Abstract
Purpose
Pulmonary surfactant (PS) replacement has been the gold standard therapy for neonatal respiratory distress syndrome; however, almost all commercial PSs contain animal proteins. We prepared a synthetic PS by using a human surfactant protein (SP) analog and evaluated its in vitro properties.
Materials and Methods
A peptide sequence (CPVHLKRLLLLLLLLLLLLLLLL) of human SP-C was chosen to develop the peptide analog (SPa-C). The new synthetic SP-C PS (sSP-C PS) was synthesized from SPa-C, dipalmitoyl phosphatidylcholine, phosphatidyl glycerol, and palmitic acid. Physical properties of the sSP-C PS were evaluated by measuring the maximum and minimum surface tensions (STs), surfactant spreading, and adsorption rate. In addition, we recorded an ST-area diagram. The data obtained on sSP-C PS were subsequently compared with those of purified natural bovine surfactant (PNBS), and the commercial product, Surfacten®.
Results
The sSP-C PS and Surfacten® were found to have maximum ST values of 32-33 mN/m, whereas that of PNBS was much lower at 19 mN/m. The minimum ST values of all three products were less than 10 mN/m. The values that were measured for the equilibrium ST of rapidly spreading sSP-C PS, Surfacten®, and PNBS were 27, 27, and 24 mN/m, respectively. The surface adsorptions were found to be the same for all three PSs (20 mN/m). ST-area diagrams of sSP-C PS and Surfacten® revealed similar properties.
Conclusion
In an in vitro experiment, the physical properties exhibited by sSP-C PS were similar to those of Surfacten®. Further study is required to evaluate the in vivo efficacy.
Keywords: Pulmonary surfactant, surfactant protein-C, synthetic peptide syntheses, respiratory distress syndrome, surface tension
INTRODUCTION
Respiratory distress syndrome (RDS) is a disease of pulmonary surfactant (PS) deficiency in newborn babies, particularly preterm infants. Although recent treatments for RDS have accomplished markedly improved outcomes, RDS remains a major cause of death among newborn babies. PS is a phospholipidprotein complex that functions in the human lung to maintain alveolar patency. Since the introduction and use of artificial PS for RDS treatment, the survival rate of preterm infants has improved markedly.1,2,3 This evolutionary change in both therapy and prognosis has been observed over the past two decades in Korea.4,5,6,7,8,9
Most of the commercially used artificial PSs are derived from animal (cow or pig) lung tissue. These animal-derived PSs contain surfactant protein (SP) from animal lung extracts, a point that raises several worrisome questions. Such PSs contain animal antigenicity, which might have potential infectious burden. Additionally, the slaughter of a large number of cows or pigs is needed to produce commercial vials, leading to increased production costs. Thus, there exists a need to produce artificial PS via completely synthetic methods without animal protein components. Lucinactant (Surfaxin®), which contains synthesized KLLLL peptide mimicking human SP-B, was developed and approved by the Food and Drug Administration in 2012.10 Recombinant SP-C based surfactant, Venticute® was also developed; however, clinical trials have been withheld.11,12 Additionally, in vitro and animal studies using synthetic analogues, such as Mini-B and CHF5633 (Chiesi Farmaceutici S.p., Parma, Italy), have recently been published.13,14
The authors conducted a synthetic peptide SP-C analogue-based surfactant study. We selected the peptide sequence CP VHLKRLLLLLLLLLLLLLLLL [SP-CL16(6-28)] for the SP-C analog using the methods of Otsubo, et al.15 We added several artificial phospholipids to prepare synthetic SP-C pulmonary surfactant (sSP-C PS). This preliminary study is the first step of our effort to determine the optimal composition with SP-CL16(6-28), for example, adding SP-B analog in the next step. Our eventual goal is to develop complete synthetic artificial surfactant and to conduct a clinical trial in the future. However, we first conducted an in vitro study to determine the physical properties of sSP-C PS prior to conducting an in vivo study with different compositions. Control subjects included purified natural bovine surfactant (PNBS)16 and a commercial product, Surfacten®, which contains bovine SPs.17 Pulsating bubble surfactometer and modified Wilhelmy balance tests were used to measure surface tension. By comparing these three groups, we evaluated the physical efficacy of sSP-C PS in vitro to assess the possibility of a next-generation synthetic surfactant.
MATERIALS AND METHODS
Preparation of sSP-C PS and PNBS
The sequence of CPVHLKRLLLLLLLLLLLLLLLL was chosen to prepare the SP-C peptide analog.15 The synthesized peptide was obtained from Anygen Co., Ltd. (Jangseong, Korea). As PS is composed mostly of phospholipids, we used dipalmitoyl phosphatidylcholine (DPPC, Sigma-Aldrich Corp., St. Louis, MO, USA), phosphatidylglycerol (PG, Sigma-Aldrich Corp., St. Louis, MO, USA), and palmitic acid (PA, Sigma-Aldrich Corp., St. Louis, MO, USA). We mixed DPPC, PG, PA, and SP-C 75:25:10:3 (w/w) and lyophilized the mixture to obtain sSP-C PS powder.18 To obtain PNBS, intact lungs of healthy cows were extracted. Normal saline (37℃) was instilled via the airway and re-suctioned after several rounds of gentle massage. Twenty liters of normal saline was used for each pair of lungs (approximately 5 kg). The obtained fluid was centrifuged at 500 g for 10 min to remove tissue remnants and blood cells. Then, a 15000 g centrifuge was applied over 60 min at 4℃ to obtain a PS pellet. To purify this crude natural surfactant (CNS), 4-5 mL of CNS pellet was diluted in 0.9% saline to be 30 mL of emulsion, and 48 mL of 26% sodium bromide solution was added. This was centrifuged at 24000 rpm for 120 minutes. In the middle, a white thin layer was obtained and mixed with normal saline. The pellet after 10 minutes of 500 g centrifugation was obtained and used as PNBS.16,19,20
Measurement of surface physical properties of sSP-C PS
A pulsating bubble surfactometer was used to measure surface tension (ST), specifically maximum and minimum ST. Modified Wilhelmy balance equipment was used to measure surface spreading rate and surface adsorption rate and to obtain an ST-area diagram. The surface spreading rate was defined as the time needed for surfactant particles to reach equilibrium ST when the PSs were placed on the fluid surface. We subsequently measured the equilibrium ST. The surface adsorption rate was defined as the time required for PSs to reach equilibrium ST within one minute of being placed under the fluid surface and rising to the surface. We measured the equilibrium ST thereafter as well.
Analysis of the surface physical properties
The measured values of the three different groups (sSP-C PS, Surfacten®, and PNBS) were compared with each other. The ideal PS physical properties included a minimum ST of less than 10 mN/m and a maximum ST of 30 mN/m measured using the pulsating bubble surfactometer and fulfilled Fujiwara's criteria based on the modified Wilhelmy balance test.21 The criteria were as follows: 1) rapid surface spreading (less than 10 seconds to reach an equilibrium ST of 24-27 mN/m), 2) rapid surface adsorption (less than one minute to reach an equilibrium ST of 27-30 mN/m), 3) a minimum ST of less than 10 mN/m with only 20-30% surface compression area, 4) a maximum ST of 27-30 mN/m with 100% surface compression area off, and 5) very low surface compressibility (less than 0.03 cm/dyne at ST 10 mN/m).
RESULTS
Minimum and maximum ST measured using pulsating bubble surfactometer
Minimum and maximum ST values measured using the pulsating bubble surfactometer are depicted in Table 1. All minimum STs measured at five minutes were less than 10 mN/m; however, the maximum STs at five minutes exhibited a different pattern. sSP-C PS and Surfacten® showed similar maximum ST values of 32-33 mN/m, and PNBS showed a maximum ST of only 18-19 mN/m.
Table 1. Comparison of Minimum and Maximum STs of Three Pulmonary Surfactant Preparations Measured Using a Pulsating Bubble Surfactometer.
| Minimum ST (mN/m) | Maximum ST (mN/m) | |||
|---|---|---|---|---|
| 1 min | 5 min | 1 min | 5 min | |
| sSP-C PS (n=10) | 8.5±0.9 | 7.7±0.8 | 33.8±2.5 | 33.9±2.4 |
| Surfacten® (n=10) | 6.4±0.7 | 5.7±0.6 | 33.2±1.7 | 32.5±1.4 |
| PNBS (n=10) | 10.2±1.2 | 8.3±1.3 | 18.6±2.2 | 19.0±1.8 |
sSP-C PS, synthetic surfactant protein C pulmonary surfactant; ST, surface tension; PNBS, purified natural bovine surfactant.
Surface spreading rate, adsorption rate, and ST-area diagram using the modified Wilhelmy balance test
Results rendered from the modified Wilhelmy balance test are depicted in Fig. 1 and Table 2. The equilibrium STs measured immediately after rapid spreading (within 10 seconds) were 27.0 mN/m (sSP-C SP), 27.0 mN/m (Surfacten®), and 24 mN/m (PNBS), suggesting that all three preparations exhibit an effective surfactant physical property. The equilibrium STs measured immediately after rapid adsorption (within one minute) for sSP-C PS and Surfacten® were both 30.0 mN/m, an effective physical property; however, that of PNBS was 31 mN/m, which was relatively ineffective. Minimum STs in the ST-area diagrams were 8.0 mN/m (sSP-C PS, effective), 6.0 mN/m (Surfacten®, effective), and 14 mN/m (PNBS, ineffective). Maximum STs were 39 mN/m (sSP-C PS, ineffective), 28.0 mN/m (Surfacten®, effective), and 27 mN/m (PNBS, effective). Surface compressibility values at 10 mN/m ST were 0.03 cm/dyne (sSP-C PS, effective), 0.03 cm/dyne (Surfacten®, effective), and 0.05 cm/dyne (PNBS, ineffective).
Fig. 1. Physical property diagrams of the three different preparations. (A) The graph shows the surface spreading rate. The equilibrium surface tension measurements obtained within 10 seconds were similar to those of Surfacten® and the synthetic surfactant protein C pulmonary surfactant (sSP-C PS); however, a lower value was obtained with purified natural bovine surfactant (PNBS). (B) The graph shows the surface adsorption rate curve. Both Surfacten® and PNBS reached rapid adsorption equilibrium surface tension within one minute, while sSP-C PS required more than one minute to reach equilibrium. (C) The graph shows the surface tension-area diagram. All three surfactant preparations exhibited reproducible hysteresis curves.
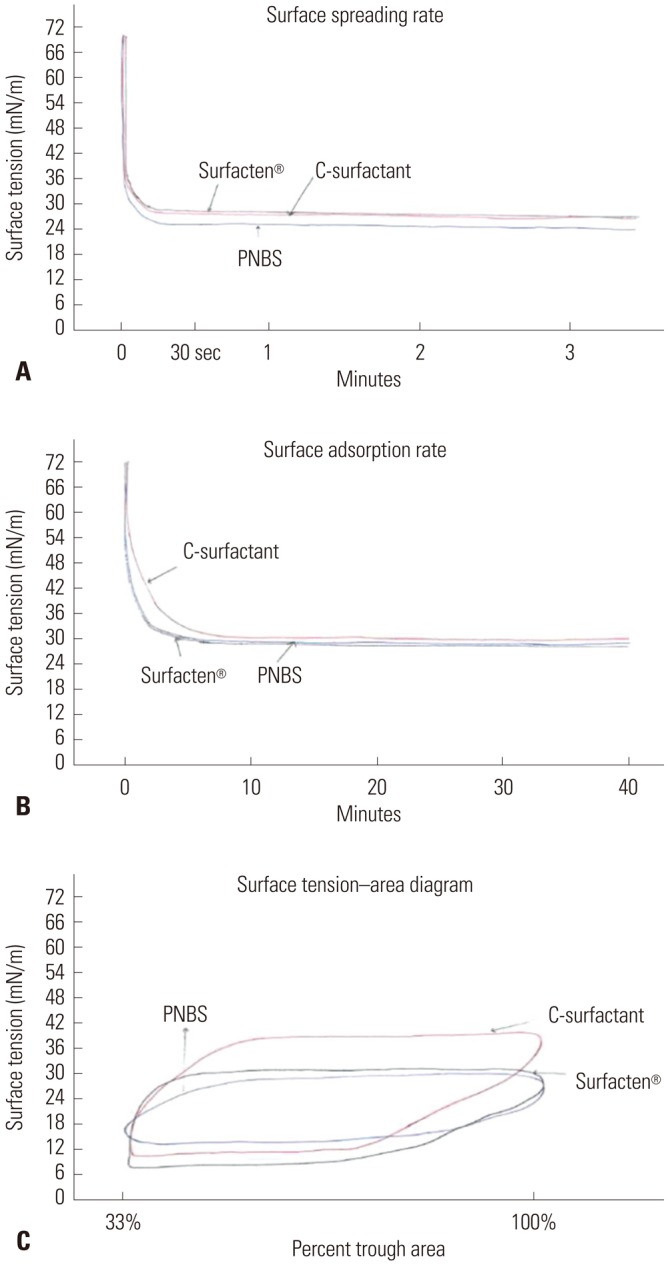
Table 2. Comparison of Findings of Three Surfactant Preparations Measured Using a Modified Wilhelmy Balance.
| sSP-C PS | Surfacten® | PNBS | |
|---|---|---|---|
| Rapid surface spreading (less than 10 sec to reach an equilibrium ST of 24-27 mN/m) (mN/m) | 27.0 (effective) | 27.0 (effective) | 24.0 (effective) |
| Rapid surface adsorption (less than 1 min to reach a equilibrium ST of 27-30 mN/m) (mN/m) | 30.0 (effective) | 30.0 (effective) | 31.0 (ineffective) |
| Minimum ST less than 10 mN/m with only 20-30% surface compression (mN/m) | 8.0 (effective) | 6.0 (effective) | 14.0 (ineffective) |
| Reproducible ST-area diagram with maximum ST of 27-30 mN/m (mN/m) | 39.0 (ineffective) | 28.0 (effective) | 27.0 (effective) |
| Very low surface compressibility of less than 0.03 cm/dyne at ST 10 mN/m (cm/dyne) | 0.03 (effective) | 0.03 (effective) | 0.05 (ineffective) |
sSP-C PS, synthetic surfactant protein C pulmonary surfactant; ST, surface tension; PNBS, purified natural bovine surfactant.
DISCUSSION
Human PS is a complex consisting of 86% phospholipids, 8% neutral lipids, and 6% SP. DPPC (50%) and PG (8%) are the most important phospholipids for the surface activity of PS. PS has four main surfactant proteins, SP-A, B, C, and D. Hydrophilic SP-A and D are removed in the manufacturing process; thus, current animal-derived PSs that are clinically used to treat neonatal RDS contain only hydrophobic SP-B and C. Therefore, we considered using SP-B alone, SP-C alone, or a mixture of both as SP in the production of a next-generation artificial PS. We prepared synthetic artificial PS (sSP-C PS) using a synthetic peptide SP-C analogue and conducted an in vitro study to assess the surface physical properties of sSP-C PS as a first step toward deciding whether it should be used alone or as a mixture with SP-B for artificial PS.
Based on the "ideal standard surface properties of artificial PS in vitro study,"21 results comparing sSP-C PS with Surfacten® and PNBS are depicted in Table 2, and the analysis of the current study may be summarized as follows. sSP-C PS and Surfacten® exhibited similar properties, as shown by the modified Wilhelmy balance test, with the exception of maximum ST. They also showed similar properties, including maximum ST, based on the pulsating bubble surfactometer test. These results suggest that sSP-C PS and Surfacten® have similar surface physical properties. PNBS differed from Surfacten®, exhibiting a minimum ST of more than 10 mN/m on both the modified Wilhelmy balance test and the pulsating bubble surfactometer test. Additionally, PNBS showed a surface compressibility of more than 0.03 at an ST of 10 mN/m on the modified Wilhelmy balance test. This finding is consistent with that when the phospholipids were added to PBNS in order to readjust components in preparation for Surfacten®. These results suggest that sSP-C PS, the subject of this study, can be used as an artificial PS that is comparable to commercialized Surfacten®.
Bovine surfactants (Surfacten®, Survanta®, Infasurf®, Alveofact®, Newfactan®) and porcine surfactants (Curosurf®), which have all been clinically proven to be effective, are used worldwide.1,2,3,22,23,24,25,26,27 According to a recent search in the Cochrane database of systematic reviews of several meta-analyses of these agents, the overall relative risk of mortality was decreased when the following procedures were observed with such agents: 1) administration of multiple doses rather than a single dose, 0.63; 2) use of animal-derived surfactant rather than synthetic surfactants, 0.86; 3) prophylactic rather than selective use, 0.61; 4) early rather than delayed selective treatment, 0.87; and 5) use of the INSURE (INtubation SURfactant Extubation) technique rather than surfactant administration followed by continued mechanical ventilation, 0.38 in preterm newborns with RDS.2,28,29,30,31 Exogenous surfactants also decreased neonatal mortality and neonatal pneumothorax by 50% and improved overall infant mortality by 6% in the United States.3 Artificial PS preparations are becoming a popular method for early prophylactic or therapeutic treatment of neonatal RDS.
However, concerns about the immunogenicity of animalderived PSs and bovine prion infection have not been clearly addressed. For this reason, human-derived PS preparations are needed. Creating artificial PS with the same structures as human PS is merely a starting point for developing human-derived PS preparations for the next generation. There exist only a small number of studies on this topic.
The progress summary for the development of synthetic surfactants is depicted in Table 3.32,33 Among these, Lucinactant (Surfaxin®) made with the KL4 peptide of SP-B was approved by the FDA in 2012.10 Development of Venticute®, a gene recombinant SP-C (rSP-C0), was stopped during clinical trials.11,12 rSP-C surfactant improved lung function in animal models; however, treatment with Venticute® in patients with acute respiratory distress syndrome (ARDS) did not improve survival, despite having a positive effect on gas exchange.34,35 Further clinical trial using Venticute® is no longer in progress; however, the ARDS trial was notably limited, as the population included only adult patients and not infants with RDS. Therefore, it is worthwhile for many researchers to continue studies regarding various combinations of synthetic analogs and phospholipid profiles. More studies are in progress as shown in Table 3; however, clinical outcomes of these synthetic surfactants are not currently being measured.
Table 3. Classification of Synthetic Surfactants Based on Peptide Content.
| Based on simplified peptides |
| WMAP10 |
| KL4 |
| KL4-surfactant (Surfaxin) |
| Poly-N-substituted glycines (peptides) with α-chiral side chains |
| Based on surfactant protein-B analogs |
| Peptides that cover the C-terminal partus |
| Peptides that cover the N-terminal partus |
| dSP-B1-25 |
| Mini-B |
| Based on SP-C analogs |
| Synthetic SP-C analogs with native poly-Val sequence |
| Poly-Val àpoly-Leu-substituted SP-C analog [SP-C(Leu)] |
| SP-C (LKS) |
| SP-C33 |
| SP-C30 |
Human SP-C consists of 35 amino acids and has an α helical structure, which is extremely hydrophobic.36 Johansson, et al.37 stressed that the poly-Val structure of synthetic SP-C attenuated the α helix unlike the native SP-C and eventually lowered surface activity. From this perspective, Nilsson, et al.38 substituted poly-Val with poly-Leu, which showed good surface activity with an α helical structure comparable to that of native SP-C. Therefore, it is beneficial to use poly-Leu instead of poly-Val when synthesizing SP-C peptide. Interestingly, Takei, et al.39 shortened the C terminal length while maintaining the poly-Leu sequence and tested several poly-Leu SP-C analog peptides. Notably, SP-CL16(6-28) showed the best biophysical activity, and this was used in our experiment (SP-C analog peptide, CPVHLKRLLLLLLLLLLLLLLLL). Otsubo and Takei40 tested various phospholipids mixed with SP-CL16(6-28) and suggested that palmitoyl-oleoyl PG (POPG) should be used instead of egg PG, as POPG consists of a single compound. In our study, we agreed with the choice of the CPVHLKRLLLLLL LLLLLLLLLL sequence; however, we nevertheless decided to use egg PG. Our results confirmed that the present sequence has good surface activity, based on the comparison with PNBS as well as Surfacten®. PNBS is a natural extract that is not used for limited clinical utility. Fujiwara, et al.17,41 developed effective preparations of Surfacten® by readjusting PNBS and adding phospholipids. In our study, PNBS also showed no significant effect.
In conclusion, the synthetic peptide SP-C analogue was mixed with DPPC, PG, PA, and SP-C (75:25:10:3, w/w) and lyophilized. It showed surface physical properties that were comparable to those of the current commercially used Surfacten®. This suggests that sSP-C PS, the subject of this study, can be used as an artificial PS and is equivalent to commercialized Surfacten®. The main limitation of this study was that it was an in vitro study and was not tested in vivo with animals. To validate our findings, pressure-volume curves and histologic percentages of alveolar areas would need to be measured using rabbit fetal RDS models by administering sSP-C PS in vivo. In this study, we present the results of the in vitro study in advance. We are planning to begin the next step of our research by assessing the properties of the preparation using SP-B alone and using a mixture of SP-B and SP-C.
ACKNOWLEDGEMENTS
This work was supported by the Kyung Hee University research fund (20110897).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Plavka R, et al. European consensus guidelines on the management of neonatal respiratory distress syndrome in preterm infants--2013 update. Neonatology. 2013;103:353–368. doi: 10.1159/000349928. [DOI] [PubMed] [Google Scholar]
- 2.Soll R, Ozek E. Multiple versus single doses of exogenous surfactant for the prevention or treatment of neonatal respiratory distress syndrome. Cochrane Database Syst Rev. 2009;(1):CD000141. doi: 10.1002/14651858.CD000141.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Halliday HL. Surfactants: past, present and future. J Perinatol. 2008;28(Suppl 1):S47–S56. doi: 10.1038/jp.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bae CW, Kwon YD, Ko SJ, Kim KS, Kim HM, Park WS, et al. Surfactant replacement therapy in neonates with respiratory distress syndrome: a collective evaluation of trials from 16 hospitals. J Korean Pediatr Soc. 1993;36:244–265. [Google Scholar]
- 5.Bae CW. Surfactant replacement therapy in RDS: a collaborative study of multi-center trials in Korea. J Korean Soc Neonatol. 1997;4:124–135. [Google Scholar]
- 6.Bae CW, Kim YM. Surfactant therapy for neonatal respiratory distress syndrome: experience in Korea over 15 years. Korean J Pediatr. 2004;47:940–948. [Google Scholar]
- 7.Bae CW, Hahn WH. Surfactant therapy for neonatal respiratory distress syndrome: a review of Korean experiences over 17 years. J Korean Med Sci. 2009;24:1110–1118. doi: 10.3346/jkms.2009.24.6.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bae CW, Hahn WH, Chang JY, Kim SM. Surfactant replacement therapy for RDS: a collaborative study of 72 multi-center trials in Korea (2010) and a review of Korean experiences over 20 years. J Korean Soc Neonatol. 2011;18:409–411. [Google Scholar]
- 9.Kim SM, Park YJ, Chung SH, Choi YS, Kim CH, Bae CW. Early prophylactic versus late selective use of surfactant for respiratory distress syndrome in very preterm infants: a collaborative study of 53 multi-center trials in Korea. J Korean Med Sci. 2014;29:1126–1131. doi: 10.3346/jkms.2014.29.8.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moya FR, Gadzinowski J, Bancalari E, Salinas V, Kopelman B, Bancalari A, et al. A multicenter, randomized, masked, comparison trial of lucinactant, colfosceril palmitate, and beractant for the prevention of respiratory distress syndrome among very preterm infants. Pediatrics. 2005;115:1018–1029. doi: 10.1542/peds.2004-2183. [DOI] [PubMed] [Google Scholar]
- 11.Davis AJ, Jobe AH, Häfner D, Ikegami M. Lung function in premature lambs and rabbits treated with a recombinant SP-C surfactant. Am J Respir Crit Care Med. 1998;157:553–559. doi: 10.1164/ajrccm.157.2.97-08019. [DOI] [PubMed] [Google Scholar]
- 12.Pfister RH, Soll RF. New synthetic surfactants: the next generation? Biol Neonate. 2005;87:338–344. doi: 10.1159/000084882. [DOI] [PubMed] [Google Scholar]
- 13.Almlén A, Walther FJ, Waring AJ, Robertson B, Johansson J, Curstedt T. Synthetic surfactant based on analogues of SP-B and SP-C is superior to single-peptide surfactants in ventilated premature rabbits. Neonatology. 2010;98:91–99. doi: 10.1159/000276980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seehase M, Collins JJ, Kuypers E, Jellema RK, Ophelders DR, Ospina OL, et al. New surfactant with SP-B and C analogs gives survival benefit after inactivation in preterm lambs. PLoS One. 2012;7:e47631. doi: 10.1371/journal.pone.0047631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Otsubo E, Irimajiri K, Takei T, Nomura M. Characterization of synthetic lung surfactant activity against proinflammatory cytokines in human monocytes. Biol Pharm Bull. 2002;25:312–317. doi: 10.1248/bpb.25.312. [DOI] [PubMed] [Google Scholar]
- 16.Bae CW, Ahn CI, Kim KL, Hahm KS, Takahashi A, Fujiwara T. Purification of bovine lung natural surfactant and assessment of surface physical properties. J Korean Med Assoc. 1991;34:534–544. [Google Scholar]
- 17.Fujiwara T, Konishi M, Chida S, Okuyama K, Ogawa Y, Takeuchi Y, et al. Surfactant replacement therapy with a single postventilatory dose of a reconstituted bovine surfactant in preterm neonates with respiratory distress syndrome: final analysis of a multicenter, double-blind, randomized trial and comparison with similar trials. The surfactant-TA study group. Pediatrics. 1990;86:753–764. [PubMed] [Google Scholar]
- 18.Takei T, Hashimoto Y, Aiba T, Sakai K, Fujiwara T. The surface properties of chemically synthesized peptides analogous to human pulmonary surfactant protein SP-C. Biol Pharm Bull. 1996;19:1247–1253. doi: 10.1248/bpb.19.1247. [DOI] [PubMed] [Google Scholar]
- 19.Kang JH, Shin SY, Maeng CY, Kim KL, Bae CW, Hahm KS. Preparation and in vitro physical activities of crude natural surfactant and artificial pulmonary surfactant containing synthetic peptide and phospholipid mixtures. J Korean Pediatr Soc. 1998;41:472–480. [Google Scholar]
- 20.Shelley SA, Paciga JE, Balis JU. Purification of surfactant from lung washings and washings contaminated with blood constituents. Lipids. 1977;12:505–510. doi: 10.1007/BF02535450. [DOI] [PubMed] [Google Scholar]
- 21.Fujiwara T, Robertson B. Pharmacology of exogenous surfactant. In: Robertson B, Van Golde LMG, Batenburg JJ, editors. Pulmonary surfactant: from molecular biology to clinical practice. 2nd ed. Amsterdam: Elservier; 1992. pp. 561–592. [Google Scholar]
- 22.Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Plavka R, et al. European consensus guidelines on the management of neonatal respiratory distress syndrome in preterm infants - 2010 update. Neonatology. 2010;97:402–417. doi: 10.1159/000297773. [DOI] [PubMed] [Google Scholar]
- 23.Ma CC, Ma S. The role of surfactant in respiratory distress syndrome. Open Respir Med J. 2012;6:44–53. doi: 10.2174/1874306401206010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramanathan R. Animal-derived surfactants: where are we? The evidence from randomized, controlled clinical trials. J Perinatol. 2009;29(Suppl 2):S38–S43. doi: 10.1038/jp.2009.31. [DOI] [PubMed] [Google Scholar]
- 25.Soll RF, Blanco F. Natural surfactant extract versus synthetic surfactant for neonatal respiratory distress syndrome. Cochrane Database Syst Rev. 2001;(2):CD000144. doi: 10.1002/14651858.CD000144. [DOI] [PubMed] [Google Scholar]
- 26.Kirsten GF, Kirsten CL, Henning PA, Smith J, Holgate SL, Bekker A, et al. The outcome of ELBW infants treated with NCPAP and In-SurE in a resource-limited institution. Pediatrics. 2012;129:e952–e959. doi: 10.1542/peds.2011-1365. [DOI] [PubMed] [Google Scholar]
- 27.Jeon GW, Oh M, Sin JB. Efficacy of surfactant-TA, calfactant and poractant alfa for preterm infants with respiratory distress syndrome: a retrospective study. Yonsei Med J. 2015;56:433–439. doi: 10.3349/ymj.2015.56.2.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seger N, Soll R. Animal derived surfactant extract for treatment of respiratory distress syndrome. Cochrane Database Syst Rev. 2009;(2):CD007836. doi: 10.1002/14651858.CD007836. [DOI] [PubMed] [Google Scholar]
- 29.Soll RF, Morley CJ. Prophylactic versus selective use of surfactant in preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev. 2001;(2):CD000510. doi: 10.1002/14651858.CD000510. [DOI] [PubMed] [Google Scholar]
- 30.Bahadue FL, Soll R. Early versus delayed selective surfactant treatment for neonatal respiratory distress syndrome. Cochrane Database Syst Rev. 2012;11:CD001456. doi: 10.1002/14651858.CD001456.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevens TP, Harrington EW, Blennow M, Soll RF. Early surfactant administration with brief ventilation vs. selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome. Cochrane Database Syst Rev. 2007;(4):CD003063. doi: 10.1002/14651858.CD003063.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim YD. New synthetic surfactants for neonates. J Korean Soc Neonatol. 2012;19:184–194. [Google Scholar]
- 33.Curstedt T, Johansson J. New synthetic surfactants--basic science. Biol Neonate. 2005;87:332–337. doi: 10.1159/000084881. [DOI] [PubMed] [Google Scholar]
- 34.Spragg RG, Lewis JF, Wurst W, Häfner D, Baughman RP, Wewers MD, et al. Treatment of acute respiratory distress syndrome with recombinant surfactant protein C surfactant. Am J Respir Crit Care Med. 2003;167:1562–1566. doi: 10.1164/rccm.200207-782OC. [DOI] [PubMed] [Google Scholar]
- 35.Spragg RG, Lewis JF, Walmrath HD, Johannigman J, Bellingan G, Laterre PF, et al. Effect of recombinant surfactant protein C-based surfactant on the acute respiratory distress syndrome. N Engl J Med. 2004;351:884–892. doi: 10.1056/NEJMoa033181. [DOI] [PubMed] [Google Scholar]
- 36.Curstedt T. Surfactant protein C: basics to bedside. J Perinatol. 2005;25(Suppl 2):S36–S38. doi: 10.1038/sj.jp.7211318. [DOI] [PubMed] [Google Scholar]
- 37.Johansson J, Nilsson G, Strömberg R, Robertson B, Jörnvall H, Curstedt T. Secondary structure and biophysical activity of synthetic analogues of the pulmonary surfactant polypeptide SP-C. Biochem J. 1995;307(Pt 2):535–541. doi: 10.1042/bj3070535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nilsson G, Gustafsson M, Vandenbussche G, Veldhuizen E, Griffiths WJ, Sjövall J, et al. Synthetic peptide-containing surfactants--evaluation of transmembrane versus amphipathic helices and surfactant protein C poly-valyl to poly-leucyl substitution. Eur J Biochem. 1998;255:116–124. doi: 10.1046/j.1432-1327.1998.2550116.x. [DOI] [PubMed] [Google Scholar]
- 39.Takei T, Hashimoto Y, Ohtsubo E, Sakai K, Ohkawa H. Characterization of poly-leucine substituted analogues of the human surfactant protein SP-C. Biol Pharm Bull. 1996;19:1550–1555. doi: 10.1248/bpb.19.1550. [DOI] [PubMed] [Google Scholar]
- 40.Otsubo E, Takei T. Effects of the human pulmonary surfactant protein-C (SP-C), SP-CL16(6-28) on surface activities of surfactants with various phospholipids. Biol Pharm Bull. 2002;25:1303–1306. doi: 10.1248/bpb.25.1303. [DOI] [PubMed] [Google Scholar]
- 41.Fujiwara T, Maeta H, Chida S, Morita T, Watabe Y, Abe T. Artificial surfactant therapy in hyaline-membrane disease. Lancet. 1980;1:55–59. doi: 10.1016/s0140-6736(80)90489-4. [DOI] [PubMed] [Google Scholar]


