Abstract
Prior work suggests there may be two distinct pathways of alcohol use disorder (AUD) risk: one associated with positive emotion enhancement and behavioral impulsivity, and one associated with negative emotion relief and coping. We sought to map these two pathways onto individual differences in neural reward and threat processing assessed using BOLD fMRI in a sample of 759 undergraduate students (426 women, mean age 19.65±1.24) participating in the Duke Neurogenetics Study. We demonstrate that problem drinking is highest in the context of stress and in those with one of two distinct neural phenotypes: 1) a combination of relatively low reward-related activity of the ventral striatum (VS) and high threat-related reactivity of the amygdala; or 2) a combination of relatively high VS activity and low amygdala reactivity. In addition, we demonstrate that the relationship between stress and problem alcohol use is mediated by impulsivity, as reflected in monetary delay discounting rates, for those with high VS-low amygdala reactivity, and by anxious/depressive symptomatology for those with the opposite neural risk phenotype. Across both neural phenotypes, we found that greater divergence between VS and amygdala reactivity predicted greater risk for problem drinking. Finally, for those individuals with the low VS-high amygdala risk phenotype we found that stress not only predicted the presence of a DSM-IV diagnosed AUD at the time of neuroimaging, but also subsequent problem drinking reported three months following study completion. These results offer new insight into the neural basis of AUD risk and suggest novel biological targets for early individualized treatment or prevention.
Introduction
With a combined lifetime prevalence of approximately 30%, alcohol abuse and dependence are among the most common and debilitating psychiatric disorders in the United States.1 Young adults represent a vulnerable population wherein emerging patterns of abuse particularly in response to stress2 can precipitate long-term dependence and associated negative sequelae in mental and physical health as well as achievement in academic and occupational settings.3–9 While viable treatment options are available, relatively high long-term relapse rates10 highlight the need for more effective prevention. Both treatment and prevention efforts have been hampered by substantial disorder heterogeneity, but subtyping individuals with alcohol use disorders (AUD) based on clinical presentation and overt behavioral traits has ultimately proven ineffective in improving long-term treatment efficacy.11 Defining distinct biological pathways of risk in highly vulnerable populations before the onset of disorder may contribute to novel subtyping paradigms that may yield more effective strategies for intervention and prevention.
Neuroimaging studies of normative or at-risk populations have proven particularly useful in determining potential biological targets for prevention of AUD. The majority of available studies have sought to map individual differences in the functioning of a corticostriatal circuit supporting reward processing and motivation onto behavioral or psychometric indices of AUD risk. This work has collectively demonstrated that reward-related activity of the ventral striatum (VS), which serves as a neural hub through which information processing is coordinated within the corticostriatal circuit, is positively correlated with risk-related behaviors including delay discounting12 and self-reported impulsivity.13, 14 Other studies have demonstrated a direct positive association between reward-related VS activity and harmful drinking patterns.15, 16 In contrast to this relative VS hyper-activity associated with risk and disorder, an extensive parallel literature has highlighted the contribution of relative VS hypo-activity to drug-seeking behaviors, possibly as a means to compensate for blunted positive incentive processing.17 Thus, both hyper- and hypo-activity of the VS in response to reward-related stimuli may confer relative risk for AUD.
A smaller emergent literature has focused on the contributions of a corticolimbic circuit supporting threat processing in the emergence of AUD risk. Several studies provide convergent evidence that relatively reduced threat-related reactivity of the amygdala, which functions as the information processing hub of the corticolimbic circuit, may increase AUD risk, possibly via diminished recognition of, and reaction to, the hazards of excessive drinking. Specifically, one prior study has demonstrated blunted threat-related amygdala reactivity in currently healthy individuals at high familial risk for AUD.18 Along similar lines, genetic liability for substance use19 has been linked to relatively reduced threat-related amygdala reactivity in a normative population of middle-aged adults.20 Conversely, we recently demonstrated that relatively increased threat-related amygdala reactivity may be a protective factor against stress-related problem drinking associated with relative VS hyper-activity in university students.21 Interestingly, heightened threat-related amygdala reactivity is characteristic of mood and anxiety disorders,22, 23 which are frequently comorbid with, and contribute to the emergence of, AUD.24, 25 Thus, as may be true with reward-related VS activity, both hypo- and hyper-reactivity of the amygdala to threat may contribute to AUD risk.
The seemingly mixed nature of these findings can be at least partially explained by results from a largely independent behavioral and psychometric literature which has suggested the existence of two major pathways of risk for problem drinking. The first is associated with behavioral disinhibition and positive emotion enhancement and the second with negative emotion relief and coping.26–28 The predominant pathway of risk for any individual may further differ as a function of biologically-based personality traits including neuroticism and extraversion.26, 29 Consistent with this notion, each pathway likely has a unique neural signature present before the development of AUD. However, no prior work has attempted to map these putative behavioral and psychological subtypes of AUD risk onto individual differences in the functioning of the neural circuits for threat and reward.
Here we use data from a sample of 759 university students to probe how differences in reward-related VS activity and threat-related amygdala reactivity might jointly contribute to distinct pathways of problem drinking and AUD risk. We focus specifically on problem drinking in the context of recent life stress, because stressful life experiences are a known risk factor for the development and persistence of AUD,30 as well as potent modulators of signaling in both neural circuits.31, 32 Based on the literature reviewed above, we hypothesized that particularly high levels of stress-related problem drinking will occur in individuals with a combination of relatively high VS and low amygdala reactivity, presumably through a positive emotion enhancement and disinhibition pathway, and those with a low VS and high amygdala reactivity, presumably through a relief/coping pathway.
Methods
Participants
Data were derived from 897 participants (513 women, mean age 19.62±1.24) who had successfully completed the ongoing Duke Neurogenetics Study (DNS) as of as of December 31, 2013. The DNS assesses a range of behavioral and biological traits among young adult, university students. The study was approved by the Duke University School of Medicine Institutional Review Board. All participants provided informed consent in accord with Duke University guidelines, and were in good general health. All participants were free of the following exclusionary criteria: (1) medical diagnoses of cancer, stroke, diabetes requiring insulin treatment, chronic kidney or liver disease, or lifetime history of psychotic symptoms; (2) use of psychotropic, glucocorticoid, or hypolipidemic medication; and (3) conditions affecting cerebral blood flow and metabolism (e.g., hypertension).
Our analyses focused on a subset of 759 participants (426 women, mean age 19.65±1.24) with BOLD fMRI data surviving a stringent multi-level quality control procedure (Supplementary Table 1). Of these 759 participants, 127 (55 women; mean age 19.79±1.21) met criteria for at least one current Axis I or select Axis II (borderline or antisocial personality disorder) diagnosis according to DSM-IV. The majority of these individuals (n=86; 35 women, mean age 19.94±1.15) were diagnosed with AUD (Supplementary Table 2). Since our study focuses on inter-individual variability in problem drinking, we did not exclude individuals with categorical disorders from analyses. Furthermore, we used presence of a current alcohol-related disorder (alcohol abuse or dependence) as a dependent variable in a subset of analyses. Notably, participants were required to pass a breathalyzer test before scanning to ensure they were not acutely intoxicated at the time of data collection. In addition, participants were asked to refrain from using any psychoactive substances while participating in the study and notified that they would be subject to a random drug screen on the day of their scan. Immediately preceding neuroimaging data collection, every 10th male participant was asked to provide a urine sample, which was tested on a QuickScreen Pro Drug Screening test (Phamatech Inc, San Diego, CA) for the presence of amphetamine, methamphetamine, opiates, cocaine and tetrahydrocannabinol (THC). Due to the pharmacokinetics of THC,33 the presence of the chemical in urine was not deemed exclusionary unless the participant was acutely intoxicated. No participant was excluded for acute THC intoxication or tested positive for any other substance.
BOLD fMRI Paradigms
Our amygdala and VS reactivity paradigms have been described in detail previously.34, 35 Briefly, the amygdala reactivity paradigm consists of 4 blocks of a face-processing task interleaved with 5 blocks of a sensorimotor control task. During task blocks, participants view a trio of faces (with neutral, angry, fearful or surprised expressions) and match 1 of 2 faces (bottom) identical to a target face (top). During control blocks, participants match simple geometric shapes. Here, we focus on the contrast of all task blocks versus control blocks (i.e., All Faces > Shapes), as all four facial expressions in our paradigm convey threat to varying degrees.36–39 Thus, we construe the All Faces > Shapes contrast as broadly threat-related. In an exploratory set of analyses we further investigated each expression-specific contrast independently to examine the extent to which amygdala reactivity to specific forms of threat (e.g., threat originating from an unspecified source in the shared environment as conveyed by fearful expressions or threat originating from a discrete source as conveyed by angry expressions) may modulate the relation between stress, VS activity and alcohol use.
Our VS reactivity paradigm consists of a number guessing task wherein participants receive predominantly positive feedback (80% correct guess), predominantly negative feedback (20% correct guess), or no feedback. There are three pseudorandomly presented blocks of each condition. Participants are unaware of the fixed outcome probabilities associated with each block and are led to believe their performance will determine a net monetary gain at the end of the scanning session. Instead, all participants receive $10. Here we focus on differential VS reactivity from Positive > Negative Feedback blocks. BOLD fMRI acquisition parameters, preprocessing and analytic techniques are described in detail in Supplementary Methods.
Self-Report and Behavioral Measures
Problem drinking over the past year was assessed using the Alcohol Use Disorder Identification Test (AUDIT).40 Responses on all items were summed to form a total score, which was then used in analyses. Recent life stress was assessed using a modified version of the Life Events Scale for Students (LESS).34 This modified version of the scale asks participants to indicate whether they experienced common stressful life events within the past 12 months; in addition, for each event that occurred participants reported on the impact it had on their lives on a 1–4 scale (with 4 being the highest). The impact scores were set to zero for events that did not occur. Based on prior research34, we focused on the highest impact metric. To ensure the specificity of our results to current life stress, we also assessed early life trauma using the Childhood Trauma Questionnaire (CTQ)41 and used CTQ Total scores as a covariate in all regression analyses. Current levels of anxiety and dysphoria were assessed using the Mood and Anxiety Symptom Questionnaire (MASQ). The MASQ provides four subscales – general distress depression (GDD) and anxiety (GAD), reflecting non-specific shared depressive and anxious symptomatology, as well as anxious arousal (AA) and anhedonia (AD), which reflect symptoms specific to anxiety and depression, respectively.42 Notably, two thirds of the items included in the AD scale are positively phrased (e.g., “I felt cheerful”, “I felt optimistic” etc). Thus we conceptualize the AD scale as tapping into positive emotion and hedonic processing more generally, rather than anhedonia specifically. Finally, behavioral impulsivity was measured using a computerized monetary delay discounting task (Supplementary Methods).12
Statistical Analyses
Linear or logistic regression models using LESS Highest Impact, amygdala reactivity, VS activity, and their interactions as independent variables, and AUDIT or AUD diagnosis as the dependent variable, respectively, were conducted in IBM SPSS Statistics 21 (IBM, Armonk, NY). Significant three-way stress x amygdala reactivity x VS activity interactions where probed by testing the significance of the slope linking LESS and AUDIT or AUD diagnosis at low (1 SD below the mean), intermediate (mean), and high (1 SD above the mean) levels of amygdala reactivity and VS activity as implemented in Model 3 of the PROCESS macro43 for SPSS. To more comprehensively depict the moderating effects of amygdala and VS reactivity across the entire range of their respective distributions, we further estimated the beta coefficient linking LESS and AUDIT at all observed amygdala and VS activity values. The distribution of AUDIT scores was slightly positively skewed (skewness = 0.963, kurtosis = 0.833). Nonetheless, skewness and kurtosis of this magnitude are still below the recommended cut-off for linear regression analysis,44 thus we have conducted all our analyses using raw AUDIT scores. Notably, however, our results did not substantially change when we repeated all analyses using a square root transformation of AUDIT resulting in a more normalized distribution (skewness = −0.307, kurtosis = −0.485; data available upon request).
To probe the potential mechanisms underlying any significant interaction effects, we constructed moderated mediation models, where we tested if current levels of anxious and depressive symptoms, as well as delay discounting, conditionally mediate the relationship between stress to AUDIT scores as a function of VS activity and amygdala reactivity. All moderated mediation analyses were conducted using Model 19 of the PROCESS macro in SPSS.43 Bootstrapped bias-corrected confidence intervals (CIs) for each indirect effect were generated using 5,000 bootstrapping iterations. Model fit was assessed using Mplus 6.12.45 Based on prior research,21 we focused all of our analyses on amygdala reactivity and VS activity in the left hemisphere. However, we also report results from exploratory analyses of the same statistical relationships in right hemisphere activation clusters (Supplementary Figure 1). The significance for all tests was set at p<0.05, two-sided. Due to the strong a priori rationale for building our statistical models, no correction for multiple testing was applied.
Results
Main Effects of Tasks and Demographics
Consistent with prior research, our amygdala and VS BOLD fMRI paradigms elicited robust threat-related amygdala reactivity (Figure 1a) and reward-related VS activity (Figure 1b), respectively. In addition, men showed higher activation in both regions (t(757) values>1.86, p values<0.064), while age was negatively correlated with VS activity bilaterally (b values<-0.06; p values<0.070). Notably these effects of age remained when controlling for gender (p values<0.05). Consistent with prior reports,46 the men in our sample reported higher AUDIT scores than women (6.36±4.74 vs. 4.35±3.77, t(737)=6.30, p<0.001). Race/ethnicity moderated AUDIT scores, such that non-Hispanic Caucasian participants reported drinking more than all other ethnic groups except for the Hispanic and multiracial group (F(3,755)=4.61, p=0.003, significant post hoc p values < 0.04 LSD-corrected). None of the other groups differed from each other (p values > 0.10 LSD-corrected). In light of these effects, all analyses were conducted with and without gender, age, and race/ethnicity, in addition to CTQ scores, as covariates.
Figure 1.
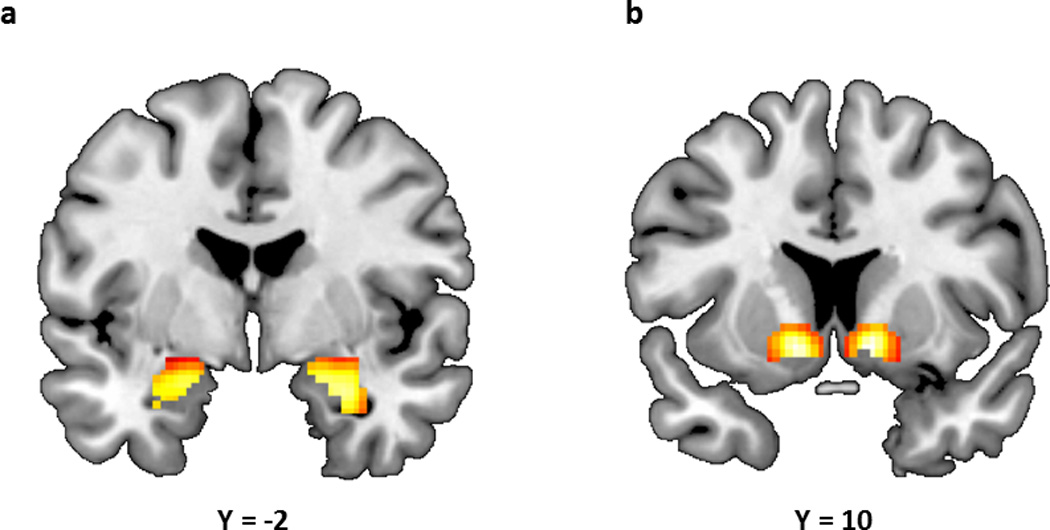
(a) Statistical parametric map illustrating mean bilateral threat-related amygdala reactivity (left: x=−22, y=−6, z=−18, t=38.64, p<0.000001, kE=167; right: x=28, y=−4, z=−20, t=42.97, p<0.000001, kE=198). (b) Statistical parametric map illustrating mean bilateral reward-related VS reactivity (left: x=−12, y=8, z=−8, t=13.12, p<0.000001, kE=303; right: x=12, y=10, z=−8, t=12.63, p<0.000001, kE=290). Activation clusters in (a) and (b) are overlaid onto canonical structural brain images in the coronal plane.
Predictors of Stress-Related Problem Drinking
LESS Highest Impact scores were positively correlated with AUDIT scores (b=0.108, p=0.003) and this effect was robust to gender, age, and race/ethnicity (b=0.132, p=0.0002). Importantly, however, the relation between LESS and AUDIT scores was moderated by amygdala and VS activation (b=−0.377, p=0.022), such that relatively increased levels of stress-related problem drinking were observed in individuals with one of two distinct neural profiles (Figure 2). The first risk profile consisted of a combination of relatively low VS activity and relatively high amygdala reactivity, while the second risk profile consisted of the opposite combination of relatively high VS activity and relatively low amygdala reactivity. Notably, while stress predicted greater problem drinking for those with intermediate (i.e., mean) levels of amygdala reactivity and VS activity as well, the relative risk (i.e., the strength of the linear relationship between stress and problem drinking) increased as the imbalance between these two neural phenotypes increased (Supplementary Figure 2). In contrast, those individuals in whom both VS activity and amygdala reactivity was either low (<1SD below the mean) or high (>1 SD above the mean) did not show any increase in problem drinking as a function of stress (b estimates< 0.240, p values>0.46). This three-way interaction was independent of gender, age, race/ethnicity, and CTQ scores (b=−0.366, p=0.022), and was not further moderated by any of these factors (all p values>0.60). Furthermore, the three-way interaction remained at trend-levels, with significant simple slopes, when non-drinkers (i.e., individuals with AUDIT scores=0; n=111) were removed from the analysis (without covariates: b=−0.302, p=0.069; with covariates: b=−0.301, p=0.060; Supplementary Figure 3).
Figure 2.
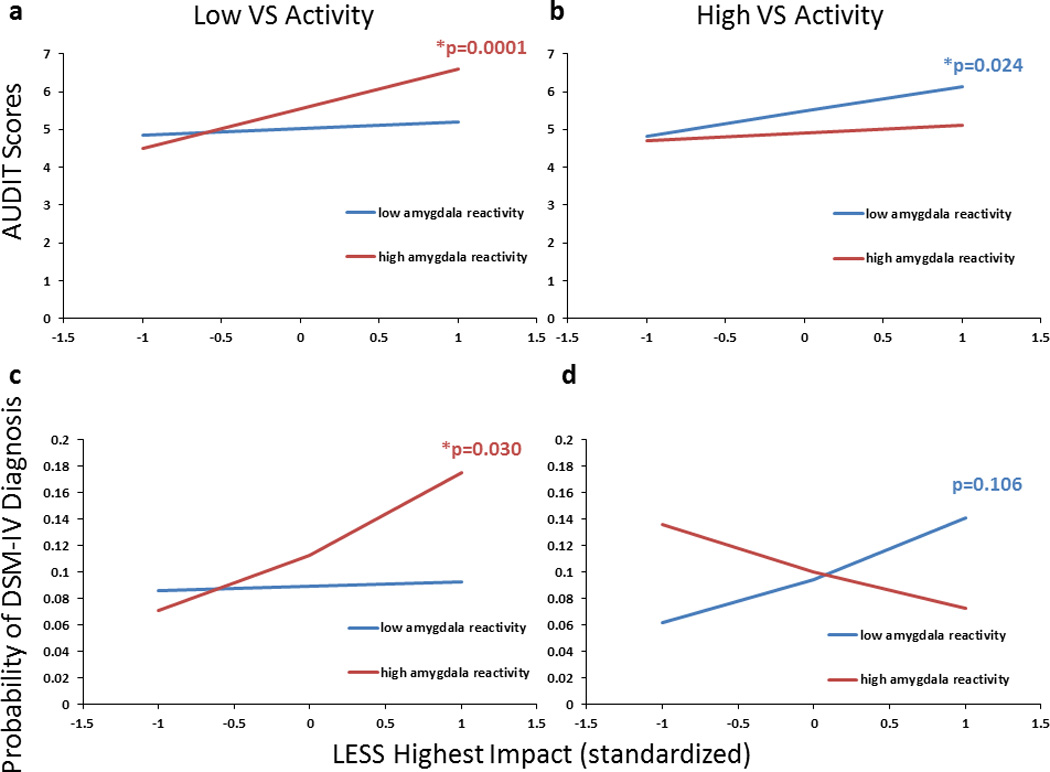
Amygdala and VS reactivity jointly moderated the relation between recent life stress and problem drinking. Slopes represent beta coefficient estimates reflecting the strength of the relation between LESS and AUDIT scores (a–b) or AUD diagnosis (c–d) at varying levels of amygdala and VS activity. High levels of stress were associated with larger increases in AUDIT scores for participants with a combination of (a) low VS (-1SD) and high amygdala (+1SD) reactivity or (b) high VS and low amygdala reactivity. High levels of stress were also associated with larger increases in the likelihood of having a concurrent AUD diagnosis for those with low VS and high amygdala reactivity (c), but not with those with high VS and low amygdala reactivity (d), for whom this relationship was only observed at 2.5 SD >mean (data not shown). Simple slopes are adjusted for gender, age, race/ethnicity and CTQ.
Predictors of Alcohol Use Disorder
Extending these results to the clinical range of problem drinking, we found that the same three-way interaction between LESS, amygdala reactivity, and VS activity predicted the likelihood of being diagnosed with an AUD, regardless of comorbidity (without covariates: b=−0.370, p=0.016; with covariates: b=−0.391, p=0.014; Figure 2c–d). Notably, stress was a more accurate predictor of the presence of a diagnosis for those with a combination of relatively low VS activity (<-1 SD) and high (>1 SD) amygdala reactivity, than for those with low amygdala reactivity and high VS activity, as in the latter group significant LESS-AUDIT correlations were only observed at larger levels of imbalance between VS activity and amygdala reactivity (e.g., ≥2.5 SD away from the mean; data not visualized). This same three-way interaction did not predict the probability of being diagnosed with a non-alcohol-related disorder (p values>0.60).
Relationship with Behavioral Traits
To evaluate whether the low VS-high amygdala risk profile is associated with drinking to reduce negative emotion, we tested a moderated mediation model with combined anxious and depressive symptomatology (sum of MASQ GDD, GDA and AA scores) as a mediator between LESS and AUDIT, and the interaction effects of VS activity and amygdala reactivity as moderators of the path between mediator and outcome (Figure 3). To ensure the results were not driven by changes in positive emotion, we removed items tapping into hedonic processing (i.e., MASQ AD scale) from our measure of depression and anxiety and included them as an independent covariate. This resulted in an acceptable model fit (χ2=38.243, df=9, p <0.001; RMSEA=0.065, CFI=0.925). Furthermore, within this model stress was strongly positively correlated with anxious and depressive symptomatology (b=0.0448, SE: 0.0079, p<0.001), which in turn mediated the relation between LESS and AUDIT scores for those individuals with relatively low VS activity and high amygdala reactivity (parameter estimate=0.1468, SE: 0.0621, 95% CI: 0.0423–0.2885), but not for those with the opposite neural risk phenotype (parameter estimate=0.1187, SE: 0.0694, 95% CI: −0.0019–0.2763, Figure 4a). Self-reported hedonic responsiveness levels (MASQ AD scale) did not mediate the relationship between LESS and AUDIT scores at any level of VS activity or amygdala reactivity, possibly due to their lack of an association with recent stress in the current sample (b=−0.0118, SE: 0.0346, p=0.73).
Figure 3.
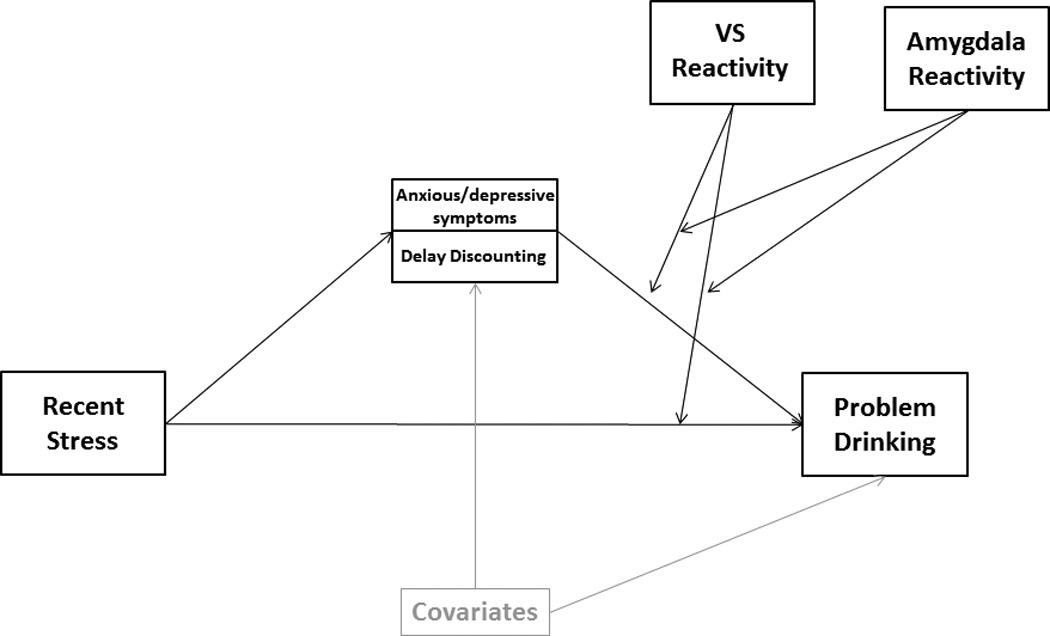
Conceptual depiction of the path analytic models tested to evaluate anxious/depressive symptomatology and delay discounting as mediators of the relation between stress and problem drinking at different levels of amygdala and VS reactivity.
Figure 4.
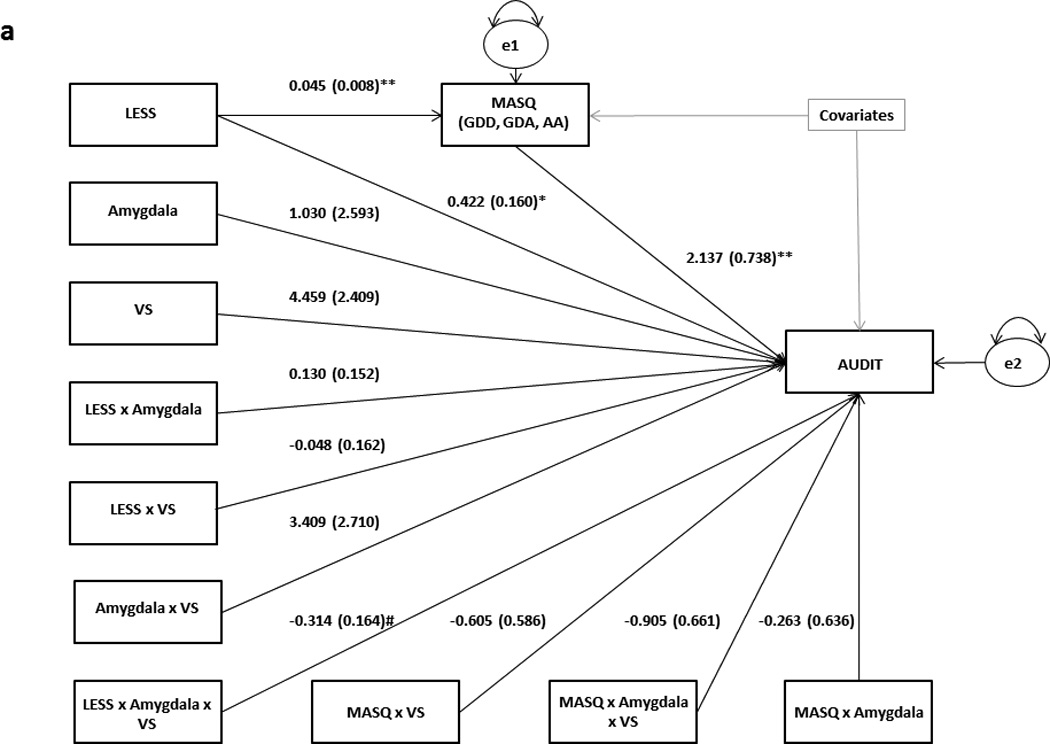
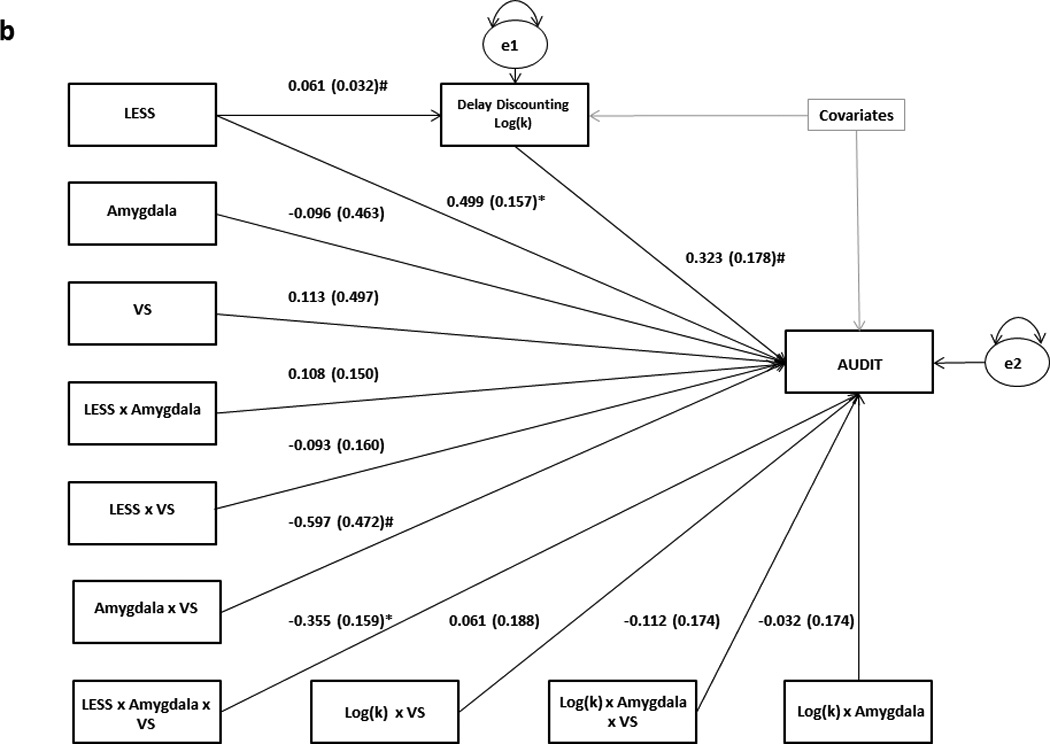
Results from path analytic moderated mediation models using (a) anxious/depressive symptoms and (b) delay discounting as mediators between LESS and AUDIT at varying levels of VS and amygdala reactivity. Raw regression coefficients are presented for each path, along with standard errors in parentheses. Covariates in both models include gender, age, race/ethnicity (dummy coded) and CTQ. MASQ AD was additionally controlled for in (a). For the sake of brevity, individual covariates and their associated paths are not depicted. Variance in the mediators and AUDIT scores unaccounted for by the model are denoted as e1 and e2, respectively.
**p<0.001
*p<0.05
#p<0.1
To further assess whether the high VS-low amygdala neural risk profile is associated with a positive emotion enhancement/behavioral disinhibition pathway to problem drinking, we tested a similar moderated mediation model using delay discounting scores as a mediator (Figure 3). The model fit the data very well (χ2=9.639, df=9, p=0.3805; RMSEA=0.010, CFI=0.995). While delay discounting was marginally positively correlated with LESS (p=0.059), it did not mediate the relationship between LESS and AUDIT scores for any amygdala reactivity or VS activity values at the 95% confidence level. However, at the 92% confidence level, delay discounting was a more reliable mediator of the relationship between LESS and AUDIT scores for those with relatively high VS activity and low amygdala reactivity (parameter estimate=0.0311, SE: 0.0271, 92% CI: 0.0001–0.1031), than for those with the opposite combination of neural traits (parameter estimate=0.0204, SE: 0.0278, 92% CI: −0.0087–0.0960; Figure 4b).
Follow-up Assessment
In a subsample of participants who completed a follow-up survey (n=232, 136 women, mean age at follow up 19.64±1.22; b=−0.469, p=0.044), amygdala reactivity and VS activity further interacted to predict the relationship between LESS and AUDIT scores reported three months following completion of the DNS protocol including neuroimaging. This relationship remained trending when accounting for CTQ scores and demographic variables including age, gender, and race/ethnicity (b=−0.441, p=0.068; Figure 5). Importantly, LESS was associated with AUDIT scores primarily in participants with the low VS-high amygdala risk phenotype (p=0.021), while the effect was less reliable for those with the high VS-low amygdala risk phenotype (p=0.080).
Figure 5.
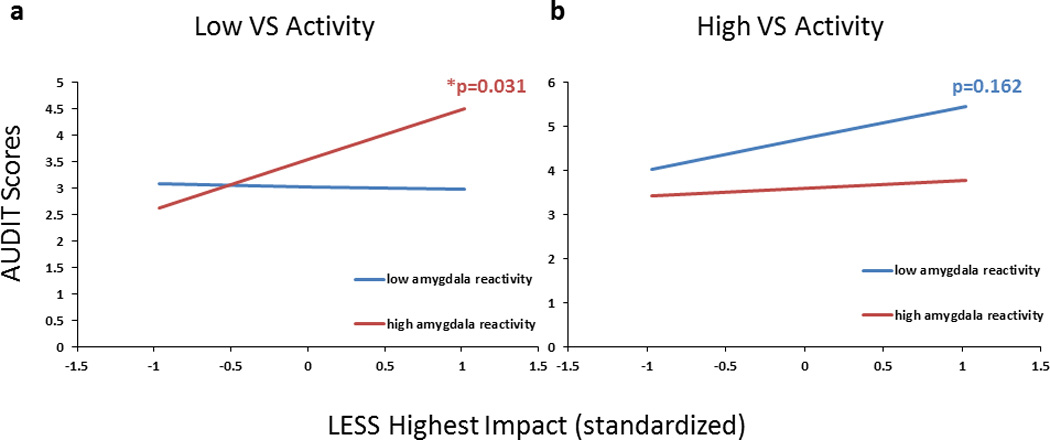
Amygdala and VS reactivity jointly moderated the relation between recent life stress and problem drinking three months following initial study completion. Slopes represent beta coefficient estimates reflecting the strength of the relation between LESS and AUDIT scores, measured at the three month follow-up assessment, as a function of varying levels of amygdala and VS activity assessed at baseline. High levels of stress were associated with larger increases in AUDIT scores for participants with a combination of low VS (-1SD) and high amygdala (+1SD) reactivity (a), but not for those with a combination of high VS and low amygdala reactivity (b). Simple slopes are adjusted for gender, age, race/ethnicity and CTQ.
Expression-specific Contrasts
All analyses were repeated with each individual expression-specific contrast. All reported effects were confirmed with respect to amygdala activity to fearful expressions using the Fear>Shapes contrast (Supplementary Figure 4–Supplementary Figure 6), but not for any of the other expressions (all p values > 0.17). Notably, in the model using the Fear>Shapes contrast, LESS predicted higher likelihood of being diagnosed with an AUD at less extreme values in the low amygdala-high VS phenotype (1 SD below and above the mean, respectively) in comparison to the model using the more general All Faces>Shapes contrast (Supplementary Figure 4d).
Discussion
Here we confirm our prior work21 by demonstrating in a larger sample that the combination of relatively low threat-related amygdala and relatively high reward-related VS reactivity represent a neural risk phenotype for stress-related problem drinking in young adult university students. We also extend this prior work by further demonstrating that the opposite pattern of relatively high threat-related amygdala and relatively low reward-related VS reactivity also predicts stress-related problem drinking. In addition, we demonstrate that the relationship between stress and problem drinking is mediated by higher impulsivity in the form of steeper monetary delay discounting for those with high VS-low amygdala reactivity and by anxious/depressive symptomatology for those with the opposite neural risk phenotype. Across both neural phenotypes, we found that greater divergence or mismatch between VS and amygdala reactivity predicted greater risk for problem drinking. In contrast, balance between VS and amygdala reactivity (i.e., either both low or both high) was protective against stress-related problem drinking. Finally, for those individuals with the low VS-high amygdala risk phenotype we found that stress not only predicted the presence of a DSM-IV diagnosed AUD at the time of neuroimaging, but also the subsequent self-reported problem drinking three months in the future.
The two distinct neural risk profiles we identify may at least partially map onto previously identified psychological pathways to problem drinking. Specifically, the high VS-low amygdala profile may be associated with a pathway characterized by positive emotion enhancement and behavioral disinhibition. The opposite risk phenotype of low VS-high amygdala reactivity may conversely map onto a pathway associated with negative emotion relief and stress coping. Notably, however, we found that unlike shared anxious/depressive symptomatology and delay discounting, self-reported positive emotion and hedonic processing did not significantly mediate the relationship between stress and problem drinking. While some studies have pointed out similarities and potential overlap between the positive emotion and disinhibition pathways to problem drinking,28, 29 others have suggested that positive emotion enhancement and lack of behavioral control may be distinct determinants of excessive alcohol consumption.27, 47, 48 Furthermore, drinking to enhance positive emotion, even when excessive and problematic, is less likely to increase as a function of recent life stress, whereas convergent data from animal models49, 50 and humans51, 52 suggests that stress can increase behavioral disinhibition and impulsive responding. Thus, perhaps the high VS-low amygdala phenotype we identify more directly shapes the risk pathway characterized by high impulsivity and behavioral disinhibition, rather than the risk pathway associated with the enhancement of positive emotion.
Relatedly, we found that across analyses the association between stress and both problem drinking and AUD diagnosis was stronger for those with the low VS-high amygdala phenotype relative to the opposite phenotype. This may reflect our focus on problem drinking specifically occurring in the context of recent stress, which is particularly important in our population of young adult university students.53 Thus, our analytic strategy may have been particularly well-suited for the discovery of risk factors predisposing to relief drinking or perhaps even neural phenotypes associated with a broader vulnerability to stress-related psychopathology. Consistent with the latter, a prior study has shown that a combination of relatively high amygdala reactivity and relatively low VS reactivity, but not the opposite combination, may be a risk factor for post-traumatic stress disorder (PTSD).54
Along similar lines and consistent with the propositions put forth by the research domain criteria (RDoC) initiative,55 it is possible that the neural risk phenotypes we identified may predispose to two distinct cross-disorder vulnerabilities: one associated with impulse control and behavioral disinhibition, and one associated with negative emotion dysregulation, broadly defined, rather than simply two distinct pathways of risk for “pure” AUD. In further support of this notion and consistent with prior literature,56, 57 more than one third of the participants meeting criteria for AUD in the current sample had a comorbid disorder (Supplementary Table 2). While the current study is underpowered to detect contributions of each neural risk phenotype to specific diagnostic comorbidities, this notion can be tested in cohorts enriched for other psychiatric disorders and/or followed longitudinally to assess lifetime comorbidity.
While our principal findings implicate broad amygdala activity to emotional facial expressions, which can convey varying degrees of threat, our subsequent expression-specific analyses revealed that amygdala activity to unspecified environmental threat as conveyed by fearful facial expressions in our paradigm specifically contributes to risk in our sample. Prior work suggests that amygdala activity to fearful facial expressions may be specifically associated with anxiety and sensitivity to distress, whereas amygdala activity to angry expressions may tap more directly into aggression and reactivity to interpersonal challenge.35, 58 Thus, the relative specificity of our results to fearful expressions is consistent with the construal of the low VS-high amygdala phenotype as predisposing to alcohol-related problems via an affective risk pathway and supports the broader conceptualization of our findings.
Our study is, of course, not without limitations. First and foremost, while AUDIT scores represented in our sample spanned a wide range (0–23), the majority of our participants scored below 8 – the generally accepted threshold for clinical significance. However, a substantial minority of participants scored 8 or above (n=197, 26%), suggesting our findings are not restricted to individuals who are merely social drinkers. Even more tellingly, we confirmed the potential clinical relevance of our model in analyses predicting clinical diagnosis of an AUD.
As a related limitation, alcohol consumption and harmful drinking patterns are known to be highest within our sample population of 18–22 year old university students with a marked decrease thereafter.59, 60 Thus, it is unclear whether problem drinking assessed at this developmental stage translates into subsequent AUD diagnosis in adulthood. Some studies, however, suggest that drinking patterns observed in college may be predictive of alcohol-related problems later in life.5, 6 Furthermore, the fact that we were able to predict the relationship between stress and problem drinking three months following study completion lends credibility to the potential utility of our neural risk phenotypes as predictors of future alcohol-related outcomes. Tracking individuals over longer periods of time would be helpful in determining the value of these neural phenotypes as predictors of long-term risk for the emergence and persistence of AUD. Gathering pertinent information from high school records or assessments and continuing to follow individuals throughout their university years and beyond could be a particularly informative and feasible strategy to implement.
As a further limitation, stress and alcohol use were assessed concurrently, using retrospective self-report measures, which creates vulnerability to reporting bias. In addition, our findings are correlational in nature and it is conceivable that rather than stress precipitating problem drinking, problem drinking is precipitating stressful life events. Even if the causality is reversed relative to our interpretation, this would not undermine the observation that individuals with greater divergence or mismatch between reward-related VS and threat-related amygdala reactivity are at increased risk. However, in this case risk would be redefined as an increased probability that problem drinking would result in the experience of more impactful stressful life events. In fact, it is possible that both causalities hold true, as stress arising from prior alcohol use could contribute to the maintenance and escalation of harmful drinking patterns, thus creating a vicious cycle.61 Larger developmental prospective longitudinal studies in at-risk and normative populations would afford the opportunity to establish risk trajectories and causality in a more definitive way.
Finally, while BOLD fMRI assessment of reward- and threat-related brain function may help subtype individuals into distinct categories of AUD risk, it is likely impractical to implement neuroimaging in a general clinical setting, especially in large groups of people. Genetic assays in contrast have become increasingly accessible in recent years and may offer a faster and more affordable way to identify at-risk individuals, while also offering valuable insight into the molecular basis of some of the observed inter-individual variability. Efforts to map reward- and threat-related brain function onto common genetic variation have been ongoing.62 Recent work from our group has highlighted promising novel strategies to increase the amount of variability accounted for by readily assayed molecular indices of these neural risk phenotypes. Specifically, we have demonstrated that a biologically-informed multilocus genetic profile reflecting the cumulative impact of five polymorphisms on dopamine signaling predicts approximately 11% of the inter-individual variability in reward-related VS reactivity.63 In addition, we recently provided evidence that epigenetic modifications impacting serotonin signaling predict a similar amount of variability in threat-related amygdala reactivity.64 Combining and refining these two strategies in future work may not only allow for a more affordable and reliable way to identify at-risk individuals, but also help uncover novel molecular targets for intervention and prevention.
Supplementary Material
acknowledgments
We thank Bartholomew Brigidi, Kelly Faig, Adam Gorka, Adrienne Romer, and Matthew Scult for their assistance in DNS data collection and analysis. The DNS is supported by Duke University and NIDA grant DA033369. Y.S.N. received support through a pre-doctoral Howard Hughes Medical Institute International Student Research fellowship. A.R.H. receives support through NIDA grants DA033369 and DA031579.
Footnotes
Disclosures
The authors report no conflicts of interest.
References
- 1.Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- 2.Rutledge PC, Sher KJ. Heavy drinking from the freshman year into early young adulthood: the roles of stress, tension-reduction drinking motives, gender and personality. J Stud Alcohol. 2001;62:457–466. doi: 10.15288/jsa.2001.62.457. [DOI] [PubMed] [Google Scholar]
- 3.Grant BF, Dawson DA. Age of onset of drug use and its association with DSM-IV drug abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1998;10:163–173. doi: 10.1016/s0899-3289(99)80131-x. [DOI] [PubMed] [Google Scholar]
- 4.Grant BF, Stinson FS, Harford TC. Age at onset of alcohol use and DSM-IV alcohol abuse and dependence: a 12-year follow-up. J Subst Abuse. 2001;13:493–504. doi: 10.1016/s0899-3289(01)00096-7. [DOI] [PubMed] [Google Scholar]
- 5.Jennison KM. The short-term effects and unintended long-term consequences of binge drinking in college: a 10-year follow-up study. Am J Drug Alcohol Abuse. 2004;30:659–684. doi: 10.1081/ada-200032331. [DOI] [PubMed] [Google Scholar]
- 6.Vaillant GE. A 60-year follow-up of alcoholic men. Addiction. 2003;98:1043–1051. doi: 10.1046/j.1360-0443.2003.00422.x. [DOI] [PubMed] [Google Scholar]
- 7.Park CL, Armeli S, Tennen H. The daily stress and coping process and alcohol use among college students. J Stud Alcohol. 2004;65:126–135. doi: 10.15288/jsa.2004.65.126. [DOI] [PubMed] [Google Scholar]
- 8.Hingson RW, Heeren T, Winter MR. Age at drinking onset and alcohol dependence: age at onset, duration, and severity. Arch Pediatr Adolesc Med. 2006;160:739–746. doi: 10.1001/archpedi.160.7.739. [DOI] [PubMed] [Google Scholar]
- 9.Bingham CR, Shope JT, Tang X. Drinking behavior from high school to young adulthood: differences by college education. Alcohol Clin Exp Res. 2005;29:2170–2180. doi: 10.1097/01.alc.0000191763.56873.c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dawson DA, Goldstein RB, Grant BF. Rates and correlates of relapse among individuals in remission from DSM-IV alcohol dependence: a 3-year follow-up. Alcohol Clin Exp Res. 2007;31:2036–2045. doi: 10.1111/j.1530-0277.2007.00536.x. [DOI] [PubMed] [Google Scholar]
- 11.Mann K, Hermann D. Individualised treatment in alcohol-dependent patients. Eur Arch Psychiatry Clin Neurosci. 2010;260(Suppl 2):116–120. doi: 10.1007/s00406-010-0153-7. [DOI] [PubMed] [Google Scholar]
- 12.Hariri AR, Brown SM, Williamson DE, Flory JD, de Wit H, Manuck SB. Preference for immediate over delayed rewards is associated with magnitude of ventral striatal activity. J Neurosci. 2006;26:13213–13217. doi: 10.1523/JNEUROSCI.3446-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forbes EE, Brown SM, Kimak M, Ferrell RE, Manuck SB, Hariri AR. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Mol Psychiatry. 2009;14:60–70. doi: 10.1038/sj.mp.4002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8:1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- 15.Nees F, Tzschoppe J, Patrick CJ, Vollstadt-Klein S, Steiner S, Poustka L, et al. Determinants of early alcohol use in healthy adolescents: the differential contribution of neuroimaging and psychological factors. Neuropsychopharmacology. 2012;37:986–995. doi: 10.1038/npp.2011.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nikolova YS, Singhi EK, Drabant EM, Hariri AR. Reward-related ventral striatum reactivity mediates gender-specific effects of a galanin remote enhancer haplotype on problem drinking. Genes Brain Behav. 2013;12:516–524. doi: 10.1111/gbb.12035. [DOI] [PubMed] [Google Scholar]
- 17.Bowirrat A, Oscar-Berman M. Relationship between dopaminergic neurotransmission, alcoholism, and Reward Deficiency syndrome. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2005;132B:29–37. doi: 10.1002/ajmg.b.30080. [DOI] [PubMed] [Google Scholar]
- 18.Glahn DC, Lovallo WR, Fox PT. Reduced amygdala activation in young adults at high risk of alcoholism: studies from the Oklahoma family health patterns project. Biol Psychiatry. 2007;61:1306–1309. doi: 10.1016/j.biopsych.2006.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sipe JC, Chiang K, Gerber AL, Beutler E, Cravatt BF. A missense mutation in human fatty acid amide hydrolase associated with problem drug use. Proc Natl Acad Sci U S A. 2002;99:8394–8399. doi: 10.1073/pnas.082235799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hariri AR, Gorka A, Hyde LW, Kimak M, Halder I, Ducci F, et al. Divergent effects of genetic variation in endocannabinoid signaling on human threat- and reward-related brain function. Biol Psychiatry. 2009;66:9–16. doi: 10.1016/j.biopsych.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nikolova YS, Hariri AR. Neural responses to threat and reward interact to predict stress-related problem drinking: A novel protective role of the amygdala. Biology of mood & anxiety disorders. 2012;2:19. doi: 10.1186/2045-5380-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. AJ Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. AJ Psychiatry. 2007;164:318–327. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- 24.Conway KP, Compton W, Stinson FS, Grant BF. Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2006;67:247–257. doi: 10.4088/jcp.v67n0211. [DOI] [PubMed] [Google Scholar]
- 25.Boschloo L, Vogelzangs N, Smit JH, van den Brink W, Veltman DJ, Beekman AT, et al. Comorbidity and risk indicators for alcohol use disorders among persons with anxiety and/or depressive disorders: findings from the Netherlands Study of Depression and Anxiety (NESDA) J Affect Disord. 2011;131:233–242. doi: 10.1016/j.jad.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 26.Verheul R, van den Brink W, Geerlings P. A three-pathway psychobiological model of craving for alcohol. Alcohol Alcohol. 1999;34:197–222. doi: 10.1093/alcalc/34.2.197. [DOI] [PubMed] [Google Scholar]
- 27.Victorio-Estrada A, Mucha RF, Stephan ER. Excessive drinking situations in German alcoholics: replication of a three-factor model used for North Americans. Drug Alcohol Depend. 1996;41:75–79. doi: 10.1016/0376-8716(96)01241-0. [DOI] [PubMed] [Google Scholar]
- 28.Colder CR, O’Connor R. Attention biases and disinhibited behavior as predictors of alcohol use and enhancement reasons for drinking. Psychology of addictive behaviors : journal of the Society of Psychologists in Addictive Behaviors. 2002;16:325–332. [PubMed] [Google Scholar]
- 29.Kuntsche E, Knibbe R, Gmel G, Engels R. Who drinks and why? A review of socio-demographic, personality, and contextual issues behind the drinking motives in young people. Addict Behav. 2006;31:1844–1857. doi: 10.1016/j.addbeh.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 30.Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- 31.Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nature reviews Neuroscience. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- 32.Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. AJ Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ellis GM, Jr, Mann MA, Judson BA, Schramm NT, Tashchian A. Excretion patterns of cannabinoid metabolites after last use in a group of chronic users. Clin Pharmacol Ther. 1985;38:572–578. doi: 10.1038/clpt.1985.226. [DOI] [PubMed] [Google Scholar]
- 34.Nikolova YS, Bogdan R, Brigidi BD, Hariri AR. Ventral striatum reactivity to reward and recent life stress interact to predict positive affect. Biol Psychiatry. 2012;72:157–163. doi: 10.1016/j.biopsych.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 35.Carre JM, Hyde LW, Neumann CS, Viding E, Hariri AR. The neural signatures of distinct psychopathic traits. Social neuroscience. 2013;8:122–135. doi: 10.1080/17470919.2012.703623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahs F, Davis CF, Gorka AX, Hariri AR. Feature-based representations of emotional facial expressions in the human amygdala. Social cognitive and affective neuroscience. 2014;9:1372–1378. doi: 10.1093/scan/nst112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phillips ML, Young AW, Senior C, Brammer M, Andrew C, Calder AJ, et al. A specific neural substrate for perceiving facial expressions of disgust. Nature. 1997;389:495–498. doi: 10.1038/39051. [DOI] [PubMed] [Google Scholar]
- 38.Somerville LH, Kim H, Johnstone T, Alexander AL, Whalen PJ. Human amygdala responses during presentation of happy and neutral faces: correlations with state anxiety. Biol Psychiatry. 2004;55:897–903. doi: 10.1016/j.biopsych.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 39.Kim H, Somerville LH, Johnstone T, Alexander AL, Whalen PJ. Inverse amygdala and medial prefrontal cortex responses to surprised faces. Neuroreport. 2003;14:2317–2322. doi: 10.1097/00001756-200312190-00006. [DOI] [PubMed] [Google Scholar]
- 40.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 41.Bernstein D, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D, Zule W. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2002;27:169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- 42.Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, McCormick RA. Testing a tripartite model: IEvaluating the convergent and discriminant validity of anxiety and depression symptom scales. J Abnorm Psychol. 1995;104:3–14. doi: 10.1037//0021-843x.104.1.3. [DOI] [PubMed] [Google Scholar]
- 43.Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. New York, NY: The Guilford Press; 2013. [Google Scholar]
- 44.Hair JF, Jr, Anderson RE, Tatham RLWCB. Multivariate Data Analysis. Fifth Edition edn. New Jersey: Prentice Hall; 1998. [Google Scholar]
- 45.Muthén LK, Muthén BO. Mplus user’s guide. 6th edn. Los Angeles, CA: Authors; pp. 1998–2010. [Google Scholar]
- 46.Harford TC, Grant BF. Prevalence and population validity of DSM-III-R alcohol abuse and dependence: the 1989 National Longitudinal Survey on Youth. J Subst Abuse. 1994;6:37–44. doi: 10.1016/s0899-3289(94)90065-5. [DOI] [PubMed] [Google Scholar]
- 47.Cannon DS, Leeka JK, Patterson ET, Baker TB. Principal components analysis of the inventory of drinking situations: empirical categories of drinking by alcoholics. Addict Behav. 1990;15:265–269. doi: 10.1016/0306-4603(90)90069-a. [DOI] [PubMed] [Google Scholar]
- 48.Magid V, Maclean MG, Colder CR. Differentiating between sensation seeking and impulsivity through their mediated relations with alcohol use and problems. Addict Behav. 2007;32:2046–2061. doi: 10.1016/j.addbeh.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsuoka Y, Furuyashiki T, Yamada K, Nagai T, Bito H, Tanaka Y, et al. Prostaglandin E receptor EP1 controls impulsive behavior under stress. Proc Natl Acad Sci U S A. 2005;102:16066–16071. doi: 10.1073/pnas.0504908102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun H, Green TA, Theobald DE, Birnbaum SG, Graham DL, Zeeb FD, et al. Yohimbine increases impulsivity through activation of cAMP response element binding in the orbitofrontal cortex. Biol Psychiatry. 2010;67:649–656. doi: 10.1016/j.biopsych.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duckworth AL, Kim B, Tsukayama E. Life stress impairs self-control in early adolescence. Frontiers in psychology. 2012;3:608. doi: 10.3389/fpsyg.2012.00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fields S, Leraas K, Collins C, Reynolds B. Delay discounting as a mediator of the relationship between perceived stress and cigarette smoking status in adolescents. Behav Pharmacol. 2009;20:455–460. doi: 10.1097/FBP.0b013e328330dcff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spear LP. College drinking, what it is and what to do about it: A review of the state of the science. J Stud Alcohol. 2002;14(Supplement No):71–81. [Google Scholar]
- 54.Admon R, Lubin G, Rosenblatt JD, Stern O, Kahn I, Assaf M, et al. Imbalanced neural responsivity to risk and reward indicates stress vulnerability in humans. Cereb Cortex. 2013;23:28–35. doi: 10.1093/cercor/bhr369. [DOI] [PubMed] [Google Scholar]
- 55.Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC medicine. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kessler RC, Crum RM, Warner LA, Nelson CB, Schulenberg J, Anthony JC. Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Arch Gen Psychiatry. 1997;54:313–321. doi: 10.1001/archpsyc.1997.01830160031005. [DOI] [PubMed] [Google Scholar]
- 57.Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, et al. Comorbidity of mental disorders with alcohol, other drug abuse Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264:2511–2518. [PubMed] [Google Scholar]
- 58.Coccaro EF, McCloskey MS, Fitzgerald DA, Phan KL. Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression. Biol Psychiatry. 2007;62:168–178. doi: 10.1016/j.biopsych.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 59.Naimi TS, Brewer RD, Mokdad A, Denny C, Serdula MK, Marks JS. Binge drinking among US adults. JAMA. 2003;289:70–75. doi: 10.1001/jama.289.1.70. [DOI] [PubMed] [Google Scholar]
- 60.Fillmore KM, Hartka E, Johnstone BM, Leino EV, Motoyoshi M, Temple MT. A meta-analysis of life course variation in drinking. Br J Addict. 1991;86:1221–1267. doi: 10.1111/j.1360-0443.1991.tb01702.x. [DOI] [PubMed] [Google Scholar]
- 61.Keyes KM, Hatzenbuehler ML, Hasin DS. Stressful life experiences, alcohol consumption, and alcohol use disorders: the epidemiologic evidence for four main types of stressors. Psychopharmacology (Berl) 2011;218:1–17. doi: 10.1007/s00213-011-2236-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hariri AR. The neurobiology of individual differences in complex behavioral traits. Annu Rev Neurosci. 2009;32:225–247. doi: 10.1146/annurev.neuro.051508.135335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nikolova YS, Ferrell RE, Manuck SB, Hariri AR. Multilocus genetic profile for dopamine signaling predicts ventral striatum reactivity. Neuropsychopharmacology. 2011;36:1940–1947. doi: 10.1038/npp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nikolova YS, Koenen KC, Galea S, Wang CM, Seney ML, Sibille E, et al. Beyond genotype: serotonin transporter epigenetic modification predicts human brain function. Nat Neurosci. 2014;17:1153–1155. doi: 10.1038/nn.3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


