Abstract
Purpose
Recent studies have revealed close relationships between hepatic injury, metabolic pathways, and gut microbiota. The microorganisms in the intestine also cause irritable bowel syndrome (IBS). The aim of this study was to examine whether IBS was associated with elevated hepatic enzyme [alanine aminotransferase (ALT) and aspartate aminotransferase (AST)], gamma-glutamyl transferase (γ-GT) levels, and metabolic syndrome (MS).
Materials and Methods
This was a retrospective, cross-sectional, case-control study. The case and control groups comprised subjects who visited our health promotion center for general check-ups from June 2010 to December 2010. Of the 1127 initially screened subjects, 83 had IBS according to the Rome III criteria. The control group consisted of 260 age- and sex-matched subjects without IBS who visited our health promotion center during the same period.
Results
Compared to control subjects, patients with IBS showed significantly higher values of anthropometric parameters (body mass index, waist circumference), liver enzymes, γ-GT, and lipid levels. The prevalences of elevated ALT (16.9% vs. 7.7%; p=0.015) and γ-GT (24.1% vs. 11.5%; p=0.037) levels were significantly higher in patients with IBS than in control subjects. A statistically significant difference was observed in the prevalence of MS between controls and IBS patients (12.7% vs. 32.5%; p<0.001). The relationships between elevated ALT levels, MS, and IBS remained statistically significant after controlling for potential confounding factors.
Conclusion
On the basis of our study results, IBS may be an important condition in certain patients with elevated ALT levels and MS.
Keywords: Irritable bowel syndrome, liver enzymes, metabolic syndrome
INTRODUCTION
Recent studies have revealed a close relationship between gut microbiota and metabolic pathways.1,2,3 Gut microbiota may play a major role in the development of hepatic injury and metabolic disorders such as insulin resistance.4,5 Additionally, these microorganisms, to some extent, also cause functional gastrointestinal disorders (FGIDs).6
One of these FGIDs is irritable bowel syndrome (IBS), which is characterized by abdominal pain, discomfort, and alteration of bowel habits in the absence of any detectable organic disease.7 The prevalence of IBS is approximately 6.6-9.6% in the Korean population8,9 and 7-30% worldwide,10,11 and it is one of the most frequently observed disorders in the general population.12 The etiology of IBS is not clearly understood, although several hypotheses have been proposed, which include alteration in the gut microbiota, dysregulation of the brain-gut axis and autonomic nervous system, genetic disposition, visceral hypersensitivity, and altered levels of gastrointestinal hormones.13,14 A potential association between small intestinal bacterial overgrowth (SIBO) and IBS has also been proposed on the basis of similar gastrointestinal symptoms15,16 and the reported prevalence of SIBO in patients with IBS.17,18
Recent emerging hypotheses suggest that altered gut microbiota may be related to gut permeability.19,20 The increased gut permeability can contribute to the pathogenesis of nonalcoholic fatty liver disease (NAFLD)21,22 and metabolic derangement,22,23 i.e., the gut-liver axis. Schnabl and Brenner24 also demonstrated that the intestinal microbiome contributes to the onset and progression of NAFLD through a breakdown of the intestinal barrier and the translocation of microbial products in animal models. Therefore, IBS with alteration of gut microbiota that is associated with the development of NAFLD through the gut-liver axis may be linked to abnormal liver and metabolic function.
Additionally, NAFLD, a hepatic manifestation of metabolic syndrome (MS), is reportedly associated with insulin resistance. Liver enzymes, especially alanine aminotransferase (ALT), are routinely measured in a useful screening assay for the detection of NAFLD in the general population.25 Accordingly, hepatic enzyme activity is associated with MS through NAFLD. This positive association between ALT level and MS is also supported by a recently published study in which a meta-analysis of prospective cohort studies was performed.26
We hypothesized that IBS may be related to abnormal liver function and metabolic parameters, and if so, such a relation should be considered as an important condition that is associated with metabolic disorders. Therefore, the aim of this study was to examine whether IBS was associated with any of the following factors: elevated hepatic enzymes [ALT and aspartate aminotransferase (AST)], elevated gamma-glutamyl transferase (γ-GT), and MS.
MATERIALS AND METHODS
Study subjects and design
From June 2010 to December 2010, data from 1127 Korean adults who underwent upper gastrointestinal (GI) endoscopy, colonoscopy, and abdominal ultrasonography at the Health Promotion Center of Ajou University Hospital, Suwon, Republic of Korea were retrospectively reviewed for inclusion in the study.
From these initial subjects, we excluded 37 with missing data values from our study. Additionally, 112 subjects were excluded for the following reasons: positive hepatitis B surface antigen, hepatitis B virus core antigen, and anti-hepatitis C virus tests; taking drugs that could affect liver function (antifungal agents, herbal remedies, and hepatotonic drugs); organic GI diseases (reflux esophagitis, peptic ulcer, diverticulitis, submucosal tumor, polyp, neoplasm, and inflammatory bowel diseases, such as Crohn's disease and ulcerative colitis); and organic hepatobiliary diseases [gallbladder stone and polyp, cholecystitis and cholangitis, hepatolithiasis, non-alcoholic steatohepatitis (NASH), liver abscess, liver cirrhosis, and hepatobiliary neoplasm]. Of these excluded subjects, we found three who had NASH (already confirmed by biopsy in gastroenterology department) overlapping with chronic hepatitis B virus infection at the time of the survey. Therefore, subjects with NASH were excluded in the present study.
The remaining 978 subjects completed a self-administered questionnaire based on the Rome III criteria for IBS evaluation. The questionnaire was translated into Korean, in accordance with guidelines suggested by a previous study.27 The translation was conducted by two translators and back-translated into English by two other translators. Among these 978 subjects, 83 who had IBS according to the Rome III criteria were enrolled after further detailed interviews by the physician; IBS subgroups were classified as IBS-C (constipation-predominant), IBS-D (diarrhea-predominant), IBS-M (mixed constipation and diarrhea), and IBS-U (unsubtyped). IBS was defined as recurrent abdominal pain or discomfort (at least 3 days per month in the last 3 months) with onset at least 6 months before diagnosis and association with two or more of the following: 1) improvement with defecation, 2) onset associated with a change in the frequency of stools, and 3) onset associated with a change in the form of stools. The control group was comprised of age- and sex-matched subjects without IBS who visited our health promotion center during the above mentioned period, and exclusion criteria were also applied to the control group. We found 260 age- and sex-matched subjects among the 978 subjects who were adequate for inclusion in the control group during the same period. Thus, 83 subjects with IBS and 260 matched controls without IBS were included in the final analyses.
Anthropometry and data collection
The height and body weight of each participant were measured while they wore light clothing without shoes. Body mass index (BMI) was calculated as the weight divided by height squared (kg/m2). Waist circumference (WC) was measured at the central part between the 12th rib and the iliac crest by a trained nurse. Blood pressure (BP) was measured after 15 minutes of rest in a sitting position using an automatic sphygmomanometer (TM-2655P, PMS Instruments, Tokyo, Japan). Overnight fasting blood samples were drawn from the antecubital vein. Fasting glucose (FG), total cholesterol (TC), triglyceride (TG), high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, total bilirubin, ALT, AST, alkaline phosphatase (ALP), and γ-GT levels were measured using an automatic analyzer (Toshiba TBA 200FR, Toshiba Medical Systems Co. Ltd., Tokyo, Japan). Data on cigarette smoking and alcohol consumption were collected using a self-reported questionnaire. Subjects who, at the time of the survey, had regularly smoked cigarettes during the past year were considered to be current smokers, and the self-reported questionnaire based alcohol consumption on the usual alcohol intake during 1 week. Alcohol consumption in subjects was calculated and then converted to weekly alcohol consumption (grams of ethanol per week) using the graduated frequency method.28 Hypertension was defined as systolic BP ≥140 mm Hg, diastolic BP ≥90 mm Hg, or the use of antihypertensive medication. Diabetes mellitus was defined as fasting blood glucose ≥126 mg/dL or the use of oral hypoglycemic agents or insulin.
The definitions of obesity, metabolic syndrome, and elevated liver-enzyme and γ-GT levels in this study
Obesity was defined as BMI ≥25 kg/m2, using the body weight and height of each subject, according to the Asian guidelines.29 In the present study, we used the definition of MS proposed by the American Heart Association/National Heart, Lung, and Blood Institute (AHA/NHLBI) Scientific Statement in 2005.30 Subjects were classified as having MS if at least three of the following five characteristics were present: 1) WC ≥90 cm in men or ≥80 cm in women (specified for Asians in the AHA/NHLBI guidelines); 2) TG level ≥150 mg/dL or use of oral medication; 3) HDL cholesterol level <40 mg/dL in men or <50 mg/dL in women or use of oral medication; 4) BP ≥130/85 mm Hg or use of oral medication; and 5) FG ≥100 mg/dL or use of oral medication or insulin.
The upper limits of normal values for serum liver enzymes and for various tests used to assess the biliary tract were defined as 41 IU/L in males and 31 IU/L in females for ALT, 40 IU/L in males and females for AST, and 66 IU/L in males and 39 IU/L in females for γ-GT, respectively, and the range for normal values was 53-128 IU/L in males and 42-98 IU/L in females for ALP, which were established as the cutoff values based on 95% confidence intervals in 705 healthy adults.31
Statistical analyses
All continuous variables are expressed as means±standard deviation, whereas categorical variables are presented as numbers and percentages. Continuous variables were analyzed using the independent t-test. Categorical variables were analyzed using the chi-square test. Given that obesity has been observed to be independently associated with ALT elevation, γ-GT elevation, and MS,32,33 in order to evaluate whether obesity affected liver function and MS in IBS patients, the prevalences of elevated liver enzymes, elevated γ-GT levels, and MS were analyzed separately according to obesity. An analysis of variance was performed to evaluate the relationship between IBS and elevated liver-enzyme and γ-GT levels according to the type of IBS. Multivariate conditional logistic analysis was performed to evaluate the odds ratio (OR) after controlling for potential confounding factors. A p-value of less than 0.05 was considered statistically significant. The statistical analysis was performed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA).
Ethics statement
The present study was approved by the Institutional Review Board at Ajou University Hospital, and written informed consent was obtained from all of the enrolled subjects.
RESULTS
General characteristics of subjects
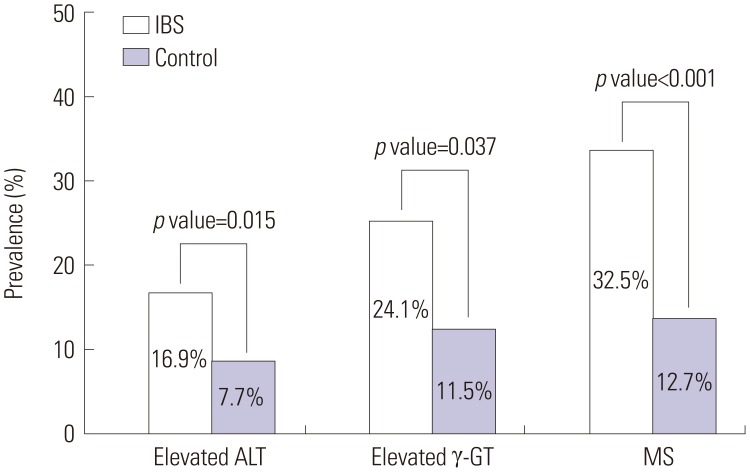
A comparison of the characteristics of patients in both the IBS group and the control group is summarized in Table 1. Eighty-three subjects were diagnosed with IBS, and among these subjects, 49.4% had IBS-D, 14.8% had IBS-C, 31.3% had IBS-M, and 4.5% had IBS-U. Age, gender, behavioral habits (alcohol consumption and smoking status), and the prevalence of diabetes mellitus and hypertension were similar between the two groups. However, compared to subjects without IBS, those with IBS had significantly higher values for BMI, WC, ALT, ALP, γ-GT, TC, TG, and LDL cholesterol. In addition, the prevalences of elevated ALT (16.9% vs. 7.7%; p=0.015), γ-GT (24.1% vs. 11.5%; p=0.037), and MS (32.5% vs. 12.7%; p<0.001) were significantly higher in subjects with IBS than in control subjects (Fig. 1).
Table 1. Comparison of Characteristics between IBS Subjects and Controls.
| IBS patients (n=83) | Controls (n=260) | p value | |
|---|---|---|---|
| Age (yrs) | 42.3±5.3 | 42.4±0.9 | 0.844* |
| Gender (male, %) | 68.7 | 68.5 | 0.971† |
| Alcohol (g/wk) | 60.0±43.9 | 63.6±95.7 | 0.730* |
| Current smoker (%) | 24.5 | 23.6 | 0.353† |
| BMI (kg/m2) | 25.1±3.3 | 24.0±2.8 | 0.010* |
| Waist circumference (cm) | 86.9±8.0 | 83.3±7.1 | <0.001* |
| SBP (mm Hg) | 119.4±14.9 | 118.4±13.8 | 0.562* |
| DBP (mm Hg) | 78.2±11.0 | 78.0±10.8 | 0.894* |
| Total bilirubin (mg/dL) | 1.0±0.4 | 1.0±0.4 | 0.633* |
| ALP (IU/L) | 69.8±19.8 | 61.7±14.9 | 0.001* |
| AST (IU/L) | 27.1±12.4 | 24.8±14.0 | 0.181* |
| ALT (IU/L) | 33.6±21.4 | 26.8±25.4 | 0.029* |
| γ-GT (IU/L) | 47.9±62.6 | 32.6±38.7 | 0.037* |
| Total cholesterol (mg/dL) | 198.4±35.9 | 182.3±30.1 | <0.001* |
| Triglycerides (mg/dL) | 144.2±83.3 | 119.6±79.4 | 0.016* |
| HDL cholesterol (mg/dL) | 48.1±11.6 | 49.2±11.5 | 0.450* |
| LDL cholesterol (mg/dL) | 121.4±33.0 | 109.3±26.2 | 0.003* |
| FG (mg/dL) | 99.4±21.2 | 96.5±14.5 | 0.245* |
| Diabetes mellitus (%) | 6 | 2.9 | 0.153† |
| Hypertension (%) | 9.6 | 6.9 | 0.416† |
IBS, irritable bowel syndrome; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; ALP, alkaline phosphatase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; γ-GT, gamma-glutamyl transferase; HDL, high-density lipoprotein; LDL, low-density lipoprotein; FG, fasting glucose.
Data are expressed as mean±standard deviation or number (percentage), as appropriate.
*p-values were calculated using the independent t-test, †p-values were calculated using the chi-square test.
Fig. 1. Prevalences of elevated ALT and γ-GT levels and metabolic syndrome in IBS patients and control subjects. Elevated ALT and γ-GT were defined as >41 IU/L for males and >31 IU/L for females for ALT and >66 IU/L for males and >39 IU/L for females for γ-GT. Metabolic syndrome (MS) was defined as the presence of three or more of the guidelines of the American Heart Association/National Heart, Lung, and Blood Institute (AHA/NHLBI) Scientific Statement published in 2005. A significant difference was observed between the two groups with respect to ALT (16.9% vs. 7.7%; p=0.015), γ-GT (24.1% vs. 11.5%; p=0.037), and MS (32.5% vs. 12.7%; p<0.001). IBS, irritable bowel syndrome; ALT, alanine aminotransferase; γ-GT, gamma-glutamyl transferase.
Prevalences of elevated ALT, elevated γ-GT levels, and MS in IBS subjects and controls with and without obesity
Regardless of whether obesity affected liver function and MS in IBS patients, as an initial step, we evaluated the prevalences of elevated ALT, elevated γ-GT levels, and MS in IBS subjects and controls with and without obesity. When the subjects were stratified according to obesity, there were no significant differences in the prevalences of elevated ALT and γ-GT levels in IBS subjects and controls with obesity (Table 2). Moreover, there were no differences in alcohol consumption between IBS and control subgroups after stratification according to obesity (data not shown). The subjects with the IBS-D subtype showed a significantly higher intake of alcohol (p=0.001) among the IBS subtype groups (data not shown), although there were no significant differences in the prevalences of elevated ALT (p=0.869), γ-GT (p=0.418), and MS (p=0.203) between the IBS subtype groups (Table 3). However, IBS subjects without obesity had a significantly higher prevalence of elevated ALT and γ-GT in comparison with matched controls (Table 2). Regardless of obesity, patients with IBS had a significantly higher prevalence of MS than control subjects.
Table 2. Prevalences of Elevated ALT and γ-GT Levels and Metabolic Syndrome in IBS Subjects and Controls with and without Obesity.
| IBS patients (n=83) | Control (n=260) | p value | |
|---|---|---|---|
| With obesity (n=130) | |||
| Elevated ALT, n (%) | 6 (13.6) | 13 (15.3) | 0.801 |
| Elevated γ-GT, n (%) | 12 (27.3) | 17 (20.0) | 0.348 |
| MS, n (%) | 23 (52.3) | 28 (32.9) | 0.033 |
| Without obesity (n=213) | |||
| Elevated ALT, n (%) | 8 (20.5) | 6 (3.5) | <0.001 |
| Elevated γ-GT, n (%) | 8 (20.5) | 13 (7.5) | 0.014 |
| MS, n (%) | 4 (10.3) | 5 (2.9) | 0.039 |
IBS, irritable bowel syndrome; ALT, alanine aminotransferase; γ-GT, gamma-glutamyl transferase; MS, metabolic syndrome.
Obesity was defined as body mass index ≥25 kg/m2. All data are expressed as percentages, and p values were calculated using the chi-square test.
Table 3. Prevalences of Elevated ALT and γ-GT Levels and Metabolic Syndrome According to IBS Subgroups.
| IBS-D (n=41) | IBS-C (n=12) | IBS-M (n=26) | IBS-U (n=4) | p value | |
|---|---|---|---|---|---|
| Elevated ALT, n (%) | 6 (14.6) | 2 (16.6) | 5 (19.2) | 1 | 0.869 |
| Elevated γ-GT, n (%) | 12 (29.3) | 2 (16.6) | 6 (23.1) | 0 | 0.418 |
| MS, n (%) | 17 (41.5) | 3 (25.0) | 7 (26.9) | 0 | 0.203 |
IBS, irritable bowel syndrome; ALT, alanine aminotransferase; γ-GT, gamma-glutamyl transferase; MS, metabolic syndrome.
p-values were calculated using the analysis of variance test.
Odds ratios (ORs) for elevated ALT, elevated γ-GT, and MS in IBS
We further investigated whether IBS status was related to elevated ALT, elevated γ-GT, and MS. To assess these associations, multiple conditional regression analysis models were employ-ed (Table 4). In the multivariate models, the adjusted ORs (and 95% confidence intervals) for elevated ALT levels and MS in the IBS group when compared with the non-IBS group were 2.300 (95% CI, 1.044-5.066) and 3.446 (95% CI, 1.977-6.007), respectively. No significant relationships were observed between IBS status and elevated γ-GT (OR, 1.647; 95% CI, 0.784-3.461) in the multivariate models after adjustment for obesity, WC, diabetes mellitus, alcohol consumption, LDL cholesterol, TC, and TG.
Table 4. OR for Elevated ALT and γ-GT Levels and MS in IBS via Multiple Conditional Regression Analysis.
| Variable | B | SE | OR | p value | 95% CI |
|---|---|---|---|---|---|
| OR for elevated ALT* | |||||
| IBS | 0.833 | 0.403 | 2.300 | 0.039 | 1.044-5.066 |
| OR for elevated γ-GT* | |||||
| IBS | 0.499 | 0.379 | 1.647 | 0.188 | 0.784-3.461 |
| OR for MS† | |||||
| IBS | 1.237 | 0.284 | 3.446 | <0.001 | 1.977-6.007 |
IBS, irritable bowel syndrome; ALT, alanine aminotransferase; γ-GT, gamma-glutamyl transferase; MS, metabolic syndrome; OR, odds ratio; LDL, low density lipoprotein; CI, confidence interval; SE, standard error.
*Adjustment for obesity, waist circumference, diabetes mellitus, alcohol consumption, LDL cholesterol, total cholesterol, and triglycerides, †Adjustment for alcohol consumption, LDL cholesterol, and total cholesterol.
DISCUSSION
In this age- and sex-matched case-control study, the prevalences of elevated ALT, elevated γ-GT, and MS were assessed in IBS patients in comparison with the control (no IBS) group. The present study showed significantly higher prevalences of elevated ALT level and MS in IBS patients compared to the controls. The relationship remained statistically significant after controlling for potential confounding factors.
There are several possible explanations for the increased prevalence of elevated ALT in IBS patients. Previous studies reported higher prevalences of altered gut microbiota and SIBO in IBS patients, establishing a link between altered gut microbiota, SIBO, and IBS.17,18,34,35,36 Both altered gut microbiota and SIBO are associated with altered tight junction and increased gut permeability,20,37,38 and these abnormalities are related to hepatic function and severity of hepatic steatosis.37,38 Indeed, a recent study showed that intestinal permeability is greater in patients with IBS.39 Thus, it is possible that IBS itself could cause elevated liver enzymes. A pathogenic role for altered gut microbiota in IBS patients with hepatitis would be strongly supported by increased intestinal permeability and increased levels of endotoxin and tumor necrosis factor-alpha. The activation of Kupffer cells by gut-derived endotoxins induces an increase in the production of pro-inflammatory cytokines and nitric oxide-related substances that, in turn, may lead to liver damage; namely, when liver injury occurs, ALT that is mainly aggregated in the cytosol of the hepatocyte is released from injured liver cells and causes a significant elevation in serum ALT activity.40,41,42 Therefore, endotoxins and possibly other gut-derived, pro-inflammatory bacterial products are involved in the development of liver disease, which may help in explaining why IBS is associated with elevated ALT levels. However, as no previous study has investigated the relationship between liver enzymes and IBS in humans, a further study is needed to confirm this hypothesis.
Until now, few epidemiological studies have assessed the relationship between IBS status and MS in an adult population, and the underlying causes of pathophysiologic changes are still not completely understood; however several in vitro studies have proposed different mechanisms to explain the lipid-lowering effects of intestinal bacteria, such as specific strains of Lactobacillus or Bifidobacterium, in humans.43,44 Such mechanisms include the physiological action of major end-products of probiotic fermentation (short-chain fatty acids), cholesterol assimilation by bacteria, enzymatic deconjugation of bile acids, and the binding of cholesterol to the bacterial cell wall. Functions of these probiotics are related to the lipid metabolism, and they may serve as a link between IBS with intestinal dysbiosis and MS. Interestingly, our study is not the first to report an association of IBS with MS in an Asian population, as we found a recent analysis among Japanese adults that also demonstrated that IBS was significantly related to a higher prevalence of MS.45 Therefore, this current study supports the positive relationship between IBS and MS. However, liver function was not included in the Japanese study.
In addition, a recent animal study suggested a possible mechanism by which the gut microbial community can contribute to obesity.46 Bacterial lipopolysaccharides (LPS) derived from gram-negative bacteria residing in the intestinal tract may act as a triggering factor, linking inflammation to high-fat diet-induced MS. The results of human studies have supported these findings. Treatment of humans with polymyxin B, an antibiotic that specifically targets gram-negative organisms, was found to reduce LPS expression and hepatic steatosis.47 A more recent study reported that patients with type 2 diabetes had higher LPS levels than a well-matched control group without diabetes.1,23 Taken together, these findings suggest that altered gut microbiota may be associated with MS and increased insulin resistance. Indeed, in morbidly obese patients, the prevalence of intestinal dysbiosis is higher than that in healthy subjects, and it is associated with severe hepatic steatosis.48,49 Such experimental evidence supports the notion that understanding the mechanisms by which the alteration in the gut microbiota produces different signaling activations, and phenotype changes may offer an interesting opportunity for the treatment of obesity and type 2 diabetes. Although the present study did not include data related to intestinal dysbiosis, our results also showed that IBS patients had significantly higher BMI and WC values than controls. On the other hand, Kang, et al.50 reported that visceral abdominal obesity, not BMI, was found to be an independent risk factor of IBS. Thus, the association between obesity and the risk of IBS may be more related to WC than to BMI.
To the best of our knowledge, no previous study has examined the relationships of liver function and MS with IBS among the general population. Thus, the strength of this study was that was the first study to show that IBS was significantly related to higher prevalences of MS and elevated hepatic enzymes among an adult population, and the results indicating the higher prevalences of elevated ALT level and MS in IBS subjects in comparison with a control group are also valuable.
There were several limitations to this study. First, this was a retrospective case-control study, and the IBS status may have affected the dietary pattern, food digestion, and nutrient absorption, which are all important factors for the prevention and treatment of MS. In addition, SIBO and intestinal dysbiosis may be only two of the suggested pathophysiologic mechanisms of IBS, NAFLD, and MS. Thus, in our study, we could not show or conclude any cause-effect relationship between altered liver function and MS in IBS patients based on the microbiota. In the future, a large-scale, prospective randomized controlled trial is needed to confirm this relationship. However, although altered gut microbiota, including intestinal dysbiosis and SIBO, may be only one of the suggested pathophysiologic mechanisms of IBS, NAFLD, and MS, it may nevertheless be clinically relevant if there is a modifiable and treatable cause unlike other causes, such as genetic disposition, age, and gender. Second, pro-inflammatory cytokine or endotoxin concentrations in the blood were not confirmed in the subjects of our study, nor was intestinal dysbiosis. Thus, as mentioned above, further studies including objective tests of the gut microbiota and pro-inflammatory cytokine or endotoxin concentration measurements might provide additional and definitive information with respect to the association between hepatic injury, MS, and IBS. Third, this study was conducted at one center, and the study population comprised only ethnic Asians, which may have caused selection bias. In this study, subjects were selected from among patients who visited the health promotion center in a tertiary hospital, and there were many male patients in their fourth decade of life. In Korea, there is a high prevalence of stomach cancer and colon cancer, and males are at higher risk. This causes male patients to actively undergo examination of the digestive organs. In recent studies that targeted Indian or Japanese patients among Asian populations, the number of male patients was higher among subjects with IBS.45,51 However, another study demonstrated that the prevalence of IBS was positively associated with female sex in Korea.52 Therefore, the sex ratio may be more related with the research method.
In conclusion, the present study suggests a possible association between elevated ALT levels, MS, and IBS, and this may be the first study in IBS patients to document this association in an Asian population. On the basis of our study, IBS may be an important condition in some patients with elevated ALT levels and MS. These results need to be confirmed by a further trial with a large sample size. Additionally, in order to establish a causal-resultant relationship (IBS and MS, liver damage), randomized controlled trials in a large population of IBS patients are needed for assessing the efficacy of the manipulation of gut microbiota.
ACKNOWLEDGEMENTS
The present study was approved by the Institutional Review Board at Ajou University Hospital, and written informed consent was obtained from all of the enrolled subjects.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Caricilli AM, Saad MJ. The role of gut microbiota on insulin resistance. Nutrients. 2013;5:829–851. doi: 10.3390/nu5030829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D'Aversa F, Tortora A, Ianiro G, Ponziani FR, Annicchiarico BE, Gasbarrini A. Gut microbiota and metabolic syndrome. Intern Emerg Med. 2013;8(Suppl 1):S11–S15. doi: 10.1007/s11739-013-0916-z. [DOI] [PubMed] [Google Scholar]
- 3.Le Roy T, Llopis M, Lepage P, Bruneau A, Rabot S, Bevilacqua C, et al. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut. 2013;62:1787–1794. doi: 10.1136/gutjnl-2012-303816. [DOI] [PubMed] [Google Scholar]
- 4.Gerritsen J, Smidt H, Rijkers GT, de Vos WM. Intestinal microbiota in human health and disease: the impact of probiotics. Genes Nutr. 2011;6:209–240. doi: 10.1007/s12263-011-0229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cholongitas E, Pipili C, Dasenaki M. Gastro-oesophageal reflux disease and irritable bowel syndrome significantly associated with metabolic syndrome. Scand J Gastroenterol. 2008;43:1405–1406. doi: 10.1080/00365520802308029. [DOI] [PubMed] [Google Scholar]
- 6.Collins SM. Translating symptoms into mechanisms: functional GI disorders. Adv Physiol Educ. 2007;31:329–331. doi: 10.1152/advan.00058.2007. [DOI] [PubMed] [Google Scholar]
- 7.Agrawal A, Whorwell PJ. Irritable bowel syndrome: diagnosis and management. BMJ. 2006;332:280–283. doi: 10.1136/bmj.332.7536.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han SH, Lee OY, Bae SC, Lee SH, Chang YK, Yang SY, et al. Prevalence of irritable bowel syndrome in Korea: population-based survey using the Rome II criteria. J Gastroenterol Hepatol. 2006;21:1687–1692. doi: 10.1111/j.1440-1746.2006.04269.x. [DOI] [PubMed] [Google Scholar]
- 9.Song SW, Park SJ, Kim SH, Kang SG. Relationship between irritable bowel syndrome, worry and stress in adolescent girls. J Korean Med Sci. 2012;27:1398–1404. doi: 10.3346/jkms.2012.27.11.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drossman DA, Li Z, Andruzzi E, Temple RD, Talley NJ, Thompson WG, et al. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci. 1993;38:1569–1580. doi: 10.1007/BF01303162. [DOI] [PubMed] [Google Scholar]
- 11.Saito YA, Locke GR, Talley NJ, Zinsmeister AR, Fett SL, Melton LJ., 3rd A comparison of the Rome and Manning criteria for case identification in epidemiological investigations of irritable bowel syndrome. Am J Gastroenterol. 2000;95:2816–2824. doi: 10.1111/j.1572-0241.2000.03192.x. [DOI] [PubMed] [Google Scholar]
- 12.American gastroenterological association medical position statement: irritable bowel syndrome. Gastroenterology. 1997;112:2118–2119. doi: 10.1053/gast.1997.1122118. [DOI] [PubMed] [Google Scholar]
- 13.Parkes GC, Brostoff J, Whelan K, Sanderson JD. Gastrointestinal microbiota in irritable bowel syndrome: their role in its pathogenesis and treatment. Am J Gastroenterol. 2008;103:1557–1567. doi: 10.1111/j.1572-0241.2008.01869.x. [DOI] [PubMed] [Google Scholar]
- 14.Ohman L, Simrén M. New insights into the pathogenesis and pathophysiology of irritable bowel syndrome. Dig Liver Dis. 2007;39:201–215. doi: 10.1016/j.dld.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 15.Di Stefano M, Corazza GR. Treatment of small intestine bacterial overgrowth and related symptoms by rifaximin. Chemotherapy. 2005;51(Suppl 1):103–109. doi: 10.1159/000081996. [DOI] [PubMed] [Google Scholar]
- 16.Singh VV, Toskes PP. Small bowel bacterial overgrowth: presentation, diagnosis, and treatment. Curr Treat Options Gastroenterol. 2004;7:19–28. doi: 10.1007/s11938-004-0022-4. [DOI] [PubMed] [Google Scholar]
- 17.Walters B, Vanner SJ. Detection of bacterial overgrowth in IBS using the lactulose H2 breath test: comparison with 14C-D-xylose and healthy controls. Am J Gastroenterol. 2005;100:1566–1570. doi: 10.1111/j.1572-0241.2005.40795.x. [DOI] [PubMed] [Google Scholar]
- 18.Pimentel M, Chow EJ, Lin HC. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am J Gastroenterol. 2000;95:3503–3506. doi: 10.1111/j.1572-0241.2000.03368.x. [DOI] [PubMed] [Google Scholar]
- 19.Szabo G, Bala S, Petrasek J, Gattu A. Gut-liver axis and sensing microbes. Dig Dis. 2010;28:737–744. doi: 10.1159/000324281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877–1887. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 21.Miele L, Beale G, Patman G, Nobili V, Leathart J, Grieco A, et al. The Kruppel-like factor 6 genotype is associated with fibrosis in nonalcoholic fatty liver disease. Gastroenterology. 2008;135:282–291. doi: 10.1053/j.gastro.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harte AL, da Silva NF, Creely SJ, McGee KC, Billyard T, Youssef-Elabd EM, et al. Elevated endotoxin levels in non-alcoholic fatty liver disease. J Inflamm (Lond) 2010;7:15. doi: 10.1186/1476-9255-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Creely SJ, McTernan PG, Kusminski CM, Fisher fM, Da Silva NF, Khanolkar M, et al. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J Physiol Endocrinol Metab. 2007;292:E740–E747. doi: 10.1152/ajpendo.00302.2006. [DOI] [PubMed] [Google Scholar]
- 24.Schnabl B, Brenner DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology. 2014;146:1513–1524. doi: 10.1053/j.gastro.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reid AE. Nonalcoholic steatohepatitis. Gastroenterology. 2001;121:710–723. doi: 10.1053/gast.2001.27126. [DOI] [PubMed] [Google Scholar]
- 26.Liu Z, Que S, Ning H, Wang L, Peng T. Elevated alanine aminotransferase is strongly associated with incident metabolic syndrome: a meta-analysis of prospective studies. PLoS One. 2013;8:e80596. doi: 10.1371/journal.pone.0080596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guillemin F, Bombardier C, Beaton D. Cross-cultural adaptation of health-related quality of life measures: literature review and proposed guidelines. J Clin Epidemiol. 1993;46:1417–1432. doi: 10.1016/0895-4356(93)90142-n. [DOI] [PubMed] [Google Scholar]
- 28.Greenfield TK. Ways of measuring drinking patterns and the difference they make: experience with graduated frequencies. J Subst Abuse. 2000;12:33–49. doi: 10.1016/s0899-3289(00)00039-0. [DOI] [PubMed] [Google Scholar]
- 29.Kim DM, Ahn CW. Definition and epidemiology of obesity. J Korean Med Assoc. 2004;47:289–297. [Google Scholar]
- 30.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American heart association/national heart, lung, and blood institute scientific statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 31.Clinical and laboratory standards institute. Defining, establishing, and verifying reference intervals in the clinical laboratory: approved guideline. 3rd ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2008. [Google Scholar]
- 32.Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98:960–967. doi: 10.1111/j.1572-0241.2003.07486.x. [DOI] [PubMed] [Google Scholar]
- 33.Suh YJ, Park SK, Choi JM, Ryoo JH. The clinical importance of serum γ-glutamyltransferase level as an early predictor of obesity development in Korean men. Atherosclerosis. 2013;227:437–441. doi: 10.1016/j.atherosclerosis.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 34.Pimentel M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome. a double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2003;98:412–419. doi: 10.1111/j.1572-0241.2003.07234.x. [DOI] [PubMed] [Google Scholar]
- 35.Lupascu A, Gabrielli M, Lauritano EC, Scarpellini E, Santoliquido A, Cammarota G, et al. Hydrogen glucose breath test to detect small intestinal bacterial overgrowth: a prevalence case-control study in irritable bowel syndrome. Aliment Pharmacol Ther. 2005;22:1157–1160. doi: 10.1111/j.1365-2036.2005.02690.x. [DOI] [PubMed] [Google Scholar]
- 36.Collins SM, Denou E, Verdu EF, Bercik P. The putative role of the intestinal microbiota in the irritable bowel syndrome. Dig Liver Dis. 2009;41:850–853. doi: 10.1016/j.dld.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 37.Ilan Y. Leaky gut and the liver: a role for bacterial translocation in nonalcoholic steatohepatitis. World J Gastroenterol. 2012;18:2609–2618. doi: 10.3748/wjg.v18.i21.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pande C, Kumar A, Sarin SK. Small-intestinal bacterial overgrowth in cirrhosis is related to the severity of liver disease. Aliment Pharmacol Ther. 2009;29:1273–1281. doi: 10.1111/j.1365-2036.2009.03994.x. [DOI] [PubMed] [Google Scholar]
- 39.Park JH, Park DI, Kim HJ, Cho YK, Sohn CI, Jeon WK, et al. The relationship between small-intestinal bacterial overgrowth and intestinal permeability in patients with irritable bowel syndrome. Gut Liver. 2009;3:174–179. doi: 10.5009/gnl.2009.3.3.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cortez-Pinto H, de Moura MC, Day CP. Non-alcoholic steatohepatitis: from cell biology to clinical practice. J Hepatol. 2006;44:197–208. doi: 10.1016/j.jhep.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 41.Portincasa P, Grattagliano I, Palmieri VO, Palasciano G. Nonalcoholic steatohepatitis: recent advances from experimental models to clinical management. Clin Biochem. 2005;38:203–217. doi: 10.1016/j.clinbiochem.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 42.Schaafsma G, Meuling WJ, van Dokkum W, Bouley C. Effects of a milk product, fermented by Lactobacillus acidophilus and with fructo-oligosaccharides added, on blood lipids in male volunteers. Eur J Clin Nutr. 1998;52:436–440. doi: 10.1038/sj.ejcn.1600583. [DOI] [PubMed] [Google Scholar]
- 43.Noh DO, Kim SH, Gilliland SE. Incorporation of cholesterol into the cellular membrane of Lactobacillus acidophilus ATCC 43121. J Dairy Sci. 1997;80:3107–3113. doi: 10.3168/jds.S0022-0302(97)76281-7. [DOI] [PubMed] [Google Scholar]
- 44.Sherman KE. Alanine aminotransferase in clinical practice. A review. Arch Intern Med. 1991;151:260–265. [PubMed] [Google Scholar]
- 45.Guo Y, Niu K, Momma H, Kobayashi Y, Chujo M, Otomo A, et al. Irritable bowel syndrome is positively related to metabolic syndrome: a population-based cross-sectional study. PLoS One. 2014;9:e112289. doi: 10.1371/journal.pone.0112289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 47.Pappo I, Becovier H, Berry EM, Freund HR. Polymyxin B reduces cecal flora, TNF production and hepatic steatosis during total parenteral nutrition in the rat. J Surg Res. 1991;51:106–112. doi: 10.1016/0022-4804(91)90078-z. [DOI] [PubMed] [Google Scholar]
- 48.Sabaté JM, Jouët P, Harnois F, Mechler C, Msika S, Grossin M, et al. High prevalence of small intestinal bacterial overgrowth in patients with morbid obesity: a contributor to severe hepatic steatosis. Obes Surg. 2008;18:371–377. doi: 10.1007/s11695-007-9398-2. [DOI] [PubMed] [Google Scholar]
- 49.Million M, Lagier JC, Yahav D, Paul M. Gut bacterial microbiota and obesity. Clin Microbiol Infect. 2013;19:305–313. doi: 10.1111/1469-0691.12172. [DOI] [PubMed] [Google Scholar]
- 50.Kang HW, Lee CG, Kim JH, Lim YJ, Lee JK, Koh MS, et al. Visceral abdominal obesity as a risk factor for irritable bowel syndrome: a case-control study. Gastroenterology. 2014;146(5 Suppl 1):S-178. [Google Scholar]
- 51.Singh SP, Kar SK, Panigrahi MK, Misra B, Pattnaik K, Bhuyan P, et al. Profile of patients with incidentally detected nonalcoholic fatty liver disease (IDNAFLD) in coastal eastern India. Trop Gastroenterol. 2013;34:144–152. doi: 10.7869/tg.118. [DOI] [PubMed] [Google Scholar]
- 52.Nam SY, Kim BC, Ryu KH, Park BJ. Prevalence and risk factors of irritable bowel syndrome in healthy screenee undergoing colonoscopy and laboratory tests. J Neurogastroenterol Motil. 2010;16:47–51. doi: 10.5056/jnm.2010.16.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]