Abstract
Songbirds exhibit significant adult neuroplasticity that, together with other neural specializations, makes them an important model system for neurobiological studies. A large body of work also points to the songbird brain as a significant target of steroid hormones, including corticosterone (CORT), the primary avian glucocorticoid. Whereas CORT positively signals the brain for many functions, excess CORT may interfere with natural neuroplasticity. Consequently, mechanisms may exist to locally regulate CORT levels in brain to ensure optimal concentrations. However, most studies in songbirds measure plasma CORT as a proxy for levels at target tissues. In this paper, we review literature concerning circulating CORT and its effects on behavior in songbirds, and discuss recent work suggesting that brain CORT levels are regulated independently of changes in adrenal secretion. We review possible mechanisms for CORT regulation in the avian brain, including corticosteroid-binding globulins, p-glycoprotein activity in the blood-brain-barrier, and CORT metabolism by the 11β hydroxysteroid dehydrogenases. Data supporting a role for CORT regulation within the songbird brain have only recently begun to emerge, suggesting that this is an avenue for important future research.
Keywords: corticosterone, neurosteroid, corticosteroid-binding globulin, p-glycoprotein, 11β hydroxysteroid dehydrogenase
Introduction
Songbirds are increasingly recognized as powerful animal models for studies in developmental neurobiology, sensory processing, the neural control of learning and memory, and the control of complex behavior. They stand out for their extensive neuroplasticity that can be retained throughout adult life (Kirn, 2010; McDonald & Kirn, 2012; Tramontin and Brenowitz, 2000). Interestingly, many of the neural attributes that make songbirds unique are influenced by sex steroid hormones (Ball et al., 2002; Brenowitz, 2013; Brenowitz, 2014; Schlinger and Brenowitz, 2009; Schlinger and Soma et al., 2004; Tramontin et al., 2003). Songbirds also possess specializations of neural sex steroid synthesis and metabolism, notably a significant capacity to synthesize estrogens in brain (Ball and Balthazart, 2009; London et al., 2009; Schlinger et al., 2014). In addition, there is increasing evidence that songbirds also possess special properties governing neural glucocorticoid physiology.
In the traditional view, steroid hormones are synthesized and released from the peripheral steroidogenic glands, the gonads and adrenals, and circulate in the bloodstream until reaching various target tissues. Steroids readily cross the blood-brain barrier (Pardridge, 1981) where they bind to receptors in the hypothalamus to initiate negative feedback control over the hypothalamo-pituitary-adrenal (HPA) and hypothalamo-pituitary-gonadal (HPG) axes, as well as receptors in numerous other brain regions important for cognition, behavior, and physiology. In songbirds, while a great deal of work has focused on the dynamics of sex-steroid availability to discrete neural circuits (see citations above), fewer studies have examined the nature of neural corticosterone (CORT) neurobiology and physiology. This is surprising, given the large amount of research devoted to understanding stress physiology, including the effects of chronic stress on CORT secretion (e.g., Cyr and Romero, 2007; Rich and Romero, 2005), behavioral effects of CORT (e.g., Breuner et al., 1998; Breuner and Wingfield, 2000; Busch et al., 2008; Loiseau et al., 2008; Pravosudov, 2003; Saldanha et al., 2000; Schoech et al., 2007; Schoech et al., 2012; Spencer and Verhulst, 2007; Wada and Breuner, 2008), the role of developmental stress in the programming of the HPA axis (Crino et al., 2014; Schoech et al., 2011; Spencer et al., 2009), and the potential fitness consequences of glucocorticoid secretion over the lifespan of the individual (Bonier et al., 2009; Breuner et al., 2008; Brown et al., 2005; Cyr and Romero, 2007; Ouyang et al., 2011). For the most part, these studies utilize measures of plasma CORT (either free or total) as indicators of CORT activity, despite the fact that local CORT synthesis may occur independently of the adrenals (Taves et al., 2011) and several mechanisms exist that alter the availability of CORT to target tissues and receptors (see below).
The purpose of this mini-review is to discuss the dynamics of CORT synthesis, release, and regulation in the songbird, with an emphasis on CORT levels and function in the songbird brain. To this end, we provide a synopsis of studies that have addressed these topics and discuss future avenues for research. Our principal goal is to outline the multiple pathways by which CORT gains access to its receptors within neural circuits in the brain and to discuss whether neural CORT levels are subject to regulation. These findings may point out ways in which the measurement of plasma CORT provides an incomplete picture of functional CORT activity in the brain. Accordingly, we first address what is known about effects of circulating CORT on songbird behavior. We then describe studies that have sought to determine the relationship between CORT levels in brain and blood. Next we discuss three sets of mechanisms for regulation of CORT access and/or activity in the songbird brain: corticosteroid-binding globulins in the periphery, p-glycoprotein-mediated transport at the blood-brain barrier, and local CORT metabolism by the 11ß hydroxysteroid dehydrogenases. As the latter mechanisms have only recently been addressed in songbirds, this stands as an exciting field for discovery. Finally, we examine the hypothesis that the adult songbird brain may retain considerable control over neural CORT levels in part to limit potentially deleterious effects of CORT on the extensive neuroplasticity that is retained in adulthood. Note that while differential expression of CORT receptors is unquestionably a primary mechanism for regulating neural effects of CORT, the focus of this paper is on less-studied CORT regulatory mechanisms in the songbird brain.
Plasma CORT and Behavior
Many studies utilize measures of circulating CORT as a proxy for CORT availability to target tissues and as an indicator of overall functioning of the HPA axis. This is particularly useful for field-based studies wherein the relatively non-invasive taking of a small blood sample permits release of the subject for relatively naturalistic study, as well as the possibility of subsequent recapture and sampling. Circulating CORT levels have been correlated with traits such as aggression (Charlier et al., 2009; Newman and Soma, 2011; Van Duyse et al., 2004) and measures of personality, such as exploratory behavior and risk-taking (Atwell et al., 2012; Carere et al., 2010; Liebl and Martin, 2012; Martins et al., 2007; Schoech et al., 2012). In addition, manipulation of CORT profiles through use of implants, patches, and oral dosing has established a causal role for CORT in the expression of various behaviors (Breuner et al., 1998; Pravosudov, 2003; Saldanha et al., 2000; Schoech et al., 2007; Spencer and Verhulst, 2007; Wada and Breuner, 2008; Wingfield and Silverin, 1986). The intracellular CORT receptors (glucocorticoid receptor (GR) and mineralocorticoid receptor (MR)) are also differentially expressed in the songbird brain (Dickens et al., 2009; Hodgson et al., 2007; Shahbazi et al., 2011) and a membrane CORT receptor has been characterized (Breuner and Orchinik, 2009). Taken together, studies documenting CORT receptor expression in brain, as well as behavioral effects of CORT, make it clear that CORT is present in and acts on the songbird brain. An important question remains, however: do CORT levels in all regions of the brain mirror levels in the circulation – i.e., are plasma CORT measures a reliable proxy for levels of CORT acting on discrete neural circuits?
The adult songbird brain exhibits remarkable plasticity that may be sensitive to the effects of CORT. Our lab and others have previously shown that excess CORT exposure inhibits neurogenesis in songbirds (Katz et al., 2008; Newman et al., 2010), and CORT can be cytotoxic to neurons in rodents (Behl et al., 1997; Magarinos and McEwen, 1995; Sapolsky et al., 1998) and birds (Newman et al., 2010). In addition, CORT can inhibit reproduction in times of chronic or extreme stress (Wingfield et al., 1983). One could therefore readily imagine that CORT levels in the songbird brain should be regulated to prevent over-exposure, and thus not all fluctuations in the songbird brain might be a mirror of the circulation. Indeed, local optimization of brain CORT levels is likely critical; even though chronic or over-exposure to CORT is detrimental, some level of CORT is necessary and permissive for many physiological and behavioral processes (Sapolsky et al., 2000). What then do we know about CORT levels in the brains of songbirds, and do they suggest that the brain is a passive recipient of fluctuations originating in the periphery?
Does Brain CORT Mirror Plasma CORT?
Studies designed to assess CORT levels in the songbird brain have to date utilized one of two techniques to measure CORT: direct steroid extraction from brain tissue, or measurement of extracellular CORT using in vivo microdialysis.
Direct Steroid Extraction from Brain Tissue
Steroid extraction from whole, discrete regions of the songbird brain is gaining interest as a technique, and our lab and others have validated this method for measures of brain estradiol, CORT, and dehydroepiandrosterone (DHEA; Chao et al., 2011; Newman et al., 2008; Taves et al., 2011). Brain CORT levels have been successfully measured in developing songbirds, notably European starlings (Sturnus vulgaris; Schmidt et al., 2009) and zebra finches (Taeniopygia guttata; Schmidt and Soma, 2008), as well as in adult song sparrow brain (Melospiza melodia; Newman and Soma, 2011).
One of the goals of studies measuring brain CORT is to determine whether or not levels are determined solely by changes in the circulation. While several studies have found no evidence for brain-specific regulation of CORT (i.e., plasma and brain CORT fluctuations were similar; Newman and Soma, 2011; Schmidt et al., 2009), a recent study found support for the hypothesis that the songbird brain regulates CORT access to discrete neural circuits. Newman and Soma (2009) investigated seasonal and stress-induced changes in plasma and brain CORT levels (as well as levels of DHEA) in adult male song sparrows. Fluctuations in multiple brain regions generally matched those in blood, with one exception. During molt, CORT levels in the hippocampus (HP) were undetectable after, but not before stress, producing a “mismatch” between the HP and the plasma. This result suggests that under certain conditions, the songbird HP is protected from exposure to acute stress-induced CORT, although the mechanism producing this effect was not investigated.
In Vivo Microdialysis to Measure Brain CORT
The studies discussed above have utilized a solid-phase extraction method to extract hormone from brain tissue. Unfortunately, measuring CORT from brain tissue as described above requires sacrifice of the animal, providing only one data point per individual. While this is certainly an informative and useful method, we have developed the use of in vivo microdialysis in the songbird brain to obtain repeated, pseudo real-time measures of extracellular steroid hormones as measured in dialysates collected from discrete brain regions (Ikeda et al., 2014; Remage-Healey et al., 2008, 2011, 2012). Microdialysis allows the researcher to manipulate the conditions that the awake animal is exposed to and to assess concurrent changes in brain steroid levels.
We recently used in vivo microdialysis to measure CORT levels over time in the songbird brain (Rensel et al., 2014). We obtained CORT measures from the HP and caudal nidopallium (cNp) over the diel cycle as well as before, during, and after restraint stress, and compared these to circulating levels. Whereas a systemic dose of as low as 5μg of CORT produced an elevation in HP CORT (plasma levels were elevated to physiologically high stress levels of ∼22ng/ml), restraint stress, which elevated plasma CORT to ∼11ng/ml, produced no measurable change in the HP or cNp (Fig. 1). In addition, while there was a robust diel rhythm in circulating CORT, brain CORT levels only showed a modest rhythm temporally shifted (∼8 hours) relative to the periphery. Finally, CORT levels were higher in the HP than the cNp, an unexpected result if the brain had uniform free access to CORT from the periphery (Rensel et al., 2014). These results, along with results from the molting song sparrow HP (Newman and Soma, 2009), raise the possibility that the adult songbird brain modulates CORT levels in a region-specific manner. How then could CORT exposure to brain be regulated? Below we consider three potential mechanisms for this regulation.
Figure 1.
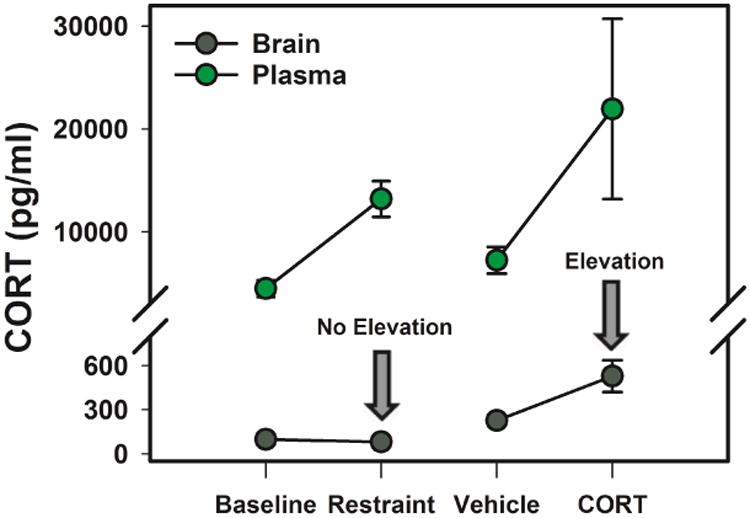
Plasma and HP CORT levels (obtained using in vivo microdialysis) at baseline and in response to an acute stressor (15min of restraint stress) and after a vehicle or 5μg intra-muscular CORT injection to obtain higher stress levels. While plasma CORT was elevated under both conditions, brain CORT levels were only elevated when high stress levels were achieved. Note that baseline and restraint stress data points are repeated samples within individuals, while vehicle and CORT-injected data points represent differences between individuals after injection. For the sake of clarity, repeated HP CORT levels are averaged across multiple time points pre and post-stress (samples 1-5 and samples 6-12 in Rensel et al., 2014) or post-injection (samples 4-6 in Rensel et al., 2014). CORT levels measured in the HP are not corrected for recovery across the microdialysis probe, leading to lower levels in dialysate than in blood.
Mechanisms of CORT Regulation
The Bloodstream: Corticosteroid-Binding Globulins
Steroid hormone binding globulins have long been recognized as a mechanism by which the availability of circulating hormones to target cells is regulated. Binding globulins are proteins, produced and secreted primarily from the liver, that circulate in the blood and bind hormone. According to the free hormone hypothesis (Mendel, 1989), the bound hormone is prevented from diffusing through the plasma membrane and binding to intracellular receptors; therefore, the “free,” or unbound hormone, is the biologically active portion. In mammals, sex steroid hormones are bound by sex hormone binding globulin (SHBG), while glucocorticoids are bound by corticosteroid binding globulin (CBG). Birds lack SHBG, however, and indeed CBGs in birds not only bind CORT but also testosterone and progesterone (Deviche et al., 2001; Wingfield et al., 1984).
The dynamics of CBGs in relation to circulating CORT in birds have been examined in numerous contexts. Some studies have utilized both free and total (bound plus free) CORT levels to examine the hypothesis that measurement of total CORT levels “masks” the effects of CBGs on the true availability of CORT to receptors. For example, Breuner et al. (2003) examined free and total circulating CORT in white-crowned sparrows (Zonotrichia leucophrys) breeding at multiple latitudes. While total stress-induced CORT levels did not differ across latitudes, free CORT levels in response to stress were significantly reduced in populations at higher latitudes; this suggests that by regulating circulating CBGs, free CORT levels were dampened and CORT-induced inhibition of reproduction limited in high-latitude populations experiencing a relatively short breeding season.
Other studies find that CBG levels are influenced by stress. After approximately 30-60 minutes of restraint stress, the CBG capacity of zebra finches and red crossbills (Loxia curvirostra) decreased (Breuner et al., 2006). In the non-songbird Japanese quail (Coturnix coturnix), this decrease in CORT-binding capacity persisted for 24 hours after the stressor (Malisch et al., 2010). One possibility is that decreased CBG levels increase the availability of CORT to receptors, as more of the circulating pool of CORT is unbound. Alternatively, CBGs may regulate CORT clearance rates; a decrease in CBG therefore permits more rapid CORT elimination. Using a CBG knockout mouse model, Petersen et al. (2006) demonstrated that a lack of CBGs led to increased CORT clearance rates and symptoms of CORT deficiency. Therefore, while binding to CBGs may make CORT inaccessible to receptors, conversely, a lack of CBGs leads to a loss of CORT availability in the body through increased excretion. A third potential function for CBGs involves their capacity to deliver CORT to the brain. Using the mouse CBG knockout model combined with in vivo microdialysis in the brain, Minni et al., (2012) showed that CBG promotes CORT delivery from the periphery to the brain, as a stressor led to an increase in plasma CORT but no change in HP CORT of knockouts (Moisan et al., 2014). Therefore, the delicate balance of CORT and CBGs may impact the concentrations of CORT that are able to reach specific circuits in the brain.
While measurement of plasma CBGs is commonly used to determine the portion of hormone that is available to receptors, the precise nature of CBG action and its utility in avian stress physiology studies has come into question (Malisch and Breuner, 2010; Schoech et al., 2013). It is difficult to determine, for example, how indicative free CORT levels are of CORT availability, given the fact that free CORT is likely to be eliminated relative to bound CORT (Petersen et al., 2006), and the affinity of CBG for CORT is sensitive to tissue-specific temperature fluctuations (Henley and Lightman, 2011). In addition, because CBGs in birds bind both testosterone and progesterone, the relative concentrations of these hormones in blood will influence the availability of CBGs for occupancy by CORT, making it difficult to infer how much CORT is in the bound form (Deviche et al., 2001). Finally, as already noted, some studies suggest that instead of blocking CORT access, CBGs may act as transporters aiding the delivery of CORT to target cells (Minni et al., 2012). For these reasons, measures of total CORT (free and bound) may be more reliable indicators of CORT exposure to the body (Schoech et al., 2013). Irrespective of whether free or total plasma CORT is measured, there are other mechanisms that might regulate CORT exposure in the brain, starting with regulation of passage through the blood-brain barrier.
The Blood-Brain Barrier: P-Glycoprotein
The blood-brain barrier (BBB) is a collection of tightly linked endothelial cells lining the vessels of the brain (Abbott et al., 2006) that restrict and/or regulate access to the brain of compounds in blood, protecting sensitive neural tissue from insult by toxins (Abbott, 2010). Those substances that do not freely diffuse through the BBB gain access via specific membrane transporters. One transporter that has been linked to glucocorticoid access to brain is p-glycoprotein (also known as mdr1 and abcb1; Cordon-Cardo et al., 1986; Pardridge, 1995, 2005). P-glycoprotein is a member of the ATP-binding cassette family of transporters, is found in peripheral tissues as well as the BBB, and has been implicated in the resistance of tumor cells to anticancer drugs (Leslie et al., 2005).
Although the capability of p-glycoprotein to regulate CORT access to the brain has been alleged for some years (Meijer et al., 1998), more recent evidence casts doubt on this function. In a mouse knockout for one isoform of p-glycoprotein (mdr1a; mice have two isoforms), cortisol entry into the brain increased relative to wild-type mice (Karssen et al., 2001; Pariante, 2008), suggesting that p-glycoprotein inhibits CORT access to brain. Interestingly, this effect was not seen for corticosterone, suggesting natural biological differences in these two glucocorticoids. Subsequent work with a knockout of both p-glycoprotein isoforms (mdr1a and mdr1b) demonstrated that corticosterone entry was indeed enhanced in knockout mice relative to controls (Uhr et al., 2002). However, a separate study failed to replicate these results (Mason et al., 2008); therefore the role of p-glycoprotein in neural CORT physiology remains unresolved. Interestingly, p-glycoprotein has been localized to neurons in addition to the cells of the blood brain barrier, indicating that its function in regulating steroid entry in brain may be regulated at multiple levels (Karssen et al., 2004).
Little is known about p-glycoprotein in avian CORT physiology. In the non-songbird chicken, p-glycoprotein has been identified in the brain (Edelmann et al., 1999). As we found evidence for differential CORT access to discrete regions of the songbird brain (described above), we tested the hypothesis that p-glycoprotein expression, presumably at the BBB, was responsible for this regional variation. To assess this possibility, we examined p-glycoprotein mRNA expression levels in the two brain regions in which microdialysis was conducted (Rensel et al., 2014). Whereas p-glycoprotein was expressed at reasonably high levels in both the HP and cNp, we found no evidence for regional differences. Thus, it appears unlikely that p-glycoprotein was responsible for the regional CORT differences we observed. Nevertheless, it remains possible that this protein participates in regulating CORT dynamics in the songbird brain, perhaps leading to the diminished stress response that we observed in both brain regions (Rensel et al., 2014). Future work with p-glycoprotein inhibitors (Srivalli and Lakshmi, 2012) in songbirds may prove useful.
Although we cannot dismiss a role for p-glycoprotein regulation of CORT access to the songbird brain, a set of mechanisms still remains at the disposal of the brain with which to regulate CORT, namely activity of the 11ß hydroxysteroid dehydrogenases.
The Brain: CORT-Metabolizing Enzymes
Metabolism of CORT by the 11ß hydroxysteroid dehydrogenases is a well-known mechanism for CORT regulation in the periphery, particularly in tissues that must balance access of CORT and aldosterone to MR (Edwards et al., 1988). The two isoforms of 11ß hydroxysteroid dehydrogenase, type 2 (11ß HSD2) and type 1 (11ß HSD1), functionally inactivate or re-activate CORT, respectively. In addition to peripheral expression, in rodents and humans, 11ß HSD1 is widely expressed in the adult brain (including in the HP), while 11ß HSD2 expression is restricted to regions involved in blood pressure and salt balance (Robson et al., 1998; Roland et al., 1995). Importantly, 11ß HSD2 is expressed at higher levels in the developing rodent brain than in the adult brain (Brown et al., 1996; Diaz et al., 1998).
In contrast with rodents, 11ß HSD2 is expressed widely in the songbird brain, and does not appear to decrease after the early post-hatching stage (Katz et al., 2010). This suggests that the songbird brain retains the capacity to inactivate CORT in a region-specific manner into adulthood. Our finding that CORT levels were higher in the HP than the cNp of male zebra finches led us to hypothesize that 11ß HSD2 may be expressed at higher levels in the cNp and that, by metabolizing CORT into 11-dehydroCORT, this enzyme produced a regional difference in CORT. We tested this hypothesis by examining 11ß HSD2 mRNA levels in the HP and cNp of adult male and female zebra finches. As predicted, we found that 11ß HSD2 mRNA expression levels were indeed higher in the cNp than the HP (Fig. 2; Rensel et al., 2014). While correlational, this result provides evidence for regional differences in neural 11ß HSD2 that may locally regulate CORT levels in discrete regions of the songbird brain.
Figure 2.
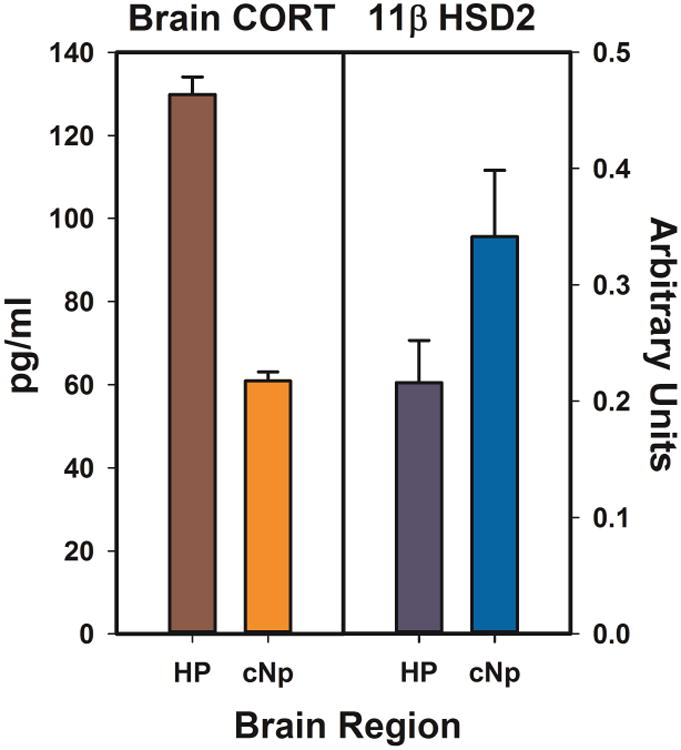
Regional differences in brain CORT obtained using in vivo microdialysis are inversely related to expression levels of the CORT-inactivating enzyme 11β hydroxysteroid dehydrogenase type 2. Enzyme expression levels were measured using qRT-PCR and are expressed relative to the reference gene glyceraldehyde 3-phosphate dehydrogenase. HP = hippocampus; cNp = caudal nidopallium. Adapted from Rensel et al., 2014.
The widespread distribution of 11ß HSD1 in the mammalian brain suggests that local reactivation of CORT is also necessary to maintain optimal CORT concentrations and availability to receptors. Like 11ß HSD2, 11ß HSD1 has received little if any experimental attention in studies of songbird stress physiology. The only studies in birds that have assessed this enzyme utilize chicken as a model system, and suggest that 11ß HSD1 is expressed in brain at lower levels than the kidney, intestine, and liver (Klusoňová et al., 2008). We recently designed PCR primers for 11ß HSD1 based on the predicted zebra finch sequence and have identified expression in a mix of kidney and liver, as well as in the HP. We are currently quantifying its expression in brain regions with varying degrees of CORT sensitivity (based on relative GR and MR abundance). In addition, it is possible that the ratio of the type 2 to type 1 isoforms of 11ß HSD is more important than absolute mRNA levels in determining local CORT levels in brain. This is an area of ongoing research in our lab.
Additional or Alternate Pathways for Glucocorticoid Regulation in Brain
Two other CORT inactivating enzymes have been identified in birds: a third isoform of 11ß HSD, known as 11ß HSD type 3, and 20 hydroxylase, which both de-activate CORT. While 20 hydroxylase activity or expression has been documented in the chicken (Bryndová et al., 2006; Kučka et al., 2006) and songbird (Katz et al., 2010) brain, 11ß HSD type 3 has only been examined in the periphery of chickens to date (Katz et al. 2008). No studies that we know of have investigated this latter enzyme in songbirds, although this is yet another mechanism by which the brain could conceivably regulate local CORT fluctuations and activity.
In addition to these pathways that inactivate CORT, the brain may regulate local CORT levels by combining any or all of the above-mentioned mechanisms with the capacity to produce CORT de novo or from circulating precursors. The rat brain expresses all of the enzymes necessary to synthesize CORT (Taves et al., 2011), and the required enzymes are present in chicken bursa and thymus (Lechner et al., 2001). No studies to date have directly examined expression of the CORT synthetic enzymes in songbird brain.
Functional Significance of CORT Regulation in the Songbird Brain
Studies that have utilized in vivo microdialysis to measure CORT in the rodent brain find, for the most part, that brain CORT fluctuates in parallel with the circulation (particularly free CORT; Qian et al., 2012; Tronche et al., 2010; but see Droste et al., 2009, Heinzmann et al., 2010) with no obvious regional differences (Dorey et al., 2012; Droste et al., 2008; Kitchener et al., 2004). In contrast, we find that the adult male zebra finch brain appears to be buffered to some extent from peripheral CORT fluctuations and can also exhibit regional differences in absolute levels of CORT (Rensel et al., 2014). Why does this species difference exist, and why might the adult songbird brain possess the capacity to locally regulate CORT?
We hypothesize that because excess glucocorticoids can interfere with neurogenesis and reduce neuronal survival (Katz et al., 2008; Newman et al., 2010), CORT levels are locally buffered in the songbird brain to protect regions undergoing significant gross neuroplasticity (as opposed to synaptic plasticity). Because such plasticity is inherently greater in the developing brain as compared to the adult brain, the brains of immature animals should express a greater set of CORT regulatory mechanisms to protect fragile developmental events. Moreover, because the brain of adult songbirds exhibits a greater degree of plasticity compared to adult rodents (Ball and Balthazart, 2004), the songbird brain should also possess a greater set of CORT regulatory mechanisms. The currently available data tends to support these expectations. As mentioned previously, the CORT-inactivating enzyme, 11ß HSD2, is expressed at greater levels in the brains of developing rodents as compared to adults (Holmes et al., 2006). Thus, when the rodent brain is experiencing elevated levels of neurogenesis and is rapidly building its vast and complex neural circuitry, it also seems capable of reducing levels of neural CORT, thereby protecting the fragile developing brain from potentially harmful levels of CORT produced when the mother is overly stressed. Songbirds are notable for retaining significant gross neuroplasticity in adulthood. Much like the brains of developing rodents (and all other vertebrates), many songbird species, including zebra finches, retain the capacity to generate new neurons throughout life, and these neurons can become incorporated into functional neural circuits (Alvarez-Borda and Nottebohm, 2002; Alvarez-Buylla and Kirn, 1997; Chen et al., 2013; Goldman et al., 1992; Nottebohm, 1987; Nottebohm, 2002). Our data suggest that like the developing rodent brain, the brain of adult songbirds also expresses significant levels of 11ß HSD2, presumably to protect against undesirable effects of excess CORT during times of stress.
Using adult male zebra finch brain slices in vitro, we previously reported that CORT impaired neurogenesis along the lateral ventricle (Katz et al., 2008) suggesting that excess CORT is detrimental to neurogenesis in songbirds. Similarly, systemic treatment of adult song sparrows with CORT led to a reduction in neurogenesis and neuron survival in the song control nucleus HVC (Newman et al., 2010). How varying degrees of stress and concomitant varying levels of CORT influence neurogenesis and neuron survival in songbirds is unknown. Moreover, it is unknown whether CORT regulatory mechanisms in brain are able to protect this plasticity under naturally stressful conditions. These are certainly areas for fruitful future research.
Conclusions
In this review, we highlight several mechanisms by which the songbird brain might regulate CORT levels independent of changes in adrenal secretion. Furthermore, we provide evidence that one of these mechanisms, CORT inactivation by 11ß HSD2, produces regional differences in CORT levels in the zebra finch brain while potentially buffering the brain from peripheral fluctuations. Multiple pathways of CORT regulation remain to be explored in songbirds, presenting promising avenues for future research. For the present, these measures remain inaccessible to field-based, long-term studies. Nevertheless, work on captive-held species like the zebra finch, where mechanisms can be explored in detail, will advance our appreciation of the ways in which the songbird brain dynamically and locally regulates glucocorticoids. No doubt, glucocorticoid signaling and function in the songbird central nervous system is complex, and determining if and how the brain participates in local management of CORT levels, or is merely a passive target of adrenal hormones, presents an important and timely avenue for research.
Acknowledgments
We dedicate this paper to Dr. Kazuyoshi Tsutsui. “Kazu” has pioneered the study of avian neurosteroidogenesis while he has also discovered novel neurochemicals of tremendous significance in vertebrate neuroendocrinology and in avian biology. His scientific enthusiasm and prowess has greatly impacted our own research, as well as the research of most investigators of avian neurobiology and neuroendocrinology. His personal warmth makes personal interactions especially enjoyable. It is very appropriate that this special issue be dedicated to Kazu, and we are honored, and extremely happy, to be able to contribute to this issue. Our research is funded by NIH grant NIMH061994 to B.A.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Abbott NJ, Patabendige AAK, Dolman DEM, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Alvarez-Borda B, Nottebohm F. Gonads and singing play separate, additive roles in new neuron recruitment in adult canary brain. J Neurosci. 2002;22:8684–8690. doi: 10.1523/JNEUROSCI.22-19-08684.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Kirn JR. Birth, migration, incorporation, and death of vocal control neurons in adult songbirds. J Neurobiol. 1997;33:585–601. [PubMed] [Google Scholar]
- Atwell JW, Cardoso GC, Whittaker DJ, Campbell-Nelson S, Robertson KW, Ketterson ED. Boldness behavior and stress physiology in a novel urban environment suggest rapid correlated evolutionary adaptation. Behav Ecol. 2012;23:960–969. doi: 10.1093/beheco/ars059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball GF, Balthazart J. Hormonal regulation of brain circuits mediating male sexual behavior in birds. Physiol Behav. 2004;83:329–346. doi: 10.1016/j.physbeh.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Ball GF, Balthazart J. Neuroendocrine regulation of reproductive behavior in birds. In: Pfaff D, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. 2nd. San Diego: Academic Press; 2009. pp. 855–895. [Google Scholar]
- Ball GF, Riters LV, Balthazart J. Neuroendocrinology of song behavior and avian brain plasticity: multiple sites of action of sex steroid hormones. Front Neuroendocrinol. 2002;23:137–78. doi: 10.1006/frne.2002.0230. [DOI] [PubMed] [Google Scholar]
- Behl C, Lezoualc'h F, Trapp T, Widmann M, Skutella T, Holsboer F. Glucocorticoids enhance oxidative stress-induced cell death in hippocampal neurons in vitro. Endocrinology. 1997;138:101–106. doi: 10.1210/endo.138.1.4835. [DOI] [PubMed] [Google Scholar]
- Bonier F, Moore IT, Martin PR, Robertson RJ. The relationship between fitness and baseline glucocorticoids in a passerine bird. Gen Comp Endocrinol. 2009;163:208–213. doi: 10.1016/j.ygcen.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Brenowitz EA. Testosterone and BDNF interactions in the avian song control system. Neuroscience. 2013;239:115–123. doi: 10.1016/j.neuroscience.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz EA. Transsynaptic trophic effects of steroid hormones in an avian model of adult brain plasticity. Front Neuroendocrinol. doi: 10.1016/j.yfrne.2014.09.003. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuner CW, Greenberg AL, Wingfield JC. Noninvasive corticosterone treatment rapidly increases activity in Gambel's white-crowned sparrows (Zonotrichia leucophrys gambelii) Gen Comp Endocrinol. 1998;111:386–94. doi: 10.1006/gcen.1998.7128. [DOI] [PubMed] [Google Scholar]
- Breuner CW, Lynn SE, Julian GE, Cornelius JM, Heidinger BJ, Love OP, Sprague RS, Wada H, Whitman BA. Plasma-binding globulins and acute stress response. Horm Metab Res. 2006;38:260–8. doi: 10.1055/s-2006-925347. [DOI] [PubMed] [Google Scholar]
- Breuner CW, Orchinik M. Plasma binding proteins as mediators of corticosteroid action in vertebrates. J Endocrinol. 2002;175:99–112. doi: 10.1677/joe.0.1750099. [DOI] [PubMed] [Google Scholar]
- Breuner CW, Orchinik M. Pharmacological characterization of intracellular, membrane, and plasma binding sites for corticosterone in house sparrows. Gen Comp Endocrinol. 2009;163:214–24. doi: 10.1016/j.ygcen.2009.01.027. [DOI] [PubMed] [Google Scholar]
- Breuner CW, Orchinik M, Hahn TP, Meddle SL, Moore IT, Owen-Ashley NT, Sperry TS, Wingfield JC. Differential mechanisms for regulation of the stress response across latitudinal gradients. Am J Physiol Regul Integr Comp Physiol. 2003;285:R594–R600. doi: 10.1152/ajpregu.00748.2002. [DOI] [PubMed] [Google Scholar]
- Breuner CW, Patterson SH, Hahn TP. In search of relationships between the acute adrenocortical response and fitness. Gen Comp Endocrinol. 2008;157:288–295. doi: 10.1016/j.ygcen.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Breuner CW, Wingfield JC. Rapid behavioral response to corticosterone varies with photoperiod and dose. Horm Behav. 2000;37:23–30. doi: 10.1006/hbeh.1999.1554. [DOI] [PubMed] [Google Scholar]
- Brown C, Brown M, Raouf S. Effects of endogenous steroid hormone levels on annual survival in cliff swallows. Ecology. 2005;86:1034–1046. [Google Scholar]
- Brown RW, Diaz R, Robson AC, Kotelevtsev YV, Mullins JJ, Kaufman MH, Seckl JR. The ontogeny of 11 beta-hydroxysteroid dehydrogenase type 2 and mineralocorticoid receptor gene expression reveal intricate control of glucocorticoid action in development. Endocrinology. 1996;137:794–79. doi: 10.1210/endo.137.2.8593833. [DOI] [PubMed] [Google Scholar]
- Bryndová J, Klusoňová P, Kučka M, Mazancová-Vagnerová K, Mikšík I, Pácha J. Cloning and expression of chicken 20-hydroxysteroid dehydrogenase. J Mol Endocrinol. 2006;37:453–462. doi: 10.1677/jme.1.02025. [DOI] [PubMed] [Google Scholar]
- Busch DS, Sperry TS, Peterson E, Do CT, Wingfield JC, Boyd EH. Impacts of frequent, acute pulses of corticosterone on condition and behavior of Gambel's white-crowned sparrow (Zonotrichia leucophrys gambelii) Gen Comp Endocrinol. 2008;158:224–33. doi: 10.1016/j.ygcen.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Carere C, Caramaschi D, Fawcett TW. Covariation between personalities and individual differences in coping with stress: Converging evidence and hypotheses. Curr Zool. 2010;56:728–741. [Google Scholar]
- Chao A, Schlinger BA, Remage-Healey L. Combined liquid and solid-phase extraction improves quantification of brain estrogen content. Front Neuroanat. 2011;5:1–7. doi: 10.3389/fnana.2011.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlier TD, Underhill C, Hammond GL, Soma KK. Effects of aggressive encounters on plasma corticosteroid-binding globulin and its ligands in white-crowned sparrows. Horm Behav. 2009;56:339–347. doi: 10.1016/j.yhbeh.2009.06.012. [DOI] [PubMed] [Google Scholar]
- Chen Z, Ye R, Goldman S. Testosterone modulation of angiogenesis and neurogenesis in the adult songbird brain. Neuroscience. 2013;239:139–148. doi: 10.1016/j.neuroscience.2012.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordon-Cardo C, O'Brien JP, Casals D, Rittman-Grauer L, Biedler JL, Melamed MR, Bertino JR. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc Natl Acad Sci U S A. 1989;86:695–698. doi: 10.1073/pnas.86.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crino OL, Driscoll SC, Breuner CW. Corticosterone exposure during development has sustained but not lifelong effects on body size and total and free corticosterone responses in the zebra finch. Gen Comp Endocrinol. 2014;196:123–129. doi: 10.1016/j.ygcen.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Cyr NE, Michael Romero L. Chronic stress in free-living European starlings reduces corticosterone concentrations and reproductive success. Gen Comp Endocrinol. 2007;151:82–9. doi: 10.1016/j.ygcen.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Deviche P, Breuner C, Orchinik M. Testosterone, corticosterone, and photoperiod interact to regulate plasma levels of binding globulin and free steroid hormone in dark-eyed juncos, Junco hyemalis. Gen Comp Endocrinol. 2001;122:67–77. doi: 10.1006/gcen.2001.7613. [DOI] [PubMed] [Google Scholar]
- Diaz R, Brown RW, Seckl JR. Distinct ontogeny of glucocorticoid and mineralocorticoid receptor and 11beta-hydroxysteroid dehydrogenase types I and II mRNAs in the fetal rat brain suggest a complex control of glucocorticoid actions. J Neurosci. 1998;18:2570–2580. doi: 10.1523/JNEUROSCI.18-07-02570.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens M, Romero LM, Cyr NE, Dunn IC, Meddle SL. Chronic stress alters glucocorticoid receptor and mineralocorticoid receptor mRNA expression in the European starling (Sturnus vulgaris) brain. J Neuroendocrinol. 2009;21:832–40. doi: 10.1111/j.1365-2826.2009.01908.x. [DOI] [PubMed] [Google Scholar]
- Dorey R, Piérard C, Chauveau F, David V, Béracochéa D. Stress-induced memory retrieval impairments: different time-course involvement of corticosterone and glucocorticoid receptors in dorsal and ventral hippocampus. Neuropsychopharmacology. 2012;37:2870–2880. doi: 10.1038/npp.2012.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droste SK, Collins A, Lightman SL, Linthorst ACE, Reul JMHM. Distinct, time-dependent effects of voluntary exercise on circadian and ultradian rhythms and stress responses of free corticosterone in the rat hippocampus. Endocrinology. 2009;150:4170–4179. doi: 10.1210/en.2009-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droste SK, de Groote L, Atkinson HC, Lightman SL, Reul JMHM, Linthorst ACE. Corticosterone levels in the brain show a distinct ultradian rhythm but a delayed response to forced swim stress. Endocrinology. 2008;149:3244–53. doi: 10.1210/en.2008-0103. [DOI] [PubMed] [Google Scholar]
- Edelmann HM, Duchek P, Rosenthal FE, Föger N, Glackin C, Kane SE, Kuchler K. Cmdr1, a chicken P-glycoprotein, confers multidrug resistance and interacts with estradiol. Biol Chem. 1999;380:231–41. doi: 10.1515/BC.1999.031. [DOI] [PubMed] [Google Scholar]
- Edwards CR, Stewart PM, Burt D, Brett L, McIntyre MA, Sutanto WS, de Kloet ER, Monder C. Localisation of 11 beta-hydroxysteroid dehydrogenase--tissue specific protector of the mineralocorticoid receptor. Lancet. 1988;2:986–989. doi: 10.1016/s0140-6736(88)90742-8. [DOI] [PubMed] [Google Scholar]
- Goldman SA, Zaremba A, Niedzwiecki D. In vitro neurogenesis by neuronal precursor cells derived from the adult songbird brain. J Neurosci. 1992;12:2532–2541. doi: 10.1523/JNEUROSCI.12-07-02532.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley DE, Lightman SL. New insights into corticosteroid-binding globulin and glucocorticoid delivery. Neuroscience. 2011;180:1–8. doi: 10.1016/j.neuroscience.2011.02.053. [DOI] [PubMed] [Google Scholar]
- Hodgson ZG, Meddle SL, Roberts ML, Buchanan KL, Evans MR, Metzdorf R, Gahr M, Healy SD. Spatial ability is impaired and hippocampal mineralocorticoid receptor mRNA expression reduced in zebra finches (Taeniopygia guttata) selected for acute high corticosterone response to stress. Proc Biol Sci. 2007;274:239–45. doi: 10.1098/rspb.2006.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MC, Seckl JR. The role of 11beta-hydroxysteroid dehydrogenases in the brain. Mol Cell Endocrinol. 2006;248:9–14. doi: 10.1016/j.mce.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Rensel MA, Schlinger BA, Remage-Healey L. In vivo detection of fluctuating brain steroid levels in zebra finches. Cold Spring Harb Protoc. 2014 doi: 10.1101/pdb.prot084616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karssen AM, Meijer OC, Pons D, De Kloet ER. Localization of mRNA expression of P-glycoprotein at the blood-brain barrier and in the hippocampus. Ann NY Acad Sci. 2004;1032:308–311. doi: 10.1196/annals.1314.048. [DOI] [PubMed] [Google Scholar]
- Karssen AM, Meijer OC, van der Sandt IC, Lucassen PJ, de Lange EC, de Boer AG, de Kloet ER. Multidrug resistance P-glycoprotein hampers the access of cortisol but not of corticosterone to mouse and human brain. Endocrinology. 2001;142:2686–94. doi: 10.1210/endo.142.6.8213. [DOI] [PubMed] [Google Scholar]
- Katz A, Heiblum R, Meidan R, Robinzon B. Corticosterone oxidative neutralization by 11-β hydroxysteroid dehydrogenases in kidney and colon of the domestic fowl. Gen Comp Endocrinol. 2008;155:814–820. doi: 10.1016/j.ygcen.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Katz A, Mirzatoni A, Zhen Y, Schlinger BA. Sex differences in cell proliferation and glucocorticoid responsiveness in the zebra finch brain. Eur J Neurosci. 2008;28:99–106. doi: 10.1111/j.1460-9568.2008.06303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz A, Oyama RK, Feng N, Chen X, Schlinger BA. 11Beta-hydroxysteroid dehydrogenase type 2 in zebra finch brain and peripheral tissues. Gen Comp Endocrinol. 2010;166:600–5. doi: 10.1016/j.ygcen.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Kirn JR. The relationship of neurogenesis and growth of brain regions to song learning. Brain Lang. 2010;115:29–44. doi: 10.1016/j.bandl.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchener P, Di Blasi F, Borrelli E, Piazza PV. Differences between brain structures in nuclear translocation and DNA binding of the glucocorticoid receptor during stress and the circadian cycle. Eur J Neurosci. 2004;19:1837–46. doi: 10.1111/j.1460-9568.2004.03267.x. [DOI] [PubMed] [Google Scholar]
- Klusoriová P, Kučka M, Ergang P, Mikšík I, Bryndová J, Pácha J. Cloning of chicken 11β-hydroxysteroid dehydrogenase type 1 and its tissue distribution. J Steroid Biochem Mol Biol. 2008;111:217–224. doi: 10.1016/j.jsbmb.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Kučka M, Vagnerová K, Klusoriová P, Mikšík I, Pácha J. Corticosterone metabolism in chicken tissues: Evidence for tissue-specific distribution of steroid dehydrogenases. Gen Comp Endocrinol. 2006;147:377–383. doi: 10.1016/j.ygcen.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Lechner O, Dietrich H, Wiegers GJ, Vacchio M, Wick G. Glucocorticoid production in the chicken bursa and thymus. Int Immunol. 2001;13:769–776. doi: 10.1093/intimm/13.6.769. [DOI] [PubMed] [Google Scholar]
- Leslie EM, Deeley RG, Cole SPC. Multidrug resistance proteins: Role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol. 2005;204:216–237. doi: 10.1016/j.taap.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Liebl AL, Martin LB. Exploratory behaviour and stressor hyper-responsiveness facilitate range expansion of an introduced songbird. Proc R Soc B Biol Sci. 2012;279:4375–4381. doi: 10.1098/rspb.2012.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loiseau C, Sorci G, Dano S, Chastel O. Effects of experimental increase of corticosterone levels on begging behavior, immunity and parental provisioning rate in house sparrows. Gen Comp. 2008;155:101–108. doi: 10.1016/j.ygcen.2007.03.004. [DOI] [PubMed] [Google Scholar]
- London S, Remage-Healey L, Schlinger B. Neurosteroid production in the songbird brain: a re-evaluation of core principles. Front Neuroendocrinol. 2009;30:302–314. doi: 10.1016/j.yfrne.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magarinos A, McEwen B. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- Malisch JL, Breuner CW. Steroid-binding proteins and free steroids in birds. Mol Cell Endocrinol. 2010;316:42–52. doi: 10.1016/j.mce.2009.09.019. [DOI] [PubMed] [Google Scholar]
- Malisch JL, Satterlee DG, Cockrem JF, Wada H, Breuner CW. How acute is the acute stress response? Baseline corticosterone and corticosteroid-binding globulin levels change 24 h after an acute stressor in Japanese quail. Gen Comp Endocrinol. 2010;165:345–350. doi: 10.1016/j.ygcen.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Martins TLF, Roberts ML, Giblin I, Huxham R, Evans MR. Speed of exploration and risk-taking behavior are linked to corticosterone titres in zebra finches. Horm Behav. 2007;52:445–453. doi: 10.1016/j.yhbeh.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Mason BL, Pariante CM, Thomas SA. A revised role for P-glycoprotein in the brain distribution of dexamethasone, cortisol, and corticosterone in wild-type and ABCB1A/B-deficient mice. Endocrinology. 2008;149:5244–5253. doi: 10.1210/en.2008-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald KS, Kirn JR. Anatomical plasticity in the adult zebra finch song system. J Comp Neurol. 2012;520:3673–3686. doi: 10.1002/cne.23120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer OC, de Lange EC, Breimer DD, de Boer AG, Workel JO, de Kloet ER. Penetration of dexamethasone into brain glucocorticoid targets is enhanced in mdr1A P-glycoprotein knockout mice. Endocrinology. 1998;139:1789–93. doi: 10.1210/endo.139.4.5917. [DOI] [PubMed] [Google Scholar]
- Mendel CM. The free hormone hypothesis a physiologically based mathematical model. Endocrine Reviews. 1989;10:232–274. doi: 10.1210/edrv-10-3-232. [DOI] [PubMed] [Google Scholar]
- Minni AM, Dorey R, Pierard C, Dominguez G, Helbling JC, Foury a, Beracochea D, Moisan MP. Critical role of plasma corticosteroid-binding-globulin during stress to promote glucocorticoid delivery to the brain: impact on memory retrieval. Endocrinology. 2012;153:4766–4774. doi: 10.1210/en.2012-1485. [DOI] [PubMed] [Google Scholar]
- Moisan MP, Minni AM, Dominguez G, Helbling JC, Foury A, Henkous N, Dorey R, Béracochéa D. Role of corticosteroid binding globulin in the fast actions of glucocorticoids on the brain. Steroids. 2014;81:109–115. doi: 10.1016/j.steroids.2013.10.013. [DOI] [PubMed] [Google Scholar]
- Newman AEM, Chin EH, Schmidt KL, Bond L, Wynne-Edwards KE, Soma KK. Analysis of steroids in songbird plasma and brain by coupling solid phase extraction to radioimmunoassay. Gen Comp Endocrinol. 2008;155:503–510. doi: 10.1016/j.ygcen.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Newman AEM, MacDougall-Shackleton SA, An YS, Kriengwatana B, Soma KK. Corticosterone and dehydroepiandrosterone have opposing effects on adult neuroplasticity in the avian song control system. J Comp Neurol. 2010;518:3662–3678. doi: 10.1002/cne.22395. [DOI] [PubMed] [Google Scholar]
- Newman A, Soma K. Corticosterone and dehydroepiandrosterone in songbird plasma and brain: effects of season and acute stress. Eur J Neurosci. 2009;29:1905–1914. doi: 10.1111/j.1460-9568.2009.06748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AEM, Soma KK. Aggressive interactions differentially modulate local and systemic levels of corticosterone and DHEA in a wild songbird. Horm Behav. 2011;60:389–396. doi: 10.1016/j.yhbeh.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. Plasticity in the adult avian central nervous system: possible relation between hormones, learning, and brain repair. Handb Physiol Nerv Syst. 1987:85–108. [Google Scholar]
- Nottebohm F. Neuronal replacement in adult brain. Brain Res Bull. 2002;57:737–749. doi: 10.1016/s0361-9230(02)00750-5. [DOI] [PubMed] [Google Scholar]
- Ouyang JQ, Sharp PJ, Dawson A, Quetting M, Hau M. Hormone levels predict individual differences in reproductive success in a passerine bird. Proc Biol Sci. 2011;278:2537–45. doi: 10.1098/rspb.2010.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge W. Transport of nutrients and hormones through the blood-brain barrier. Diabetologia. 1981;20:246–254. doi: 10.1007/BF00254490. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. Transport of small molecules through the blood-brain biology and methodology barrier: biology and methodology. Adv Drug Deliv Rev. 1995:5–36. [PubMed] [Google Scholar]
- Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2:3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariante CM. The role of multi-drug resistance p-glycoprotein in glucocorticoid function: Studies in animals and relevance in humans. Eur J Pharmacol. 2008;583:263–271. doi: 10.1016/j.ejphar.2007.11.067. [DOI] [PubMed] [Google Scholar]
- Petersen HH, Andreassen TK, Breiderhoff T, Bräsen JH, Schulz H, Gross V, Gröne HJ, Nykjaer A, Willnow TE. Hyporesponsiveness to glucocorticoids in mice genetically deficient for the corticosteroid binding globulin. Mol Cell Biol. 2006;26:7236–7245. doi: 10.1128/MCB.00400-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravosudov VV. Long-term moderate elevation of corticosterone facilitates avian food-caching behaviour and enhances spatial memory. Proc Biol Sci. 2003;270:2599–604. doi: 10.1098/rspb.2003.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Droste SK, Lightman SL, Reul JMHM, Linthorst ACE. Circadian and ultradian rhythms of free glucocorticoid hormone are highly synchronized between the blood, the subcutaneous tissue, and the brain. Endocrinology. 2012;153:4346–53. doi: 10.1210/en.2012-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Dong SM, Chao A, Schlinger BA. Sex-specific, rapid neuroestrogen fluctuations and neurophysiological actions in the songbird auditory forebrain. J Neurophysiol. 2012;107:1621–31. doi: 10.1152/jn.00749.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Dong S, Maidment NT, Schlinger BA. Presynaptic control of rapid estrogen fluctuations in the songbird auditory forebrain. J Neurosci. 2011;31:10034–8. doi: 10.1523/JNEUROSCI.0566-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Maidment N, Schlinger B. Forebrain steroid levels fluctuate rapidly during social interactions. Nat Neurosci. 2008;11:1327–1334. doi: 10.1038/nn.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rensel MA, Comito D, Kosarussavadi S, Schlinger BA. Region-specific neural corticosterone patterns differ from plasma in a male songbird. Endocrinology. 2014;155:3572–3581. doi: 10.1210/en.2014-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich EL, Romero LM. Exposure to chronic stress downregulates corticosterone responses to acute stressors. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1628–36. doi: 10.1152/ajpregu.00484.2004. [DOI] [PubMed] [Google Scholar]
- Robson AC, Leckie CM, Seckl JR, Holmes MC. 11 Beta-hydroxysteroid dehydrogenase type 2 in the postnatal and adult rat brain. Brain Res Mol Brain Res. 1998;61:1–10. doi: 10.1016/s0169-328x(98)00161-2. [DOI] [PubMed] [Google Scholar]
- Roland BL, Krozowski ZS, Funder JW. Glucocorticoid receptor, mineralocorticoid receptors, 11ß-hydroxysteroid dehydrogenase-1 and -2 expression in rat brain and kidney: In situ studies. Mol Cell Endocrinol. 1995;111:R1–R7. doi: 10.1016/0303-7207(95)03559-p. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Schlinger BA, Clayton NS. Rapid effects of corticosterone on cache recovery in mountain chickadees (Parus gambeli) Horm Behav. 2000;37:109–15. doi: 10.1006/hbeh.2000.1571. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Packan DR, Vale WW. Glucocorticoid toxicity in the hippocampus: in vitro demonstration. Brain Res. 1988;453:367–371. doi: 10.1016/0006-8993(88)90180-1. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schlinger BA, Brenowitz EA. Neural and hormonal control of birdsong. In: Pfaff D, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. 2nd. San Diego: Academic Press; 2009. pp. 897–942. [Google Scholar]
- Schlinger BA, Remage-Healey L, Rensel M. Establishing regional specificity of neuroestrogen action. Gen Comp Endocrinol. 2014;205:235–241. doi: 10.1016/j.ygcen.2014.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt KL, Chin EH, Shah AH, Soma KK. Cortisol and corticosterone in immune organs and brain of European starlings: developmental changes, effects of restraint stress, comparison with zebra finches. Am J Physiol Regul Integr Comp Physiol. 2009;297:R42–51. doi: 10.1152/ajpregu.90964.2008. [DOI] [PubMed] [Google Scholar]
- Schmidt KL, Soma KK. Cortisol and corticosterone in the songbird immune and nervous systems: local vs systemic levels during development. Am J Physiol Regul Integr Comp Physiol. 2008;295:R103–10. doi: 10.1152/ajpregu.00002.2008. [DOI] [PubMed] [Google Scholar]
- Schoech SJ, Bowman R, Bridge ES, Morgan GM, Rensel MA, Wilcoxen TE, Boughton RK. Corticosterone administration does not affect timing of breeding in Florida scrub-jays (Aphelocoma coerulescens) Horm Behav. 2007;52:191–6. doi: 10.1016/j.yhbeh.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Schoech S, Rensel M, Heiss R. Short-and long-term effects of developmental corticosterone exposure on avian physiology, behavioral phenotype, cognition, and fitness: A review. Curr Zool. 2011;57:514–530. [Google Scholar]
- Schoech S, Rensel M, Wilcoxen T. Here today, not gone tomorrow: long-term effects of corticosterone. J Ornithol. 2012;153:S217–S226. [Google Scholar]
- Schoech SJ, Romero LM, Moore IT, Bonier F. Constraints, concerns and considerations about the necessity of estimating free glucocorticoid concentrations for field endocrine studies. Funct Ecol. 2013;27:1100–1106. [Google Scholar]
- Shahbazi M, Schmidt M, Carruth LL. Distribution and subcellular localization of glucocorticoid receptor-immunoreactive neurons in the developing and adult male zebra finch brain. Gen Comp Endocrinol. 2011;174:354–61. doi: 10.1016/j.ygcen.2011.09.017. [DOI] [PubMed] [Google Scholar]
- Soma KK, Tramontin AD, Featherstone J, Brenowitz EA. Estrogen contributes to seasonal plasticity of the adult avian song control system. J Neurobiol. 2004;58:413–422. doi: 10.1002/neu.10288. [DOI] [PubMed] [Google Scholar]
- Spencer KA, Evans NP, Monaghan P. Postnatal stress in birds: A novel model of glucocorticoid programming of the hypothalamic-pituitary-adrenal axis. Endocrinology. 2009;150:1931–1934. doi: 10.1210/en.2008-1471. [DOI] [PubMed] [Google Scholar]
- Spencer KA, Verhulst S. Delayed behavioral effects of postnatal exposure to corticosterone in the zebra finch (Taeniopygia guttata) Horm Behav. 2007;51:273–80. doi: 10.1016/j.yhbeh.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Srivalli KM, Lakshmi PK. Overview of P-glycoprotein inhibitors: A rational outlook. Brazilian J Pharm Sci. 2012;48:353–367. [Google Scholar]
- Taves MD, Gomez-Sanchez CE, Soma KK. Extra-adrenal glucocorticoids and mineralocorticoids: evidence for local synthesis, regulation, and function. Am J Physiol Endocrinol Metab. 2011;301:E11–24. doi: 10.1152/ajpendo.00100.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramontin AD, Brenowitz EA. Seasonal plasticity in the adult brain. Trends Neurosci. 2000;23:251–258. doi: 10.1016/s0166-2236(00)01558-7. [DOI] [PubMed] [Google Scholar]
- Tramontin AD, Wingfield JC, Brenowitz EA. Androgens and estrogens induce seasonal-like growth of song nuclei in the adult songbird brain. J Neurobiol. 2003;57:130–140. doi: 10.1002/neu.10263. [DOI] [PubMed] [Google Scholar]
- Tronche C, Piérard C, Coutan M, Chauveau F, Liscia P, Béracochéa D. Increased stress-induced intra-hippocampus corticosterone rise associated with memory impairments in middle-aged mice. Neurobiol Learn Mem. 2010;93:343–51. doi: 10.1016/j.nlm.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Uhr M, Holsboer F, Müller MB. Penetration of endogenous steroid hormones corticosterone, cortisol, aldosterone and progesterone into the brain is enhanced in mice deficient for both mdr1a and mdr1b P-glycoproteins. J Neuroendocrinol. 2002;14:753–9. doi: 10.1046/j.1365-2826.2002.00836.x. [DOI] [PubMed] [Google Scholar]
- Van Duyse E, Arckens L, Pinxten R, Eens M, Darras V. Opposite changes in plasma testosterone and corticosterone levels following a simulated territorial challenge in male great tits. Behaviour. 2004;141:451–467. [Google Scholar]
- Wada H, Breuner CW. Transient elevation of corticosterone alters begging behavior and growth of white-crowned sparrow nestlings. J Exp Biol. 2008;211:1696–703. doi: 10.1242/jeb.009191. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Matt KS, Farner DS. Physiologic properties of steroid hormone-binding proteins in avian blood. Gen Comp Endocrinol. 1984;53:281–92. doi: 10.1016/0016-6480(84)90254-5. [DOI] [PubMed] [Google Scholar]
- Wingfield J, Moore M, Farner D. Endocrine responses to inclement weather in naturally breeding populations of white- crowned sparrows (Zonotrichia leucophrys pugetensis) Auk. 1983;100:56–62. [Google Scholar]
- Wingfield JC, Silverin B. Effects of corticosterone on territorial behavior of free-living male song sparrows Melospiza melodia. Horm Behav. 1986;20:405–417. doi: 10.1016/0018-506x(86)90003-6. [DOI] [PubMed] [Google Scholar]


