/ajhb.20661. [DOI] [PubMed] [Google Scholar]
- Case TJ. On the evolution and adaptive significance of postnatal growth rates in the terrestrial vertebrates. Quarterly Review of Biology. 1978:243–282. doi: 10.1086/410622. [DOI] [PubMed] [Google Scholar]
- Cavalli-Sforza LL. African pygmies. Academic Pr; 1986. [Google Scholar]
- Cepon-Robins TJ, Liebert MA, Gildner TE, Urlacher SS, Colehour AM, Snodgrass JJ, Madimenos FC, Sugiyama LS. Soil-Transmitted Helminth Prevalence and Infection Intensity Among Geographically and Economically Distinct Shuar Communities in the Ecuadorian Amazon. Journal of Parasitology. 2014;100:598–607. doi: 10.1645/13-383.1. [DOI] [PubMed] [Google Scholar]
- Charnov E, Berrigan D. Why do female primates have such long lifespans and so few babies? Or life in the slow lane. Evolutionary Anthropology: Issues, News, and Reviews. 1993;1(6):191–194. [Google Scholar]
- CODENPE. Consejo de Desarrollo de las Nacionalidades y Pueblos del Ecuador. 2012. Nacionalidad Shuar. [Google Scholar]
- Cole TJ, Green PJ. Smoothing reference centile curves: the LMS method and penalized likelihood. Statistics in medicine. 1992;11(10):1305–1319. doi: 10.1002/sim.4780111005. [DOI] [PubMed] [Google Scholar]
- Colehour A, Meadow JF, Cepon-Robins TJ, Gildner TE, Liebert MA, Urlacher SS, Bohannan BJ, Snodgrass JJ, Sugiyama LS. Local domestication of microbes via cassava beer fermentation. PeerJ PrePrints. Report nr. 2014:2167–9843. doi: 10.7717/peerj.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton DWT, Nesheim M. Nutritional impact of intestinal helminthiasis during the human life cycle. Annual Review of Nutrition. 2002;22(1):35–59. doi: 10.1146/annurev.nutr.22.120501.134539. [DOI] [PubMed] [Google Scholar]
- Dangour A. Growth of upper-and lower-body segments in Patamona and Wapishana Amerindian children (cross-sectional data) Annals of human biology. 2001;28(6):649–663. doi: 10.1080/03014460110047982. [DOI] [PubMed] [Google Scholar]
- De Onis M. Development of a WHO growth reference for school-aged children and adolescents. Bulletin of the World Health Organization. 2007;85(09):660–667. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Onis M, Garza C, Victora CG, Onyango AW, Frongillo EA, Martines J. The WHO Multicentre Growth Reference Study: planning, study design, and methodology. Food and nutrition bulletin. 2004;25(1 Suppl):S15–26. doi: 10.1177/15648265040251S103. [DOI] [PubMed] [Google Scholar]
- Descola P, Lloyd J. The spears of twilight: life and death in the Amazon jungle. new Press; Nueva york: 1996. [Google Scholar]
- Diamond JM. Anthropology. Why are pygmies small? Nature. 1991;354(6349):111–112. doi: 10.1038/354111a0. [DOI] [PubMed] [Google Scholar]
- Dufour DL. Nutritional ecology in the tropical rain forests of Amazonia. American Journal of Human Biology. 1992;4(2):197–207. doi: 10.1002/ajhb.1310040205. [DOI] [PubMed] [Google Scholar]
- Elizondo S. Age at menarche: its relation to linear and ponderal growth. Annals of human biology. 1992;19(2):197–199. doi: 10.1080/03014469200002072. [DOI] [PubMed] [Google Scholar]
- Ellison PT. Prediction of age at menarche from annual height increments. American journal of physical anthropology. 1981;56(1):71–75. doi: 10.1002/ajpa.1330560108. [DOI] [PubMed] [Google Scholar]
- Eveleth P, Tanner J. World variation in human growth. 1990. [Google Scholar]
- Foster Z, Byron E, Reyes-Garc aV, Huanca T, Vadez V, Apaza L, Prez E, Tanner S, Gutierrez Y, Sandstrom B, et al. Physical growth and nutritional status of Tsimane' Amerindian children of lowland Bolivia. American journal of physical anthropology. 2005;126(3):343–351. doi: 10.1002/ajpa.20098. [DOI] [PubMed] [Google Scholar]
- Gasser T, Köhler W, Müller HG, Largo R, Molinari L, PRADER A. Human height growth: correlational and multivariate structure of velocity and acceleration. Annals of human biology. 1985;12(6):501–515. doi: 10.1080/03014468500008081. [DOI] [PubMed] [Google Scholar]
- Gleiss A, Lassi M, Blumel P, Borkenstein M, Kapelari K, Mayer M, Schemper M, Hausler G. Austrian height and body proportion references for children aged 4 to under 19 years. Ann Hum Biol. 2013;40(4):324–332. doi: 10.3109/03014460.2013.776110. [DOI] [PubMed] [Google Scholar]
- Godoy RA, Leonard WR, Reyes-García V, Goodman E, McDade T, Huanca T, Tanner S, Vadez V. Physical stature of adult Tsimane’ Amerindians, Bolivian Amazon in the 20th century. Economics & Human Biology. 2006;4(2):184–205. doi: 10.1016/j.ehb.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Goodman KJ, Correa P, Mera R, Yepez MC, Cerón C, Campo C, Guerrero N, Sierra MS, Bravo LE. Effect of Helicobacter pylori Infection on Growth Velocity of School-age Andean Children. Epidemiology. 2011;22(1):118–126. doi: 10.1097/EDE.0b013e3181fe7e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedes DP, De Matos JA, Lopes VP, Ferreirinha JE, Silva AJ. Physical growth of schoolchildren from the Jequitinhonha Valley, Minas Gerais, Brazil: Comparison with the CDC-2000 reference using the LMS method. Ann Hum Biol. 2010;37(4):574–584. doi: 10.3109/03014460903524469. [DOI] [PubMed] [Google Scholar]
- Gurven M, Kaplan H, Supa AZ. Mortality experience of Tsimane Amerindians of Bolivia: regional variation and temporal trends. American Journal of Human Biology. 2007;19(3):376–398. doi: 10.1002/ajhb.20600. [DOI] [PubMed] [Google Scholar]
- Gurven M, Walker R. Energetic demand of multiple dependents and the evolution of slow human growth. Proceedings of the Royal Society of London Series B: Biological Sciences. 2006;273(1588):835–841. doi: 10.1098/rspb.2005.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas JD, Campirano F. Interpopulation variation in height among children 7 to 18 years of age. Food and nutrition bulletin. 2006;27(4):S212–S223. doi: 10.1177/15648265060274S505. [DOI] [PubMed] [Google Scholar]
- Hakeem R, Shaikh AH, Asar F. Assessment of linear growth of affluent urban Pakistani adolescents according to CDC 2000 references. Ann Hum Biol. 2004;31(3):282–291. doi: 10.1080/03014460310001658800. [DOI] [PubMed] [Google Scholar]
- Harner MJ. The Jívaro, people of the sacred waterfalls. Univ of California Press; 1984. [Google Scholar]
- Hasan M, Batieha A, Jadou H, Khawaldeh A, Ajlouni K. Growth status of Jordanian schoolchildren in military-funded schools. European journal of clinical nutrition. 2001;55(5):380–386. doi: 10.1038/sj.ejcn.1601167. [DOI] [PubMed] [Google Scholar]
- Hauspie RC, Molinari L. CAMBRIDGE STUDIES IN BIOLOGICAL AND EVOLUTIONARY ANTHROPOLOGY. 2004. Parametric models for postnatal growth; pp. 205–233. [Google Scholar]
- Healy M, Rasbash J, Yang M. Distribution-free estimation of age-related centiles. Annals of human biology. 1988;15(1):17–22. doi: 10.1080/03014468800009421. [DOI] [PubMed] [Google Scholar]
- Henn BM, Gignoux CR, Jobin M, Granka JM, Macpherson J, Kidd JM, Rodríguez-Botigué L, Ramachandran S, Hon L, Brisbin A. Hunter-gatherer genomic diversity suggests a southern African origin for modern humans. Proceedings of the National Academy of Sciences. 2011;108(13):5154–5162. doi: 10.1073/pnas.1017511108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiernaux J, Froment A. The correlations between anthropobiological and climatic variables in sub-Saharan Africa: revised estimates. Human Biology. 1976:757–767. [PubMed] [Google Scholar]
- Hill KR, Hurtado AM. Ache life history: The ecology and demography of a foraging people. Transaction Publishers; 1996. [Google Scholar]
- Hodge LG, Dufour DL. Cross-sectional growth of young Shipibo Indian children in Eastern Peru. American journal of physical anthropology. 1991;84(1):35–41. doi: 10.1002/ajpa.1330840104. [DOI] [PubMed] [Google Scholar]
- Houck K, Sorensen MV, Lu F, Alban D, Alvarez K, Hidobro D, Doljanin C, Ona AI. The effects of market integration on childhood growth and nutritional status: The dual burden of under- and over-nutrition in the Northern Ecuadorian Amazon. American Journal of Human Biology. 2013:n/a–n/a. doi: 10.1002/ajhb.22404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indrayan A. Demystifying LMS and BCPE Methods of Centile Estimation for Growth and Other Health Parameters. Indian pediatrics. 2014;51(1):37–43. doi: 10.1007/s13312-014-0310-6. [DOI] [PubMed] [Google Scholar]
- Jokisch BD, McSweeney K. Informe sobre los Resultados del Diagnóstico de la Situacíon de Salud y de los Servicios de Salud de las Nacionalidades Shuar y Achuar FICSH-FIPSE-FINAE 2005. University of Ohio, Ohio State University; 2006. [Google Scholar]
- Jokisch BD, McSweeney K. Assessing the Potential of Indigenous-Run Demographic/Health Surveys: the 2005 Shuar Survey, Ecuador. Human Ecology. 2011;39(5):683–698. [Google Scholar]
- Karafet TM, Osipova LP, Gubina MA, Posukh OL, Zegura SL, Hammer MF. High levels of Y-chromosome differentiation among native Siberian populations and the genetic signature of a boreal hunter-gatherer way of life. Human biology. 2002;74(6):761–789. doi: 10.1353/hub.2003.0006. [DOI] [PubMed] [Google Scholar]
- Karlberg J, Kwan E, Lam B, Low L. The timing of early postnatal catch-up growth in normal, full-term infants born short for gestational age. Hormone research in paediatrics. 1997;48(Suppl 1):17–24. doi: 10.1159/000191279. [DOI] [PubMed] [Google Scholar]
- Karsten R. Societas Scientiarum Fennica, Commentationes Humanarum LitterarumVIII. 1935. The Head-Hunters of Western Amazonas: The Life and Culture of the Jibaro Indians of Eastern Ecuador and Peru; p. 1. [Google Scholar]
- Kent S. Cultural diversity among twentieth-century foragers: An African perspective. Cambridge university press; 1996. [Google Scholar]
- Khandelwal P, Jain V, Gupta AK, Kalaivani M, Paul VK. Association of early postnatal growth trajectory with body composition in term low birth weight infants. Journal of Developmental Origins of Health and Disease. 2014;5(3):189–196. doi: 10.1017/S2040174414000178. [DOI] [PubMed] [Google Scholar]
- Kramer KL, Greaves RD, Ellison PT. Early reproductive maturity among Pumé foragers: Implications of a pooled energy model to fast life histories. American Journal of Human Biology. 2009;21(4):430–437. doi: 10.1002/ajhb.20930. [DOI] [PubMed] [Google Scholar]
- Kuang-Yao Pan W, Erlien C, Bilsborrow RE. Morbidity and mortality disparities among colonist and indigenous populations in the Ecuadorian Amazon. Social science & medicine. 2010;70(3):401–411. doi: 10.1016/j.socscimed.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL. 2000 CDC Growth Charts for the United States: methods and development. Vital and health statistics Series 11, Data from the national health survey. 2002;(246):1–190. [PubMed] [Google Scholar]
- Liebert MA, Snodgrass JJ, Madimenos FC, Cepon TJ, Blackwell AD, Sugiyama LS. Implications of market integration for cardiovascular and metabolic health among an indigenous Amazonian Ecuadorian population. Annals of human biology. 2013;40(3):228–242. doi: 10.3109/03014460.2012.759621. [DOI] [PubMed] [Google Scholar]
- Lohman TG, Roche AF, Martorell R. Anthropometric standardization reference manual. 1988. [Google Scholar]
- Lu F. Integration into the market among indigenous peoples: A cross-cultural perspective from the Ecuadorian Amazon. Current Anthropology. 2007;48(4):593–602. [Google Scholar]
- Madimenos FC, Snodgrass JJ, Liebert MA, Cepon TJ, Sugiyama LS. Reproductive effects on skeletal health in Shuar women of Amazonian Ecuador: A life history perspective. American Journal of Human Biology. 2012;24(6):841–852. doi: 10.1002/ajhb.22329. [DOI] [PubMed] [Google Scholar]
- Marwaha RK, Tandon N, Singh Y, Aggarwal R, Grewal K, Mani K. A study of growth parameters and prevalence of overweight and obesity in school children from Delhi. Indian pediatrics. 2006;43(11):943. [PubMed] [Google Scholar]
- Mcdade TW, Tallman PS, Madimenos FC, Liebert MA, Cepon TJ, Sugiyama LS, Snodgrass JJ. Analysis of variability of high sensitivity C-reactive protein in lowland Ecuador reveals no evidence of chronic low-grade inflammation. American Journal of Human Biology. 2012;24(5):675–681. doi: 10.1002/ajhb.22296. [DOI] [PubMed] [Google Scholar]
- Migliano AB, Vinicius L, Lahr MM. Life history trade-offs explain the evolution of human pygmies. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(51):20216–20219. doi: 10.1073/pnas.0708024105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffatt MF, Schou C, Faux JA, Abecasis GR, James A, Musk AW, Cookson WO. Association between quantitative traits underlying asthma and the HLA-DRB1 locus in a family-based population sample. European journal of human genetics : EJHG. 2001;9(5):341–346. doi: 10.1038/sj.ejhg.5200636. [DOI] [PubMed] [Google Scholar]
- Mushtaq MU, Gull S, Mushtaq K, Abdullah HM, Khurshid U, Shahid U, Shad MA, Akram J. Height, weight and BMI percentiles and nutritional status relative to the international growth references among Pakistani school-aged children. BMC pediatrics. 2012;12(1):31. doi: 10.1186/1471-2431-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy P, Kovacs E, Moreno LA, Veidebaum T, Tornaritis M, Kourides Y, Siani A, Lauria F, Sioen I, Claessens M, et al. Percentile reference values for anthropometric body composition indices in European children from the IDEFICS study. 2014;38(S2):S15–S25. doi: 10.1038/ijo.2014.131. [DOI] [PubMed] [Google Scholar]
- Neyzi O, Furman A, Bundak R, Gunoz H, Darendeliler F, Bas F. Growth references for Turkish children aged 6 to 18 years. Acta paediatrica. 2006;95(12):1635–1641. doi: 10.1080/08035250600652013. [DOI] [PubMed] [Google Scholar]
- Orr CM, Dufour DL, Patton JQ. A comparison of anthropometric indices of nutritional status in Tukanoan and Achuar Amerindians. American Journal of Human Biology. 2001;13(3):301–309. doi: 10.1002/ajhb.1053. [DOI] [PubMed] [Google Scholar]
- Piperata BA, Spence JE, Da-Gloria P, Hubbe M. The nutrition transition in amazonia: rapid economic change and its impact on growth and development in Ribeirinhos. American journal of physical anthropology. 2011;146(1):1–13. doi: 10.1002/ajpa.21459. [DOI] [PubMed] [Google Scholar]
- Rigby R, Stasinopoulos D. Generalized additive models for location, scale and shape. Journal of the Royal Statistical Society: Series C (Applied Statistics) 2005;54(3):507–554. [Google Scholar]
- Rigby RA, Stasinopoulos DM. Smooth centile curves for skew and kurtotic data modelled using the Box–Cox power exponential distribution. Statistics in medicine. 2004;23(19):3053–3076. doi: 10.1002/sim.1861. [DOI] [PubMed] [Google Scholar]
- Roberts DF. Body weight, race and climate. American journal of physical anthropology. 1953;11(4):533–558. doi: 10.1002/ajpa.1330110404. [DOI] [PubMed] [Google Scholar]
- Rowland M, Rowland S, Cole T. Impact of infection on the growth of children from 0 to 2 years in an urban West African community. American Journal of Clinical Nutrition. 1988;47(1):134. doi: 10.1093/ajcn/47.1.134. [DOI] [PubMed] [Google Scholar]
- Rubenstein S. Colonialism, the Shuar Federation, and the Ecuadorian state. Environment and Planning D. 2001;19(3):263–294. [Google Scholar]
- Saari A, Sankilampi U, Hannila M-L, Kiviniemi V, Kesseli K, Dunkel L. New Finnish growth references for children and adolescents aged 0 to 20 years: length/height-for-age, weight-for-length/height, and body mass index-for-age. Annals of medicine. 2011;43(3):235–248. doi: 10.3109/07853890.2010.515603. [DOI] [PubMed] [Google Scholar]
- Sackey M-E, Weigel MM, Armijos RX. Predictors and nutritional consequences of intestinal parasitic infections in rural Ecuadorian children. Journal of tropical pediatrics. 2003;49(1):17–23. doi: 10.1093/tropej/49.1.17. [DOI] [PubMed] [Google Scholar]
- Samaras K, Botelho NK, Chisholm DJ, Lord RV. Subcutaneous and visceral adipose tissue gene expression of serum adipokines that predict type 2 diabetes. Obesity. 2010;18(5):884–889. doi: 10.1038/oby.2009.443. [DOI] [PubMed] [Google Scholar]
- Santos RV, Coimbra CE., Jr Socioeconomic transition and physical growth of Tupi-Monde Amerindian children of the Aripuana Park, Brazilian Amazon. Human Biology. 1991:795–819. [PubMed] [Google Scholar]
- Schell LM, Magnus PD. Is there an elephant in the room? Addressing rival approaches to the interpretation of growth perturbations and small size. American Journal of Human Biology. 2007;19(5):606–614. doi: 10.1002/ajhb.20669. [DOI] [PubMed] [Google Scholar]
- Sellen DW. Growth patterns among seminomadic pastoralists (Datoga) of Tanzania. American journal of physical anthropology. 1999;109(2):187–209. doi: 10.1002/(SICI)1096-8644(199906)109:2<187::AID-AJPA5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Shrimpton R, Victora CG, de Onis M, Lima RC, Blossner M, Clugston G. Worldwide Timing of Growth Faltering: Implications for Nutritional Interventions. PEDIATRICS. 2001;107(5):e75–e75. doi: 10.1542/peds.107.5.e75. [DOI] [PubMed] [Google Scholar]
- Stasinopoulos DM, Rigby RA. Generalized additive models for location scale and shape (GAMLSS) in R. Journal of Statistical Software. 2007;23(7):1–46. [Google Scholar]
- Stearns SC. The evolution of life histories. Oxford University Press; Oxford: 1992. [Google Scholar]
- Stephensen C. Burden of infection on growth failure. Journal of Nutrition. 1999;129(2):534. doi: 10.1093/jn/129.2.534S. [DOI] [PubMed] [Google Scholar]
- Stini WA. Nutritional stress and growth: sex difference in adaptive response. American journal of physical anthropology. 1969;31(3):417–426. doi: 10.1002/ajpa.1330310316. [DOI] [PubMed] [Google Scholar]
- Stinson S. Physical growth of Ecuadorian Chachi Amerindians. American Journal of Human Biology. 1989;1(6):697–707. doi: 10.1002/ajhb.1310010607. [DOI] [PubMed] [Google Scholar]
- Stinson S. Variation in body size and shape among South American Indians. American Journal of Human Biology. 1990;2(1):37–51. doi: 10.1002/ajhb.1310020105. [DOI] [PubMed] [Google Scholar]
- Stinson S. Human Biology: An Evolutionary and Biocultural Perspective. 2. 2012. Growth variation: biological and cultural factors; pp. 587–635. [Google Scholar]
- Stirling MW. Historical and ethnographical material on the Jivaro Indians. US Government Printing Office; 1938. [Google Scholar]
- Tanner JM. A history of the study of human growth. Cambridge University Press; 1981. [Google Scholar]
- Tobias P. Physique and body composition in Southern Africa. Journal of human evolution. 1972;1(4):339–343. [Google Scholar]
- Ulijaszek S. Between-population variation in pre-adolescent growth. European journal of clinical nutrition. 1994;48:S5–13. discussion S13–14. [PubMed] [Google Scholar]
- Ulijaszek S. Growth and body size: In Human energetics in biological anthropology Cambridge Studies in Biological Anthropology. Vol. 16. Cambridge: Cambridge University Press; 1995. [Google Scholar]
- Ulijaszek S. Ethnic differences in patterns of human growth in stature; 2001. Philadelphia: Lippincott-Raven; 1999. pp. 1–20. [Google Scholar]
- Ulijaszek SJ. The International Growth Standard for Children and Adolescents Project: Environmental influences on preadolescent and adolescent growth in weight and height. Food and nutrition bulletin. 2006;27(4):S279–S294. doi: 10.1177/15648265060274S510. [DOI] [PubMed] [Google Scholar]
- Victora CG. The association between wasting and stunting: an international perspective. The Journal of nutrition. 1992;122(5):1105–1110. doi: 10.1093/jn/122.5.1105. [DOI] [PubMed] [Google Scholar]
- Walker R, Gurven M, Hill K, Migliano A, Chagnon N, De Souza R, Djurovic G, Hames R, Hurtado AM, Kaplan H, et al. Growth rates and life histories in twenty-two small-scale societies. American Journal of Human Biology. 2006;18(3):295–311. doi: 10.1002/ajhb.20510. [DOI] [PubMed] [Google Scholar]
- Wang S, Lewis CM, Jr, Jakobsson M, Ramachandran S, Ray N, Bedoya G, Rojas W, Parra MV, Molina JA, Gallo C. Genetic variation and population structure in Native Americans. PLoS genetics. 2007;3(11):e185. doi: 10.1371/journal.pgen.0030185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells JCK, Cortina-Borja M. Different associations of subscapular and triceps skinfold thicknesses with pathogen load: An ecogeographical analysis. American Journal of Human Biology. 2013 doi: 10.1002/ajhb.22418. [DOI] [PubMed] [Google Scholar]
- WHO. Physical status: The use of and interpretation of anthropometry, Report of a WHO Expert Committee. 1995. [PubMed] [Google Scholar]
- WHO. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatrica (Oslo, Norway: 1992) Supplement. 2006;450:76. doi: 10.1111/j.1651-2227.2006.tb02378.x. [DOI] [PubMed] [Google Scholar]
- WHO. Life tables for WHO Member States. 2011;2011 W\ lV who inl/gho/counlries/chn/en. [Google Scholar]
- Wilson WM, Bulkan J, Piperata BA, Hicks K, Ehlers P. Nutritional status of Makushi Amerindian children and adolescents of Guyana. Annals of human biology. 2011;38(5):615–629. doi: 10.3109/03014460.2011.588248. [DOI] [PubMed] [Google Scholar]
- Zonta M, Oyhenart E, Navone G. Nutritional status, body composition, and intestinal parasitism among the Mbyá-Guaraní communities of Misiones, Argentina. American Journal of Human Biology. 2010;22(2):193–200. doi: 10.1002/ajhb.20977. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.