Abstract
Purpose
This study aimed to investigate whether Müllerian inhibiting substance (MIS) in combination with calcitriol modulates proliferation and apoptosis of human ovarian cancer (OCa) cell lines (SKOV3, OVCAR3, and OVCA433) and identify the signaling pathway by which MIS mediates apoptosis.
Materials and Methods
OCa cell lines were treated with MIS in the absence or presence of calcitriol. Cell viability and proliferation were evaluated using the Cell Counting Kit-8 assay and apoptosis was evaluated by DNA fragmentation assay. Western blot and enzyme-linked immunosorbent assay were used to determine the signaling pathway.
Results
The cells showed specific staining for the MIS type II receptor. Treatment of OCa cells with MIS and calcitriol led to dose- and time-dependent inhibition of cell growth and survival. The combination treatment significantly suppressed cell growth, down-regulated the expression of B-cell lymphoma 2 (Bcl-2), and up-regulated the expressions of Bcl-2 associated X protein, caspase-3, and caspase-9 through the extracellular signal-regulated kinase signaling pathway.
Conclusion
These results, coupled with a much-needed decrease in the toxic side effects of currently employed therapeutic agents, provide a strong rationale for testing the therapeutic potential of MIS, alone or in combination with calcitriol, in the treatment of OCa.
Keywords: Ovarian cancer, Müllerian inhibiting substance, calcitriol, antiproliferation, apoptosis
INTRODUCTION
Epithelial ovarian cancer (OCa) affects nearly 25000 women in North America every year and is the fifth most common malignancy in women with a five-year mortality of over 70%.1 Because the symptoms are often not observed until the cancer has spread extensively, less than 25% of women are diagnosed at early stage of the disease. Combined surgery and cytotoxic therapy produce favorable clinical responses in 50% to 80% of patients; however, the majority of patients relapse. Therefore, it is crucial to search for new biologically targeted treatment modalities.2,3
The Müllerian duct, which is formed from the coelomic epithelium, develops into the Fallopian tubes, uterus, cervix, proximal vagina, and the surface epithelium of the ovaries in females.4 Müllerian inhibiting substance (MIS), also referred to as anti-Müllerian hormone or AMH, is a glycoprotein composed of two identical 535 amino acid residues subunits with a combined molecular weight of 140 kDa. MIS belongs to the transforming growth factor-β (TGF-β) family along with bone morphogenetic protein, activin, and inhibin.5 Several studies have suggested that MIS inhibits the growth of cell lines and tissues of MIS receptor-expressing gynecological malignancies such as breast, endometrial, cervical, and ovarian cancer.6,7,8,9,10
Calcitriol (1, 25-dihydroxycholecalciferol), the hormonally active form of Vitamin D (Vit D), has long been known as an important regulator of calcium homeostasis and bone metabolism through its activity in the intestines, bones, kidneys, and the parathyroid glands.11 Emerging evidence indicates that calcitriol may be implicated in the regulation of other important biological processes such as insulin secretion, immune response, pro-differentiation, anti-proliferation, pro-apoptosis, anti-angiogenesis, inhibition of invasion and metastasis, and antiinflammation.12 It has recently been suggested that MIS constitutes a novel target regulated by calcitriol in prostate cells, and that induction of MIS expression may play an important role in the anti-cancer activity of calcitriol.13,14
Growing evidence suggesting that MIS and calcitriol can act synergistically to inhibit the growth of tumor cells prompted us to examine the effects of MIS in combination with calcitriol on OCa cell lines. Therefore, the purpose of this study was to investigate whether MIS in combination with calcitriol modulates the proliferation and apoptosis of human OCa cell lines (SKOV3, OVCAR3, and OVCA433) and to identify the signaling pathway by which MIS and calcitriol mediate proliferation and apoptosis.
MATERIALS AND METHODS
Cell culturing
Human epithelial OCa cell lines (SKOV3, OVCAR3, and OVCA 433) were cultured in RPMI-1640 medium (Gibco-BRL, Gaithersburg, MD, USA) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 µg/mL streptomycin. The cells were incubated in a humidified atmosphere containing 5% CO2 at 37℃.
MIS type II receptor (MISRII) detection
MIS type II receptor (MISRII) expression was examined in SKOV3, OVCAR3, and OVCA433 cell lines by western blot analysis, performed with rabbit polyclonal antihuman MISRII antiserum (Abcam, Cambridge, MA, USA).
Cell viability and proliferation assay
Cell viability and proliferation were measured using the Cell Counting Kit-8 (CCK-8) assay (Dojindo Laboratories, Kumamoto, Japan). Cells were seeded in a 96-well flat-bottomed plate (2×103 cells in 100 µL per well), incubated overnight to allow for cell attachment and recovery, and then exposed to MIS (USCN Life Science Inc., Wuhan, Hubei, China) and calcitriol (Sigma, St. Louis, MO, USA) dissolved in methanol for 24, 48, 72, and 96 h. Next, CCK-8 solution (10 µL) was added to each well, and the cells were incubated for an additional two hours. Absorbance at 450 nm was measured with a microplate reader.
Treatment of cells with inhibitors
Cells were seeded at a density of 5×103 cells/100-mm dish. Following the 48-h incubation, they were washed with a serum-free medium and then transferred into media without FBS at least 16 h prior to the start of the experiments. Cells were pretreated with 20 µM each of the following inhibitors: SB203580 (Sigma), a p38 mitogen-activated protein kinase (p38 MAPK) inhibitor; PD98059 (Sigma), an extracellular signal-regulated kinase (ERK) inhibitor; LY294002, a phosphoinositide 3-kinase (PI3K) inhibitor; and SP600125 (Sigma), a c-Jun amino-terminal kinase (JNK) inhibitor.
Western blot analysis
Following treatment, cells were collected and centrifuged and whole-cell lysates were prepared using a lysis buffer. Total protein concentration was determined using a DC protein assay kit (Bio-Rad, Hercules, CA, USA). Thirty micrograms of protein was directly separated by 10% SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to polyvinylidene difluoride (PVDF, Bio-Rad Laboratories). Blocked membranes were then incubated with primary antibodies overnight at 4℃. B-cell lymphoma 2 (Bcl-2), Bcl-2 associated X protein (BAX), ERK, phospho-ERK, JNK, and phosphor-JNK purchased from Cell signaling Technology (Beverly, MA, USA); and caspase-3 and caspase-9 from Biovision (Milpitas, CA, USA) were used at a 1:1000 dilution. Subsequent to washing, the membranes were incubated with a horseradish peroxidase-conjugated anti-rabbit or anti-mouse secondary antibody (Santa Cruz, Biotechnology, Santa Cruz, CA, USA). The blots were re-probed with anti-β-actin antibody as a loading control. Protein bands were visualized using an enhanced chemiluminescence system (ECL™; Amersham, Little Chalfont, UK) and band intensities were quantified using the Luminescent image analyzer (LAS 4000 mini; Fujifilm, Uppsala, Sweden). All experiments were independently repeated three times.
DNA fragmentation assay
The DNA fragmentation assay was performed using enzyme-linked immunosorbent assay (ELISA) with a DNA fragmentation Kit (Roche Applied Science, Indianapolis, IN, USA). Cells were seeded at a density of 1×105 cells per well in 96-well plates. Subsequent to 24 h growth, the medium was changed to a serum-free one, and cells were grown for an additional 24 h. In order to label the DNA, the medium was replaced with 10% FBS-Dulbecco's modified Eagle medium, 10 µM 5-bromo-2'-deoxyuridine was added to each well, and cells were incubated for 24 h. Cells were treated with calcitriol for 4 h and then incubated with MIS for an additional 96 h. Cells were then lysed in 200 µL of incubation buffer, and soluble DNA fragments were quantified using the Cellular DNA fragmentation ELISA kit according to the manufacturer's instructions. All experiments were performed in triplicate.
Statistical analysis
Data were analyzed by Student's t-test or ANOVA for each of the repeated experiments. For all analyses, statistically significant differences were designated as p<0.05. Results are shown as mean±SD.
RESULTS
MISRII is expressed in ovarian cancer cell lines
Before evaluating the response of OCa cells to MIS, we first determined whether the cells expressed MISRII. Thus, expression of MISRII was examined in SKOV3, OVCAR3, and OVCA433 cell lines by western blot analysis, using a rabbit polyclonal antihuman MISRII antiserum. All cell lines showed specific staining for MISRII, although expression in OVCAR3 was higher than in SKOV3 and OVCA433 (data not shown).
Growth inhibitory concentrations for MIS and calcitriol
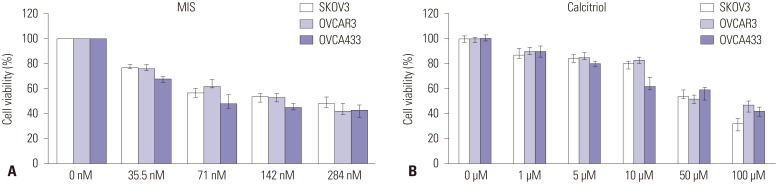
OCa cells were treated with a range of MIS (35.5-284 nM) and calcitriol concentrations (1-100 µM) to determine the IC50 for each cell line. For all subsequent experiments, MIS and calcitriol concentrations were held constant at or near their IC50, 71 nM and 50 µM, respectively (Fig. 1).
Fig. 1. Effects of MIS and calcitriol on the viability of ovarian cancer (OCa) cells. (A) Cells were treated with a range of MIS (35.5-284 nM) to determine the IC50 for each cell line. (B) Cells were treated with a range of calcitriol concentrations (1-100 µM) to determine the IC50 for each cell line. Values are presented as a percentage of the control and were calculated using the following equation: [(mean absorbance of treated cells)/(mean absorbance of control cells)]×100. Data are expressed as mean±SD from three independent experiments. For all subsequent experiments, MIS and calcitriol concentrations were held constant at or near their IC50, 71 nM and 50 µM, respectively. MIS, Müllerian inhibiting substance.
Cooperative effects of MIS and calcitriol on suppression of ovarian cancer cell proliferation
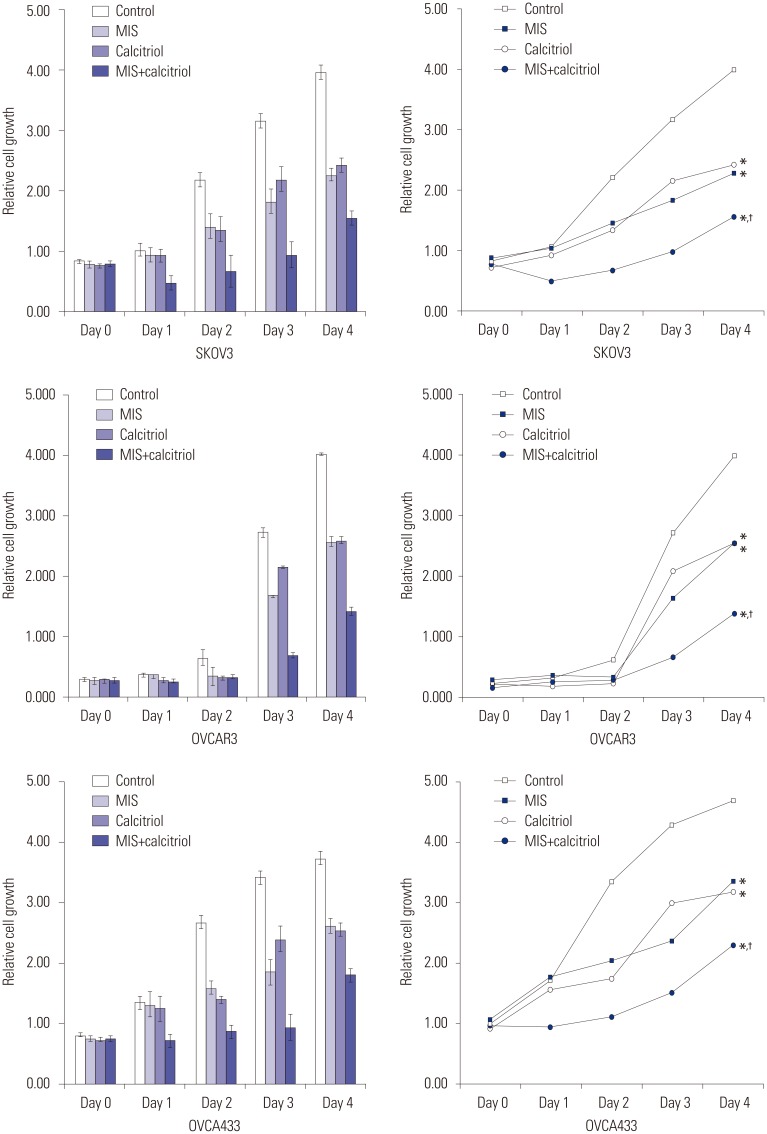
SKOV3, OVCAR3, and OVCA433 cells were grown in plates for 48 h, and then treated with 71 nM MIS and 50 µM calcitriol. After 24, 48, 72, and 96 h of treatment, the cells were incubated with CCK-8 solution and absorbance was read at 450 nm. As shown in Fig. 2, cell proliferation decreased significantly following 72 h of exposure to MIS combined with calcitriol.
Fig. 2. Viability and proliferation assays conducted with ovarian cancer (OCa) cell lines treated with MIS and calcitriol (relative cell growth as a function of time). MIS and calcitriol concentrations were held constant at or near their IC50; 71 nM MIS and 50 µM calcitriol. Cells were treated with vehicles (control), 71 nM MIS (MIS), 50 µM calcitriol (calcitriol), or both agents (MIS+calcitriol). Combined use of calcitriol and MIS reduces cell proliferation of human OCa cell lines (SKOV3, OVCAR3, and OVCA433). Cell growth was monitored for 96 h post treatment. For the combination treatment, cells were pretreated with calcitriol (50 µM) for 4 h prior to the addition of MIS (71 nM). Cell proliferation was measured using the Cell Counting Kit-8 solution as described in the Materials. Results are representative of three experiments. Data in the bar graph represent mean±SD. *p<0.05 vs. control, †p<0.05 vs. MIS. MIS, Müllerian inhibiting substance.
Calcitriol enhanced MIS-induced apoptosis in ovarian cancer cell lines
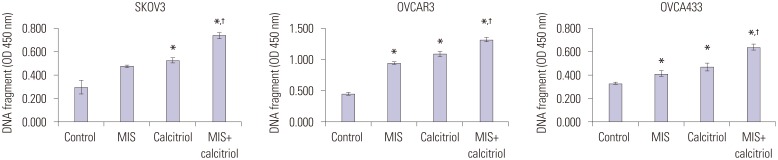
The degree of apoptosis was analyzed by measuring the level of cellular DNA fragmentation using ELISA. A significant increase in DNA fragmentation was apparent in cells treated with MIS (71 nM) combined with calcitriol (50 µM), compared with the control. In addition, the results for the combination treatment were significantly different from MIS alone treatment in all cell lines (Fig. 3).
Fig. 3. Calcitriol enhances MIS-induced apoptosis in OCa cell lines. Cells (1×105/well) were pretreated with or without calcitriol (50 µM) for 4 h prior to the addition of MIS (71 nM). Apoptosis was measured as cellular DNA fragmentation determined by ELISA. Results are representative of three experiments. Significant inhibition relative to the control (MIS 0 nM) is indicated by an asterisk and the cross indicates a significant difference relative to the MIS treatment (MIS 71 nM). Data are presented as mean±SD. *p<0.05 vs. control, †p<0.05 vs. MIS. MIS, Müllerian inhibiting substance; ELISA, enzyme-linked immunosorbent assay; OD, optical density.
MIS and calcitriol alter the expression of regulatory proteins in SKOV3
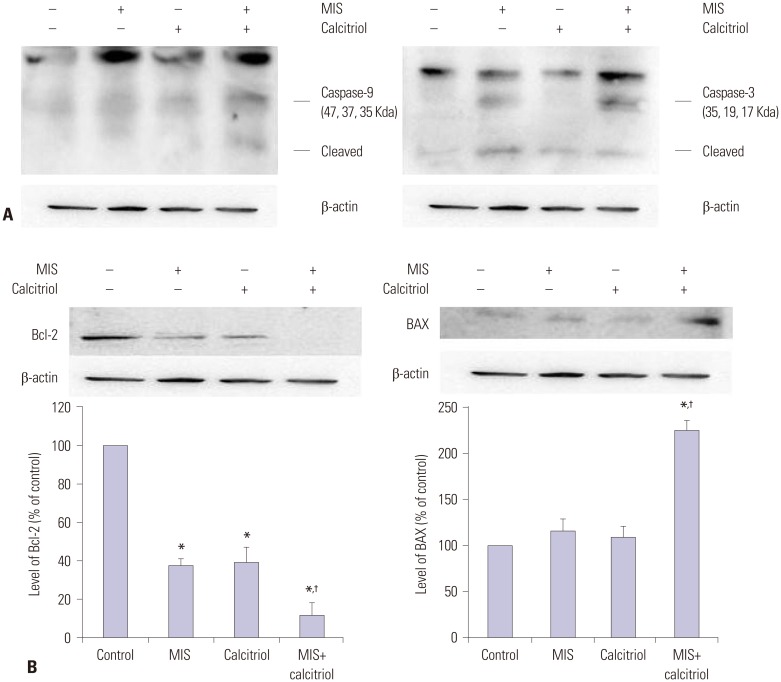
To demonstrate that MIS and calcitriol-reduced cell proliferation was due to apoptotic activity, the levels of Bcl-2, BAX, caspase-3, and caspase-9 were examined. SKOV3 cells were pretreated with or without calcitriol (50 µM) for 4 h prior to the addition of MIS (71 nM). Following 48 h of incubation, cells were analyzed by western blot analysis with anti-caspase-9 antibody and anti-caspase-3 antibody; the expression of the apoptosis-related proteins Bcl-2 and BAX were also evaluated. Treatment of SKOV3 with MIS plus calcitriol induced apoptosis, as evidenced by an increase in the levels of BAX, caspase-3, and caspase-9 and a decrease in the levels of Bcl-2 (Fig. 4).
Fig. 4. MIS and calcitriol alter the expression of regulatory proteins in SKOV3. Cells were treated with vehicles (control), 71 nM MIS (MIS), 50 µM calcitriol (calcitriol), or both (MIS+calcitriol). For the combined treatment, cells were incubated in the presence or absence of calcitriol (50 µM) for 4 h followed by stimulation with MIS (71 nM) for 48 h. (A) Western blot analysis of caspase-9 and caspase-3. (B) Western blot analysis of Bcl-2 and BAX. Band intensities were quantitated and data are presented as mean±SD. *p<0.05 vs. control, †p<0.05 vs. MIS. MIS, Müllerian inhibiting substance; Bcl-2, B-cell lymphoma 2; BAX, Bcl-2 associated X protein.
The effects of MIS plus calcitriol on the phosphorylation of ERK in SKOV3
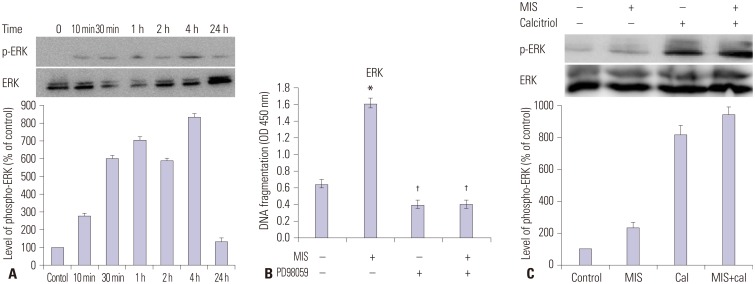
Potential molecular mechanisms underlying the synergistic effect of calcitriol and MIS were assessed by western blot analysis following treatment with chemical inhibitors; p38 MAPK activation was inhibited with 20 µM SB203580, PI3K was inhibited with 20 µM LY294002, ERK was inhibited with 20 µM PD98059, and JNK signaling was inhibited with 20 µM SP600125. Although MIS did not activate the p38 MAPK, PI3K, or JNK pathway (data not shown), Fig. 5A indicates that the ERK pathway is activated by MIS. SKOV3 cells were then treated with 71 nM MIS, 20 µM PD98059, or both for 2 h and then the degree of apoptosis was analyzed by measuring the level of cellular DNA fragmentation using ELISA. A significant increase in fragmentation was observed for the MIS treatment vs. the control (p<0.05) and MIS vs. PD98059 (p<0.05) (Fig. 5B). Next, cells were incubated with or without calcitriol (50 µM) for 4 h prior to the addition of MIS (71 nM). Following 48 h of incubation, cells were harvested and ERK activation was analyzed by western blotting using an anti-phospho-ERK antibody. The combination treatment resulted in the most intense specific band compared to control, MIS alone, and calcitriol alone (Fig. 5C).
Fig. 5. Calcitriol increases MIS-induced ERK phosphorylation in SKOV3 cells. (A) Western blot analysis of ERK and phosphorylated ERK levels in SKOV3 cells treated with MIS. Bands were detected with anti-ERK and anti phospho-ERK antibodies. (B) SKOV3 cells were treated with 71 nM MIS, 20 µM PD98059, or both for 2 h and the degree of apoptosis was analyzed by ELISA measuring the level of cellular DNA fragmentation. Significant increase relative to controls (MIS 0 nM) is indicated by asterisks and crosses indicate a significant difference (p<0.05) compared with MIS treatment (MIS 71 nM). (C) SKOV3 cells were incubated with or without calcitriol (50 µM) for 4 h prior to the addition of MIS (71 nM). Subsequent to 48 h of incubation, cells were harvested and ERK activation was analyzed by western blot analysis using anti-phospho-ERK antibody. *p<0.05 vs. control, †p<0.05 vs. MIS. MIS, Müllerian inhibiting substance; ERK, extracellular signal-regulated kinase; ELISA, enzyme-linked immunosorbent assay; OD, optical density.
DISCUSSION
The clinical use of cytotoxic drugs has had a significant impact on neoplastic diseases. However, their therapeutic effectiveness is limited because of their narrow therapeutic index and the onset of chemoresistance. Therefore, many efforts are currently being directed at finding new therapeutic options that may overcome these problems.
Our present study was aimed at further elucidating the molecular mechanisms underlying the anti-proliferative and cancer preventive effects of MIS and calcitriol in order to develop strategies to improve OCa treatment. The results suggested that calcitriol enhanced the antitumor activity of MIS in OCa cells by down-regulating the expression of Bcl-2 and up-regulating the expression of BAX, caspase-3, and caspase-9 through the ERK signaling pathways.
Initial in vitro studies using human OCa cell lines or tissues and several follow-up studies have revealed that MIS inhibits the growth of human cancer cells including breast, cervical, endometrial, prostate cancer, and ocular melanoma.6,8,9,15,16,17 In addition, a recent study indicates that MIS also plays a role in cell cycle arrest and apoptosis of endometriosis.18
The prophylactic and therapeutic activities of Vit D against the most common types of cancer have been extensively investigated both in vitro and in vivo.19,20,21 The most striking results have been obtained from studies on breast cancer, prostate cancer, and colorectal cancer.19,22 Experimental observations suggest that the chemopreventive effects of Vit D are due mainly to its ability to modulate important biological functions such as cell proliferation, cell differentiation, growth factor gene expression, signal transduction, and apoptosis.23,24 Interestingly, recent studies have shown that calcitriol may also affect OCa cell proliferation by decreasing human telomerase reverse transcriptase mRNA through a small non-coding RNA.25
Vit D and TGF-β have similar effects on cell growth and differentiation. Experimental stress studies indicate that Vit D may increase the expression levels of TGF-β and its receptors or TGF-β secretion in certain cell types.26,27,28 The Feldman research group has demonstrated that MIS, a TGF-β family member, constitutes a novel target gene regulated by calcitriol in prostate cells.14 Exposing prostate cancer cells to calcitriol for 24 h resulted in a considerable increase in the expression of MIS mRNA. In addition, HeLa cells transfected with an MIS promoter-luciferase construct and a Vit D receptor expression vector demonstrated a significant (two- to four-fold) induction of MIS promoter-luciferase following treatment with calcitriol, suggesting that the MIS promoter is responsive to calcitriol.13,14
Determining the utility of MIS as an anticancer drug would most likely involve administering MIS to patients as an adjuvant in combination with other drugs. Therefore, elucidating the anti-proliferation and apoptosis signaling mechanisms downstream of MIS is necessary before combining MIS with commonly used cytotoxic drugs. Moreover, it is important to test for synergy or additivity between MIS and other drugs to ensure that they do not counteract with each other. Since little is known as for the signaling pathways by which MIS mediates proliferation inhibition and apoptosis in OCa cell lines, we investigated several potential molecular mechanisms using chemical inhibitors of the ERK, p38 MAPK, PI3K, and JNK signaling pathways. Our results demonstrated that MIS is not dependent on the p38 MAPK, PI3K, or JNK pathways, but that the ERK pathway is activated by MIS. Consistent with our findings, Renlund, et al.29 reported that MIS does not activate the JNK pathway. In addition, they identified the JNK inhibitor, SP600125, as an activator of the MIS signal transduction pathway.
Numerous case studies have demonstrated that serum MIS levels can be increased >1000-fold above the normal range without any significant adverse reactions; therefore, the therapeutic administration of MIS to cancer patients may be well tolerated.10 However, purified recombinant MIS is difficult and expensive to obtain, and the clinical use of calcitriol is limited, because of the adverse effects of hypercalcemia. Thus, several important issues remain to be resolved prior to clinical use, including indication, appropriate doses, blood concentration, adverse effects, resistance, drug interactions, and effectiveness in vivo.
Despite these issues, our findings indicate that treatment with MIS in combination with calcitriol may be an effective clinical strategy for treating ovarian cancer, since combination of the two agents enhances the anti-proliferative and apoptotic effects of each agent alone. These results, coupled with the need for a decrease in the toxic side effects of currently employed therapeutic agents, provide a strong rationale for testing the therapeutic potential of MIS, alone or in combination with calcitriol, in the treatment of OCa. Future studies should address the exact biological functions of MIS and of the extent of the MIS-stimulated anti-proliferative and apoptotic activities of calcitriol.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Berkenblit A, Cannistra SA. Advances in the management of epithelial ovarian cancer. J Reprod Med. 2005;50:426–438. [PubMed] [Google Scholar]
- 3.Salom E, Almeida Z, Mirhashemi R. Management of recurrent ovarian cancer: evidence-based decisions. Curr Opin Oncol. 2002;14:519–527. doi: 10.1097/00001622-200209000-00008. [DOI] [PubMed] [Google Scholar]
- 4.MacLaughlin DT, Teixeira J, Donahoe PK. Perspective: reproductive tract development--new discoveries and future directions. Endocrinology. 2001;142:2167–2172. doi: 10.1210/endo.142.6.8262. [DOI] [PubMed] [Google Scholar]
- 5.Coughlin JP, Donahoe PK, Budzik GP, MacLaughlin DT. Müllerian inhibiting substance blocks autophosphorylation of the EGF receptor by inhibiting tyrosine kinase. Mol Cell Endocrinol. 1987;49:75–86. doi: 10.1016/0303-7207(87)90065-7. [DOI] [PubMed] [Google Scholar]
- 6.Gupta V, Carey JL, Kawakubo H, Muzikansky A, Green JE, Donahoe PK, et al. Mullerian inhibiting substance suppresses tumor growth in the C3(1)T antigen transgenic mouse mammary carcinoma model. Proc Natl Acad Sci U S A. 2005;102:3219–3224. doi: 10.1073/pnas.0409709102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Segev DL, Ha TU, Tran TT, Kenneally M, Harkin P, Jung M, et al. Mullerian inhibiting substance inhibits breast cancer cell growth through an NFkappa B-mediated pathway. J Biol Chem. 2000;275:28371–28379. doi: 10.1074/jbc.M004554200. [DOI] [PubMed] [Google Scholar]
- 8.Renaud EJ, MacLaughlin DT, Oliva E, Rueda BR, Donahoe PK. Endometrial cancer is a receptor-mediated target for Mullerian Inhibiting Substance. Proc Natl Acad Sci U S A. 2005;102:111–116. doi: 10.1073/pnas.0407772101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barbie TU, Barbie DA, MacLaughlin DT, Maheswaran S, Donahoe PK. Mullerian Inhibiting Substance inhibits cervical cancer cell growth via a pathway involving p130 and p107. Proc Natl Acad Sci U S A. 2003;100:15601–15606. doi: 10.1073/pnas.2636900100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacLaughlin DT, Donahoe PK. Müllerian inhibiting substance/anti-Müllerian hormone: a potential therapeutic agent for human ovarian and other cancers. Future Oncol. 2010;6:391–405. doi: 10.2217/fon.09.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ponsonby AL, Lucas RM, Lewis S, Halliday J. Vitamin D status during pregnancy and aspects of offspring health. Nutrients. 2010;2:389–407. doi: 10.3390/nu2030389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giammanco M, Di Majo D, La Guardia M, Aiello S, Crescimannno M, Flandina C, et al. Vitamin D in cancer chemoprevention. Pharm Biol. 2015;53:1399–1434. doi: 10.3109/13880209.2014.988274. [DOI] [PubMed] [Google Scholar]
- 13.Malloy PJ, Peng L, Wang J, Feldman D. Interaction of the vitamin D receptor with a vitamin D response element in the Mullerian-inhibiting substance (MIS) promoter: regulation of MIS expression by calcitriol in prostate cancer cells. Endocrinology. 2009;150:1580–1587. doi: 10.1210/en.2008-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krishnan AV, Moreno J, Nonn L, Malloy P, Swami S, Peng L, et al. Novel pathways that contribute to the anti-proliferative and chemopreventive activities of calcitriol in prostate cancer. J Steroid Biochem Mol Biol. 2007;103:694–702. doi: 10.1016/j.jsbmb.2006.12.051. [DOI] [PubMed] [Google Scholar]
- 15.Hoshiya Y, Gupta V, Segev DL, Hoshiya M, Carey JL, Sasur LM, et al. Mullerian Inhibiting Substance induces NFkB signaling in breast and prostate cancer cells. Mol Cell Endocrinol. 2003;211:43–49. doi: 10.1016/j.mce.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 16.Parry RL, Chin TW, Epstein J, Hudson PL, Powell DM, Donahoe PK. Recombinant human mullerian inhibiting substance inhibits human ocular melanoma cell lines in vitro and in vivo. Cancer Res. 1992;52:1182–1186. [PubMed] [Google Scholar]
- 17.Kim HS, Sung YJ, Paik S. Cancer Cell Line Panels Empower Genomics-Based Discovery of Precision Cancer Medicine. Yonsei Med J. 2015;56:1186–1198. doi: 10.3349/ymj.2015.56.5.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Namkung J, Song JY, Jo HH, Kim MR, Lew YO, Donahoe PK, et al. Mullerian inhibiting substance induces apoptosis of human endometrial stromal cells in endometriosis. J Clin Endocrinol Metab. 2012;97:3224–3230. doi: 10.1210/jc.2012-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leyssens C, Verlinden L, Verstuyf A. Antineoplastic effects of 1,25(OH)2D3 and its analogs in breast, prostate and colorectal cancer. Endocr Relat Cancer. 2013;20:R31–R47. doi: 10.1530/ERC-12-0381. [DOI] [PubMed] [Google Scholar]
- 20.Pereira F, Larriba MJ, Muñoz A. Vitamin D and colon cancer. Endocr Relat Cancer. 2012;19:R51–R71. doi: 10.1530/ERC-11-0388. [DOI] [PubMed] [Google Scholar]
- 21.Swami S, Krishnan AV, Feldman D. Vitamin D metabolism and action in the prostate: implications for health and disease. Mol Cell Endocrinol. 2011;347:61–69. doi: 10.1016/j.mce.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krishnan AV, Feldman D. Molecular pathways mediating the antiinflammatory effects of calcitriol: implications for prostate cancer chemoprevention and treatment. Endocr Relat Cancer. 2010;17:R19–R38. doi: 10.1677/ERC-09-0139. [DOI] [PubMed] [Google Scholar]
- 23.Gocek E, Studzinski GP. Vitamin D and differentiation in cancer. Crit Rev Clin Lab Sci. 2009;46:190–209. doi: 10.1080/10408360902982128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haussler MR, Whitfield GK, Kaneko I, Haussler CA, Hsieh D, Hsieh JC, et al. Molecular mechanisms of vitamin D action. Calcif Tissue Int. 2013;92:77–98. doi: 10.1007/s00223-012-9619-0. [DOI] [PubMed] [Google Scholar]
- 25.Kasiappan R, Shen Z, Tse AK, Jinwal U, Tang J, Lungchukiet P, et al. 1,25-Dihydroxyvitamin D3 suppresses telomerase expression and human cancer growth through microRNA-498. J Biol Chem. 2012;287:41297–41309. doi: 10.1074/jbc.M112.407189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daniel C, Schroder O, Zahn N, Gaschott T, Steinhilber D, Stein JM. The TGFbeta/Smad 3-signaling pathway is involved in butyrate-mediated vitamin D receptor (VDR)-expression. J Cell Biochem. 2007;102:1420–1431. doi: 10.1002/jcb.21361. [DOI] [PubMed] [Google Scholar]
- 27.Tu H, Flanders WD, Ahearn TU, Daniel CR, Gonzalez-Feliciano AG, Long Q, et al. Effects of calcium and vitamin D3 on transforming growth factors in rectal mucosa of sporadic colorectal adenoma patients: a randomized controlled trial. Mol Carcinog. 2015;54:270–280. doi: 10.1002/mc.22096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bizzarri M, Cucina A, Valente MG, Tagliaferri F, Borrelli V, Stipa F, et al. Melatonin and vitamin D3 increase TGF-beta1 release and induce growth inhibition in breast cancer cell cultures. J Surg Res. 2003;110:332–337. doi: 10.1016/s0022-4804(03)00040-4. [DOI] [PubMed] [Google Scholar]
- 29.Renlund N, Pieretti-Vanmarcke R, O'Neill FH, Zhang L, Donahoe PK, Teixeira J. c-Jun N-terminal kinase inhibitor II (SP600125) activates Mullerian inhibiting substance type II receptor-mediated signal transduction. Endocrinology. 2008;149:108–115. doi: 10.1210/en.2007-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]