Abstract
Objectives
Up to 70% of endometrioid endometrial cancers carry PTEN gene deletions that can upregulate mTOR activity. Investigational mTOR kinase inhibitors may provide a novel therapeutic approach for these tumors. Using a xenograft tumor model of endometrial cancer, we assessed the activity of mTOR and downstream effector proteins in the mTOR translational control pathway after treatment with a dual mTOR Complex 1 and 2 (mTORC1/2) catalytic inhibitor (PP242) compared to that of an allosteric mTOR Complex 1 (mTORC1) inhibitor (everolimus, RAD001).
Methods
Grade 3 endometrioid endometrial cancer cells (AN3CA) were xenografted into nude mice. Animals were treated with PP242; PP242 and carboplatin; carboplatin; RAD001; RAD001 and carboplatin. Mean tumor volume was compared across groups by ANOVA. Immunoblot analysis was performed to assess mTORC1/2 activity using P-Akt, P-S6 and P-4E-BP1.
Results
The mean tumor volume of PP242 + carboplatin was significantly lower than in all other treatment groups, P<0.001 (89% smaller). The RAD001 + carboplatin group was also smaller, but this did not reach statistical significance (P=0.097). Immunoblot analysis of tumor lysates treated with PP242 demonstrated inhibition of activated P-Akt.
Conclusions
Catalytic mTORC1/2 inhibition demonstrates clear efficacy in tumor growth control that is enhanced by the addition of a DNA damage agent, carboplatin. Targeting mTORC1/2 leads to inhibition of Akt activation and strong downregulation of effectors of mTORC1, resulting in downregulation of protein synthesis. Based on this study, mTORC1/2 kinase inhibitors warrant further investigation as a potential treatment for endometrial cancer.
Keywords: mTOR pathway, endometrial cancer, rapamycin, preclinical study, translational regulation, eIF4E, 4E-BP
Background
Endometrial cancer is the most common gynecologic malignancy, with almost 50,000 new diagnoses and more than 8,000 deaths estimated to occur in the United States in 2013 [1]. While most women with endometrial cancer are diagnosed at an early stage owing to symptoms of irregular bleeding, approximately 15% of diagnoses are made at stages III or IV, with five-year survival rates ranging from 20–50%. Patients with advanced or recurrent disease have limited treatment options. Despite many recent advances in cancer therapy, there has been little improvement in survival for this patient population over the past 30 years [2]. Current treatment standards include surgical cytoreduction followed by adjuvant chemotherapy and/or radiation, with a possible addition of hormonal therapy. In recurrent disease, a variety of cytotoxic chemotherapy agents with or without radiation can be used for systemic or local disease control. Few, if any, of the treatments presently regarded as “standard of care” exploit known molecular alterations common to endometrial cancer as a target for therapy, [2, 3] with the exception of hormonal therapy. However, hormonal agents tend to have limited efficacy in poorly differentiated cancers, which comprise the majority of advanced and recurrent cases [2, 3].
Most endometrial cancers are of endometrioid histology [4]. Endometrioid and nonendometrioid endometrial cancers have distinct molecular alterations that provide potential new therapeutic targets [2, 3]. Up to 83% of endometrioid endometrial cancers have mutations in the tumor suppressor phosphatase and tensin homologue (PTEN) pathway [4], making proteins in this pathway natural targets in the treatment of these cancers. The protein phosphatase encoded by the PTEN gene has multiple anti-cancer activities. It maintains cell cycle arrest at the G1/S checkpoint, upregulates pro-apoptotic pathways controlled by the protein kinase Akt, and downregulates pro-survival anti-apoptotic pathways. When functioning normally, PTEN also prevents focal adhesion formation and cell spread, and serves as an inhibitor of mTOR pathway activation [5]. Therefore, loss of normal PTEN function results in aberrant cell proliferation, apoptotic escape, and abnormal cell spread [6], as well as increased mTOR activation [7–9]. This increase in mTOR activation subsequently increases protein synthesis necessary to sustain these aberrant, pro-proliferative activities in endometrial cancer [5]. In molecular terms, loss of PTEN activity results in increased phosphorylated and activated Akt, which can hyper-activate mTOR and stimulate mRNA translation. This in turn results in an overall moderate increase in protein synthesis, and a selective larger increase in the translation of angiogenic, DNA damage and repair, survival and pro-proliferative mRNAs [10]. Thus, restoring normalcy to the mTOR pathway, which is upregulated or hyperactivated in many endometrioid endometrial cancers, represents an attractive molecular target for treatment.
mTOR forms two protein complexes, mTOR Complex 1 (mTORC1) and mTOR Complex 2 (mTORC2) [11]. mTORC1 directly regulates mRNA translation. Rapalogs such as sirolimus and temsirolimus are allosteric mTOR inhibitors that block only mTORC1 activity. Rapalogs have been shown to have activity in endometrial cancer in both cell culture [9, 12, 13] and clinical studies [14–16]. However, rapalogs as anti-cancer agents represent a compromised approach. They only weakly inhibit mTORC1, and they have been linked to increased Akt activity by positive feedback regulation from loss of mTORC1 suppression of PI3K/Akt by IRS1, and from mTORC2 itself [14, 15]. Thus, suppression of only mTORC1 may ultimately upregulate cancer cell proliferative pathways. An agent that dually inhibits both mTORC1 and 2, such as a catalytic mTOR inhibitor, would theoretically result in improved anti-tumor activity compared to a rapalog [5].
Our study was designed to evaluate the anti-tumor activity of a catalytic mTORC1/2 inhibitor (PP242) compared to everolimus (RAD001), a rapalog, in an animal model of endometrioid endometrial cancer. Using a xenograft tumor model of endometrial cancer, we assessed the activity and expression of downstream proteins in the mTOR pathway after treatment with an mTORC1/2 inhibitor (PP242) or an allosteric mTORC1 (RAD001) inhibitor, with and without concurrent DNA damage chemotherapy with carboplatin. This paper presents our preclinical data in support of the clinical development of mTORC1/2 inhibitors for the treatment of endometrioid endometrial cancers.
Materials and Methods
Cell lines
Pilot studies of multiple endometrial cancer cell lines were conducted to establish tumorigenicity of various lines. These studies demonstrated enhanced tumorigenicity of the AN3CA cell line compared to other endometrial cancer cell lines tested (Hec1A and Hec1B) based on increased mean tumor volume 26 days after injection (Hec1A 122 mm3; Hec1B 123 mm3; AN3CA 1232 mm3). Further, AN3CA cells, isolated from a metastatic lesion in the lymph node of a patient with advanced endometrial cancer, produce poorly differentiated and platinum-resistant malignant tumors, and mirror more closely the clinical-pathologic characteristics of more aggressive endometrial cancers that would have a higher propensity toward recurrent or metastatic disease. Based on these findings, the human grade 3 endometrial adenocarcinoma cell line AN3CA was chosen for study. This cell line has previously been demonstrated to be PTEN negative, reflective of the majority of recurrent and metastatic endometrial cancers [16]. Cells were cultured at 37°, 5% CO2 in Minimum Essential Medium (Cellgro) supplemented with 10% fetal bovine serum, sodium pyruvate, and penicillin/streptomycin.
Tissue culture cell treatment protocols
AN3CA cells were plated in 6-well plates, then treated for 5 or 10 h with: (1) control vehicle; (2) carboplatin, 125 µg/ml; (3) PP242, 2.5 µM; (4) PP242 (2.5 µM) + carboplatin (125 µg/ml); (5) RAD001, 100 nM; (6) RAD001 (100 nM) + carboplatin (125 µg/ml). After treatment, immunoblot analyses were performed on cell lysates to assess activation of the mTOR pathway.
Xenograft animal tumor model
All studies were approved by the NYU School of Medicine Institutional Animal Care and Use Committee (IACUC), and conducted in accordance with IACUC guidelines. Female BALB/c nu/nu mice, age 5 weeks, were obtained from Taconic Farms, Inc. 2×106 cells were injected subcutaneously in the right flank in a total volume of 100 µl (50 µl RPMI and 50 µl Matrigel), using a 26-gauge needle. When mean tumor volume was approximately 160 mm3 calculated using external calipers and the standard formula for volume of an ellipsoid (π/6 × (larger diameter) × (smaller diameter)), mice were randomized into 6 groups, stratifying for average tumor volume. Each treatment group consisted of 7–8 mice and studies were repeated twice.
Xenograft tumor model treatment protocols
Treatment was conducted for 4 weeks. RAD001 and PP242 were administered on days 1–5 of each week. In groups in which carboplatin was also administered, this was injected on day 2 of the cycle. Mice were randomized into the following treatment groups: (1) control, treated by oral gavage with vehicle, 100 µl polyvinylpyrrolidone (PVP) + 5% N-methylpyrrolidone; (2) single agent PP242, 100 mg/kg PP242 in 100 µl by oral gavage (suspended in 15% PVP + 5% N-methylpyrrolidone as per manufacturer instructions); (3) PP242/carboplatin, treated as in group 2 with the addition of weekly carboplatin, 50 mg/kg injected on day 2 of weekly treatment cycle in total volume of 125 µl; (4) carboplatin, weekly at 50 mg/kg; (5) RAD001, at 2.5 mg/kg RAD001 in 100 µl by oral gavage (suspended in PBS + 10% DMSO); and (6) RAD001 + carboplatin, treated as in group 5 with the addition of weekly carboplatin as in group 4.
Assessment of xenograft tumor treatment response and toxicity
Tumor size was monitored twice per week as described above. Mice were weighed weekly and percentage weight change was used as a standard measure of toxicity.
Immunoblot analyses of tumor mTORC1/2 pathway response to treatment
Following treatments of tissue culture cells, cells were washed twice in ice-cold PBS and lysed at 4°C or 0.5% SDS lysis buffer [17]. NP-40 lysates were clarified by centrifugation at 13,000×g for 10 min and protein concentrations were determined for each sample by Bradford assay (Bio-Rad, Hercules, CA). To determine the total levels and phosphorylation status of specific proteins, equal amounts of protein were resolved by SDS-PAGE and analyzed by Western immunoblotting with specific antibodies as indicated. The phosphorylation status of most proteins was determined by immunoblotting membrane first with phospho-specific antibody then stripping the membranes using Restore Western blot stripping buffer (Pierce), followed by re-probing membranes with non-phospho-specific antibodies.
For tumor immunoblotting studies, at 2 h following the last treatment, mice were sacrificed and tumors were rapidly harvested into RIPA buffer [17]. Tumors were extracted by homogenization in RIPA buffer using a Tekmar tissumizer followed by centrifugation at 4°C for 10 min at 13,000 × g to remove insoluble material. Protein concentration was determined using the Biorad DC Protein Assay. To determine the total levels of specific proteins, equal amounts of protein from lysates were resolved by SDS-PAGE and analyzed by immunoblotting with specific antibodies.
Antibodies
For immunoblotting, the following antibodies were used. Rabbit anti-Akt, rabbit antiphospho- Akt S473, rabbit anti-S6, rabbit anti-phospho S6 (S240/244), rabbit anti-4E-BP1, rabbit anti-phospho-4E-BP1 (S65), all used at 1:1000 dilution (Cell Signaling); mouse anti-α-actin, 1:2000 (BD Pharmingen). Samples were separated by SDS-PAGE and transferred to PVDF membranes (Millipore). The Enhanced Chemiluminescence (ECL, GE Healthcare) procedure was used to detect protein signals as described by the manufacturer.
[35S]-Methionine incorporation assay
Cells were labeled with 50 µCi of [35S]-methionine/cysteine per mL (Easytag Express Protein Labeling Mix, Perkin Elmer) in Met/Cys-free DMEM with 5% FBS for 20 min, lysates prepared and specific activity of [35S]-methionine/cysteine incorporation into nascent protein was determined by trichloroacetic acid (TCA) precipitation onto GF/C filters and liquid scintillation counting. Studies were repeated three times and data presented as mean with standard error of the mean (SEM).
Statistical analyses
Mean tumor volume in each group was calculated at each measurement date, and these values were normalized to the mean starting tumor volume in each respective group. This allowed for assessment of relative tumor growth, independent of starting volume. Tumor growth curves were plotted, and SEM was calculated and applied to these graphs. Tumor volume was compared between groups using ANOVA.
Results
Catalytic inhibition of mTORC1/2 provides more effective blockade of mTOR downstream signaling and protein synthesis than rapalog inhibition of mTORC1
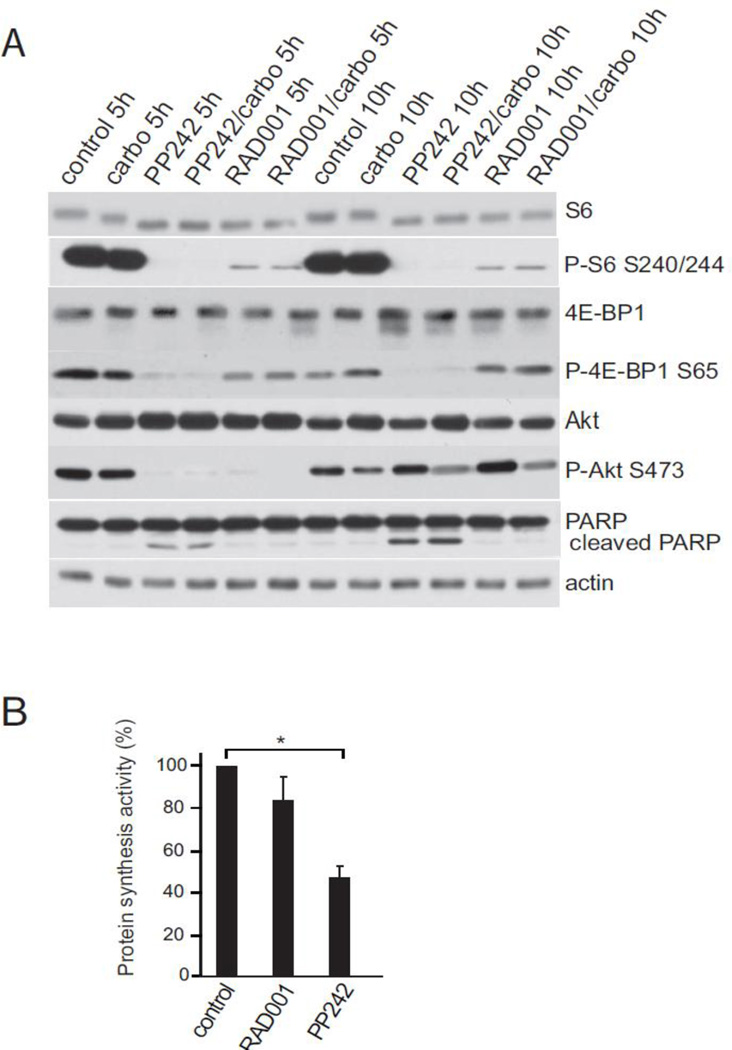
We assessed the effect of catalytic and allosteric inhibition of the mTOR pathway on treatment of AN3CA cells in tissue culture. Because phosphorylation of mTOR itself is considered an unreliable marker of its activity [11], we utilized two downstream phosphorylation targets and effectors of the mTORC1 pathway, ribosomal protein S6 and the eIF4E inhibiting protein 4E-BP1. While many studies rely solely on analysis of S6 or S6 kinase (S6K) phosphorylation as a marker for mTORC1 activity, these are considered less reliable than 4E-BP1 because they more poorly interact with mTORC1 and are more readily inhibited with small reductions in mTORC1 activity [11, 18]. Cells were treated for 5 h or 10 h with 2.5 µM PP242 or 100 mM RAD001. Analysis of P-S6 levels showed effective inhibition by both RAD001 and PP242, whereas only catalytic inhibition by PP242 showed strong downregulation of 4E-BP1 phosphorylation (Fig. 1A). The inability of rapalog RAD001 to strongly block mTORC1 activity was observed at both 5 h and 10 h time points, indicating that this is a steady-state response, regardless of co-treatment with carboplatin at DNA damaging levels (125 ng/ml). There was no change in total protein levels for any of the biomarker proteins. In addition, the PP242 treatment groups demonstrated a marked decrease in levels of phosphorylated Akt, not seen in cells treated with RAD001 or in the control or carboplatin treatment groups. We note as well that PARP cleavage, a marker of induced cell death, was strongly increased only in cells treated with the catalytic mTORC1/2 inhibitor.
Fig. 1.
Effect of PP242 catalytic and RAD001 allosteric rapalog inhibitors on mTOR downstream effector protein phosphorylation and protein synthesis in endometriod cancer cells in culture. (A) AN3CA cells in tissue culture were treated for 5 h or 10 h with 2.5 µM PP242 or 100 mM RAD001, considered maximal inhibitory doses based on IC50 studies in a variety of cell types. Cells were lysed, equal amounts of soluble cell protein extracts resolved by SDS-PAGE and analyzed by protein immunoblotting with specific antibodies as shown. Phosphorylation immunoblotting of proteins was determined by immunoblotting membrane first with phospho-specific antibody then stripping the membranes followed by re-probing with non-phospho-specific antibodies. ECL was used for detection. Results shown are representative of three independent experiments. (B) Protein synthesis rates were determined 5 h after treatment of AN3CA cells with 2.5 µM PP242 or 100 mM RAD001, by [35S]-methionine incorporation. Standard activity of label incorporation into nascent protein was determined by TCA precipitation and liquid scintillation counting. Results were normalized to control untreated cells. Standard errors of the mean (SEM) shown. *, P<0.05 by t-test.
The greater inhibition of mTORC1 by catalytic compared to allosteric rapalog inhibitors also was associated with greater reduction in protein synthesis (Fig. 1B). Cells treated for 5 h with PP242 at 2.5 µM or RAD001 at 100 mM were subjected to metabolic labeling with 35S-methionine and protein specific activities determined to measure de novo protein synthesis activity. RAD001 reduced overall protein synthesis by approximately 15%, in keeping with typical results in the literature, whereas PP242 reduced protein synthesis activity by almost half, consistent with the greater phosphorylation and activation of the eIF4E/translation inhibitor, 4E-BP1.
Effective control of endometrial tumor growth by mTORC1/2 inhibition in a xenotransplant animal model
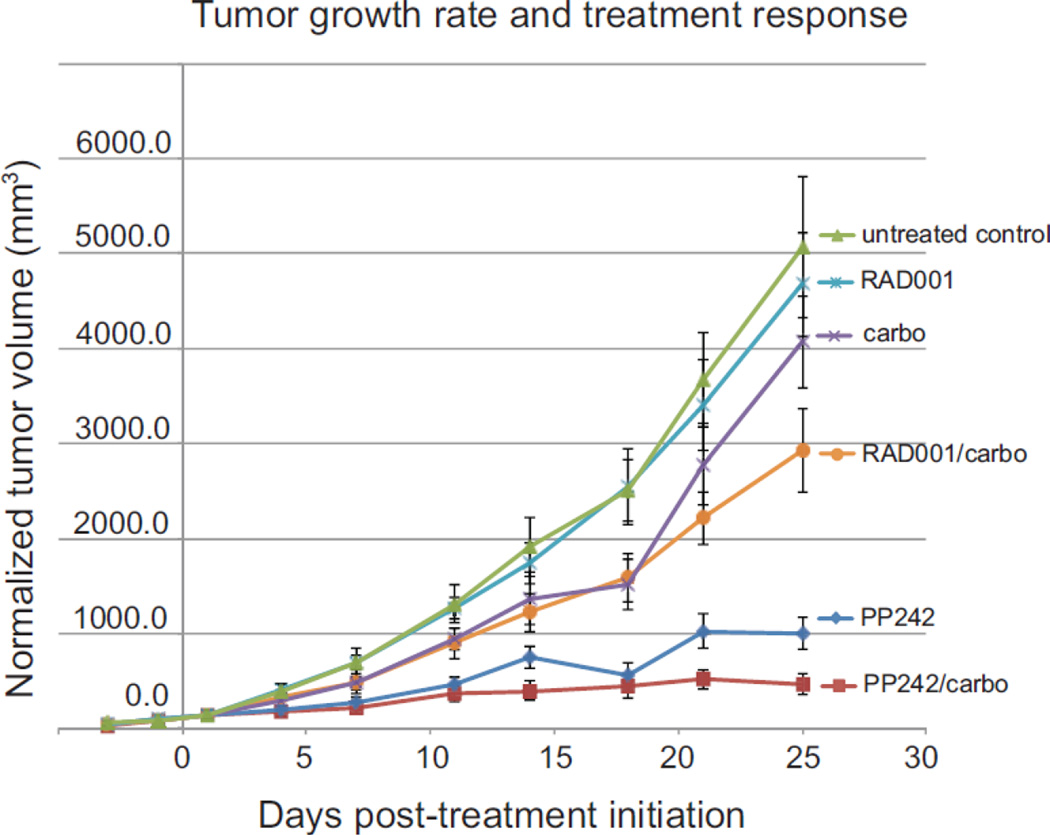
We utilized a xenotransplant animal tumor model of endometrioid endometrial cancer and a clinically relevant co-treatment strategy involving weekly carboplatin to assess the effectiveness of catalytic compared to rapalog inhibition of the mTOR pathway in tumor control. Animals were injected subcutaneously in the flank with equal numbers of AN3CA tumor cells, then randomized into treatment groups when the average tumor volume reached 160 mm3. The mean tumor volume in the treatment groups ranged from 122 mm3 to 191 mm3. Animals were treated as described in Methods for four weeks and tumor volumes measured throughout that period. At treatment completion, the average smallest tumor volume was in the PP242/carboplatin group, at 553 +/− 130 mm3, compared to an average of about 4800 +/− 554 mm3 in the untreated control and single agent RAD001 groups (Fig. 2, Table 1). The difference in mean tumor volume was statistically significant when comparing tumor volume in the PP242/carboplatin group with mean tumor volumes in all other treatment groups at the completion of treatment (P <0.001).
Fig. 2.
AN3CA endometrial tumor average response to treatment in a xenotransplant mouse model. Female BALB/c nu/nu mice were injected subcutaneously in the right flank with 2 × 106 AN3CA cells, then mice randomized into treatment groups when tumors were 160 mm3. RAD001 (2.5 mg/kg) and PP242 (100 mg/kg) were administered by gavage on days 1–5 of each week, carboplatin (50 mg/kg, i.p.) was given on day 2 of the weekly cycle. Tumor sizes were determined by precision caliber twice weekly. Results represent the mean with SEM of two independent studies of 7–8 mice per treatment arm.
Table 1.
Xenograft endometrial tumor response to treatment on day 20
| Treatment group | Treatment | Mean tumor volume (mm3) +/− SEM |
|---|---|---|
| 1 | Control | 4814 +/− 704 |
| 2 | PP242 | 1280 +/− 212 |
| 3 | PP242/carboplatin | 553 +/− 130 |
| 4 | carboplatin | 4588 +/−545 |
| 5 | RAD001 | 4725 +/− 554 |
| 6 | RAD001/carboplatin | 2385 +/− 359 |
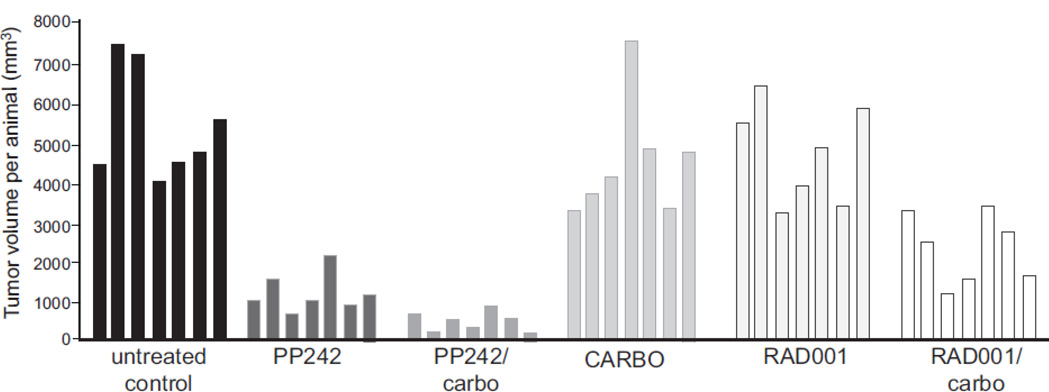
As shown in both the averaged data (Fig. 2) and individual tumor treatment responses by waterfall plot analysis for a representative set of studies (Fig. 3), the PP242/carboplatin treatment group had the largest treatment effect with smallest tumor volume at the end of treatment. The group treated with PP242 alone also demonstrated a marked effect, but with tumors approximately twice as large as those in the combination PP242/carboplatin group at the end of treatment. Single agent RAD001 had no effect on tumor size and was not statistically different from the untreated controls. There was some antitumor activity in the RAD001/carboplatin group, with mean tumor reduction of almost half, though not nearly as striking as seen in the PP242/carboplatin group. Single agent carboplatin was ineffective and not statistically different from the untreated control group. The treatment effect seen in the PP242/carboplatin group was statistically significant when compared with the other treatment groups combined. This treatment effect was also clinically significant, as tumors in the PP242/carboplatin group exhibited a 90% reduction in mean tumor volume compared to those in the control group at the completion of treatment (P<0.001).
Fig. 3.
Waterfall plot of individual AN3CA tumor responses to treatment with PP242, RAD001 without or with concurrent carboplatin. The data shown in Figure 3 were replotted to demonstrate individual final treatment responses per animal at the end of the 25 day treatment cycle. Each column represents one individual mouse corresponding to the different treatment groups.
Comparison of treatment toxicities for catalytic and allosteric mTOR inhibitors in a xenotransplant animal tumor model
Toxicity of the different treatment protocols was measured by percentage of animal weight change during treatment (Table 2). Mice in the group with the greatest treatment effect (PP242/carboplatin) exhibited a mean −3.0% weight change compared to mice in the group with the least treatment effect (control), which gained the most weight (+13.8%), a part of which was tumor weight.
Table 2.
Toxicity treatment protocols in xenograft endometrial animal tumor model.
| Treatment group | Treatment | % Weight Change |
|---|---|---|
| 1 | Control | +13.8 |
| 2 | PP242 | +12.8 |
| 3 | PP242/Carboplatin | −3.0 |
| 4 | Carboplatin | +6.4 |
| 5 | RAD001 | +12.2 |
| 6 | RAD001/Carboplatin | +8.6 |
Effect of catalytic mTORC1/2 or allosteric mTORC1 inhibition on the downstream mTOR effector pathway in xenotransplant tumor tissues
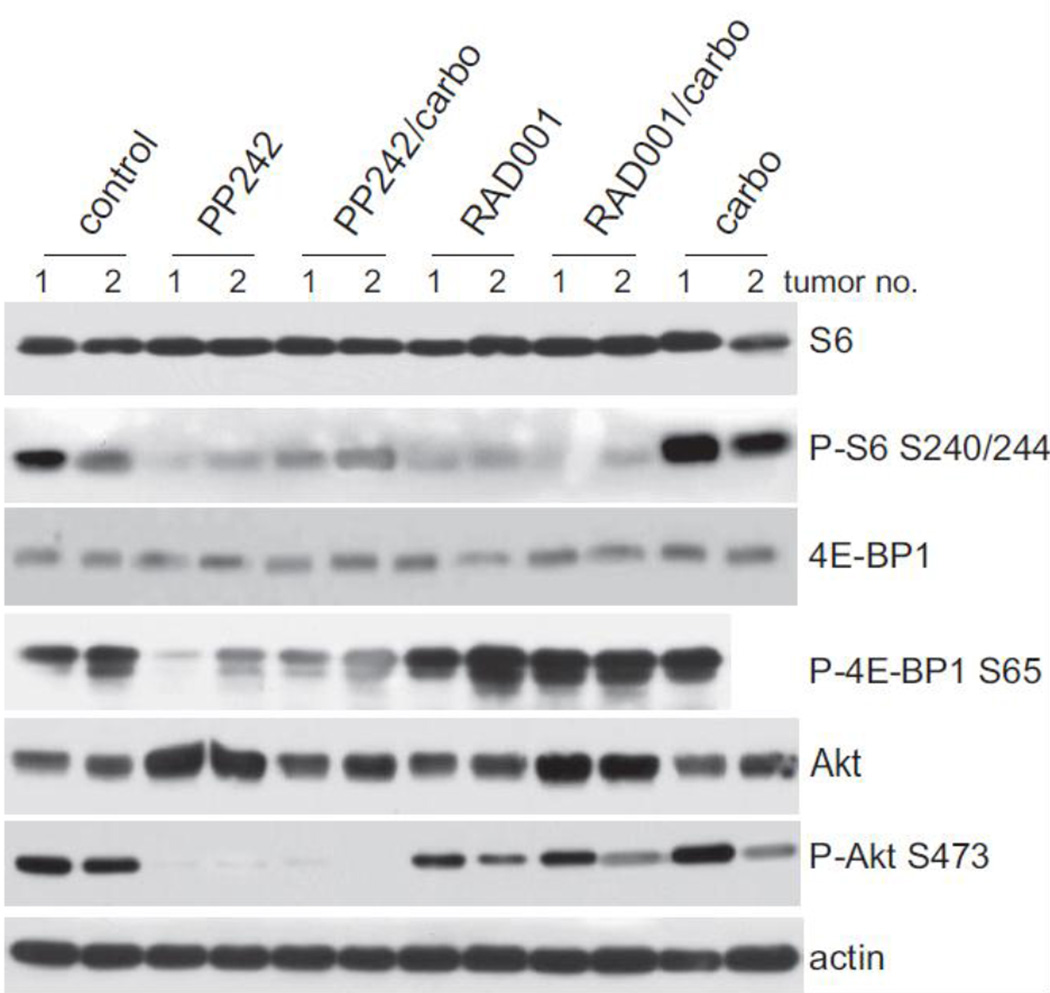
Analysis of the tumors in mice across treatment groups demonstrated the superior blockade by mTORC1/2 inhibitors on downstream effectors of the mTOR pathway. Representative immunoblot analyses were performed on excised tumors taken at 2 h post-final treatment (Fig. 4). While there is greater variability in response to treatment of tumors compared to tissue culture cells, as expected, representative tumors provided several key findings. First, the inadequacy of relying on reduced S6 protein phosphorylation as a measure of mTOR inhibition was apparent. S6 protein phosphorylation was strongly reduced in tumor lysates from both RAD001 and PP242 treated samples independently of tumor treatment response. In contrast, only mTORC1/2 inhibition with PP242, and not mTORC1 inhibition with RAD001, strongly reduced phosphorylation of 4E-BP1, a more stringent measure of mTORC1 inhibition and consistent with much greater tumor response. These data confirm those of tissue culture. Second, only catalytic mTORC1/2 inhibition (PP242) resulted in strong downregulation of AKT activation, shown by the striking reduction in P-AKT at Ser 473, which was not observed in rapalog treated tumors under any conditions. Thus, these data provide evidence for the importance of inhibiting mTORC2 and blockade of the Akt upregulatory loop in tumor control. Third, neither mTORC1/2 inhibition with PP242, nor mTORC1 inhibition with RAD001, resulted in reduction of the steady state levels of mTORC1 effector proteins. These data suggest the importance of achieving significant inhibition of mTOR activity through catalytic inhibition and its association with strong tumor treatment response.
Fig. 4.
Effect of PP242 catalytic and RAD001 allosteric rapalog inhibitors on mTOR downstream effector protein phosphorylation in AN3CA endometriod tumors. Equal amounts of soluble protein extracts obtained from individual representative tumors were resolved by SDS-PAGE and analyzed by protein immunoblotting with specific antibodies as shown. Phosphorylation immunoblotting of proteins was determined by immunoblotting membrane first with phospho-specific antibody then stripping the membranes followed by re-probing with nonphospho- specific antibodies. ECL was used for detection. Extracts were prepared 2 h following the last drug treatment. Two tumors closest to the mean of tumor response in each treatment arm were chosen for analysis, identified as samples 1 and 2.
Discussion
There is a clear need for more effective, biomarker-directed treatment strategies in advanced and recurrent cases of endometrioid endometrial cancer [2, 3]. The use of PI3K inhibitors relies on evidence suggesting that activating mutations in PI3K/Akt pathway (PIK3CA) and inactivation of p53 confer a poor prognosis in endometrial cancer [19, 20]. However, the clinical implementation of PI3K/Akt inhibitors has been limited by toxicity and resistance [3, 21]. Given that hyperactivation and possibly overexpression of mTOR occurs in many endometrial cancers and cell lines [5, 7, 8, 22, 23], and that many of these tumors have mTOR activating loss of PTEN [5, 24], interest has grown in the use of mTOR inhibitors in the treatment of endometrial cancer. mTOR inhibition is further downstream than PI3K/Akt inhibition and therefore may be better tolerated. Cell culture and tumor explant studies using rapalogs such as rapamycin and its esters, support their modest efficacy in endometrial cancer cell inhibition [9, 12]. A small clinical trial of temsirolimus (CCI-779), an ester of rapamycin, in treatment naïve or prior chemotherapy treated women with endometrial cancer, showed encouraging single agent activity that was higher in the treatment naïve group [25]. Completion of a recent Phase II trial with single agent everolimus (RAD001) in patients with recurrent or metastatic endometrial cancer refractory to chemotherapy showed on average a 36% rate of response and stable disease for 3 months [26], similar in response and duration to several other studies of rapalog mTOR inhibitors in advanced endometrial cancer [27–31].
Inhibition of the mTOR pathway with concurrent chemotherapy and/or anti-hormonal therapy remains an attractive approach for control of advanced endometrial cancer. Nevertheless, it is apparent that potentially intractable features of rapamycin-based therapy limit efficacy. We therefore explored the novel class of dual mTORC1/2 catalytic inhibitors in a preclinical model of endometrioid endometrial cancer.
Treatment with a dual mTOR inhibitor resulted in notable tumor growth inhibition in this xenograft model of high grade, PTEN-negative endometrial cancer. The addition of a DNA damaging agent, carboplatin, potentiated this effect. In comparing the activity of a dual mTORC1/2 inhibitor (PP242) with that of the clinically available rapalog everolimus (RAD001) that acts only on mTORC1, PP242 exhibited superior antitumor activity as single agent and with concurrent carboplatin. Notably, single-agent carboplatin had minimal anti-tumor activity in our model. The platinum-refractory nature of our model is a reflection of the high grade of the cell line that we chose and its recurrent metastatic presentation. Despite this, therapy with PP242 or RAD001 in combination with carboplatin resulted in significant tumor growth inhibition. These results demonstrate potentiation of the anti-tumor effect that PP242 and RAD001 have on abnormal cell proliferation by DNA damaging agents including carboplatin, improved over the cytotoxic activity that genotoxic DNA damaging agents have when used alone.
Immunoblot analyses of lysates of cells treated with PP242 vs. RAD001, with or without carboplatin, demonstrated that dual mTORC1/2 inhibition strongly reduces activation of effector proteins in the mTOR pathway, particularly 4E-BP1. Further, this abolished any evidence for the upregulation of Akt activity, which likely is the mediator of rapalog-related treatment efficacy concerns. Importantly, we also demonstrated that reliance solely on S6 phosphorylation as a surrogate measure of mTOR activity is unreliable. Use of S6 phosphorylation to measure mTORC1 activity is standard practice in many preclinical and clinical studies. Improved inhibition of mTORC1 by catalytic mTOR inhibition much more strongly blocked its activity and in turn reduced protein synthesis than is achievable with an allosteric, rapamycin-based mTOR inhibitor. Hypophosphorylation and the inhibition of Akt upregulation as a result of inhibition of mTORC2 is also likely responsible for the striking tumor response seen in PP242 treated cells, as Akt is both an activator of mTORC1 and a direct downstream effector of mTORC2 [32].
Immunoblot analysis of tumors from mice in the different treatment groups confirmed findings noted in cell culture, that activation (phosphorylation) of Akt is much better inhibited by a dual mTORC1/2 inhibitor than by a rapalog. Despite the greater complexity of an animal model than tissue culture studies, the clear decrease in phosphorylated Akt in animals treated with PP242, and the much greater reduction in mTORC1 activity compared to animals treated with RAD001, likely accounts for much of the improved anti-tumor response and benefit of addition of carboplatin.
Clinical trials have demonstrated that PTEN status is an inconsistent and possibly irrelevant predictor of efficacy for mTOR inhibitors. Further study is warranted to assess the activity of dual mTORC1/2 kinase inhibitors in those endometrial cancers with normal PTEN phenotype, although this constitutes a minority of recurrent and high grade clinical presentations. Future studies of interest may involve use of an intraperitoneal tumor model and employ imaging modalities to assess treatment response. From the present study, it is clear that dual mTORC1/2 kinase inhibition results in significant, superior anti-tumor activity in a high grade, PTEN-negative xenograft model of endometrioid endometrial cancer compared to rapalog treatment, even with the addition of concurrent carboplatin. In fact, in this model, treatment with a rapalog did not even outperform the control untreated group. Based on our current study, dual mTORC1/2 kinase inhibitors warrant further investigation as a potential treatment for endometrioid endometrial cancers.
Highlights.
Catalytic mTORC1/2 inhibition demonstrates clear efficacy over rapamycin approaches in endometrial tumor models
Targeting mTORC1/2 inhibits Akt activation in endometrial tumors, aiding tumor control
mTORC1/2 kinase inhibitors warrant further investigation as a treatment for endometrial cancer
Acknowledgments
This work was supported by the following sources: Avon Foundation for Women (R.J.S.), Breast Cancer Research Foundation (R.J.S.). Supported in part by grant UL1 TR000038 from the National Center for the Advancement of Translational Science (NCATS), National Institutes of Health (F.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
All authors declare no conflicts of interest.
We, the authors of this manuscript, have no financial or personal relationships to disclose that could inappropriately influence or bias this work.
References
- 1.Cancer Facts and Figures 2013. Atlanta: American Cancer Society; 2013. [Google Scholar]
- 2.Sorosky JI. Endometrial cancer. Obstet Gynecol. 2008;111:436–447. doi: 10.1097/AOG.0b013e318162f690. [DOI] [PubMed] [Google Scholar]
- 3.Salvesen HB, Haldorsen IS, Trovik J. Markers for individualised therapy in endometrial carcinoma. Lancet Oncol. 2012;13:e353–e361. doi: 10.1016/S1470-2045(12)70213-9. [DOI] [PubMed] [Google Scholar]
- 4.Hecht JL, Mutter GL. Molecular and pathologic aspects of endometrial carcinogenesis. J Clin Oncol. 2006;24:4783–4791. doi: 10.1200/JCO.2006.06.7173. [DOI] [PubMed] [Google Scholar]
- 5.Korets SB, Czok S, Blank SV, Curtin JP, Schneider RJ. Targeting the mTOR/4E-BP pathway in endometrial cancer. Clin Cancer Res. 2011;17:7518–7528. doi: 10.1158/1078-0432.CCR-11-1664. [DOI] [PubMed] [Google Scholar]
- 6.Bansal N, Yendluri V, Wenham RM. The molecular biology of endometrial cancers and the implications for pathogenesis, classification, and targeted therapies. Cancer Control. 2009;16:8–13. doi: 10.1177/107327480901600102. [DOI] [PubMed] [Google Scholar]
- 7.Choi CH, Lee JS, Kim SR, Kim TJ, Lee JW, Kim BG, Bae DS. Clinical significance of pmTOR expression in endometrioid endometrial carcinoma. Eur J Obstet Gynecol Reprod Biol. 2010;153:207–210. doi: 10.1016/j.ejogrb.2010.07.038. [DOI] [PubMed] [Google Scholar]
- 8.Torres A, Torres K, Pesci A, Ceccaroni M, Paszkowski T, Cassandrini P, Zamboni G, Maciejewski R. Deregulation of miR-100, miR-99a and miR-199b in tissues and plasma coexists with increased expression of mTOR kinase in endometrioid endometrial carcinoma. BMC Cancer. 2012;12:369. doi: 10.1186/1471-2407-12-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bae-Jump VL, Zhou C, Boggess JF, Whang YE, Barroilhet L, Gehrig PA. Rapamycin inhibits cell proliferation in type I and type II endometrial carcinomas: a search for biomarkers of sensitivity to treatment. Gynecol Oncol. 2010;119:579–585. doi: 10.1016/j.ygyno.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silvera D, Formenti SC, Schneider RJ. Translational control in cancer. Nat Rev Cancer. 2010;10:254–266. doi: 10.1038/nrc2824. [DOI] [PubMed] [Google Scholar]
- 11.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 12.Bae-Jump VL, Zhou C, Boggess JF, Gehrig PA. Synergistic effect of rapamycin and cisplatin in endometrial cancer cells. Cancer. 2009;115:3887–3896. doi: 10.1002/cncr.24431. [DOI] [PubMed] [Google Scholar]
- 13.Shafer A, Zhou C, Gehrig PA, Boggess JF, Bae-Jump VL. Rapamycin potentiates the effects of paclitaxel in endometrial cancer cells through inhibition of cell proliferation and induction of apoptosis. Int J Cancer. 2010;126:1144–1154. doi: 10.1002/ijc.24837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, Egia A, Sasaki AT, Thomas G, Kozma SC, Papa A, Nardella C, Cantley LC, Baselga J, Pandolfi PP. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118:3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL, Baselga J, Rosen N. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu KH, Wu W, Dave B, Slomovitz BM, Burke TW, Munsell MF, Broaddus RR, Walker CL. Loss of tuberous sclerosis complex-2 function and activation of mammalian target of rapamycin signaling in endometrial carcinoma. Clin Cancer Res. 2008;14:2543–2550. doi: 10.1158/1078-0432.CCR-07-0321. [DOI] [PubMed] [Google Scholar]
- 17.Silvera D, Arju R, Darvishian F, Levine PH, Zolfaghari L, Goldberg J, Hochman T, Formenti SC, Schneider RJ. Essential role for eIF4GI overexpression in the pathogenesis of inflammatory breast cancer. Nat Cell Biol. 2009;11:903–908. doi: 10.1038/ncb1900. [DOI] [PubMed] [Google Scholar]
- 18.Kang SA, Pacold ME, Cervantes CL, Lim D, Lou HJ, Ottina K, Gray NS, Turk BE, Yaffe MB, Sabatini DM. mTORC1 phosphorylation sites encode their sensitivity to starvation and rapamycin. Science. 2013;341:1236566. doi: 10.1126/science.1236566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Catasus L, Gallardo A, Cuatrecasas M, Prat J. Concomitant PI3K-AKT and p53 alterations in endometrial carcinomas are associated with poor prognosis. Mod Pathol. 2009;22:522–529. doi: 10.1038/modpathol.2009.5. [DOI] [PubMed] [Google Scholar]
- 20.Catasus L, Gallardo A, Cuatrecasas M, Prat J. PIK3CA mutations in the kinase domain (exon 20) of uterine endometrial adenocarcinomas are associated with adverse prognostic parameters. Mod Pathol. 2008;21:131–139. doi: 10.1038/modpathol.3800992. [DOI] [PubMed] [Google Scholar]
- 21.Dedes KJ, Wetterskog D, Ashworth A, Kaye SB, Reis-Filho JS. Emerging therapeutic targets in endometrial cancer. Nat Rev Clin Oncol. 2011;8:261–271. doi: 10.1038/nrclinonc.2010.216. [DOI] [PubMed] [Google Scholar]
- 22.Darb-Esfahani S, Faggad A, Noske A, Weichert W, Buckendahl AC, Muller B, Budczies J, Roske A, Dietel M, Denkert C. Phospho-mTOR and phospho-4EBP1 in endometrial adenocarcinoma: association with stage and grade in vivo and link with response to rapamycin treatment in vitro. J Cancer Res Clin Oncol. 2009;135:933–941. doi: 10.1007/s00432-008-0529-5. [DOI] [PubMed] [Google Scholar]
- 23.Lu KH, Wu W, Dave B, Slomovitz BM, Burke TW, Munsell MF, Broaddus RR, Walker CL. Loss of tuberous sclerosis complex-2 function and activation of mammalian target of rapamycin signaling in endometrial carcinoma. Clin Cancer Res. 2008;14:2543–2550. doi: 10.1158/1078-0432.CCR-07-0321. [DOI] [PubMed] [Google Scholar]
- 24.Wahl H, Daudi S, Kshirsagar M, Griffith K, Tan L, Rhode J, Liu JR. Expression of metabolically targeted biomarkers in endometrial carcinoma. Gynecol Oncol. 2010;116:21–27. doi: 10.1016/j.ygyno.2009.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oza AM, Elit L, Tsao MS, Kamel-Reid S, Biagi J, Provencher DM, Gotlieb WH, Hoskins PJ, Ghatage P, Tonkin KS, Mackay HJ, Mazurka J, Sederias J, Ivy P, Dancey JE, Eisenhauer EA. Phase II study of temsirolimus in women with recurrent or metastatic endometrial cancer: a trial of the NCIC Clinical Trials Group. J Clin Oncol. 2011;29:3278–3285. doi: 10.1200/JCO.2010.34.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ray-Coquard I, Favier L, Weber B, Roemer-Becuwe C, Bougnoux P, Fabbro M, Floquet A, Joly F, Plantade A, Paraiso D, Pujade-Lauraine E. Everolimus as second- or third-line treatment of advanced endometrial cancer: ENDORAD, a phase II trial of GINECO. Br J Cancer. 2013;108:1771–1777. doi: 10.1038/bjc.2013.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slomovitz BM, Lu KH, Johnston T, Coleman RL, Munsell M, Broaddus RR, Walker C, Ramondetta LM, Burke TW, Gershenson DM, Wolf J. A phase 2 study of the oral mammalian target of rapamycin inhibitor, everolimus, in patients with recurrent endometrial carcinoma. Cancer. 2010;116:5415–5419. doi: 10.1002/cncr.25515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Columbo N, MMeekin S, Schwartz P, Kostka J, Sessa C, Gehig P, Halloway R, Braly P, Matei D, Einstein M. A phase II trial of the mTOR inhibitor AP23573 as a single agent in advanced endometrial cancer. J Clin Oncol. 2007;25 Abstr. 5516. [Google Scholar]
- 29.Fleming GF, Filiaci VL, Hanjani P, Burke JJ, II, Davidson SA, Leslie KK, Zaino RJ. Hormonal therapy plus temsirolimus for endometrial carcinoma (EC): Gynecological Group Trial #248. J Clin Oncol. 2011;29 Abstr. 5014. [Google Scholar]
- 30.Oza AM, Poveda A, Clamp AR, Pignata S, Scambia G, Del Campo JM, McCormack M, Sevcik L, Schwartz BM, Guan S, Lee R, Cheng JD, Haluska FG. A randomized phase II (RP2) trial of ridaforolimus (R) compared with progestin (P) or chemotherapy (P) in female adult patients with advanced endometrial carcinoma. J Clin Oncol. 2011;29 doi: 10.1200/JCO.2014.58.8871. Abstr. 5009. [DOI] [PubMed] [Google Scholar]
- 31.Mackay HJ, Welch S, Tsao MS, Biagi JJ, Elit L, Ghatage P, Martin LA, Tonkin KS, Ellard S, Lau SK, McIntosh L, Eisenhauer EA, Oza AM. Phase II study of oral ridaforolimus in patients with metastatic and/or locally advanced recurrent endometrial cancer. J Clin Oncol. 2011;29 doi: 10.1016/j.ygyno.2014.06.033. Abstr. 5013. [DOI] [PubMed] [Google Scholar]
- 32.Cybulski N, Hall MN. TOR complex 2: a signaling pathway of its own. Trends Biochem Sci. 2009;34:620–627. doi: 10.1016/j.tibs.2009.09.004. [DOI] [PubMed] [Google Scholar]