Abstract
Purpose
Cholecystectomy in patients with an intact gallbladder after endoscopic removal of stones from the common bile duct (CBD) remains controversial. We conducted a case-control study to determine the risk of recurrent CBD stones and the benefit of cholecystectomy for prevention of recurrence after endoscopic removal of stones from the CBD in Korean patients.
Materials and Methods
A total of 317 patients who underwent endoscopic CBD stone extraction between 2006 and 2012 were included. Possible risk factors for the recurrence of CBD stones including previous cholecystectomy history, bile duct diameter, stone size, number of stones, stone composition, and the presence of a periampullary diverticulum were analyzed.
Results
The mean duration of follow-up after CBD stone extraction was 25.4±22.0 months. A CBD diameter of 15 mm or larger [odds ratio (OR), 1.930; 95% confidence interval (CI), 1.098 to 3.391; p=0.022] and the presence of a periampullary diverticulum (OR, 1.859; 95% CI, 1.014 to 3.408; p=0.045) were independent predictive factors for CBD stone recurrence. Seventeen patients (26.6%) in the recurrence group underwent elective cholecystectomy soon after endoscopic extraction of CBD stones, compared to 88 (34.8%) in the non-recurrence group; the difference was not statistically significant (p=0.212).
Conclusion
A CBD diameter of 15 mm or larger and the presence of a periampullary diverticulum were found to be potential predictive factors for recurrence after endoscopic extraction of CBD stones. Elective cholecystectomy after clearance of CBD stones did not reduce the incidence of recurrent CBD stones in Korean patients.
Keywords: Cholecystectomy, gallstones, common bile duct, recurrence
INTRODUCTION
Since its introduction in 1974, endoscopic biliary sphincterotomy (EST) has been the standard therapy for the removal of bile duct stones.1,2 The safety of EST has been well established, and its use has become widespread. There have been many reports of bile duct stone recurrence after EST, although the range of reported recurrence rates is wide (3% to 24%).3,4,5,6,7,8 The suggested causes of recurrent bile duct stones after EST are cholecystolithiasis, mechanical lithotripsy, pneumobilia, a dilated common bile duct (CBD), periampullary diverticulum, angulation of the CBD (≤145°) on endoscopic retrograde cholangiopancreatography (ERCP), bile stasis, biliary stricture, and papillary stenosis.6,7,9,10
Cholecystectomy in patients with an intact gallbladder (GB) after endoscopic removal of stones from the CBD remains a matter of debate.11 Several prospective randomized trials have shown that cholecystectomy after endoscopic treatment of bile duct stones reduces recurrent biliary events and is recommended.12,13 Several previous trials recommended elective cholecystectomy after EST in cases of GB calculi, preexisting cholangitis, acute biliary pancreatitis, complete opacification of the GB during ERCP, and non-visualization of the GB after EST;14,15 however, other studies have shown that elective cholecystectomy after EST does not reduce the incidence of recurrent biliary complications.16,17,18,19,20 Although cholecystectomy after endoscopic removal of CBD stones reduced recurrent CBD stones in some studies of Caucasian patients, the data was inconclusive in Asians due to differences in stone composition. We conducted a case-control study to determine the risk of recurrent CBD stones and to evaluate the need for subsequent cholecystectomy in order to prevent recurrence after endoscopic removal of stones from the CBD in Korean patients.
MATERIALS AND METHODS
Patients
Patients enrolled in this study met the following criteria: 1) ERCP with EST performed between January 2006 and January 2012 at Severance Hospital, 2) the presence of CBD stones and their complete clearance documented by cholangiography, 3) GB in situ documented by abdominal ultrasonography or abdominal computed tomography at the time of initial ERCP, 4) no evidence of underlying advanced malignancy, 5) no evidence of intrahepatic duct stones or bile duct stricture, and 6) follow-up longer than 3 months after ERCP.
Sixty-four patients with recurrent bile duct stones after CBD stone extraction by ERCP (recurrence group) between 2006 and 2012 were included in this study. For each patient of this recurrence group, four propensity-score age- and sex-matched control patients (non-recurrence group) were enrolled. A total of 317 patients who underwent endoscopic CBD stone extraction were studied.
Definitions and main outcome measures
Patient clinical data were obtained prospectively from a patient database. Possible risk factors for the recurrence of CBD stones including history of cholecystectomy, previous Billroth-II operation, bile duct diameter, stone size, number of stones, stone composition, periampullary diverticulum, mechanical lithotripsy, multiple ERCP sessions, and use of ursodeoxycholic acid (UDCA) and/or Rowachol® (Rowa Pharmaceuticals Ltd., Newtown, Bantry, Co. Cork, Ireland) medication were analyzed. The entry date was the time of complete endoscopic removal of CBD stones. The follow-up durations were determined from the entry date to the last visit date. The recurrence-free duration was determined from the entry date to the occurrence of a recurrent bile duct stone or the last visit date. The following data were noted before EST: gender, age, body mass index (BMI), total bilirubin level, jaundice, the presence or absence of a periampullary diverticulum, bile duct diameter, and the number and sizes of stones. A periampullary diverticulum was defined as the presence of a diverticulum within a radius of 2-3 cm from the ampulla of vater and was divided into the following three types according to the position between the major papilla and diverticulum: type 1, papilla located deep within the diverticulum (intra-diverticular papilla); type 2, papilla located on the inner rim of the diverticulum (juxta-papillary diverticulum) in such a way that the papillary orifice was not visible endoscopically; and type 3, papilla located outside the diverticulum (extra-diverticular papilla) in such a way that the papillary orifice was easily detectable.7,21,22 The recurrence of bile duct stones was defined as development of stones not earlier than 3 months after the complete removal of previous bile duct stones. The diagnosis of recurrent choledocholithiasis was made based on clinical symptoms, the laboratory alteration, and the radiological visualization of bile duct stones. All recurrent CBD stones were confirmed by ERCP.
ERCP procedures
All ERCP procedures were performed using side-viewing endoscopes (TJF-260V or TJF-240; Olympus Optical Co., Tokyo, Japan). These ERCPs were carried out by experienced endoscopists (J.B.C., S.Y.S., S.W.P., S.M.B., J.Y.P., and M.J.C.). A pull-type sphincterotome (Clever-cut; Olympus, Athens, Greece) or needle knife (MicroKnife™ XL, Boston Scientific, Marlborough, MA, USA) with or without a guide wire (Jagwire, Microvasive, Boston Scientific, Marlborough, MA, USA) was applied in cases of difficult CBD cannulation. For stones that were too large for removal in one session, an endoscopic mechanical lithotripsy was attempted in order to fragment the stones. When incomplete stone removal was suspected at the end of the procedure, an endoscopic nasobiliary drainage or plastic stent was inserted in order to prevent cholangitis.
Statistical analysis
Categorical variables were analyzed with chi-squared tests, while continuous variables (expressed as means with standard deviations) were analyzed using Student's t-test. We conducted the Mann-Whitney U test for nonparametric statistical analyses to identify statistical differences in recurrence risk factors. Potential risk factors were assessed by a multivariate logistic regression model. Cumulative recurrence rates of bile duct stones during the follow-up intervals were compared using the Kaplan-Meier method, and the statistical significance of the difference between them was examined by the log-rank test. A p-value of less than 0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 20.0 (IBM SPSS, New York, USA).
RESULTS
Patient characteristics
Characteristics of the 317 patients are shown in Table 1. The mean age was 65.6±12.1 years; there were 173 women (54.6%) and 144 men (45.4%). Mean BMI was 23.3±3.1 kg/m2. Most of the patients (90.5%) had taken the UDCA and/or Rowachol® after endoscopic removal of bile duct stones. Patients were followed-up for a mean period of 25.4±22.0 months. The incid-ence of cholelithiasis was higher in the cholecystectomy group before cholecystectomy than in the non-cholecystectomy group (99.0% vs. 39.2%; p<0.001).
Table 1. Patient Characteristics and ERCP Findings at Initial ERCP.
| Characteristic | All (n=317) | Cholecystectomy, n=105 (33.1%) | Non-cholecystectomy, n=212 (66.9%) | p value |
|---|---|---|---|---|
| Age (mean±SD, yrs) | 65.6±12.1 | 63.0±12.0 | 66.9±11.9 | 0.006 |
| Gender (male/female) | 144/173 (45.4/54.6) | 51/54 (48.6/51.4) | 93/119 (43.9/56.1) | 0.429 |
| BMI (mean±SD, kg/m2) | 23.3±3.1 | 23.8±3.0 | 23.0±3.1 | 0.038 |
| Total bilirubin (mean±SD, mg/dL) | 1.8±2.5 | 1.8±2.0 | 1.8±2.7 | 0.955 |
| Jaundice (yes/no) | 60/257 (18.9/81.1) | 21/84 (20/80) | 39/173 (18.4/81.6) | 0.732 |
| UDCA and/or Rowachol® medication (yes/no) | 287/30 (90.5/9.5) | 103/2 (98.1/1.9) | 184/28 (86.8/13.2) | 0.001 |
| Cholelithiasis (yes/no) | 187/130 (59/41) | 104/1 (99/1) | 83/129 (39.2/60.8) | <0.001 |
| CBD diameter (mean±SD, mm) | 15.3±4.9 | 14.5±4.0 | 15.6±5.2 | 0.128 |
| Bile duct stone diameter ≥15 mm (yes/no) | 77/240 (24.3/75.7) | 22/83 (21/79) | 55/157 (25.9/74.1) | 0.329 |
| Bile duct stone number ≥5 (yes/no) | 113/204 (35.6/64.4) | 41/64 (39/61) | 72/140 (34/66) | 0.374 |
| Stone composition (brown pigment/cholesterol) | 159/158 (50.2/49.8) | 55/50 (52.4/47.6) | 104/108 (49.1/50.9) | 0.577 |
| Previous Billroth-II operation (yes/no) | 17/300 (5.4/94.6) | 4/101 (3.8/96.2) | 13/199 (6.1/93.9) | 0.388 |
| Periampullary diverticulum (yes/no) | 81/236 (25.6/74.4) | 33/72 (31.4/68.6) | 48/164 (22.6/77.4) | 0.091 |
| Mechanical lithotripsy (yes/no) | 19/298 (6/94) | 3/102 (2.9/97.1) | 16/196 (7.5/92.5) | 0.098 |
| Multiple sessions of ERCP (≥2, yes/no) | 60/257 (18.9/81.1) | 15/90 (14.3/85.7) | 45/167 (21.2/78.8) | 0.138 |
| Recurrence-free duration (mean±SD, months) | 24.4±21.5 | 27.9±24.4 | 22.6±19.8 | 0.223 |
| Follow-up duration (mean±SD, months) | 25.4±22.0 | 28.2±24.3 | 24.0±20.6 | 0.365 |
ERCP, endoscopic retrograde cholangiopancreatography; SD, standard deviation; BMI, body mass index; UDCA, ursodeoxycholic acid; CBD, common bile duct.
Risk factors for recurrence of CBD stones
Possible risk factors associated with recurrence of bile duct stones, including age, gender, BMI, total bilirubin level, jaundice, use of UDCA and/or Rowachol® medication after endoscopic removal of bile duct stones, cholelithiasis, the size of the CBD, the number, sizes, and types of stones, cholecystectomy, Billroth II gastrectomy, periampullary diverticulum, mechanical lithotripsy, and multiple ERCP sessions are listed in Table 2. The mean duration of follow-up after CBD stone extraction was 26.4±17.8 months in the recurrence group and 25.2±22.9 months in the non-recurrence group (p=0.088). Of the 64 patients in the recurrence group, 17 (26.6%) underwent elective cholecystectomy soon after endoscopic extraction of CBD stones compared to 88 (34.8%) of the 253 patients in the non-recurrence group, a difference which was not statistically significant (p=0.212). There was no significant difference between the recurrence group and the non-recurrence group in terms of the presence of gallstones (p=0.286).
Table 2. Univariate Analysis of Risk Factors for Recurrent Bile Duct Stones in Terms of Patient Clinical Characteristics and ERCP Findings between the Recurrence Group and the Non-Recurrence Group at Initial ERCP.
| Risk factor | Patients with recurrent stone, n=64 (20.2%) | Patients without recurrent stone, n=253 (79.8%) | p value |
|---|---|---|---|
| Age (mean±SD, yrs) | 67.8±12.2 | 65.1±12.0 | 0.134 |
| Gender (male/female) | 26/38 (40.6/59.4) | 118/135 (46.6/53.4) | 0.388 |
| BMI (mean±SD, kg/m2) | 23.2±3.0 | 23.3±3.1 | 0.805 |
| Total bilirubin (mean±SD, mg/dL) | 1.4±1.4 | 1.9±2.7 | 0.227 |
| Jaundice (yes/no) | 9/55 (14.1/85.9) | 51/202 (20.2/79.8) | 0.266 |
| UDCA and/or Rowachol® medication (yes/no) | 59/5 (92.2/7.8) | 228/25 (90.1/9.9) | 0.613 |
| Cholelithiasis (yes/no) | 34/30 (53.1/46.9) | 153/100 (60.5/39.5) | 0.286 |
| CBD diameter (mean±SD, mm) | 16.1±4.1 | 15.0±5.1 | 0.046 |
| CBD diameter ≥15 mm (yes/no) | 38/26 (59.4/40.6) | 109/144 (43.1/56.9) | 0.020 |
| Bile duct stone diameter ≥15 mm (yes/no) | 18/46 (28.1/71.9) | 59/194 (23.3/76.7) | 0.423 |
| Bile duct stone number ≥5 (yes/no) | 20/44 (31.2/68.8) | 93/160 (36.8/63.2) | 0.411 |
| Stone composition (brown pigment/cholesterol) | 36/28 (56.2/43.8) | 123/130 (48.6/51.4) | 0.275 |
| Cholecystectomy (yes/no) | 17/47 (26.6/73.4) | 88/165 (34.8/65.2) | 0.212 |
| Previous Billroth-II operation (yes/no) | 2/62 (3.1/96.9) | 15/238 (5.9/94.1) | 0.540 |
| Periampullary diverticulum (yes/no) | 22/42 (34.4/65.6) | 59/194 (23.3/76.7) | 0.070 |
| Mechanical lithotripsy (yes/no) | 4/60 (6.2/93.8) | 15/238 (5.9/94.1) | 0.999 |
| Multiple sessions of ERCP (≥2, yes/no) | 16/48 (25/75) | 44/209 (17.4/82.6) | 0.165 |
| Recurrence-free duration (mean±SD, months) | 21.2±14.6 | 25.2±22.9 | 0.844 |
| Follow-up duration (mean±SD, months) | 26.4±17.8 | 25.2±22.9 | 0.088 |
ERCP, endoscopic retrograde cholangiopancreatography; SD, standard deviation; BMI, body mass index; UDCA, ursodeoxycholic acid; CBD, common bile duct.
On univariate analysis, the diameter of the CBD was a significant contributor to the recurrence of CBD stones. The mean diameter of the CBD in the recurrence group was 16.1±4.1 mm, whereas that in the non-recurrence group was 15.0±5.1 mm (p=0.046). A CBD diameter of 15 mm or larger was identified as a predictive factor for the recurrence of CBD stones (p= 0.020). Of the 317 patients, 159 (50.2%) had brown pigment stones at initial ERCP. Such brown pigment stones (39 cases, 60.9%) were more common in cases of recurrence than cholesterol stones (25 cases, 39.1%).
Multivariate analysis determined that a CBD diameter of 15 mm or larger [odds ratio (OR), 1.930; 95% confidence interval (CI), 1.098 to 3.391; p=0.022] and the presence of a periampullary diverticulum (OR, 1.859; 95% CI, 1.014 to 3.408; p=0.045) were independent predictive factors for CBD stone recurrence (Table 3). Elective cholecystectomy was not a risk factor for recurrence (p=0.198).
Table 3. Multivariate Analysis of Predictive Factors for the Recurrence of CBD Stones Identified Using a Logistic Regression Model.
| Variables | p value | OR (95% CI) |
|---|---|---|
| CBD diameter ≥15 mm | 0.022 | 1.930 (1.098-3.391) |
| Cholecystectomy | 0.198 | 0.663 (0.354-1.240) |
| Periampullary diverticulum | 0.045 | 1.859 (1.014-3.408) |
OR, odds ratio; CI, confidence interval; CBD, common bile duct.
Comparison of the cholecystectomy and non-cholecystectomy groups
During the follow-up period, 105 (33.1%) of 317 patients with GB in situ underwent cholecystectomy after ERCP with EST (cholecystectomy group), and the remaining 212 patients (66.9%) did not undergo cholecystectomy (non-cholecystectomy group). There were no significant differences between the two groups in terms of gender distribution, total bilirubin level, jaundice, CBD size, or the number, sizes, and types of stones at initial ERCP. The patients in the cholecystectomy group (mean, 63.0±12.0 years) were younger than those in the non-cholecystectomy group (mean, 66.9±11.9 years; p=0.006).
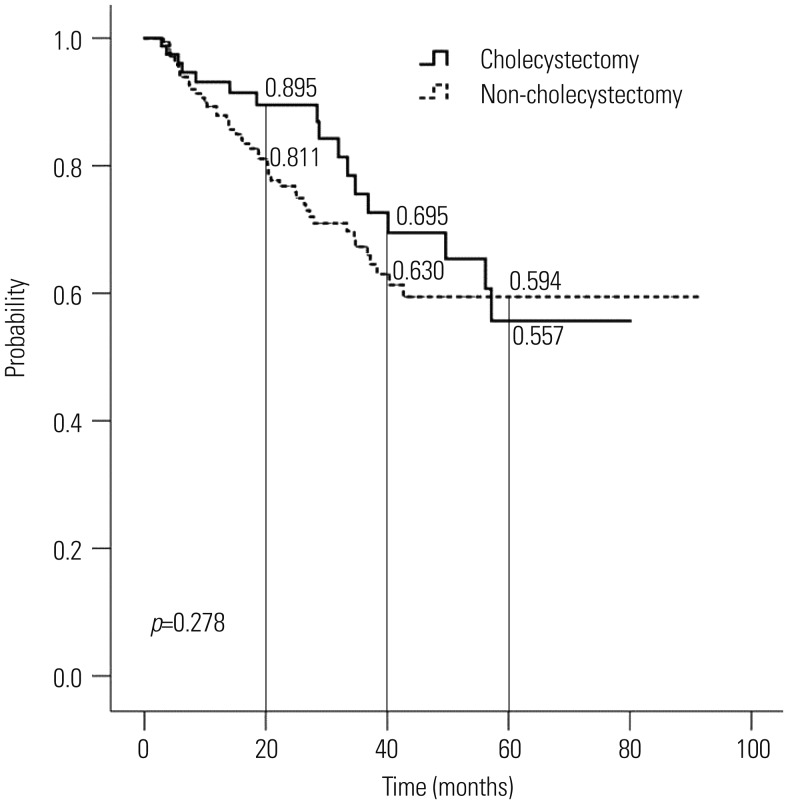
Kaplan-Meier analysis revealed that the cumulative recurrence rate of CBD stones between the cholecystectomy group and the non-cholecystectomy group was not significantly different (p=0.278) (Table 4, Fig. 1). At 5 years, the cumulative probabilities of CBD stone recurrence in the cholecystectomy and the non-cholecystectomy groups were 44.3% and 40.6%, respectively. In patients with cholelithiasis, there was no difference in the cumulative recurrence rate of CBD stones between the cholecystectomy group and the non-cholecystectomy group (p=0.279) (Table 4).
Table 4. Cumulative Recurrence Rates of Common Bile Duct Stones in Patients Who Underwent Cholecystectomy and Those Who Did Not.
| No. of patients (%) | Cumulative recurrence rate | p value | ||||
|---|---|---|---|---|---|---|
| 1 yr | 2 yrs | 3 yrs | 5 yrs | |||
| Total | 0.278 | |||||
| Cholecystectomy | 105 (33.1) | 0.069 | 0.105 | 0.245 | 0.443 | |
| Non-cholecystectomy | 212 (66.9) | 0.121 | 0.232 | 0.327 | 0.406 | |
| Cholelithiasis (+) | 0.279 | |||||
| Cholecystectomy | 104 (55.6) | 0.069 | 0.105 | 0.245 | 0.443 | |
| Non-cholecystectomy | 83 (44.4) | 0.147 | 0.234 | 0.340 | 0.379 | |
Fig. 1. Probability of remaining free of common bile duct stone recurrence after endoscopic common bile duct stone extraction in patients who underwent cholecystectomy and those who did not, as determined by Kaplan-Meier analysis.
DISCUSSION
Previous studies reported risk factors for the recurrence of bile duct stones in post-EST patients.6,7,9,10 Significant risk factors were an intact GB with cholecystolithiasis, mechanical lithotripsy, pneumobilia, dilated CBD, periampullary diverticulum, angulation of the CBD (≤145°) on ERCP, bile stasis, biliary stricture, and papillary stenosis. In the present study, significant risk factors were bile duct diameter and the presence of a periampullary diverticulum. The diameter of the bile duct is already known to be an important predictor for bile duct stone recurrence. Some authors have identified a bile duct diameter ≥15 mm as a risk factor for recurrent stones.8,23 The diameter of the dilated bile duct does not decrease after removal of the stone, as the elasticity of the duct wall is lost due to chronic inflammation and fibrosis. A dilated bile duct may result in bile stasis and bacterial infection, which are potential risk factors for recurrent stones.23,24 In addition, periampullary diverticula compress the distal CBD anatomically, leading to bile stasis and bacterial infection of the bile duct via the orifice of the sphincter of Oddi.21 Bile stasis associated with a type 1 or 2 diverticulum could be caused by either mechanical factors or the presence of coexisting motility disorders involving the sphincter of Oddi.7,25 In addition, large stones often require mechanical lithotripsy. Mechanical lithotripsy is likely to increase the risk of recurrence, as even a few missed small stone fragments may act as nidi for stone reaggregation.8 However, in the present study, large stones and mechanical lithotripsy were not significant risk factors for recurrence.
CBD stones are classified as primary and secondary stones based on etiology and pathogenesis. A primary stone in the CBD indicates that it was formed within the bile duct, whereas the term secondary indicates a stone that has migrated from the original site where it was formed. Primary bile duct stones can form in the bile duct several years after cholecystectomy, and most are brown pigment stones (calcium bilirubinate stones). On the contrary, secondary bile duct stones usually form in the GB and consist predominately of cholesterol.25 In recent studies, prophylactic cholecystectomy did not reduce the formation of recurrent CBD stones or additional recurrent cholangitis after CBD stone removal by EST in Korean patients, in whom brown pigment stones are more common.19,20 Similarly, in the present study, cholecystectomy was not associated with a reduction in the recurrence rate of bile duct stones. The cumulative recurrence rate of CBD stones between the cholecystectomy group and the non-cholecystectomy group was not significantly different. This finding can be explained by the fact that bile duct stone recurrence occurred more frequently due to brown pigment stones than due to cholesterol stones in this study. Brown pigment stones more commonly form in the bile duct, rather than migrate from the GB; thus, cholecystectomy would not affect the risk of bile duct stone recurrence in this setting.
In a recent study, patients with a calculous GB had a significantly higher rate of recurrent biliary complication than the acalculous GB group or the cholecystectomy group.26 In another study, the recurrence rates of CBD stones were not significantly different between the patients with and without gallstones within the GB in situ group.19 In the present study, there was no association of the relationship between gallstones and recurrent CBD stones in patients with choledocholithiasis after endoscopic treatment. This can indicates that the majority of CBD stones are likely secondary stones that migrated from the GB in Western patients, whereas primary stones are likely to be formed within the bile duct in Korean patients.
UDCA is currently used in the medical treatment of gallstones, particularly in patients with mild symptoms (i.e., without pancreatitis, cholecystitis, or cholangitis) and a small stone size (<10 mm), as well as those with cholesterol stones and a normally functioning GB. Rowachol® is an essential oil preparation that increases biliary lipid secretion; used alone, it has only weak litholytic properties, although it might have advantages when combined with UDCA.27 Gallstone composition among Asians is known to be different from that of Caucasians. Calcium bilirubinate stones are not rare in Koreans, and such stones show radiolucency on abdominal X-ray imagery. The complete dissolution rate of radiolucent GB stones by UDCA is reported to be lower in Asians, despite the efficacy of dissolutive agents such as UDCA and Rowachol® in the setting of cholesterol stones, which account for most bile duct stones in Caucasians.28 In the present study, use of UDCA and/or Rowachol® medication did not significantly reduce the recurrence of bile duct stones. Given the fact that pigment stones are the most common type of bile duct stone in Korea, this discrepancy could be due to geographic and ethnic differences in CBD stone composition.
In conclusion, a CBD diameter of 15 mm or larger and the presence of a periampullary diverticulum were significant predictive factors for recurrence after endoscopic extraction of CBD stones. For patients with risk factors for bile duct stone recurrence, periodic surveillance may be recommended. In addition, prophylactic cholecystectomy after clearance of CBD stones does not appear to reduce the incidence of recurrent CBD stones in Korean patients, in whom pigment stones are more common. Further prospective studies are needed to investigate long-term outcomes in these patients.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Classen M, Demling L. [Endoscopic sphincterotomy of the papilla of vater and extraction of stones from the choledochal duct (author's transl)] Dtsch Med Wochenschr. 1974;99:496–497. doi: 10.1055/s-0028-1107790. [DOI] [PubMed] [Google Scholar]
- 2.Kawai K, Akasaka Y, Murakami K, Tada M, Koli Y. Endoscopic sphincterotomy of the ampulla of Vater. Gastrointest Endosc. 1974;20:148–151. doi: 10.1016/s0016-5107(74)73914-1. [DOI] [PubMed] [Google Scholar]
- 3.Bergman JJ, van der Mey S, Rauws EA, Tijssen JG, Gouma DJ, Tytgat GN, et al. Long-term follow-up after endoscopic sphincterotomy for bile duct stones in patients younger than 60 years of age. Gastrointest Endosc. 1996;44:643–649. doi: 10.1016/s0016-5107(96)70045-7. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka M, Takahata S, Konomi H, Matsunaga H, Yokohata K, Takeda T, et al. Long-term consequence of endoscopic sphincterotomy for bile duct stones. Gastrointest Endosc. 1998;48:465–469. doi: 10.1016/s0016-5107(98)70086-0. [DOI] [PubMed] [Google Scholar]
- 5.Prat F, Malak NA, Pelletier G, Buffet C, Fritsch J, Choury AD, et al. Biliary symptoms and complications more than 8 years after endoscopic sphincterotomy for choledocholithiasis. Gastroenterology. 1996;110:894–899. doi: 10.1053/gast.1996.v110.pm8608900. [DOI] [PubMed] [Google Scholar]
- 6.Ando T, Tsuyuguchi T, Okugawa T, Saito M, Ishihara T, Yamaguchi T, et al. Risk factors for recurrent bile duct stones after endoscopic papillotomy. Gut. 2003;52:116–121. doi: 10.1136/gut.52.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim DI, Kim MH, Lee SK, Seo DW, Choi WB, Lee SS, et al. Risk factors for recurrence of primary bile duct stones after endoscopic biliary sphincterotomy. Gastrointest Endosc. 2001;54:42–48. doi: 10.1067/mge.2001.115335. [DOI] [PubMed] [Google Scholar]
- 8.Sugiyama M, Atomi Y. Risk factors predictive of late complications after endoscopic sphincterotomy for bile duct stones: long-term (more than 10 years) follow-up study. Am J Gastroenterol. 2002;97:2763–2767. doi: 10.1111/j.1572-0241.2002.07019.x. [DOI] [PubMed] [Google Scholar]
- 9.Keizman D, Shalom MI, Konikoff FM. An angulated common bile duct predisposes to recurrent symptomatic bile duct stones after endoscopic stone extraction. Surg Endosc. 2006;20:1594–1599. doi: 10.1007/s00464-005-0656-x. [DOI] [PubMed] [Google Scholar]
- 10.Cheon YK, Lehman GA. Identification of risk factors for stone recurrence after endoscopic treatment of bile duct stones. Eur J Gastroenterol Hepatol. 2006;18:461–464. doi: 10.1097/00042737-200605000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Lee SH, Jung MJ, Hwang HK, Kang CM, Lee WJ. The first experiences of robotic single-site cholecystectomy in Asia: a potential way to expand minimally-invasive single-site surgery? Yonsei Med J. 2015;56:189–195. doi: 10.3349/ymj.2015.56.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boerma D, Rauws EA, Keulemans YC, Janssen IM, Bolwerk CJ, Timmer R, et al. Wait-and-see policy or laparoscopic cholecystectomy after endoscopic sphincterotomy for bile-duct stones: a randomised trial. Lancet. 2002;360:761–765. doi: 10.1016/S0140-6736(02)09896-3. [DOI] [PubMed] [Google Scholar]
- 13.Lau JY, Leow CK, Fung TM, Suen BY, Yu LM, Lai PB, et al. Cholecystectomy or gallbladder in situ after endoscopic sphincterotomy and bile duct stone removal in Chinese patients. Gastroenterology. 2006;130:96–103. doi: 10.1053/j.gastro.2005.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammarstrom LE, Holmin T, Stridbeck H. Endoscopic treatment of bile duct calculi in patients with gallbladder in situ: long-term outcome and factors. Scand J Gastroenterol. 1996;31:294–301. doi: 10.3109/00365529609004881. [DOI] [PubMed] [Google Scholar]
- 15.Lai JH, Wang HY, Chang WH, Chu CH, Shih SC, Lin SC. Recurrent cholangitis after endoscopic lithotripsy of common bile duct stones with gallstones in situ: predictive factors with and without subsequent cholecystectomy. J Laparoendosc Adv Surg Tech A. 2012;22:324–329. doi: 10.1089/lap.2011.0353. [DOI] [PubMed] [Google Scholar]
- 16.Hill J, Martin DF, Tweedle DE. Risks of leaving the gallbladder in situ after endoscopic sphincterotomy for bile duct stones. Br J Surg. 1991;78:554–557. doi: 10.1002/bjs.1800780512. [DOI] [PubMed] [Google Scholar]
- 17.Lai KH, Lin LF, Lo GH, Cheng JS, Huang RL, Lin CK, et al. Does cholecystectomy after endoscopic sphincterotomy prevent the recurrence of biliary complications? Gastrointest Endosc. 1999;49(4 Pt 1):483–487. doi: 10.1016/s0016-5107(99)70047-7. [DOI] [PubMed] [Google Scholar]
- 18.Kwon SK, Lee BS, Kim NJ, Lee HY, Chae HB, Youn SJ, et al. Is cholecystectomy necessary after ERCP for bile duct stones in patients with gallbladder in situ? Korean J Intern Med. 2001;16:254–259. doi: 10.3904/kjim.2001.16.4.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui ML, Cho JH, Kim TN. Long-term follow-up study of gallbladder in situ after endoscopic common duct stone removal in Korean patients. Surg Endosc. 2013;27:1711–1716. doi: 10.1007/s00464-012-2662-0. [DOI] [PubMed] [Google Scholar]
- 20.Heo J, Jung MK, Cho CM. Should prophylactic cholecystectomy be performed in patients with concomitant gallstones after endoscopic sphincterotomy for bile duct stones? Surg Endosc. 2015;29:1574–1579. doi: 10.1007/s00464-014-3844-8. [DOI] [PubMed] [Google Scholar]
- 21.Lobo DN, Balfour TW, Iftikhar SY, Rowlands BJ. Periampullary diverticula and pancreaticobiliary disease. Br J Surg. 1999;86:588–597. doi: 10.1046/j.1365-2168.1999.01121.x. [DOI] [PubMed] [Google Scholar]
- 22.Boix J, Lorenzo-Zúñiga V, Añaños F, Domènech E, Morillas RM, Gassull MA. Impact of periampullary duodenal diverticula at endoscopic retrograde cholangiopancreatography: a proposed classification of periampullary duodenal diverticula. Surg Laparosc Endosc Percutan Tech. 2006;16:208–211. doi: 10.1097/00129689-200608000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Pereira-Lima JC, Jakobs R, Winter UH, Benz C, Martin WR, Adamek HE, et al. Long-term results (7 to 10 years) of endoscopic papillotomy for choledocholithiasis. Multivariate analysis of prognostic factors for the recurrence of biliary symptoms. Gastrointest Endosc. 1998;48:457–464. doi: 10.1016/s0016-5107(98)70085-9. [DOI] [PubMed] [Google Scholar]
- 24.Geenen DJ, Geenen JE, Jafri FM, Hogan WJ, Catalano MF, Johnson GK, et al. The role of surveillance endoscopic retrograde cholangiopancreatography in preventing episodic cholangitis in patients with recurrent common bile duct stones. Endoscopy. 1998;30:18–20. doi: 10.1055/s-2007-993722. [DOI] [PubMed] [Google Scholar]
- 25.Kim MH, Myung SJ, Seo DW, Lee SK, Kim YS, Lee MH, et al. Association of periampullary diverticula with primary choledocholithiasis but not with secondary choledocholithiasis. Endoscopy. 1998;30:601–604. doi: 10.1055/s-2007-1001363. [DOI] [PubMed] [Google Scholar]
- 26.Tsai TJ, Lai KH, Lin CK, Chan HH, Wang EM, Tsai WL, et al. The relationship between gallbladder status and recurrent biliary complications in patients with choledocholithiasis following endoscopic treatment. J Chin Med Assoc. 2012;75:560–566. doi: 10.1016/j.jcma.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Leiss O, von Bergmann K. Effect of Rowachol on biliary lipid secretion and serum lipids in normal volunteers. Gut. 1985;26:32–37. doi: 10.1136/gut.26.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baek YH, Kim HJ, Park JH, Park DI, Cho YK, Sohn CI, et al. [Risk factors for recurrent bile duct stones after endoscopic clearance of common bile duct stones] Korean J Gastroenterol. 2009;54:36–41. doi: 10.4166/kjg.2009.54.1.36. [DOI] [PubMed] [Google Scholar]